Abstract
Background/Objective
Primary adrenal lymphoma (PAL) is an aggressive form of lymphoma associated with adrenal insufficiency (AI) in most cases. It requires a histologic confirmation unlike other cases of primary AI.
Case Report
We report a case of a 66-year-old man who presented with AI with symptomatic hypotension and hypo-osmolar hyponatremia. Ultrasound and computed tomography scans revealed bilateral bulky adrenal masses that were avid on fluorodeoxyglucose positron emission tomography scan. The diagnosis of PAL was confirmed with adrenal biopsy. He was treated with rituximab-based chemotherapy, which was complicated by several endocrine challenges, including worsening diabetes, multiple adrenal crises, prolonged hyponatremia, and refractory hypokalemia requiring spironolactone. He eventually developed central nervous system disease and was treated with palliative intent.
Discussion
AI in the setting of PAL can constitute both diagnostic and therapeutic challenges, including significant electrolyte imbalances as discussed in this case report.
Conclusion
It is important to have a high suspicion for PAL, especially in the presence of bilateral adrenal masses and AI. Early adrenal biopsy is required for diagnosis. Multidisciplinary care is vital to manage complications that arise during the disease course and treatment.
Key words: primary adrenal lymphoma, bilateral adrenal masses, adrenal insufficiency, hypocortisolism, adrenal crisis
Abbreviations: AI, adrenal insufficiency; CNS, central nervous system; CT, computed tomography; DLBCL, diffuse large B cell lymphoma; PAL, primary adrenal lymphoma; R-CHOP, rituximab-cyclophosphamide, doxorubicin, vincristine, and prednisone
Highlights
-
•
Although PAL is a rare entity, it should be considered in the differential diagnosis of bilateral adrenal masses and early diagnosis can potentially improve survival rates.
-
•
Adrenal insufficiency is associated with a poor prognosis and there needs to be a high clinical suspicion for this. Appropriate titration of hydrocortisone is important at the time of presentation as well as throughout the course of chemotherapy.
-
•
Adrenal crisis poses a great risk throughout the course of chemotherapy and as described in our case, even after chemotherapy has concluded.
-
•
Electrolyte imbalances including hypokalaemia and hyponatremia can be difficult to manage
Clinical relevance summary
Cases of endocrine complications of chemotherapy in patients with primary adrenal insufficiency are sparse in the existing literature. This case highlights the importance of monitoring electrolytes in this setting and considering use of spironolactone for persistent hypokalaemia during chemotherapy.
Introduction
Primary adrenal lymphoma (PAL) is a rare and aggressive subtype of lymphoma. Patients often present late with features of adrenal insufficiency (AI), which is associated with PAL in approximately two thirds of cases. Imaging often reveals unilateral or bilateral adrenal masses, and a core adrenal biopsy is required to confirm the diagnosis. Over 95% of cases are of B cell lineage, and thus, treatment most commonly involves a rituximab-containing chemotherapy regimen. Prognosis is poor with a 12-month survival of approximately 20%. The presence of AI and central nervous system (CNS) involvement are both strongly correlated with worse outcomes.1 We present a case of PAL presenting with AI and explore the complex interplay between the endocrine and hematologic manifestations of the disease and their influence on management and prognosis.
Case Report
A 66-year-old male patient with a past medical history of type 2 diabetes managed with insulin, hypertension, and alcohol-related chronic liver disease presented with severe epigastric pain on a background of fatigue, anorexia, and weight loss of 10 kg evolving over several months. Examination was significant for low-grade fever, profound symptomatic hypotension, generalized hyperpigmentation, and epigastric tenderness. Biochemistry demonstrated hypo-osmolar hyponatremia with urine sodium wasting. Ultrasound of the abdomen demonstrated bilateral hypoechoic suprarenal masses, confirmed on a computed tomography (CT) scan as adrenal masses measuring 98 × 42 × 56 and 96 × 52 × 61 mm on the left and right sides, respectively (Fig. 1).
Fig. 1.
A, Coronal contrast-enhanced computed tomography showing bilateral adrenal masses measuring 98 × 42 × 56 mm (33 HU) and 96 × 52 × 61 mm (28 HU) on the left and right sides, respectively. B, Axial computed tomography demonstrating bilateral heterogeneous adrenal masses with significant compression of the inferior vena cava (arrow).
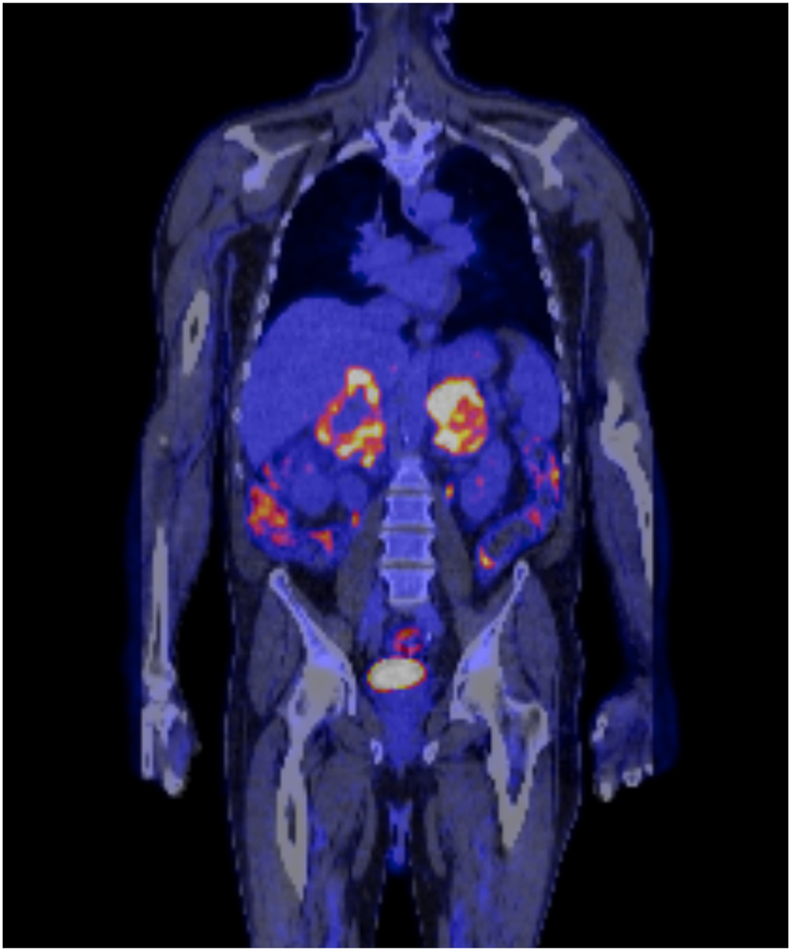
A diagnosis of primary AI was suspected and subsequently confirmed with a low cortisol and elevated adrenocorticotropic hormone levels (Table). He concurrently had elevated plasma and urine metanephrine levels, which normalized with hydrocortisone and fludrocortisone replacement. Bilateral adrenal metastases from an unknown primary carcinoma were considered, and an 18F-fluorodeoxyglucose positron emission tomography scan confirmed these masses to be intensely avid with numerous surrounding avid lymph nodes with no other evidence of metastatic disease (Fig. 2).
Table.
Biochemistry Results Before and After Steroid Replacement
| Investigation | Initial results | After steroid replacement | Reference range |
|---|---|---|---|
| Sodium | 123 mEq/L | 134 mEq/L | 135-145 mEq/L |
| Plasma potassium | 4.2 mEq/L | 4.8 mEq/L | 3.5-5.2 mEq/L |
| Plasma chloride | 93 mEq/L | 99 mEq/L | 95-110 mEq/L |
| Plasma creatinine | 0.61 mg/dL | 0.79 mg/dL | 0.7-1.2 mg/dL |
| Plasma osmolality | 260 mOsm/kg | 289 mOsm/kg | 275-295 mOsm/kg |
| Urine sodium | 162 mEq/L | N/A | <20 mEq/L |
| Urine osmolality | 428 mOsm/kg | N/A | 40-1400 mOsm/kg |
| Plasma glucose | 203 mg/dL | 160 mg/dL | 54-140 mg/dL |
| Plasma cortisol | 3.7 μg/dL | 26.9 μg/dL | 5-25 μg/dL |
| Plasma adrenocorticotropic hormone | 444.5 pg/mL | 1.05 pg/mL | 6-76 pg/mL |
| Plasma normetanephrine | 566.6 pg/mL | 162.6 pg/mL | <245.16 pg/mL |
| Plasma metanephrine | 19.06 pg/mL | 4.01 pg/mL | <136.2 pg/mL |
| 24-h urine normetanephrine | 1.95 mg/24 h | N/A | 0.06-0.39 mg/24 h |
| 24-h urine metanephrine | <0.04 mg/24 h | N/A | 0.04-0.3 mg/24 h |
| Plasma dehydroepiandrosterone sulfate | N/A | <142.8 ng/mL | <2142 ng/mL |
| Plasma testosterone | N/A | 147 ng/dL | 300-1000 ng/dL |
| Plasma renin | N/A | 216 mIU/L | 9.8-23.8 mIU/L |
| Plasma aldosterone | N/A | 0.25 ng/dL | 0-50 ng/dL |
| Plasma lactate dehydrogenase | N/A | 883 U/L | 120-250 U/L |
Some results were not available (N/A).
Fig. 2.
Positron emission tomography demonstrating bilateral large fluorodeoxyglucose avid adrenal lesions with numerous surrounding malignant fluorodeoxyglucose avid lymph nodes near the upper abdominal aorta and inferior vena cava.
The patient proceeded to have a CT-guided core biopsy, and histopathology revealed a high-grade B cell lymphoma with a high proliferation index (Ki-67 approaching 100%). Immunohistochemistry demonstrated CD20+, CD10−, BCL2+, BCL6−, and MUM1+ lymphocytes consistent with a diffuse large B cell lymphoma (DLBCL) of nongerminal center B cell–like subtype. The bone marrow and CNS were not involved.
The patient was assessed to have Ann Arbor stage IVB disease with a CNS International Prognostic Index in Diffuse Large B cell Lymphoma of 5, which placed him at a high risk of CNS progression. He was treated with 6 cycles of rituximab-cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) chemotherapy with intrathecal methotrexate at the time of staging. He was deemed unfit for high-dose methotrexate for CNS prophylaxis because of severe derangement of his liver function tests in the setting of underlying cirrhosis.
Several complications were encountered during his treatment, including decompensation of diabetes due to intermittent high-dose glucocorticoids, severe mucositis limiting oral intake, and febrile neutropenia, which delayed his chemotherapy cycles. He also had intermittent hyponatremia, 2 episodes of adrenal crises associated with hypotension, hyponatremia, and hyperkalemia requiring intensive care unit admission and prolonged stress dosing of his glucocorticoids. Persistent hypokalemia following cycle 2 of R-CHOP and lasting several months necessitated cessation of fludrocortisone and commencement of spironolactone (Fig. 3).
Fig. 3.
Serum potassium and sodium levels between November 2019 and November 2020.
Positron emission tomography-CT after 4 and 6 cycles of chemotherapy showed complete metabolic response. Six months after completing R-CHOP chemotherapy, the patient presented with a 1-week history of progressive generalized headache, diplopia, gait ataxia, and speech disturbance. CT scan indicated a cerebellar bleed with an underlying right-sided mass lesion. He underwent stereotactic craniotomy and debulking of the cerebellar lesion, which confirmed relapse of his lymphoma. Owing to his comorbidities and complications encountered during therapy, he was deemed unsuitable for salvage chemotherapy. He was treated with palliative intent whole brain radiation therapy. He died 3 months later, 16 months following the initial diagnosis.
Discussion
We present a case of an aggressive form of adrenal B cell lymphoma in a patient who presented initially with AI and had bilateral bulky adrenal masses. His management was complicated by multiple endocrine issues, including worsening diabetes, hyponatremia, adrenal crises, and hyperkalemia.
Because primary AI is often immune mediated and associated with atrophic adrenal glands, its occurrence in the presence of large (>6 cm) adrenal masses strongly favors a narrowed differential diagnosis of infection (bilateral tuberculosis or fungal infections), bilateral adrenal hemorrhage, and malignancy (metastatic cancer, disseminated lymphoma, or PAL). PAL is one of the very few indications for urgent core biopsy of the adrenal gland, and existing literature puts a strong emphasis on excluding pheochromocytoma prior to biopsy, either as the underlying etiology or as concurrent subclinical disease.1, 2, 3 This situation is presumably very rare, and to our knowledge, only a single case report has described PAL and pheochromocytoma coexisting in the same adrenal gland in a patient with AI.4
As highlighted in our case, serum free metanephrine measurements can occasionally be problematic, especially if performed prior to adequate steroid replacement, because compensatory norepinephrine production occurs in the setting of hypocortisolism, which then leaves the clinician dealing with much-dreaded biochemical equivocality. In this setting, measuring 24-hour urinary catecholamines is a more specific test and can be useful, obviating the need for further expensive tests, such as an iodine 123-metaiodobenzylguanidine scan.
In the systematic review of 187 cases of PAL by Rashidi and Fisher,1 AI is predicted by the presence of hyperpigmentation and is seen most commonly in older patients and those with bilateral disease. It is strongly correlated with worse outcomes. The reasons for this are unclear; however, AI may reflect more aggressive disease and, as seen in our case, predisposes patients to the added threat of an adrenal crisis throughout chemotherapy. It is important to consider the need for stress doses of hydrocortisone during and even following completion of chemotherapy. Our patient presented with adrenal crises twice, 1 and 2 months following completion of his sixth cycle of R-CHOP chemotherapy.
Primary AI occurs more commonly in PAL than with any other nonlymphomatous metastatic cancer affecting both adrenal glands, suggesting that there are additional mechanisms driving AI. In further support of this, 20% of cases of PAL associated with AI occurred with unilateral disease, and tumor size appeared to have little correlation with the presence of AI. This suggests that direct tissue infiltration and destruction by the lymphoma, a mechanism recognized with other cancers metastasizing to the adrenal glands, do not account for the high frequency of AI observed, and an as-yet-uncharacterized cytokine-related paracrine effect on the adrenal biochemical microenvironment has been proposed as a possible explanation.1
Hyponatremia is seen in nearly all cases of primary AI; however, consideration of syndrome of inappropriate antidiuretic hormone secretion as a contributing cause is important in the setting of lymphoma, particularly if sodium fails to correct despite adequate corticosteroid and mineralocorticoid replacement. Lymphoma can further drive hyponatremia, either indirectly via nonosmotic posterior pituitary activation or because of volume depletion from prolonged anorexia or rarely via paraneoplastic secretion of antidiuretic hormone by lymphoma cells themselves.5,6 Despite initial hyponatremia resolving with steroid replacement, our patient experienced ongoing intermittent hyponatremia, which was not associated with adrenal crises and may have been mediated by the aforementioned mechanisms (Fig. 3).
Refractory hypokalemia was a major challenge in our case, contributing to prolonged admissions for intravenous replacement (Fig. 3). The etiology of hypokalemia was unclear but was likely multifactorial due to poor oral intake and diarrhea from severe mucositis, large doses of insulin for hyperglycemia, and fludrocortisone replacement. The hypokalemia persisted despite correction of these factors, eventually responding to low-dose spironolactone and cessation of fludrocortisone implying a mineralocorticoid-dependent process. Although recovery of the renin-aldosterone axis can rarely occur in the setting of metabolic remission and reduction in the renin levels, it was deemed later unlikely because the renin levels remained elevated. Possible explanations of the hypokalemia include mineralocorticoid-like effect from high stress doses of hydrocortisone during chemotherapy cycles and administration of R-CHOP chemotherapy. A case report by Lieber et al7 describes hypokalemia secondary to functional Bartter syndrome in the setting of cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy use, characterized by renal potassium wasting with elevated plasma renin levels, metabolic alkalosis, secondary hyperaldosteronism, and normal blood pressure, which bears significant resemblance to our case. Hypokalemia is also noted as a frequent adverse effect of R-CHOP chemotherapy in the PRIMEUR-IVL trial.8
Our patient had an initial good response to chemotherapy but eventually developed CNS recurrence, which has been described in existing literature. Lymphoma cells may enter the CNS via hematogenous spread, direct extension from adjacent bone metastases, growth along neurovascular bundles, or retroperitoneal lymph nodes, or through the intervertebral venous plexus.9 Although the incidence of CNS relapse in DLBCL has been reported to be low (≤5%), studies1,10, 11, 12, 13 have reported the incidence of CNS relapse in PAL to be higher at 13%, highlighting the importance of CNS prophylaxis, which is recommended in patients with high CNS International Prognostic Index in Diffuse Large B cell Lymphoma scores, elevated lactate dehydrogenase level, and widely disseminated disease.
In addition to CNS involvement and AI at time of presentation, other factors of poor prognosis in this case include older age, elevated serum lactate dehydrogenase level, nongerminal center B cell–like classification, high Ki-67 proliferation index, larger size of tumor, and bilateral adrenal involvement.10,13,14 Further treatment was limited in the presence of significant comorbidities and the ensuing challenges of managing disease progression with the complications of his therapy and recurrent adrenal crises.
Conclusion
PAL should be considered in the differential diagnosis of a patient presenting with primary AI and bilateral adrenal masses. An early histopathologic diagnosis with core biopsy after exclusion of pheochromocytoma is vital to expedite treatment. AI confers a poor prognosis and requires added considerations in management and monitoring. Electrolyte imbalances, including hyponatremia and hypokalemia, can be very challenging to manage. Spironolactone may be effective in managing hypokalemia induced by high stress doses of hydrocortisone despite absent endogenous mineralocorticoid synthesis. Patients with PAL have a higher rate of CNS relapse and worse outcomes than patients with other forms of DLBCL.
Disclosure
The authors have no multiplicity of interest to disclose.
References
- 1.Rashidi A., Fisher S.I. Primary adrenal lymphoma: a systematic review. Ann Hematol. 2013;92(12):1583–1593. doi: 10.1007/s00277-013-1812-3. [DOI] [PubMed] [Google Scholar]
- 2.Joseph F.G., Cook S., Gowda D. Primary adrenal lymphoma with initial presentation concerning for bilateral adrenal pheochromocytomas. BMJ Case Rep. 2017;2017 doi: 10.1136/bcr-2017-220549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sweeney A.T., Srivoleti P., Blake M.A. Management of the patient with incidental bilateral adrenal nodules. J Clin Transl Endocrinol Case Rep. 2021;20 [Google Scholar]
- 4.Babinska A., Peksa R., Sworczak K. Primary malignant lymphoma combined with clinically "silent" pheochromocytoma in the same adrenal gland. World J Surg Oncol. 2015;13:289. doi: 10.1186/s12957-015-0711-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kobayashi K., Yokote T., Akioka T., Takubo T., Tsuji M., Hanafusa T. Inappropriate antidiuretic hormone production in diffuse large B-cell lymphoma. Br J Haematol. 2008;143(1):2. doi: 10.1111/j.1365-2141.2008.07255.x. [DOI] [PubMed] [Google Scholar]
- 6.Itaya M., Nagata S., Ogino S., et al. A case of primary adrenal diffuse large B cell lymphoma presenting with severe hyponatremia. CEN Case Rep. 2016;5(1):91–94. doi: 10.1007/s13730-015-0200-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lieber I.H., Stoneburner S.D., Floyd M., McGuffin W.L. Potassium-wasting nephropathy secondary to chemotherapy simulating Bartter’s syndrome. Cancer. 1984;54(5):808–810. doi: 10.1002/1097-0142(19840901)54:5<808::aid-cncr2820540507>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 8.Shimada K., Yamaguchi M., Atsuta Y., et al. Rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone combined with high-dose methotrexate plus intrathecal chemotherapy for newly diagnosed intravascular large B-cell lymphoma (PRIMEUR-IVL): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020;21(4):593–602. doi: 10.1016/S1470-2045(20)30059-0. [DOI] [PubMed] [Google Scholar]
- 9.Levitt L.J., Dawson D.M., Rosenthal D.S., Moloney W.C. CNS involvement in the non-Hodgkin’s lymphomas. Cancer. 1980;45(3):545–552. doi: 10.1002/1097-0142(19800201)45:3<545::aid-cncr2820450322>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 10.Kim Y.R., Kim J.S., Min Y.H., et al. Prognostic factors in primary diffuse large B-cell lymphoma of adrenal gland treated with rituximab-CHOP chemotherapy from the Consortium for Improving Survival of Lymphoma (CISL) J Hematol Oncol. 2012;5:49. doi: 10.1186/1756-8722-5-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laurent C., Casasnovas O., Martin L., et al. Adrenal lymphoma: presentation, management and prognosis. QJM. 2017;110(2):103–109. doi: 10.1093/qjmed/hcw174. [DOI] [PubMed] [Google Scholar]
- 12.Majidi F., Martino S., Haase M., et al. Multicenter case series of primary adrenal lymphoma (PAL) Blood. 2017;130(1):1562. [Google Scholar]
- 13.Peng F., Guo L., Yao W.K., et al. Identification of prognostic factors in patients with diffuse large B-cell lymphoma. Indian J Pathol Microbiol. 2017;60(1):87–91. doi: 10.4103/0377-4929.200056. [DOI] [PubMed] [Google Scholar]
- 14.Yang Y., Xie W., Ren Y., Tian H., Chen T. A case report of primary adrenal lymphoma: a rare but aggressive and invasive disease. Medicine (Baltimore) 2020;99(28) doi: 10.1097/MD.0000000000020938. [DOI] [PMC free article] [PubMed] [Google Scholar]