Abstract
Objectives
Platelet-rich concentrates, namely platelet-rich plasma (PRP) and platelet-rich fibrin (PRF), have recently shown potential roles in accelerating orthodontic tooth movement (OTM) and reducing treatment duration. Our study aims to systematically evaluate the effect of platelet-rich concentrates on OTM.
Materials and methods
An electronic search of 11 databases, followed by a hand search of reference lists of eligible studies and related reviews, was conducted up to January 2022. Randomized controlled trials investigating OTM of patients with platelet-rich concentrates were included. Risk of bias was assessed by version 2 of Cochrane tool (RoB 2) for assessing risk of bias in randomized trials.
Results
Among 715 records initially identified, 9 studies were included, of which 3 used PRP and the other 6 applied PRF. 7 studies supported a positive relationship between platelet-rich concentrates and OTM, but the other 2 studies reported a null and a negative effect of PRF, respectively. The overall qualities of evidence were moderate to high.
Conclusions
Platelet-rich concentrates as PRP and PRF seem to be effective in accelerating OTM at early stages, while their long-term efficacy remains controversial. Repeated application of platelet concentrates may increase the accelerated stability of OTM.
Keywords: Orthodontic treatment, Platelet-rich plasma, Platelet-rich fibrin, Tooth movement
Orthodontic treatment; Platelet-rich plasma; Platelet-rich fibrin; Tooth movement.
1. Introduction
Duration of orthodontic treatment, one major concern for many patients, often takes more than 2 years [1]. Lengthy orthodontic treatment not only decreases patients’ desire and compliance for clinical therapeutics, but also results in a high risk of adverse effects like white spot lesions, root resorption and periodontal diseases [2]. Therefore, attempts to accelerate tooth movement and reduce treatment duration are of great significance to both orthodontists and patients.
Over the past few years, approaches including surgical and non-surgical techniques have been proposed as promising ways to shorten the treatment time. However, surgery-assisted procedures like corticotomy, micro-osteoperforation and piezocision are considered to be invasive, requiring complicated procedures [3]. On the other hand, non-surgical methods show conflicting results regarding tooth movement acceleration, and some biological agents like parathyroid hormone and prostaglandin may lead to irreversible systemic complications [4, 5]. Thus, there is a need for studies investigating a less invasive remedy without compromising systemic safety.
Platelet-rich concentrates comprise four subtypes as pure platelet-rich plasma (P-PRP), leukocyte- and platelet-rich plasma (L-PRP), pure platelet-rich fibrin (P-PRF), leukocyte- and platelet-rich fibrin (L-PRF) [6]. Among them, platelet-rich plasma (PRP) is the first generation of autologous concentrations of platelets in a small volume of plasma. Platelet-rich fibrin (PRF), as the second generation, holds the advantages of easier preparations and longer effects [7, 8]. Platelet-rich concentrates are rich in variable growth factors and cytokines such as transforming growth factor β, vascular endothelial growth factor, interleukin, interferon and tumor necrosis factor α. These characteristics support their roles in multitudinous biological processes, including inflammation, angiogenesis, osteoblastogenesis and osteoclastogenesis, and as a result, motivate wound healing and bone regeneration [9, 10, 11, 12]. Nowadays, platelet-rich concentrates are proved effective in regenerative dentistry and oral surgery such as tooth extraction, implantology, and periodontal therapy [13, 14]. Orthodontic tooth movement (OTM) refers to the tooth movement of patients under orthodontic treatment. It occurs by remodeling of alveolar bone and periodontal ligament under orthodontic force produced by orthodontic appliances like archwires and springs. Thus, platelet-rich concentrates show a prospect in orthodontic practice.
In a recently published systematic review, studies have suggested the potential function of platelet-rich concentrates in the OTM acceleration of animal models [15]. However, their roles in the OTM of humans remain unclear. As clinical studies accumulate, demands for summarized and comprehensive knowledge of OTM after the application of platelet-rich concentrates arise. Here, our study aims to systematically evaluate the effect of platelet-rich concentrates on OTM for further application.
2. Materials and methods
2.1. Protocol and registration
The review was conducted and presented according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [16] and registered in the International Prospective Register of Systematic Reviews database (CRD42021258838).
2.2. Eligibility criteria
The eligible criteria were based on the PICOS formula: (1) participants: patients receiving fixed orthodontic treatment; (2) intervention: platelet-rich concentrates including PRP and PRF; (3) comparison: placebo or no intervention; (4) outcomes: primary outcome was the rate of OTM, secondary outcomes were the duration of treatment, pain and periodontal health; (5) study design: randomized controlled study (RCT).
The exclusion criteria were: (1) patients with periodontal diseases, craniofacial syndromes such as cleft lip or palate, systemic diseases related to bone metabolism, or a previous orthodontic treatment history; (2) non-randomized, non-prospective, or non-comparative studies as cohort studies, cross-sectional studies, case reports, review, abstracts, editorials, and opinions.
2.3. Information sources, search strategy and study selection
PubMed, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, Embase, Web of Science, Scopus, China National Knowledge Infrastructure, Chinese Biomedical Literature Database, ProQuest Dissertation & Theses Database, Systematic for Information on Grey Literature in Europe, ClinicalTrials.gov were searched up to January 2022 without language restriction. The reference lists of eligible studies and related reviews were checked for additional studies. The details of database search are presented in Supplementary Table 1. Two reviewers selected studies independently and in duplicate. Any disagreement was resolved by discussion with two senior authors.
2.4. Data collection and data items
Two authors extracted data from eligible studies using piloted forms: author and year, country of study, study design, patient characteristics, treatment protocols, outcome measurements, results, conclusions and details of intervention with platelet-rich concentrates.
2.5. Risk of bias in individual studies
The risk of bias (RoB) of RCTs was evaluated by version 2 of Cochrane tool for assessing risk of bias in randomized trials (RoB 2) [17] by two authors independently and in duplicate. Disagreements were resolved by two senior authors.
2.6. Summary measures and synthesis of results
Meta-analysis to determine the pooled estimates of platelet-rich concentrates was preplanned. However, the heterogeneity in methodology and clinical features precluded quantitative analysis. As a result, the results were qualitatively analyzed.
2.7. Risk of bias across studies and additional analysis
Analyses for small-study effects, publication bias and subgroup analysis were not conducted although originally planned owing to an insufficient number of studies identified. The quality of evidence was appraised with the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach [18].
3. Results
3.1. Study selection
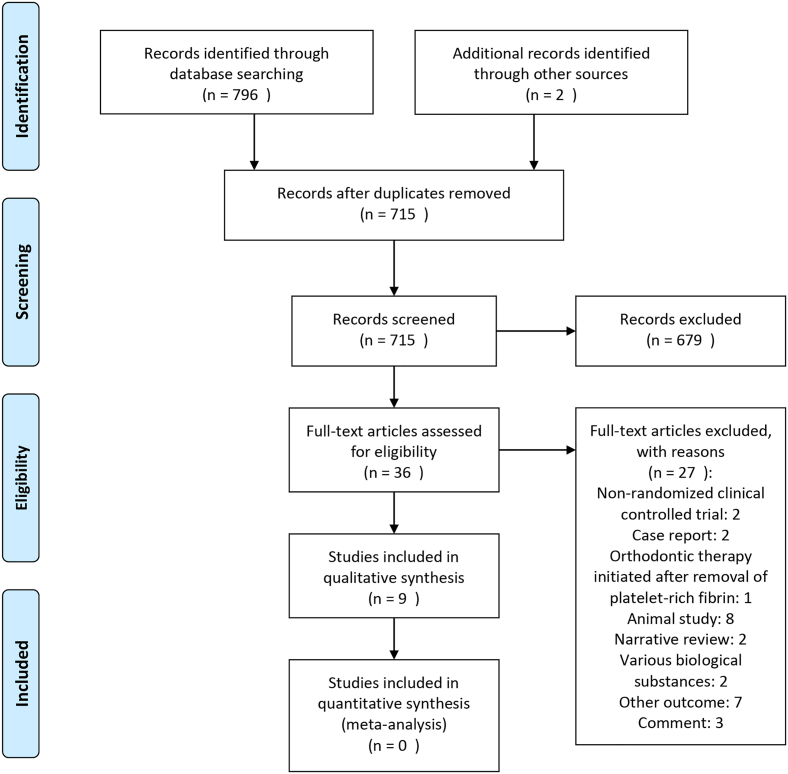
A total of 715 studies were initially identified. Then, 36 articles remained after screening the titles and abstracts. 27 studies were excluded with reasons (Supplementary Table 2) and 9 were included in the systematic review (Figure 1) [19, 20, 21, 22, 23, 24, 25, 26, 27].
Figure 1.
PRISMA flow-chart diagram of literature selection.
3.2. Study characteristics
Table 1 shows the characteristics of included studies. A total of 158 patients were examined, with mean age ranging from 16.4522 to 33 years [21]. Among 9 RCTs included, 8 adopted split-mouth design [19, 20, 21, 22, 23, 25, 26, 27]. 3 studies tested PRP [20, 26, 27], and the other 6 investigated PRF, of which 1 applied PRF clot [19], 1 applied PRF membrane [21], and 4 applied PRF injection [22, 23, 24, 25]. The OTM rate was measured by space closure in 1 study [19], incisor retraction in 1 study [24] and canine distalization in another 7 studies [20, 21, 22, 23, 25, 26, 27], with periods lasting from 4 weeks [24] to 1.5 years [26]. Table 2 presents the details of the intervention with platelet-rich concentrates.
Table 1.
Characteristics of included studies.a
| Study ID | Origin | Study design | Participants | Malocclusion | Study groups | Intervention | Outcomes | |
|---|---|---|---|---|---|---|---|---|
| 1 | Tehranchi 2018 [19] | Iran | Split-mouth design | 8 patients, 3 females, 5 males (30 extraction sockets). Age: 17.37 ± 12.48 years, range 12–25 years. |
Not recorded | Experimental side: extraction socket with L-PRF. Control side: no intervention, secondary healing. |
L-PRF clot | Space closure: horizontal linear distance between mid-marginal ridges of adjacent teeth, measured on study casts using a digital caliper. |
| 2 | El-Timamy 2020 [20] | Egypt | Split-mouth design | 15 female patients. Age: range 18 ± 3 years. |
Severe crowding or protrusion requiring first premolars extractions. | Experimental side: PRP injection with 10% CaCl2 activating solution. Control side: 10% CaCl2 injection only. |
PRP injection | Canine distalization: rate of canine retraction detected by change in canine position in superimposed models |
| 3 | Pacheco 2020 [21] | Brazil | Split-mouth design | 17 patients, 12 females, 5 males. Age: mean of 33 years, range 20–45 years. |
Angle Class I (14) or Class II Division 1 (3) malocclusion needing extraction of maxillary first premolars. | Experimental side: alveolus with L-PRF membranes. Control side: no intervention. |
L-PRF membrane | Canine distalization rate: monthly distalization rate of maxillary canines measured using a flexible ruler placed at dental midline from maxillary central incisors to mesial surface of canines. |
| 4 | Çağlı Karcı 2021 [22] | Turkey | Split-mouth design | 12 patients, 7 females, 5 males. Age: 16.45 ± 0.27 years. |
Angle Class II malocclusion with dentoalveolar protrusion or moderate crowding. | Experimental side: PRF injection. Control side: no intervention. |
PRF injection | Canine distalization and space closure: amount of canine distal movement and closure extraction space in superimposed dental model scans. |
| 5 | Erdur 2021 [23] | Turkey | Split-mouth design | 20 patients, 12 females, 8 males. Age: 21.4 ± 2.9 years. |
Angle Class II Division 1 malocclusion requiring maxillary first premolar extraction. | Experimental side: PRF injection. Control side: sham injection. |
PRF injection | Canine distalization: distance between midpoints of vertical lines drawn from incisal edge to cervical line over marginal ridge of lateral and canine teeth on dental cast measured by digital caliper. |
| 6 | Karakasli 2021 [24] | Turkey | Parallel | 40 patients, 23 females, 17 males. Age: 20.7 ± 1.45 years. |
Angle Class II Division 1 malocclusion requiring maxillary first premolar extraction and incisor retraction. | Experimental group: PRF injection. Control group: no intervention. |
PRF injection | Incisor retraction: linear distance between distal contact point of lateral incisor and mesial contact point of canine on plaster models recorded with a digital caliper. |
| 7 | Zeitounlouian 2021 [25] | Syria | Split-mouth design | 21 patients, 15 females, 6 males. Age: 20.85 ± 3.85 years, range 16–28 years. |
Angle Class II Division 1 requiring extraction of maxillary first premolars. | Experimental side: PRF injection. Control side: no intervention. |
PRF injection | Canine distalization: distance between medial end of third palatal rugae and cusp tip of upper canine. |
| 8 | Joy 2021 [26] | India | Split-mouth design | 15 patients, 9 females, 6 males. Age: 21.7 ± 2.52 years. |
Any malocclusion requiring lower first premolar extraction and canine retraction. | Experimental side: PRP injection. Control side: no intervention. |
PRP injection | Canine distalization: distance between mandibular canine cusp tip and first molar central fossa evaluated using digital Vernier caliper. |
| 9 | Angel 2022 [27] | India | Split-mouth design | 10 patients, 6 females, 4 males. Age: 19.05 ± 3.3 years, range 16–24 years. |
Bimaxillary protrusion or Angle Class II division 1 malocclusion requiring maxillary first premolar extraction. | Experimental side: PRP injection. Control side: no intervention. |
PRP injection | Canine distalization: rate of canine movement assessed using digital model superimposition. |
| Study ID | Measurement time | Primary results | Additional outcomes | Conclusions | Level of evidence | |
|---|---|---|---|---|---|---|
| 1 | Tehranchi 2018 [19] | T0: before placement of L-PRF. T1-T8: every 2 weeks during 2–16 weeks after study commencement. |
The linear distance decreased more in experimental side, experimental group showed higher rate of OTM (P = 0.006). | Not recorded | Application of L-PRF may accelerate OTM, particularly in extraction case. | Moderate |
| 2 | El-Timamy 2020 [20] | T0: before canine retraction. T1-T4: monthly until 4th month. |
Rate of canine retraction was faster on intervention side in first 2 months (T0-T1: P = 0.049, T1-T2: P = 0.772), but slower in 3rd month (P = 0.02). Total distances of both groups in 4 months were similar (P = 0.895) | 1. Canine rotation was comparable, with a mean difference of 1.036° (P = 0.71). 2. Pain increased following each injection without difference between two groups. |
PRP injection increased OTM during early stages (first 2 months), but did not exhibit long-term acceleration effects. Repeated PRP injection to maintain a steady accelerated OTM warrant further investigation. | High |
| 3 | Pacheco 2020 [21] | T1: beginning of retraction phase. T2: end of fifth month. |
Mean distalization rate was 0.909 mm/mo (95% CI, 0.8–1 mm) in control side, while 0.668 mm/mo (95% CI, 0.6–0.7 mm) in experimental side (P = 0.004). Difference was 0.23 mm/mo (95% CI, 0.07–0.39 mm). | 1. Canine inclination was greater on control side (8.57 ± 3.07°) than experimental side (5.81 ± 3.09°) treated with L-PRF (P = 0.001). | L-PRF decreased the distalization rate of maxillary canines in young adult patients. | Moderate |
| 4 | Çağlı Karcı 2021 [22] | T0: first premolar extraction and onset of canine distalization. T1-T6: every 2 weeks of 12 weeks after onset of canine distalization. |
Experimental side exhibited greater canine distal movement than control side (P = 0.011), as the same tendency as the amount of closure extraction space at T0-T1 (P = 0.018) and T0-T6 (P = 0.049) | 1. There were no difference in molar mesialization, canine rotation, transversal measurements (P>0.05). 2. Periodontal parameters (plaque index, gingival index, probing depth) showed no difference (P>0.05). |
PRF accelerated OTM. | Moderate |
| 5 | Erdur 2021 [23] | T0: before tooth extraction. T1-T4: 1, 4, 8, 12 weeks from beginning of distalization. |
Study group has higher rates of canine movement at all time points, and a higher total movement (6.06 ± 0.29 mm) than control group (3.89 ± 0.34 mm) (P<0.001). Mean movement increased in weeks that PRF was injected (P<0.001). | Not recorded | PRF injection facilitated OTM and shortened treatment duration by stimulating expression of inflammatory cytokines. | Moderate |
| 6 | Karakasli 2021 [24] | T0: before incisor retraction. T1–T4: 1–4 weeks after incisor retraction initiation. |
Study group showed higher weekly and total incisor movement than control group (P<0.001). Incisors moved faster in T1-T0 and T3-T2 intervals (P<0.05) | Not recorded | PRF injection increased rate of maxillary incisor retraction and shortened treatment duration. | Moderate |
| 7 | Zeitounlouian 2021 [25] | T0: before canine retraction. T1-T5: monthly up to 5 months. |
Monthly rate of canine retraction on experimental side were greater at T2, T3 and T4, but only significant at T2 (P = 0.018). Total movements were comparable (P = 0.918). | 1. Molar mesialization and canine rotation showed no difference at all time points (P>0.05). 2. Overall duration of canine retraction between experimental (3.28 ± 1.00 months) and control (3.57 ± 1.16 months) sides was not significant. |
Rate of canine retraction was not significantly greater on experimental side than control side except at 2 nd month. Repeated PRF injection might be needed but merit more researches. | High |
| 8 | Joy 2021 [26] | T0: before canine retraction. T1: at completion of retraction. |
Rate of canine retraction for PRP group and control group was 0.87 ± 0.12 and 0.7 ± 0.13 mm/mo, respectively (P<0.001). | 1.Overral duration canine retraction for PRP group and control group was 5.96 ± 0.94 and 7.42 ± 1.12 months, respectively. | PRP injection accelerated OTM rate by 1.24 times. | Moderate |
| 9 | Angel 2022 [27] | T0: before canine retraction. T2-T3: 30 or 60 days. |
OTM rate on PRP side increased by 35% in first month (P = 0.001) and by 14% at the end of second month (P = 0.015). | Not recorded | Local administration of PRP increased OTM rate during 60-day period. | High |
L-PRF, leukocyte platelet-rich fibrin; OTM, orthodontic tooth movement; PRF, platelet-rich fibrin; PRP, platelet-rich plasma.
Table 2.
Details of platelet-rich concentrates.a
| Study ID | Intervention | Preparation protocol | Application method | Sites | Dose | Application interval | |
|---|---|---|---|---|---|---|---|
| 1 | Tehranchi 2018 [19] | L-PRF clot | 2700 rpm, 12 min | L-PRF plugs were placed gently into sockets, and sutured using 4-0 Vicryl sutures | Extraction socket of premolars | Not recorded | only once immediately after premolar extraction (day 0) |
| 2 | El-Timamy 2020 [20] | PRP injection | Not recorded | Intraligamental injection | Middle, distobuccal and distopalatal areas of distal surface of canines, together with submucosal injections buccally and palatally | 25 units | 0, 21, 42 days |
| 3 | Pacheco 2020 [21] | L-PRF membrane | 2700 rpm, 14 min | The experimental alveolus was preserved with L-PRF membranes and sutured with a 4–0 nylon suture | Alveoli after first premolars extractions | Not recorded | only once 15 days before retraction initiation |
| 4 | Çağlı Karcı 2021 [22] | PRF injection | 800 rpm, 3 min | Submucosal injection | Buccal, palatal and distal surfaces of maxillary canine | 0.7 mL | 0, 4, 8 weeks |
| 5 | Erdur 2021 [23] | PRF injection | 700 rpm, 3 min | Intraligamental injection | Distobuccal and distopalatal sides of canine tooth | 4 mL | 0, 2 weeks |
| 6 | Karakasli 2021 [24] | PRF injection | 700 rpm, 3 min | Intraligamental injection | Periodontal ligament space of incisors | 2–3 mL | 0, 2 weeks |
| 7 | Zeitounlouian 2021 [25] | PRF injection | 700 rpm, 3 min | Submucosal injection | Buccal and palatal sides through attached gingiva | 3 mL | 0, 1 month |
| 8 | Joy 2021 [26] | PRP injection | 1000 rpm, 12 min | Submucosal injection | Attached buccal gingiva and lingual mucosa of canine and first premolar extraction site. | 1 mL | Only once at same appointment as canine retraction initiation (day 0). |
| 9 | Angel 2022 [27] | PRP injection | Not recorded | Submucosal injection | Buccal, palatal and distal sites around canine. | 1.8 mL | Only once at same appointment as canine retraction initiation (day 0). |
L-PRF, leukocyte platelet-rich fibrin; PRF, platelet-rich fibrin; PRP, platelet-rich plasma.
3.3. Risk of bias within studies
RoB 2 was used to assess the RoB of included studies. 5 studies [20, 22, 24, 25, 27] were determined to be low RoB, and 4 were of having some concerns for different reasons [19, 21, 23, 26]. Table 3 summarized the details of RoB assessment.
Table 3.
Risk of bias assessment by the version 2 of Cochrane tool for assessing risk of bias in randomized trials (RoB 2).
| Study ID | Bias arising from the randomization process | Bias due to deviations from intended interventions | Bias due to missing outcome data | Bia in measurement of the outcome | Bias in selection of the reported result | Overall assessment | |
|---|---|---|---|---|---|---|---|
| 1 | Tehranchi 2018 [19] | Low risk | Some concerns | Low risk | Low risk | Some concerns | Some concerns |
| 2 | El-Timamy 2020 [20] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| 3 | Pacheco 2020 [21] | Low risk | Low risk | Low risk | Some concerns | Some concerns | Some concerns |
| 4 | Çağlı Karcı 2021 [22] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| 5 | Erdur 2021 [23] | Low risk | Low risk | Low risk | Some concerns | Low risk | Some concerns |
| 6 | Karakasli 2021 [24] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| 7 | Zeitounlouian 2021 [25] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| 8 | Joy 2021 [26] | Some concerns | Low risk | Low risk | Low risk | Low risk | Some concerns |
| 9 | Angel 2022 [27] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
3.4. Effect of PRP on OTM
Angel et al. [27] examined the OTM after PRP injection, finding that OTM rate increased by 35% in the first month (2.06 ± 0.36 mm vs. 1.34 ± 0.28 mm, P = 0.001) and by 14% in the second month (1.12 ± 0.32 mm vs. 0.96 ± 0.2 mm, P = 0.015). Joy et al. [26] also found that the OTM rate in PRP group was 1.24 times faster than that of control group (0.87 ± 0.12 mm/month vs. 0.7 ± 0.13 mm/month, P<0.001). Interestingly, El-Timamy et al. [20] showed that the rate of canine retraction was faster in the first 2 months (1.55 ± 0.63 mm vs. 1.35 ± 0.62 mm, P = 0.049; 1.33 ± 0.87 mm vs. 1.27 ± 0.40 mm, P = 0.772), but slower at the 3rd month (0.59 ± 0.96 mm vs. 1.01 ± 0.63 mm, P = 0.020) on the intervention side. As a result, the total distance during 4 months of study period was similar in both groups (4.57 ± 1.32 mm vs. 4.53 ± 1.12 mm, P = 0.895). In addition, canine rotation was comparable, with a mean difference of 1.036° (P = 0.710) [20].
3.5. Effect of PRF on OTM
6 studies focused on the effect of PRF on OTM, of which 4 supported the acceleration of OTM by PRF application. Tehranchi et al. [19] reported that linear distance between mid-marginal ridges of adjacent teeth decreased more on the experimental side during 4 months of study, revealing teeth with L-PRF placed in extraction sockets moved faster than the control side (P = 0.006). Çağlı Karcı et al. [22] found that the experimental side with PRF injection exhibited greater canine distal movement (P = 0.011) and amount of extraction space closure (P = 0.049) after 12 weeks, while Erdur et al. [23] concluded that PRF injection facilitated canine tooth movement at all time points within 12 weeks and mean movement increased in weeks after PRF injection (P<0.001). Karakasli et al. [24] noted that the study group with PRF injection had faster incisor movement than the control group and the values were higher within the first week after PRF application.
However, the other 2 studies with a period of 5 months had different perspectives. Zeitounlouian et al. [25] found that the rate of canine retraction on experimental side with PRF injection was only statistically greater in the 2 nd month than the control side (P = 0.018) and the overall movements were comparable between two groups (P = 0.918). Pacheco et al. [21], using L-PRF membrane, reported that PRF decreased canine distalization rate, based on the results that mean distalization rates were 0.67 mm and 0.90 mm per month on experimental and control sides, respectively (P = 0.004).
As for other types of tooth movements, Pacheco et al. [21] reported that canine inclination was greater on the control side (P = 0.001) and was weakly correlated with distalization rate. Çağlı Karcı et al. [22] reported that PRF had no effect on molar mesialization, canine rotation or transversal measurements. Zeitounlouian et al. [25] reported that there was no difference between the two sides in molar mesialization and canine rotation.
3.6. Additional outcomes as treatment duration, pain and periodontal health
Joy et al. [26] demonstrated that the mean time to complete canine retraction in the PRP group and control group were 5.96 ± 0.94 and 7.42 ± 1.12 months, while Zeitounlouian et al. [25] showed that overall duration on the experimental side with PRF injection (3.28 ± 1.00 month) was similar to that in control side (3.57 ± 1.16 month). According to El-Timamy et al. [20], pain increased following each PRP injection, with no difference between two sides. Çağlı Karcı et al. [22] found that there was no difference in periodontal parameters as probing depth, plaque index or gingival index between PRF and control sides.
3.7. Risk of bias across studies and additional analysis
Small-study effects, publication bias, or subgroup analysis were not analyzed. The quality of evidence, graded with GRADE system, ranged from moderate to high (Table 4, Supplementary Table 3).
Table 4.
GRADE Summary of Findings Table of the effects of PRP and PRF on OTM.b
| Outcome (No. of studies) | Impact | Certainty |
|---|---|---|
| PRP (first 2 months) (3) | PRP injection increases OTM during early stage (first 2 months). | ⊕⊕⊕⊕ HIGH |
| PRF (first 3 months) (5) | PRF accelerates OTM in early stages. | ⊕⊕⊕◯ MODERATEa |
2 studies are evaluated as having some concerns.
GRADE, Grading of Recommendations, Assessment, Development, and Evaluation; OTM, orthodontic tooth movement; PRF, platelet-rich fibrin; PRP, platelet-rich plasma.
4. Discussion
Platelet-rich concentrates, namely platelet-rich plasma and platelet-rich fibrin, have shown great potential in regenerative medicine, owing to their superior characteristics as reservoirs and scaffolds for platelets, leucocytes, growth factors and cytokines. Recently, numerous studies have been performed to explore their merits in various aspects of orthodontic treatment, including post-orthodontic stability [28], rapid maxillary expansion [29], root resorption [30], temporomandibular joint disorders [31] and cleft lip or palate [32]. However, their actual effects on OTM need to be clarified. Here, we conducted an evidence-based review on this topic.
According to the studies included, debatable conclusions were presented. 7 studies [19, 20, 22, 23, 24, 26, 27] supported a positive acceleration of OTM by PRP or PRF, while the other 2 studies [21, 25] showed different ideas. Zeitounlouian et al. [25] reported no benefit of PRF to tooth movement. Pacheco et al. [21] even claimed that PRF decreased the rate of canine distalization. These controversial results may attribute to the different intervention procedures they adopted in dosage, delivery methods (plug, membrane or injection), concentrate presentation (PRP, PRF or L-PRF) and observation periods.
El-Timamy [20] and Angel [27] showed that PRP injection exerted a promising role in accelerating OTM at the early stages (first 2 months) of tooth movement (Supplementary Table 4). The short-term positive results were proved by animal studies. For example, Güleç et al. [33] reported that tooth movement on the high-concentration PRP side was 1.4 times faster than that in the moderate-concentration group, and 1.7 times faster than that in the control group detected on day 21. Rashid et al. [34] found that, after 63 days of retraction, first premolars with repeated PRP injection showed greater tooth movement than those on the control side by 2.13 times. In a long-term observation, Joy [26] reported a 0.17067 mm/month accelerated OTM rate in the PRP group, while El-Timamy [20] found no difference after 4 months. Although, Liou [35] stated that PRP could last for 5–6 months after a single injection in clinical applications, with a fastest rate of acceleration during 2–4 months. The difference may be partially explained by different preparation procedures and doses they choose, as different techniques can result in different concentrations of leukocytes, platelets and growth factors [36, 37], and PRP functions in a dose-dependent manner [38, 39].
It is inadvisable to draw a conclusion by simply synthesizing the ultimate conclusion of each included study due to their varied periods of trials, but we can gain a deeper insight into the effect of PRF by time-dependent analysis of the data shown in every study (Supplementary Table 4). Firstly, within a short term as first 3 months, PRF seemed to be effective in accelerating OTM, with the highest rate of acceleration at around the 2nd monthly interval. All of the four studies [19, 22, 23, 25] that reported data during the first 3 months supported an accelerating effect of PRF on OTM. Meanwhile, three of them [19, 23, 25] showed a maximal difference in OTM rate between the experimental side and control side in the 2 nd month after PRF application, implying that PRF exerted the greatest effect on OTM acceleration after the first month.
However, no consensus was reached from a long-term view over 4 months. Two studies [19, 25] reported comparable amounts of tooth movement between intervention and control groups in the end, while Pacheco et al. [21] indicated a decreased OTM rate after 5 months. It seems that after the accelerative period at the early stage, OTM decreases as time goes on. In another prospective cohort study, Nemtoi et al. [40] demonstrated that PRF placed in extraction socket still showed a positive efficiency in accelerating OTM after 6 months. Taken together, we tend to believe that single-dosage PRF exerts no, or even a negative effect on OTM in the long term, as several studies [19, 22, 25] reported comparable values or decreasing tendencies in the PRF group compared with the control group regarding the OTM rate at the late stages. As a result, repeated intervention with PRF might help maintain the accelerated OTM, but more studies are needed to clarify its effectiveness and repetition procedures like time interval between applications and dosage in each usage [20, 25]. Though the underlying mechanism remains unclear, we can assume that 1) immediately after PRF application, increased cells and cytokines enhance bone remodeling and then accelerate OTM [23], 2) after a specific period, as PRF degrades and exogenous growth factors and cytokines lower, autogenous growth factors and cytokines may decrease OTM through a negative feedback mechanism [20]. But the actual effects and mechanism of PRF, as well as PRP, need to be elucidated by further well-designed studies with standard protocols and PRP/PRF parameters.
This review systematically analyzes the time-dependent effect of platelet-rich concentrates on OTM in clinical trials, and the results may provide a reference for clinical applications and future research. Overall, dentists could use PRP or PRF, by a repetition method, to accelerate OTM and reduce the duration of orthodontic treatment. For those patients seeking orthodontic treatment who need multidisciplinary therapy like tooth extraction and periodontal surgery, platelet-rich concentrates can be applied in operation, as it not only supports periodontal tissue regeneration, but also accelerates OTM. However, limitations should not be neglected. Although nine RCTs were included, the heterogeneity in study design and platelet-rich concentrate preparation makes it hard to quantitatively analyze the results. Hence, more studies with standardized designs are needed to illustrate their effects.
5. Conclusions
Our study provides a valued insight into the time-dependent effects of these autologous derivatives. The findings indicate a prospect for clinical use of platelet-rich concentrates, and direct future research to investigate their long-term efficacy and treatment protocols. Platelet-rich concentrates as platelet-rich plasma (PRP) and platelet-rich fibrin (PRF), exhibit a short-term acceleration on orthodontic tooth movement (OTM) at early stages, while their long-term effects have yet to be concluded. Repeated application may help maintain a steady accelerated OTM. Standard protocols for the preparation and application of PRP and PRF, as well as more well-designed and standardized studies, are needed to facilitate understanding of their effects and development of clinical applications.
Declarations
Author contribution statement
Ke Yao, Yongzhi Wu and Jingyi Cai: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Yigan Wang: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Yu Shen: Conceived and designed the experiments; Analyzed and interpreted the data.
Dian Jing and Zhihe Zhao: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by the National Natural Science Foundation of China (grant number 81901041), the Science and Technology Program of Sichuan Province (grant number 2022ZDZX0031), and the Research and Development Program of West China Hospital of Stomatology, Sichuan University (grant number RD-03-202012).
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interest’s statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Contributor Information
Dian Jing, Email: janetjingdian@hotmail.com.
Zhihe Zhao, Email: zhzhao@scu.edu.cn.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Moresca R. Orthodontic treatment time: can it be shortened? Dental Press J. Orthod. 2018;23:90–105. doi: 10.1590/2177-6709.23.6.090-105.sar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Dboush R., Esfahani A.N., El-Bialy T. Impact of photobiomodulation and low-intensity pulsed ultrasound adjunctive interventions on orthodontic treatment duration during clear aligner therapy: a retrospective study. Angle Orthod. 2021;91:619–625. doi: 10.2319/112420-956.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang J., Chen P.J., Dutra E.H., Nanda R., Yadav S. The effect of the extent of surgical insult on orthodontic tooth movement. Eur. J. Orthod. 2019;41:601–608. doi: 10.1093/ejo/cjz006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santana L.G., Duarte-Rodrigues L., Alves-Duarte A.C., Galvão E.L., Douglas-de-Oliveira D.W., Marques L.S., et al. Systematic review of biological therapy to accelerate orthodontic tooth movement in animals: translational approach. Arch. Oral Biol. 2020;110 doi: 10.1016/j.archoralbio.2019.104597. [DOI] [PubMed] [Google Scholar]
- 5.Arqub S.A., Gandhi V., Iverson M.G., Ahmed M., Kuo C.L., Mu J., et al. The effect of the local administration of biological substances on the rate of orthodontic tooth movement: a systematic review of human studies. Prog. Orthod. 2021;22:5. doi: 10.1186/s40510-021-00349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dohan Ehrenfest D.M., Rasmusson L., Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF) Trends Biotechnol. 2009;27:158–167. doi: 10.1016/j.tibtech.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 7.Castro A.B., Cortellini S., Temmerman A., Li X., Pinto N., Teughels W., et al. Characterization of the leukocyte- and platelet-rich fibrin block: release of growth factors, cellular content, and structure. Int. J. Oral Maxillofac. Implants. 2019;34:855–864. doi: 10.11607/jomi.7275. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi E., Flückiger L., Fujioka-Kobayashi M., Sawada K., Sculean A., Schaller B., et al. Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clin. Oral Invest. 2016;20:2353–2360. doi: 10.1007/s00784-016-1719-1. [DOI] [PubMed] [Google Scholar]
- 9.Yin W., Qi X., Zhang Y., Sheng J., Xu Z., Tao S., et al. Advantages of pure platelet-rich plasma compared with leukocyte- and platelet-rich plasma in promoting repair of bone defects. J. Transl. Med. 2016;14:73. doi: 10.1186/s12967-016-0825-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernandez-Moure J.S., Van Eps J.L., Cabrera F.J., Barbosa Z., Medrano Del Rosal G., Weiner B.K., et al. Platelet-rich plasma: a biomimetic approach to enhancement of surgical wound healing. J. Surg. Res. 2017;207:33–44. doi: 10.1016/j.jss.2016.08.063. [DOI] [PubMed] [Google Scholar]
- 11.Everts P., Onishi K., Jayaram P., Lana J.F., Mautner K. Platelet-rich plasma: new performance understandings and therapeutic considerations in 2020. Int. J. Mol. Sci. 2020;21:7794. doi: 10.3390/ijms21207794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strauss F.J., Nasirzade J., Kargarpoor Z., Stähli A., Gruber R. Effect of platelet-rich fibrin on cell proliferation, migration, differentiation, inflammation, and osteoclastogenesis: a systematic review of in vitro studies. Clin. Oral Invest. 2020;24:569–584. doi: 10.1007/s00784-019-03156-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Francisco I., Fernandes M.H., Vale F. Platelet-rich fibrin in bone regenerative strategies in orthodontics: a systematic review. Materials. 2020;13:1866. doi: 10.3390/ma13081866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu J., Gou L., Zhang P., Li H., Qiu S. Platelet-rich plasma and regenerative dentistry. Aust. Dent. J. 2020;65:131–142. doi: 10.1111/adj.12754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Z., Zhou J., Chen S. The effectiveness of locally injected platelet-rich plasma on orthodontic tooth movement acceleration. Angle Orthod. 2021;91:391–398. doi: 10.2319/061320-544.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sterne J.A.C., Savović J., Page M.J., Elbers R.G., Blencowe N.S., Boutron I., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 18.Guyatt G.H., Oxman A.D., Schünemann H.J., Tugwell P., Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J. Clin. Epidemiol. 2011;64:380–382. doi: 10.1016/j.jclinepi.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 19.Tehranchi A., Behnia H., Pourdanesh F., Behnia P., Pinto N., Younessian F. The effect of autologous leukocyte platelet rich fibrin on the rate of orthodontic tooth movement: a prospective randomized clinical trial. Eur. J. Dermatol. 2018;12:350–357. doi: 10.4103/ejd.ejd_424_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Timamy A., El Sharaby F., Eid F., El Dakroury A., Mostafa Y., Shaker O. Effect of platelet-rich plasma on the rate of orthodontic tooth movement: a split-mouth randomized trial. Angle Orthod. 2020;90:354–361. doi: 10.2319/072119-483.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pacheco A.A.R., Collins J.R., Contreras N., Lantigua A., Pithon M.M., Tanaka O.M. Distalization rate of maxillary canines in an alveolus filled with leukocyte-platelet-rich fibrin in adults: a randomized controlled clinical split-mouth trial. Am. J. Orthod. Dentofacial Orthop. 2020;158:182–191. doi: 10.1016/j.ajodo.2020.03.020. [DOI] [PubMed] [Google Scholar]
- 22.Çağlı Karcı İ., Baka Z.M. Assessment of the effects of local platelet-rich fibrin injection and piezocision on orthodontic tooth movement during canine distalization. Am. J. Orthod. Dentofacial Orthop. 2021;160:29–40. doi: 10.1016/j.ajodo.2020.03.029. [DOI] [PubMed] [Google Scholar]
- 23.Erdur E.A., Karakasli K., Oncu E., Ozturk B., Hakki S. Effect of injectable platelet-rich fibrin (i-PRF) on the rate of tooth movement: a randomized clinical trial. Angle Orthod. 2021;91:285–292. doi: 10.2319/060320-508.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karakasli K., Erdur E.A. The effect of platelet-rich fibrin (PRF) on maxillary incisor retraction rate. Angle Orthod. 2021;91:213–219. doi: 10.2319/050820-412.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeitounlouian T.S., Zeno K.G., Brad B.A., Haddad R.A. Effect of injectable platelet-rich fibrin (i-PRF) in accelerating orthodontic tooth movement: a randomized split-mouth-controlled trial. J. Orofac. Orthop. 2021;82:268–277. doi: 10.1007/s00056-020-00275-x. [DOI] [PubMed] [Google Scholar]
- 26.Joy N.V., Jyothikiran H., Raghunath N.R. Comparison of mini-implant-supported mandibular canine retraction with and without submucosal injection of platelet-rich plasma: a split-mouth study. World J. Dent. 2021;12:446–452. [Google Scholar]
- 27.Angel S.L., Samrit V.D., Kharbanda O.P., Duggal R., Kumar V., Chauhan S.S., et al. Effects of submucosally administered platelet-rich plasma on the rate of tooth movement. Angle Orthod. 2022;92:73–79. doi: 10.2319/011221-40.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alhasyimi A.A., Pudyani P.P., Asmara W., Ana I.D. Enhancement of post-orthodontic tooth stability by carbonated hydroxyapatite-incorporated advanced platelet-rich fibrin in rabbits. Orthod. Craniofac. Res. 2018;21:112–118. doi: 10.1111/ocr.12224. [DOI] [PubMed] [Google Scholar]
- 29.Alomari E.B., Sultan K. Efficacy of injectable platelet-rich plasma in reducing alveolar bone resorption following rapid maxillary expansion: a cone-beam computed tomography assessment in a randomized split-mouth controlled trial. Angle Orthod. 2019;89:705–712. doi: 10.2319/091018-661.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeitounlouian T.S., Zeno K.G., Brad B.A., Haddad R.A. Three-dimensional evaluation of the effects of injectable platelet rich fibrin (i-PRF) on alveolar bone and root length during orthodontic treatment: a randomized split mouth trial. BMC Oral Health. 2021;21:92. doi: 10.1186/s12903-021-01456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh A.K., Sharma N.K., Kumar P.G.N., Singh S., Mishra N., Bera R.N. Evaluation of arthrocentesis with and without platelet-rich plasma in the management of internal derangement of temporomandibular joint: a randomized controlled trial. J. Maxillofac Oral Surg. 2021;20:252–257. doi: 10.1007/s12663-019-01320-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giudice G., Cutrignelli D.A., Leuzzi S., Robusto F., Sportelli P., Nacchiero E. Autologous bone grafting with platelet-rich plasma for alveolar cleft repair in patient with cleft and palate. Ann. Ital. Chir. 2016;87:5–12. [PubMed] [Google Scholar]
- 33.Güleç A., Bakkalbaşı B., Cumbul A., Ü Uslu, Alev B., Yarat A. Effects of local platelet-rich plasma injection on the rate of orthodontic tooth movement in a rat model: a histomorphometric study. Am. J. Orthod. Dentofacial Orthop. 2017;151:92–104. doi: 10.1016/j.ajodo.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 34.Rashid A., ElSharaby F.A., Nassef E.M., Mehanni S., Mostafa Y.A. Effect of platelet-rich plasma on orthodontic tooth movement in dogs. Orthod. Craniofac. Res. 2017;20:102–110. doi: 10.1111/ocr.12146. [DOI] [PubMed] [Google Scholar]
- 35.Liou E.J.W. The development of submucosal injection of platelet rich plasma for accelerating orthodontic tooth movement and preserving pressure side alveolar bone. APOS Trends Orthod. 2016;6:5–11. [Google Scholar]
- 36.Pachito D.V., Bagattini  M., de Almeida A.M., Mendrone-Júnior A., Riera R. Technical procedures for preparation and administration of platelet-rich plasma and related products: a scoping review. Front. Cell Dev. Biol. 2020;8 doi: 10.3389/fcell.2020.598816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dohan Ehrenfest D.M., Pinto N.R., Pereda A., Jiménez P., Corso M.D., Kang B.S., et al. The impact of the centrifuge characteristics and centrifugation protocols on the cells, growth factors, and fibrin architecture of a leukocyte- and platelet-rich fibrin (L-PRF) clot and membrane. Platelets. 2018;29:171–184. doi: 10.1080/09537104.2017.1293812. [DOI] [PubMed] [Google Scholar]
- 38.Weibrich G., Hansen T., Kleis W., Buch R., Hitzler W.E. Effect of platelet concentration in platelet-rich plasma on peri-implant bone regeneration. Bone. 2004;34:665–671. doi: 10.1016/j.bone.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 39.Choi B.H., Zhu S.J., Kim B.Y., Huh J.Y., Lee S.H., Jung J.H. Effect of platelet-rich plasma (PRP) concentration on the viability and proliferation of alveolar bone cells: an in vitro study. Int. J. Oral Maxillofac. Surg. 2005;34:420–424. doi: 10.1016/j.ijom.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 40.Nemtoi A., Sirghe A., Nemtoi A., Haba D. The effect of a plasma with platelet-rich fibrin in bone regeneration and on rate of orthodontic tooth movement in adolescents. Rev. Chem. 2018;69:3727–3730. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supp. material/referenced in article.