Abstract
Purpose
Radium-223 has been demonstrated in clinical trials to improve survival in castration-resistant prostate cancer (CRPC) patients with bone metastases. However, its performance in routine use remains to be fully characterized. This study aims to describe patient outcomes in the real world as well as identify factors associated with completion of the 6-dose regimen and alkaline phosphatase (ALP) response.
Methods
Thirty-six patients who received at least one dose of radium-223 at the Jewish General Hospital in Montréal, Canada, were analysed in a retrospective manner. Using logistic regression, the primary analysis aimed to identify factors associated with treatment completion, and the secondary analysis aimed to identify factors associated with ALP response.
Results
Twenty-one out of 36 patients received all 6 doses of radium-223. Fifteen patients had an ALP response, defined as a 30% decrease in ALP from baseline values. On primary analysis, baseline ALP > 120 U/L and prostate-specific antigen (PSA) > 50 μg/L were significantly associated with lower therapy completion rates (OR = 0.10, p = 0.004; OR = 0.18, p = 0.022 respectively). On adjustment for confounders, only ALP remained significant (OR = 0.14, p = 0.021). Clinical disease progression was the most common reason for treatment non-completion, and it was also associated with elevated baseline ALP (OR = 6.00, p = 0.044). On secondary analysis, previous chemotherapy for CRPC was a negative predictor of ALP response (OR = 0.15, p = 0.034).
Conclusion
Elevated baseline ALP and PSA were associated with a lower rate of radium-223 regimen completion; receiving chemotherapy for CRPC prior to radium-223 was associated with a lower rate of ALP response.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13139-022-00760-8.
Keywords: Radiopharmaceutical, Radium, Prostate cancer, Metastatic, Alkaline phosphatase
Introduction
Prostate cancer is one of the most common cancers affecting men worldwide [1]. In cases of metastatic prostate cancer, bone metastases are a major cause of mortality and morbidity [2].
Radium-223 dichloride is the first, and at present, only, alpha-emitting therapeutic radiopharmaceutical approved for use in humans. Radium-223 targets bone metastases by acting as a calcium mimetic, localizing to regions with high bone turnover. The emitted alpha-particles then induce double-stranded deoxyribonucleic acid (DNA) breaks in the cells of the adjacent tumour environment. Its inherent bone targeting properties, high energy emission, and low penetration make radium-223 an effective and specific therapeutic modality for bone metastases in prostate cancer [3].
In 2013, the Alpharadin in Symptomatic Prostate Cancer (ALSYMPCA) randomized control trial showed positive results for radium-223, demonstrating a survival benefit of 3.6 months in the treatment arm as well as a favourable safety profile [4]. This led to the approval of the product for use in patients with symptomatic bone metastases by the United States Food and Drug Administration, Health Canada, and the European Medicines Agency. Radium-223 is currently used in a regimen of 6 doses given intravenously every 4 weeks.
However, since this pivotal trial, there have been numerous real-world reports that outcomes are not as favourable as seen in ALSYMPCA, finding lower overall survival as well as lower rates of treatment completion in the community [5–10]. As there is an association between treatment non-completion and poorer patient outcomes, there is significant interest in finding any pre-treatment factors that may predict completion to better guide patient selection [6].
In addition, methods to assess and monitor patient response throughout therapy are lacking with radium-223. Although no biochemical marker has been validated as a surrogate for predicting survival in patients treated with radium-223, serum alkaline phosphatase (ALP) has shown promise in this regard, where a decrease following therapy has been associated with better survival [11, 12]. A reflection of bone metabolism, baseline ALP has also been found to be a good indicator of patient prognosis in metastatic prostate cancer in several studies [7, 12–15]. As such, it is commonly examined as an endpoint, and factors associated with its dynamics may be of interest.
In this paper, we report the demographic data, baseline status, and outcomes of all patients who have undergone radium-223 therapy at the Jewish General Hospital in Montréal, Canada. We use these data to determine possible predictive factors for treatment completion and ALP responses.
Methods
Participants and Data Collection
This study includes all 36 patients who started a radium-223 regimen at the Jewish General Hospital between November 2015 and January 2020. Patients were eligible to receive radium-223 if they were diagnosed with castration-resistant prostate cancer (CRPC), symptomatic bone metastases, and no soft-tissue metastases except for lymph nodes less than 3 cm in short axis. Eligible patients were assessed by clinicians to determine suitability and offered therapy as per standard clinical practice. All patients who received at least one dose were included in the study with no excluded patients for any reason. All patients included in the study had either finished or discontinued radium-223 therapy by the time of analysis.
Data were retrospectively gathered from electronic medical records including oncology clinical notes, routine oncology labs, and imaging reports. Demographic data and previous therapies, as well as baseline clinical, biochemical, and radiographic status, were collected. In addition, clinical, biochemical, and radiographic progress throughout the treatment was collected. Reasons for discontinuation were noted when applicable. For chemotherapy prior to radium-223, only chemotherapy received during castration resistant disease was considered.
Treatment Completion
The primary endpoint was the completion of the six-dose regimen. Completion was defined as receiving 6 doses of radium-223; anything less was considered incomplete.
Biochemical Response
A biochemical response in prostate specific antigen (PSA), lactate dehydrogenase (LDH), or ALP was defined as a drop of > 30% from the pre-treatment baseline at any point during the radium-223 regimen [5, 7, 8]. Laboratory values were generally obtained at monthly intervals from regular oncology clinic visits. The pre-treatment baseline is defined as the most recent set of laboratory results dated prior to the first dose of radium-223. Laboratory values were followed until the first result after the final dose of radium-223 received, which occurred approximately one month after the final dose. For patients without any values after the final dose, the results were followed to the last set of values available during the regimen.
Clinical Response
Clinical response was determined based on the oncology clinical notes regarding the patient’s ability to perform activities and self-care. Patients with consistently worsening or consistently improving functional statuses were considered to be progressing or responding, respectively. Patients with minimal or inconsistent changes in functional status were considered clinically stable.
Statistical Analyses
Clinical and demographic data were presented as median and interquartile range for non-normal continuous variables. Frequencies and proportions were presented for categorical variables. Mann–Whitney and chi-square tests were used to compare the continuous and categorical variables, respectively.
For the primary analysis, we constructed univariate and multivariable logistic regression models to assess the associations between clinical factors and treatment completion. Specifically, multivariable models were constructed for the associations between (i) treatment completion and baseline PSA, (ii) treatment completion and baseline ALP, and (iii) treatment completion and baseline hemoglobin (Hb). We selected potential factors and confounders based on prior knowledge regarding the relationship between the hypothesized factors and completion of treatment [6–8, 16]. In the multivariable models, Gleason score and previous chemotherapy were selected as confounders. Patient age was not included as it was similar between the two groups. A similar approach was taken for the analysis of ALP responses which included age and previous chemotherapy as confounders. The number of covariates included in the regression models satisfied the minimum sample size requirement (5 or more events per variable) suggested by Vittinghoff et al. [17].
Sensitivity analyses were conducted to assess the robustness of the estimated coefficients. An E-value was used to assess for the possibility of spurious correlation due to unconsidered confounders [18]. For all analyses, a p-value of < 0.05 was considered as statistically significant. Statistical analyses were performed in R version 4.1.0.
Results
Thirty-six patients were included in this study, and their baseline characteristics are summarized in Table 1. A total of 21 (58%) patients received all 6 doses of radium-223. Of the remainder, 1 (3%), 2 (6%), 3 (8%),7 (19%), and 2 (6%) patient(s) received 5, 4, 3, 2, and 1 dose(s), respectively. All patients received androgen deprivation therapy (ADT) concurrently with radium-223. All patients except one received hormone therapy with at least one of abiraterone or enzalutamide prior to radium-223 therapy. Of the patients that did, five continued this therapy during radium-223, and the remainder stopped. There is an association between treatment non-completion (receiving 1–5 doses) of radium-223 and elevated baseline PSA or ALP.
Table 1.
Baseline patient characteristics
| Treatment complete 6 doses |
Treatment incomplete 1–5 doses |
p-value | |
|---|---|---|---|
| n = 21 | n = 15 | ||
| Age (years), median (IQR) | 75 (69, 77) | 74 (68, 79) | 0.748 |
| Delay from time of diagnosis to first dose (years), median (IQR) | 2.4 (1.9, 3.4) | 2.7 (1.6, 4.0) | 0.898 |
| Gleason score, median (IQR) | 9 (8, 9) | 9 (7, 9) | 0.643 |
| Previous chemotherapy for CRPC (%) | 1.000 | ||
| Yes | 7 (33.3) | 5 (33.3) | |
| No | 13 (61.9) | 10 (66.7) | |
| Unknown | 1 (4.8) | 0 (0.0) | |
| ECOG performance status (%) | n/a | ||
| 0–1 | 11 (52.4) | 7 (46.7) | |
| 2–3 | 3 (14.3) | 3 (20.0) | |
| Unknown | 7 (33.3) | 5 (33.3) | |
| Tumor burden (%) | 0.568 | ||
| 1–6 | 2 (9.5) | 1 (6.7) | |
| 6–20 | 10 (47.6) | 4 (26.7) | |
| > 20 | 8 (38.1) | 9 (60.0) | |
| Superscan | 1 (4.8) | 1 (6.7) | |
| PSA (μg/L) (%) | 0.043 | ||
| < 50 | 14 (66.7) | 4 (26.7) | |
| > 50 | 7 (33.3) | 11 (73.3) | |
| LDH (U/L) (%) | 0.427 | ||
| < 210 | 11 (52.4) | 5 (33.3) | |
| > 210 | 10 (47.6) | 10 (66.7) | |
| ALP (U/L) (%) | 0.007 | ||
| < 120 | 15 (71.4) | 3 (20.0) | |
| > 120 | 6 (28.6) | 12 (80.0) | |
| Hemoglobin (g/L) (%) | 0.558 | ||
| < 100 | 17 (81.0) | 10 (66.7) | |
| > 100 | 4 (19.0) | 5 (33.3) | |
| WBC count (109/L) (%) | 1.000 | ||
| < 4.5 | 20 (95.2) | 14 (93.3) | |
| > 4.5 | 1 (4.8) | 1 (6.7) | |
| Platelet count (109/L) (%) | 0.760 | ||
| < 145 | 20 (95.2) | 13 (86.7) | |
| > 145 | 1 (4.8) | 2 (13.3) |
Bolded indicates p < 0.05
Abbreviations: CRPC castration-resistant prostate cancer, ECOG Eastern Cooperative Oncology Group, PSA prostate-specific antigen, LDH lactate dehydrogenase, ALP alkaline phosphatase, WBC white blood cell, IQR interquartile range
Biochemical Responses
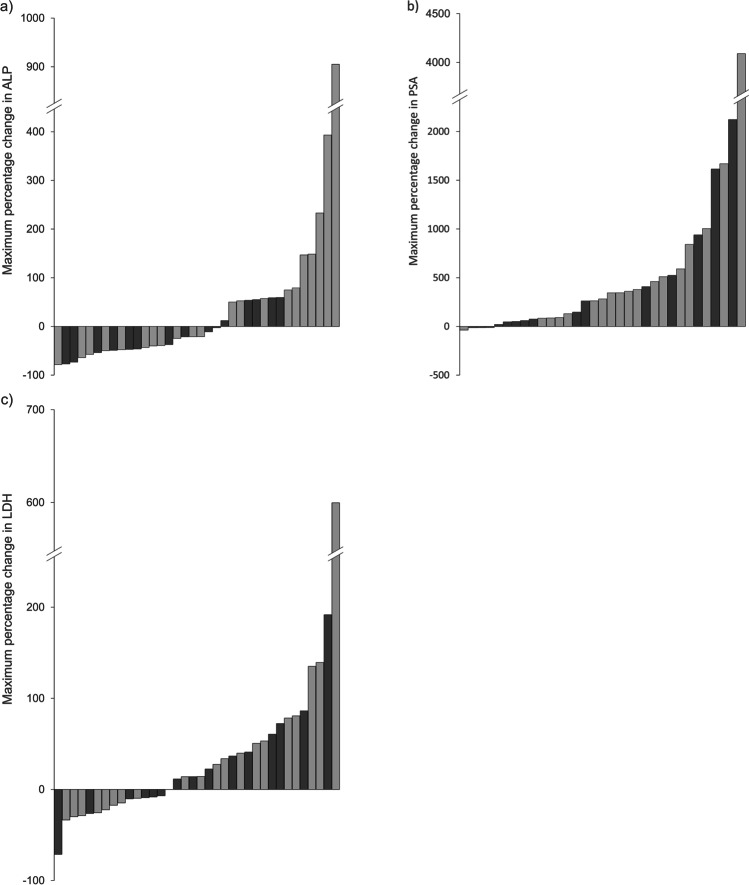
An ALP response, defined as a drop of > 30% in ALP at any point in the regimen, was observed in a total of 15 (42%) patients, 8 of whom received 6 doses of radium-223. A > 30% LDH response was observed in 3 (8%) patients, and a > 30% PSA response was observed in 2 (6%) patients. Notably, no patients had a > 50% PSA response. The data are summarized in the waterfall plots in Fig. 1.
Fig. 1.
Waterfall plot showing maximum percentage change during therapy in a alkaline phosphatase (ALP), b prostate specific antigen (PSA), and c lactate dehydrogenase (LDH). Light gray = 6 doses of radium-223 received; dark gray = less than 6 doses of radium-223 received. Note that the last data point in all three series has been truncated for scaling purposes
Clinical Response
Nine (26%) patients were deemed to be clinically responding as per review of clinical notes. Sixteen (46%) were deemed to be clinically stable, and 10 (29%) were deemed to be clinically progressing.
Treatment Completion
Table 2 summarizes the results of univariate and multivariate logistic regression analyses of pre-treatment parameters as predictors of treatment completion. Eastern Cooperative Oncology Group (ECOG) performance status was excluded due to missing data. On univariate analysis, baseline ALP and PSA were identified to be associated with lower odds of treatment completion (OR = 0.10, p = 0.004; OR = 0.18, p = 0.022 respectively). On multivariate analysis, only ALP remained significant (OR = 0.14, p = 0.021) after adjusting for relevant covariates. All multivariable models were adjusted for chemotherapy and Gleason score whose estimated effect sizes are presented in Appendix A. Sensitivity analysis resulted in an E-value of 4.78 for the association between ALP and treatment completion, indicating that an unaccounted confounder would need to be associated with both baseline ALP and treatment completion with an odds-ratio of 4.78 to explain away the finding [18].
Table 2.
Logistic regression models for treatment completion
| Unadjusted | Adjusted | ||||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | Model | |
| Age | 1.02 | 0.94–1.10 | 0.676 | ||||
| Delay from diagnosis | 0.91 | 0.70–1.19 | 0.486 | ||||
| Gleason score | 1.23 | 0.62–2.43 | 0.560 | ||||
| Prior chemotherapy for CRPC | 1.08 | 0.26–4.42 | 0.918 | ||||
| PSA > 50 μg/L | 0.18 | 0.04–0.78 | 0.022 | 0.22 | 0.05–1.03 | 0.055 | [TC1] |
| ALP > 120 U/L | 0.10 | 0.02–0.49 | 0.004 | 0.14 | 0.03–0.75 | 0.021 | [TC2] |
| Hb < 100 g/L | 0.47 | 0.10–2.17 | 0.334 | 0.22 | 0.03–1.61 | 0.135 | [TC3] |
Multivariate analysis was adjusted for prior chemotherapy and Gleason score
Abbreviations: OR odds ratio, CI confidence interval, CRPC castration-resistant prostate cancer, PSA prostate-specific antigen, ALP alkaline phosphatase, Hb hemoglobin, TC1-3 multivariate models for treatment completion 1–3 (Appendix A)
Reasons for Discontinuation
Of the 15 patients who discontinued the regimen prior to completion, the reasons were disease progression in 8 patients (53%), bone marrow toxicity in 4 patients (27%), increased pain in 1 patient (7%), and unknown in 2 patients (13%).
Given that the main reasons for treatment discontinuation were disease progression and bone marrow toxicity, further analysis was done to determine whether baseline ALP would also be associated with these events. The results are summarized in Tables S1 and S2 in the supplementary material. There was a significant correlation between elevated baseline ALP and clinical progression of disease (OR = 6.00, p = 0.044), but no significant correlation between baseline ALP and bone marrow toxicity.
ALP Response
Table 3 summarizes the results of univariate and multivariate logistic regression analyses of pre-treatment parameters as predictors of ALP response. Age at time of treatment, previous chemotherapy for castration resistant disease, and baseline ALP were found to be significantly associated with ALP response on univariate analysis (OR = 0.9, p = 0.037; OR = 0.15, p = 0.034; OR = 5.5, p = 0.022 respectively). Multivariate analyses are adjusted for age and previous chemotherapy, and the effect sizes of the adjusted covariates are presented in Appendix A. Sensitivity analysis found an E-value of 13.6 for the association between chemotherapy and ALP response.
Table 3.
Logistic regression models for ALP response
| Unadjusted | Adjusted | ||||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | Model | |
| Age | 0.9 | 0.82–0.99 | 0.037 | ||||
| Delay from diagnosis | 1.01 | 0.78–1.31 | 0.942 | ||||
| Gleason score | 1.34 | 0.65–2.72 | 0.427 | ||||
| Prior chemotherapy for CRPC | 0.15 | 0.03–0.87 | 0.034 | ||||
| PSA > 50 μg/L | 2.00 | 0.52–7.69 | 0.313 | 1.67 | 0.28–9.95 | 0.571 | [ALP1] |
| ALP > 120 U/L | 5.5 | 1.28–23.7 | 0.022 | 92.8 | 2.31–3730 | 0.016 | [ALP2] |
Multivariate analysis was adjusted for age and prior chemotherapy
Abbreviations: OR odds ratio, CI confidence interval, CRPC castration-resistant prostate cancer, PSA prostate-specific antigen, ALP alkaline phosphatase, ALP1-2 multivariate models for ALP responses 1–2 (Appendix A)
Discussion
Radium-223 is a therapy for bone metastases from castration-resistant prostate cancer proven to improve overall survival [4]. However, data collected from use in the real world have not shown the same favourable outcomes as the initial clinical trial [5–10]. This has sparked interest in further characterizing causes for these differences as well as highlighted the importance of developing pre-treatment criteria to optimize outcomes in patients selected for treatment.
Compared to the ALSYMPCA trial, our patients were slightly older with a median age of 74 years vs 71 years. Our patients had less previous use of chemotherapy, with only 33% having received chemotherapy prior to radium-223 treatment compared to 57% [4]. Our patients had lower baseline ALP and PSA values than in ALSYMPCA, with median values of 120 U/L and 50 µg/L respectively. This is in line with that of many post-approval studies, which were also generally lower than in ALSYMPCA [5, 8, 10, 11].
Similar to many other studies evaluating patient responses in the community, we found lower response rates and lower treatment completion rates than ALSYMPCA [4, 5, 9, 10, 19]. Our ALP response rate was 42% compared to 47% in ALSYMPCA. This may be in part due to lower baseline ALP levels. The treatment completion rate was 58% compared to 63%. The main reason for discontinuation in our study was progression of disease.
It has been established that completion of therapy is associated with better overall survival in both large trials and smaller studies [5, 7, 8, 10, 20]. Thus, it has been of interest to determine possible predictive factors for radium-223 therapy completion. In our study, the factors associated with treatment completion were lower baseline ALP and PSA, a finding which is in line with several other publications [5, 8, 19–21]. Baseline ALP was significant on both multivariate and univariate analysis, whereas baseline PSA was only significant on univariate analysis. This is also in accordance with the ALSYMPCA trial, and continues to suggest that patients with less advanced disease are more likely to complete and benefit from radium-223 therapy.
As we found that baseline ALP was associated with treatment completion, it was decided to investigate whether it would also be associated with disease progression or bone marrow toxicity, the two main reasons for treatment non-completion in our study. We found that baseline ALP is significantly associated with clinical progression of disease. This correlation reinforces the possibility of a mechanism for the association between elevated ALP and treatment non-completion whereby the more advanced disease status reflected by the ALP leads to higher rates of disease progression and functional decline, which is known to be associated with treatment non-completion [6, 7, 20]. As poor functional status is also linked with shorter overall survival, it would not be unexpected for higher baseline ALP to be related to higher mortality via this mechanism as well [22, 23].
Given the importance of ALP as a prognostic factor in patients with metastatic prostate cancer, we also sought to see what factors may be predictive of ALP response. There was an association between receiving chemotherapy prior to radium-223 for CRPC and lower rates of ALP response on logistic regression analysis. To our knowledge, this is the first report of such a finding. If validated, this information could be useful in establishing the most advantageous sequencing of chemotherapy and radium-223 therapy.
Our study holds the advantage of reflecting outcomes in real-world usage of radium-223. The major shortcomings of our study are its observational and retrospective nature and the limited sample size. Thus, our findings are hypothesis generating and would need to be confirmed in prospective, preferably randomized, studies.
Conclusion
In patients undergoing radium-223 therapy for metastatic CRPC, we found that higher baseline PSA and ALP were associated with lower rates of treatment completion. The most common reason for treatment non-completion was clinical disease progression, and this was significantly associated with elevated baseline ALP. We also found that use of chemotherapy for CRPC prior to radium-223 is a negative predictor of ALP response.
Supplementary Information
Below is the link to the electronic supplementary material.
Author Contribution
The study was designed by Richard Liu, Stephan Probst, and Cristiano Ferrario. Material preparation and data collection were performed by Richard Liu. The data analysis was performed by Richard Liu and Lamin Juwara. The first draft of the manuscript was written by Richard Liu, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by a grant of $3000 by Bayer.
Data Availability
Contact the corresponding author for data requests.
Declarations
Competing Interests
Stephan Probst declares having previously received a research grant from Bayer for a clinical trial related to radium-223. The remaining authors, Richard Liu, Lamin Juwara, and Cristiano Ferrario, declare that they have no competing interests.
Ethics Approval
The study was approved by the institutional review board of the Jewish General Hospital. The requirement for written consent was waived as the study was retrospective in nature. The study was performed in accordance with the Helsinki declaration as revised in 2013 and its later amendments.
Consent for Publication
Not applicable.
Disclaimer
The funders had no role in study design, data collection, analysis, interpretation, or preparation of the manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Richard F. Liu, Email: richard.liu@mail.mcgill.ca
Lamin Juwara, Email: lamin.juwara@mail.mcgill.ca.
Cristiano Ferrario, Email: cristiano.ferrario@mcgill.ca.
Stephan M. Probst, Email: stephan.probst@mcgill.ca
References
- 1.Teo MY, Rathkopf DE, Kantoff P. Treatment of advanced prostate cancer. Annu Rev Med. 2019;70:479–499. doi: 10.1146/annurev-med-051517-011947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gartrell BA, Saad F. Managing bone metastases and reducing skeletal related events in prostate cancer. Nat Rev Clin Oncol. 2014;11:335–345. doi: 10.1038/nrclinonc.2014.70. [DOI] [PubMed] [Google Scholar]
- 3.Sartor O, Sharma D. Radium and other alpha emitters in prostate cancer. Transl Androl Urol. 2018;7:436–444. doi: 10.21037/tau.2018.02.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parker C, Nilsson S, Heinrich D, Helle SI, O'Sullivan JM, Fosså SD, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–223. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 5.Cheng S, Arciero V, Goldberg H, Tajzler C, Manganaro A, Kozlowski N, et al. Population-based analysis of the use of radium-223 for bone-metastatic castration-resistant prostate cancer in ontario, and of factors associated with treatment completion and outcome. Cancer Manag Res. 2019;11:9307–9319. doi: 10.2147/CMAR.S213051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Doelen MJ, Mehra N, Hermsen R, Janssen MJR, Gerritsen WR, van Oort IM. Patient selection for radium-223 therapy in patients with bone metastatic castration-resistant prostate cancer: New recommendations and future perspectives. Clin Genitourin Cancer. 2019;17:79–87. doi: 10.1016/j.clgc.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Parikh S, Murray L, Kenning L, Bottomley D, Din O, Dixit S, et al. Real-world outcomes and factors predicting survival and completion of radium 223 in metastatic castrate-resistant prostate cancer. Clin Oncol. 2018;30:548–555. doi: 10.1016/j.clon.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Parimi S, Tsang E, Alexander A, McKenzie M, Bachand F, Sunderland K, et al. A population-based study of the use of radium 223 in metastatic castration-resistant prostate cancer: factors associated with treatment completion. Can Urol Assoc J. 2017;11:350–355. doi: 10.5489/cuaj.4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lavelli V, Nappi AG, Caputo P, Asabella AN, Fanelli M, Sardaro A, et al. Impact of pre-treatment variables on the completion of (223)radium-dichloride therapy in mCRPC patients with bone metastases. Hell J Nucl Med. 2019;22(Suppl 2):153–163. [PubMed] [Google Scholar]
- 10.van der Doelen MJ, Kuppen MCP, Jonker MA, Mehra N, Janssen MJR, van Oort IM, et al. 223Ra therapy in patients with advanced castration-resistant prostate cancer with bone metastases: Lessons from daily practice. Clin Nucl Med. 2018;43:9–16. doi: 10.1097/RLU.0000000000001904. [DOI] [PubMed] [Google Scholar]
- 11.van der Doelen MJ, Stockhaus A, Ma Y, Mehra N, Yachnin J, Gerritsen WR, et al. Early alkaline phosphatase dynamics as biomarker of survival in metastatic castration-resistant prostate cancer patients treated with radium-223. Eur J Nucl Med Mol Imaging. 2021;48:3325–3334. doi: 10.1007/s00259-021-05283-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sartor O, Coleman RE, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM, et al. An exploratory analysis of alkaline phosphatase, lactate dehydrogenase, and prostate-specific antigen dynamics in the phase 3 ALSYMPCA trial with radium-223. Ann Oncol. 2017;28:1090–1097. doi: 10.1093/annonc/mdx044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heinrich D, Bruland Ø, Guise TA, Suzuki H, Sartor O. Alkaline phosphatase in metastatic castration-resistant prostate cancer: reassessment of an older biomarker. Future Oncol. 2018;14:2543–2556. doi: 10.2217/fon-2018-0087. [DOI] [PubMed] [Google Scholar]
- 14.Wong WW, Anderson EM, Mohammadi H, Daniels TB, Schild SE, Keole SR, et al. Factors associated with survival following radium-223 treatment for metastatic castration-resistant prostate cancer. Clin Genitourin Cancer. 2017;15:e969–e975. doi: 10.1016/j.clgc.2017.04.016. [DOI] [PubMed] [Google Scholar]
- 15.Dizdarevic S, Jessop M, Begley P, Main S, Robinson A. 223Ra-dichloride in castration-resistant metastatic prostate cancer: Improving outcomes and identifying predictors of survival in clinical practice. Eur J Nucl Med Mol Imaging. 2018;45:2264–2273. doi: 10.1007/s00259-018-4083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49:1373–1379. doi: 10.1016/S0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- 17.Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol. 2007;165:710–718. doi: 10.1093/aje/kwk052. [DOI] [PubMed] [Google Scholar]
- 18.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: Introducing the E-value. Ann Intern Med. 2017;167:268–274. doi: 10.7326/M16-2607. [DOI] [PubMed] [Google Scholar]
- 19.Alva A, Nordquist L, Daignault S, George S, Ramos J, Albany C, et al. Clinical correlates of benefit from radium-223 therapy in metastatic castration resistant prostate cancer. Prostate. 2017;77:479–488. doi: 10.1002/pros.23286. [DOI] [PubMed] [Google Scholar]
- 20.Saad F, Gillessen S, Heinrich D, Keizman D, O'Sullivan JM, Nilsson S, et al. Disease characteristics and completion of treatment in patients with metastatic castration-resistant prostate cancer treated with radium-223 in an international early access program. Clin Genitourin Cancer. 2019;17:348–55.e5. doi: 10.1016/j.clgc.2019.05.012. [DOI] [PubMed] [Google Scholar]
- 21.McKay RR, Jacobus S, Fiorillo M, Ledet EM, Cotogna PM, Steinberger AE, et al. Radium-223 use in clinical practice and variables associated with completion of therapy. Clin Genitourin Cancer. 2017;15:e289–e298. doi: 10.1016/j.clgc.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 22.Chen WJ, Kong DM, Li L. Prognostic value of ECOG performance status and Gleason score in the survival of castration-resistant prostate cancer: a systematic review. Asian J Androl. 2021;23:163–169. doi: 10.4103/aja.aja_53_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vidal M, Delgado A, Martinez C, Correa JJ, Durango IC. Overall survival prediction in metastatic castration-resistant prostate cancer treated with radium-223. Int Braz J Urol. 2020;46:599–611. doi: 10.1590/s1677-5538.ibju.2019.0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Contact the corresponding author for data requests.