Abstract
A novel β-glucosidase (Thglu3) was identified from Thermotoga sp. which had biotransformation activity for notoginsenoside R1 (NR-R1). Sequence analysis of Thglu3 revealed that it could be classified into glycoside hydrolase family 3 (GH3). The gene encoding a 719-amino acid protein was cloned and expressed in Escherichia coli. The recombinant enzyme was purified, and its molecular weight was approximately 81 kDa. The recombinant Thglu3 exhibited an optimal activity at 75 °C and pH 6.4. The β-glucosidase had high selectivity for cleaving the outer glucose moiety at the C20 position of NR-R1, which produced the more pharmacologically active notoginsenoside R2 (NR-R2). Under the optimal reaction conditions for gram-scale production, 30 g NR-R1 was transformed to NR-R2 using 20 g crude enzyme at pH 6.4 and 75 °C within 1 h with a molar yield of 93%. This study was the first report of the highly efficient and selective gram-scale transformation of NR-R2 from NR-R1 by a thermophilic β-glucosidase.
Keywords: Biotransformation, β-Glucosidase, Notoginsenoside, Glycoside hydrolase family, Thermotoga
Introduction
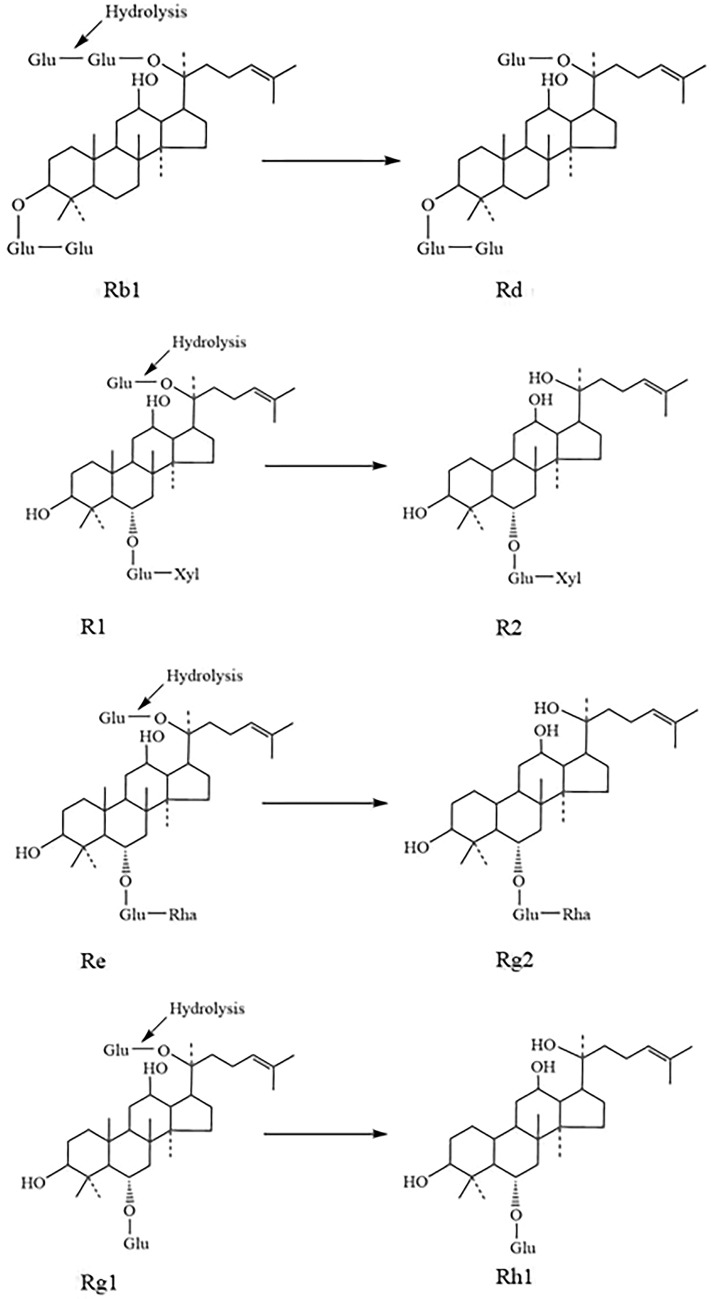
Panax notoginseng (PN), a member of the family Araliaceae, has been widely used as a kind of valuable Chinese-traditional medicine for thousands of years. The pharmacological activities include maintaining the micro-circulatory homeostasis, and anti-inflammatory, anti-angiogenetic, anti-cancer and antiviral effects (Hawthorne et al. 2022; Jiang et al. 2022; Zheng et al. 2022). Notoginsenosides (NGs), also named saponins, are the principal components responsible for the diverse and significant effects of PN. More than 20 NGs presenting different pharmaceutical activities have been identified from PN. They include NG-R1, -R2, -R3, -R4, -R6, -Fa, -Fc, and -Fe, and ginsenoside Rg1, -Rg2, -Rb1, -Rb2, -Rb3, -Rc, -Rd, -Re, -Rh2, -Rh1, and -F2. As seen in Fig. 1, these NGs, mainly belong to triterpenoidal saponins, comprise a nonsugar constituent of dammarane skeleton and a sugar constituent including 1–4 molecule glycosides such as glucose, arabinopyranose, arabinofuranose, xylose, and rhamnose. According to the type of the aglycone structure, notoginsenosides are divided into two groups: protopanaxadiol (PPD)-saponins and protopanaxatriol (PPT)-type saponins.
Fig. 1.
Chemical structures of notoginsenosides and ginsenosides. a PPD type. b PPT type. Glu β-d-glucopyranosyl, Arap α-l-arabinopyranosyl, Araf α-l-arabinofuranosyl, Xyl β-d-xylopyranosyl, and Rha α-l-rhamnopyranosyl
NG-R1, the third most abundant saponin in PN, belongs to PPT-type saponin. It possesses one glucose linked on C20 position and two sugar moieties including one inner glucose and one outer xylose attached on C6 position of PPT-aglycone. Increasing evidence shows that NG-R1 exhibits various pharmaceutical activities, including cardiovascular protection (Wang et al. 2021a, b), promoting angiogenesis (Zhong et al. 2020), neuroprotection (Wang et al. 2021a, b), brain protection (Zou et al. 2017), anti-diabetes (Zhou et al. 2019), liver protection (Gong et al. 2022), gastrointestinal protection (Li et al. 2014), lung protection (He et al. 2022), bone protection (Zhao et al. 2017), renal protection (Fan et al. 2020), and anti-cancer (Qin et al. 2021). However, NG-R1 exhibits poor membrane permeability and relatively low bioavailability, whose application in the clinic has been greatly affected. It has been demonstrated that sugar moieties in saponins lead to large polarity and lower bioavailability. Moreover, deglycosylated saponins are proven to be more pharmaceutically active because of their smaller size, better permeability across the cell membrane and higher bioavailability (Ku 2016). In our previous work, we demonstrated that deglycosylated saponins (Rd, GypXVII, and PPT) had significantly greater anti-inflammatory activity than their glycosylated precursors (Rb1, Re and Rg1) (Yu et al. 2017). Liu et al. reported that NG-R2, deglycosylated from NG-R1 using Cordyceps sinensis, exhibited elevated cardioprotective effect against DOX-induced cell injury (Liu et al. 2022). Hence, transformation of NG-R1 through hydrolysis of the sugar moieties linked to aglycone has attracted wide attention recently.
Transformation approaches include physiochemical methods such as heating, acid treatment and alkali treatment, and bioconversion methods using microorganisms and enzymes (Park et al. 2010). Among these methods, the enzymatic conversion is the most promising method because of its high substrate specificity and stability, low levels of by-products, and high production yields. To minimize processing time and production cost, recombinant enzymes obtained from E. coli strains are commonly used. Moreover, higher productivity was achieved when recombinant thermophilic enzymes were applied to transform notoginsenosides. For instance, the thermophilic β-xylosidase from Dictyoglomus thermophilum could convert NR-R1 into Rg1 with a molar conversion rate of approximately 100% (Li et al. 2022).
Until now, thermophilic β-glucosidases have been explored to hydrolyze the sugar moieties linked to the C3, C6, and C20 positions in glycosylated notoginsenosides. They belong to GH 1 and GH 3 family. Different β-glucosidase has different hydrolytic activities on the structural differences of sugar moieties attached to notoginsenosides, such as linked positions, inner and outer residues and types of sugar moieties. For instance, the GH1 thermophilic β-glucosidase from P. furiosus DSMZ 3638 transformed NR-R1, NR-R2, Re, Rg2, and Rf into Rg1 and Rh1 and exhibited specifically hydrolytic activity for the outer glycoside at the C6 position (Oh et al. 2014). The GH3 thermophilic β-glucosidase from Dictyoglomus turgidum specifically hydrolyzed the xylose at the C6 position and the glucoses attached to the C6 and C20 position of R1 along the following pathway: R1 → R2 → Rh1 → PPT (Lee et al. 2014). Considering the complex and diverse structures of notoginsenosides, the number of thermophilic β-glucosidases is still limited. And the biotransformation activity, specificity, and thermostability of most enzymes still do not meet the industrial demands. It is, therefore, meaningful to explore novel β-glucosidases with good thermostability, high catalytic efficiency and specificity.
In this paper, a novel thermophilic β-glucosidase from Thermotoga sp. was cloned, purified and characterized. It showed high specificity that hydrolyzed only the outer glucose at the C20 position in NG-R1, and biotransformed the NG-R1 into the deglycosylated NG-R2. This study was the first report of the highly efficient and selective gram-scale transformation of NG-R1 to NG-R2 by a thermophilic β-glucosidase.
Materials and methods
Materials and strains
Escherichia coli strains were incubated at 37 °C in Luria–Bertani (LB) medium (10 g/L tryptone, 5 g/L yeast extract, and 10 g/L NaCl) with 50 mg/L kanamycin. Authentic saponin Rb1, Rb2, Rc, Rd, F2, Re, Rg1, Rg2, Rh1, R1 and R2 were purchased from Shanghai Yuanye Biological Technology Co. Ltd. (Shanghai, China). p-Nitrophenyl (pNP)-α-d-glucopyranoside, pNP-α-l-arabinofuranoside, pNP-β-d-galactopyranoside, pNP-α-l-arabinopyranoside, pNP-β-d-glucopyranoside, pNP-α-l-rhamnopyranoside, pNP-β-d-xylopyranoside, o-Nitrophenyl (oNP)-β-d-glucopyranoside and oNP-β-d-galactopyranoside were purchased from Sigma-Aldrich (St Louis, MO, USA).
Phylogenetic analysis of Thglu3
The theoretical molecular weight (Mw) and isoelectric point (pI) of Thglu3 were estimated on the ExPasy server (http://web.expasy.org/compute pi/). Homologs of Thglu3 (Genbank No. WP_165276387.1) were searched with the BLASTp program on NCBI (https://blast.ncbi.nlm.nih.gov/Blast.cgi). The glycoside hydrolases in GH3 family were chosen at the CAZy web (http://www.cazy.org/fam/GH3.html), and the sequence information of those proteins were collected by means of the CAZy web page links (Drula et al. 2022; Henrissat and Davies 1997). Multiple alignments of Thglu3 and three characterized β-glucosidases from the GH3 family were performed using the ClustalX program (Larkin et al. 2007). The phylogenetic tree of Thglu3 and the enzymes chosen from GH3 family with notoginsenoside-transforming activities was constructed using the neighbor-joining method (Saitou and Nei 1987) with default parameters and 1000 bootstrap in the MEGA4 Program (Felsenstein 1985).
Molecular cloning, expression and purification of Thglu3
The β-glucosidase gene, Thglu3, from Thermotoga sp. was obtained via gene synthesis (Sangon, Shanghai). The DNA fragment was inserted into the pET28a vector digested with BamHI and XhoI. The recombinant pET28a-Thglu3 with a His-tag at N terminal was transformed into E. coli BL21 (DE3), which was induced with 1 mM isopropyl β-d-thiogalactopyranoside (IPTG) at 16 °C for an additional 12 h.
The recombinant strains were collected by centrifugation at 6,000 × g for 20 min and resuspended in a lysis buffer (50 mM Tris–HCl, pH 7.1). The cells were then sonicated and centrifuged at 14,000 × g for 30 min at 4 °C to remove the debris. The supernatants containing the target proteins were loaded onto a Ni–NTA affinity chromatography column (GE Healthcare) and purified using a 20–100 mM imidazole gradient. The purified enzyme was dialyzed against 50 mM phosphate–citrate buffer (pH 6.4) and concentrated to 1.0 mg/mL. The protein homogeneity was confirmed by 8% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE).
β-glucosidase activity of Thglu3
The pNPG was used to test the β-glucosidase activity of Thglu3. Hydrolysis of pNPG was measured at 75 °C in 50 mM phosphate–citrate buffer (pH 6.4). The activity was determined by measuring the increase in absorbance at 405 nm due to the release of pNP. One unit (IU) of activity was defined as the amount of enzyme liberating 1 μmol of p-nitrophenol per min.
Enzyme characterization of Thglu3
Using pNPG as the substrate, the effects of temperature and pH on enzyme activity were investigated by assaying the β-glucosidase activity according to the method described previously. The pH optima of Thglu3 was tested over the pH range of 3.0–8.0 in 50 mM phosphate–citrate buffer at 60 °C. The temperature optima of Thglu3 was measured between 50 and 90 °C (5 °C intervals) at the optimum pH. Thermal stability of the enzyme was studied by incubating about 1.0 mg/mL purified enzyme solutions at different temperatures ranging from 50 to 90 °C for 1 h in 50 mM phosphate–citrate buffer (pH 6.4). The residual activity on pNPG was determined at 75 °C in 50 mM phosphate–citrate buffer (pH 6.4).
The effects of metal ions (Ba2+, Co2+, Mn2+, Ni2+, Mg2+, Na+, K+, Ca2+, Zn2+, Fe2+ and Hg2+), and EDTA on enzyme activity were investigated. The enzyme activity with no addition for the control was set as 100%. After incubation, the enzyme with various metal ions (1 mM and 10 mM), and EDTA (5 mM and 10 mM) at room temperature for 30 min, the residual activity was measured according to the method described previously using pNPG as substrate at pH 6.4 and 75 °C.
The substrate specificity of Thglu3 was investigated using the following substrates: pNP-α-d-glucopyranoside, pNP-α-l-arabinofuranoside, pNP-β-d-galactopyranoside, pNP-α-l-arabinopyranoside, pNP-β-d-glucopyranoside, pNP-α-l-rhamnopyranoside, pNP-β-d-xylopyranoside, oNP-β-d-glucopyranoside and oNP-β-d-galactopyranoside. The enzyme activities were measured according to the method described previously. One unit (IU) of activity was defined as the amount of enzyme liberating 1 μmol of p-nitrophenol per min.
The substrate specificity of Thglu3 towards different notoginsenosides was also studied. Saponins Rb1, Rb2, Rc, Rd, F2, Re, Rg1, Rg2, Rh1, R1 and R2 were used as substrates. Enzyme solution (1 mg/mL) was reacted with equal volume of substrate solution (1 mg/mL) in 50 mM phosphate–citrate buffer (pH 6.4) at 75 °C for 5–30 min. The reaction solution without enzyme served as blank control. The enzymatic products were subsequently extracted with H2O-saturated n-butanol. After evaporation of solvents, the products were dissolved in methanol and analyzed via HPLC. One unit (IU) of activity was defined as the amount of enzyme catalyzing the conversion of 1 μmol of notoginsenoside substrate per min.
Gram-scale production of NG-R2 from NG-R1 by Thglu3
High cell density fermentation was performed to produce the recombinant Thglu3. The LB medium supplemented with kanamycin (50 mg/mL) was used to cultivate the E. coli BL21 (DE3) harboring pET28a-Thglu3 in a 5 L tank reactor (FS-05-D05D05P, Winpact, USA) with a 2 L working volume. At the initial stage, fermentation was performed at 37 ℃, pH 7.0 and at stirring speed of 200 rpm. When dissolved oxygen was lower than 30%, the speed increased by 10 rpm. When the speed reached 900 rpm, the dissolved oxygen coupling was closed. After the nutrition exhausted, the dissolved oxygen feedback feeding was carried out by flow feeding to ensure the dissolved oxygen range at 20–40%. When OD600 reached 30, cells harboring pET28a-Thglu3 were induced at 25 °C for 8 h and were then harvested via centrifugation at 5000 rpm for 20 min at 4 °C. Cell pellets were resuspended in 10 volumes (w/v) of 50 mM phosphate–citrate buffer (pH 6.4) and disrupted by sonication and the supernatant was used as the crude enzyme for biotransformation reaction.
To optimize the enzyme and substrate concentrations, crude enzyme concentrations from 5 to 20 mg/ml (at 15 mg/ml NG-R1) and substrate concentrations from 0 to 40 mg/ml (at 20 mg/ml enzyme) were evaluated. The time course reactions of NG-R2 production were performed at 75 °C in 50 mM citrate/phosphate buffer (pH 6.4) containing 30 mg/ml NG-R1 and 20 mg/ml crude enzyme. For all biotransformation reactions, samples were collected at regular intervals and HPLC was used to monitor the transformation process.
HPLC analysis
Chromatographic separation was performed on an Agilent 1200 series HPLC system (Agilent Technologies, Santa Clara, CA, USA) coupled with a Syncronis C18 chromatographic column (5 cm × 3.0 mm, 2.7 μm, Supelco. USA). The column oven temperature was maintained at 35 °C, and the mobile phases A and B were water with 0.1% formic acid and acetonitrile, respectively. The gradient elution program was determined as follows: 0–2.5 min, 19% (B); 2.55 min, 19–30% (B); 5–11 min, 30–33% (B); 11–20 min, 33–45% (B); 20–25 min, 45–65% (B). The injection volume was 5 μL, and the flow rate was 0.4 mL/min.
Results
Sequence analysis of Thglu3 from Thermotoga sp.
The β-glucosidase gene (Thglu3) consisted of 2157 bp encoding 719 amino acids with a theoretical molecular mass of 81.58 kDa and a theoretical pI value of 5.77. The amino acid sequence of Thglu3 (Genbank No. WP_165276387.1) exhibited highest similarity with that of GH 3 protein from Thermotoga caldifontis (93.6% identity, Genbank No. WP_041075862.1). The Thermotoga caldifontis protein has not yet been characterized. The nearest characterized glycoside hydrolase was GH3 β-glucosidase from Thermotoga neapolitana DSM 4359 (77.2% identity, Genbank No. ACM22846.1).
As seen in Fig. 2, three GH3 glycoside hydrolases were chosen for multiple amino acid sequence alignment with Thglu3. The β-glucosidase from Thermotoga petrophila DSM 13,995 (Genbank No. ABQ46916) displayed high sequence identity with Thglu3 (76% identity). The other two glycoside hydrolases from Thermotoga neapolitana DSM 4359 (77.2% identity, Genbank No. ACM22846.1) and Dictyoglomus turgidum (39% identity, Genbank No. WP_012582633) have been structurally characterized. Based on the structure analysis of these two glycoside hydrolases (Varghese et al. 1999; Pozzo et al. 2010), the catalytic residues were clearly investigated. We predicted that the conserved residues D241 and E456 of Thglu3 are the typical catalytic nucleophile and catalytic acid of the GH3 enzyme, respectively.
Fig. 2.
Multiple amino acid sequence alignment of Thglu3 with several characterized glycoside hydrolases from GH3. The accession numbers of the aligned sequences are for the following organisms: ABQ46916, β-glucosidase gene from Thermotoga petrophila DSM 13,995; ACM22846, β-glucosidase from Thermotoga neapolitana DSM 4359; WP_012582633, β-glycosidase from Dictyoglomus turgidum. The accession numbers were indicated to the left of the amino acid sequences. Identical residues are indicated by a red background. Symbols: ↑ amino acids forming a catalytic residues
Until now, 11 β-glucosidases from GH3 family were identified to display notoginsenoside-transforming activities (Table 1). To gain a better understanding of the evolutionary position of Thglu3, the phylogenetic tree of Thglu3 and these 11 enzymes was constructed using the neighbor-joining method in the MEGA4 program with bootstrap values based on 1,000 replications. The resulting consensus tree is presented in Fig. 3. Thglu3 formed a separate, well-supported clade with β-glucosidases from T. neapolitana (Genbank No. ACM22846.1) and T. petrophila (ABQ46916), indicating that it had close kinship with T. neapolitana β-glucosidase and T. petrophila β-glucosidase. Thglu3 had 77.2% and 76% sequence homology with T. neapolitana β-glucosidase and T. petrophila β-glucosidase, respectively. Besides, they displayed similar enzyme characteristics. Based on the literature reports and our lab data, Thglu3, T. neapolitana glucosidase (Bi et al. 2019) and T. petrophila (Xie et al. 2015) glucosidase are thermophilic enzymes with hydrolytic activities for Re and Rg1.
Table 1.
β-glucosidases exhibiting notoginsenoside-transforming activities from GH3 family
| Organism | Reaction conditions | Genbank No | Product | Cleavage site | References |
|---|---|---|---|---|---|
| L. brevis | pH 7.0, 30℃ | BAN05876 | Rb1 → Rd; F2 → CK | C3, C20 | Zhong et al. (2016) |
| M. esteraromaticum | pH 7.0, 37℃ | AEX88467 | Rb2 → CY → CK; Rb1 → Rd → CK | C3, C20 | Quan et al. (2012a, b, c, d) |
| A. mirum | pH 7.0, 37℃ | WP_015801787 |
Rb1 → GypXVII → GypLXXV; Rb2 → CO → CY; Rc → CMc1 → CMc; Rd → F2 → Rh2 → PPD; Re → Rg2; Rg1 → Rh1 → PPT |
C3, C6, C20 | Cui et al. (2013a, b) |
| S. keddieii | pH 8.0, 25℃ | ACZ20402 |
Rb1 → GypXVII → GypLXXV → CK; Rb2 → CO → CY; Rc → CMc1 → CMc; Rd → F2 → CK; Re → Rg2 /F1; Rg1 → F1 |
C3, C6, C20 |
Kim et al. (2012) |
| T. neapolitana | pH 5.0, 85℃ | ACM22846 | Re → Rg2; Rg1 → PPT | C6, C20 | Quan et al. (2012a, b, c, d) |
| D. turgidum | pH 5.5, 80℃ | WP_012582633 |
R1 → R2 → Rh1 → PPT; Rg1 → Rh1 → PPT; Re → Rg2 |
C6, C20 | Lee et al. 2014 |
| F. johnsoniae | pH 6.0, 37℃ | ABQ03809 | Rb1 → Rd; GypXVII → F2 | C20 | Hong et al. 2012 |
| M. esteraromaticum | pH 7.0, 37℃ | AEX88466 | Re → Rg2; Rg1 → Rh1; Rb1 → Rd → Rg3 | C20 | Quan et al. (2012a, b, c, d) |
| T. petrophila | pH 6.0, 90℃ | ABQ46916 | Rb1 → Rd → Rg3; Re → Rg2; Rg1 → Rh1 | C20 | Xie et al. (2015) |
| M. sp. Strain QM49 | pH 8.0, 30℃ | AFS34656 | Re → Rg2; Rg1 → Rh1 | C20 | Cui et al. (2013a, b) |
| P. aculeatum | pH 4.5, 70℃ | AGA96121 | Rb1 → Rd → F2 → CK; Rb2 → CO → CY; Rc → CMc1 → CMc; Rg1 → F1; Rf → Rh1 → PPT | C3, C6 | Lee et al. (2013) |
Fig. 3.

Phylogenetic analysis of Thglu3, and notoginsenoside-transforming glycoside hydrolases from GH3. The units at the bottom of the tree indicate numbers of substitution events
Expression, purification and characterization of Thglu3
The putative β-glucosidase (Thglu3) from Thermotoga sp. was cloned and expressed in Escherichia coli under the control of the IPTG-inducible promoter T7. After being induced under 16 °C for 12 h with 1 mM IPTG, the recombinant enzyme was solubly overexpressed in E.coli cells. The recombinant Thglu3 was purified by His-trap affinity chromatography. The specific activity of purification was 4.1-fold higher than that of the crude soluble fraction, and the purification yield was approximately 56.2%. The expressed enzyme was determined as a single band by SDS-PAGE, with a molecular mass of approximately 81 kDa (Fig. 4), which was almost consistent with the molecular weight of 81,585 Da calculated from 719 amino acids.
Fig. 4.

Purification of Thglu3. Lane M, molecular mass marker; Lane 1, Thglu3 after Ni–NTA affinity chromatography purification
The optimum temperature and pH of the purified Thglu3 were determined using pNPG as the substrate. The optimal temperature for Thglu3 activity was 75 °C, while it also displayed high activity between 50 and 90 °C. As seen in Fig. 5a, the enzyme was very stable at temperatures ranging from 50 to 75 °C. After incubation for 1 h, thermostability tended to decrease at or above 80 °C. Hence, Thglu3 was identified to be a thermophilic enzyme. The maximum activity was observed at pH 6.4, and the β-glucosidase activity was higher than 60% of the maximum activity (specific activity of 320 U/mg) at the pH range from 3.0 to 7.2. While from pH 7.2, the enzyme activity decreased swiftly (Fig. 5b).
Fig. 5.
a Effect of temperature on enzyme activity (black square) and stability (black circle). b Effect of pH on enzyme activity
The effects of metal ions, and EDTA on Thglu3 activity were also investigated (Table 2). Ba2+, Co2+ and Mn2+ were able to significantly increase enzyme activity under the high concentration of 10 mM. Moreover, Ni2+, Mg2+, Na+, K+, Ca2+, and Zn2+ slightly enhanced the enzyme activity under concentrations of 1 mM and 10 mM. And Fe2+ and Hg2+ were able to inhibit enzyme activity under concentrations of 1 mM and 10 mM. The chelating agent EDTA did not inhibit the activity, which indicated that Thglu3 was not metalloprotein.
Table 2.
Effects of metal ions and EDTA on the enzyme activity of Thglu3
| Additives | Relative activity (%) | |
|---|---|---|
| Control | 100 | 100 |
| Metal ions | 1 mM | 10 mM |
| Ni2+ | 106 ± 1.6 | 112 ± 3.3 |
| Mg2+ | 121 ± 1.9 | 112 ± 2.9 |
| Na+ | 120 ± 3.1 | 109 ± 1.6 |
| Ba2+ | 115 ± 0.9 | 134 ± 0.8 |
| K+ | 106 ± 2.2 | 109 ± 1.3 |
| Mn2+ | 140 ± 1.7 | 150 ± 0.6 |
| Ca2+ | 98 ± 0.4 | 111 ± 2.5 |
| Zn2+ | 106 ± 3.6 | 114 ± 3.4 |
| Co2+ | 145 ± 2.8 | 193 ± 1.1 |
| Fe2+ | 89 ± 1.1 | 95 ± 0.7 |
| Hg2+ | 9 ± 0.1 | 5 ± 0.4 |
| Inhibitors | 5 mM | 10 mM |
| EDTA | 101 ± 1.5 | 98 ± 1.3 |
The substrate specificity of Thglu3 was investigated using pNP and oNP glycosides with α and β configurations including pNP-α-d-glucopyranoside, pNP-α-l-arabinofuranoside, pNP-β-d-galactopyranoside, pNP-α-l-arabinopyranoside, pNP-β-d-glucopyranoside, pNP-α-l-rhamnopyranoside, pNP-β-d-xylopyranoside, oNP-β-d-glucopyranoside and oNP-β-d-galactopyranoside. As seen in Table 3, Thglu3 displayed high activity towards pNP-β-d-glucopyranoside, and less activity toward oNP-β-d-glucopyranoside. In addition, no activity was detected with pNP-α-L-arabinofuranoside, pNP-β-d-galactopyranoside, pNP-α-l-arabinopyranoside, pNP-α-d-glucopyranoside, pNP-α-l-rhamnopyranoside, pNP-β-d-xylopyranoside and oNP-β-d-galactopyranoside. Hence, Thglu3 was identified to be β-glucosidase.
Table 3.
Enzyme specificity of Thglu3 on various substrates
| Substrate | Relative activity(%) |
|---|---|
| pNP-α-d-glucopyranoside | NDb |
| pNP-α-l-arabinofuranoside | ND |
| pNP-β-d-galactopyranoside | ND |
| pNP-α-l-arabinopyranoside | ND |
| pNP-β-d-glucopyranoside | 100 ± 2.1a |
| pNP-α-l-rhamnopyranoside | ND |
| pNP-β-d-xylopyranoside | ND |
| oNP-β-d-glucopyranoside | 11 ± 0.5 |
| oNP-β-d-galactopyranoside | ND |
aThe activity against pNP-β-D-glucopyranoside was assumed to be 100%, and corresponded to a specific activity of 1781 U/mg
bNot detected, specific activity is not detected by the analytical methods used in this study
The substrate specificity of Thglu3 on notoginsenosides was also measured. According to the type of the aglycone structure, notoginsenosides are divided into two groups: PPD-type saponins and PPT-type saponins. As seen in Table 4, sugar moieties including glucose, arabinopyranose, and arabinofuranose linked to C3 and C20 position in PPD-type saponins. And sugar moieties including glucose, xylose, and rhamnose linked to C6 and C20 position of PPT-type saponins. For PPD-type saponins, Thglu3 only acted on Rb1 with relative activity of 27%. For PPT-type saponins, the relative activity of Thglu3 was in the order R1 > Rg1 > Re. The enzyme exhibited no activity on Rg2 and Rh1.
Table 4.
Substrate specificity of Thglu3 on PPD- and PPT-type saponins
| Type | Substratea | C3 | C6 | C20 | Product | Relative activity (%) (mean % ± SD)b |
|---|---|---|---|---|---|---|
| PPD- | Rb1 | Glu(1 → 2)Glu- | Glu(1 → 6)Glu- | Rd | 27 ± 2.4 | |
| Rb2 | Glu(1 → 2)Glu- | Arap(1 → 6)Glu- | NDc | |||
| Rc | Glu(1 → 2)Glu- | Araf(1 → 6)Glu- | ND | |||
| Rd | Glu(1 → 2)Glu- | Glu- | ND | |||
| F2 | Glu- | Glu- | ND | |||
| PPT- | R1 | Xyl(1 → 2)Glu- | Glu- | R2 | 100 ± 0.5b | |
| Re | Rha(1 → 2)Glu- | Glu- | Rg2 | 42 ± 1.2 | ||
| R2 | Xyl(1 → 2)Glu- | H- | ND | |||
| Rg1 | Glu- | Glu- | Rh1 | 61 ± 0.7 | ||
| Rg2 | Rha(1 → 2)Glu- | H- | ND | |||
| Rh1 | Glu- | H- | ND |
aFinal concentration of substrate was 1.0 mg/ml
bThe activity against R1 was assumed to be 100%, and corresponded to a specific activity of 105 U/mg
cNot detected, specific activity is not detected by the analytical methods used in this study
For the verification of the biotransformation pathways of Rb1, R1, Re and Rg1 using Thglu3, the HPLC analyses were performed. The chemical structures of transformed products (peaks 1–4) were identified by comparison of their retention times with standards. As seen in Fig. 6a–d, deglycosylated products of Rb1, R1, Re and Rg1 were identified to be Rd, R2, Rg2 and Rh1, respectively. Based on the structural analysis, Rd, R2, Rg2 and Rh1 were generated by hydrolysis of the outer glucose linked to the C20 position of aglycone (Fig. 7). As Thglu3 displayed the highest activity towards R1, the large-scale transformation of R1 into R2 was further studied.
Fig. 6.
HPLC analysis of saponin Rb1, R1, Re and Rg1 during biotransformation process using Thglu3. Saponins standards were indicated on the peaks. Numbers were used to indicate the product peaks
Fig. 7.
Biotransformation pathways of saponin Rb1, R1, Re and Rg1 using Thglu3
Gram-scale production of R2 from R1 by Thglu3
To facilitate scaling up of the production, the recombinant E. coli BL21 cells were obtained by high cell density fermentation. High cell density fermentation is a relative concept, which refers to the application of certain culture technology and devices to improve the fermentation density of bacteria, so that the cell density is significantly higher than that of ordinary fermentation culture, and finally improve the specific productivity of specific products.
Cell pellets were resuspended and disrupted by sonication. The soluble fraction collected by centrifugation was treated as crude enzyme. For large-scale biotransformation, the crude enzyme was not further purified by His-trap affinity chromatography.
To reduce the production cost, substrate R1 (15 mg/ml) and crude enzyme solution were added to the reaction mixture (2L) in equal volume. The effect of crude enzyme concentration (5–20 mg/ml) on R2 production was studied. As seen in Fig. 8a, the R2 production increased with increasing the enzyme concentration. The conversion of R1 reached 100% using 20 mg/ml enzyme within 30 min, indicating that the crude enzyme concentration was optimal at 20 mg/ml.
Fig. 8.

a Effect of the concentrations of crude enzyme including 5 mg/ml enzyme (black square), 10 mg/ml enzyme (black circle), 15 mg/ml enzyme (black triangle) and 20 mg/ml enzyme (black inverted triangle) on R2 production using Thglu3. b Effect of the concentrations of substrate R1 on R2 production using Thglu3. c Effect of the transformation time on R2 production (black circle) from R1 (black square) using Thglu3
In consideration of decreasing the reactor volume, the effect of substrate concentration on R2 production was assessed in the reaction mixture (2 L) by varying the concentrations of R1 from 0 to 40 mg/ml and reacting with equal volume of crude enzyme (20 mg/ml) for 10 min. The R2 production increased with increasing R1 concentration and reached a plateau above 30 mg/ml (Fig. 8b). Thus, the optimal substrate concentration was determined to be 30 mg/ml.
To determine the most appropriate reaction time, the time course of the biotransformation of R1 to R2 was monitored via HPLC analyses. The reaction mixture (2L) contains equal volumes of 30 mg/ml R1 and 20 mg/ml Thglu3. As seen in Fig. 8c, the production of R2 reached its highest level after 1 h biotransformation, and the R1 was almost transformed completely.
Hence, the scaled-up biotransformation was performed in a 5.0 L glass bottle (2.0 L working volume) under optimal conditions (shaking 200 rpm for 1 h at pH 6.4 and 75 °C). The crude recombinant Thglu3 (20 mg/ml, 1 L) was reacted with an equal volume of R1 (30 mg/ml, 1 L). Under the optimal reaction conditions, 30 g R1 was transformed to 26 g R2 with a molar yield of 93% and a productivity of 13 g L−1 h−1.
Discussion
Biotransformation of specific saponins in the valuable medical plants to increase their bioavailability and pharmaceutical activities has attracted more and more attention. Until now, transformation of notoginsenosides using glycosidases has been attempted. Moreover, glycosidases from GH3 family are the main source of notoginsenoside-transforming enzymes. As seen in Table 1, we summarized the notoginsenoside-transforming β-glucosidases from GH3 family. These β-glucosidases display different enzyme characterization such as catalytic activity and specificity. They can be divided into three groups according to the difference of hydrolysis activity of sugar moieties at different positions in saponins. Group I enzymes catalyze the simultaneous hydrolysis of C3/C6 and C20 sugars in saponins. For instance, M. esteraromaticum β-glucosidase first hydrolyzed the inner glucose moiety attached to the C3 position and then the arabinopyranose moiety attached to the C20 position of Rb2 (Quan et al. 2012a, b, c, d). Group II enzymes catalyze the hydrolysis of sugars linked to C3 or C6 position in PPD-type or PPT-type saponins, respectively. Until now, β-glucosidase from P. aculeatum (Lee et al. 2013) was the only GH3 enzyme belonging to Group II. Group III β-glucosidases only catalyze the hydrolysis of C20 sugar moieties in PPD-type or PPT-type saponins, For instance, F. johnsoniae glucosidase transformed Rb1 and GypXVII into Rd and F2, respectively, by hydrolyzing the outer glucose attached to C20 position of aglycone (Hong et al. 2012). In addition, large-scale production of notoginsenosides using GH3 glycosidases was also achieved. For instance, β-glucosidase from Thermotoga petrophila DSM 13,995 hydrolyzed 10 g/L of Rb1 into 6.93 g/L of Rg3 within 90 min, with a corresponding molar conversion of 97.9% (Xie et al. 2015). However, considering the complex and diverse structures of notoginsenosides, the number of notoginsenoside-transforming glycosidases is still limited. It is, therefore, meaningful to explore novel glycosidases with good thermostability, high catalytic efficiency and specificity from GH3 family.
The ubiquitous characteristic of Thermotoga species is likely the result of genomic and metabolic versatility, which includes an extraordinary array of enzymes involved in diverse carbohydrate utilization pathways. The α-glucosidases and β-glucosidases with high activities and stabilities were identified from Thermotoga species, such as α-glucosidase from Thermotoga neapolitana (Yun et al. 2011) and β-glucosidase from Thermotoga petrophila (Haq et al. 2012). We believe that there are still a lot of glycosidase resources in Thermotoga species to be developed.
In this study, the Thglu3 gene was obtained from Thermotoga sp. and was cloned in E. coli BL21 (DE3). Based on the multiple amino acid sequence alignment of Thglu3 and other three GH3 glycoside hydrolases (Thermotoga neapolitana β-glucosidase, Dictyoglomus turgidum β-glucosidase and Thermotoga petrophila β-glucosidase), the conserved residues D241 and E456 of Thglu3 were identified as the typical catalytic nucleophile and catalytic acid of the GH3 enzyme, respectively. Besides, based on the literature reports and our lab data, the substrate selectivity differences between these enzymes were analyzed. The T. neapolitana β-glucosidase catalyzed the hydrolysis of the glucose moieties attached at C6 and C20 position of the aglycone to produce Rg2 and PPT from Re, Rf and Rg1 (Bi ta al. 2019). The glycoside hydrolase from D. turgidum specifically hydrolyzed the xylose at the C6 position and the glucose in PPT-type saponins (Lee et al. 2014). The β-glucosidase from T. petrophila hydrolyzed Rb1 along the pathway Rb1 → Rd → Rg3, suggesting hydrolysis of the outer glucose molecules at position C20, followed by hydrolysis of the inner glucose molecules at the same position (Xie et al. 2015). Compared to these three β-glucosidases, Thglu3 exhibited different specificity, Thglu3 exhibited high substrate specificity in hydrolysis of only outer glucose linked to C20 position in PPD- and PPT-type saponins whereas the enzyme did not hydrolyze the glucose at the C3 and C6 position of aglycone. In the future, we will try to solve the crystal structure of Thglu3 and find out key amino acid residues responsible for its unique enzymatic specificity.
Thglu3 with an optimal temperature of 75 °C was identified to be a thermophilic β-glucosidase. Thermostable enzymes serve as ideal catalysts for biotransformation application because high temperatures improve the saponins solubility, enhance the substrate conversion and reduce the need for expensive cooling process. For instance, β-glucosidase from Thermotoga thermarum (Pei et al. 2015) transformed Rb1 into Rg3 with a corresponding molar conversion of 97.8% within 60 min at 85 °C. Similarly, Thglu3 transformed 30 g R1 into R2 at 75 °C within 1 h with a molar yield of 93%.
Thglu3 was most active against pNP-β-d-glucopyranoside, followed by oNP-β-d-glucopyranoside, while no enzyme activity was detected against pNP-β-d-galactopyranoside, pNP-β-d-xylopyranoside, pNP-α-l-arabinofuranoside, pNP-α-l-arabinopyranoside and pNP-α-l-rhamnopyranoside. These results indicated that Thglu3 had high selectivity on residual glucose and has a strong affinity to aryl-β-glucose. This explained why Thglu3 did not have catalytic activity against Rb2 and Rc which had outer arabinopyranose and arabinofuranose moiety at the C20 position of aglycone, respectively. It was also the reason that R2 and Rg2, which had outer xylose and rhamnose moiety at the C6 position of aglycone, respectively, were not further hydrolyzed.
As for notoginsenosides and ginsenosides, Thglu3 exhibited high hydrolytic activity on R1, low activity on Rb1, Re, and Rg1, no activity on Rb2, Rc, Rd, F2, R2, Rg2 and Rh1. It transformed R1, Rb1, Re and Rg1 into R2, Rd, Rg2 and Rh1, respectively. For PPD-type saponins, Rb1, Rb2, Rc, and Rd share the same protopanaxadiol skeletons and a disaccharide (glucose-β-(1 → 2)-glucose) substitution at C3 site. The main difference among them is the number and type of sugar moieties attached at C20 of aglycone. The results demonstrated that Thglu3 specifically cleaved the outer glucose moiety at the C20 position of aglycone, but does not hydrolyze sugar moieties linked to C3 position of aglycone. As Rd was not further transformed, Thglu3 did not hydrolyze the inner glucose at the C20 position of aglycone. For PPT-type saponins, R1, Re, and Rg1 share the same protopanaxatriol skeletons and one glucose substitution at C20 site. The main difference among them is the number and type of sugar moieties attached at C6 position of aglycone. Similarly, Thglu3 specifically cleaved the glucose moiety at the C20 position of aglycone, but displayed no activity on the sugar moieties linked to C6 position of aglycone. Hence, Thglu3 had high selectivity to β-(1 → 6)-glucosidic linkage and it hydrolyzed the outer glucose at the C20 position in PPD- and PPT-type saponins whereas the enzyme did not hydrolyze the glucose at the C3 and C6 position of aglycone.
Based on the specificity analysis, Thglu3 can be grouped into Group III enzymes and catalyzed the hydrolysis of Rb1, Re and Rg1 via the following pathway: Rb1 → Rd, Re → Rg2, Rg1 → Rh1. Other Group III enzymes, for instance, β-glucosidases from M. esteraromaticum (Quan et al. 2012a, b, c, d) and T. petrophila (Xie et al. 2015) hydrolyzed Rb1 through the following pathway: Rb1 → Rd → Rg3. They possessed the hydrolysis activity on outer and inner glucose attached on C20 position of aglycone. However, Thglu3 exhibited high substrate specificity in hydrolysis of only outer glucose linked to C20 position of aglycone. The β-glucosidase from M. sp. Strain QM49 exhibited the same hydrolysis behavior on Re and Rg1 as Thglu3 (Cui et al. 2013a, b). However, Thglu3 and the M. sp. Strain QM49 glucosidase possessed different physicochemical properties, especially optimal temperature. Thglu3 was thermophilic β-glucosidase, while M. sp. Strain QM49 glucosidase was mesophilic β-glucosidase. As we mentioned before, thermophilic enzymes have more advantages in industrial application than mesophilic enzymes. Hence, Thglu3 with good thermostability would be a promising enzyme used for saponins biotransformation.
The R1 and R2 belong to protopanaxatriol (PPT)-type notoginsenosides. R1 is the third most abundant saponin in PN. While the concentration of R2 is quite low in PN. R1 and R2 share the same dammarane skeleton and possess the same sugar moieties linked to C6 position of aglycone. The structural difference lies in the sugar number linked to C20 position, that NG-R2 has no glucose attached on the C20 position of aglycone. Based on the structural similarity, R2 can be generated by cleaving the outer glucose moiety at the C20 position of R1. Until now, transformation activities towards R1 have been identified using β-glucosidases from D. turgidum (Lee et al. 2014) and T. petrophila (Xie et al. 2015). However, D. turgidum β-glucosidase catalyzed the hydrolysis of R1 via the following pathway: R1 → R2 → Rh1 → PPT, that R2 was further transformed. Furthermore, the specific activity of D. turgidum β-glucosidase towards R1 was 0.067 U/mg. The transformation of R2 from R1 using T. petrophila β-glucosidase lacks quantitative data. Based on our lab data, the specific activity of Thglu3 on R1 was 1.56 U/mg. Until now, Thglu3 was the first enzyme used for gram-scale production of R2.
In conclusion, in the current study, a novel GH3 β-glucosidase (Thglu3) was successfully cloned and expressed in Escherichia coli. The recombinant Thglu3 exhibited optimal activity at pH 6.4 and 75 °C. Thglu3 displayed high specificity for biotransformation of R1 into R2. The large-scale preparation of R2 will lay a foundation for the future pharmacological activity research and application development.
Acknowledgements
The authors acknowledge funding from Scientific and Technological Development Program of Jilin province of China (grant number 20210204046YY).
Author contributions
SSY designed the research; PZ conducted the biochemistry and molecular experiments, and wrote the manuscript; YX conducted the qualitatively and quantitative investigation of the transformation process by high-performance liquid chromatography, and data analysis; KLZ, HXZ, and NW involved in data analysis, and co-wrote the manuscript. All authors approved the final version of the manuscript.
Funding
This work was financially supported by funding from Scientific and Technological Development Program of Jilin province of China (Grant number 20210204046YY).
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article.
Declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- Bi YF, Wang XZ, Jiang S, et al. Enzymatic transformation ofginsenosides Re, Rg1, and Rf to ginsenosides Rg2 and aglycon PPT using β-glucosidase from Thermotoga neapolitana. Biotechnol Lett. 2019;41(4–5):613–623. doi: 10.1007/s10529-019-02665-7. [DOI] [PubMed] [Google Scholar]
- Cui CH, Kim SC, Im WT. Characterization of the ginsenoside-transforming recombina-nt beta-glucosidase from Actinosynnema mirum and bioconversion of major ginsenosides into minor ginsenosides. Appl Microbiol Biotechnol. 2013;97:649–659. doi: 10.1007/s00253-012-4324-5. [DOI] [PubMed] [Google Scholar]
- Cui CH, Liu QM, Kim JK, et al. Identification and characterization of a Mucilaginiba-cter sp. strain QM49 beta-glucosidase and its use in the production of the pharmaceutic-ally active minor ginsenosides (S)-Rh1 and (S)-Rg2. Appl Environ Microbiol. 2013;79:5788–5798. doi: 10.1128/AEM.01150-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drula E, Garron ML, Dogan S, et al. The carbohydrate-active enzyme database: functions and literature. Nucleic Acids Res. 2022;50(D1):D571–D577. doi: 10.1093/nar/gkab1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan C, Chen Q, Ren J, et al. Notoginsenoside R1 suppresses inflammatory signaling a-nd rescues renal ischemia-reperfusion injury in experimental rats. Med Sci Monit. 2020;26:1234–1010. doi: 10.12659/msm.920442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J. Confidence limits of phylogenies: an approach using the bootstrap. Evol-Ution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Gong X, Shan L, Cao S, et al. Notoginsenoside R1, an active compound from Panax notoginseng, inhibits hepatic stellate cell activation and liver fibrosis via MAPK signalin-g pathway. Am J Chin Med. 2022;50:511–523. doi: 10.1142/s0192415x22500197. [DOI] [PubMed] [Google Scholar]
- Haq IU, Khan MA, Muneer B, et al. Cloning, characterization and molecular docking of a highly thermostable β-1,4-glucosidase from Thermotoga petrophila. Biotechnol Lett. 2012;34(9):1703–1709. doi: 10.1007/s10529-012-0953-0. [DOI] [PubMed] [Google Scholar]
- Hawthorne B, Lund K, Freggiaro S, et al. The mechanism of the cytotoxic effect of P-anax notoginseng extracts on prostate cancer cells. Biomed Pharmacother. 2022;149:112887. doi: 10.1016/j.biopha.2022.112887. [DOI] [PubMed] [Google Scholar]
- He J, Liu MW, Wang ZY, Shi RJ. Protective effects of the notoginsenoside R1 on acute lung injury by regulating the miR-128-2-5p/Tollip signaling pathway in rats with se-vere acute pancreatitis. Innate Immun. 2022;28:19–36. doi: 10.1177/175342592110687-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrissat B, Davies G. Structural and sequence-based classification of glycoside hydrol-ases. Curr Opin Struct Biol. 1997;7(5):637–644. doi: 10.1016/s0959-440x(97)80072-3. [DOI] [PubMed] [Google Scholar]
- Hong H, Cui CH, Kim JK, et al. Enzymatic biotransformation of ginsenoside Rb1 an-d gypenoside XVII into ginsenosides Rd and F2 by recombinant beta-glucosidase from Flavobacterium johnsoniae. J Ginseng Res. 2012;36:418–424. doi: 10.5142/jgr.2012.36.4.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Li H, Huang P, et al. Panax notoginseng saponins protect PC12 cells against Aβ induced injury via promoting parkin-mediated mitophagy. J Ethnopharmacol. 2022;285:114859. doi: 10.1016/j.jep.2021.114859. [DOI] [PubMed] [Google Scholar]
- Kim JK, Cui CH, Yoon MH, et al. Bioconversion of major ginsenosides Rg1 to mino-r ginsenoside F1 using novel recombinant ginsenoside hydrolyzing glycosidase cloned fr-om Sanguibacter keddieii and enzyme characterization. J Biotechnol. 2012;161:294–301. doi: 10.1016/j.jbiotec.2012.06.021. [DOI] [PubMed] [Google Scholar]
- Ku S. Finding and producing probiotic glycosylases for the biocatalysis of ginsenoside-s: a mini review. Molecules. 2016;21:1420–3049. doi: 10.3390/molecules21050645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, et al. Clustal W and Clustal X version 2.0. B-Ioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Lee GW, Yoo MH, Shin KC, et al. Beta-glucosidase from Penicillium aculeatum hydr-olyzes exo-, 3-O-, and 6-O-beta-glucosides but not 20-O-beta-glucoside and other glycosi-des of ginsenosides. Appl Microbiol Biotechnol. 2013;97:6315–6324. doi: 10.1007/s00253-013-4828-7. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Shin KC, Lee GW, Oh DK. Production of aglycone protopanaxatriol from gi-nseng root extract using Dictyoglomus turgidum beta-glycosidase that specifically hydroly-zes the xylose at the C-6 position and the glucose in protopanaxatriol-type ginsenosides. Appl Microbiol Biotechnol. 2014;98:3659–3667. doi: 10.1007/s00253-013-5302-2. [DOI] [PubMed] [Google Scholar]
- Li C, Li Q, Liu YY, et al. Protective effects of notoginsenoside R1 on intestinal ische-mia-reperfusion injury in rats. Am J Physiol Gastrointest Liver Physiol. 2014;306:G111–G122. doi: 10.1152/ajpgi.00123.2013. [DOI] [PubMed] [Google Scholar]
- Li Q, Wang L, Fang X, Zhao L. Highly efficient biotransformation of notoginsenoside R1 into ginsenoside Rg1 by Dictyoglomus thermophilum β-xylosidase Xln-DT. J Microb-Iol Biotechnol. 2022;32:447–457. doi: 10.4014/jmb.2111.11020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Xin Y, Qiu Z, et al. Cordyceps sinensis-mediated biotransformation of notoginse-noside R1 into 25-OH-20(S/R)-R2 with elevated cardioprotective effect against DOX-ind-uced cell injury. RSC Adv. 2022;12:12938–12946. doi: 10.1039/d2ra01470j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh HJ, Shin KC, Oh DK. Production of ginsenosides Rg1 and Rh1 by hydrolyzing the outer glycoside at the C-6 position in protopanaxatriol-type ginsenosides using β-glucosidase from Pyrococcus furiosus. Biotechnol Lett. 2014;36(1):113–119. doi: 10.1007/s10529-013-1331-2. [DOI] [PubMed] [Google Scholar]
- Park CS, Yoo MH, Noh KH, Oh DK. Biotransformation of ginsenosides by hydrolyzi-ng the sugar moieties of ginsenosides using microbial glycosidases. Appl Microbiol Biot-Echnol. 2010;87:9–19. doi: 10.1007/s00253-010-2567-6. [DOI] [PubMed] [Google Scholar]
- Pei J, Xie J, Yin R, et al. Enzymatic transformation of ginsenoside Rb1 to ginsenosid-e 20(S)-Rg3 by GH3 β-glucosidase from Thermotoga thermarum DSM 5069T. J Mol Ca-Tal B-Enzym. 2015;113:104–109. doi: 10.1016/j.molcatb.2014.12.012. [DOI] [Google Scholar]
- Pozzo T, Pasten JL, Karlsson EN, et al. Structural and functional analyses of beta-glucosidase 3B from Thermotoga neapolitana: a thermostable three-domain representative of glycoside hydrolase 3. J Mol Biol. 2010;397(3):724–739. doi: 10.1016/j.jmb.2010.01.072. [DOI] [PubMed] [Google Scholar]
- Qin HL, Wang XJ, Yang BX, et al. Notoginsenoside R1 attenuates breast cancer progr-ession by targeting CCND2 and YBX3. Chin Med J (engl) 2021;134:546–554. doi: 10.1097/cm9.0000000000001328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan LH, Jin Y, Wang C, et al. Enzymatic transformation of the major ginsenoside R-b2 to minor compound Y and compound K by a ginsenoside-hydrolyzing beta-glycosida-se from Microbacterium esteraromaticum. J Ind Microbiol Biotechnol. 2012;39:1557–1562. doi: 10.1007/s10295-012-1158-1. [DOI] [PubMed] [Google Scholar]
- Quan LH, Min JW, Jin Y, et al. Enzymatic biotransformation of ginsenoside Rb1 to c-ompound K by recombinant beta-glucosidase from Microbacterium esteraromaticum. J A-Gric Food Chem. 2012;60:3776–3781. doi: 10.1021/jf300186a. [DOI] [PubMed] [Google Scholar]
- Quan LH, Min JW, Sathiyamoorthy S, et al. Biotransformation of ginsenosides Re and Rg1 into ginsenosides Rg2 and Rh1 by recombinant beta-glucosidase. Biotechnol Lett. 2012;3–4:913–917. doi: 10.1007/s10529-012-0849-z. [DOI] [PubMed] [Google Scholar]
- Quan LH, Min JW, Yang DU, et al. Enzymatic biotransformation of ginsenoside Rb1 to 20(S)-Rg3 by recombinant beta-glucosidase from Microbacterium esteraromaticum. Ap-Pl Microbiol Biotechnol. 2012;94:377–384. doi: 10.1007/s00253-011-3861-7. [DOI] [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phyl-ogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Varghese JN, Hrmova M, Fincher GB. Three-dimensional structure of a barley beta-D-glucan exohydrolase, a family 3 glycosyl hydrolase. Structure. 1999;7(2):179–190. doi: 10.1016/s0969-2126(99)80024-0. [DOI] [PubMed] [Google Scholar]
- Wang D, Gao B, Yang T, et al. Protective effect of NGR1 against glutamate-induced cytotoxicity in HT22 hippocampal neuronal cells by upregulating the SIRT1/Wnt/β-cateni-n pathway. Evid Based Complement Alternat Med. 2021 doi: 10.1155/2021/4358163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Lv L, Xu Y, et al. Cardioprotection of Panax Notoginseng saponins against acute myocardial infarction and heart failure through inducing autophagy. Biomed Pharmacother. 2021;136:111287. doi: 10.1016/j.biopha.2021.111287. [DOI] [PubMed] [Google Scholar]
- Xie J, Zhao D, Zhao L, et al. Overexpression and characterization of a Ca(2+) activate-d thermostable beta-glucosidase with high ginsenoside Rb1 to ginsenoside 20(S)-Rg3 bio-conversion productivity. J Ind Microbiol Biotechnol. 2015;42:839–850. doi: 10.1007/s10295-015-1608-7. [DOI] [PubMed] [Google Scholar]
- Yu S, Zhou X, Li F, et al. Microbial transformation of ginsenoside Rb1, Re and Rg1 and its contribution to the improved anti-inflammatory activity of ginseng. Sci Rep. 2017;7:138. doi: 10.1038/s41598-017-00262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun BY, Jun SY, Kim NA, et al. Crystal structure and thermostability of a putative α-glucosidase from Thermotoga neapolitana. Biochem Biophys Res Commu. 2011;416(1–2):92–98. doi: 10.1016/j.bbrc.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Zhao S, Yan L, Li X, et al. Notoginsenoside R1 suppresses wear particle-induced oste-olysis and RANKL mediated osteoclastogenesis in vivo and in vitro. Int Immunopharma-Col. 2017;47:118–125. doi: 10.1016/j.intimp.2017.03.018. [DOI] [PubMed] [Google Scholar]
- Zheng YR, Fan CL, Chen Y, et al. Anti-inflammatory, anti-angiogenetic and antiviral activities of dammarane-type triterpenoid saponins from the roots of Panax notoginseng. Food Funct. 2022;13:3590–3602. doi: 10.1039/d1fo04089h. [DOI] [PubMed] [Google Scholar]
- Zhong FL, Ma R, Jiang M, et al. Cloning and characterization of ginsenoside-hydrolyz-ing beta-glucosidase from Lactobacillus brevis that transforms ginsenosides Rb1 and F2 into ginsenoside Rd and compound K. J Microbiol Biotechnol. 2016;26:1661–1667. doi: 10.4014/jmb.1605.05052. [DOI] [PubMed] [Google Scholar]
- Zhong J, Lu W, Zhang J, et al. Notoginsenoside R1 activates the Ang2/Tie2 pathway to promote angiogenesis. Phytomedicine. 2020;78:153302. doi: 10.1016/j.phymed.2020.153302. [DOI] [PubMed] [Google Scholar]
- Zhou P, Xie W, Meng X, et al. Notoginsenoside R1 ameliorates diabetic retinopathy t-hrough PINK1-dependent activation of mitophagy. Cells. 2019;8:2073–4409. doi: 10.3390/cells8030213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou S, Zhang M, Feng L, et al. Protective effects of notoginsenoside R1 on cerebral i-schemia-reperfusion injury in rats. Exp Ther Med. 2017;14:6012–6016. doi: 10.3892/etm.2017.5268. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article.