Abstract
Through the long-term activation of the sympathetic nervous system and the hypothalamic-pituitary-adrenal axis, chronic psychosocial stress can compromise mental and bodily health. Psychosocial stress is determined by the perception of social interactions as ego-threatening, and thus strongly influenced by individual social processing capacities. In the current study, we investigated whether three key components of social processing are linked to how individuals respond to the experience of acute psychosocial stress exposure. Empathy, compassion, and Theory of Mind (ToM) were assessed using a state-of-the-art paradigm, the EmpaToM. Participants (N = 118) also underwent the Trier Social Stress Test (TSST), a standardized psychosocial laboratory stress test. Stress responses were measured in terms of salivary cortisol and alpha-amylase, heart-rate, high-frequency heart-rate variability (HF-HRV), and subjective stress experience. ToM performance correlated with different aspects of the acute psychosocial stress response. More specifically, higher levels of ToM were linked to increased alpha-amylase and reduced HF-HRV sensitivity to stress. Empathy and compassion levels had no influence on stress sensitivity. We conclude that ToM performance has a stable albeit contradictory association with acute psychosocial stress, while empathy and compassion tendencies appear to be largely unrelated. Overall, the relationship between EmpaToM-derived empathy, compassion, and ToM characteristics with stress sensitivity in the TSST is relatively weak.
Keywords: Psychosocial stress, Cortisol, Empathy, Theory of mind, Compassion
Highlights
-
•
Empathy and compassion showed no associations with psychosocial stress-sensitivity.
-
•
ToM was linked to increased sympathetic and decreased parasympathetic reactivity.
-
•
Socio-affective and -cognitive abilities are loosely linked to psychosocial stress.
1. Introduction
Acute psychosocial stress, although often unpleasant, is a healthy response to everyday adversities. However, the experience of chronic psychosocial strain can have detrimental effects on physical and emotional health, leading to a range of physiological and psychological pathologies [1,2]. When confronted with psychosocial stress, two main systems are activated. The sympathetic nervous system (SNS) prompts the release of epinephrine and norepinephrine from the adrenal glands, and the hypothalamic-pituitary-adrenal (HPA) axis triggers the release of the main stress hormone cortisol [1,2].
Research has shown that the capacity to understand and control one's own emotions has an important impact on an individual's stress vulnerability [3]. However, we know very little about associations between stress vulnerability and the social capacity to understand and share the emotions and mental states of others. Because psychosocially stressful situations are defined by their socio-evaluative and uncontrollable nature [4], their perception as such is determined by social cognitions. In other words, the mechanisms underlying our ability to understand and interact with our social environment shape the very information upon which a given psychosocial stress response is based [5]. We therefore hypothesize that our abilities to share the emotions of others (empathy; [6]), to generate positive feelings of concern and prosocial motivation towards others (compassion; [7]), and to understand their thoughts and believes (Theory of Mind; [17]) affect the way in which we react to psychosocial stress. Previous work on the topic has mostly focused on the causal effect of how psychosocial stress influences subsequent social processes [[8], [9], [10]]. In the current study, we contrarily explored how individual differences in measures of socio-affective and socio-cognitive abilities are linked to adaptively coping with a psychosocially stressful situation.
Empathy has been defined as the process of generating an isomorphic affective state of another in oneself, while realizing that the source of that state lies in the other [6]. Only few studies have investigated how empathy relates to psychosocial stress. One found no association between self-reported empathy and stress-induced cortisol release [8]. The other revealed elevated subjective stress in individuals with higher self-reported empathic concern [11].
While empathy is broadly understood as feeling with somebody else [6], compassion is about feeling for another person. More precisely, compassion is characterized by feelings of warmth and concern, as well as the motivation to help the other [7]. While empathic distress is a self-related negative state, often resulting in withdrawal to avoid negative affect, compassion is other-related, positive, linked to reward- and affiliation-related brain activity, and acts as a drive to care for the welfare of others [7].
Associations of compassion with psychosocial stress responsivity remain mostly unexplored. One study found stress-buffering effects of trait compassion - if participants were stressed by individuals acting in a supportive rather than neutral manner [12]. In the context of a longitudinal mental training study (the ReSource Project; [19]), our group observed that regularly performed compassion-based mental training over a duration of three months increased compassion in the EmpaToM, a socio-cognitive computer task simultaneously assessing reactive measures of empathy, compassion and theory of mind [13], and reduced subjective and cortisol stress reactivity to acute psychosocial stress [14]. Next to an increase in positive emotions in everyday life [15], one other study showed a decrease in cortisol stress reactivity after eight weeks of loving-kindness meditation for participants adhering to high training frequency [16].
In ToM [17] an individual infers and reasons about the mental states of others. Another's affective state would therefore not be reproduced and experienced but represented conceptually. One study which investigated the specific effects of mentalizing on psychosocial stress reactivity, found elevated cortisol and heart-rate reactivity in association with higher mentalizing accuracy in the Reading the Mind in the Eyes Test (RMET; [11]). In two further studies stemming from the ReSource Project, a 3-month mental training module cultivating perspective taking on self and others specifically improved ToM performance in the EmpaToM [18], and, like the compassion-based training, had a buffering effect on subjective and cortisol reactivity to acute psychosocial stress [14].
Our current study was embedded in the large-scale ReSource Project [19]. The utilized data stem from the training-free retest control cohort, and from the pre-training baseline measurements of the experimental cohorts. In detail, we investigated in N = 118 healthy male and female participants whether individual differences in the tendency and ability to engage in certain social capacities such as empathy, compassion and Theory of Mind (ToM), were linked to individual differences in acute psychosocial stress sensitivity. Empathy and compassion tendencies as well as ToM capacities were measured using the computerized EmpaToM task [13]. These social capacities were subsequently related to self-reported and physiological reactivity to, and recovery from, a standardized psychosocial laboratory stressor, the Trier Social Stress Test (TSST; [20]).
Based on the presented literature in non-clinical healthy samples, we derived the following hypotheses: Because a higher tendency to share others’ negative affective states, especially if experiencing empathic distress, is linked to higher psychosocial stress sensitivity, we expected positive associations between higher levels in empathy and the acute psychosocial stress response. The compassion-based training literature suggests a stress-buffering role of compassion. Thus, we expected higher levels of compassion to go along with lower psychosocial stress sensitivity. With regard to ToM, mental training cultivating perspective taking abilities has been linked to a blunted psychosocial stress response [14] (see Ref. [11] for contradictory results). We therefore suggest that the ability to take others perspectives may already be beneficial for stress reduction in untrained individuals.
2. Materials and methods
2.1. Participants
Participants were recruited in the context of the ReSource Project, a multi-method longitudinal mental training intervention study [19]. Out of the 332 ReSource participants, a subsample of N = 130 was subjected to the Trier Social Stress Test without prior training exposure (either at the baseline measurement time point of the project, or as part of a retest control cohort that did not undergo any training). Twelve individuals were excluded from the current analysis because they were missing EmpaToM data points. This resulted in a sample of N = 118 participants (65 women; age M = 40.10, SD = 9.03, age range = 22–55). Although the stress data reported here have previously been published in the context of other research questions [14,21,22], none of these studies linked measures of acute stress responsivity to measures of socio-emotional and socio-cognitive abilities. Data derived from the EmpaToM has also been published elsewhere [13,18,[23], [24], [25], [26], [27], [28], [29], [51]].
Before commencement of the study, volunteers underwent a comprehensive face-to-face mental health diagnostic interview with a trained clinical psychologist. The interview included a computer-assisted German version of the Structured Clinical Interview for DSM-IV Axis-I disorders, the SCID-I DIA-X [30], and a personal interview for Axis-II disorders, the SCID-II [31]. Volunteers were excluded if they fulfilled criteria for an Axis-I disorder within the past two years, or for schizophrenia, psychotic disorder, bipolar disorder, substance dependency, or an Axis-II disorder at any time in their life. Volunteers taking medication influencing the HPA axis were also excluded. Details of the multistep recruitment procedure, inclusion/exclusion criteria, and the final sample description of the ReSource Project can be found in Ref. [19]. At the testing time point reported here, all participants were training-naive. Female hormonal status on the day of stress testing was assessed through self-report. Thirty-four women had a natural menstrual cycle, 11 took hormonal contraceptives, and 20 reported to have no cycle, either due to menopause or polycystic ovary syndrome.
The ReSource Project was registered with the Protocol Registration System of ClinicalTrial.gov under the title “Plasticity of the Compassionate Brain” (Identifier NCT01833104). It was approved by the Research Ethics Boards of Leipzig University (ethic number: 376/12-ff) and Humboldt University Berlin (ethic number: 2013–20, 2013–29, 2014–10). Participants gave their written informed consent, could withdraw from the study at any time, and were financially compensated for the testing sessions. The current study was not preregistered.
2.2. Experimental design and procedure
TSST and EmpaToM testing took place on two separate occasions, on average 14 days apart (range = 1-73 days). Stress testing was performed between 12pm and 6pm in one 130-min session, taking place either at the MPI-CBS in Leipzig or at an affiliated laboratory in Berlin. Upon arrival at the laboratory, participants received a snack and a glass of juice to equalize blood sugar levels. During testing, participants were only allowed to drink water. At 15 min after arrival, the baseline subjective stress questionnaire and saliva sample for cortisol and alpha-amylase measurements were collected (at −55 min before stressor onset). After a 30 min resting phase, participants were provided with the stress testing instructions. Following 10 min of stress anticipation, the subjective stress questionnaire was administered for a second time (at −5 min prior to stressor onset, resulting in an anticipation phase of 15 min duration), followed by the TSST stress phase. Subjective stress questionnaires and saliva samples were again collected immediately after the stress phase (between 10 and 12 min after stressor onset), and throughout the 50-min recovery phase (at 20, 30 and 55 min after stressor onset). An electrocardiogram (ECG) to assess sympathetic and parasympathetic nervous system activity was continuously recorded from 30 min prior until 25 min after stressor onset (see Fig. 1 for the testing timeline). Next to the above markers, three blood samples for the measurement of oxytocin were drawn throughout the stress session. Oxytocin data was not included in the current analysis.
Fig. 1.
TSST testing timeline. Baseline subjective stress, cortisol and alpha-amylase samples were collected at −55 min before stressor onset. Participants received testing instructions 15 min before stressor onset, resulting in 10 min of stress anticipation. The subjective stress questionnaire was administered for a second time at −5 min prior to stressor onset. Subjective stress questionnaires and saliva samples were again collected at 10, 20, 30 and 55 min after stressor onset. An electrocardiogram to assess sympathetic and parasympathetic nervous system activity was continuously recorded from 30 min prior until 25 min after stressor onset.
Behavioral EmpaToM data was collected inside the scanner during a 140-min magnetic resonance imaging session at the MPI-CBS in Leipzig. Next to the EmpaToM, several cognitive tasks and resting state scans were acquired at this testing appointment. As our research question solely focuses on the relationship of behavioral EmpaToM data and psychosocial stress, these data are also not subject to the current analysis.
2.3. Stress induction
Participants underwent the Trier Social Stress Test (TSST; Kirschbaum et al., 1993), a standardized psychosocial stress paradigm, which reliably induces physiological and psychological stress responses. Briefly, the TSST entails an anticipation phase of flexible duration, an audio- and videotaped mock job talk (5 min), as well as a difficult mental arithmetic task (5 min). These challenges are performed in front of a gender-mixed committee of two alleged behavioral analysts, who are trained to be non-empathic to the struggles of the participant. The efficacy of the TSST to induce psychosocial stress is based on the components of novelty, unpredictability, uncontrollability, and social-evaluative threat [4].
2.4. The EmpaToM task: Empathy, compassion and theory of mind
The EmpaToM task is a computer-based behavioral paradigm used to induce and capture tendencies in empathic and compassionate involvement as well as mentalizing abilities [13]. The EmpaToM measures have been validated against neural activations using standard imaging paradigms and behavioral assessments from other published empathy, compassion and theory of mind paradigms [13,26]. In the EmpaToM, participants are initially presented with a fixation cross (1–3s), followed by the name of a person (1s) subsequently speaking in one of altogether forty-eight 15s video clips. The allegedly autobiographic stories told in these video clips by actors vary in emotional content (neutral vs. negative) and in the kind of question posed about video content (factual vs. ToM-related). After each clip, participants are asked to rate their own subjective feeling (How do you feel, ranging from negative to positive?), and the degree to which they feel compassionate for the person they have observed (How much compassion do you feel, ranging from none to very much?) on a continuous scale from ranging from 0 to 6. Following another fixation cross (1–3s), participants are presented with a three-answer multiple choice question either requiring a ToM inference of the speaker's mental state or factual reasoning about the content of the shown video clip, depending on condition (nonToM vs. ToM) (14s). The chosen answer is highlighted and shown for another second, after which a fixation cross (0–2s) is presented. While compassion is directly reported, empathy is operationalized as an isomorphic emotional state in the observer, that is, ratings of affect in the emotional minus the neutral video condition. By using the difference score of affect ratings between emotional and neutral trials as an approximation of empathy, general mood during the task is controlled for. A higher average empathy difference score reflects a higher tendency to empathize. Compassion ratings were averaged across all trials types. A composite ToM score was calculated by z-standardizing accuracy and reaction times, subtracting reaction time means from accuracy ratings, and dividing the result by two, providing a measure of ToM capacity controlling for individual differences in speed and/or accuracy driven response strategies.
2.5. Measures of acute stress reactivity
Cortisol and alpha-amylase. Cortisol and alpha-amylase were captured as proxies of HPA axis and sympathetic nervous system activity. They were collected in saliva using Salivettes (Sarstedt, Nümbrecht, Germany). Participants placed the saliva collection swabs in their mouth and refrained from chewing for 2 min. Salivettes were stored at −30 °C until assay (at the Department of Biological and Clinical Psychology, University of Trier, Germany). For determination of cortisol activity (nmol/l), a time-resolved fluorescence immunoassay with intra- and interassay variabilities of less than 10% and 12% was used [32]. Alpha-amylase activity (expressed in U/ml) was determined using an enzyme kinetic method [33]. Because one or more measurements were missing, three participants were excluded from cortisol and six participants were excluded from alpha-amylase analysis.
Autonomic nervous system activity. To gauge autonomic activity, heart-rate and high frequency heart-rate-variability (HF-HRV) were continuously sampled at a frequency of 250 Hz using the Zephyr Bioharness 3 (Zephyr Technology, Annapolis, Maryland, USA). Heart-rate is a measure of predominantly sympathetic activity, with higher frequencies relating to increased activity. HF-HRV measures the variability of heart-rate in the respiration frequency range of 0.15–0.4 Hz, and is considered a reliable marker of parasympathetic, specifically vagus nerve, activity [34]. The current 65-min electrocardiography recording was split into four time-phases: A 10-min timeframe before stressor onset (from −30 to −20 min), a 20-min timeframe before and during the anticipatory phase (from −20 to 0 min), a 10-min timeframe right after stressor onset (from 0 to 10 min), and a 15-min timeframe in the recovery phase (from 10 to 25 min). Because of variation in the transitions between phases, only the middle 8-min ECG sequences of baseline and stress phases were included into the analysis (i.e., the first and last minutes per phase were excluded). For the same reason, only the final 8-min sequence of the recovery phase was included. Raw ECG data was extracted using Matrix Laboratory (Matlab; version R2014a) and manually checked for artifacts using in-house software of the MPI CBS. Twenty participants were excluded from analysis due to unusable or missing data in one of the time phases. Lastly, using Artiifact [35], beats per min (heart-rate) and square milliseconds (HF-HRV) were averaged per participant in each time-frame.
Self-reported stress. Subjective stress experience throughout the stress session was assessed using the 20-item state scale of the State Trait Anxiety Inventory [36] which targets feelings of apprehension, nervousness, tension, worry, and activation/arousal of the autonomic nervous system. The state scale of the STAI has been validated as a measure of subjective stress experience across numerous studies [37].
2.6. Statistical analysis
Analyses were performed using R 4.1.2 [38]. All physiological measures were ln-transformed and winsorized to 3 standard deviations to handle skewness and normalize the data for subsequent analysis.
Model building. To capture cortisol, alpha-amylase, STAI, heart-rate and HF-HRV stress sensitivity, Area under the curve with respect to increase (AUCi) was calculated. AUCi is a measure of overall stress-sensitivity, as the recovery period is included in the calculation [39]. Subsequently, stress marker AUCi were predicted by EmpaToM-derived empathy, compassion and ToM using linear multiple regression modeling.
Concerning covariates, age and gender were added as control variables in the cortisol models. Because a female “hormonal status” factor with four levels (male, natural cycle, hormonal contraceptives or no cycle) did not significantly improve model fit in comparison to participant gender, it was not included into the cortisol model. Age, gender and BMI were included in the alpha-amylase, heart-rate and HF-HRV models. In the STAI model, only age and gender were included as controlling factors. Empathy, compassion and ToM were added as single fixed effects. Standardized regression coefficients (β), confidence intervals (CI) and p-values are reported.
Because TSST and EmpaToM data acquisition was not realized on the same day, we conducted follow-up analyses while controlling for the number of days between testing sessions. Only for the alpha-amylase model, an additional significant interaction of number of days between testing and compassion emerged. All other effects remained unaffected. For a more detailed description of the follow-up analysis, see the Supplementary Results.
Missing data. As the calculation of AUCi requires complete data sets, participants missing single data points were excluded from analysis via list-wise deletion. In our sample of N = 118 participants, N = 3 were missing at least one cortisol data-point, N = 4 were missing at least one alpha-amylase data-point and N = 3 were missing at least one STAI data point. N = 20 participants were missing at least one heart-rate data point and N = 21 participants were missing at least one HF-HRV data-point. Furthermore, N = 3 participants were missing BMI data-points, further reducing the available observations for the alpha-amylase, heart-rate and HF-HRV models. The number of observations in each model can be found in the model summaries (see Table 2).
Table 2.
Multiple regression model summaries.
| AUCi |
Cortisol |
Alpha-amylase |
STAI |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Predictors | β | CI | Β | CI | β | CI | |||
| (Intercept) | 0.05 | −0.24–0.34 | −0.17 | −0.46–0.11 | −0.10 | −0.38–0.19 | |||
| Age | −0.21 * | −0.40–−0.01 | 0.11 | −0.10–0.31 | −0.14 | −0.33–0.06 | |||
| Gender [Women] | −0.09 | −0.49–0.31 | 0.31 | −0.09–0.71 | 0.18 | −0.22–0.58 | |||
| Empathy | −0.13 | −0.35–0.09 | 0.02 | −0.19–0.23 | 0.06 | −0.16–0.28 | |||
| Compassion | 0.13 | −0.09–0.34 | −0.03 | −0.24–0.18 | 0.00 | −0.21–0.22 | |||
| ToM | 0.08 | −0.11–0.28 | 0.23 * | 0.04–0.41 | −0.05 | −0.25–0.14 | |||
| BMI | −0.18 | −0.38–0.01 | |||||||
| Observations | 115 | 111 | 115 | ||||||
| R2/R2 adjusted |
0.057/0.014 |
0.146/0.097 |
0.029/−0.016 |
||||||
| AUCi | Heart-rate | HF-HRV | |||||||
| Predictors | β | CI | β | CI | |||||
| (Intercept) | −0.13 | −0.46–0.19 | 0.30 | −0.00–0.61 | |||||
| Age | −0.17 | −0.41–0.08 | −0.05 | −0.28–0.17 | |||||
| Gender [Women] | 0.24 | −0.21–0.70 | −0.55 * | −0.98–−0.12 | |||||
| BMI | 0.01 | −0.23–0.24 | 0.03 | −0.19–0.25 | |||||
| Empathy | −0.06 | −0.30–0.19 | −0.05 | −0.28–0.18 | |||||
| Compassion | 0.03 | −0.21–0.26 | 0.09 | −0.13–0.31 | |||||
| ToM | −0.12 | −0.33–0.10 | 0.33 ** | 0.13–0.53 | |||||
| Observations | 95 | 94 | |||||||
| R2/R2 adjusted | 0.036/−0.030 | 0.157/0.099 | |||||||
Note. Multiple linear regression coefficients for AUCi cortisol, alpha-amylase, STAI, heart-rate and HF-HRV models. Standardized beta-coefficients (β), confidence intervals (CI) are presented. Significant regression weights are depicted as * p < 0.05, **p < 0.01, ***p < 0.001.
3. Results
3.1. Descriptive results
Descriptive statistics and intercorrelations of the employed measures and variables are presented in Table 1. Age and BMI showed a positive association, suggesting higher BMI values with increasing age (r = 0.33, CI [0.16, 0.49]). Compassion ratings were moderately correlated with empathy ratings (r = 0.49, CI [0.34, 0.62]). Regarding the assessed stress markers (all operationalized as AUCis) heart-rate and HF-HRV showed a negative correlation (r = −0.47, CI [-0.61, −0.30]) and heart-rate and cortisol showed a positive correlation (r = 0.22, CI [0.01, 0.40]). The remaining stress markers showed no associations. ToM was positively correlated with both alpha-amylase (r = 0.27, CI [0.09, 0.43]) and HF-HRV (r = 0.26, CI [0.06, 0.44]). We found no gender differences in terms of empathy (d = −0.32, CI [-0.69, 0.07] or compassion (d = −0.33, CI [-0.72, 0.05]). Women had moderately higher ToM scores than men (d = 0.40, CI [-0.77, −0.01]).
Table 1.
Means, standard deviations, and correlations of covariates, EmpaToM variables and stress marker AUCi.
| Variable | M | SD | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 Age | 40.10 | 9.03 | |||||||||
| 2 BMI | 23.69 | 2.79 | .33** | ||||||||
| 3 Empathy | 1.52 | 0.80 | −.14 | −.17 | |||||||
| 4 Compassion | 3.37 | 0.77 | .10 | −.06 | .49** | ||||||
| 5 ToM | 0.00 | 0.80 | −.04 | −.10 | .13 | .05 | |||||
| 6 Cortisol | 38.71 | 46.26 | −.19* | .03 | −.04 | .04 | .07 | ||||
| 7 Alpha-amylase | 27.97 | 34.04 | .06 | −.19* | .02 | −.01 | .27** | −.03 | |||
| 8 STAI | 465.52 | 552.33 | −.12 | −.04 | .09 | .03 | −.02 | .04 | −.00 | ||
| 9 Heart-rate | 6.25 | 3.92 | −.13 | −.05 | −.04 | −.02 | −.08 | .22* | −.06 | .06 | |
| 10 HF-HRV | −19.56 | 38.42 | −.10 | .02 | .02 | .06 | .26* | .02 | .16 | −.14 | −.47** |
Note. Mean (M) and standard deviation (SD). *p < 0.05. **p < 0.01.
3.2. Stress induction
N = 87 (73,73%) participants exhibited an average cortisol increase of at least 1.5 nmol/L from baseline levels, a threshold previously defined as a relevant physiological stress response [40]. Stress marker trajectories for all participants across the testing session are presented in Fig. 2.
Fig. 2.
Overview of TSST stress marker trajectories. Trajectories of raw cortisol (A), alpha-amylase (B), subjective stress (C), heart-rate (D) and logarithmized HF-HRV (E) across the TSST testing session with standard errors for each measurement point.
3.3. Association of stress markers with empathy, compassion and theory of mind
In our main models, cortisol, alpha-amylase, heart-rate, HF-HRV and STAI stress sensitivity were modeled using AUCis as dependent variables. EmpaToM-derived empathy, compassion and ToM were entered into the models as predictors. Regression estimates and fit indices are summarized in Table 2.
Cortisol. Regarding cortisol stress sensitivity, we found no significant effects of empathy (β = −0.13, CI [−0.34, 0.12], p = 0.228), compassion (β = 0.13, CI [−0.09, 0.34], p = 0.244) or ToM (β = 0.08, CI [−0.11, 0.28], p = 0.383) on cortisol stress sensitivity. Of the control variables, only age had a significant effect on the cortisol AUCi (β = −0.21, CI [−0.40, −0.01], p = 0.039), suggesting lower cortisol AUCi with higher age.
Alpha-amylase. There was a significant effect of ToM (β = 0.23, CI [0.04, 0.41], p = 0.018) on alpha-amylase stress-sensitivity. In detail, higher ToM levels predicted higher alpha-amylase stress-sensitivity (see Fig. 3). Empathy (β = 0.02, CI [−0.19, 0.23], p = 0.840) and compassion (β = −0.03, CI [−0.24, 0.18], p = 0.777), or any of the control variables, showed no significant effects.
Fig. 3.
High ToM increases alpha-amylase reactivity. Effects of EmpaToM-derived ToM differences (median split) on logarithmized alpha-amylase. Higher ToM capabilities were associated with greater alpha-amylase AUCi, indicating that participants with higher ToM abilities showed an increased alpha-amylase stress response.
STAI. There were no significant effects of empathy (β = 0.06, CI [−0.16, 0.28], p = 0.579), compassion (β = 0.00, CI [−0.21, 0.22], p = 0.968), ToM (β = −0.05, CI [−0.25, 0.14], p = 0.586), or of any of the control variables on subjective stress sensitivity.
Heart-rate. There were no significant effects of empathy (β = −0.06, CI [−0.30, 0.19], p = 0.644), compassion (β = 0.03, CI [−0.21, 0.26], p = 0.817), ToM (β = −0.12, CI [−0.33, 0.10], p = 0.289), or of any of the control variables on heart-rate stress sensitivity.
HF-HRV. There was a significant effect of ToM on HF-HRV stress-sensitivity (β = 0.33, CI [0.13, 0.31], p = 0.002). In detail, higher ToM levels predicted lower HF-HRV stress-sensitivity (see Fig. 4; keep in mind that heart-rate variability decreases when under stress). Empathy (β = −0.05, CI [−0.28, 0.18], p = 0.689) and compassion (β = 0.09, CI [−0.13, 0.31], p = 0.401) were unaffected by ToM levels Also, there was a significant effect of gender on HF-HRV (β = −0.55, CI [−0.98, −0.12], p = 0.013), suggesting greater HF-HRV stress sensitivity in women.
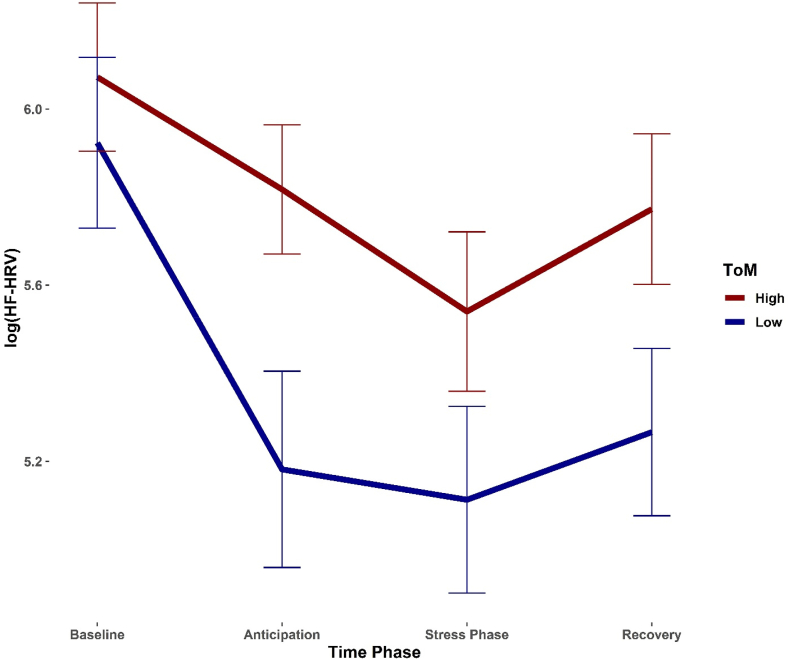
Fig. 4.
High ToM reduces HF-HRV reactivity. Effects of EmpaToM-derived ToM differences (median split) on logarithmized HF-HRV. Higher ToM capabilities were associated with higher HF-HRV AUCi, indicating that participants with higher ToM abilities showed a blunted HF-HRV stress response.
4. Discussion
The current study investigated the link between social capacities and the propensity to react to a psychosocial stressor. More specifically, we explored associations of behaviorally collected individual differences in empathy, compassion, and ToM, assessed with the EmpaToM [13], and subjective, autonomic and hormonal stress markers collected during a psychosocial laboratory stress test, the TSST. Stress sensitivity was operationalized as the area under the curve with respect to increase for all stress markers. Based on previous findings [14], we expected a link between higher levels of compassion and ToM with reduced stress sensitivity. In contrast, higher empathy ratings when exposed to others’ suffering were expected to go along with stronger stress responses.
We found associations of higher levels in ToM performance with decreased HF-HRV stress-sensitivity and increased alpha-amylase stress-sensitivity. Cortisol, subjective and heart-rate stress-sensitivity remained unaffected.
Contrasting our expectations, empathy, the ability to share the feelings and emotions of others, had no significant effects on any of the assessed stress-markers. Thus, other than previously reported [11], subjective stress reactivity was not increased with higher levels of empathy. The most plausible explanation for this discrepancy is that empathy assessments greatly differed in our and Tollenaar and Overgaauw's studies. While in the EmpaToM, empathy is gauged as an isomorphic momentary affective state, Tollenaar & Overgaauw assessed self-reported trait empathy using the Interpersonal Reactivity Index (IRI) [41]. In detail, the previously reported association of empathy with subjective stress was driven by the empathic concern subscale of the IRI, which is defined as the experience of emotions of warmth, sympathy, and concern for others. Furthermore, in our model [7], we differentiate between a healthy empathic response and empathic distress, whereby only the latter represents a non-adaptive response to others' suffering. It may well be, that the EmpaToM videos elicited healthy empathic responses, which were too weak to activate the threat-system.
Like empathy, compassion, the ability to feel for another individual in need, showed no association with any of the stress markers. It seems plausible that in a situation of ongoing stress, in which people suffer due to the behavior of others (rather than witnessing suffering in others), and which, like the TSST, is deprived of social feedback cues, compassion is not an intuitive strategy for stress regulation. This reasoning is in line with results by Cosley and colleagues [12] who found stress-buffering effects of trait compassion in a TSST-like paradigm only if participants were stressed by individuals behaving in a supportive, rather than neutral, manner. If individuals learn to cultivate compassion as a strategy against adversity in the context of mental training interventions, they may employ it more consciously in phases of acute stress. Consequently, higher levels of trained compassion would show effects on physiological stress sensitivity [14] that are not discernible when focusing on naïve compassion abilities.
ToM, the ability to conceptualize others’ mental states, was associated with sympathetic and parasympathetic stress sensitivity. Interestingly, higher ToM performance predicted both greater alpha-amylase and lower HF-HRV stress responses, suggesting inverse associations of ToM and psychosocial stress sensitivity, depending on the underlying autonomic stress marker.
A positive association of mentalizing abilities and sympathetic (i.e., alpha-amylase) stress sensitivity confirms the previously reported relationship of increased heart-rate reactivity with higher levels of ToM [11]. It also matches research in patients with Autism Spectrum Disorder (ASD), who generally suffer from substantial ToM deficits and show blunted HR and cortisol responses to psychosocial stress [42]. The found association of higher mentalizing abilities with lower parasympathetic (i.e., HF-HRV) stress sensitivity, does not support this line of research, however. Although stress exposure may perturb the ability to infer other's feelings in general, higher ToM abilities may also be helpful to interpret the TSST committee's behavior (i.e., their intention to induce stress). This insight would likely make the committee members easier to relate to, and the situation less threatening altogether. Previously, associations of ToM and heart-rate variability have been found in terms of higher resting state heart-rate variability and better mentalizing abilities (for a meta-analysis see Ref. [43]).
Given their cooperation in the regulation of bodily functions [44], a linear relationship of sympathetic and parasympathetic activity with ToM would have been anticipated. However, both at baseline and after challenge, different stress markers often show only weak associations [45], especially if measured through different methodology [22]. Accordingly, alpha-amylase and HF-HRV stress-sensitivity showed no significant correlation in the current sample. In this context, it is important to realize that the different components of the autonomic stress system, while collectively reacting to a stressor, serve different purposes [46]. In detail, average mentalizing abilities could be linked to greater energy allocation via salivary enzymes, while at the same time favoring cardio-vascular adaptability in terms of a blunted HF-HRV reaction. Overall, contradictory associations of individual differences with different stress markers illustrate the complexity of the human stress system [22].
There are several limitations to the current study. First, since no experimental manipulation was employed, all findings are solely of correlational nature. Thus, no conclusions about a causal link between socio-affective and socio-cognitive capacities with acute psychosocial stress can be drawn. Second, a large number of participants were missing heart-rate and HF-HRV data points, leading to possible power issues concerning small effect sizes. Third, EmpaToM data acquisition was realized during an fMRI testing session, possibly influencing empathy and compassion ratings as well as ToM performance in comparison to a more natural testing environment. Last, TSST and EmpaToM data collection was not realized on the same day. To assure reproducibility of the results, follow-up analysis were conducted controlling for the differences in time between testing sessions.
In summary, we find that while training-naïve empathy and compassion tendencies show no associations, mentalizing abilities are differentially linked to different aspects of the acute psychosocial stress response. Inconsistencies of these findings with the previous literature suggest that the relationship of social processing abilities with stress sensitivity is relatively weak if assessed at baseline. The targeted training of empathy, compassion and ToM in the context of meditation-based interventions, however, seems to boost associations (for a meta-analysis see Ref. [47]). Given the functional complexity of the different branches of the stress system, the collection of numerous stress markers, even if indexing highly interconnected stress components, is of great importance to obtain a fine-grained representation of the acute stress response [48].
Funding
This study forms part of the ReSource Project headed by T.S. Data for this project were collected between 2013 and 2016 at the former Department of Social Neuroscience at the MPI CBS Leipzig. T.S. (Principal Investigator) received funding for the ReSource Project from the European Research Council (ERC) under the European Community's Seventh Framework Program (FP7/2007–2013) ERC grant agreement number 205557. The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author contributions
J.U.B. analysed the data and drafted the manuscript. A.B., F-M.T. and P.K. developed the EmpaToM and supported the data analysis. V.E. designed the experiment and supported data analysis and drafting of the manuscript. T.S. initiated and developed the ReSource Project and secured all funding. She also co-developed the design for the EmpaToM and all stress-related measures as PI of the ReSource project and was leader of all ReSource related meetings with her entire staff including meetings with the teachers, the researchers, the ReSource support and testing staff. All authors critically revised the manuscript.
Declarations of competing interest
None.
Acknowledgements
We are thankful to the members of the Social Neuroscience Department involved in the ReSource Project over many years, in particular to Astrid Ackermann, Christina Bochow, Matthias Bolz and Sandra Zurborg for managing the large-scale longitudinal study, to Elisabeth Murzik, Nadine Otto, Sylvia Tydecks, and Kerstin Träger for help with recruiting and data archiving, to Henrik Grunert for technical assistance, and to Hannes Niederhausen and Torsten Kästner for data management.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cpnec.2022.100159.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Chrousos G. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 2009;5(7):374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- 2.Sapolsky R.M. Social status and health in humans and other animals. Annu Rev Anthropol. 2004;33:393–418. doi: 10.1146/annurev.anthro.33.070203.144000. [DOI] [Google Scholar]
- 3.Wang M., Saudino K.J. Emotion regulation and stress. J Adult Dev. 2011;18(2):95–103. doi: 10.1007/s10804-010-9114-7. [DOI] [Google Scholar]
- 4.Dickerson S.S., Kemeny M.E. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130(3):355. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- 5.Frith C., Frith U. Mechanisms of social cognition. Annu Rev Psychol. 2012;63(1):287–313. doi: 10.1146/annurev-psych-120710-100449. [DOI] [PubMed] [Google Scholar]
- 6.De Vignemont F., Singer T. The empathic brain: how, when and why? Trends Cogn. Sci. 2006;10(10):435–441. doi: 10.1016/j.tics.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 7.Singer T., Klimecki O.M. Empathy and compassion. Curr. Biol. 2014;24(18):R875–R878. doi: 10.1016/j.cub.2014.06.054. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez-Liencres C., Breidenstein A., Wolf O.T., Brüne M. Sex-dependent effects of stress on brain correlates to empathy for pain. Int J Psychophysiol. 2016;105:47–56. doi: 10.1016/j.ijpsycho.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 9.Smeets T., Dziobek I., Wolf O.T. Social cognition under stress: differential effects of stress-induced cortisol elevations in healthy young men and women. Horm Behav. 2009;55(4):507–513. doi: 10.1016/j.yhbeh.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 10.Tomova L., von Dawans B., Heinrichs M., Silani G., Lamm C. Is stress affecting our ability to tune into others? Evidence for gender differences in the effects of stress on self-other distinction. Psychoneuroendocrinology. 2014;43:95–104. doi: 10.1016/j.psyneuen.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Tollenaar M.S., Overgaauw S. Empathy and mentalizing abilities in relation to psychosocial stress in healthy adult men and women. Heliyon. 2020;6(8) doi: 10.1016/j.heliyon.2020.e04488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cosley B.J., McCoy S.K., Saslow L.R., Epel E.S. Is compassion for others stress buffering? Consequences of compassion and social support for physiological reactivity to stress. J. Exp. Soc. Psychol. 2010;46(5):816–823. doi: 10.1016/j.jesp.2010.04.008. [DOI] [Google Scholar]
- 13.Kanske P., Böckler A., Trautwein F.-M., Singer T. Dissecting the social brain: introducing the empatom to reveal distinct neural networks and brain–behavior relations for empathy and theory of mind. Neuroimage. 2015;122:6–19. doi: 10.1016/j.neuroimage.2015.07.082. [DOI] [PubMed] [Google Scholar]
- 14.Engert V., Kok B.E., Papassotiriou I., Chrousos G.P., Singer T. Specific reduction in cortisol stress reactivity after social but not attention-based mental training. Sci. Adv. 2017;3(10) doi: 10.1126/sciadv.1700495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fredrickson B., Cohn M., Coffey K., Pek J., Finkel S. Open hearts build lives: positive emotions, induced through loving-kindness meditation, build consequential personal resources. J Pers Soc Psychol. 2008;95(5):1045–1062. doi: 10.1037/a0013262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pace T.W., Negi L.T., Adame D.D., Cole S.P., Sivilli T.I., Brown T.D., Issa M.J., Raison C.L. Effect of compassion meditation on neuroendocrine, innate immune and behavioral responses to psychosocial stress. Psychoneuroendocrinology. 2009;34(1):87–98. doi: 10.1016/j.psyneuen.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frith C., Frith U. Theory of mind. Curr. Biol. 2005;15(17):R644–R645. doi: 10.1016/j.cub.2005.08.041. [DOI] [PubMed] [Google Scholar]
- 18.Trautwein F.M., Kanske P., Böckler A., Singer T. Differential benefits of mental training types for attention, compassion, and theory of mind. Cognition. 2020;194 doi: 10.1016/j.cognition.2019.104039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singer T., Kok B.E., Bornemann B., Zurborg S., Bolz M., Bochow C. second ed. Max Planck Institute for Human Cognitive and Brain Sciences; Leipzig: 2016. The ReSource Project: Background, Design, Samples, and Measurements; pp. 46–55. [Google Scholar]
- 20.Kirschbaum C., Pirke K.-M., Hellhammer D.H. The ‘trier social stress test’–a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28(1–2):76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- 21.Engert V., Koester A.M., Riepenhausen A., Singer T. Boosting recovery rather than buffering reactivity: higher stress-induced oxytocin secretion is associated with increased cortisol reactivity and faster vagal recovery after acute psychosocial stress. Psychoneuroendocrinology. 2016;74:111–120. doi: 10.1016/j.psyneuen.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 22.Engert V., Kok B.E., Puhlmann L.M., Stalder T., Kirschbaum C., Apostolakou F.…Singer T. Exploring the multidimensional complex systems structure of the stress response and its relation to health and sleep outcomes. Brain, Behavior, and Immunity. 2018;73:390–402. doi: 10.1016/j.bbi.2018.05.023. [DOI] [PubMed] [Google Scholar]
- 23.Böckler A., Herrmann L., Trautwein F.M., Holmes T., Singer T. Know thy selves: learning to understand oneself increases the ability to understand others. J. cogn. enhanc. 2017;1(2):197–209. doi: 10.1007/s41465-017-0023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanske P., Böckler A., Trautwein F.M., Parianen Lesemann F.H., Singer T. Are strong empathizers better mentalizers? Evidence for independence and interaction between the routes of social cognition. Soc Cogn Affect Neurosci. 2016;11(9):1383–1392. doi: 10.1093/scan/nsw052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Molenberghs P., Trautwein F.M., Böckler A., Singer T., Kanske P. Neural correlates of metacognitive ability and of feeling confident: a large-scale fMRI study. Soc Cogn Affect Neurosci. 2016;11(12):1942–1951. doi: 10.1093/scan/nsw093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tholen M.G., Trautwein F.M., Böckler A., Singer T., Kanske P. Functional magnetic resonance imaging (fMRI) item analysis of empathy and theory of mind. Hum Brain Mapp. 2020;41(10):2611–2628. doi: 10.1002/hbm.24966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valk S.L., Bernhardt B.C., Böckler A., Trautwein F.M., Kanske P., Singer T. Socio-cognitive phenotypes differentially modulate large-scale structural covariance networks. Cereb. Cortex. 2016:bhv319. doi: 10.1093/cercor/bhv319. [DOI] [PubMed] [Google Scholar]
- 28.Valk S.L., Bernhardt B.C., Böckler A., Kanske P., Singer T. Substrates of metacognition on perception and metacognition on higher‐order cognition relate to different subsystems of the mentalizing network. Hum Brain Mapp. 2016;37(10):3388–3399. doi: 10.1002/hbm.23247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valk S.L., Kanske P., Park B.Y., Hong S.J., Boeckler-Raettig A., Trautwein F.M.…Singer T. bioRxiv; 2020. A Low-Dimensional Connectome Manifold Governs the Organization and Plasticity of Social Brain Functions in Humans. [DOI] [Google Scholar]
- 30.Wittchen H.U., Pfister H. Swets & Zeitlinger; Frankfurt: 1997. Diagnostisches Expertensystem für psychische Störungen (DIA-X) [Google Scholar]
- 31.Wittchen H.U., Zaudig M., Fydrich T. Hogrefe; Göttingen: 1997. SKID – Strukturiertes Klinisches Interview für DSM-IV. Achse I und Achse II. [Google Scholar]
- 32.Dressendörfer R., Kirschbaum C., Rohde W., Stahl F., Strasburger C. Synthesis of a cortisol-biotin conjugate and evaluation as a tracer in an immunoassay for salivary cortisol measurement. J. Steroid Biochem. Mol. Biol. 1992;43(7):683–692. doi: 10.1016/0960-0760(92)90294-s. [DOI] [PubMed] [Google Scholar]
- 33.Lorentz K., Gütschow B., Renner F. Evaluation of a direct alpha-amylase assay using 2-chloro-4-nitrophenyl-alpha-d-maltotrioside. Clin. Chem. Lab. Med. 1999;37(11–12):1053–1062. doi: 10.1515/CCLM.1999.154. [DOI] [PubMed] [Google Scholar]
- 34.Berntson G.G., Thomas Bigger J., Jr., Eckberg D.L., Grossman P., Kaufmann P.G., Malik M., Nagaraja H.N., Porges S.W., Saul J.P., Stone P.H. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997;34(6):623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- 35.Kaufmann T., Sütterlin S., Schulz S.M., Vögele C. Artiifact: a tool for heart rate artifact processing and heart rate variability analysis. Behav Res Methods. 2011;43(4):1161–1170. doi: 10.3758/s13428-011-0107-7. [DOI] [PubMed] [Google Scholar]
- 36.Spielberger C. Press; Palo Alto, CA: 1983. Manual of the State-Trait Anxiety Inventory (STAI) [Google Scholar]
- 37.Campbell J., Ehlert U. Acute psychosocial stress: does the emotional stress response correspond with physiological responses? Psychoneuroendocrinology. 2012;37(8):1111–1134. doi: 10.1016/j.psyneuen.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 38.R Core Team R: a language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria. 2020. https://www.R-project.org
- 39.Pruessner J.C., Kirschbaum C., Meinlschmid G., Hellhammer D.H. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28(7):916–931. doi: 10.1016/S0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- 40.Miller R., Plessow F., Kirschbaum C., Stalder T. Classification criteria for distinguishing cortisol responders from nonresponders to psychosocial stress: evaluation of salivary cortisol pulse detection in panel designs. Psychosom Med. 2013;75(9):832–840. doi: 10.1097/PSY.0000000000000002. [DOI] [PubMed] [Google Scholar]
- 41.Davis M.H. Measuring individual differences in empathy: evidence for a multidimensional approach. J Pers Soc Psychol. 1983;44(1):113. doi: 10.1037/0022-3514.44.1.113. [DOI] [Google Scholar]
- 42.Hollocks M.J., Howlin P., Papadopoulos A.S., Khondoker M., Simonoff E. Differences in hpa-axis and heart rate responsiveness to psychosocial stress in children with autism spectrum disorders with and without co-morbid anxiety. Psychoneuroendocrinology. 2014;46:32–45. doi: 10.1016/j.psyneuen.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 43.Zammuto M., Ottaviani C., Laghi F., Lonigro A. The heart in the mind: a systematic review and meta-analysis of the association between theory of mind and cardiac vagal tone. Front. Physiol. 2021;12 doi: 10.3389/fphys.2021.611609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCorry L.K. Physiology of the autonomic nervous system. American journal of pharmaceutical education. 2007;71(4) doi: 10.5688/aj710478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nater U.M., Rohleder N., Gaab J., Berger S., Jud A., Kirschbaum C., Ehlert U. Human salivary alpha-amylase reactivity in a psychosocial stress paradigm. Int J Psychophysiol. 2005;55(3):333–342. doi: 10.1016/j.ijpsycho.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 46.Pruessner J.C., Ali N. In: Neuroendocrinology of Stress. Russell J.A., Shipston M.J., editors. John Wiley & Sons; 2015. Neuroendocrine mechanisms of stress regulation in humans; pp. 121–142. [Google Scholar]
- 47.Pascoe M.C., Thompson D.R., Jenkins Z.M., Ski C.F. Mindfulness mediates the physiological markers of stress: systematic review and meta-analysis. J Psychiatr Res. 2017;95:156–178. doi: 10.1016/j.jpsychires.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 48.Ali N., Nater U.M. Salivary alpha-amylase as a biomarker of stress in behavioral medicine. Int J Behav Med. 2020;27(3):337–342. doi: 10.1007/s12529-019-09843-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Favre P., Kanske P., Engen H., Singer T. Decreased emotional reactivity after 3-month socio-affective but not attention-or meta-cognitive-based mental training: a randomized, controlled, longitudinal fMRI study. NeuroImage. 2021;237 doi: 10.1016/j.neuroimage.2021.118132. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.