Abstract
The idea of using engineered bacteria as prospective living therapeutic agents for the treatment of different diseases has been raised. Nevertheless, the development of safe and effective treatment strategies remains essential to the success of living bacteria-mediated therapy. Hydrogels have presented great promise for the delivery of living bacterial therapeutics due to their tunable physicochemical properties, good bioactivities, and excellent protection of labile payloads. In this review, we summarize the hydrogel design strategies for living bacteria-mediated therapy and review the recent advances in hydrogel-based living bacterial agent delivery for the treatment of typical diseases, including those for digestive health, skin fungal infections, wound healing, vaccines, and cancer, and discuss the current challenges and future perspectives of these strategies in the field. It is believed that the importance of hydrogel-based living bacteria-mediated therapy is expected to further increase with the development of both synthetic biology and biomaterials science in the future.
Keywords: Hydrogel, Living bacterial therapeutics, Drug delivery, Bacteriotherapy
Graphical abstract

1. Introduction
Bacteria, a main microbial species that closely participate in human health and disease development, have been raised as emerging living therapeutic candidates for disease treatment and health maintenance in recent decades, including digestive diseases [1], skin wounds [2], genitourinary tract diseases [3], living vaccines [4], and cancer therapy [5,6]. Despite the increasing interest in the field of bacteriotherapy, some essential challenges are still hard to ignore for future clinical applications [7]. How to selectively limit the in vivo clearance and inactivation of living bacterial therapeutics and maintain sufficient local responses after administration are main challenges for living bacteria-mediated therapy [8]. In addition, uncertain toxicity and deleterious side effects are also required for bacterial therapy according to results from preclinical and clinical studies [6]. Therefore, innovative strategies are urgently needed to address these challenges.
Controlling colonization and delivery can not only improve the local concentration of bacterial therapeutics and reduce toxicity to normal tissues but also help the microorganism effectively bypass the key challenges associated with bacteriotherapy, which is considered one of the effective approaches for enhancing this treatment modality [7]. For instance, to overcome the above challenges, the strategy of modifying the bacterial surface by physical, chemical or biological techniques has commonly been used to enhance the extra capacities of the bacteria, improve their delivery behaviors and promote the efficiency of bacteriotherapy [9,10]. Despite considerable advances in living bacteria-associated disease treatments, numerous obstacles (including bacterial viability, the first-pass effect, systemic infection risks, and so on) continue to limit the clinical translation of surface-modified bacteria [8]. Local administration is another important option that can effectively improve the concentration of engineered therapeutic bacteria at the target site and reduce the microbial clearance rate as well as the potential risk of systemic infection [7,11]. Notably, the use of living bacteria hydrogel formulations has emerged as a promising method for the local delivery of engineered therapeutic bacterial strains and bacteriotherapy [[12], [13], [14]].
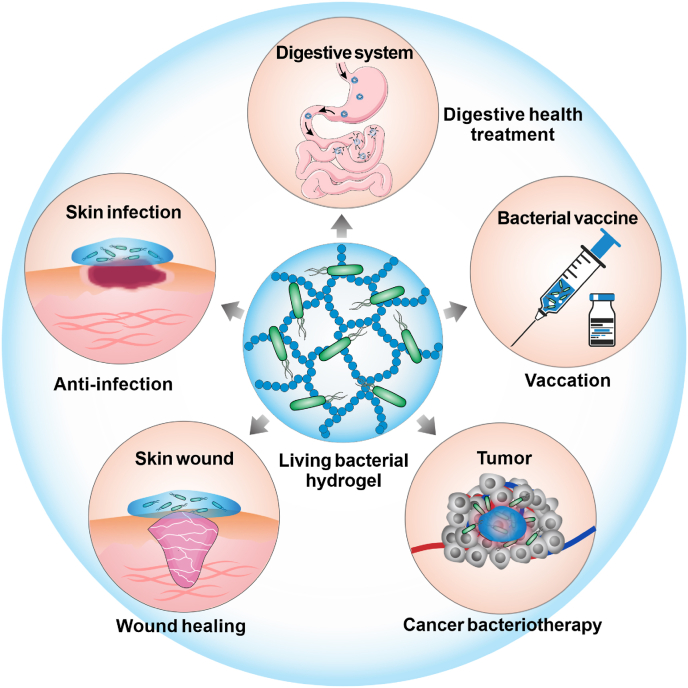
In this review, we will summarize the main strategies of hydrogel design for living bacteria-mediated therapy and review the recent advances in hydrogel-based living bacterial therapeutic delivery and typical disease treatments, including bacterial-promoted wound healing, cancer bacteriotherapy, bacterial vaccines, probiotic-mediated digestive health treatment, and so on (Fig. 1). The promises and challenges of these strategies will also be discussed.
Fig. 1.
Schematic illustration of hydrogels in living bacteria-mediated therapy, primarily those involved with digestive health treatment, skin fungal infections, wound healing, living bacterial vaccines, and cancer bacteriotherapy.
2. Hydrogels for the delivery of living bacteria therapeutics
2.1. Advantages of hydrogels for living bacteria delivery
Hydrogels with a three-dimensional (3D) structure and high water content are an attractive class of biomaterials owing to their tunable physicochemical and biological properties [15]. These semisolid materials have been widely used for cell culture [16], tissue engineering [17], medical implants [18], biosensors [13,19], drug delivery [20], 3D-printed living materials [21] and so on [22]. In particular, hydrogels have drawn much attention as potential platforms in the field of drug delivery, because they can not only control the release of various therapeutics in a spatial and temporal manner but also offer a simple administration method for complicated drug regimens [[23], [24], [25], [26], [27], [28]]. Furthermore, hydrogels have presented many unique advantages for bioactive therapeutic delivery [29]. For example, these materials have good biocompatibility, tunable chemical permeability, and excellent mechanical compliance that is similar to the extracellular matrix and beneficial to maintaining the biomedical activities of bioactive therapeutics [15,30]. In light of the above advantages, novel hydrogel-based bioactive drug delivery systems have been constantly emerging in recent decades to enhance the treatment of various diseases [24,[31], [32], [33]]. In particular, there has been growing interest in the development of hydrogel systems to deliver living bacteria.
Compared with other carriers, hydrogels present many unique properties to meet the demands of the delivery of living bacterial therapeutics [34]. First, these macroscopic soft materials can provide sufficient space to accommodate the bacteria even though they have a much larger volume than small molecule drugs [26,30]. Second, the good biocompatibility and tunable physicochemical properties of hydrogels are conducive to maintaining bacterial vitality as well as improving treatment efficacy [35,36]. Third, their adjustable pore structure and mechanical properties provide physical barriers that can not only protect the living bacteria from potential clearance and inactivation but also deliver the bacterial therapeutic agents in a controllable and safe manner [13,26,37]. Moreover, the adhesion and release behaviors of the bacteria can be regulated by changing the biodegradability, internal charge, and chain hydrophilicity of the hydrogels [13,34,38]. These inherent qualities make hydrogels promising candidates for the delivery of living bacterial therapeutics.
2.2. Hydrogel design strategies for living bacteria-mediated therapy
Rational hydrogel design is the key in the development of such living bacteria delivery systems, and several important factors should be taken into account when designing the target hydrogels. First, selecting suitable materials is the most basic need in bacteria-compatible hydrogel design. In general, polymers (both natural and synthetic) are the largest class of materials currently used in both the gel and solid states [39,40]. According to published studies, different types of polymeric hydrogels have been employed to develop living bacterial delivery systems. In particular, polysaccharide-derived hydrogels, such as alginate, cellulose, chitosan, dextran, hyaluronic acid, and their derivatives, have been widely used to deliver such therapeutics due to their good biocompatibility and economical cost [39]. In addition, synthetic polymers are another class of materials that are used for the fabrication of living bacterial hydrogel systems, such as Pluronic F-127, poly (ethylene glycol) (PEG), synthetic polypeptides, and glycolide dimethacrylate [41,42]. Furthermore, a number of biomacromolecule-based hydrogels with low immunogenicity have been used to deliver microbial agents, including fibrinogen, collagen, and albumin [15,43,44]. In addition, a reported biofilm-inspired cell-generated living hydrogel showed the unique capabilities of self-replication, self-replenishment, and self-healing when used as a hydrogel matrix, which endowed the system with potential for efficient living bacteria delivery [34,44]. Due to their good biocompatibility, the above materials are safe candidates for use in hydrogel-based living bacteria therapies [45]. Nevertheless, cationic hydrogels are generally considered to be unsuitable carriers for living bacterial delivery systems because of the microbial lysis effects of the cationic charge [46].
Second, the hydrogel fabrication strategy is another key factor affecting the activity and efficient delivery of the encapsulated bacteria [34]. Hydrogels can be classified as physical or chemical gels based on their formation method. Physical hydrogels depend on intermolecular interactions and have been widely used to fabricate living bacterial delivery systems due to their mild solution phase transition behaviors and easy operation, as well as the generation of fewer byproducts during the gelation process [20]. As an example, alginate-derived hydrogels ionically crosslinked by Ca2+ have been widely employed to construct living bacterial delivery systems due to their excellent cell compatibility and gentle gelation behaviors [[47], [48], [49], [50]]. Thermogelling hydrogels have also been used in previous works to deliver living bacteria to their convenience during both the preparation of the bacteria-encapsulating hydrogel precursor solutions and in vivo administration [41,[51], [52], [53]]. However, the relatively high critical concentration and low mechanical strength of physical hydrogels are unfavorable for the exchange of substances and long-term bacterial survival during the treatment process. In addition, it is difficult for these gels, which merely depend on intermolecular interactions, to form a strong physical containment barrier and prevent microbial leakage during in vivo bacterial transmission. Compared to physical hydrogels, hydrogels crosslinked by chemical bonds can form a sparse and strong network and require a relatively lower critical gelation concentration, higher mechanical strength and physical stability, and prolonged degradation behavior, which may be conducive to controlling the long-term encapsulation and transmission behaviors of the bacterial therapeutics [54]. Nevertheless, possible limitations resulting from some potential toxic agents (e.g., uncrosslinked monomers, residual catalysts or initiators, and organic solvents) from gel formation should also be considered for living bacterial delivery in vivo, which may influence the activity of the encapsulated bacterial therapeutic or harm normal organs [20]. Mild chemical crosslinking reactions without toxic byproducts (such as biorthogonal click chemistry and long wavelength-triggered high efficiency photoreactions) during gel formation are expected to be used for living bacterial delivery, as they facilitate bacterial viability and positive therapeutic outcomes [55]. Overall, good biodegradability and biocompatibility are significant for living bacteria encapsulation and delivery, especially during the gelation and degradation processes. Furthermore, regulation of the charge or hydrophilicity of the hydrogel can notably affect the microbe-hydrogel interface as well as influence cell attachment and adhesion behavior, which are crucial for improving bacterial viability and the efficiency of bacteriotherapy [34].
Third, hydrogel system design also needs to consider the physiological characteristics of the particular disease to be treated to regulate the bacterial release behavior and enhance living bacteria-mediated therapy. For example, as a probiotic oral delivery vehicle, the hydrogel is required to bypass the key challenges associated with oral delivery (including resistance to the acidic environment of stomach acid, bile salts, and digestive enzymes) for targeted delivery of probiotics to the intestine [39]. While, as a carrier for living bacterial cancer therapy, hydrogel design not only needs to consider how to evade therapeutic microbial clearance and inactivation by the immune system but also toward achieving the on-demand release of living bacterial agents in a safety pattern for efficient cancer therapy [50]. It can be seen that distinct characteristics of diseases set different requirements for hydrogel design as well as related living bacterial therapy. The principal findings of such investigations will be presented in detail in the following sections.
Besides, it is necessary that the strategy for biological containment be considered in the design of hydrogel systems, as it will undoubtedly enhance people's confidence in the biosafety of bacterial therapy involving hydrogels [6]. Presently, efforts in this regard are already being made. For instance, Zhao and coworkers demonstrated that a well-designed hydrogel-based encapsulation system could not only protect the activity of encapsulated genetically modified microorganisms (GMMs) but also effectively avoid GMM escape and environmental insults [13]. In another work, Han et al. also demonstrated that chitosan-derived hydrogel microcapsules prepared in tripolyphosphate solution could notably prevent leakage of engineered E. coli according to hematoxylin and eosin (H&E) and inflammatory factor assay results [56].
In recent years, a number of chemical design strategies have been successfully used to engineer hydrogels for cell culture, which will bring new development opportunities to hydrogel-living bacterial delivery systems [57].
3. Recent advances in the use of hydrogels for living bacteria-mediated therapy
3.1. Living bacterial hydrogels for intestinal health
The human body hosts complex microbial communities that play significant roles in physiology and disease [58]. Research has shown that the constant interaction between the intestinal microbiota and host is associated with certain types of inflammatory intestinal diseases, including ulcerative colitis and Crohn's disease [59,60]. Probiotic microorganisms (Lactobacillus species, Clostridium butyricum, Bifidobacterium, etc.) are becoming increasingly popular in digestive health due to their widely recognized health benefits to the host [61]. Despite oral administration being considered as the best way to take probiotics, how to maintain the activity of these microorganisms during storage and gastrointestinal transit remains a challenge [61].
In the most recent decade, the encapsulation of probiotics within biocompatible hydrogels has been demonstrated to be an encouraging approach for improving survival and efficacy for the oral delivery of probiotics [[61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76]]. In these studies, natural polysaccharides (e.g., agarose, alginate, cellulose, chitosan, dextran, and starch) have been widely used to develop hydrogel-based probiotic delivery systems due to their easy biodegradability, good biocompatibility and economic practicability [39]. In addition to the above advantages, their structures can be easily modified to form functional gel matrixes with diverse architectures and mechanical properties, which benefit the maintenance of biotherapeutic activity. Not only that, natural polysaccharides are also required to be resistant to the acidic environment of the stomach but disintegration under neutral-basic pH conditions for the targeted delivery of probiotics to the intestine [43,72,75]. According to actual demands, these matrixes are usually designed to generate hydrogel/microgel formations for probiotic delivery [63,66,67,75]. The recent progress in hydrogel-based probiotic delivery systems is discussed in the following section based on these two perspectives.
Hydrogels have generated considerable interest in the past few years as carriers for the protection and delivery of probiotics [66,75,76]. For example, Risbo and coworkers developed a chitosan and dextran sulfate-based physical and chemical crosslinked hydrogel for probiotic encapsulation targeting the gastrointestinal tract [66]. The results showed that the swelling behavior of this hydrogel was mainly dependent on the material composition but slightly influenced by the pH of the media. Although culturability tests showed that the viability of Lactobacillus acidophilus in the gel decreased by approximately 3.6 log CFU/mL compared to the number of viable native cells because of the considerable amount of entrapped viable cells in the hydrogel network; however, this material is still an appealing biomatrix for probiotic encapsulation. Recently, pH-sensitive hydrogels have attracted increasing interest in the field of probiotic delivery [72,75,76]. In particular, alginate-based hydrogels not only protect the loaded bacterial cells under simulated stomach conditions but also release the loaded viable probiotic payload in a timely manner under neutral-basic pH conditions [43,72]. For instance, Yuan and coworkers demonstrated that a fabricated pH-sensitive propylene glycol alginate-based hydrogel could greatly reduce the chemical degradation of curcumin and increase the survival of Lactobacillus under both simulated gastrointestinal tract conditions and after long-term storage [75]. Although the above works suggested that these hydrogels are promising carriers for improving probiotic delivery, some of the present results still need functional verification in vivo.
Microgels, water-swollen networks of crosslinks with diameters ranging from 1–1000 μm, have great potential as biomacromolecule delivery platforms [14,[77], [78], [79], [80]]. In terms of oral probiotic delivery, microgels exhibit several advantages compared to traditional hydrogels. First, the smaller size of the microgels not only allows them to be easily injected and swallowed but also gives them a better ability to cross the biological barriers in the digestive system (including low pH, bile salts, enzymes, etc.), which is beneficial for the development of safe and efficient oral probiotic delivery systems [80]. Second, after surface functionalization, microgels can maintain better intestinal adhesion and release the encapsulated probiotics at the target sites in a sustained manner, which is favorable for the long-term treatment of intestinal diseases. The above advantages of microgels have attracted significant interest in the community of oral probiotic delivery [[68], [69], [70],73,81,82]. For example, the work of Li et al. showed that encapsulating Lactobacillus rhamnosus GG (LGG) with polysaccharide-based hydrogel beads increased the tolerance of LGG under acidic conditions and enhanced P40 protein (an LGG-derived protein that contributes to the ability of LGG to maintain intestinal homeostasis) production, which had greater effects on the prevention of dextran sulfate sodium-induced colonic injury and colitis than free LGG in mice [63]. In another work, an LGG-loaded exfoliated bentonite/alginate nanocomposite hydrogel was developed by Yoo and coworkers for effective intestinal probiotic delivery in vivo. The results demonstrated that the survival rate of LGG within the nanocomposite hydrogels notably improved, and the viable counts of LGG were sixfold higher than those in the control groups in the mouse fecal recovery experiment [47]. The above findings indicate that microgels can be engineered into a favorable platform for the intestinal delivery of probiotics.
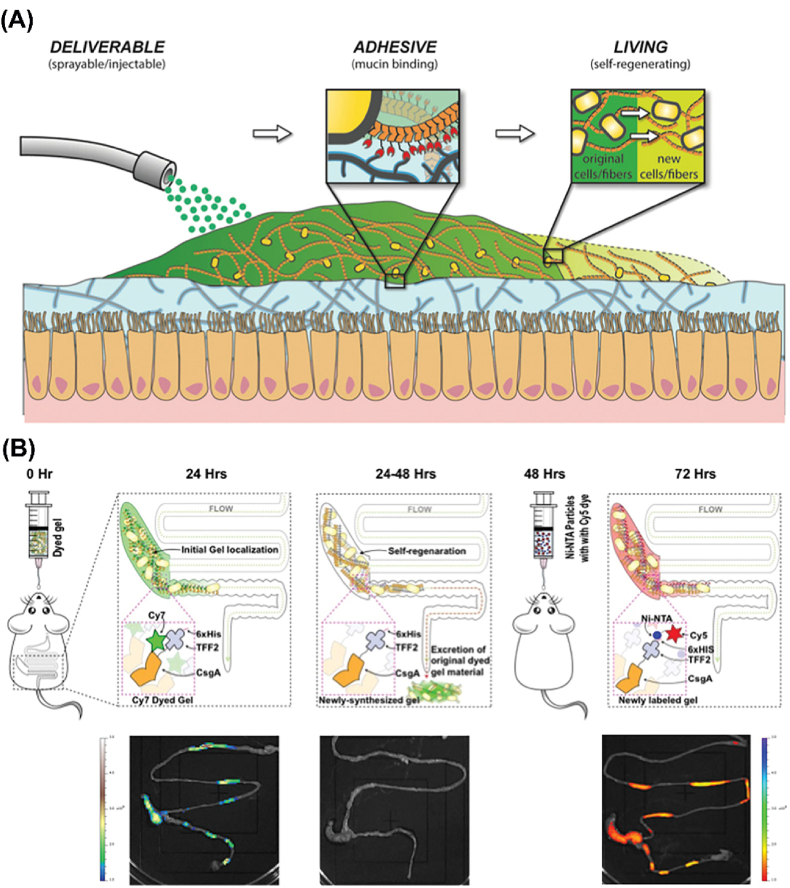
In addition to their benefits for digestive health, the use of living bacterial hydrogels also appears to be a reliable solution for intestinal disease treatment. For example, Joshi and coworkers developed a genetically programmable self-regenerating living bacterial curli hydrogel as a mucoadhesive patch for potential medical applications in the gut (Fig. 2) [44]. This living material was created with Escherichia coli (E. coli) through protein secretion and self-assembly processes, which resulted in tunable rheological properties and tissue adhesion by genetically encoded factors and processing steps. Importantly, this living hydrogel can persist for several days in the mammalian gastrointestinal tract, benefiting from its excellent regeneration capacity. The above advantages make this living material a potential platform for future intestinal disease treatment with a prolonged residence time, such as for the treatment of gastric ulcers, postoperative wound healing, and local anticancer drug release [83].
Fig. 2.
(A) Schematic illustration of the delivery, adhesion, and self-regeneration of an E. coli-generated living mucoadhesive hydrogel in the gut. (B) Functionality investigation of the E. coli-generated curli hydrogel labeled by Cy5-Ni-NTA dye in vivo [44]. Copyright 2019. Reproduced with permission from Wiley-VCH.
3.2. Hydrogel-derived living bacterial wound dressings
Skin wound repair is an important biological process for the restoration of skin integrity after injury. The dressing of wounds is a general and efficient method for promoting wound repair. Recently, various wound dressings have been developed for improving wound healing efficiency, including semipermeable membranes/foams, hydrocolloids, and hydrogels [84]. In particular, hydrogels have attracted much interest in wound care due to their specific advantages, including conserving moisture, preventing dehydration, protecting underlying tissues from infection, reducing pain by cooling and accelerating the healing process [[85], [86], [87]]. Although numerous hydrogel-based wound dressings have appeared in the clinic, novel hydrogel-based smart wound dressings are still desirable in this field today [17]. Favorably, the recent developments in the development of engineered living materials has brought promise for designing hydrogel-based bioactive wound dressings [36,88,89]. In particular, living bacterial hydrogel dressings with creative designs have been demonstrated to promote wound healing in mouse models [51,55,90,91].
For instance, traditional antibacterial wound dressings cannot avoid the suppression of the growth of beneficial bacteria in the process of killing pathogenic bacteria escape disturbing the balance of the local microbial ecosystem [92]. Nevertheless, the modality by which living Bacillus subtilis-incorporated hydrogels continuously produce antifungal agents to combat fungal infection has been indicated to be a safe and cost-efficient therapeutic option [41]. Given this, Liu and coworkers fabricated a living Lactobacillus-encapsulated hydrogel scaffold to promote infected wound healing [55]. In this work, probiotics were encapsulated in methacrylate-modified gelatin-based microgels and further immobilized in a hyaluronic acid-based hydrogel network. The results showed that this living bacterial hydrogel scaffold had excellent capabilities to eliminate harmful bacterial infection and inflammation, thus accelerating wound healing and local tissue repair. In another work, a living Lactococcus-loaded heparin-poloxamer thermoresponsive hydrogel delivery system was fabricated by Deng and coworkers to regulate therapeutic angiogenesis and enhance diabetic wound healing [51]. The results indicated that this living bacterial wound dressing scaffold could bioengineer the wound microenvironment and promote angiogenesis in diabetic wounds by protecting vascular endothelial growth factor (VEGF), promoting the proliferation of endothelial cells and secreting lactic acid to polarize macrophages into an anti-inflammatory phenotype. Additionally, the fabricated hydrogel scaffolds in the above works could not only protect living bacteria from immune clearance but also restrict unwanted extravasation and growth, thereby minimizing potential threats [51,55].
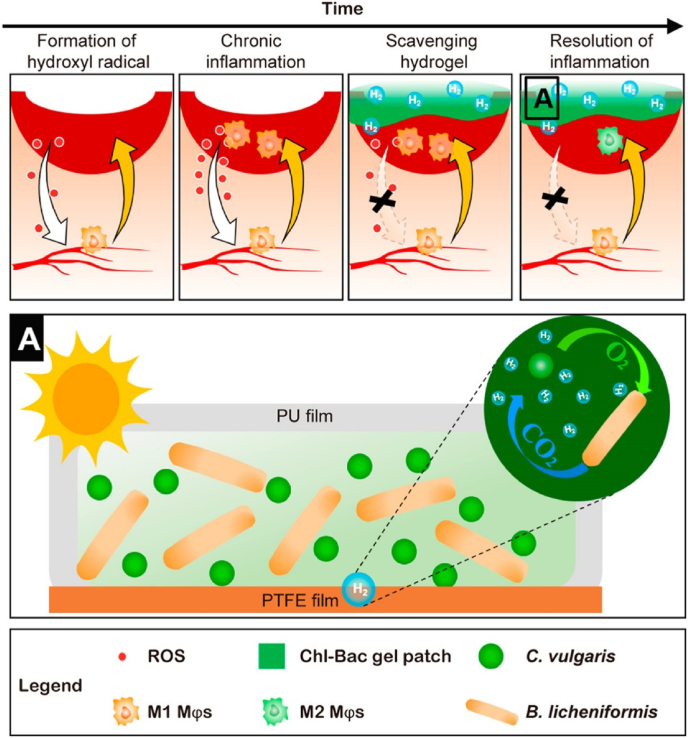
Photosynthetic bacteria (PSB), with the original photoenergy synthesis system, have applications in human healthcare due to their biological activities [93]. In recent years, PSBs have been encapsulated in hydrogels for wound healing applications [90,91]. For example, a living PSB (Spirulina platensis, SP)-loaded carboxymethyl chitosan-based hydrogel scaffold was developed to promote infected wound healing by producing and locally delivering O2 to hypoxic tissues [90]. The results indicated that the hydrogel not only maintained the O2-generating capability of SP but also enhanced its adhesion to the infection. Under 650 nm laser irradiation, the generated reactive oxygen species (ROS) from the loaded SP caused the photodynamic death of bacteria in the infected area and enhanced wound healing progress. All of the evidence demonstrated that this combined wound treatment strategy possessed the capability to accelerate the healing of an infected wound. In another work, a symbiotic algae-bacteria hydrogel was fabricated for the local production of hydrogen as well as acceleration of diabetic wound healing [91]. The results showed that this hydrogel continuously produced hydrogen over 3 days, which could selectively consume the highly toxic species •OH and ONOO−, subsequently reducing wound inflammation (Fig. 3). This symbiotic algae-bacteria hydrogel-based wound dressing with good biocompatibility and ROS scavenging features has promising potential for clinical use.
Fig. 3.
Schematic illustration of the symbiotic Algae-Bacteria hydrogel patch that reduces oxidative stress and inflammation in chronic diabetic wounds [91]. This hydrogel patch can scavenge hydroxyl radicals and neutralize chemokines by releasing hydrogen. The sequestration of chemokines by the hydrogel scaffold reduces immune cell invasion, which in turn lowers the levels of inflammatory chemokines and leads to the resolution of inflammation. Copyright 2022, American Chemical Society.
In summary, hydrogel-derived living bacterial dressing materials have shown considerable opportunities for smart wound care. Nevertheless, this field is still in its infancy, and investigations of the in-depth mechanism behind living bacterial hydrogel-induced wound healing is needed in the future.
3.3. Hydrogel-based living bacterial vaccines
Vaccines are one of the most important medical interventions, as they provide active acquired immunity for preventing diseases [9,94]. In particular, live bacterial vaccines have been shown to stimulate durable humoral and cellular immunity as a consequence of their mimicry of natural infection and intrinsic adjuvant properties [95,96]. Several bacteria, such as Listeria, lactic acid bacteria, and Salmonella, have been engineered for use as live vaccines against infectious diseases and cancer [[97], [98], [99], [100]]. Nevertheless, safety concerns are the main problem owing to the risks of infection or sepsis from the living bacterial products in immunocompromised patients [9]. Inoculation of attenuated bacterial vaccines via genetic engineering and recombinant technology is considered an effective method to overcome the above limitations [101,102]. In addition, diversified delivery strategies could also increase delivery efficiency and safety [96]. Among them, hydrogels have become an important platform for living bacterial vaccine development, benefiting from their spatiotemporal control during therapeutic delivery [26,103].
In recent decades, different types of hydrogel-based vaccines have been explored for prophylactic or therapeutic treatments [104]. As a delivery vehicle, hydrogels can provide a local antigen depot, prolong the residence time of antigens, and continuously induce a high immune response [105,106]. For example, a recombinant elastin-like polypeptide-based tetanus toxoid-loaded hydrogel produced high levels of tetanus antibodies with good avidity for over 4 months after a single injection [105]. In another study, a silk fibroin hydrogel-based mucosal vaccine broadened the distribution of gastric intraepithelial CD4+ tissue-resident memory T cells, which induced optimal immune protection against Helicobacter felis [107]. Hydrogel-based therapeutic tumor vaccines have attracted significant attention in the field of cancer therapy [[108], [109], [110], [111], [112]]. In these studies, different types of drugs (such as tumor antigens, cytokine granulocyte-macrophage colony-stimulating factor (GM-CSF), Toll-like receptor 9 agonists, activate dendritic cells (DCs), cytosine-phosphodiester-guanine (CpG), a BRD4 inhibitor, tumor cell lysates (TLs), etc.) were selectively encapsulated in hydrogel carriers with different designs [[109], [110], [111], [112]]. These hydrogel-based tumor vaccines induced long-lasting antitumor immunity and enhanced tumor immunotherapy by providing sustained release of the immunomodulators, antigens and other components (Fig. 4) [110].
Fig. 4.
Scheme of the preparation of a tumor-penetrable peptide (Fmoc-KCRGDK) hydrogel personalized cancer vaccine for cancer immunotherapy [110]. (A) Preparation of hydrogels involved in personalized cancer vaccines. (B) Mechanism of vaccine-mediated cancer immunotherapy against postoperative tumor recurrence and metastasis. Copyright 2018, Nature Publishing Group.
Hydrogels have also been employed to develop effective platforms for living bacterial vaccines [42,113,114]. For instance, Grainger and coworkers developed a photopolymerized PEG-crosslinked hydrogel-based live bacterial vaccine (Brucella abortus strain RB51) ballistic delivery [42]. They found that the RB51 live vaccine showed excellent viability after photopolymerized encapsulation within the gel matrixes. After being loaded within the hydrogel vaccine, the biobullet showed ballistic properties similar to those of commercially available biobullets. Then, its vaccine performance was evaluated in bison calves in another work [113]. The results indicated that this hydrogel biobullet produced increased immunologic responses when compared to the control bison groups that were vaccinated with saline, parenteral SRB51 alone, or ballistically with compressed SRB51. Further investigation showed that the living RB51 bacterial vaccine remained highly viable in model animals after hydrogel polymerization, lyophilization, and storage [114]. Additionally, hydrogel-based living bacterial vaccines have gained increasing attention for the treatment of cancers. For example, engineered E. coli with a production-lysis circuit were encapsulated into chitosan-based hydrogel microcapsules to durably produce and release self-assembled protein nanovaccines that were based on bacterial microcomponents fused with the antigen ovalbumin, which activated specific immunity in mice and achieved obvious tumor prevention [56]. In another work, Liu and coworkers also found that a hydrogel-loaded living engineered attenuated Salmonella typhimurium strain could activate antitumor immunity to inhibit tumor metastasis and rechallenge [50].
Although related research on hydrogel-based living bacterial vaccines is relatively scarce, there is a strong possibility that the situation will improve with the cross-development of both synthetic biology and materials science in the near future [115].
3.4. Hydrogel-based cancer bacteriotherapy
Bacteriotherapy possesses many unique advantages for cancer treatment compared with conventional strategies for cancer therapy, such as tumor hypoxia targeting motility, good tissue penetration, anticancer toxin production, and the induction of an antitumor immune response [5,9,116,117]. Despite this, finding more potent bacterial agents with less off-target toxicity is still a main challenge for cancer bacterial therapy [117]. Engineering commensal bacterial species (e.g., Salmonella, Clostridium and E. coli) is considered to be an efficient method for cancer treatment [5,7]. Presently, some of these bacterial agents have shown promising effects in controlling tumor growth and promoting survival in experimental models [118]. Nevertheless, several studies have also demonstrated that the use of bacteria alone (whether engineered or not) was improbable to stamp out tumors [5,119]. Engineered bacterial multimodality therapy was previously thought to be a promising strategy to improve bacterial anticancer efficiency [4,120]. In particular, combined immune regulatory treatment and bacterial therapy would likely be a curative therapeutic approach for cancer therapy [121].
On the other hand, how to selectively evade therapeutic microbial clearance and inactivation by the immune system is another key challenge for cancer bacteriotherapy [117]. As such, local administration offers an important way to overcome the abovementioned adverse effects [11,50]. For example, Danino and coworkers fabricated a novel engineered probiotic system for local cancer treatment by coupling immunotherapeutic expression with an optimized lysing mechanism [11]. The engineered E. coli could home to the hypoxic tumor core and release the programmed cell death ligand 1 antibody (anti-PD-L1) and CTLA-4 antibody (anti-CTLA-4) through probiotic lysis within the tumor microenvironment (TME). The sustained therapeutic nanobodies expressed and delivered by the bacteria not only enhanced the antitumor activity in different tumor-bearing mouse models but also restrained the bacteria to the tumor site and minimized the risk of toxic systemic effects to some degree. However, the potential risk of uncontrolled toxicity associated with local therapeutic infection still remains for these strategies. Therefore, a rational, controlled delivery system is essential for improving the efficiency of cancer bacteriotherapy.
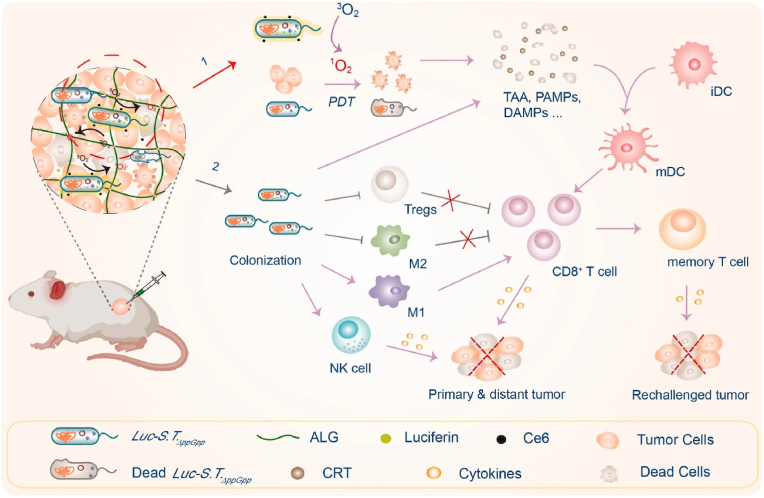
Hydrogels are considered an effective platform for the construction of living bacterial antitumor systems [36,89]. These platforms can not only provide sufficient space for bacterial survival and therapeutic biosynthesis as biofactories but also achieve the on-demand release of both living bacterial agents and other therapeutic payloads for multimodal cancer therapy [2,14,50,88,122]. For example, Campo and coworkers developed a novel strategy by embedding an engineered optogenetic E. coli into an agarose hydrogel for opto-regulating protein release [122]. In this system, the engineered bacteria expressed and secreted a red fluorescent protein under a low dose of blue light irradiation, which displayed dose-dependent release behavior over several weeks within the bacterial hydrogel. In another work, an active endotoxin-free E. coli strain was encapsulated into an agarose hydrogel by Campo and coworkers for the light-regulated secretion of the antimicrobial and antitumoral drug deoxyviolacein [123]. Benefiting from the sustained-release effect of the gel depot, the production and release of deoxyviolacein was maintained at a meaningful level for at least 6 weeks. Recently, an engineered bioluminescent bacterium was developed by transforming an attenuated S. typhimurium strain with a firefly luciferase-expressing plasmid to locally illuminate tumors and boost photodynamic therapy (PDT). Once fixed into the tumor with an alginate hydrogel, this colonized bioluminescent bacterium provided sufficient luciferase that continually emitted light to activate the photosensitizer chlorin e6 (Ce6) and improve PDT (Fig. 5) [50]. This strategy is a general and highly effective synergistic cancer treatment approach for different tumors affected by light-penetration that can not only suppress different types of tumors but also evoke potent antitumor immunity.
Fig. 5.
Schematic illustrating the coloading of engineered bioluminescent bacteria and Ce6 into hydrogel depots for self-activated PDT and systemic anticancer immunity boost [50]. Copyright 2022. Reproduced with permission from Elsevier Science Ltd.
Furthermore, designed hydrogel formulations can coregulate the human microbiota and enhance cancer immunotherapy [31,124]. It has been demonstrated that a phospholipid-based phase separation hydrogel could sustain the release of the anti-programmed cell death-1 peptide AUNP-12 for at least 6 weeks, which maintained an immunopermissive TME and increased bacteria-mediated immunotherapy [124]. In another work, Zhang and coworkers found that silver nanoparticles (NPs) containing adhesive hydrogels could not only inhibit competing bacterial growth but also assist with exogenous Peptostreptococcus anaerobius to upregulate the intratumoral level of Peptostreptococcus in vivo. This combination strategy of oral microbiota modulation has been proven to notably enhance the efficacy of oral squamous cell carcinoma (OSCC) immunotherapy after synergy with checkpoint inhibition [31].
Accordingly, hydrogels have enviable advantages as carriers and biofactories in local cancer bacteriotherapy. With the cross-development of both synthetic biology and cancer immunotherapy, hydrogels as promising living bacterial delivery platforms may have more opportunities to contribute to this field.
3.5. Living bacterial hydrogels for the treatment of other diseases
In recent years, living bacterial hydrogels have also been investigated for the treatment of other diseases. For instance, Mizrahi and coworkers developed a novel living B. subtilis-incorporated Pluronic thermogel for treating skin fungal infections [41]. The bacterial-containing Pluronic solution (18% w/v) could transform into a semisolid B. subtilis cultural depot and continuously secrete antifungal agents after administration to the fungi-infected skin. This system showed strong antifungal activity and completely inhibited Candida growth, which was clinically comparable to the effects of ketoconazole. Furthermore, the tunable mechanical properties and penetrability of this living bacterial agent increased its potential for antifungal applications. Moreover, their subsequent study revealed that B. subtilis in Pluronic F-127 has the capability to modulate the composition of the skin microbiota, which is beneficial for skin disease treatment [52].
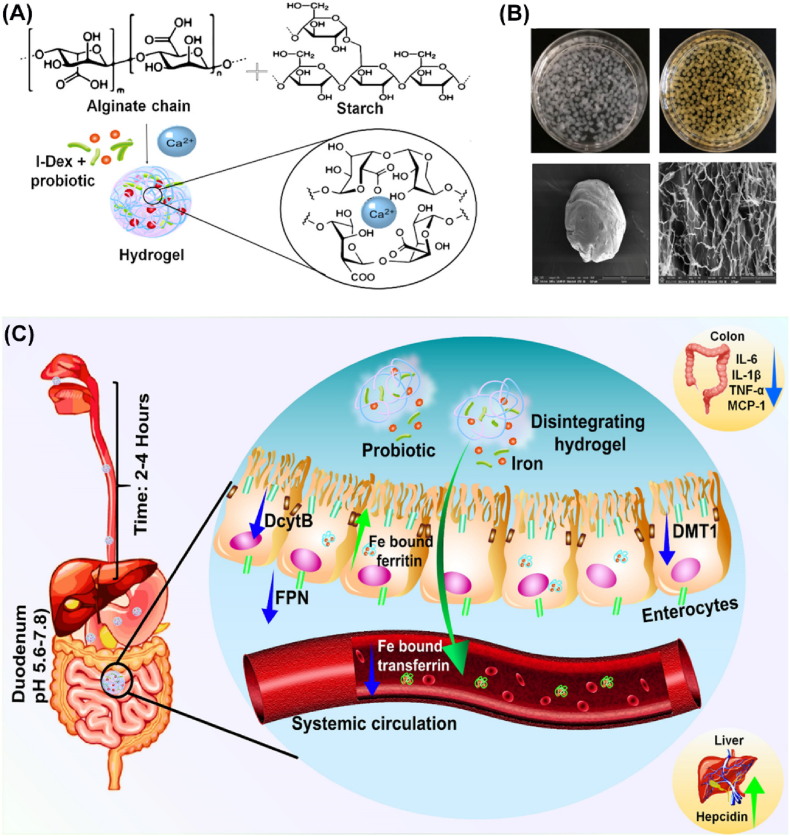
In another work, Sagar et al. developed an iron-containing and anti-inflammatory probiotic bacteria coencapsulated alginate/starch hydrogel to improve the efficiency of treating iron deficiency anemia (IDA) orally (Fig. 6) [48]. In this system, the drug-loaded alginate/starch hydrogel remained protected in the acidic environment of the stomach but displayed sustained release of the encapsulated iron and probiotics with the pH increase in the intestine. The released probiotics continuously stimulated the healthy gut bacteria and reduced the intestinal inflammatory response as well as improved iron absorption with minimal side effects. Such treatment strategies may contribute to the development of oral IDA treatment.
Fig. 6.
Schematic illustration of iron and anti-inflammatory probiotic bacteria coencapsulated in alginate/starch hydrogels to improve the efficiency of IDA treatment [48]. (A) Ionic crosslinked iron and probiotic bacteria coloaded in the hydrogel formation, (B) physical appearance of the formed hydrogel, and (C) scheme of hydrogel disintegration and payload release at intestinal pH. Copyright 2021. Reproduced with permission from American Chemical Society.
4. Conclusions and future perspectives
In this review, we briefly summarized the recent advances in hydrogels involved in living bacteria-mediated therapy for typical diseases and conditions, including digestive health treatment, skin fungal infections, wound healing, living bacterial vaccines, and cancer bacteriotherapy (Table 1). All of these studies indicate that hydrogels are attractive platforms for living bacterial medicine delivery because of their high similarity to physiological tissue and adjustable physicochemical properties. Hydrogels can not only protect and smartly release living bacterial payloads to the lesion site but also offer a type of versatile carrier for the codelivery of various therapeutics and effective combination therapies.
Table 1.
Representative formulations of the hydrogels involved in living bacteriotherapy described in this review.
| Disease Type | Hydrogel Base | Therapeutic(s) | Administration Route | Treatment Strategy | Model | Ref. |
|---|---|---|---|---|---|---|
| Intestinal disease treatment | Pectin hydrogel beads | Lactobacillus rhamnosus GG | Oral administration | Colitis therapy | Mouse colitis model | [63] |
| Alginate and chitosan microgel | Bifidobacterium longum | Oral administration | Probiotic delivery | Simulated gastric/intestinal fluid | [61] | |
| Pectin/starch hydrogel | Lactobacillus plantarum | Oral administration | Probiotic colon delivery | Simulated gastric/intestinal fluid | [64] | |
| Cellulose-based microgel | Lactobacillus plantarum | Oral administration | Probiotic intestinal delivery | Simulated intestinal fluid | [65] | |
| Chitosan-dextran sulfate hydrogel | Lactobacillus acidophilus | Oral administration | Probiotic encapsulation | Simulated intestinal fluid | [66] | |
| Ca-alginate/chitosan microgel | Lactobacillus plantarum | Oral administration | Probiotic encapsulation | Simulated gastrointestinal conditions | [67] | |
| EDTA-Ca-alginate-based hydrogel | Lactobacillus rhamnosus | Oral administration | Probiotic intestinal delivery | Simulated gastrointestinal conditions | [68] | |
| Cellulose/chitosan-based microgel | Lactobacillus rhamnosus GG | Oral administration | Probiotic intestinal delivery | Simulated gastrointestinal conditions | [69] | |
| Oil-induced biphasic microgel | Bifidobacterium pseudocatenulatum | Oral administration | Probiotic encapsulation | Simulated digestion | [70] | |
| Alginate and carrageenan-based hydrogel beads | Lactobacillus acidophilus | Oral administration | Probiotic encapsulation | Simulated gastrointestinal and thermal conditions | [71] | |
| Alginate-based hydrogel | Lactobacillus casei | Oral administration | Probiotic intestinal delivery | Simulated stomach acid and intestinal fluid conditions | [72] | |
| Alginate-based double network hydrogel | Lactobacillus rhamnosus GG | Oral administration | Probiotic intestinal delivery | Simulated sequential gastrointestinal digestion | [73] | |
| Alginate/pectin-based hydrogel bilayer beads | Lactobacillus bulgaricus | Oral administration | Probiotic encapsulation | Simulated saliva, gastric juice, and intestinal juice | [81] | |
| Bentonite/alginate nanocomposite hydrogel | Lactobacillus rhamnosus GG | Oral administration | Probiotic intestinal delivery | Male ICR mice | [47] | |
| Fish gelatin/alginate double network gel | Bifidobacterium longum | Oral administration | Probiotic encapsulation | Simulated gastric fluid | [43] | |
| Ca-alginate hydrogel | Lactobacillus Plantarum | Oral administration | Probiotic colon-targeted release | Simulated stomach acid and intestinal fluid conditions | [82] | |
| Sodium tripolyphosphate gel | Lactobacillus plantarum | Oral administration | Probiotic intestinal delivery | Acidic environment of simulated gastric juice | [76] | |
| Propylene glycol alginate/β-lactoglobulin composite hydrogel | Lactobacillus rhamnosus GG and curcumin | Oral administration | Probiotic and drug intestinal codelivery | Simulated gastrointestinal tract conditions | [75] | |
|
E. coli-generated curli hydrogel |
E. coli |
Oral administration |
Probiotic intestinal delivery |
C57BL/6 mice |
[44] |
|
| Wound healing | 3D-printed agarose hydrogel patch | Bacillus subtilis | Wound patching | Infected wound healing | Infected mouse wounds | [36] |
| Carboxymethyl chitosan-based hydrogel | Spirulina platensis | Wound patching and laser irradiation (650 nm) | Infected wound healing | Infected mouse wounds | [90] | |
| Methacrylate-modified hyaluronic acid crosslinked hydrogel | Lactobacillus reuteri | Wound patching | Infected wound healing | Infected mouse wounds | [55] | |
| Heparin poloxamer thermoresponsive hydrogel | Lactococcus | Wound patching | Diabetic wound healing | Diabetic mouse wounds | [51] | |
| Symbiotic algae-bacteria hydrogel |
Chlorella and Bacillus licheniformis |
Wound patching |
Diabetic wound healing |
Diabetic mouse wounds |
[91] |
|
| Bacterial vaccines | Photopolymerized PEG-crosslinked hydrogel | Brucella abortus strain RB51 | Ballistic delivery | Prevention of infection | Live elk and bison calves | [42] |
| Photopolymerized PEG-based hydrogel | Brucella abortus strain RB51 | Ballistic delivery | Prevention of infection | Bison calves | [113] | |
| Lyophilized PEG-glycolide dimethacrylate crosslinked hydrogel | Brucella abortus strain RB51 | Ballistic delivery | Prevention of infection | Bison | [114] | |
| Polymer Gantrez AN 119 and Pluronic F-127 composite hydrogel |
Shigella flexneri outer membrane vesicle antigen |
Intranasal administration |
Mucosal active immunization |
BALB/c mice |
[106] |
|
| Cancer therapy | Alginate-based hydrogel | E. coli | Subcutaneous implantation | Cell therapy | PC3 tumor-bearing mice | [49] |
| Alginate-based hydrogel | Firefly luciferase-expressing Salmonella typhimurium and chlorin e6 | Intratumoral injection and laser irradiation | Cancer photodynamic therapy and immunotherapy | B16 and CT26 tumor-bearing mice, VX2 tumor-bearing rabbits | [50] | |
| Polyaldehyde dextran and chitosan viscous hydrogel |
Exogenous P. anaerobius, Ag NPs and anti-PD-1 |
Local administration |
Immunotherapy |
OSCC-bearing mice |
[31] |
|
| Skin infection treatment | Pluronic F-127 thermoresponsive hydrogel | Bacillus subtilis | Local administration | Local fungal infection treatment | Mice with cutaneous fungal infection | [41] |
| Pluronic F-127 thermoresponsive hydrogel |
Bacillus subtilis |
Local administration |
Skin microbiota dysbiosis |
Mouse skin microbiota |
[52] |
|
| Iron deficiency anemia | Alginate/starch hydrogel | Lactobacillus fermentum and iron dextran | Oral administration | Iron deficiency anemia | Iron-depleted mouse model | [48] |
Despite this, the field of hydrogel-based living bacteria-mediated therapy is still in its infancy, and there remain many improvements that should be made before the translation from bench to bedside. First, living bacteriotherapy has not reached its full potential based on existing hydrogel technologies, and more custom-built properties for advanced hydrogel design are still required to mediate the complex treatment processes of different diseases. These challenges may be addressed well following the future cross-development of synthetic biology and materials science [115]. Second, these living bacterial therapeutics do not follow the conventional small molecule pharmacokinetic and dose‒response patterns in vivo; thus, choosing the correct starting dose and schedule for administration can be challenging [9]. Generally, dose escalation is standard practice in drug clinical trials, which applies to this field. Fortunately, it has been well demonstrated by previous studies that hydrogel formulations could improve the local concentration of therapeutics for local disease treatment [26]. This advantage may reduce the required efficacious dose of the therapeutic bacteria and lower the side effects, thereby enhancing the therapeutic index of living bacterial medicines. Third, the systematic biosafety of these hydrogel-based living bacterial agents should be considered. Thus, using recombination to modify bacterial strains with controllable inactivation mechanisms is an encouraging way to improve the therapeutic role and biosafety of living bacteria during disease treatment [6,56]. Furthermore, the careful design of physical containment in hydrogel vehicles to inhibit unnecessary bacterial escape and minimize infection risk should be a practical approach to improve the biosafety of hydrogel-based bacteriotherapy [13,56,125]. Last, a therapeutic approach using live bacteria as a monotherapy has been demonstrated to be insufficient for the treatment of complex diseases, and how to efficiently combine this strategy with other treatments to achieve an efficient therapeutic response is also a challenge. Pleasantly, as a macroscale platform, hydrogels can not only provide sufficient biological space for bacteria to survive and “work” but they can also leverage the release needs of different types of payloads, which benefits the development of multimodal bacterial-involved disease treatment [21,26].
As this field is still growing, future advances rely on a deep understanding of living bacteria-mediated therapy, the advanced design of hydrogel-based drug delivery systems, and effective collaborations between relevant fields. The exciting application of hydrogel-involved living bacteria-mediated therapy to treat various diseases is very promising with the further cross-development of both synthetic biology and materials science in the future.
CRediT author statement
Shuangjiang Yu: Conceptualization, Methodology, Preparation, Writing - Original Draft, Funding acquisition. Hongcheng Sun: Software, Data Curation, Funding acquisition. Yongguang Li: Investigation, Funding acquisition. Shu Wei: Writing - Review & Editing, Project administration, Visualization. Jiayun Xu: Data Curation, Resources. Junqiu Liu: Formal analysis, Supervision, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 22075065, 22001054), the National Key R&D Program of China (No. 2020YFA0908500, 2018YFA0901600), the Provincial Natural Science Foundation of China (No. LR22B010001), and Research Start-up Fund from Hangzhou Normal University (No. 2019QDL025, 2019QDL026, 4095C5022121604).
Contributor Information
Shuangjiang Yu, Email: yusj@hznu.edu.cn.
Shu Wei, Email: shuwei@hznu.edu.cn.
Data availability
The authors do not have permission to share data.
References
- 1.Cao Z., et al. Nat. Commun. 2019;10:5783. doi: 10.1038/s41467-019-13727-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nguyen P.Q., et al. Adv. Mater. 2018;30 doi: 10.1002/adma.201704847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zmora N., et al. Sci. Transl. Med. 2019;11 doi: 10.1126/scitranslmed.aaw1815. eaaw1815. [DOI] [PubMed] [Google Scholar]
- 4.Li Z., et al. Adv. Mater. 2021 [Google Scholar]
- 5.Forbes N.S. Nat. Rev. Cancer. 2010;10:785. doi: 10.1038/nrc2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charbonneau M.R., et al. Nat. Commun. 2020;11:1738. doi: 10.1038/s41467-020-15508-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forbes N.S., et al. J. Immunother. Cancer. 2018;6:78. doi: 10.1186/s40425-018-0381-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu F., Liu J. Adv. Drug Deliv. Rev. 2022;188 doi: 10.1016/j.addr.2022.114443. [DOI] [PubMed] [Google Scholar]
- 9.Zhou S., et al. Nat. Rev. Cancer. 2018;18:727. doi: 10.1038/s41568-018-0070-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng D.-W., et al. Nat. Commun. 2018;9:1680. doi: 10.1038/s41467-018-03233-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gurbatri C.R., et al. Sci. Transl. Med. 2020;12 doi: 10.1126/scitranslmed.aax0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mettu S., et al. J. Agric. Food Chem. 2021;69:4946. doi: 10.1021/acs.jafc.1c00468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang T.C., et al. Nat. Chem. Biol. 2021;17:724. doi: 10.1038/s41589-021-00779-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daly A.C., et al. Nat. Rev. Mater. 2020;5:20. doi: 10.1038/s41578-019-0148-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seliktar D. Science. 2012;336:1124. doi: 10.1126/science.1214804. [DOI] [PubMed] [Google Scholar]
- 16.Chrisnandy A., et al. Nat. Mater. 2022;21:479. doi: 10.1038/s41563-021-01136-7. [DOI] [PubMed] [Google Scholar]
- 17.Bertsch P., et al. Chem. Rev. 2022 doi: 10.1021/acs.chemrev.2c00179. [DOI] [Google Scholar]
- 18.Zhang J., et al. Adv. Mater. 2022 [Google Scholar]
- 19.Dobashi Y., et al. Science. 2022;376:502. doi: 10.1126/science.aaw1974. [DOI] [PubMed] [Google Scholar]
- 20.Yu S., et al. Macromol. Biosci. 2018;18 doi: 10.1002/mabi.201800240. [DOI] [PubMed] [Google Scholar]
- 21.Liu X., et al. Adv. Mater. 2018;30 [Google Scholar]
- 22.Cascone S., Lamberti G. Int. J. Pharm. 2020;573 doi: 10.1016/j.ijpharm.2019.118803. [DOI] [PubMed] [Google Scholar]
- 23.Wang C., et al. Sci. Transl. Med. 2018;10 doi: 10.1126/scitranslmed.aan3682. [DOI] [PubMed] [Google Scholar]
- 24.Yu S., et al. Adv. Mater. 2018;30 [Google Scholar]
- 25.Vermonden T., et al. Chem. Rev. 2012;112:2853. doi: 10.1021/cr200157d. [DOI] [PubMed] [Google Scholar]
- 26.Li J.Y., Mooney D.J. Nat. Rev. Mater. 2016;1 doi: 10.1038/natrevmats.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Q., et al. Nat. Nanotechnol. 2019;14:89. doi: 10.1038/s41565-018-0319-4. [DOI] [PubMed] [Google Scholar]
- 28.Yu S., et al. Biomacromolecules. 2017;18:4341. doi: 10.1021/acs.biomac.7b01374. [DOI] [PubMed] [Google Scholar]
- 29.Yao Y., et al. Acc. Mater. Res. 2022 doi: 10.1021/accountsmr.2c00094. [DOI] [Google Scholar]
- 30.Liu X.Y., et al. Mater. Today. 2020;36:102. [Google Scholar]
- 31.Zheng D.W., et al. Nat. Biomed. Eng. 2022;6:32. doi: 10.1038/s41551-021-00807-9. [DOI] [PubMed] [Google Scholar]
- 32.Chen W.H., et al. Sci. China Chem. 2022;65:1. doi: 10.1007/s11426-022-1243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lv Q., et al. Adv. Ther. 2020;3 [Google Scholar]
- 34.Liu X., et al. Adv. Mater. 2022 [Google Scholar]
- 35.Rivera-Tarazona L.K., et al. Adv. Funct. Mater. 2021:32. [Google Scholar]
- 36.Gonzalez L.M., et al. Nat. Chem. Biol. 2020;16:126. doi: 10.1038/s41589-019-0412-5. [DOI] [PubMed] [Google Scholar]
- 37.Kamdar S., et al. Nature. 2022;603:819. doi: 10.1038/s41586-022-04509-3. [DOI] [PubMed] [Google Scholar]
- 38.An Y.H., Friedman R.J. J. Biomed. Mater. Res. 1998;43:338. doi: 10.1002/(sici)1097-4636(199823)43:3<338::aid-jbm16>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 39.Rial-Hermida M.I., et al. ACS Biomater. Sci. Eng. 2021;7:4102. doi: 10.1021/acsbiomaterials.0c01784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang J., et al. ACS Nano. 2021;15:4294. doi: 10.1021/acsnano.0c07291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lufton M., et al. Adv. Funct. Mater. 2018;28 [Google Scholar]
- 42.Christie R.J., et al. Vaccine. 2006;24:1462. doi: 10.1016/j.vaccine.2005.05.048. [DOI] [PubMed] [Google Scholar]
- 43.Liu J., et al. J. Sci. Food Agric. 2021;101:4398. doi: 10.1002/jsfa.11081. [DOI] [PubMed] [Google Scholar]
- 44.Duraj-Thatte A.M., et al. Adv. Mater. 2019;31 doi: 10.1002/adma.201901826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang J., et al. Nat. Chem. Biol. 2019;15:34. doi: 10.1038/s41589-018-0169-2. [DOI] [PubMed] [Google Scholar]
- 46.Li P., et al. Nat. Mater. 2011;10:149. doi: 10.1038/nmat2915. [DOI] [PubMed] [Google Scholar]
- 47.Kim J., et al. Carbohydr. Polym. 2021;272 doi: 10.1016/j.carbpol.2021.118459. [DOI] [PubMed] [Google Scholar]
- 48.Sagar P., et al. ACS Appl. Bio Mater. 2021;4:7467. doi: 10.1021/acsabm.1c00720. [DOI] [PubMed] [Google Scholar]
- 49.Hui L., et al. Adv. Healthcare Mater. 2022;11 [Google Scholar]
- 50.Yang Z., et al. Biomaterials. 2022;281 doi: 10.1016/j.biomaterials.2021.121332. [DOI] [PubMed] [Google Scholar]
- 51.Lu Y.F., et al. Adv. Funct. Mater. 2021;31 [Google Scholar]
- 52.Moskovicz V., et al. BMC Microbiol. 2021;21:231. doi: 10.1186/s12866-021-02295-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moon H.J., et al. Chem. Soc. Rev. 2012;41:4860. doi: 10.1039/c2cs35078e. [DOI] [PubMed] [Google Scholar]
- 54.Alavarse A.C., et al. Int. J. Biol. Macromol. 2022;202:558. doi: 10.1016/j.ijbiomac.2022.01.029. [DOI] [PubMed] [Google Scholar]
- 55.Ming Z., et al. Adv. Sci. 2021;8 doi: 10.1002/advs.202102545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Han C., et al. Biomaterials. 2022;287 doi: 10.1016/j.biomaterials.2022.121619. [DOI] [PubMed] [Google Scholar]
- 57.Lou J., Mooney D.J. Nat. Rev. Chem. 2022 doi: 10.1038/s41570-022-00420-7. [DOI] [PubMed] [Google Scholar]
- 58.Sonnenburg J.L., Fischbach M.A. Sci. Transl. Med. 2011;3:78ps12. doi: 10.1126/scitranslmed.3001626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blaser M.J. Proc. Natl. Acad. Sci. USA. 2010;107:6125. doi: 10.1073/pnas.1002112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marques T.M., et al. Innovat. Food Sci. Emerg. Technol. 2014;22:239. [Google Scholar]
- 61.Yeung T.W., et al. Front. Microbiol. 2016;7:494. doi: 10.3389/fmicb.2016.00494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen S., et al. J. Microencapsul. 2013;30:103. doi: 10.3109/02652048.2012.700959. [DOI] [PubMed] [Google Scholar]
- 63.Li R., et al. J. Contr. Release. 2016;230:79. doi: 10.1016/j.jconrel.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dafe A., et al. Int. J. Biol. Macromol. 2017;97:536. doi: 10.1016/j.ijbiomac.2017.01.060. [DOI] [PubMed] [Google Scholar]
- 65.Li W., et al. Food Hydrocolloids. 2017;62:66. [Google Scholar]
- 66.Yucel Falco C., et al. Carbohydr. Polym. 2017;172:175. doi: 10.1016/j.carbpol.2017.04.047. [DOI] [PubMed] [Google Scholar]
- 67.Zaeim D., et al. Food Bioprod. Process. 2017;102:250. [Google Scholar]
- 68.Zheng H., et al. Carbohydr. Polym. 2017;155:329. doi: 10.1016/j.carbpol.2016.08.096. [DOI] [PubMed] [Google Scholar]
- 69.Singh P., et al. Food Hydrocolloids. 2018;82:457. [Google Scholar]
- 70.Alehosseini A., et al. Food Hydrocolloids. 2019;87:487. [Google Scholar]
- 71.Afzaal M., et al. Int. J. Food Prop. 2020;23:1899. [Google Scholar]
- 72.Enck K., et al. Curr. Pharmaceut. Des. 2020;26:3134. doi: 10.2174/1381612826666200210111925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Qi X., et al. Int. J. Biol. Macromol. 2020;165:1675. doi: 10.1016/j.ijbiomac.2020.10.028. [DOI] [PubMed] [Google Scholar]
- 74.Sun M., et al. Biomacromolecules. 2020;21:1351. doi: 10.1021/acs.biomac.0c00071. [DOI] [PubMed] [Google Scholar]
- 75.Su J., et al. Carbohydr. Polym. 2021;254 doi: 10.1016/j.carbpol.2020.117446. [DOI] [PubMed] [Google Scholar]
- 76.Zhang A.Q., et al. Food Hydrocolloids. 2021;120 [Google Scholar]
- 77.Zhang X., et al. Chem. Soc. Rev. 2015;44:1948. doi: 10.1039/c4cs00341a. [DOI] [PubMed] [Google Scholar]
- 78.Malmsten M., et al. Curr. Opin. Colloid. In. 2010;15:435. [Google Scholar]
- 79.Jeevarathinam A.S., et al. Mater. Today Bio. 2021;9 doi: 10.1016/j.mtbio.2020.100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kittel Y., et al. Adv. Healthcare Mater. 2022;11 [Google Scholar]
- 81.Hu X., et al. Int. J. Biol. Macromol. 2021;193:1050. doi: 10.1016/j.ijbiomac.2021.11.076. [DOI] [PubMed] [Google Scholar]
- 82.Qin X.-S., et al. Food Hydrocolloids. 2021;113 [Google Scholar]
- 83.Cui C.Y., Liu W.G. Prog. Polym. Sci. 2021;116 [Google Scholar]
- 84.Liang Y., et al. ACS Nano. 2021;15:12687–12722. doi: 10.1021/acsnano.1c04206. [DOI] [PubMed] [Google Scholar]
- 85.Koehler J., et al. Eur. Polym. J. 2018;100:1. [Google Scholar]
- 86.Lohmann N., et al. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aai9044. [DOI] [PubMed] [Google Scholar]
- 87.Li S., et al. Adv. Sci. 2018;5 [Google Scholar]
- 88.Shang L., et al. Acc. Chem. Res. 2020;2:59. [Google Scholar]
- 89.Liu X., et al. Proc. Natl. Acad. Sci. USA. 2017;114:2200. [Google Scholar]
- 90.Li W.L., et al. Adv. Ther. 2021;4 [Google Scholar]
- 91.Chen H., et al. Nano Lett. 2022;22:229. doi: 10.1021/acs.nanolett.1c03693. [DOI] [PubMed] [Google Scholar]
- 92.Di Domizio J., et al. Nat. Immunol. 2020;21:1034. doi: 10.1038/s41590-020-0721-6. [DOI] [PubMed] [Google Scholar]
- 93.Raja R., et al. Crit. Rev. Microbiol. 2016;42:394. doi: 10.3109/1040841X.2014.957640. [DOI] [PubMed] [Google Scholar]
- 94.Guan X., et al. Biomaterials. 2018;171:198. doi: 10.1016/j.biomaterials.2018.04.039. [DOI] [PubMed] [Google Scholar]
- 95.Detmer A., Glenting J. Microb. Cell Factories. 2006;5:23. doi: 10.1186/1475-2859-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ding C., et al. Immunol. Lett. 2018;197:70. doi: 10.1016/j.imlet.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 97.Brockstedt D.G., et al. J. Immunother. Cancer. 2013;1:P203. [Google Scholar]
- 98.Frey S.E., et al. Vaccine. 2013;31:4874. doi: 10.1016/j.vaccine.2013.07.049. [DOI] [PubMed] [Google Scholar]
- 99.Bruhn K.W., et al. Microb. Infect. 2007;9:1226. doi: 10.1016/j.micinf.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 100.Wang M., et al. Appl. Microbiol. Biotechnol. 2016;100:5691. doi: 10.1007/s00253-016-7557-x. [DOI] [PubMed] [Google Scholar]
- 101.Garmory H.S., et al. J. Drug Target. 2003;11:471. doi: 10.1080/10611860410001670008. [DOI] [PubMed] [Google Scholar]
- 102.Hu Q., et al. Nano Lett. 2015;15:2732. doi: 10.1021/acs.nanolett.5b00570. [DOI] [PubMed] [Google Scholar]
- 103.Kim J., et al. Nat. Biotechnol. 2015;33:64. doi: 10.1038/nbt.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Basu P., et al. Polymer. 2021;230 [Google Scholar]
- 105.Asai D., et al. Macromol. Biosci. 2019;19 doi: 10.1002/mabi.201900167. [DOI] [PubMed] [Google Scholar]
- 106.Pastor Y., et al. Int. J. Pharm. 2020;579 doi: 10.1016/j.ijpharm.2020.119154. [DOI] [PubMed] [Google Scholar]
- 107.Hu C., et al. Emerg. Microb. Infect. 2020;9:2289. doi: 10.1080/22221751.2020.1830719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Luo Z., et al. Adv. Mater. 2017;29 [Google Scholar]
- 109.Shah N.J., et al. Nat. Biomed. Eng. 2020;4:40. doi: 10.1038/s41551-019-0503-3. [DOI] [PubMed] [Google Scholar]
- 110.Wang T., et al. Nat. Commun. 2018;9:1532. doi: 10.1038/s41467-018-03915-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shih T.Y., et al. Adv. Healthcare Mater. 2018;7 [Google Scholar]
- 112.Yang F., et al. Appl. Mater. Today. 2020;19 [Google Scholar]
- 113.Olsen S.C., et al. Vaccine. 2006;24:1346. doi: 10.1016/j.vaccine.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 114.Falconer J.L., et al. Int. J. Pharm. 2016;498:187. doi: 10.1016/j.ijpharm.2015.12.040. [DOI] [PubMed] [Google Scholar]
- 115.Tang T.C., et al. Nat. Rev. Mater. 2021;6:332. [Google Scholar]
- 116.Zitvogel L., et al. Science. 2018;359:1366. doi: 10.1126/science.aar6918. [DOI] [PubMed] [Google Scholar]
- 117.Zhou S. Nature. 2016;536:33. doi: 10.1038/nature18915. [DOI] [PubMed] [Google Scholar]
- 118.Song W., et al. Nat. Nanotechnol. 2019;14:1093. doi: 10.1038/s41565-019-0589-5. [DOI] [PubMed] [Google Scholar]
- 119.Din M.O., et al. Nature. 2016;536:81. doi: 10.1038/nature18930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lawn J.E., et al. Lancet. 2016;387:587. doi: 10.1016/S0140-6736(15)00837-5. [DOI] [PubMed] [Google Scholar]
- 121.Sepich-Poore G.D., et al. Science. 2021:371. doi: 10.1126/science.abc4552. eabc4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sankaran S., Del Campo A. Adv. Biosyst. 2019;3 doi: 10.1002/adbi.201800312. [DOI] [PubMed] [Google Scholar]
- 123.Sankaran S., et al. Small. 2019;15 doi: 10.1002/smll.201804717. [DOI] [PubMed] [Google Scholar]
- 124.Chen W.F., et al. Adv. Funct. Mater. 2020;30 doi: 10.1002/adfm.201908349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jeong Y., Irudayaraj J. Chem. Commun. 2022;58:8584. doi: 10.1039/d2cc01187e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors do not have permission to share data.