Abstract
A 56-year-old man presented with vague upper abdominal pain for more than 4 months. His abdominal ultrasound and MRI showed thickening of the neck and base of the gallbladder and nodule formation at the base of the gallbladder. 18F-FDG PET/CT revealed intense FDG uptake in the base of the gallbladder and multiple lymph nodes. 68 Ga-FAPI-04 PET/CT not only showed intense FAPI uptake in the above mentioned FDG-avid lesions but also showed intense FAPI uptake in the neck lesion of the gallbladder and some other additional lymph nodes. Finally, histopathological examination confirmed poorly differentiated tubular adenocarcinoma of the neck and base of the gallbladder. Our case illustrated that 68 Ga-FAPI-04 PET/CT may outperform 18F-FDG PET/CT in the detection of gallbladder cancer primary and metastatic lesions.
Keywords: Gallbladder cancer, 18F-FDG, 68 Ga-FAPI-04, PET/CT
Introduction
Gallbladder cancer is the most common bile duct malignancy, and epidemiological studies have shown that the global incidence of gallbladder cancer is about 2/100,000 [1]. The onset of gallbladder cancer is insidious, and most patients have developed to an advanced stage when they are treated, with a poor prognosis. Therefore, early diagnosis and early treatment are the keys to improve the prognosis of patients with gallbladder cancer. 18F-fluorodeoxyglucose (FDG) PET/CT is a useful diagnostic imaging method for gallbladder cancer, but there were also positive results (such as inflammatory gallbladder disease) and false negative results (such as small size and/or low-grade tumors) that need attention [2–4]. Many studies have shown that 68 Ga-labelled fibroblast activation protein inhibitor (FAPI) is a promising PET tracer that has shown a superior diagnostic efficacy than 18F-FDG for the diagnosis of primary and metastatic lesions in patients with various types of cancer [5–8]. Here is a case report which demonstrates the superiority of 68 Ga-FAPI-04 PET/CT over 18F-FDG PET/CT.
Case Report
A 56-year-old man presented with vague upper abdominal pain for more than 4 months, which was persistent and slightly worse after eating and drinking. Physical examination revealed slight tenderness in the upper abdomen. Gastrointestinal tumor markers showed elevated ferritin (418.70 ng/ml), CA19-9 (43.51 U/ml), and CA50 (28.60 IU/ml). His abdominal ultrasound showed thickening of the gallbladder wall. MRI showed thickening of the neck and base of the gallbladder, nodule formation at the base of the gallbladder, and isointensity on T1-weighted image (T1WI), and hyperintensity on T2-weighted image (T2WI), diffusion-weighted imaging (DWI), and SPAIR, and inhomogeneous enhancement on enhanced scans. He was referred to perform 18F-FDG PET/CT scan and then recruited in our 68 Ga-FAPI PET/CT trial (AHSWMU-2020–035) approved by the institutional review board at our hospital. A written informed consent was signed by the patient. 18F-FDG PET/CT revealed intense FDG uptake (SUVmax 11.0) at the base of the gallbladder and multiple lymph nodes (SUVmax 14.9) in the hepatic hilar region, retroperitoneal region, and para-abdominal aorta areas (Fig. 1). 68 Ga-FAPI-04 PET/CT showed intense FAPI uptake not only in the above FDG-avid lesions, including the base of the gallbladder (SUVmax 12.0) and multiple lymph nodes (SUVmax 18.0) in the region described above, but also in the neck lesion of the gallbladder (SUVmax 12.0) and some other lymph nodes (SUVmax 12.2) in the above region (Fig. 2). Therefore, cancer of the gallbladder neck and gallbladder base with lymph node metastases was considered. The patient had a surgical indication, was in fair health, and the patient and his family had a strong desire for surgery and prolonged survival. The patient then underwent laparoscopic radical cholecystectomy for gallbladder cancer, i.e., cholecystectomy, lymph node dissection, wedge resection of the liver, and tissue biopsy of the gallbladder duct. Tumor tissues were observed at the base of the gallbladder and the neck of the gallbladder, respectively, measuring approximately 2.7 cm × 2.2 cm × 1.5 cm and 0.8 cm × 0.5 cm × 0.7 cm, and histopathological examination confirmed poorly differentiated tubular adenocarcinoma of both the neck and base of the gallbladder (Fig. 3). After surgery, the patient underwent chemotherapy.
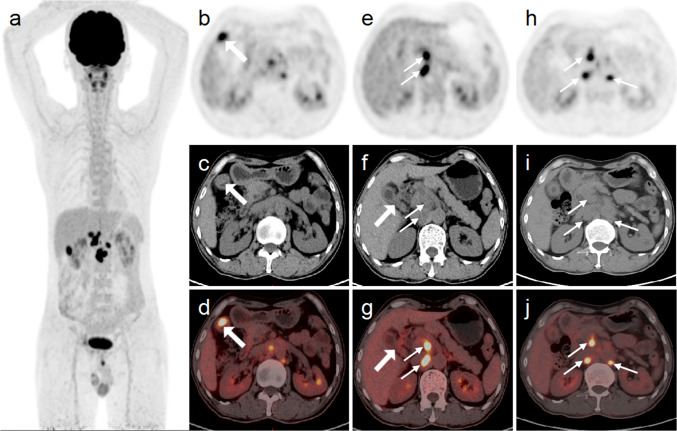
Fig. 1.
The MIP of 18F-FDG PET (a) showed multiple hypermetabolic lesions in the upper abdomen. Abdominal axial images (b–d) showed thickening of the base of the gallbladder with nodule formation and intense 18F-FDG uptake (thick arrows). Abdominal axial images (e–g) showed thickening of the neck of the gallbladder without increased 18F-FDG uptake (thick arrows). Additionally, there were multiple hypermetabolic lymph nodes (thin arrows) in the hepatic hilar region, retroperitoneal region, and para-abdominal aorta areas (a–j)
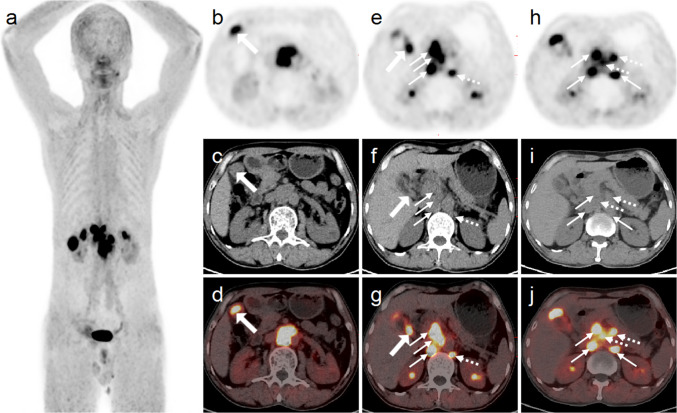
Fig. 2.
In 68 Ga-FAPI-04 PET/CT, the MIP of PET (a) showed more lesions with high uptake of FAPI in the upper abdomen than 18F-FDG PET/CT. In the corresponding axial fusion images (b–j), intense 68 Ga-FAPI-04 uptake was also noted in the above FDG-avid lesions. In addition, gallbladder neck lesions without increased 18F-FDG uptake showed strong 68 Ga-FAPI-04 uptake (e–g, thick arrows) in abdominal axial images and showed more lymph nodes with high FAPI uptake (e–j; dotted arrow)
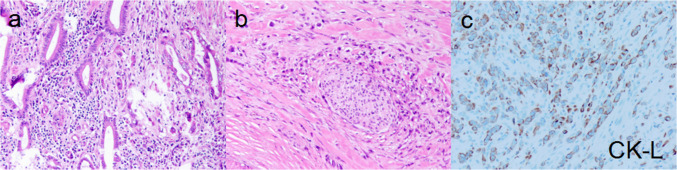
Fig. 3.
Figures a and b were stained by HE, and figure c was stained by ELPS, with CK-L being the low molecular CK. Histopathological examination revealed poorly differentiated tubular adenocarcinoma of the neck and base of the gallbladder
Discussion
18F-FDG, an analogue of glucose, reflects the glucose metabolism and the expression of glucose transporter protein of the lesion [9], but 68 Ga-FAPI-04 reflects the expression of the fibroblast activation protein (FAP) in the tumor stroma [10]. In our case, gallbladder neck lesions with strong FAPI uptake did not have increased uptake of FDG. The false negative result for gallbladder neck tumor on 18F-FDG PET/CT may be associated with small size and/or low-grade tumors [2–4]. FAP is highly expressed on CAFs (cancer-associated fibroblasts) present in > 90% of human epithelial tumors [11]. The uptake of FAPI was higher in gallbladder neck tumors due to the possibility of a significant degree of fibrosis and high expression of FAP. Compared with 18F-FDG PET/CT, the 68 Ga-FAPI-04 PET/CT detected more primary and metastatic lesions and delineated these lesions more clearly, illustrating that 68 Ga-FAPI-04 may outperform 18F-FDG in the detection of gallbladder cancer lesions. 68 Ga-FAPI-04 PET/CT provides a more comprehensive and clearer understanding of the site of lesion involvement and could guide intraoperative surgical maneuvers such as avoiding clamping of tumors in the neck of the gallbladder, and intraoperative tumor suppression has been reported to increase the number of circulating tumor cells (CTCs), which can lead to recurrence [12–14]. In addition, 68 Ga-FAPI-04 PET/CT can be used for prognostic assessment, and usually high expression of FAP is associated with poor prognosis [10], as well as for postoperative efficacy monitoring, which may be more sensitive than 18F-FDG PET/CT. Further investigations should be done for the potential of 68 Ga-FAPI-04 PET/CT in the diagnosis of gallbladder cancer.
Acknowledgements
The authors are grateful to the members of the Department of Nuclear Medicine, The Affiliated Hospital, Southwest Medical University, and Nuclear Medicine and Molecular Imaging Key Laboratory of Sichuan Province for their technical guidance, cooperation, and assistance in completing this research project.
Author Contribution
Zhanwen Huang collected this case. Chunmei Guo and Dengsai Peng make the same contribution. Ya Liu and Liming Chen made comments on this case.
Data Availability
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Declarations
Conflict of Interest
Chunmei Guo, Dengsai Peng, Ya Liu, Liming Chen, and Zhanwen Huang declare no conflict of interest.
Ethical Approval and Consent to Participate
The study was approved by the institutional review board of the Affiliated Hospital of Southwest Medical University (AHSWMU-2020–035), and informed consent was obtained from all individual participants included in the study. All procedures performed in studies involving human participants were in accordance with the Helsinki declaration as revised in 2013 and its later amendments.
Consent for Publication
The participants signed consent regarding publishing their data and photographs.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hori M, Saito E. Gallbladder cancer incidence rates in the world from the Cancer Incidence in Five Continents XI. Jpn J Clin Oncol. 2018;48:866–867. doi: 10.1093/jjco/hyy119. [DOI] [PubMed] [Google Scholar]
- 2.Annunziata S, Pizzuto DA, Caldarella C, Galiandro F, Sadeghi R, Treglia G. Diagnostic accuracy of fluorine-18-fluorodeoxyglucose positron emission tomography in gallbladder cancer: a meta-analysis. World J Gastroenterol. 2015;21:11481–11488. doi: 10.3748/wjg.v21.i40.11481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramos-Font C, Gómez-Rio M, Rodríguez-Fernández A, Jiménez-Heffernan A, Sánchez Sánchez R, Llamas-Elvira JM. Ability of FDG-PET/CT in the detection of gallbladder cancer. J Surg Oncol. 2014;109:218–224. doi: 10.1002/jso.23476. [DOI] [PubMed] [Google Scholar]
- 4.Moradi F, Iagaru A. The role of positron emission tomography in pancreatic cancer and gallbladder cancer. Semin Nucl Med. 2020;50:434–446. doi: 10.1053/j.semnuclmed.2020.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Kratochwil C, Flechsig P, Lindner T, Abderrahim L, Altmann A, Mier W, et al. 68Ga-FAPI PET/CT: tracer uptake in 28 different kinds of cancer. J Nucl Med. 2019;60:801–805. doi: 10.2967/jnumed.119.227967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen H, Pang Y, Wu J, Zhao L, Hao B, Wu J, et al. Comparison of [68Ga]Ga-DOTA-FAPI-04 and [18F] FDG PET/CT for the diagnosis of primary and metastatic lesions in patients with various types of cancer. Eur J Nucl Med Mol Imaging. 2020;47:1820–1832. doi: 10.1007/s00259-020-04769-z. [DOI] [PubMed] [Google Scholar]
- 7.Chen H, Zhao L, Ruan D, Pang Y, Hao B, Dai Y, et al. Usefulness of [68Ga]Ga-DOTA-FAPI-04 PET/CT in patients presenting with inconclusive [18F]FDG PET/CT findings. Eur J Nucl Med Mol Imaging. 2021;48:73–86. doi: 10.1007/s00259-020-04940-6. [DOI] [PubMed] [Google Scholar]
- 8.Sharma P, Singh SS, Gayana S. Fibroblast activation protein inhibitor PET/CT:a promising molecular imaging tool. Clin Nucl Med. 2021;46:e141–e150. doi: 10.1097/RLU.0000000000003489. [DOI] [PubMed] [Google Scholar]
- 9.Boellaard R, Delgado-Bolton R, Oyen WJ, Giammarile F, Tatsch K, Eschner W, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42:328–54. doi: 10.1007/s00259-014-2961-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loktev A, Lindner T, Mier W, Debus J, Altmann A, Jäger D, et al. A tumor-imaging method targeting cancer-associated fibroblasts. J Nucl Med. 2018;59:1423–1429. doi: 10.2967/jnumed.118.210435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imlimthan S, Moon ES, Rathke H, Afshar-Oromieh A, Rösch F, Rominger A, et al. New frontiers in cancer imaging and therapy based on radiolabeled fibroblast activation protein inhibitors: a rational review and current progress. Pharmaceuticals (Basel). 2021;14 [DOI] [PMC free article] [PubMed]
- 12.Gall TM, Jacob J, Frampton AE, Krell J, Kyriakides C, Castellano L, et al. Reduced dissemination of circulating tumor cells with no-touch isolation surgical technique in patients with pancreatic cancer. JAMA Surg. 2014;149:482–485. doi: 10.1001/jamasurg.2013.3643. [DOI] [PubMed] [Google Scholar]
- 13.Hashimoto M, Tanaka F, Yoneda K, Takuwa T, Matsumoto S, Okumura Y, et al. Significant increase in circulating tumour cells in pulmonary venous blood during surgical manipulation in patients with primary lung cancer. Interact Cardiovasc Thorac Surg. 2014;18:775–783. doi: 10.1093/icvts/ivu048. [DOI] [PubMed] [Google Scholar]
- 14.Li X, Liu S, Li H, Guo L, Zhang B, Lin Z, et al. Proper hepatic pedicle clamping during hepatectomy is associated with improved postoperative long-term prognosis in patients with AJCC stage IIIB hepatocellular carcinoma. Oncotarget. 2016;7:24623–24632. doi: 10.18632/oncotarget.8331. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.