Abstract
Ductus arteriosus aneurysm (DAA) is a rare cardiovascular anomaly, and thrombosis of DAA is even less common. The management of asymptomatic DAA with a thrombus is controversial. We here report a neonate with a thrombus from a DAA that grew rapidly into the pulmonary artery. The thrombus was detected incidentally in the main pulmonary artery by routine screening echocardiography. There was no clinical evidence of its presence until a few days after birth. The thrombus grew rapidly, despite administration of heparin. Six days after birth, the patient became cyanotic and had developed right ventricular pressure overload as a result of obstruction of the left pulmonary artery. The thrombus was immediately removed and the DAA resected. The patient was discharged home without any complications. Complications related to thrombus of a DAA can be critical and therefore require careful monitoring.
Learning objective
A thrombus extending from a ductus arteriosus aneurysm into the pulmonary artery can have serious consequences; thus, careful monitoring is required. Any signs of such complications should prompt immediate consideration of removal of the aneurysm and thrombus.
Keywords: Ductus arteriosus aneurysm, Thrombus, Neonate
Introduction
Ductus arteriosus aneurysm (DAA), a rare cardiovascular anomaly, is characteristically identified in infants. DAA can reportedly be associated with serious complications, such as rupture, erosion into an airway, infection, and compression of adjacent structures [1], [2]. In this report, we describe a neonate who required emergency surgery for a massive, rapidly growing thrombus that extended into the pulmonary artery from a DAA.
Case report
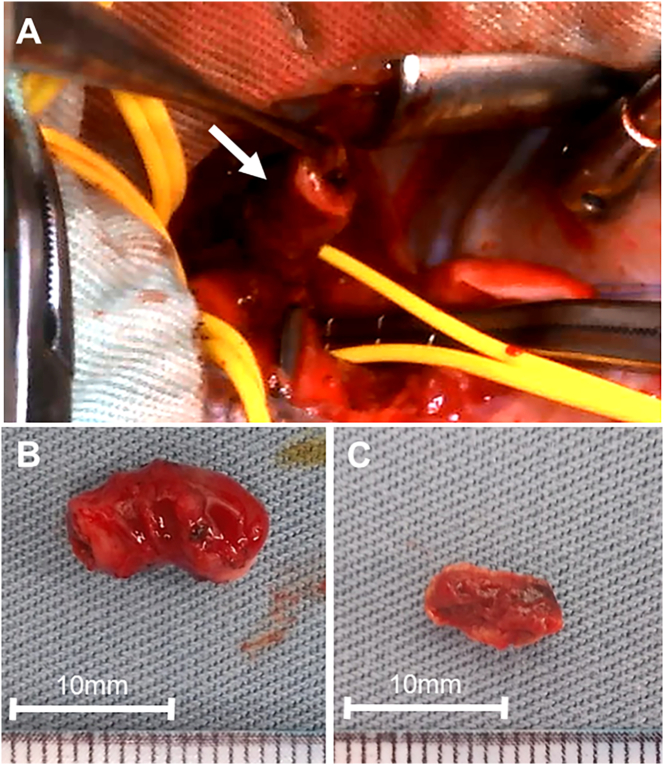
A 2.4-kg full-term boy was born with 1 and 5 min Apgar scores of 6 and 7, respectively, in a regional hospital. Cardiac abnormalities were not detected during the fetal period. After birth, he was transferred to the neonatal intensive care unit (NICU) in Kitasato University Hospital because of respiratory disorders, possibly due to neonatal asphyxia. After initiation of respiratory support, the patient immediately improved and did not show any signs of hypoxia, dehydration, or infection. Screening by echocardiography on admission showed a solid mass in the left pulmonary artery (Fig. 1A) and normal blood flow velocity. Computed tomography showed a large, low-density mass between the main pulmonary artery and descending aorta (Fig. 2A, B), suggestive of a DAA with thrombus. Systemic heparinization was started to prevent thromboembolism, and the activated partial thromboplastin time (APTT) was kept within 1.5–2.0 times of that control. Daily echocardiographic screening performed by pediatric cardiologists showed gradual extension of the thrombus into the main pulmonary artery without any clinical signs. Six days after birth, the patient became cyanotic, and echocardiography showed that the thrombus had occluded the left pulmonary artery (Fig. 1B). On the same day, emergency surgery to remove the thrombus and DAA was performed by pediatric cardiac surgeons. The operation was performed through median sternotomy. Cardiopulmonary bypass was established using bicaval cannulations with regional cerebral perfusion (RCP). The patient was cooled down to 28 °C. The ascending aorta was cross-clamped and cardiac arrest was achieved with antegrade cardioplegia. The main pulmonary artery was opened and a thrombus in the left pulmonary was completely removed. A dilated DAA was found between the left pulmonary artery and the descending aorta. The DAA was transected at the pulmonary artery side and this side was closed (Fig. 3A). The DAA and descending aorta were carefully dissected to avoid left recurrent laryngeal nerve injury. Under RCP, the descending aorta was clamped, and the DAA was completely removed. The aortic end of the DAA was stenotic, and therefore, the defect of the descending aorta was directly closed. The patient was weaned from cardiopulmonary bypass uneventfully. The aortic cross-clamping time was 47 min and the RCP time was 8 min. The DAA was 11 mm in length and 6 mm outer diameter, and the diagnosis was confirmed by histological findings (Fig. 3B, C). Elastica van Gieson staining showed partial rupture of the internal elastic lamina in the DAA, and the thrombus contained fibrin, red blood cells, and inflammatory cells. The patient had an uneventful postoperative course and was discharged 18 days after surgery without any complications. Postoperative echocardiography showed no pulmonary artery stenosis. There has been no evidence of thromboembolism during follow-up and the patient is currently well.
Fig. 1.
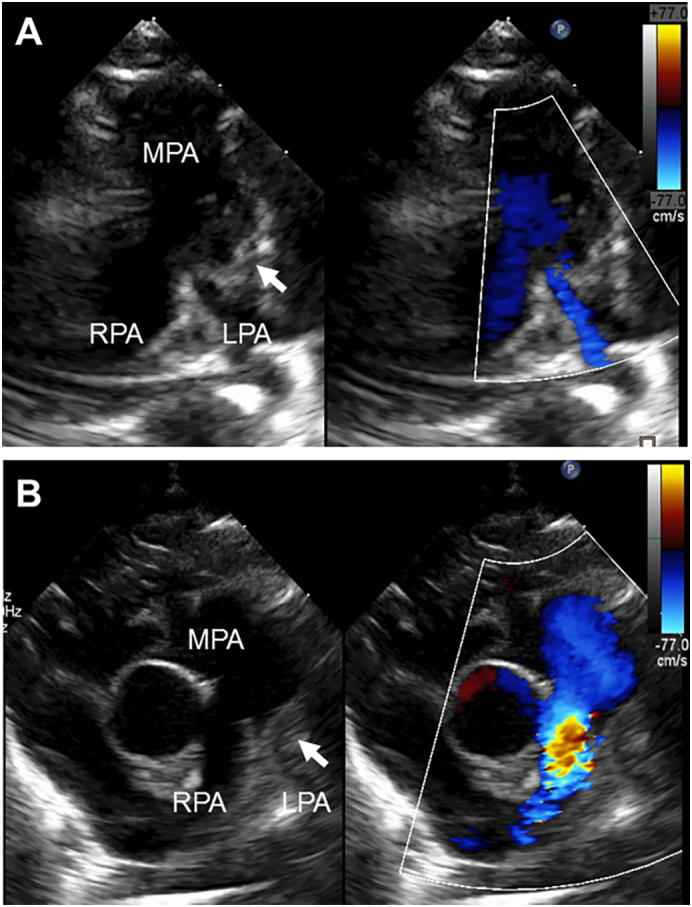
Two-dimensional color Doppler echocardiographic short-axis views showing the thrombus (arrow) within the MPA. (A) Six hours after birth. (B) Six days after birth.
LPA, left pulmonary artery; MPA, main pulmonary artery; RPA, right pulmonary artery.
Fig. 2.
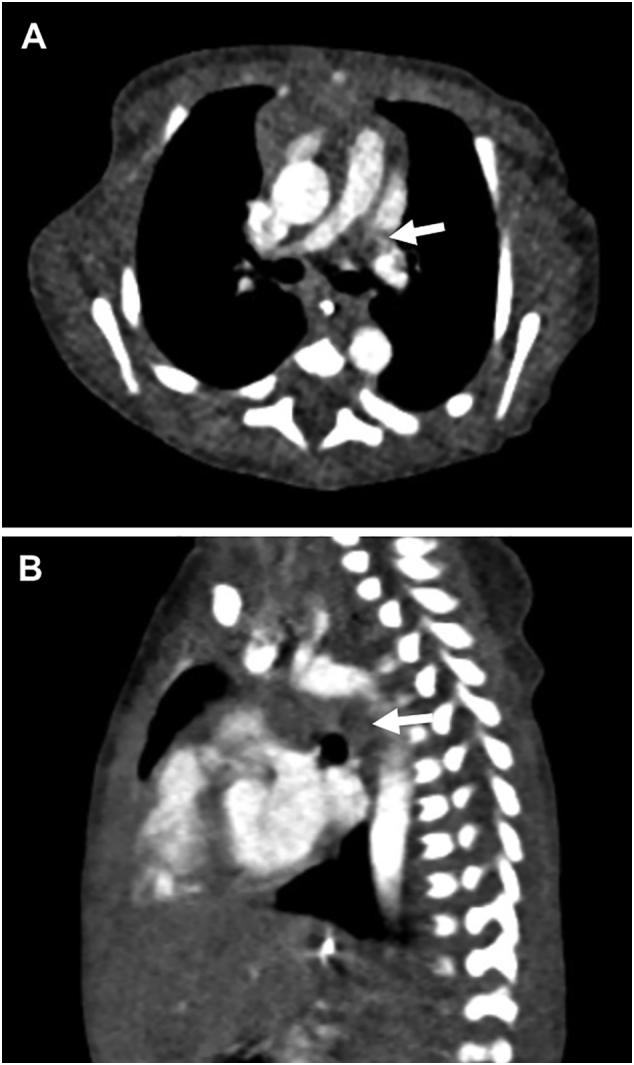
Computed tomography images showing dilated ductus arteriosus occupied by thrombus (arrow). (A) Axial view. (B) Sagittal view.
Fig. 3.
(A) Intraoperative view of the ductus arteriosus aneurysm (arrow), which was transected from the pulmonary artery side. Specimens after complete removal of (B) the ductus arteriosus aneurysm and (C) the thrombus.
Discussion
The pathogenesis of DAA is unclear. The following possibilities have been proposed: delayed closure at the aortic end of the ductus arteriosus, congenital weakness of the ductal wall, increased flow through the ductus arteriosus, intrauterine ductal constriction and post-stenotic dilatation of ductus arteriosus, abnormal intimal cushion formation, and defective elastin in the ductus arteriosus [1], [3], [4]. In our case, histological findings showed partial rupture in the DAA, which supports the contention that congenital weakness of the ductal wall contributes to the pathogenesis of DAA. The incidence of neonatal DAA is reportedly 0.8 %–8.8 % [2], [3], [4], [5], [6]. Jan et al. reported that there was no clinical evidence of its presence or complications in 48 neonates with DAA [5]. In our case, the DAA with thrombus was identified incidentally during routine cardiac assessment and there was no clinical evidence of it at the time of birth. The thrombus contained fibrin, red blood cells, and inflammatory cells, suggesting that fresh thrombus had formed after birth and that neonatal asphyxia may have contributed to its formation [7].
A previous study proposed surgical indications for clinically evident DAA [2]. However, management of asymptomatic DAA with a thrombus is controversial. Conservative management with anticoagulation therapy may result in resolution of a thrombus, obviating the need for surgery [8]. There is a risk of thromboembolism; however, cardiac surgery in a neonate is risky. We initially chose to minimize the risk of thromboembolism by conservative management in the form of heparinization. Surprisingly, despite heparinization, the thrombus extended into the pulmonary artery and grew rapidly. This may have been attributable to the difficulties associated with anticoagulant therapy in neonates. Frequent blood sampling for measuring APTT is invasive, whereas excessive anticoagulation carries a risk of intracranial bleeding [9]. Thus, daily, non-invasive echocardiographic screening played an important role in monitoring our patient. Thromboembolism is a critical situation and surgical embolectomy is always challenging because of a risk of distal embolization. In our case, removal of the thrombus and DAA was performed using RCP. A previous study showed that RCP was a safe and easy technique for aortic arch repair in neonates to avoid neurological complications [10].
DAA with a thrombus is rare but may have critical complications. We could detect the growth of thrombus by serial echocardiography and used the findings to determine the indications for, and optimal timing of, surgery. In conclusion, careful monitoring in the NICU, accurate diagnosis by pediatric cardiologists and immediate surgery by pediatric cardiac surgeons were associated with successful outcome in this case.
Informed consent
We obtained parental written informed consent for publishing the details of this case.
Acknowledgments
Acknowledgment
None.
Conflict of interest
None.
References
- 1.Lund J.T., Jensen M.B., Hjelms E. Aneurysm of the ductus arteriosus: a review of the literature and the surgical implications. Eur J Cardiothorac Surg. 1991;5:566–570. doi: 10.1016/1010-7940(91)90220-e. [DOI] [PubMed] [Google Scholar]
- 2.Dyamenahalli U., Smallhorn J.F., Geva T., Fouron J.C., Cairns P., Jutras L., Hughes V., Rabinovitch M., Mason C.A., Hornberger L.K. Isolated ductus arteriosus aneurysm in the fetus and infant: a multi-institutional experience. J Am Coll Cardiol. 2000;36:262–269. doi: 10.1016/s0735-1097(00)00707-5. [DOI] [PubMed] [Google Scholar]
- 3.Lund J.T., Hansen D., Brocks V., Jensen M.B., Jacobsen J.R. Aneurysm of the ductus arteriosus in the neonate: three case reports with a review of the literature. Pediatr Cardiol. 1992;13:222–226. doi: 10.1007/BF00838780. [DOI] [PubMed] [Google Scholar]
- 4.Acherman R.J., Siassi B., Wells W., Goodwin M., DeVore G., Sardesai S., Wong P.C., Ebrahimi M., Pratti-Madrid G., Castillo W., Ramanathan R. Aneurysm of the ductus arteriosus: a congenital lesion. Am J Perinatol. 1998;15:653–659. doi: 10.1055/s-2007-999298. [DOI] [PubMed] [Google Scholar]
- 5.Jan S.L., Hwang B., Fu Y.C., Chai J.W., Chi C.S. Isolated neonatal ductus arteriosus aneurysm. J Am Coll Cardiol. 2002;39:342–347. doi: 10.1016/s0735-1097(01)01736-3. [DOI] [PubMed] [Google Scholar]
- 6.Falcone M.W., Perloff J.K., Roberts W.C. Aneurysm of the nonpatent ductus arteriosus. Am J Cardiol. 1972;29:422–426. doi: 10.1016/0002-9149(72)90542-5. [DOI] [PubMed] [Google Scholar]
- 7.Makatsariya A., Bitsadze V., Khizroeva J., Vorobev A., Makatsariya N., Egorova E., Mischenko A., Mashkova T., Antonova A. Neonatal thrombosis. J Matern Fetal Neonatal Med. 2022;35:1169–1177. doi: 10.1080/14767058.2020.1743668. [DOI] [PubMed] [Google Scholar]
- 8.Pereira A.G., Teixeira A., Martins F.M. Spontaneous thrombosis of the ductus arteriosus. Rev Port Cardiol. 2011;30:537–540. [PubMed] [Google Scholar]
- 9.Monagle P., Newall F. Anticoagulation in children. Thromb Res. 2012;130:142–146. doi: 10.1016/j.thromres.2012.03.028. [DOI] [PubMed] [Google Scholar]
- 10.Miyaji K., Miyamoto T., Kohira S., Itatani K., Tomoyasu T., Inoue N., Ohara K. Regional high-flow cerebral perfusion improves both cerebral and somatic tissue oxygenation in aortic arch repair. Ann Thorac Surg. 2010;90:593–599. doi: 10.1016/j.athoracsur.2010.03.113. [DOI] [PubMed] [Google Scholar]