Abstract
Transcriptional selectivity derives, in large part, from the sequence-specific DNA-binding properties of the ς subunit of RNA polymerase. There are 17 ς factors in Bacillus subtilis which, in general, recognize distinct sets of promoters. However, some ς factors have overlapping promoter selectivity. We hypothesize that the overlap between the regulons activated by the ςX and ςW factors can be explained by overlapping specificity for the −10 region: ςX recognizes −10 elements with the sequence CGAC and ςW recognizes CGTA, while both can potentially recognize CGTC. To test this model, we mutated the ςX-specific autoregulatory site (PX), containing the −10 element CGAC, to either CGTC or GCTA. Conversely, the ςW autoregulatory site (PW) was altered from CGTA to CGTC or CGAC. Transcriptional analyses, both in vitro and in vivo, indicate that changes to the −10 element are sufficient to switch a promoter from the ςX to the ςW regulon or, conversely, from the ςW to the ςX regulon, but context effects clearly play an important role in determining promoter strength. It seems likely that these subtle differences in promoter selectivity derive from amino acid differences in conserved region 2 of ς, which contacts the −10 element. However, we were unable to alter promoter selectivity by replacements of two candidate recognition residues in ςW.
While the sequencing of bacterial genomes is proceeding at a rapid pace, functional annotation remains a formidable challenge. Typically, half or more of all predicted open reading frames encode proteins which have no functionally characterized homologs or which are related only to large classes of proteins with a wide range of functions (e.g., transporters or oxidoreductases). Additional clues to gene function can often be gleaned from careful analysis of operon organization (13, 23) or by identifying groups of genes (stimulons) that are coordinately activated under specific conditions (12, 19). Interpreting global transcriptional profiles requires that genes be grouped into regulons that share a common regulatory factor. Regulons activated by secondary ς factors are often a significant component of the stimulons activated in bacteria under specific stress conditions or in response to changing environmental conditions (3–5).
Sequencing of the Bacillus subtilis genome revealed genes for seven previously unidentified ς factors, all belonging to the extracytoplasmic-function (ECF) subfamily (15). Mutants with alterations in these genes are viable and do not have obvious phenotypes, although the mutant strains are often somewhat more sensitive to selected stress conditions (7, 8). Therefore, to define the roles of the ECF ς factors in B. subtilis, we have sought to identify target genes dependent on ECF ς factors for their expression (10, 11).
ECF ς factors often positively regulate their own synthesis (16, 21). The identification of the corresponding autoregulatory promoters provides useful clues to promoter selectivity which can then be used to search the genome for additional target sites. To develop this strategy, we analyzed a large collection of point mutations in the ςX-dependent autoregulatory promoter (PX) to define bases critical for activity (11). As expected for a ς70 class holoenzyme, the critical bases are clustered near −35 and −10 relative to the transcription start point (tGtAACN17CGaC; bases with no allowable substitutions are in uppercase). Using this information, we were able to identify a number of promoters that are recognized by ςX both in vivo and in vitro. However, some of the target genes we identified were still transcribed, from the same start point, even in a sigX null mutant (11). This suggested that at least one other holoenzyme has an overlapping specificity with ςX.
In parallel with these studies of the ςX regulon, we also initiated an analysis of the ςW regulon. Like sigX, sigW is transcribed from an autoregulatory promoter element, PW (TGAAACN16CGTA) (9). Analysis of the genome revealed 15 additional operons with candidate promoters identical to PW (in the −35 and −10 elements and spacer length), and all 15 of these sites are ςW dependent both in vivo and in vitro (10). Thus, promoter sequence comparisons have proven to be a valuable approach to defining ς factor regulons.
Despite considerable sequence similarity between PW and PX, these promoters are exclusively recognized by the cognate ς in vivo and in vitro (9–11). Sequence comparisons, in conjunction with the mutational analysis of PX, suggested that this selectivity might derive from the −10 region sequences. PX contains the −10 element sequence CGAC, while PW contains CGTA (9). Characterization of the ςX and ςW regulons also identified several promoters with the −10 region sequence CGTC. In vitro, these promoters seem to be recognized by both holoenzyme forms (9). In vivo, these promoters seem to depend primarily upon ςX, but often both ςX and ςW contribute to expression.
It is difficult to accurately predict promoters based solely on −35 and −10 consensus sequences. Context effects may play a large role in promoter selectivity, and important discriminatory elements may residue outside the classically defined −35 and −10 elements. As a test of our model for promoter recognition by ςX and ςW, we have engineered mutations within the −10 element to convert PX into a ςW-dependent promoter and, conversely, to convert PW into a ςX-dependent promoter. Our results indicate that changes to the −10 region are sufficient for altering holoenzyme selectivity both in vivo and in vitro. These observations support the notion that the −10 region is a critical selectivity determinant for these two ECF ς factors.
MATERIALS AND METHODS
Bacterial strains, growth media, and antibiotics.
All B. subtilis and Escherichia coli strains used in this work are listed in Table 1. Strains were grown at 37°C with vigorous shaking in Luria broth (LB) medium unless otherwise indicated. In E. coli, ampicillin resistance was selected by using 100 μg of ampicillin/ml. In B. subtilis, antibiotics used for selection were neomycin at 8 μg/ml, spectinomycin at 100 μg/ml, kanamycin at 20 μg/ml, and macrolides-lincomycin-streptogramin B at 25 μg/ml (lincomycin) and 1 μg/ml (erythromycin).
TABLE 1.
Strains and plasmids used in this work
| Strain or plasmid | Description | Source or derivation |
|---|---|---|
| E. coli | ||
| DH5α | supE44Δ lacU169 (φ80lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | Lab stock |
| BL21 | F−ompT hsdSB(rB− mB−) gal dcm | Novagen |
| BL21/DE3 | BL21 with λ DE3 (T7 RNAP under lac control) | Novagen |
| HE4524 | BL21/DE3 containing pJQ27 | This work |
| HE4525 | BL21/DE3 containing pJQ28 | This work |
| B. subtilis | ||
| CU1065 | W168 trpC2 attSPβ | Lab stock |
| ZB307A | W168 SPβc2Δ2::Tn971::pSK10Δ6 (MLSr) | 29 |
| HB7007 | CU1065 sigX::spc (Spcr) SigX− | 8 |
| HB7013 | CU1065::pVA29 (MLSr) RsiX− | 8 |
| HB7022 | CU1065 SPβ7019 (MLSr Neor) PX-cat-lacZ | 8 |
| HB0010 | CU1065 sigW::kan (Kmr) RsiW− | |
| HB0020 | CU1065 sigW::erm (MLSr) SigW− | |
| HB0030 | HB0020 sigX::spc (MLSr Spcr) SigX− SigW− | |
| HB7019 | ZB307A SPβ7019 (MLSr Neor) PX-cat-lacZ (wild type; CGACta) | 8 |
| HB4506 | ZB307A × (pJQ1 × ScaI) PX-cat-lacZ with −10 CGTAaa (X3) | This work |
| HB4507 | ZB307A × (pJQ2 × ScaI) PX-cat-lacZ with −10 CGTAta (X2) | This work |
| HB4508 | ZB307A × (pJQ3 × ScaI) PX-cat-lacZ with −10 CGTCta (X1) | This work |
| HB4521 | ZB307A × (pJQ8 × ScaI) PX-cat-lacZ (wild type; CGTA) | This work |
| HB4522 | ZB307A × (pJQ9 × ScaI) PX-cat-lacZ with −10 CGTC (W1) | This work |
| HB4523 | ZB307A × (pJQ10 × ScaI) PX-cat-lacZ with −10 CGAC (W2) | This work |
| Plasmids | ||
| pJPM122 | Integrational plasmid for cat-lacZ fusion construction | 26 |
| pET17b | Expression vector under the control of T7 promoter | Novagen |
| pJQ1 | PX variant with a CGTAaa −10 element in pJPM122 | This work |
| pJQ2 | PX variant with a CGTAta −10 element in pJPM122 | This work |
| pJQ3 | PX variant with a CGTCaa −10 element in pJPM122 | 11 |
| pJQ8 | PW with the consensus CGTA −10 element in pJPM122 | This work |
| pJQ9 | PW variant with a CGTC −10 element in pJPM122 | This work |
| pJQ10 | PW variant with a CGAC −10 element in pJPM122 | This work |
| pJQ27 | The sigW gene with R75S mutation cloned into pET17b | This work |
| pJQ28 | The sigW gene with R75S N79H mutations in pET17b | This work |
Mutagenesis of PX and PW.
To introduce mutations into PX, 1 nmol of oligonucleotide 313 (−10 region, CGTAaa [−10 consensus bases are in uppercase]) or 314 (−10 region, CGTAta) were mixed with 1 nmol of the reverse oligonucleotide XH135 in 50 μl of TMED buffer (10 mM Tris-HCl [pH 8.0], 10 mM MgCl2, 1 mM EDTA, 1 mM dithiothreitol [DTT], 50 mM NaCl), heated at 95°C, and cooled slowly to allow annealing. Deoxynucleoside triphosphates (10 μl) at 25 mM and 2 μl of Sequenase, version 2, were added, and the oligonucleotides were extended at 37°C for 1 h. The duplex product was purified using a Qiagen purification kit, digested with HindIII and BamHI, and ligated into pJPM122 to construct pJQ1 and pJQ2, respectively. After transformation into E. coli DH5α with selection for ampicillin resistance, plasmids were recovered and the sequence of the promoter region was verified by DNA sequencing. A plasmid containing a −10 element of CGTCaa was obtained from the saturation mutagenesis studies by Huang and Helmann (11) and renamed pJQ3. The plasmids pJQ1, pJQ2, and pJQ3 contain the −44 to +11 regions of PX variants.
To introduce mutations into PW, PW and its variants were amplified using the forward primer XH180 (with a HindIII site) and one of three reverse primers (416 to 418) (Table 2). The reverse primers carry a BamHI site and the indicated −10 element (Table 2). The resulting PCR fragments were then cloned into pJPM122 (26) to construct pJQ8, pJQ9, and pJQ10, following the procedures described above. The plasmids pJQ8, pJQ9, and pJQ10 contain the −79 to +6 regions of PW and its variants.
TABLE 2.
Oligonucleotides used in this study
| Oligonucleotide | Sequence (5′-3′)a | Function |
|---|---|---|
| 313 | GCATAGAAGCTTAGTTGTAATGTAACTTTTCAAGCTATTCATACGTAAAAAAAGTGAACGGAG | PX mutagenic primer (X2) |
| 314 | GCATAGAGAGCTTAGTTGTAATGTAACTTTTCAAGCTATTCATACGTATAAAAAGTGAACGGAG | PX mutagenic primer (X3) |
| XH135 | CGCGGATCCCCTCCGTTCACTTTT | PX reverse primer |
| XH180 | ACGAATAAGCTTCTACACCCTGCCAAA | PW forward primer |
| 416 | CGCGGATCCGGTCTGTATGTATACGAGCTTCGTTTCAAAAG | PW primer (WT) |
| 417 | CGCGGATCCGGTCTGTATGTAGACGAGCTTCGTTTCAAAAG | PW mutagenic primer (W1) |
| 418 | CGCGGATCCGGTCTGTATGTAGTCGAGCTTCGTTTCAAAAG | PW mutagenic primer (W2) |
| 452 | ACATCTAGACCGGTGAAGGCAGAGG | sigW forward (XbaI) |
| 453 | TGTTCACTCGAGCTCATTTCATCACCCCAC | 3′ end of sigW (Xho) |
| 454 | GGCTTTATAGTATCGCGACCAATTTG | R75 mutagenic (sense) |
| 455 | CAAATTGGTCGCGATACTATAAAGCCAAG | R75 mutagenic (antisense) |
| 456 | GGCTTTATAGTATCGCGACCCATTTGACCATTG | R75 and N79 mutagenic (sense) |
| 457 | GGTCAAATGGGTCGCGATACTATAAAGCCAAG | R75 and N79 mutagenic (antisense) |
| 435 | CATAGAAGCTTAGTTGTAATGTAACTTTTCAAG | PX primer for pJPM122 derivatives |
| 366 | ACTCTCCGTCGCTATTGTAACCAG | cat gene; for promoter amplification for runoffs |
Introduced restriction sites used for cloning are in italics, −10 region sequences (and relevant flanking bases for PX) are underlined, and codons altered by mutagenesis are double underlined.
Construction and analysis of SPβ reporter phage.
To recombine the promoter-cat-lacZ fusions into the SPβ prophage, each pJPM122 derivative was linearized by digestion with ScaI and transformed into ZB307A [W168 SPβc2Δ2::Tn917::pSK10Δ6 (MLSr)] (29) with selection for neomycin resistance. To transduce each reporter fusion into various genetic backgrounds, SPβ lysates were prepared by heat induction at 50°C for 10 min followed by continued incubation at 37°C for 90 min (1). The resulting lysates were used to transduce recipient strains using standard techniques (Table 1).
Expression levels for each reporter fusion were determined by β-galactosidase assays of cultures grown in LB media. Each strain was grown overnight in LB medium containing appropriate antibiotics and diluted 100-fold into LB medium without antibiotics. Samples of cells were taken after growth at 37°C for 3 h, harvested, and frozen at −80°C. β-Galactosidase activity was assayed as described by Miller (20).
Overproduction and purification of ςW mutants.
Mutagenesis of sigW was achieved by two rounds of PCR with Vent DNA polymerase (New England Biolabs). Primer 452 is located at the 5′ end of sigW and contains a ribosome binding site and an XbaI restriction site, and primer 453 is located at the 3′ end of sigW and contains a XhoI restriction site. Primers 454 and 455 introduce mutations in the codon of Arg75 on the sense and antisense strands, respectively. Primer 456 and 457 introduce mutations in the codons for both Arg75 and Asn79. In the first round of PCR, primer 452 and primer 455 or 457 were used to amplify the mutated 5′ fragments of the sigW gene from B. subtilis chromosomal DNA, and primer 453 and primer 454 or 456 were used to amplify the mutated 3′ fragments of sigW gene. In the second round of PCR, the products from the first round were used as templates and oligonucleotides 452 and 453 were used as primers. The amplified fragments from the second round were digested with XbaI and XhoI and cloned into pET17b to construct pJQ27 and pJQ28. The sequences of both cloned mutant sigW genes were confirmed by DNA sequencing.
For purification of ςW mutants, pJQ27 and pJQ28 were transformed into E. coli BL21/DE3 to generate strains HE4524 and HE4525. Both strains were grown to mid-logarithmic phase at 37°C in 500 ml of LB medium with 100 μg of ampicillin/ml. Isopropyl-β-d-thiogalactopyranoside (IPTG) was added to 0.4 mM, and cells were harvested after further incubation for 3 h. After centrifugation, the cell pellets were suspended in 20 ml of disruption buffer (50 mM Tris-HCl [pH 8.0], 2 mM EDTA, 0.1 mM DTT, 1 mM β-mercaptoethanol, 233 mM NaCl, 10% glycerol) and lysed by sonication, and the inclusion bodies were recovered by centrifugation. The inclusion bodies were washed twice with 100 ml of TEDG buffer (50 mM Tris-HCl [pH 8.0], 0.1 mM EDTA, 1 mM DTT, 10% glycerol) containing 0.5% (vol/vol) Triton X-100 and 10 mM EDTA and then dissolved in 10 ml of TEDG–6 M guanidine hydrochloride. A portion (2.5 ml) was gradually diluted to 250 ml with TEDGX (TEDG containing 0.01% Triton X-100) to allow renaturation of ςW mutants and then loaded onto a 10-ml heparin-Sepharose CL-6B column equilibrated with TEDGX. After being washed with 80 ml TEDGX–0.2 M NaCl, the ςW mutant proteins were eluted with TEDGX–0.5 M NaCl. The peak fractions were concentrated with Centricon 10 from Amicon and stored at −80°C.
In vitro transcription assays.
Runoff transcription assays were performed with DNA products from PCR as the templates. PX and its variants were amplified from B. subtilis HB7022 [CU1065 SPβ7019 PX-cat-lacZ (MLSr Neor)] chromosomal DNA or plasmid pJQ1, pJQ2, or pJQ3 with primers 435 and 366. PW and its variants were amplified from plasmid pJQ8, pJQ9, or pJQ10 with primers XH180 and 366. Primer 366 is located within the cat gene, and the PCR-amplified products contain the promoter regions and a 263-bp 5′ fragment of the cat gene.
B. subtilis core RNA polymerase (RNAP) (14), ςX (8), ςW (9), and δ (17) preparations have been described previously. Typical transcription reaction mixtures (20 μl) contained 0.36 pmol of core RNAP, 4.5 pmol of ς, 4.2 pmol of δ, and 0.04 pmol of template DNA in transcription buffer (20 mM Tris-HCl [pH 8.0], 10 mM MgCl2, 50 mM KCl, 0.5 mM DTT, 0.1 mg of bovine serum albumin/ml, 5% [vol/vol] glycerol, and the RNase inhibitor RNasin from Promega at 0.8 U/reaction), to which were added nucleoside triphosphate mixtures containing 10 nmol of ATP, GTP, and CTP, 1 nmol of UTP, and 0.6 pmol of [α-32P]UTP (3,000 Ci/mmol). Core RNAP, ς, and δ were mixed on ice for 15 min to form RNAP holoenzyme before the addition of template DNA and incubation at 37°C for 10 min to allow promoter binding. Nucleoside triphosphates were added, and transcription was allowed to proceed for 7 min at 37°C prior to addition of 6 μg of heparin/reaction and an additional 11 min of incubation. Reactions were terminated by the addition of 80 μl of stop solution (2.5 M NH4 acetate, 10 mM EDTA, and 0.1 mg of glycogen/ml), extracted with phenol-chloroform, and precipitated with ethanol. The pellets were dissolved in 8 μl of loading buffer (20 μg of xylene cyanol FF/ml, 20 μg of bromophenol blue/ml, and 60 mg of urea/ml in 1× Tris-borate-EDTA buffer) and subjected to 8 M urea–6% polyacrylamide gel electrophoresis. Reaction products were visualized by using a Molecular Dynamics PhosphorImager system and ImageQuant software.
Experiments to test the selectivity of ςW mutant proteins were performed as described above or using a template competition assay containing both PW and the CGTC variant. To distinguish the RNA products from the two fragments, the PCR product carrying the CGTC PW variant was digested with DdeI. This reduces the size of the PCR fragment from 353 to 281 bp and leads to a concomitant decrease in the length of the runoff transcript. Transcripts were quantified using a Molecular Dynamics PhosphorImager system and ImageQuant software, and the molar ratios were calculated after taking into account the difference in UMP content of each runoff RNA.
RESULTS
Activity of PX and PW variants in vitro.
PX (the ςX-dependent promoter preceding sigX) and PW (the ςW-dependent promoter preceding sigW) have very similar sequences in the −10 and −35 elements, yet EςX cannot recognize PW nor can EςW recognize PX. Comparison of PX with PW, in the vicinity of the −35 and −10 elements, reveals seven sequence differences that might account for the mutually exclusive recognition of these two promoters (Fig. 1). Previous mutational analysis of PX indicates that changing the sequences at five of these positions does not eliminate promoter activity (11), and alignment of known ςX-dependent promoters supports the idea that these are unlikely to be key selectivity determinants (11). Nor is the difference in spacer length between PX and PW likely to be an important factor: both ςX and ςW can recognize promoters with either 16- or 17-base spacer regions (10, 11). These observations led us to focus our attention on the −10 element.
FIG. 1.
Sequence comparison of PX and PW. The sequence of the ςX-dependent autoregulatory site, PX, is shown, with critical bases, as judged by mutational analysis (11), in bold. Alignment of PX with PW reveals a high degree of sequence similarity with related −35 and −10 sequences, also in bold. The numbers represent seven positions that might account for the mutually exclusive recognition of these two promoters. Positions 1, 2, 4, and 7 are viewed as unlikely discriminatory features, since the corresponding mutations in PX retain at least 25% of wild-type activity in vivo (11). A change of A to T at position 5 also retains activity in vivo and this activity is eliminated in a sigX mutant. A variant of PX with a single base deletion (Δ), to generate a 16-bp spacer as in PW, retains activity, although at a considerably lower level than PX. This analysis, together with alignment of promoters shown previously to depend on ςX, ςW, or both, suggests a critical role for position 6 (located at −9 relative to the most common transcriptional start point) in the discrimination of PX from PW (9).
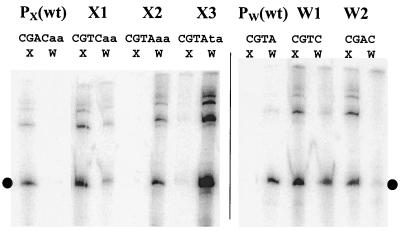
To determine whether the −10 element is the key determinant that distinguishes between EςX and EςW, we mutated the −10 elements so that PX acquired the −10 element CGTC or CGTA and PW acquired the −10 element CGTC or CGAC (Table 3). Reconstituted RNAP holoenzyme was then used to determine promoter activity in runoff transcription assays (Fig. 2). Under our reaction conditions, core RNAP alone (ββ′α2δ) could not recognize or transcribe from either PX or PW, and enzyme reconstituted with ςA recognized a ςA-dependent promoter with high activity but did not recognize either PX or PW (data not shown). Consistent with previous reports (9), reconstituted EςX directs transcription from PX, but not PW, while EςW directs transcription from PW, but not PX.
TABLE 3.
Sequences of PX, PW and variants used in this study
| Promoter | Sequencea | IVTb | β-Galc |
|---|---|---|---|
| PX | aaTGTAACttttcaagctattcataCGACaaaaaagtgaag | X | X |
| X1 | aaTGTAACttttcaagctattcataCGTCaaaaaagtgaacg | X, w | X |
| X2 | aaTGTAACttttcaagctattcataCGTAaaaaaagtgaacg | W | NA |
| X3 | aaTGTAACttttcaagctattcataCGTAtaaaaagtgaacg | W | W |
| PW | atTGAAACcttttgaaa-cgaagctCGTAtacatacagaccg | W | W |
| W1 | atTGAAACcttttgaaa-cgaagctCGTCtacatacagaccg | X, W | X, w |
| W2 | atTGAAACcttttgaaa-cgaagctCGACtacatacagaccg | X | X, w |
Conserved −35 and −10 elements are in uppercase. Bases altered by mutagenesis are in bold. The experimentally mapped transcription start sites are underlined.
The activity of each promoter in in vitro transcription (IVT) assays is indicated. A lowercase letter indicates weak activity.
The activity of each promoter in vivo is summarized. NA, no activity was detected.
FIG. 2.
In vitro transcription of PX, PW, and their variants by EςX and EςW holoenzymes. The −10 elements of the promoters are indicated. Downstream flanking bases are shown for the ςX −10 element, since one base was altered between the X2 and X3 variants. The dot indicates the expected runoff products. wt, wild type.
Alteration of the PX −10 element from CGAC to CGTC, a single base change, allows in vitro recognition by the EςW holoenzyme, but the level of transcription is still less than that achieved with EςX. One additional base change, to generate a −10 region of sequence CGTA results in a promoter that can only be recognized by EςW. Introduction of an adjacent T residue (corresponding to position 7) (Fig. 1) results in a much stronger promoter while retaining the strong preference for the EςW holoenzyme. Thus, two or three base changes in the PX −10 element are sufficient to switch this sequence from one exclusively recognized by ςX to one preferentially recognized by ςW.
Similar results were found with the PW variants. When the −10 element of PW was changed from CGTA to CGTC, the resulting promoter could be recognized by both EςX and EςW with nearly equal activity. One additional base change, to generate a −10 sequence CGAC, results in a PW variant that is preferentially recognized by EςX. Thus, the −10 elements of PX and PW determine whether the promoter is ςX and/or ςW dependent in vitro.
Activity of PX variants in vivo.
To test the promoter activities of the PX and PW variants in vivo, we cloned the promoters into an SPβ-derived prophage to generate promoter lacZ operon fusions. The resulting reporter fusions were transduced into the wild-type strain (CU1065), mutant strains altered in sigX (HB7007), rsiX (HB7013), sigW (HB0020), or rsiW (HB0010), or the double sigX sigW mutant (HB0030).
Consistent with previous work (8), PX is active in CU1065 but not in the sigX mutant (Fig. 3). As expected for a ςX-dependent promoter, activity increases in the rsiX anti-ς factor mutant. Unexpectedly, activity of PX is also reduced severalfold in a sigW mutant. The origin of this effect is unclear, since previous analyses failed to reveal a comparable effect. Indeed, activity of most ςX-dependent promoters is slightly elevated in sigW mutant strains (M. Cao and J. D. Helmann, unpublished data). The basis for this discrepancy is not clear.
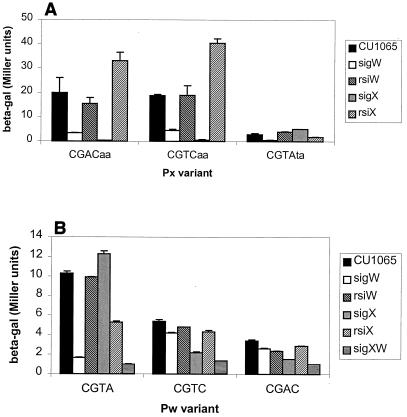
FIG. 3.
In vivo expression driven by PX, PW, and their variants. β-Galactosidase activities (beta-gal) were measured for strains carrying the indicated promoter variant. (A) Results for wild-type PX (CGACaa) and its −10 region variants. Note that the CGTAta variant used (X3) was altered at three positions (underlined), since the X2 variant (Table 3) did not have detectable activity in vivo. (B) Results for wild-type PW (CGTA) and its −10 region variants. Error bars represent the standard deviations from at least two assays.
The CGTC PX variant has an in vivo profile virtually indistinguishable from PX: activity is reduced to background levels in the sigX mutant strain (Fig. 3). This suggests that in vivo ςW does not contribute significantly to expression, despite the fact that EςW can recognize, albeit weakly, this promoter in vitro.
The CGTAaa PX variant (bases flanking the four −10 consensus positions are in lowercase) did not show any measurable activity in any recipient strain. Notice that the CGTAaa PX variant has a much weaker activity than the CGTAta PX variant in vitro, although it is also ςW dependent (Fig. 2). Consistent with the in vitro transcription results, the CGTAta PX variant is no longer dependent on ςX for expression, and instead, activity is reduced to background levels (<1 Miller unit) in the sigW mutant. As expected for a ςW-dependent promoter, activity is elevated slightly in an rsiW mutant. In addition, activity is elevated in the sigX mutant and decreased slightly in an rsiX background. The latter results are consistent with previous observations that activities of ςW and ςX promoters are often mutually antagonistic: increased activity of one leads to decreased activity of the other (9, 10). The basis for this effect is not yet understood. Thus, despite the fact that the in vivo activity of the PX CGTA variant is low (∼5 Miller units), it has all the hallmarks of a ςW-dependent promoter sequence.
Activity of PW variants in vivo.
As expected, PW directs the synthesis of β-galactosidase in the wild type (CU1065), but activity is completely lost in the sigW mutant and actually increases slightly in the sigX mutant strain. With the PW variant with a CGTC sequence in the −10 region, mutation of sigW has only a small effect on activity while a sigX mutation leads to a significant decrease in activity. In the double sigX sigW mutant, activity is reduced further still, to near background. Thus, this single base change has converted a ςW-dependent promoter to a one primarily dependent on ςX in vivo (Fig. 3), consistent with in vitro transcription results (Fig. 2). Similar results are seen with the PW variant with a CGAC −10 region.
Taken together, the in vitro and in vivo expression studies demonstrate that sequence changes localized to the −10 region can convert a ςX-dependent to a ςW-dependent promoter and vice versa. However, in each case the total promoter activity is significantly reduced. Thus, there are likely to be other sequence features within the promoter region that, while not essential for recognition, nevertheless help optimize the promoter for activity with the cognate holoenzyme. In addition, the in vivo activity of these promoters may be affected by regulatory proteins not present in the purified in vitro system.
Promoter specificity of ςW region 2 mutants.
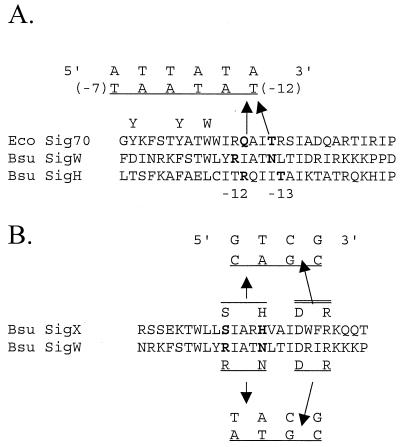
Numerous previous studies indicate that region 2 of ς factors interacts with the −10 element of promoter DNA (see references 3, 5, and 22 for reviews). Sequence comparisons of ςX, ςW, and other ς factors identify two amino acids that are candidates for contacting the −10 region consensus element. Wilson modeled this region of ECF ς factors as an alpha helix, based on the three-dimensional structure of this domain in E. coli ς70 (28), and noted that there is a correlation between the identity of surface-exposed amino acids and the −10 element sequences recognized by the corresponding holoenzymes (Fig. 4) (28). Specifically, she hypothesized that His64 and Ser60 of ςX recognize the critical AC in the −10 element of PX, while Asn79 and Arg75 of ςW recognize the TA in the −10 element of PW. To test this model, we mutated the sigW gene to allow expression of ςW variants having either one or both of these candidate selectivity determinants replaced with the corresponding residues from ςX. The R75S and R75S N79H mutants were expressed in E. coli and purified using procedures similar to that used for wild-type ςW (9).
FIG. 4.
Alignment of the −10 recognition domain (region 2) of ς factors and a model for promoter recognition. (A) The portion of conserved ς region 2 corresponding to the DNA −10 region recognition helix (helix 14 in the crystal structure of a fragment of ς70) (18) is shown. In E. coli ς70, residues Q437 and T440 have been implicated in recognition of the T residue at the −12 position of the −10 region consensus (25, 27). Note that the −10 region is written in inverted orientation (the transcriptional start site would be to the left and the −35 region to the right). Genetic experiments with B. subtilis ςH also support a role for this region in −10 region recognition and contribute to a model orienting the recognition helix with its amino-terminal end at the downstream end of the −10 region (2). The amino-terminal end of this helix contains multiple aromatic amino acids (two Y and two W) that form a single-stranded DNA-binding surface that makes contributions both to promoter melting and to −10 region recognition (reviewed in reference 6). The corresponding region of ςW is shown based on a multiple-sequence alignment of ECF ς family members against other ς70 family members (16). N79 of ςW is a candidate recognition residue that aligns with ς70 Q437, while R75 aligns with the conserved W residues in ς70. (B) Modeling region 2 of ςW and ςX as an α helix reveals four amino acids that might contribute to −10 region recognition as shown (28). In each case, the conserved R and D residues would recognize the upstream CG dinucleotide common to both ςX and ςW promoters while the two amino-terminal residues would distinguish between the downstream portion of the −10 element.
We used in vitro transcription assays to determine if the ςW mutations affected promoter recognition. Like ςW, both the R75S and the R75S N79H ςW mutant proteins recognize PW, but not PX, in vitro. Neither mutant holoenzyme is able to recognize the CGAC PW variant. Thus, substitution of either or both of these amino acids failed to confer a ςX-like selectivity on the resulting holoenzyme. Both mutants, like wild-type ςW, can recognize the CGTC PW variant. Reasoning that perhaps transcriptional selectivity had been altered only marginally by these amino acid changes, we set up a mixed-template transcription system containing both the wild-type PW and the CGTC variant (see Materials and Methods). The molar ratios of transcripts from PW to those from the CGTC PW variant were 7.2, 9.9, and 9.8 for the wild-type ςW, the R75S mutant, and the R75S N79H mutant, respectively. Thus, these mutations did not allow ςW to recognize the CGTC −10 region better than the wild type, as might have been expected if the mutant proteins had a selectivity more similar to that of ςX.
DISCUSSION
We have used in vitro transcription and β-galactosidase assays to investigate the role of the PX and PW −10 elements in recognition by EςX and EςW. Altering the −10 element of PX from CGAC to CGTA makes the promoter ςW dependent, while changing the −10 element of PW from CGTA to either CGAC or CGTC makes the promoter recognizable by EςX. This indicates that the −10 region is the major sequence element that distinguishes a ςX-dependent from a ςW-dependent promoter. In contrast, the −35 elements of promoters under ECF ς control often have similar sequence features, including a conserved AAC trinucleotide motif (21). Comparisons of known ςW- and ςX-dependent promoters are consistent with this model for the −10 region as a key selectivity determinant and have failed to reveal any plausible discriminatory sequences in the −35 region.
Several lines of evidence suggest that other sequence elements also function in promoter recognition and also play a major role in determining promoter strength. For example, the B. subtilis genome contains 27 perfect matches to TGAAACN16CGTA, including at least 16 active, ςW-dependent promoters. However, the remaining 11 sites are not positioned upstream of genes and are thus unlikely to be active promoters (10). Additional sequence elements are postulated to help distinguish the 16 active ςW-dependent promoters from the other 11 sites with identical −35 and −10 elements. Previously, we suggested that these additional sequence features might include upstream promoter elements between −40 and −70, a pyrimidine-rich region just downstream of the −35 element (Fig. 1), and additional sequence determinants near the −10 element (10). The complexity of promoter recognition is also apparent from the observation that mutating the −10 element of PW from CGTA to CGAC results in a ςX-dependent promoter, but one much less active than PX (at least in vivo). Conversely, mutating the −10 element of PX from CGAC to CGTA results in a weak ςW-dependent promoter. Thus, while the −10 element plays a dominant role in promoter selectivity, overall activity of the promoter is strongly influenced by context. The origins of these context effects on promoter strength are not yet clear. It is possible that regulatory proteins present in vivo, but lacking in our in vitro system, also play an important role in governing both promoter strength and selectivity.
In previous studies, we found that promoters with a CGTC −10 element could be transcribed by either the EςX or EςW holoenzyme, but with variable efficiency (9). In the present study, we found that in the context of PX, the CGTC variant is much more active with EςX than with EςW and in vivo activity is completely eliminated in a sigX mutant. In contrast, when the CGTC −10 element occurs in the context of PW, the resulting promoter is recognized about equally by the two holoenzymes in vitro and both appear to contribute to in vivo expression. Thus, it is difficult to predict which holoenzyme will play a dominant role in expression of candidate promoters with a CGTC −10 element, although in most cases it appears to be ςX. Two promoters of this class, both dependent in vivo on EςX, are found upstream of the dltABCD and the pssA operons and control expression of genes involved in modification of teichoic acids and phospholipid biosynthesis, respectively (M. Cao, J. Qiu, and J. D. Helmann, unpublished data). Other examples include promoters preceding the abh, divIC, ywbN, and yrhH genes (9) and a promoter identified by Petersohn et al. upstream of the yjbC gene (10, 11, 24).
While our studies lend strong support to our model for DNA determinants that distinguish ςX- from ςW-dependent promoters, we were not successful in identifying the corresponding amino acid determinants in ς factor. A model, based on alignment of ς factor sequences (16) and analysis of mutations known to affect −10 region recognition, was developed in which two residues in an α-helical portion of conserved region 2 were proposed to contact the −10 element (28). However, replacement of these residues in ςW with the corresponding residues from ςX did not alter promoter selectivity in vitro. This may indicate that these residues play no role in promoter recognition. Alternatively, these residues may recognize the conserved portions of the −10 element, such as the initial CG dinucleotide, and other residues, not tested in this study, may discriminate between the CGAC (ςX-specific) and CGTA (ςW-specific) −10 elements.
Understanding the promoter selectivity of ς factors is essential for the use of consensus-directed approaches to defining ς factor regulons, which in turn provides important insights into biological function (10, 11, 24). One limitation of the consensus-directed methods is that they tend to identify those promoters, and only those promoters, that closely match a predefined consensus. This limitation can be circumvented, however, through the use of DNA arrays to define ς factor regulons. Indeed, knowledge of ς factor regulons will be key to the interpretation of genome-scale transcriptional profiling experiments in general, which often reveal the activation and repression of multiple regulons in response to stress conditions or environmental stimuli.
ACKNOWLEDGMENTS
We thank Megan Wilson and Iain Lamont for communication of unpublished results.
This work was supported by grant GM47446 from the NIH.
REFERENCES
- 1.Cutting S M, VanderHorn P B. Genetic analysis. In: Harwood C R, Cutting S M, editors. Molecular biological methods for bacillus. Chichester, United Kingdom: John Wiley and Sons, Ltd.; 1990. pp. 27–74. [Google Scholar]
- 2.Daniels D, Zuber P, Losick R. Two amino acids in an RNA polymerase sigma factor involved in the recognition of adjacent base pairs in the −10 region of a cognate promoter. Proc Natl Acad Sci USA. 1990;87:8075–8079. doi: 10.1073/pnas.87.20.8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gross C A, Lonetto M, Losick R. Bacterial sigma factors. In: McKnight S L, Yamamoto K R, editors. Transcriptional regulation. Vol. 1. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. pp. 129–176. [Google Scholar]
- 4.Haldenwang W G. The sigma factors of Bacillus subtilis. Microbiol Rev. 1995;59:1–30. doi: 10.1128/mr.59.1.1-30.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Helmann J D. Bacterial sigma factors. In: Conaway R C, Conaway J, editors. Transcription: mechanisms and regulation. Vol. 3. New York, N.Y: Raven Press; 1994. pp. 1–17. [Google Scholar]
- 6.Helmann J D, deHaseth P L. Protein-nucleic acid interactions during open complex formation investigated by systematic alteration of the protein and DNA binding partners. Biochemistry. 1999;38:5959–5967. doi: 10.1021/bi990206g. [DOI] [PubMed] [Google Scholar]
- 7.Horsburgh J M, Moir A. Sigma M, an ECF RNA polymerase sigma factor of Bacillus subtilis 168, is essential for growth and survival in high concentrations of salt. Mol Microbiol. 1999;32:41–50. doi: 10.1046/j.1365-2958.1999.01323.x. [DOI] [PubMed] [Google Scholar]
- 8.Huang X, Decatur A, Sorokin A, Helmann J D. The Bacillus subtilis ςX protein is an extracytoplasmic function sigma factor contributing to the survival of high temperature stress. J Bacteriol. 1997;179:2915–2921. doi: 10.1128/jb.179.9.2915-2921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang X, Fredrick K L, Helmann J D. Promoter recognition by Bacillus subtilis ςW: autoregulation and partial overlap with the ςX regulon. J Bacteriol. 1998;180:3765–3770. doi: 10.1128/jb.180.15.3765-3770.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang X, Gaballa A, Cao M, Helmann J D. Identification of target promoters for the Bacillus subtilis extracytoplasmic function ς factor, ςW. Mol Microbiol. 1999;31:361–371. doi: 10.1046/j.1365-2958.1999.01180.x. [DOI] [PubMed] [Google Scholar]
- 11.Huang X, Helmann J D. Identification of target promoters for the Bacillus subtilis ςX factor using a consensus-directed search. J Mol Biol. 1998;279:165–173. doi: 10.1006/jmbi.1998.1765. [DOI] [PubMed] [Google Scholar]
- 12.Hughes J D, Estep P W, Tavazoie S, Church G M. Computational identification of cis-regulatory elements associated with groups of functionally related genes in Saccharomyces cerevisiae. J Mol Biol. 2000;296:1205–1214. doi: 10.1006/jmbi.2000.3519. [DOI] [PubMed] [Google Scholar]
- 13.Huynen M, Snel B, Lathe III W, Bork P. Predicting protein function by genomic context: quantitative evaluation and qualitative inferences. Genome Res. 2000;10:1204–1210. doi: 10.1101/gr.10.8.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Juang Y L, Helmann J D. The delta subunit of Bacillus subtilis RNA polymerase. An allosteric effector of the initiation and core-recycling phases of transcription. J Mol Biol. 1994;239:1–14. doi: 10.1006/jmbi.1994.1346. [DOI] [PubMed] [Google Scholar]
- 15.Kunst F, Ogasawara N, Moszer I, Albertini A M, Alloni G, Azevedo V, Bertero M G, Bessieres P, Bolotin A, Borchert S, Borriss R, Boursier L, Brans A, Braun M, Brignell S C, Bron S, Brouillet S, Bruschi C V, Caldwell B, Capuano V, Carter N M, Choi S K, Codani J J, Connerton I F, Danchin A, et al. The complete genome sequence of the Gram-positive bacterium Bacillus subtilis. Nature. 1997;390:249–256. doi: 10.1038/36786. [DOI] [PubMed] [Google Scholar]
- 16.Lonetto A M, Brown K L, Rudd K E, Buttner M J. Analysis of the Streptomyces coelicolor sigE gene reveals the existence of a subfamily of eubacterial s factors involved in the regulation of extracytoplasmic functions. Proc Natl Acad Sci USA. 1994;91:7573–7577. doi: 10.1073/pnas.91.16.7573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopez de Saro F J, Woody A Y, Helmann J D. Structural analysis of the Bacillus subtilis delta factor: a protein polyanion which displaces RNA from RNA polymerase. J Mol Biol. 1995;252:189–202. doi: 10.1006/jmbi.1995.0487. [DOI] [PubMed] [Google Scholar]
- 18.Malhotra A, Severinova E, Darst S A. Crystal structure of a ς70 subunit fragment from E. coli RNA polymerase. Cell. 1996;87:127–136. doi: 10.1016/s0092-8674(00)81329-x. [DOI] [PubMed] [Google Scholar]
- 19.McGuire A M, Hughes J D, Church G M. Conservation of DNA regulatory motifs and discovery of new motifs in microbial genomes. Genome Res. 2000;10:744–757. doi: 10.1101/gr.10.6.744. [DOI] [PubMed] [Google Scholar]
- 20.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1972. pp. 352–355. [Google Scholar]
- 21.Missiakas D, Raina S. The extracytoplasmic function sigma factors: role and regulation. Mol Microbiol. 1998;28:1059–1066. doi: 10.1046/j.1365-2958.1998.00865.x. [DOI] [PubMed] [Google Scholar]
- 22.Moran C P., Jr . RNA polymerase and sigma factors. In: Sonenshein A L, Hoch J A, Losick R, editors. Bacillus subtilis and other gram-positive bacteria. Washington, D.C.: ASM Press; 1993. pp. 653–667. [Google Scholar]
- 23.Overbeek R, Fonstein M, D'Souza M, Pusch G D, Maltsev N. The use of gene clusters to infer functional coupling. Proc Natl Acad Sci USA. 1999;96:2896–2901. doi: 10.1073/pnas.96.6.2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petersohn A, Bernhardt J, Gerth U, Hoper D, Koburger T, Volker U, Hecker M. Identification of ςB-dependent genes in Bacillus subtilis using a promoter consensus-directed search and oligonucleotide hybridization. J Bacteriol. 1999;181:5718–5724. doi: 10.1128/jb.181.18.5718-5724.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siegele D A, Hu J C, Walter W A, Gross C A. Altered promoter recognition by mutant forms of the sigma 70 subunit of Escherichia coli RNA polymerase. J Mol Biol. 1989;206:591–603. doi: 10.1016/0022-2836(89)90568-8. [DOI] [PubMed] [Google Scholar]
- 26.Slack F J, Mueller J P, Sonenshein A L. Mutations that relieve nutritional repression of the Bacillus subtilis dipeptide permease operon. J Bacteriol. 1993;175:4605–4614. doi: 10.1128/jb.175.15.4605-4614.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waldburger C, Gardella T, Wong R, Susskind M M. Changes in conserved region 2 of Escherichia coli sigma 70 affecting promoter recognition. J Mol Biol. 1990;215:267–276. doi: 10.1016/s0022-2836(05)80345-6. [DOI] [PubMed] [Google Scholar]
- 28.Wilson M J. Ph.D. thesis. Dunedin, New Zealand: University of Otago; 2000. [Google Scholar]
- 29.Zuber P, Losick R. Role of AbrB and SpoOA- and SpoOB-dependent utilization of a sporulation promoter in Bacillus subtilis. J Bacteriol. 1987;169:2223–2230. doi: 10.1128/jb.169.5.2223-2230.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]