Abstract
Subjectively reported sleepiness and objectively measured vigilance are often used to assess and monitor operating performance. Evidence suggests that the response patterns of the two measures are independent of each other. However, the neural mechanism underlying this phenomenon remains unclear. This study aimed to investigate whether subjective sleepiness and objective vigilance were associated with each other. Thirty-three participants were subjected to 34 h of acute sleep deprivation. We collected sleepiness, vigilance, and resting-state fMRI data. We also located the neural mechanism of isolation of object and subject parameters. Firstly, the correlation analysis showed that there was no statistically significant correlation between the changes in vigilance and sleepiness during the sleep deprivation period. Then, implementing the support vector machine algorithm through functional connectivities as features, we found that different functional connectivity patterns underline the isolation of these two factors during sleep deprivation. The functional connectivities involved in characterizing the vulnerability of objective vigilance are more extensive, involving the connectivities within the sensorimotor network, between the subcortical and cortical network, and among multiple cortical networks. The functional connectivity involved in characterizing the vulnerability of subjective sleepiness is limited to the communication between the subcortical thalamus and the somatosensory cortex. In addition, we found that implementing global signal regression would reduce the model’s power to predict vigilance and sleepiness. This work contributes to our understanding of how sleep deprivation affects individual cognition and behavior, and will be of use in the evaluation and prediction of cognitive performance during sleep loss.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11571-021-09772-0.
Keywords: Sleep deprivation, Vulnerability, Resting-state, Vigilance, Sleepiness
Introduction
Sleep deprivation has extensive and complex effects on cognitive functions (Krause et al. 2017). ‘Dose-dependence’ is related to the duration of deprivation, while ‘form-dependence’ is related to the type of deprivation, such as acute sleep deprivation, chronic sleep deprivation, REM sleep deprivation, or NREM sleep deprivation (Rechtschaffen et al. 1999). In addition, the impact of sleep deprivation on different individuals and different cognitive tasks varies and is known as ‘individual-dependence’ and ‘cognitive domains-dependence’ (Tkachenko and Dinges 2018).
Previous studies have found that different cognitive domains have different vulnerability parameters to sleep deprivation, and this phenomenon is called sleep deprivation vulnerability. Sleep deprivation vulnerability varies between subjects but is stable within subjects, in the sense that there is a stable vulnerability parameter even under different periods of sleep deprivation. Olga Tkachenkoa and David F Dinges characterized sleep deprivation vulnerability as ‘ubiquitous and unexplained’. ‘Ubiquitous’ means that the effects of sleep deprivation cover almost all cognitive domains, and vulnerability to these effects is also different from each other. ‘Unexplained’ means that there is limited understanding of the mechanisms underlying the differences in the observed vulnerability and the issue remains controversial (Tkachenko and Dinges 2018).
Researchers usually evaluate the individual’s basic operating performance in two ways. One is to measure the individual’s level of wakefulness or sleepiness through oral reports, and the other is to measure the individual’s vigilance movement response time through behavioral tasks. Performance assessed using both methods is significantly damaged with the increase in waking time. At the same time, there are rhythmic fluctuations in performance with changes of the biological clock (Jewett et al. 1999; Krause et al. 2017). However, previous studies have found that the effects of sleep deprivation on subjectively reported sleepiness and objectively measured vigilance performance are independent of each other, that is, there is no statistically significant correlation between these parameters (Berka et al. 2005; Jewett et al. 1999; Leproult et al. 2003). Leproult et al. investigated whether the differences in subjective reported performance reflected changes in cognitive ability after sleep deprivation and found that after 27 h and 40 h of sleep deprivation, there was no significant correlation between changes in subjective performance and changes in objective performance (Leproult et al. 2003). A study of military personnel with continuous sleep deprivation for 25 h revealed that self-reported sleepiness and psychomotor vigilance performance showed different response patterns to sleep loss (Chandler et al. 2013). These studies support the conclusion that the individual’s perception of impaired subjective wakefulness has no association with impaired objective vigilance. Further investigation, using 13 neurobehavioral measures to analyze the responses from individuals exposed to 36 h of sleep deprivation on three occasions, revealed three independent orthogonal factors: the subjective assessment of sleepiness; cognitive processing; and objective vigilance (Van Dongen et al. 2004). These three independent factors have different patterns of response to sleep deprivation.
Although previous studies have found that sleep deprivation vulnerability of subjective sleepiness and objective vigilance are independent of each other, the underlying mechanism remains unclear. This study used 34-h sleep deprivation and multi-time sampling to determine how sleep deprivation affects subjective sleepiness and objective vigilance and whether these two measures co-vary within an individual. Resting-state functional connectivity functional magnetic resonance imaging (rsfc-fMRI) has been used to investigate the neural mechanism underlying the lack of association between subjective sleepiness and objective vigilance in the context of sleep deprivation. Considering the complex interactions of functional brain networks that are involved in psychological behavior characteristics (Liegeois et al. 2019), this study did not use the traditional approach of mass univariate analysis; rather, it adopted the multivariate machine learning method. By implementing a machine learning model that uses functional connectivity as feature values, we aimed to predict the impairment on subjective sleepiness and objective vigilance caused by sleep deprivation and locate the most predictive connectivities. We hypothesized that the functional connectivities involved in characterizing vulnerability of subjective sleepiness and objective vigilance are different. This research will contribute to our understanding of the mechanisms underlying the effects of sleep deprivation on cognition and behavior and has practical significance for the evaluation and prediction of work performance under prolonged wakefulness.
Methods
Participants
Participants were recruited from college students in Dalian, China, mainly through advertisements on campus. The experiment was approved by the Ethics Committee of Southwest University. The inclusion criteria were as follows: Native Chinese speakers; normal or corrected vision; sleep on a regular schedule in the month preceding the study; no history of brain trauma; no history of mental illness; no tobacco or alcohol abuse; no metal implants in the body; no other Magnetic Resonance Imaging (MRI) contraindications.
A total of 33 participants (19 women; overall average age, 21.15 years) participated in the sleep deprivation experiment. Two participants interrupted the experiment and left the laboratory due to physical discomfort. Each participant received a monetary reward of 200–360 RMB after the experiment.
Protocol
Participants were required to attend two visits to the laboratory with a one-week interval between visits, and to ban caffeine and alcohol intake 24 h before arriving in the laboratory. To avoid potential sequence effects, the sequence of the two visits to the laboratory was counter-balanced. Participants were randomly divided into two groups. One group completed the ‘well-rested’ experiment session first, followed by the ‘sleep deprivation’ session. The other group completed the ‘sleep deprivation’ part first, followed by the ‘well-rested’ session, with an interval of approximately one week (Fig. 1). The one-week interval was set to avoid the impact of sleep debt caused by deprivation on baseline measurements. The participants were required to fill in a sleep diary every day during the week before the experiment and the interval between two visits to the laboratory. This information was only used to confirm participant sleep status before the experiment, and was not used in the subsequent analysis.
Fig. 1.
The protocol of this study. ARSQ Amsterdam Resting-State Questionnaire, PVT psychomotor vigilance test. In order to avoid potential sequence effects, the sequence of the two visits to the laboratory was counter-balanced. One week interval was set to avoid the impact of sleep debt caused by deprivation on baseline measurement
When conducting the well-rested experiment, participants were required to sleep for more than 7 h the night before the experiment and arrive at the laboratory at 08:00 h. Participants were required to complete the following tests: the Amsterdam Resting-State Questionnaire (ARSQ, Diaz et al. 2013), the psychomotor vigilance test (PVT, Dinges et al. 1997), and the MRI scan. The PVT is a 10-min standard version based on E-prime 2.0, with an inter-stimulus interval of 2–10 s. The MRI scan includes three neuroimaging modalities: structural imaging scan, resting-state functional imaging scan, and diffusion-weighted imaging scan. Measures were taken to prevent the participant from falling asleep during scanning. First, we talked to the participant during the scanning intervals to ensure that he/she was not asleep and reminded the participant to keep his/her eyes open. Second, we required verbal confirmation from the participant that he/she was awake in the scanner after completion of the scanning. Last, task-based sessions requiring keypress feedback from participants were conducted while scanning. We did not describe these sessions in the methods section because this part of the task is not within the scope of this study. By checking the keystrokes and task performance records, we could assess the participant's waking state.
A subsequent visit required participants to arrive before 20:00 h, with the sleep deprivation monitoring session commencing at approximately 22:00 h. Therefore, this experiment required approximately a total of 34 h for each participant, including 12 h of awake time during the day. Participants were required to maintain their regular sleep schedule without napping during the day. After arrival at the laboratory, assistants involved in the experiments monitored participants until the completion of the experiment. The laboratory performed a sunlight-shading treatment for the entire duration of the experiment. The indoor light intensity remained constant (~ 100 lx). Participants were allowed to perform many relaxation activities, such as listening to music, reading, and watching movies; but they were not allowed to perform strenuous exercises or play video games. At eight fixed time points (TP), within 24 h, participants were required to cooperate to complete the PVT test. At 09:00 h and 17:00 h on the experiment day, participants were required to complete the ARSQ and undergo an MRI scan (consistent with the well-rested session). Participants were free to leave the premises after the MRI scan. No monitored restorative sleep was arranged due to limited laboratory conditions. The detailed experimental protocol is presented in Fig. 1.
MRI acquisition
The MRI images were acquired by the General Electric (GE) DISCOVERY MR750 3.0 Tesla scanner with an 8-channel head coil. The participant’s head was fixed with a sponge to reduce any possible movement. Noise-reducing earplugs and headphones were worn to minimize noise interference during scanner operation. The resting-state images were acquired with the Echo Planar Imaging (EPI) sequence. The scanning acquisition parameters were as follows: repetition time (TR) = 2000 ms; echo time (TE) = 30 ms, flip angle = 90°, acquisition matrix = 64 × 64, in-plane resolution = 3.0 * 3.0 mm2, field of view (FOV) = 240 × 240 mm2, number of slices = 33 (interval scanning from the bottom), thickness = 3.5 mm, gap = 0.7 mm, and scanning time = 410 s. A total of 205 images were acquired. Participants was required to keep their eyes open and look at the white cross (the background color was black) in the center of the screen, and not to deliberately carry out systematic thinking. Subsequently, high-resolution T1-weighted image data was acquired through the gradient-echo (GR) sequence, which was applied to the resting-state image space preprocessing. The scanning acquisition parameters were as follows: TR/TE = 6.6/2.9 ms, FOV = 240 × 240 mm2, flip angle = 12°, acquisition matrix = 256 × 256 mm2, thickness/gap = 1/0 mm, slices = 192, and scanning time = 5 min. Diffusion-weighted images were not within the scope of this study.
Resting-state images analysis
Resting-state fMRI data analysis was performed using the functional connectivity toolbox (Whitfield-Gabrieli and Nieto-Castanon 2012), usually abbreviated as Conn. The processing flow included preprocessing, denoising, and functional connectivity calculation (Fig. 2). Of the 33 participants, two participants withdrew from the experiment, and in one case the MRI images were damaged, so the final analysis included data from the remaining 30 participants.
Fig. 2.
Data analysis pipelines. Significant connectivities were determined through F-test, then changes of these connectivities were extracted to be features to predict matched changes of each behavior indicator separately. Machine learning regression models used support vector machines based on the linear kernel function. An additional pipeline with GSR was used for cross-validation. + represents the day after one night of sleep deprivation. ROI region of interest; GSR global signal regression
Data preprocessing included image format conversion, data scrubbing, slice timing correction, motion correction, and spatial normalization. Spatial smoothing was not performed to avoid potential signal contamination from nearby regions when extracting ROI BOLD signals. Data preprocessing was performed as follows. (1) Image format conversion: the dicom2niix software (Li et al. 2016) was used to convert the images acquired by the scanner in Dicom format to the Nifti format. (2) Scrubbing: the first 5 bold images were scrubbed to avoid magnetic field instability; this involves establishing 5 regressors (box function) for the first 5 time points and adding these regressors to the linear model as confounds in the subsequent denoising process. (3) Slice timing correction: slice timing correction could correct signal changes caused by sampling time differences. (4) Motion correction: rigid body transformation was used to align each volume with the reference image and obtain six head motion parameters, including three translation parameters and three rotation parameters. The volume obtained from the first TR was selected as the reference image. (5) Spatial normalization: the high-resolution T1-weighted image for each participant was used to normalize the resting-state images to the standardized Montreal Neurological Institute (MNI) image space by nonlinear alignment.
The exclusion standard for the head movement was set to 2 mm or 2°. No participant was excluded because the maximum head movement of all participants was only 1.54 mm.
Denoising was performed after preprocessing. Denoising aims to remove noise from the signal, enhance the signal-to-noise ratio, and improve the statistical effectiveness of subsequent analysis. This includes the following steps: (1) Motion control: six head movement parameters and their first derivatives were regressed out. (2) aCompCor: aCompCor implements principal component analysis (PCA) to extract the first five principal components from white matter and cerebrospinal fluid signals, respectively, and add them to the linear model as confounds, which can effectively remove noise, such as head movement, respiration, and cardiac pulse (Behzadi et al. 2007; Muschelli et al. 2014). The bold signal was extracted from the eroded white matter and cerebrospinal fluid mask to avoid mixing with gray matter signals. Without erosion, the mixing of gray matter signals will produce an effect similar to the global signal regression (GSR) (Power et al. 2017). (3) Artifact detection: Artifact Detection Tools (ART, http://web.mit.edu/swg/software.htm) was used to detect outliers in bold signal and head movements. The detection threshold was defined as a scan-to-scan mean global signal change exceeding 5 standard deviations, or scan-to-scan composite motion exceeding 0.9 mm (composite motion describes the maximum motion of any voxel within the bounding box of the brain). (4) Detrending: was used to remove the linear trend in the bold signal. (5) Bandpass filter: the band was set to 0.008–0.09 Hz to remove low frequency and high frequency noise.
The brain functional atlas used in functional connectivity calculation contains 264 regions of interest (ROIs). The radius of each ROI was defined as 5 mm. This atlas was selected because it used the functional organization information and anatomical information of the brain comprehensively to determine the coordinates and attribution network of 264 ROIs (Power et al. 2011). These ROIs were partitioned into ten brain networks, namely the default mode network (DMN, 58 ROIs), sensorimotor network (SMN, 35 ROIs), visual network (VN, 31 ROIs), frontoparietal task control network (FPN, 24 ROIs), saliency network (SN, 18 ROIs), cingulo-opercular task control network (CON, 14 ROIs), auditory network (AN, 13 ROIs), subcortical network (Sub, 13 ROIs), dorsal attention network (DAN, 11 ROIs), ventral attention network (VAN, 9 ROIs), and uncertain regions (UN, 38 ROIs). Subsequently, the signal of each ROI was obtained by extracting the first principal component after PCA decomposition. Finally, based on the extracted ROI signals, the ROI-ROI bivariate correlation was calculated by the weighted general linear model. After Fisher-Z transformation, a functional connectivity matrix of size 264 × 264 was obtained.
GSR could cause distortion of connectivity values and negative deviations of correlation structures (Murphy et al. 2009), although GSR could also improve denoising performance (Power et al. 2015) and enhance the association between behavioral measures and resting-state functional connectivity (Li et al. 2019). Therefore, an additional pipeline with GSR was added to cross-validate the results of this analysis, while other analysis details remained consistent (Fig. 2).
Behavior measures
Of the 30 participants with valid fMRI data, 7 participants had missing time points for PVT and ARSQ data. Therefore, only 23 participants had matched full data.
PVT (Dinges et al. 1997) is a popular paradigm used to test an individual’s vigilance. Reaction time (RT) was used to characterize vigilance in this study. RT was calculated using the median of the reaction times of valid trials, which has RT between 100 and 500 ms.
ARSQ was used to measure resting-state thinking (Diaz et al. 2013), with a score of 0 to 4 for each item. This study used the first version of ARSQ, which covers seven dimensions: (1) discontinuity of mind, (2) theory of mind, (3) self, (4) planning, (5) sleepiness, (6) comfort, and (7) somatic awareness. Participants completed the questionnaire outside the MRI cabin. The instructions were consistent with the instructions for the resting-state scan. After 5 min of rest, participants completed the ARSQ based on their feelings during the rest-activity. The ASRQ sleepiness dimension score was adopted in this study because, compared with other scales, it can better match the brain state during resting state scanning. The sleepiness dimension scores range from 0 to 12, the higher the score, the higher the degree of sleepiness. The corresponding items of the sleepiness dimension are attached in the Supplementary materials.
First, for each indicator obtained from PVT and ARSQ, one-way repeated measures analysis of variance (ANOVA) was performed to test whether the indicator changed significantly during the 34 h of sleep deprivation. Post-hoc multiple comparisons were then performed to test whether there were significant differences between each time point.
Subsequently, correlation analysis was performed to test whether there was a correlation between the changes of sleepiness and vigilance caused by sleep deprivation, that is, whether the sleep deprivation vulnerability of sleepiness was associated with the vulnerability of vigilance. Specifically, Pearson correlation was carried out separately in two conditions (B-A and C-B), that is, from 09:00 h on the day after sleep deprivation (time point B) to well-rested (time point A), and from 17:00 h on the day after sleep deprivation (time point C) to 09:00 h on the day after sleep deprivation (time point B).
Support vector machine (SVM)
Next, a statistical learning model was built to use functional connectivity values to predict the changes in vigilance and sleepiness during sleep deprivation. First, we needed to enter valid features to be incorporated into the model. Specifically, the values that the model needed to output or predict were the changes in each behavior indicator for two conditions (B minus A and C minus B). The input features of the model were the changes of the functional connectivity that contains valid information in the corresponding conditions. The criterion for containing valid information is whether the functional connectivity has changed significantly between the three time points of resting-state scanning after the F-test (contrast matrix in three time points: [− 1, 1, 0; 0, − 1, 1], see Fig. 2). As this step was mainly performed to select effective features, a loose multiple comparison strategy was adopted, that is, p < 0.05 after false discover rate (FDR) correction.
After performing the F-test to obtain the significant functional connectivities, the changes of these functional connectivities were extracted in the corresponding period and passed as training features to the SVM for prediction. The two time periods were multiplied by 23 participants, resulting in a sample size of 46.
The machine learning algorithm used in this research was the Support Vector Machine. SVM has the advantages of clear theoretical assumptions, rapid convergence, and decent performance in small samples, so it has been widely used in neuroscience. The linear kernel regression model was selected based on the Libsvm toolbox Version 3.23 (Chang and Lin 2011). Recent work found that the linear model could give the best predictions for functional connectome-based predictive models for resting-state fMRI (Dadi et al. 2019). In general, the sample size of this model was 46 (23 participants × 2 time periods), the features were the changes of functional connectivities between the three time points of resting-state scanning, and the data to fit and predict was the changes of sleepiness and vigilance during the matching period. One model was generated for each behavior outcome (objective vigilance and subjective sleepiness, respectively), so a total of two models were created in the analysis. Leave-one-out cross-validation was performed, that is, 46-fold cross-validation. The explanation coefficient (R2) was used to evaluate the performance of this model. No specific hyperparameter tuning was performed on SVM models.
Due to the complex characteristics of machine learning algorithms and data-driven characteristics, interpretation of the results and determination and filtering of effective features requires caution. To establish a threshold for the initial model weights to obtain significant predictive connectivities, we used a method based on obtaining the maximum statistics from the zero-distribution generated by the permutation test (Nichols and Hayasaka 2003). However, because the sample size was relatively limited (46 samples in this study), the weights of the model obtained may have a large variance. To identify the most predictive and stable connectivity, a permutation test combined with bootstrapping was adopted (Milazzo et al. 2016; Ng et al. 2014). The method was introduced as follows: when predicting a certain behavior indicator, such as sleepiness score, this indicator was first randomly permutated 10,000 times. Subsequently, in each permutation, we sampled 1000 bootstrap samples and applied SVM for each sample. Then, the normalized mean weight of each feature obtained by 1000 bootstrap samples was calculated. The maximum weight among these samples was stored as the weight obtained by this permutation. A total of 10,000 maximum weights were obtained to generate the adjusted zero-distribution, while the distribution of the minimum weight was also obtained through the same method. Statistical significance was set at two-tailed p < 0.05.
Results
Demographic information
The demographic information of the 33 participants (including 19 women with an average age of 21.15 years) participating in this experiment is shown in Table 1. Participants' SDS scores were not above 63 (this value is generally used as a cut-off for moderate depression), and their total PSQI scores were not above 7 (this value is generally used as a criterion to exclude participants with poor sleep quality). The MEQ scores of participants in this experiment were all in the range of 30–70.
Table 1.
Demographic variables of participants (N = 33)
| Variable | Mean | Std | Range |
|---|---|---|---|
| Age | 21.15 | 3.32 | 18–30 |
| BMI | 21.92 | 2.42 | 18.29–27.70 |
| MEQ | 50.17 | 7.65 | 36–65 |
| PSQI | 3.88 | 1.80 | 1–7 |
| SDS | 41.29 | 7.68 | 25–62 |
BMI Body Mass Index, MEQ Morningness-Eveningness Questionnaire, PSQI Pittsburgh Sleep Quality Index, SDS Self-Rating Depression Scale
Behavior measures
For vigilance, significant change was found in reaction time (F = 9.272, p < 0.0001, df = 8) through one-way ANOVA. For sleepiness, the score of the sleepiness dimension in ARSQ changed significantly (F = 35.74, p < 0.0001, df = 2). The results are presented in Table S1 and Figure S1.
For other dimensions of the ARSQ scale, significant changes were found in the score of the planning (F = 6.537, p = 0.0044, df = 2) and comfort (F = 11.91, p < 0.0001, df = 2) dimensions. In the other four dimensions (including discontinuity of mind, theory of mind, somatic awareness, and self), no significant F-effect was found. Full results of one-way repeated measures ANOVA are presented in Table S1. Please refer to the supplementary materials for further post-hoc multiple comparison results for indicators of interest (Table S2). The Bonferroni multiple comparison correction was used.
Detailed, participants’ vigilance performance reached its lowest point at 07:00 h the next morning. During the following day, participants’ vigilance showed a U-shaped trend of recovery first and deterioration then (Figure S1).
In the following correlation analysis, no significant correlation was found between changes in the sleepiness score and changes in reaction time for the two conditions (Fig. 3), i.e., 09:00 h on the day after sleep deprivation to well-rested (condition B–A, r = 0.0319, p = 0.3789, df = 22), and 17:00 h on the day after sleep deprivation to 09:00 h on the day after sleep deprivation (condition C–B, r = 0.3789, p = 0.0746, df = 22). In addition, we used another wide-accepted metric of PVT paradigm, lapses, to perform the same analysis. Lapses were calculated using the number of trials from 500 to 3000 ms (trials exceeding 3000 ms were considered invalid). We obtained comparable results (condition B–A, r = 0.3789, p = 0.0746, df = 22; condition C–B, r = 0.0186, p = 0.9330, df = 22; Figure S2). No multiple comparison correction was used.
Fig. 3.
Main results of behavioral measures. A Post-hoc multiple comparison results of reaction time (ms) at three main time points. B Post-hoc multiple comparison results of sleepiness scores of ARSQ. C Changes of reaction time (ms) between three time points. D Changes of sleepiness score between three time points. The red line represents the mean value. To highlight the deficits of reaction, scores of the reference time are all fixed at zero (cyan square). E Correlation result of the time period between well-rested and 9:00 on the day after sleep deprivation. F, Correlation result of the time period between 9:00 on the day after sleep deprivation and 17:00 on the day after sleep deprivation. No significant correlation result was found even under no multiple comparison correction. Note + represents the day after one night of sleep deprivation; *, **, ***represent p < 0.05, p < 0.01, p < 0.001 separately. PVT Psychomotor Vigilance Test, ARSQ Amsterdam Resting-State Questionnaire
SVM
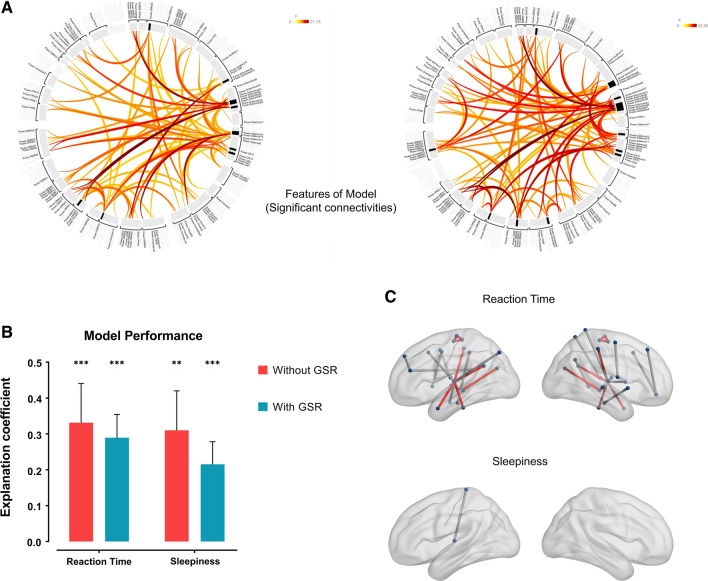
The model achieved a decent performance in predicting reaction time of PVT (R2 = 0.3313, p = 0.0004). A similar performance was shown in predicting sleepiness score of ARSQ (R2 = 0.3101, p = 0.0001).
In the pipeline with GSR for validation, model performance was slightly worse than without GSR. Despite the models still performed significantly in predicting reaction time of PVT (R2 = 0.2895, p = 0.0014) and sleepiness score of ARSQ (R2 = 0.2153, p = 0.0004). All the statistical results in this part are generated by the permutation test (Fig. 4).
Fig. 4.
Results of the prediction model. A The features of the SVM model, which were significant connectivities after FDR p < 0.05 through the F-test. The left graph represents results without GSR (55 connectivities), while the right graph represents results with GSR (62 connectivities). B Explanation coefficient of the model. The primary model had achieved a decent performance in predicting reaction time of PVT (R2 = 0.3313, p = 0.0004) and sleepiness score of ARSQ (R2 = 0.3101, p = 0.0001). In the pipeline with GSR, model performance was slightly worse for reaction time (R2 = 0.2895, p = 0.0014) and sleepiness (R2 = 0.2153, p = 0.0004). The vertical line represents the standard deviation. ** and *** represents p < 0.01 and 0.001 separately. All the statistical results in this part are generated by the permutation test. C Significant connectivities to predict reaction time and sleepiness. Red lines represent overlapped results both in the pipeline without GSR and the pipeline with GSR. The left brain represents results without GSR, while the right-brain represents results with GSR. There is no significant connectivity to predict sleepiness score in the pipeline with GSR
Significant predictive connectivity
Overall, 55 connectivities were found to be significant after FDR correction through the F-test to be passed to the model as features. After permutation test with bootstrapping, 18 connectivities were found to significantly predict reaction time, while only 1 connectivity was found to significantly predict sleepiness. For the pipeline with GSR, 62 connectivities were found to be significant in the F-test to be passed to the model as features. After permutation test with bootstrapping, 21 connectivities were found to significantly predict reaction time y (Table S3), while 9 of these coincided with the results of the pipeline without GSR. No connectivity was found to significantly predict sleepiness. See Fig. 4 and Table 2 for details.
Table 2.
Functional connectivities with significant predictive power in the primary model
| Indicator | ROI 1 | ROI 2 | Weight | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Coordinates (mm) | Anatomical Label | Network | Coordinates (mm) | Anatomical Label | Network | ||||||
| Reaction time | − 7 | − 21 | 65 | Paracentral Lobule L | SMN | 2 | − 28 | 60 | Paracentral Lobule R | SMN | 3.0829 |
| − 7 | − 21 | 65 | Paracentral Lobule L | SMN | 3 | − 17 | 58 | Supp Motor Area R | SMN | 3.3326 | |
| 2 | − 28 | 60 | Paracentral Lobule R | SMN | 3 | − 17 | 58 | Supp Motor Area R | SMN | 2.2871 | |
| − 20 | 45 | 39 | Superior Frontal L | DMN | − 42 | 38 | 21 | Middle Frontal L | FPN | 2.4380 | |
| 42 | 0 | 47 | Precentral R | SN | − 2 | − 13 | 12 | Thalamus L | Sub | 3.0550 | |
| − 39 | − 75 | 44 | Angular L | DMN | − 10 | − 18 | 7 | Thalamus L | Sub | 2.5139 | |
| − 7 | − 55 | 27 | Precuneus L | DMN | − 10 | − 18 | 7 | Thalamus L | Sub | 3.1485 | |
| − 10 | − 18 | 7 | Thalamus L | Sub | 12 | − 17 | 8 | Thalamus R | Sub | 5.0652 | |
| 6 | − 72 | 24 | Cuneus R | VN | − 5 | − 28 | − 4 | Undefined | Sub | 3.6051 | |
| − 21 | − 22 | − 20 | Undefined | Un | − 2 | − 37 | 44 | Middle Cingulum L | DMN | − 3.5860 | |
| − 37 | − 29 | − 26 | Fusiform L | Un | − 10 | − 18 | 7 | Thalamus L | Sub | − 3.1483 | |
| 65 | − 12 | − 19 | Middle Temporal R | DMN | − 10 | − 18 | 7 | Thalamus L | Sub | − 2.3006 | |
| − 53 | 3 | − 27 | Middle Temporal L | DMN | − 10 | − 18 | 7 | Thalamus L | Sub | − 2.4742 | |
| − 1 | 15 | 44 | Supp Motor Area L | SN | − 10 | − 18 | 7 | Thalamus L | Sub | − 2.4733 | |
| 10 | − 2 | 45 | Supp Motor Area R | SMN | 23 | 10 | 1 | Putamen R | Sub | − 2.3158 | |
| − 42 | 38 | 21 | Middle Frontal L | FPN | 54 | − 43 | 22 | Superior Temporal R | VAN | − 4.7612 | |
| 31 | − 14 | 2 | Putamen R | Sub | 51 | − 29 | − 4 | Middle Temporal R | VAN | − 3.5644 | |
| − 10 | − 18 | 7 | Thalamus L | Sub | 47 | − 30 | 49 | Postcentral R | SMN | − 3.2523 | |
| Sleepiness | − 7 | − 33 | 72 | Paracentral Lobule L | SMN | − 10 | − 18 | 7 | Thalamus L | Sub | − 2.7996 |
The results in this table were from the pipeline without GSR. The coordinates are based on MNI standard space. SMN: Sensory/somatomotor network; FPN: Fronto-parietal network; DMN: Default mode network; SN: Salience network; Sub: Subcortical network; Un: Uncertain; VAN: Ventral attention network; VN: Visual network; L: Left brain; R: Right brain; Supp: Supplementary. Anatomical location referenced from the Automated Anatomical Labeling (AAL) template. Network partition was based on power (2011)
Discussion
We measured participant deficits of objective vigilance and subjective-reported sleepiness over approximately 34 h of sleep deprivation. Although similar fluctuation trends were found at the group level, the subsequent correlation analysis did not find any significant correlation between the deficits of the two measures during sleep deprivation. This further validates the findings of previous studies, that sleep deprivation vulnerability of objective vigilance and subjective sleepiness are independent of each other. Subsequently, through the method of multivariate machine learning, we used functional connectivity to predict the deficits of vigilance and sleepiness during sleep deprivation and found that there are different connectivity representations behind the two measures. The functional connectivities involved in characterizing the vulnerability of objective vigilance include the connectivities within the SMN network, between the subcortical and cortical network, and among multiple cortical networks. Only one connectivity between the subcortical network (thalamus) and the SMN network (paracentral lobule) was found to significantly predict sleepiness.
Deficits of vigilance after sleep deprivation
From the recording after the ‘well-rested’ session, the reaction time of PVT continued to increase with the accumulation of sleep pressure. At 07:00 h the next morning, participant vigilance dropped to a minimum and then began to rebound. After 15:00 h, participants' vigilance began to decline again. The findings are consistent with previous studies (Krause et al. 2017). As the duration of wakefulness increases, the individual's vigilance gradually decreases, but this process is not completely linear and exhibits rhythmic characteristics. It is generally believed that in the case of total sleep deprivation of no longer than 36 h, the individual's vigilance reaches the lowest level around dawn of the next day and recovers after sunrise. After lunch, the individual's vigilance will be further impaired (Muto et al. 2016).
In the subsequent analysis, SVM was used to predict individual vigilance and a decent predictive performance was achieved. After permutation test with bootstrapping, 18 functional connectivities, involving 7 functional networks, were found to be significantly predictive of the deficits of vigilance, which indicates that the deficits of vigilance in sleep deprivation may be a complicated process and involve interaction between multiple brain networks.
PVT contains three main components, encoding time, decision time, and response execution time. Previous studies have found that after one night of TSD, the increase in RT mainly occurs in non-decision time, that is, stimulation coding and exercise execution. It may be the reason why multiple brain networks are involved in PVT deficits (Patanaik et al. 2014).
Aggravation of sleepiness after sleep deprivation
Through the sleepiness scores at the three time points obtained from the ARSQ scale, it can be seen that the average sleepiness score measured in the morning when participants first arrived in the laboratory was the lowest. In the corresponding time point of the next day, the sleepiness score increased significantly. Although the sleepiness score obtained in the last measurement further increased, there was no statistically significant difference between the sleepiness score measured on the morning after sleep deprivation and at night after sleep deprivation (Table S1).
Only one connectivity between the left paracentral lobule of somatosensory network and left thalamus of subcortical network survived after the statistical analysis, which contributed the most predictive power, indicating that the neural mechanism of the aggravation of subjective-reported sleepiness after sleep deprivation is relatively simple, mainly involving the processing of somatosensory information and the subcortical arousal system. The paracentral lobule lies between the precentral and postcentral gyrus, which controls the motor and sensory functions (Spasojevic et al. 2013). The thalamus controls basic wakefulness level and swift of sleep–wake states, which is a crucial brain region that drives the subcortical system to wake up the cortical cerebral (Gent et al. 2018; Sherman 2001).
Differential sleep deprivation vulnerability of vigilance and sleepiness
Although at the group level, sleep deprivation has similar effects on vigilance and sleepiness, there is no significant correlation between the deficits of individual subjective sleepiness and objective vigilance. Sleep deprivation vulnerability of subjective sleepiness and objective vigilance are independent of each other. This indicates that there are different underlying neural mechanisms.
Both sleepiness and vigilance are affected by sleep pressure and circadian rhythm (Goel et al. 2013; Van Dongen and Dinges 2000), but the fact that sleep deprivation vulnerability is independent indicates that the degree of individual vigilance and sleepiness affected by the dual processes may not be consistent. Individual subjective sleepiness is sensitive to the accumulation of sleep pressure, which does not mean that objective vigilance is also sensitive to the accumulation of sleep pressure. The sensitivity of the two processes may be independent of each other.
From the perspective of the neurophysiological mechanisms, sleep pressure and biological rhythms both have an impact on the functional connectivity of the brain, but different connectivities have different degrees of sensitivity to the dual process of sleep (Blautzik et al. 2013; Samann et al. 2010). The connectivity patterns involved in characterizing deficits of sleepiness and vigilance after sleep deprivation are different, explaining the independence of sleep deprivation vulnerability of vigilance and sleepiness.
Global signal regression
We speculate that there are two possible reasons for the decreased performance in predicting vigilance and sleepiness after GSR. First, the disbenefits caused by the distortion of the correlation structure may outweigh the benefits of noise control, thereby reducing the performance of the model. The second possible reason is that the global signal contains valuable information including vigilance (Huang et al. 2019; Wong et al. 2013), and the loss of information after regression resulted in a decline in prediction performance.
Limitations
This study has the following limitations. First, the experimental sample size is small. Of the 33 participants that participated in the experiment, complete data from only 23 participants were available for analysis. However, the within-subject design and repeated measurement, covering multiple time points, compensated for this problem to some extent, coupled with the strict multiple comparison methods. Second, the features’ dimension of the validation model was different from the primary model. The reason for this is that the number of dimensions was controlled within a reasonable range, and the dimensions are relatively close (55 in the primary model VS 62 in the validation model). In this study, we maintained the same analysis details of the validation process except for GSR, so we did not force intervention in the process to keep the dimension of features consistent. Third, the samples were derived from the corresponding changes involving the same participants in two chronological periods, so there may be a problem with the non-independent sample. However, the sample covering non-overlapping two time periods can enrich the information about the model. This is a trade-off to improve the generalization of the model and avoid overfitting. Fourth, it is difficult to separate the effects of the sleep pressure process and circadian process through the protocol of this research. The specific impact on each process of behavior and functional connectivity requires further delicate research in the future. Last, there is a lack of some more real-time ways to monitor the participants' wakefulness. The application of monitoring equipment such as eye tracker will help improve the effectiveness of sleep deprivation studies in a more objective and immediate way in the future.
Conclusion
This research confirms that sleep deprivation vulnerability of objective vigilance and subjective sleepiness are independent of each other, because the underlying functional connectivities are different. The functional connectivities involved in characterizing the vulnerability of objective vigilance include the connectivities within the SMN network, those between the subcortical and cortical network, and those among multiple cortical networks. The functional connectivity involved in characterizing the vulnerability of subjective sleepiness is limited to the communication between the subcortical thalamus and the somatosensory cortex. In addition, we found that implementing global signal regression would reduce the model’s power to predict vigilance and sleepiness. This work contributes to our understanding of the mechanisms of how sleep deprivation affects an individual’s cognition and behavior, and demonstrates that it is important to select the appropriate measurement method when evaluating operating performance. Furthermore, when using the resting-state to study other cognitive functions, we can predict the fluctuations of sleepiness and vigilance and study their impact on the research topic or isolate these effects as confounding factors.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This research was supported by grants from the National Nature Science Foundation of China (31971028).
Author contributions
CX conceived and planned the experiments with input from XL. YT and CX carried out the experiment. YT designed and performed the analysis. YT wrote the manuscript with support from CX and XL. XL supervised the project.
Funding
National Natural Science Foundation of China (31971028).
Data and code available statement
The code is available at https://github.com/WolkeTian/sleep_deprivation_Vigilance. The data is available from the corresponding author upon request.
Declarations
Conflict of interest
The authors declared that they have no conflicts of interest in this work.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37(1):90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berka C, Levendowski D, Westbrook P, Davis G, Lumicao M, Olmstead R, Popovic M, Zivkovic V, Ramsey C (2005) EEG quantification of alertness: methods for early identification of individuals most susceptible to sleep deprivation, vol 5797. SPIE. 10.1117/12.597503
- Blautzik J, Vetter C, Peres I, Gutyrchik E, Keeser D, Berman A, Kirsch V, Mueller S, Poppel E, Reiser M, Roenneberg T, Meindl T. Classifying fMRI-derived resting-state connectivity patterns according to their daily rhythmicity. Neuroimage. 2013;71:298–306. doi: 10.1016/j.neuroimage.2012.08.010. [DOI] [PubMed] [Google Scholar]
- Chandler JF, Arnold RD, Phillips JB, Turnmire AE. Predicting individual differences in response to sleep loss: application of current techniques. Aviat Space Environ Med. 2013;84(9):927–937. doi: 10.3357/asem.3581.2013. [DOI] [PubMed] [Google Scholar]
- Chang C-C, Lin C-J. Libsvm. ACM Trans Intell Syst Technol. 2011;2(3):1–27. doi: 10.1145/1961189.1961199. [DOI] [Google Scholar]
- Dadi K, Rahim M, Abraham A, Chyzhyk D, Milham M, Thirion B, Varoquaux G. Benchmarking functional connectome-based predictive models for resting-state fMRI. Neuroimage. 2019;192:115–134. doi: 10.1016/j.neuroimage.2019.02.062. [DOI] [PubMed] [Google Scholar]
- Diaz BA, Van Der Sluis S, Moens S, Benjamins JS, Migliorati F, Stoffers D, Den Braber A, Poil SS, Hardstone R, Van't Ent D, Boomsma DI, De Geus E, Mansvelder HD, Van Someren EJ, Linkenkaer-Hansen K. The Amsterdam Resting-State Questionnaire reveals multiple phenotypes of resting-state cognition [Original Research] Front Hum Neurosci. 2013;7(446):446. doi: 10.3389/fnhum.2013.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinges DF, Pack F, Williams K, Gillen KA, Powell JW, Ott GE, Aptowicz C, Pack AI. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4–5 hours per night. Sleep. 1997;20(4):267–277. doi: 10.1093/sleep/20.4.267. [DOI] [PubMed] [Google Scholar]
- Gent TC, Bandarabadi M, Herrera CG, Adamantidis AR. Thalamic dual control of sleep and wakefulness. Nat Neurosci. 2018;21(7):974–984. doi: 10.1038/s41593-018-0164-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel N, Basner M, Rao H, Dinges DF. Circadian rhythms, sleep deprivation, and human performance. Prog Mol Biol Transl Sci. 2013;119:155–190. doi: 10.1016/B978-0-12-396971-2.00007-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Long Z, Lei X. Electrophysiological signatures of the resting-state fMRI global signal: a simultaneous EEG-fMRI study. J Neurosci Methods. 2019;311:351–359. doi: 10.1016/j.jneumeth.2018.09.017. [DOI] [PubMed] [Google Scholar]
- Jewett ME, Dijk DJ, Kronauer RE, Dinges DF. Dose-response relationship between sleep duration and human psychomotor vigilance and subjective alertness. Sleep. 1999;22(2):171–179. doi: 10.1093/sleep/22.2.171. [DOI] [PubMed] [Google Scholar]
- Krause AJ, Simon EB, Mander BA, Greer SM, Saletin JM, Goldstein-Piekarski AN, Walker MP. The sleep-deprived human brain [Review Article] Nat Rev Neurosci. 2017;18(7):404–418. doi: 10.1038/nrn.2017.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leproult R, Colecchia EF, Berardi AM, Stickgold R, Kosslyn SM, Van Cauter E. Individual differences in subjective and objective alertness during sleep deprivation are stable and unrelated. Am J Physiol Regul Integr Comp Physiol. 2003;284(2):R280–290. doi: 10.1152/ajpregu.00197.2002. [DOI] [PubMed] [Google Scholar]
- Li X, Morgan PS, Ashburner J, Smith J, Rorden C. The first step for neuroimaging data analysis: DICOM to NIfTI conversion. J Neurosci Methods. 2016;264:47–56. doi: 10.1016/j.jneumeth.2016.03.001. [DOI] [PubMed] [Google Scholar]
- Li J, Kong R, Liegeois R, Orban C, Tan Y, Sun N, Holmes AJ, Sabuncu MR, Ge T, Yeo BTT. Global signal regression strengthens association between resting-state functional connectivity and behavior. Neuroimage. 2019;196:126–141. doi: 10.1016/j.neuroimage.2019.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liegeois R, Li J, Kong R, Orban C, Van De Ville D, Ge T, Sabuncu MR, Yeo BTT. Resting brain dynamics at different timescales capture distinct aspects of human behavior. Nat Commun. 2019;10(1):2317. doi: 10.1038/s41467-019-10317-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milazzo AC, Ng B, Jiang H, Shirer W, Varoquaux G, Poline JB, Thirion B, Greicius MD. Identification of mood-relevant brain connections using a continuous, subject-driven rumination paradigm. Cereb Cortex. 2016;26(3):933–942. doi: 10.1093/cercor/bhu255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44(3):893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschelli J, Nebel MB, Caffo BS, Barber AD, Pekar JJ, Mostofsky SH. Reduction of motion-related artifacts in resting state fMRI using aCompCor. Neuroimage. 2014;96:22–35. doi: 10.1016/j.neuroimage.2014.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto V, Jaspar M, Meyer C, Kusse C, Chellappa SL, Degueldre C, Balteau E, Shaffii-Le Bourdiec A, Luxen A, Middleton B, Archer SN, Phillips C, Collette F, Vandewalle G, Dijk DJ, Maquet P. Local modulation of human brain responses by circadian rhythmicity and sleep debt. Science. 2016;353(6300):687–690. doi: 10.1126/science.aad2993. [DOI] [PubMed] [Google Scholar]
- Ng B, Dressler M, Varoquaux G, Poline JB, Greicius M, Thirion B. Transport on Riemannian manifold for functional connectivity-based classification. Med Image Comput Comput Assist Interv. 2014;17(Pt 2):405–412. doi: 10.1007/978-3-319-10470-6_51. [DOI] [PubMed] [Google Scholar]
- Nichols T, Hayasaka S. Controlling the familywise error rate in functional neuroimaging: a comparative review. Stat Methods Med Res. 2003;12(5):419–446. doi: 10.1191/0962280203sm341ra. [DOI] [PubMed] [Google Scholar]
- Patanaik A, Zagorodnov V, Kwoh CK, Chee MW. Predicting vulnerability to sleep deprivation using diffusion model parameters. J Sleep Res. 2014;23(5):576–584. doi: 10.1111/jsr.12166. [DOI] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, Vogel AC, Laumann TO, Miezin FM, Schlaggar BL, Petersen SE. Functional network organization of the human brain. Neuron. 2011;72(4):665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Schlaggar BL, Petersen SE. Recent progress and outstanding issues in motion correction in resting state fMRI. Neuroimage. 2015;105:536–551. doi: 10.1016/j.neuroimage.2014.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Plitt M, Laumann TO, Martin A. Sources and implications of whole-brain fMRI signals in humans. Neuroimage. 2017;146:609–625. doi: 10.1016/j.neuroimage.2016.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechtschaffen A, Bergmann BM, Gilliland MA, Bauer K. Effects of method, duration, and sleep stage on rebounds from sleep deprivation in the rat. Sleep. 1999;22(1):11–31. doi: 10.1093/sleep/22.1.11. [DOI] [PubMed] [Google Scholar]
- Samann PG, Tully C, Spoormaker VI, Wetter TC, Holsboer F, Wehrle R, Czisch M. Increased sleep pressure reduces resting state functional connectivity [journal article] MAGMA. 2010;23(5–6):375–389. doi: 10.1007/s10334-010-0213-z. [DOI] [PubMed] [Google Scholar]
- Sherman SM. A wake-up call from the thalamus. Nat Neurosci. 2001;4(4):344–346. doi: 10.1038/85973. [DOI] [PubMed] [Google Scholar]
- Spasojevic G, Malobabic S, Pilipovic-Spasojevic O, Djukic-Macut N, Malikovic A. Morphology and digitally aided morphometry of the human paracentral lobule. Folia Morphol (warsz) 2013;72(1):10–16. doi: 10.5603/fm.2013.0002. [DOI] [PubMed] [Google Scholar]
- Tkachenko O, Dinges DF. Interindividual variability in neurobehavioral response to sleep loss: a comprehensive review. Neurosci Biobehav Rev. 2018;89:29–48. doi: 10.1016/j.neubiorev.2018.03.017. [DOI] [PubMed] [Google Scholar]
- Van Dongen HPA, Dinges DF. Circadian rhythms in fatigue, alertness, and performance. Princ Pract Sleep Med. 2000;20:391–399. [Google Scholar]
- Van Dongen HP, Baynard MD, Maislin G, Dinges DF. Systematic interindividual differences in neurobehavioral impairment from sleep loss: evidence of trait-like differential vulnerability. Sleep. 2004;27(3):423–433. [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2(3):125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Wong CW, Olafsson V, Tal O, Liu TT. The amplitude of the resting-state fMRI global signal is related to EEG vigilance measures. Neuroimage. 2013;83:983–990. doi: 10.1016/j.neuroimage.2013.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The code is available at https://github.com/WolkeTian/sleep_deprivation_Vigilance. The data is available from the corresponding author upon request.