Abstract
Critical-sized cranial bone defect remains a great clinical challenge. With advantages in regenerative medicine, injectable hydrogels incorporated with bioactive molecules show great potential in promoting cranial bone repair. Recently, we developed a dual delivery system by sequential release of bone morphogenetic protein 2 (BMP2) followed by insulin-like growth factor 1 (IGF1) in microparticles (MPs), and an injectable alginate/collagen (alg/col)-based hydrogel. In this study, we aim to evaluate the effect of dual delivery of BMP2 and IGF1 in MPs through the injectable hydrogel in critical-sized cranial bone defect healing. The gelatin MPs loaded with BMP2 and poly(lactic-co-glycolic acid)-poly(ethylene glycol)-carboxyl (PLGA-PEG-COOH) MPs loaded with IGF1 were prepared, respectively. The encapsulation efficiency and release profile of growth factors in MPs were measured. A cranial defect model was applied to evaluate the efficacy of the dual delivery system in bone regeneration. Adult Sprague Dawley rats were subjected to osteotomy to make an ⌀8-mm cranial defect. The injectable hydrogel containing MPs loaded with BMP2 (2 μg), IGF1 (2 μg), or a combination of BMP2 (1 μg) and IGF1 (1 μg) were injected to the defect site. New bone formation was evaluated by microcomputed tomography, histological analysis, and immunohistochemistry after 4 or 8 weeks. Data showed that dual delivery of the low-dose BMP2 and IGF1 in MPs through alg/col-based hydrogel successfully restored cranial bone as early as 4 weeks after implantation, whose effect was comparable to the single delivery of high-dose BMP2 in MPs. In conclusion, this study suggests that dual delivery of BMP2 and IGF1 in MPs in alg/col-based hydrogel achieves early bone regeneration in critical-sized bone defect, with advantage in reducing the dose of BMP2.
Impact Statement
Sequential release of bone morphogenetic protein 2 (BMP2) followed by insulin-like growth factor 1 (IGF1) in two different microparticles promotes critical-sized bone defect healing. This dual delivery system reduces the dose of BMP2 by supplementing IGF1, which may diminish the potential side effects of BMP2.
Keywords: craniomaxillofacial bone defect, bone morphogenetic protein, insulin-like growth factor, hydrogel, bone formation
Introduction
Craniomaxillofacial bone defect could be caused by congenital defects or acquired injuries to the face and jaw.1 In most of the cases, critical-sized cranial bone cannot repair spontaneously. The patients ultimately require surgical reconstruction of the cranial bone to protect brain and restore facial skeleton esthetically and functionally.1 Surgical methods such as cranial bone transport have been proposed to restore such bone defects.2,3 However, the complexity of the cranial bone transport surgery may require specific professional surgical skills and instruments. Besides the bone transport, autograft or alloplastic biomaterials, including titanium mesh, polyetheretherketone (PEEK), poly-methyl methacrylate (PMMA), or hydroxyapatite cement have been applied.4 Those alloplastic biomaterials may provide temporary protection to the brain. However, complications, such as erosion, mechanical failure, migration, and infection, may cause damage to the brain and removal of the implant. Recent years, injectable hydrogels incorporated with bioactive molecules show great potential in promoting cranial bone repair.
A variety of natural and synthetic materials have been applied as carriers for the delivery of therapeutic agents, including stem cells, growth factors, or small molecules to tissue defect site in regenerative medicine.5,6 Hydrogels made of various polymers contain large water content, porous microstructure, which provide a stable three-dimensional (3D) microenvironment for cell growth.6 Shear-thinning injectable hydrogels become more and more attractive as they are partially or fully crosslinked before injection, which makes them readily infill and adapt to and stay in tissue defect site with cell encapsulation, without leaking out or diluting by body fluid.5
In our previous study, we successfully developed a facile method to make shear-thinning injectable bone morphogenetic protein 2 (BMP2)-laden alginate/collagen (alg/col) hydrogels.7 BMP2 protein loaded into the hydrogels showed sustained release (SR) in 14 days. This BMP2-laden hydrogel remarkably promotes cranial bone defect healing after 8 weeks of implantation in rats. Besides BMP2, insulin-like growth factor 1 (IGF1) is another growth factor, which exerts promotive effect on cell proliferation and differentiation of osteoblasts.8,9
Previous studies have provided the evidence that combined delivery of BMP2 and IGF1 have better effect than single growth factor, and sequential release of BMP2 followed by IGF1 synergistically promoted osteogenesis of mesenchymal stem cells (MSCs).10 However, the release periods of BMP2 and IGF1 in those dual delivery systems are relatively short, which is not satisfied for translation. Recently, our group has developed a dual delivery system through gelatin-based and poly(lactic-co-glycolic acid)-poly (ethylene glycol)-carboxyl (PLGA-PEG-COOH)-based microparticles (MPs) to achieve stepwise release pattern of BMP2 and IGF1, respectively.11 Sequential release of BMP2 followed by IGF1 was achieved by the different degradation rates of gelatin MPs (gMPs) and PLGA-PEG-COOH MPs (pMPs), leading to a synergistical effect on osteogenic differentiation of MSCs. In our current study, we aim to further evaluate the efficacy of the dual delivery system on cranial bone defect healing in an animal model, through a sequential release of BMP2 followed by IGF1 in the injectable alg/col hydrogels.
Methods
Materials
Type-B gelatin (Mw 40000), N-(3-Dimethylaminopropyl)-N-ethylcarbodiimide hydrochloride (EDC), N-Hydroxysulfosuccinimide (NHS), glyoxal, sodium metabisulfite, 2-(N-Morpholino) ethanesulfonic acid (MES), calcium sulfate (CaSO4), poly(ethylene glycol) (PEG) (Mw 1000), and human BMP-2 ELISA Kit were purchased from Sigma-Aldrich (St. Louis, MO). PLGA-PEG-COOH (50:50, PLGA Mw10kDa, PEG Mw2000) was purchased from Nanosoft polymers (Winston-Salem, NC). Phosphate-buffered saline (PBS, 0.01 M), sodium hydroxide (NaOH), and ethanol were purchased from Thermo Fisher Scientific (Waltham, MA). Rat tail collagen type I was purchased from Corning, Inc., (Corning, NY). BMP2 and IGF1 were purchased from PeproTech (Rocky Hill, NJ). Sodium alginate (alginate, 500GM) was purchased from Pfaltz & Bauer, Inc., (Waterbury, CT).
Fabrication of gMPs and pMPs
Methods of fabrication of gMPs and pMPs, and growth factor loading have been reported previously.11 For the gMPs, 200 mg of gelatin was dissolved in 20 mL of deionized (DI) distilled water. The pH was adjusted to 7.00 with 0.2 M sodium hydroxide. gMPs were formed by displacement of the water with ethanol under stirring. The prepared gMPs were then crosslinked after adding glyoxal. Sodium metabisulfite aqueous solution was added to quench the unreacted aldehyde groups of glyoxal. gMPs were separated after centrifugation, washing, and lyophilization. For the pMPs, 50 mg PLGA-PEG-COOH was dissolved in 8 mL dimethyl sulfoxide, then loaded in a dialysis tube. The mixture was dialyzed against DI water for 24 h for assembly, with a change of water every 2 to 4 h. After self-assembly, the pMPs were obtained after centrifugation and lyophilization. COOH− of pMPs was activated in MES buffer supplemented with EDC and NHS salt for 1 h at room temperature.11–13
Loading of growth factors
Ten milligrams of gMPs or pMPs were dialyzed against DI water overnight, then added to the 1 mL BMP2 solution (20 μg/mL) or IGF1 solution (20 μg/mL) at 4°C to react overnight, respectively. The BMP2-grafted gMPs or IGF1-grafted pMPs were centrifuged and freeze dried. BMP2-gMPs and IGF1-pMPs were sterilized by E-beam with the dosage of 25 kGy (ISO 11137).11 The encapsulation efficiency (EE) and loading capacity (LC) of BMP2 and IGF1 were calculated based on the total amount and leftover amount in supernatant and wash fluid accordingly to the previous study.11,12 Briefly, the EE was defined as the percentages of the amount of grafted growth factors in the MPs to the total amount of growth factors added. The LC was defined as the percentages of the amount of grafted growth factors to the MPs in the total amount of MPs added. The EE and LC were measured before and after E-beam sterilization. After sterilization, the growth factor-grafted MPs were washed in sterilized PBS solution and shaken at 100 rpm at 37°C before lyophilization. The accumulative release profile of BMP2 or IGF1 from the gMPs or pMPs were determined on day 0, 1, 3, 5, 7, 9, 14, 21, and 28 by ELISA Kits, respectively.
Hydrogel preparation
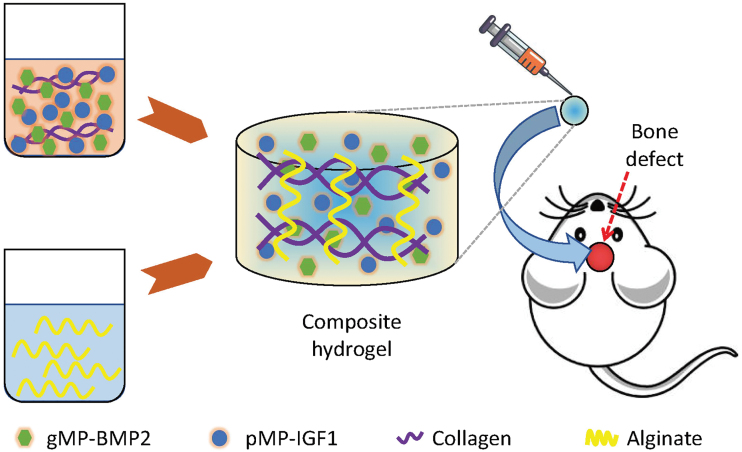
The schematic figure of the study design is shown in Figure 1. The method of preparing alg/col hydrogels has been reported previously.7 One percent (w/v) alginate solution was prepared in calcium-free DMEM , then filtered through a 0.22 μm PES filter (Millex, Millipore). Alginate precursor solution was prepared by a mixture of the filtered alginate solution and 1N NaOH (16 μL/mL), then sterilized by another filtration. Calcium sulfate (CaSO4) was sterilized using E-beam at 25 kGy as previously described.14 One hundred milligrams of sterile CaSO4 was added into 1 mL sterile DI water, and vortexed for 5 min.
FIG. 1.
Schematic figure of the experimental design. Gelatin microparticles (gMPs) and PLGA-PEG-COOH MPs (pMPs) were incorporated with BMP2 (gMP-BMP2) and IGF1 (pMP-IGF1), respectively. These MPs were loaded into the collagen precursor. The collagen precursor solution was gradually added to the alginate precursor solution (1:1, v/v) and mixed thoroughly to form the injectable alg/col-based hydrogel. The hydrogel encapsulating with gMP-BMP2, pMP-IGF1, or a combination of gMP-BMP2 and pMP-IGF1 were implanted into the ⌀8-mm cranial defect rat model. alg/col, alginate/collagen; BMP2, bone morphogenetic protein 2; IGF1, insulin-like growth factor 1; PLGA-PEG-COOH, poly(lactic-co-glycolic acid)-poly(ethylene glycol)-carboxyl. Color images are available online.
The BMP2-grafted gMPs (20 mg), IGF1-grafted pMPs (20 mg), or their mixture (10 mg BMP2-grafted gMPs and 10 mg IGF1-grafted pMPs) were first uniformly dispersed in calcium-free DMEM (0.25 mL) using sonication followed by the addition of 0.25 mL collagen stock solution (4 mg/mL). Fifteen microliters of CaSO4 solution was supplemented to the resultant collagen solution (0.5 mL) and mixed thoroughly to make collagen precursor solutions.15 Finally, the MPs-laden collagen precursor solution was gradually added to the alginate precursor solution (1:1 volume ratio) and mixed thoroughly to form the injectable hydrogel (Fig. 1).
In vivo cranial bone defect repair
Ten-week-old male Sprague Dawley rats (Charles River Laboratories, Wilmington, MA) were used in this study. The animal experimental protocol was approved by the Institutional Animal Care and Use Committee (IACUC) at Stanford University (Approval No.: APLAC-26885) and was in accordance with the National Institute of Health (NIH)'s guidelines.16 Surgical procedures were under anesthesia and conducted based on the previous publications.7,17,18 Briefly, an incision was made along the sagittal midline. The periosteum was carefully incised along the sagittal suture to expose the calvarial bone. A trephine drill (Dentium, Suwon, Korea) was used to create a circle defect with 8-mm diameter under normal saline irrigation, with its center in the middle of the sagittal suture.
Then, alg/col hydrogel (100 μL) loading with MPs, which were grafted by 2 μg BMP2 (gMP-BMP2),7 2 μg IGF1 (pMP-IGF1), or a combination of 1 μg BMP2 and 1 μg IGF1 (MP-BMP2/IGF1) were injected into the cranial defect site by the 23G syringes (n = 6 per time point). The rats without treatment were regarded as blank controls (Blank, n = 6 per time point). Buprenorphine SR was administered subcutaneously to minimize the suffering of the animals after operations. After 4 or 8 weeks, the rats were sacrificed, and cranial bone samples were harvested for microcomputed tomography (micro-CT) analysis and histological analysis.
Micro-CT analysis
All the cranial samples were fixed in 10% neutral buffered formalin and then preserved in 70% ethanol before scanning using a Skyscan 1276 micro-CT (Bruker, Kontich, Belgium) as previously described.7,19,20 A custom isotropic resolution was set at 20 μm per voxel with a voltage of 70 kV and a current of 200 μA. A 0.5 mm Al filter was applied for beam hardening correction. The two-dimensional (2D) images were reconstructed using an NRecon software (version 1.0.7.0.; Bruker) for image analysis. After 2D reconstruction, the images were further analyzed using a CTAn software (version 1.17; Bruker). The 8-mm cranial defect site was regarded as the region of interest (ROI) with the middle of sagittal suture as the center. Twenty-five slices in the ROI were used to create a volume of interest of approximately π × 4 × 4 mm for bone volume/total volume fraction (BV/TV) and percentages of new bone area measurement by CTAn. Three-dimensional images were reconstructed using the MicroView 3D Image Viewer (Version 2.5.0; Parallax Innovations, Inc., Ilderton, Canada) with Hounsfield units threshold >10,000.
Histology
After micro-CT scanning, all the samples were proceeded to histological analysis. The samples were decalcified in 10% EDTA solution for 4 weeks and embedded in optimal cutting temperature compound (Fisher HealthCare, Waltham, MA). Five-micron sections were made by a HM525 NX rotary cryostat (Thermo Fisher, Waltham, MA) along the sagittal suture. The sections were stained with Hematoxylin and Eosin (H&E; Sigma-Aldrich) or the Masson's Trichrome Staining Kit (Abcam, Cambridge, UK) for analysis under a light microscope (Leica, Germany).21 The proportion of new regenerated bone (red), hydrogel residuals (blue), and other tissues in the cranial defect sites (n = 6 per time point) were analyzed using the ImageJ software (NIH, MD).
Immunohistochemistry
The sections were incubated with the primary antibodies against osteocalcin (OCN, 1:200; Santa Cruz) or osteopontin (OPN, 1:200; Santa Cruz) overnight at 4°C. A horseradish peroxidase–streptavidin detection system (Dako, Carpinteria, CA) was applied to develop the positive signals. The randomly selected areas (5 areas in each slide, n = 6 per time point) were selected under a light microscope for analysis. The positive areas were defined by the threshold between 140 and 195 in the images. The ratio of positively stained area in the total defect area was determined by the ImageJ software.7
Statistical analysis
The sample sizes (n = 6) in animal studies are determined by G-Power (Universität Düsseldorf, p < 0.05 with a 90% probability based on the regenerated bone volume in preliminary studies). Data were shown as mean and standard deviation. All quantitative parameters were analyzed using two-way analysis of variance and post hoc Tukey's test. Statistical analysis was performed using a SPSS software (version 16.0; IBM, Armonk, NY). A p-value smaller than 0.05 (p < 0.05) was considered statistically significant.
Results
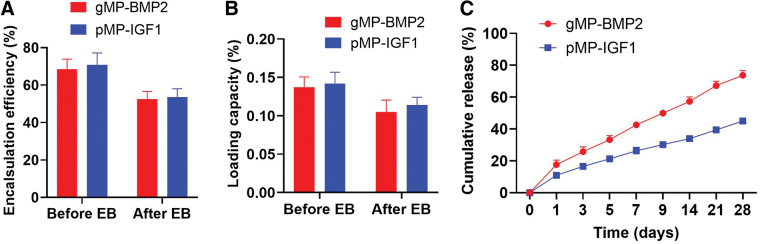
In our recent studies, we have reported the physical and biological properties of the BMP2-grafted gMPs, IGF1-grafted pMPs, and the alg/col hydrogel.7,11 In this study, we also found the similar results in the encapsulation and release pattern. After E-beam sterilization, the EE of BMP2 or IGF1 is 52.46% or 53.48%, and LC of BMP2 or IGF1 is 0.10% or 0.11%, respectively (Fig. 2A, B and Supplementary Table S1). Since the reduction in the EE after E-beam sterilization, the final concentration of BMP2 and IGF1 in alg/col hydrogels was decreased from 40 to around 20 μg/mL. Results also suggested that the release rate of BMP2 is much faster compared with IGF1, showing a sequential SR pattern during the 28 days (Fig. 2C). Our study also revealed that alg/col hydrogel is injectable, biocompatible, bioresorbable, making it a suitable carrier for biomolecules for tissue engineering.7
FIG. 2.
In vitro characterization of BMP2-grafted gelatin MPs (gMP-BMP2) and IGF1-grafted PLGA-PEG-COOH MPs (pMP-IGF1). (A) EE of BMP2 or IGF1 in gMPs or pMPs. The EE was defined as the percentages of the amount of grafted growth factors in the MPs in the total amount of growth factors added. (B) LC of BMP2 or IGF1 in 10 mg gMPs or pMPs. The LC was defined as the percentages of the amount of grafted growth factors in the MPs in the total amount of MPs added. The EE and LC were measured before and after E-beam sterilization. (C) The cumulative release profile of BMP2 and IGF1 for the MPs after E-beam sterilization. Data are shown as mean ± SD (n = 3). EE, encapsulation efficiency; LC, loading capacity; SD, standard deviation. Color images are available online.
A rat cranial bone defect model was adopted to evaluate the effect of dual delivery of BMP2 and IGF1 in the composite hydrogels. This col/alg hydrogel showed shear-thinning and injectable through a 23G syringe, which covered the cranial defect completely and stably, without any leakage (Supplementary Fig. S1).
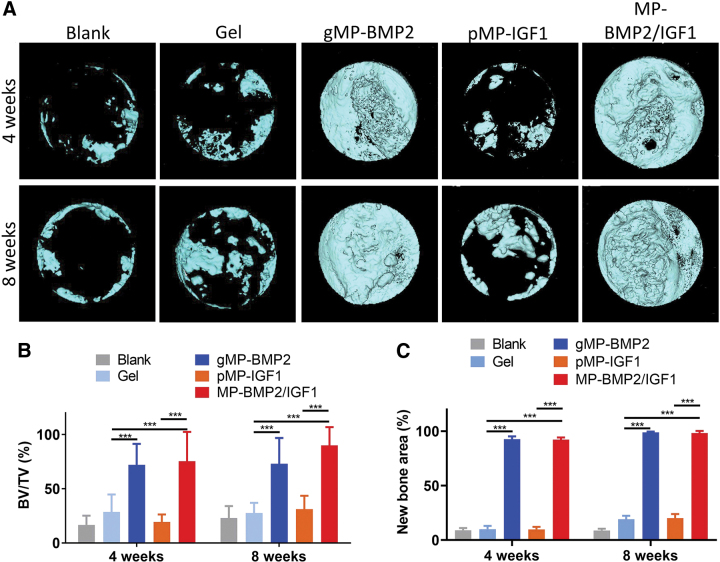
Micro-CT analysis was conducted to quantitatively measure the new bone regeneration at the bone defect site (Fig. 3 and Supplementary Fig. S2). BV/TV and newly regenerated bone area covering the defect sites were analyzed and compared. From the micro-CT data, we found the defect sites were almost empty in the blank control group, and a few bone islands in the hydrogel only group after 4 or 8 weeks of implantation (Fig. 3A). Interestingly, cranial bone healing was significantly enhanced in the gMP-BMP2 group (Fig. 3A), with 152.6% (p < 0.001) or 163.8% (p < 0.001) increase in BV/TV, or with 82.8% (p < 0.001) or 79.9% (p < 0.001) increase in new bone area after 4 or 8 weeks, respectively, compared with the hydrogel only group (Fig. 3B, C). However, the hydrogel loaded with pMP-IGF1 did not show any promotive effect on the bone healing (Fig. 3A–C).
FIG. 3.
Micro-CT results of regenerated bone in the cranial defect sites after 4 or 8 weeks of operation. (A) Representative 3D images of newly regenerated bone in cranial defect sites captured on top of the samples; (B) Quantitative BV/TV, and (C) new regenerated bone area in the defect sites. The animals were treated with with alg/col hydrogel only (Gel), the hydrogel loaded with BMP2 in microparticles (MP-BMP2), or IGF1 in microparticles (MP-IGF1), or a combination of MP-BMP2 and MP-IGF1 (MP-BMP2/IGF1). The animal model without treatment was regarded as blank control (Blank). Data are shown as mean ± SD (n = 6 per time point). ***p < 0.001. 3D, three-dimensional; BV/TV, bone volume/total volume fraction; micro-CT, microcomputed tomography. Color images are available online.
Dual delivery of gMP-BMP2 and pMP-IGF1 in hydrogel significantly enhanced cranial bone healing, with 164.2% (p < 0.001) or 225.5% (p < 0.001) increase in BV/TV, or with 82.3% (p < 0.001) or 79.3% (p < 0.001) increase in regenerated bone area, after 4 or 8 weeks, respectively, compared with the hydrogel only group (Fig. 3B). Compared with the MP-BMP2 only group, the dual delivery of gMP-BMP2 and pMP-IGF1 did not show significant increase in new bone regeneration in the defect sites (Fig. 3A–C).
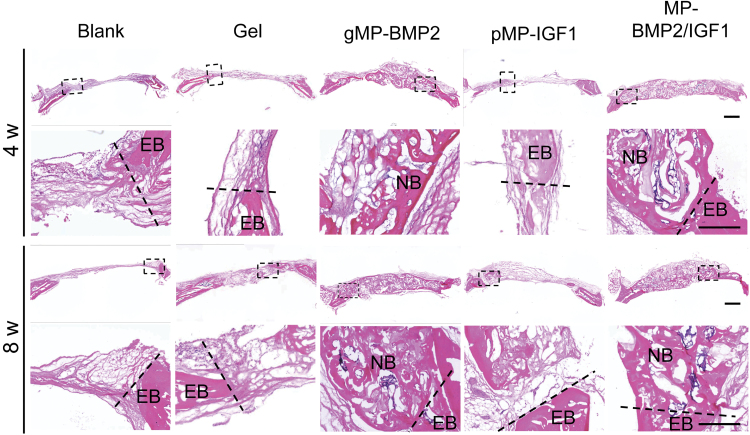
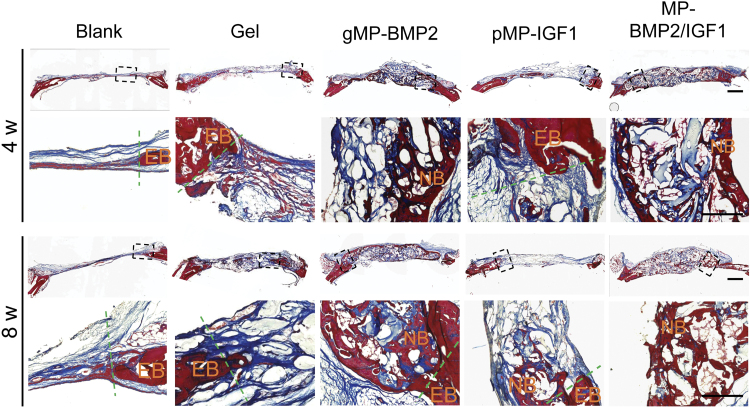
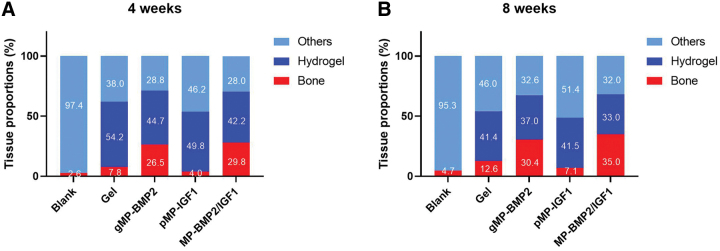
Histological results from H&E staining and Masson's Trichrome staining showed newly regenerated tissue in the cranial bone defect sites and their integration with the existing native bone (Figs. 4 and 5). We found there was mainly fibrous tissue in the defect sites, with very small amount of newly regenerated bone in the blank control group (2.6% or 4.7%) or hydrogel only group (7.8% or 12.6%) at 4 or 8 weeks after treatments (Fig. 6).
FIG. 4.
Representative images of H&E staining of cranial defect sites with native bone at 4 or 8 weeks after operation. The sections were made in coronal axial. Black dash frames indicate the locations of the images in higher magnification. Dash lines indicate the margin dividing EB and the newly formed tissue in cranial defect sites. Scale bar: 1 mm. EB, existing bone; H&E, Hematoxylin and Eosin; NB, new bone. Color images are available online.
FIG. 5.
Representative images of Masson's Trichrome staining of cranial defect sites with existing native bone at 4 or 8 weeks after operation. The sections were made in coronal axial. Black dash frames indicate the locations of the images in higher magnification. Dash lines indicate the margin dividing EB and the newly formed tissue in cranial defect sites. Scale bar: 1 mm. Color images are available online.
FIG. 6.
Semiquantitative data of the proportion of new regenerated bone, hydrogel residuals, and other tissues in the cranial defect sites. Data are shown as mean percentages (5 randomly selected area in each sample, n = 6 per time point). (A) 4 weeks or (B) 8 weeks after treatments. Color images are available online.
Interestingly, a large amount of newly regenerated bone was found at the defect sites with seamless integration to the native bone in both MP-BMP2 group and MP-BMP2/MP-IGF1 group after 4 or 8 weeks of operation (Figs. 4–6). The newly formed bone after 8 weeks of operation seems more mature than that after 4 weeks in the MP-BMP2/MP-IGF1 group as shown by the Masson's Trichrome staining (Fig. 5). However, we found that the newly regenerated bone in both gMP-BMP2 group and MP-BMP2/MP-IGF1 group was still cancellous structure rather than compact bone, indicating bone remodeling was still ongoing after 8 weeks of treatments. Moreover, hydrogel degradation seems fastest in the MP-BMP2/MP-IGF1 group, exhibiting 42.2% or 33.0% hydrogel residues at 4 or 8 weeks after treatments (Fig. 6).
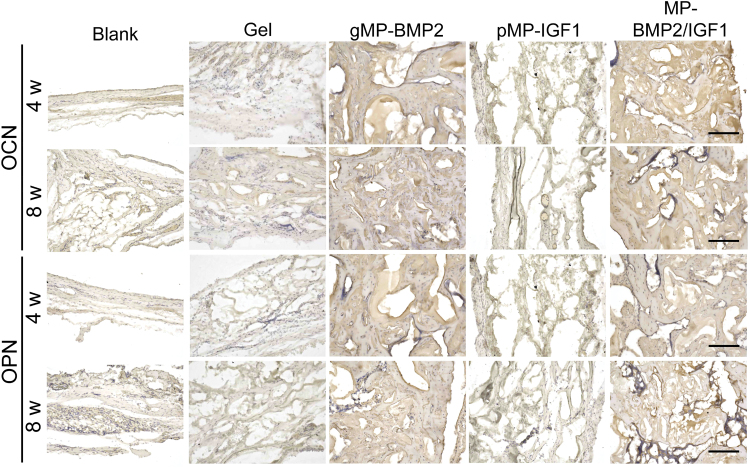
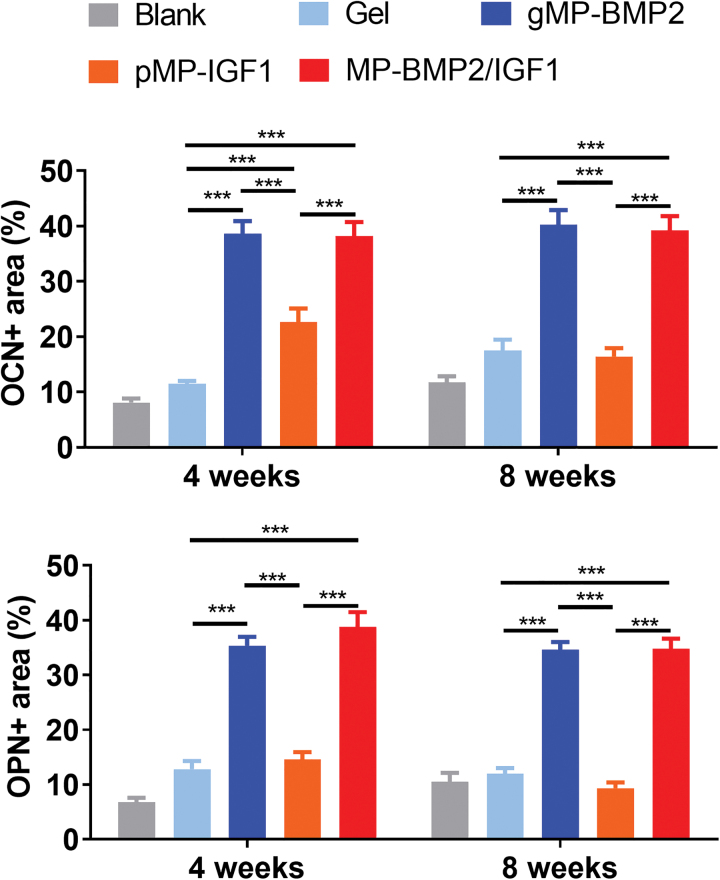
Immunohistochemistry was conducted to measure the new bone formation markers, including OCN and OPN at the bone defect sites (Fig. 7). From the results, we found that both OCN and OPN were highly expressed in the new regenerated bone in the gMP-BMP2 group or MP-BMP2/IGF1 group at 4 or 8 weeks after operation, compared with the hydrogel only group (Figs. 7 and 8). However, pMP-IGF1 did not show significant effect on those bone formation markers, compared with the blank control or hydrogel only group (Figs. 7 and 8).
FIG. 7.
Representative images of bone formation markers in the newly regenerated tissue in cranial defect sites measured by immunohistochemistry at 4 or 8 weeks after operation. Expression of osteocalcin (OCN) or osteopontin (OPN) was determined. Scale bar: 200 μm. Color images are available online.
FIG. 8.
Semiquantitative results of bone formation markers in the newly regenerated tissue in cranial defect sites measured by immunohistochemistry at 4 or 8 weeks after operation. OCN or OPN expression area was determined by ImageJ, normalized by the total cranial defect area. Data are shown as mean ± SD (n = 6 per time point per group). ***p < 0.001. Color images are available online.
Discussion
The shear-thinning, microporous, and injectable alg/col-based hydrogels have been reported in our recent publication, showing favorable properties in growth factor retention, release, and cell viability.7 In this study, the hydrogel was made from collagen precursor and alginate precursor in 1:1 volume ratio, which has been optimized in our previous study.7 In this animal study, we found that the injectable hydrogel could be easily formed after mixing the collagen and alginate precursor solutions and extruded while retaining structural integrity. When the animals were treated with col/alg hydrogel without loading any growth factors, loose fibrous tissue could be seen in majority of the defect sites after 4 or 8 weeks, and no inflammation could be found in the hydrogel implant, indicating a good biocompatibility.
However, only very limited amount of newly formed bone could be found after 8 weeks in the hydrogel-treated animals, showing no significant change compared with the surgical controls. These results revealed that this alg/col-based hydrogel itself could not significantly promote bone healing, while it could be an appropriate carrier for osteoinductive growth factors.
Furthermore, we found that this alg/col-based hydrogel could degrade overtime, with some residuals in all the groups after 8 weeks of implantation. Previous study also found that the degradation of alg/col-based hydrogel was tunable. Among these different volume ratios, the hydrogel could be gradually degraded in PBS solution added with collagenase and citrate buffer, and the remaining 20.5% of the mass after 21 days.7 From our histological results, we still found some hydrogel residuals, indicating the total degradation in vivo should be longer than 8 weeks. Our results show that the degradation rate of the alg/col hydrogel was favorable for the new bone regeneration over the 8 weeks. An appropriate degradation of materials is critical for bone regeneration.22 From the view of regenerative medicine, degradation rate of scaffolds should cope with the pace of tissue regeneration to allow complete replacement with host tissue.23 A slow or fast degradation of hydrogel may retard bone regeneration.24
pMP-IGF1 (2 μg IGF1) only in the alg/col hydrogel did not show significant improvement in the cranial defect healing. Previous studies also showed that IGF1 may mainly contribute to cell proliferation. However, the osteogenic effect of IGF1 in MSCs or bone regeneration in animal models is very limited, and only happens in high dose.25 A combination of IGF1 with other growth factors, such as BMP2,10,11 transforming growth factor-β1,26,27 and platelet-derived growth factor28,29 have shown synergistic effect on osteogenic differentiation or new bone formation. Hence, it is reasonable to make use of the IGF1 with their promotive effect on cell proliferation, combining another growth factor with strong effect on cell differentiation. With a potent osteoinductive effect, BMP2 has been approved for the enhancement of spinal fusion30 and treatment of nonunion.31
In our study, we further characterized the pMP- and gMP-based dual delivery system for sequential release of BMP2 and IGF1. Although BMP2-grafted gMPs and IGF1-grafted pMPs experienced decreased bioactivity to ∼23.5% or 24.5% after E-beam sterilization, the EE of BMP2 or IGF1 was as high as 52.46% or 53.48%, respectively, indicating that over 50% of the total amount of growth factors remained active in the MPs.
In our previous study, we found that a sequential releasing of BMP2 and IGF1 from MPs significantly enhanced osteogenic differentiation of MSCs compared with those of the single delivery of either pMP-IGF1 or gMP-BMP2, or simultaneous administration of IGF1- and BMP2-containing culture medium.11 In this study, gMP-BMP2 (2 μg BMP2) and a combination of gMP-BMP2 (1 μg BMP2) and pMP-IGF1 (1 μg IGF1) in the alg/col hydrogel successfully restored the 8-mm (in diameter) cranial defect as early as 4 weeks after implantation, indicating a comparable effect on bone regeneration between the two groups. It suggests that supplemental IGF1 (1 μg) to the lower dose of BMP2 (1 μg) could be as effective as higher dose of BMP2 (2 μg). The effect of local delivery of BMP2-loading hydrogel has been verified in previous studies.7,32,33
Considering possible side effects of BMP2 in high doses, a supplemental IGF1 may reduce the use of BMP2, which helps in minimizing the risk of causing side effects.34 Although bone mass increased overtime from 4 to 8 weeks, no significant increase in the bone mass could be found in the BMP2 or dual delivery of BMP2/IGF1 treatment group, indicating bone formation was active at the early 4 weeks, and gradually became less active afterward. However, their mechanical properties were not investigated. Considering the importance of mechanical properties in cranial defect healing, push-out testing should be considered in future studies.35
Our team previously developed chitosan/gMP-based dual delivery system for sequential release of BMP2 and IGF1, showing significantly higher osteogenic differentiation potential compared with single growth factor10 in vitro. By developing another novel pMP- and gMP-based dual delivery system, we demonstrated the combined delivery of BMP2 and IGF1 that induced fast release of BMP2 followed by slow release of IGF1, which synergistically promoted osteogenesis compared with single growth factor delivery.11 In this study, we further verified the results in animal experiments and confirmed a synergistic effect of low-dose BMP2 and IGF1 using this dual delivery system in bone regeneration in cranial defect model.
Previous studies have also provided the evidence of co-delivery of BMP2 and IGF1 promoting osteogenesis or bone repair. Zhang et al. showed poly(lactide-co-glycolide) (PLGA) and HA porous composite scaffolds coated with polydopamine, which immobilized BMP2 and IGF1.36 A synergistic effect on bone regeneration in the radius defect model was found by the X-ray imaging. However, a lack of quantitative measurement in the bone mass and histological evidence makes this study inconclusive. Lu et al. made macromer oligo(poly(ethylene glycol) fumarate) (OPF) bilayered hydrogels to deliver BMP2 and IGF1 in gMPs in two separate layers.37 Results showed that subchondral bone formation was synergistically enhanced by the dual delivery of IGF1 and BMP2 in separate layers. However, the release pattern of the growth factors in the bilayered hydrogels were not elaborated.
Overall, this current study found that dual delivery of low-dose BMP2 and IGF1 through alg/col-based hydrogel successfully promoted cranial bone defect healing as early as 4 weeks after implantation. Dual delivery of low-dose BMP2 and IGF1 significantly enhanced bone regeneration, whose effect is comparable to the high-dose BMP2 treatment group. In conclusion, our study suggests that dual delivery of BMP2 and IGF1 in alg/col-based hydrogel has beneficial effect on critical-sized cranial bone defect healing.
One main limitation of this study is that we did not repeat the ex vivo study to characterize the release pattern and the cellular responses of BMP2 and IGF1 in the dual delivery system. In our previous study, we showed the physical properties of gMPs or pMPs, including the morphology, particle size, and growth factor encapsulation and release profiles in MPs and in poly(ethylene glycol) hydrogel.11 Although this study has also shown similar results in terms of EE, LC, and accumulative release, the growth factors were encapsulated in MPs without any hydrogel carrier. We expect that alg/col-based hydrogel may prolong the release of the growth factors encapsulated in MPs, which may be more favorable in promoting bone regeneration. Another limitation is that we did not include a group treated with BMP2 in 1 μg, although previous studies suggested that critical-sized bone defect (8 mm in diameter) in rat could be repaired by BMP2 in a minimal dose of 2.5 μg in 4 weeks.38
Data Availability
All original data, including loading and release of growth factors, micro-CT, and histological data, are available upon request to the corresponding author.
Supplementary Material
Acknowledgments
The authors thank Dr. Masahiro Maruyama, Dr. Alexander Stahl, Dr. Jiannan Li, Dr. Liming Zhao, and Dr. Ning Zhang for their technical support in animal surgeries, micro-CT analysis, and histological analysis. They also thank Stanford Center for Innovation in In vivo Imaging (SCi3) small animal imaging center and Dr. Timothy C. Doyle for providing imaging facilities for this project.
Authors' Contributions
Y.P.Y. designed the study. S.M. and Y.B. prepared hydrogel and microparticles. Y.P., U.L., and S.L. implanted materials into rats and analyzed the samples. S.L., S.K., J.H., N.F.H., and Y.P.Y. wrote and revised the article. Y.P.Y. conceived the study.
Disclosure Statement
No competing financial interests exist.
Funding Information
National Institutes of Health grant U01AR069395 to Y.P.Y., R01AR072613 to Y.P.Y., R01AR074458 to Y.P.Y., and NIH1S10OD02349701 to Timothy C. Doyle. Department of Defense grant W81XWH-20-1-0343 to Y.P.Y. Yonsei University School of Dentistry Intramural Faculty Research Grant 6-2020-0029 to Y.P., and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education NRF-2020R 1F 1A 104997812: 2021-31-0016 to Y.P.
Supplementary Material
References
- 1. Szpalski, C., Barr, J., Wetterau, M., Saadeh, P.B., and Warren, S.M.. Cranial bone defects: current and future strategies. Neurosurg Focus 29, E8, 2010. [DOI] [PubMed] [Google Scholar]
- 2. Elbanoby, T., Aly, G.M., Abdelfattah, U., Choi, J.W., Power, H.A., and Abd El Fattah, Y.A.. Bone transport distraction osteogenesis in the reconstruction of pediatric posttraumatic calvarial defects. Plast Reconstr Surg Glob Open 7, e2201, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gerety, P.A., Wink, J.D., Sherif, R.D., Clarke, N., Nah, H.D., and Taylor, J.A.. Treatment of large calvarial defects with bone transport osteogenesis: a preclinical sheep model. J Craniofac Surg 25, 1917, 2014. [DOI] [PubMed] [Google Scholar]
- 4. Wang, S., Zhao, Z., Yang, Y., et al. A high-strength mineralized collagen bone scaffold for large-sized cranial bone defect repair in sheep. Regen Biomater 5, 283, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dimatteo, R., Darling, N.J., and Segura, T.. In situ forming injectable hydrogels for drug delivery and wound repair. Adv Drug Deliv Rev 127, 167, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Slaughter, B.V., Khurshid, S.S., Fisher, O.Z., Khademhosseini, A., and Peppas, N.A.. Hydrogels in regenerative medicine. Adv Mater 21, 3307, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moeinzadeh, S., Park, Y., Lin, S., and Yang, Y.P.. In-situ stable injectable collagen-based hydrogels for cell and growth factor delivery. Materialia (Oxf) 15, 100954, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lagumdzija, A., Ou, G., Petersson, M., Bucht, E., Gonon, A., and Pernow, Y.. Inhibited anabolic effect of insulin-like growth factor-I on stromal bone marrow cells in endothelial nitric oxide synthase-knockout mice. Acta Physiol Scand 182, 29, 2004. [DOI] [PubMed] [Google Scholar]
- 9. Fiedler, J., Brill, C., Blum, W.F., and Brenner, R.E.. IGF-I and IGF-II stimulate directed cell migration of bone-marrow-derived human mesenchymal progenitor cells. Biochem Biophys Res Commun 345, 1177, 2006. [DOI] [PubMed] [Google Scholar]
- 10. Kim, S., Kang, Y., Krueger, C.A., et al. Sequential delivery of BMP-2 and IGF-1 using a chitosan gel with gelatin microspheres enhances early osteoblastic differentiation. Acta Biomater 8, 1768, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bai, Y., Moeinzadeh, S., Kim, S., et al. Development of PLGA-PEG-COOH and gelatin-based microparticles dual delivery system and E-beam sterilization effects for controlled release of BMP-2 and IGF-1. Part Part Syst Charact 37, 2000180, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kang, F., Yi, Q., Gu, P., et al. Controlled growth factor delivery system with osteogenic-angiogenic coupling effect for bone regeneration. J Orthop Translat 31, 110, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kuo, W.T., Huang, J.Y., Chen, M.H., et al. Development of gelatin nanoparticles conjugated with phytohemagglutinin erythroagglutinating loaded with gemcitabine for inducing apoptosis in non-small cell lung cancer cells. J Mater Chem B 4, 2444, 2016. [DOI] [PubMed] [Google Scholar]
- 14. Bruyas, A., Moeinzadeh, S., Kim, S., Lowenberg, D.W., and Yang, Y.P.. Effect of electron beam sterilization on three-dimensional-printed polycaprolactone/beta-tricalcium phosphate scaffolds for bone tissue engineering. Tissue Eng Part A 25, 248, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bendtsen, S.T., and Wei, M.. Synthesis and characterization of a novel injectable alginate-collagen-hydroxyapatite hydrogel for bone tissue regeneration. J Mater Chem B 3, 3081, 2015. [DOI] [PubMed] [Google Scholar]
- 16. National Research Council (U.S.). Committee for the Update of the Guide for the Care and Use of Laboratory Animals., Institute for Laboratory Animal Research (U.S.), National Academies Press (U.S.). Guide for the care and use of laboratory animals, 8th ed. National Academies Press, 2011:xxv, 220 p. [Google Scholar]
- 17. Zhang, K., Lin, S., Feng, Q., et al. Nanocomposite hydrogels stabilized by self-assembled multivalent bisphosphonate-magnesium nanoparticles mediate sustained release of magnesium ion and promote in-situ bone regeneration. Acta Biomater 64, 389, 2017. [DOI] [PubMed] [Google Scholar]
- 18. Liu, C., Lin, C., Feng, X., et al. A biomimicking polymeric cryogel scaffold for repair of critical-sized cranial defect in a rat model. Tissue Eng Part A 25, 1591, 2019. [DOI] [PubMed] [Google Scholar]
- 19. Liu, C.N., Morin, J., Dokmanovich, M., et al. Nanoparticle contrast-enhanced micro-CT: a preclinical tool for the 3D imaging of liver and spleen in longitudinal mouse studies. J Pharmacol Toxicol Methods 96, 67, 2019. [DOI] [PubMed] [Google Scholar]
- 20. Buytaert, J., Goyens, J., De Greef, D., Aerts, P., and Dirckx, J.. Volume shrinkage of bone, brain and muscle tissue in sample preparation for micro-CT and light sheet fluorescence microscopy (LSFM). Microsc Microanal 20, 1208, 2014. [DOI] [PubMed] [Google Scholar]
- 21. Shi, G.S., Li, Y.Y., Luo, Y.P., et al. Bioactive PLGA/tricalcium phosphate scaffolds incorporating phytomolecule icaritin developed for calvarial defect repair in rat model. J Orthop Translat 24, 112, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang, F., Wang, J., Cao, L., Chen, R., Tang, L., and Liu, C.. Injectable and redox-responsive hydrogel with adaptive degradation rate for bone regeneration. J Mater Chem B 2, 295, 2014. [DOI] [PubMed] [Google Scholar]
- 23. Stevens, B., Yang, Y., Mohandas, A., Stucker, B., and Nguyen, K.T.. A review of materials, fabrication methods, and strategies used to enhance bone regeneration in engineered bone tissues. J Biomed Mater Res B Appl Biomater 85, 573, 2008. [DOI] [PubMed] [Google Scholar]
- 24. Patterson, J., Siew, R., Herring, S.W., Lin, A.S., Guldberg, R., and Stayton, P.S.. Hyaluronic acid hydrogels with controlled degradation properties for oriented bone regeneration. Biomaterials 31, 6772, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Qi, Z., Xia, P., Pan, S., et al. Combined treatment with electrical stimulation and insulin-like growth factor-1 promotes bone regeneration in vitro. PLoS One 13, e0197006, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Srouji, S., Blumenfeld, I., Rachmiel, A., and Livne, E.. Bone defect repair in rat tibia by TGF-beta1 and IGF-1 released from hydrogel scaffold. Cell Tissue Bank 5, 223, 2004. [DOI] [PubMed] [Google Scholar]
- 27. Blumenfeld, I., Srouji, S., Lanir, Y., Laufer, D., and Livne, E.. Enhancement of bone defect healing in old rats by TGF-beta and IGF-1. Exp Gerontol 37, 553, 2002. [DOI] [PubMed] [Google Scholar]
- 28. Ortolani, E., Guerriero, M., Coli, A., Di Giannuario, A., Minniti, G., and Polimeni, A.. Effect of PDGF, IGF-1 and PRP on the implant osseointegration. An histological and immunohistochemical study in rabbits. Ann Stomatol (Roma) 5, 66, 2014. [PMC free article] [PubMed] [Google Scholar]
- 29. Stefani, C.M., Machado, M.A., Sallum, E.A., Sallum, A.W., Toledo, S., and Nociti, H.. Platelet-derived growth factor/insulin-like growth factor-1 combination and bone regeneration around implants placed into extraction sockets: a histometric study in dogs. Implant Dent 9, 126, 2000. [DOI] [PubMed] [Google Scholar]
- 30. Garrison, K.R., Donell, S., Ryder, J., et al. Clinical effectiveness and cost-effectiveness of bone morphogenetic proteins in the non-healing of fractures and spinal fusion: a systematic review. Health Technol Assess 11, 1, 2007. [DOI] [PubMed] [Google Scholar]
- 31. Emara, K.M., Diab, R.A., and Emara, A.K.. Recent biological trends in management of fracture non-union. World J Orthop 6, 623, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mumcuoglu, D., Fahmy-Garcia, S., Ridwan, Y., et al. Injectable BMP-2 delivery system based on collagen-derived microspheres and alginate induced bone formation in a time- and dose-dependent manner. Eur Cell Mater 35, 42, 2018. [DOI] [PubMed] [Google Scholar]
- 33. Seo, B.B., Koh, J.T., and Song, S.C.. Tuning physical properties and BMP-2 release rates of injectable hydrogel systems for an optimal bone regeneration effect. Biomaterials 122, 91, 2017. [DOI] [PubMed] [Google Scholar]
- 34. James, A.W., LaChaud, G., Shen, J., et al. A review of the clinical side effects of bone morphogenetic protein-2. Tissue Eng Part B Rev 22, 284, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Spicer, P.P., Kretlow, J.D., Young, S., Jansen, J.A., Kasper, F.K., and Mikos, A.G.. Evaluation of bone regeneration using the rat critical size calvarial defect. Nat Protoc 7, 1918, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang, J., Li, J., Jia, G., et al. Improving osteogenesis of PLGA/HA porous scaffolds based on dual delivery of BMP-2 and IGF-1 via a polydopamine coating. 10.1039/C7RA12062A. RSC Adv 7, 56732, 2017. [Google Scholar]
- 37. Lu, S., Lam, J., Trachtenberg, J.E., et al. Dual growth factor delivery from bilayered, biodegradable hydrogel composites for spatially-guided osteochondral tissue repair. Biomaterials 35, 8829, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pelaez, M., Susin, C., Lee, J., et al. Effect of rhBMP-2 dose on bone formation/maturation in a rat critical-size calvarial defect model. J Clin Periodontol 41, 827, 2014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All original data, including loading and release of growth factors, micro-CT, and histological data, are available upon request to the corresponding author.