Abstract
Background/Objective
Familial hypocalciuric hypercalcemia (FHH) is an uncommon cause of hypercalcemia; however, it is important to consider and rule out in patients with suspected primary hyperparathyroidism (PHPT), ideally, before proceeding with surgery. Herein, we present a patient where this process identified a calcium-sensing receptor gene (CASR) sequence variant currently labeled as a variant of unknown significance (VUS), yet the patient’s family pedigree suggests that it is in fact a pathogenic CASR sequence variant.
Case Report
A 35-year-old woman was referred to the Endocrine Surgery clinic for evaluation of “recurrent PHPT” and need for reoperative parathyroidectomy. Before referral, she was treated with subtotal parathyroidectomy for the presumed diagnosis of PHPT-related symptomatic hypercalcemia. Postoperatively, she had persistent symptoms. Upon referral, additional relevant information was elicited that suspected FHH instead of PHPT, including a family history of hypercalcemia with CASR VUS in multiple family members and hypocalciuria in the patient. She underwent genetic testing revealing a missense CASR VUS in exon 3 c.392C>A (p.Ala110Asp), the same as in her mother. Medical management instead of reoperation was advised for the diagnosis of FHH.
Discussion
To our knowledge, this CASR sequence variation has not been previously reported in the literature. Reporting newly discovered sequence variations with the context of a family’s medical history is important because it allows for the recognition of new pathogenic variants. This expands the registry of already known sequence variations and their associated clinical pathology for future patients undergoing genetic testing.
Conclusion
This CASR variant represents a novel pathogenic sequence variation causing FHH.
Key words: familial hypocalciuric hypercalcemia (FHH), primary hyperparathyroidism (PHPT), genetic testing, Sherloc classification system
Abbreviations: CaSR, calcium-sensing receptor; CCCR, calcium creatinine clearance ratio; FHH, familial hypocalciuric hypercalcemia; PHPT, primary hyperparathyroidism; PTH, parathyroid hormone; RR, reference range; VUS, variant of unknown significance
Highlights
-
•
Establish importance that familial hypocalciuric hypercalcemia (FHH) should always be on the differential diagnosis of patients being evaluated for hypercalcemia
-
•
Illustrate and review the nuances of diagnostic scrutiny that can differentiate potential FHH from primary hyperparathyroidism
-
•
Educate clinicians that direct submissions of clinical information to the genetic testing companies for review and published case reports regarding specific mutations are important avenues to share knowledge that can change our understanding of variants of unknown significance and therefore clinical management in future patients with the same mutation
Clinical Relevance
Our index patient had a CASR mutation variant of unknown significance (VUS). We found the same mutation in her mother as well as CASR VUS in her maternal uncle and aunt which we suspect is the same mutation, concluding it is pathogenic resulting in FHH. Publication is needed to disseminate this new knowledge as it changes clinical/surgical management for future patients with the same mutation.
Introduction
Familial hypocalciuric hypercalcemia (FHH) is an uncommon cause of hypercalcemia because of inactivating sequence variations in the CASR, GNA11, or AP2S1 genes, which control important signaling pathways necessary for maintaining calcium homeostasis.1,2 There are 3 types of FHH categorized based on the gene sequence variation. Type 1 FHH is the most common, accounting for >65% of cases, and is caused by sequence variation of the CASR gene on chromosome 3, thus affecting the calcium-sensing receptor (CaSR).2,3 Over 200 pathogenic CASR sequence variations are currently known,1,3,4 and the vast majority are missense sequence variations.2 Type 2 and 3 FHHs are caused by sequence variations of the GNA11 and AP2S1 genes, respectively, both on chromosome 19, leading to dysfunction of the calcium signaling pathway via the G-protein subunit α-11 and adaptor-related protein complex 2, σ-1 subunit.1,2 Autosomal dominant inheritance is seen with near 100% penetrance.5 The reported prevalence of FHH is 1:10 000 to 1:100 000.4 The prevalence is probably underestimated because patients remain undiagnosed given the generally asymptomatic disease course and diagnosis is considered only after routine laboratory tests identify incidental hypercalcemia.
There are several etiologies for hypercalcemia, with primary hyperparathyroidism (PHPT) being the most common. During workup for PHPT, FHH should be considered in the differential diagnosis because their biochemical profiles overlap but are managed quite differently. Unlike PHPT, parathyroidectomy is ineffective for patients with FHH as persistent hypercalcemia is noted postoperatively.5,6 It is estimated that 10% to 23% of all “unsuccessful” parathyroidectomies are attributed to misdiagnosis of PHPT in patients with FHH.4,7 Herein, we present a patient who underwent parathyroidectomy for suspected PHPT but was later found to have a CASR variant of unknown significance (VUS) and family history consistent with FHH. The goals of highlighting this patient’s clinical case are twofold. One goal is to illustrate the nuances of diagnostic scrutiny that can differentiate potential FHH from PHPT because there is ongoing need to reiterate this process for the benefit of clinicians of all specialties who encounter patients with hypercalcemia. The second goal is to contribute relevant new knowledge in this field. This family’s pedigree suggests that the CASR variant in exon 3 c.392C>A (p.Ala110Asp) is a new pathogenic CASR sequence variation. Important details related to this discovery are reviewed here.
Case Report
A 35-year-old woman was referred to the Endocrine Surgery clinic for evaluation of “recurrent PHPT.” Before our evaluation, she had presented to a team of primary care physicians and endocrinologists with complaints of persistent fatigue; hypercalcemia was detected on screening laboratory analysis (calcium, 11.4 mg/dL; reference range [RR], 8.5-10.5 mg/dL). She was unaware of a personal history of hypercalcemia and denied other signs and symptoms associated with hypercalcemia (eg, cognitive dysfunction, anxiety, depression, gastrointestinal complaints, nephrolithiasis, pancreatitis, and low bone density or fractures). Her family history was significant for hypercalcemia in multiple relatives, including her mother, maternal uncle, and maternal aunt. Her mother underwent parathyroidectomy over 20 years prior with persistent postoperative hypercalcemia leading to genetic testing and identification of a CASR VUS. It is unclear whether the patient shared this historical information with her initial team of physicians and surgeons.
Further evaluation by her initial team included total serum vitamin D 25(OH) (40 ng/mL; RR, 30-85 ng/mL), intact parathyroid hormone (PTH, 31 pg/mL; RR, 10-65 pg/mL), and 24-hour urine calcium (117 mg/day; RR, 50-300 mg/day). A dual-energy x-ray absorptiometry scan of the lumbar spine, left total hip, left femoral neck, and left wrist revealed z-scores of −1.1, −0.5, −1.1, and −0.3 and bone mineral density values of 0.92, 0.88, 0.71, and 0.67 g/cm2, respectively. Neck ultrasound identified a hypoechoic structure in the region of the left inferior thyroid pole interpreted by radiologists as “possible parathyroid adenoma versus lymph node.” A subsequent technetium 99m sestamibi scan was nonlocalizing. Given the ultrasound findings in the setting of her hypercalcemia, she was referred for surgical evaluation for hypothesized PHPT. She underwent subtotal parathyroidectomy by the surgical team at the original institution with excision of left inferior (76 mg), right superior (42 mg), and right inferior (77 mg) parathyroid glands. The left superior gland appeared normal and remained in situ. Intraoperative PTH was reported to drop into the normal range, from 87 pg/mL before incision to 34 pg/mL at 20 minutes after excision of the glands. The pathology report described “enlarged parathyroids” with weights of each specimen as included earlier. Postoperatively, the patient’s fatigue did not improve, and 2 months later, she was referred to our tertiary care center for further evaluation because of concern for persistent PHPT and need for reoperative parathyroidectomy.
At the time of our evaluation, physical examination was unrevealing and showed a healthy-appearing young woman with a well-healed transverse cervical scar. No abnormal parathyroid glands were evident on surgeon-performed point-of-care ultrasound in clinic. Repeat biochemical testing showed levels of serum calcium, ionized calcium, and intact PTH of 10.2 mg/dL (RR, 8.5-10.5 mg/dL), 5.79 mg/dL (RR, 4.52-5.28 mg/dL), and 39 pg/mL (RR, 10-65 pg/mL), respectively, and, this time, undetectable 24-hour urine calcium. Additionally, total vitamin D 25(OH), PTH-related protein, phosphorus, magnesium, and serum protein electrophoresis were all normal. Computed tomography of the abdomen and pelvis was also obtained because she complained of intermittent abdominal and flank pain after surgery. This detected punctate bilateral signals (<2 mm) without hydronephrosis and was interpreted as nonobstructing nephrolithiasis, which was unlikely to be the etiology of her abdominal pain. Given her young age of presentation, significant family history of hypercalcemia with a CASR VUS, and low 24-hour urine calcium, she was referred to genetic counseling and underwent germline gene testing (Invitae Corporation) for 50 endocrine diseases and cancer susceptibility genes associated with hypercalcemia (Common Hereditary Cancers Panel https://www.invitae.com/en/physician/tests/01102/ and Hyperparathyroidism Panel https://www.invitae.com/en/physician/tests/01303/).
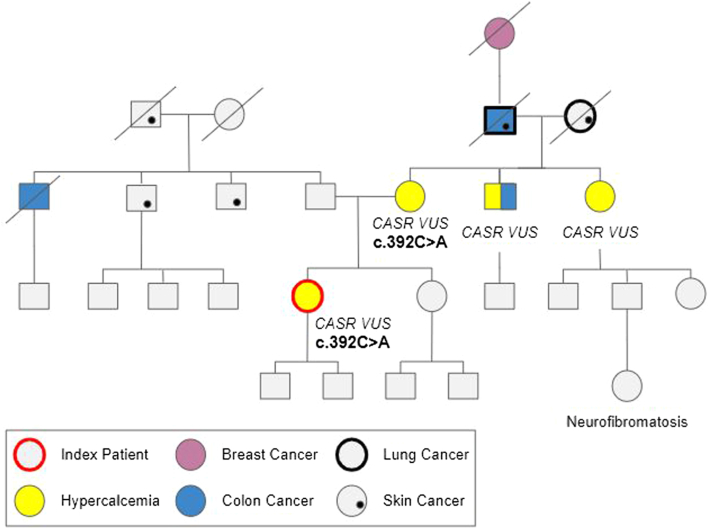
The patient was found to have a heterozygous CASR VUS in exon 3 c.392C>A (p.Ala110Asp). Records were obtained from her mother’s genetic testing originally performed several years prior by Athena Diagnostics, which revealed the same CASR VUS in exon 3 c.392C>A (p.Ala110Asp). The maternal uncle and maternal aunt had also been genetically tested before our patient’s presentation with both reporting CASR VUS; however, the report with the testing laboratory and specific sequence variation could not be obtained. Genetic testing was offered to all first- and second-degree members of the family and any relative with hypercalcemia, including confirmatory tests for all afflicted family members previously tested; however, none followed up for testing. Figure provides the patient’s family pedigree. No further surgical intervention was recommended to our patient because the authors were concerned that she actually manifested a diagnosis of FHH instead of PHPT. The patient complied with ongoing follow-up via the endocrinology team at our tertiary center. Biochemical evaluation 8 months after her parathyroidectomy showed the same pattern of laboratory results as she had prior to parathyroidectomy: serum calcium level mildly elevated at 10.6 mg/dL (RR, 8.5-10.5 mg/dL) and midnormal range of intact PTH at 32 pg/mL (RR, 10-65 pg/mL).
Fig.
Patient family pedigree. CASR = calcium-sensing receptor gene; VUS = variant of unknown significance.
Discussion
This case report focuses on a 35-year-old young woman with clinical findings that required thoughtful discrimination between FHH and PHPT, including unusual phenotypes of PHPT.8 When FHH was first described in 1966 by Jackson et al, 9 the cause was unknown, and diagnosis was challenging.10 Recognition of FHH occurred after failure of subtotal parathyroidectomy in patients found to have hypercalcemia without hypercalciuria, normal PTH, and multiple family members with hypercalcemia.6 The discovery of CASR in 199310 and that inactivating sequence variations in CASR result in FHH by Pollak et al11 now allows genetic confirmation of FHH diagnosis.
The most recent published consensus guidelines recommend preoperative 24-hour urine calcium to calculate the calcium creatinine clearance ratio (CCCR) as the first step in the algorithms differentiating FHH from PHPT and who should be genetically tested.12 There is wide acceptance within the literature that a CCCR of <0.01 portends a high risk of FHH, whereas that of >0.02 is generally indicative of PHPT.12, 13, 14, 15 CCCR in this algorithm is used as a screening tool to help select which patients should be referred for genetic testing. Genetic testing is recommended to confirm the diagnosis of FHH when a CCCR is <0.01 and rule out FHH when the CCCR is equivocal (0.01-0.02).12,14 Several studies have questioned these cutoffs given the degree of biochemical overlap between the 2 entities because there are patients with FHH who have a CCCR of >0.02 and patients with PHPT who have a CCCR of <0.01.13,15 Because of this, some groups have transitioned to use CCCR very selectively or essentially abandoned it altogether as a primary screening tool.16,17 A statistical predictive model “pro-FHH” has been developed for patients with hypercalcemia with normal PTH but has yet to be validated in a larger, heterogeneous population.18 This may become particularly useful in situations where clinicians are attempting to distinguish between FHH and atypical, rare variants of PHPT that have been described, such as normohormonal PHPT.8,19 Genetic testing should also be considered in patients, even with suspected PHPT, if they are young, have multigland disease (>2) or parathyroid carcinoma, have relatives with hypercalcemia, or demonstrate any clinical manifestations of syndromic/familial hyperparathyroidism.12 Genes that should be tested for include MEN1, CASR, AP2S1, GNA11, HRPT2, CDKN, RET, and PTH.12 Any of these etiologies could have been relevant to our patient; the CASR variant happened to be the abnormality that was detected.
Clinicians should also be cognizant that FHH can have atypical presentations that further obscure the correct diagnosis. While it was long thought that FHH was a “benign” or asymptomatic condition characterized by mild hypercalcemia, there have been case reports of severe and symptomatic hypercalcemia (even requiring parathyroidectomy), FHH with concurrent parathyroid adenoma causing PHPT, and FHH associated with pancreatitis or nephrolithiasis.1,3,10,20, 21, 22 These cases serve as reminders that the collective understanding of parathyroid disease is incomplete and to challenge the paradigm that FHH and PHPT always follow distinct patterns.
This specific case report illustrates several key points in the workup of patients referred for surgical management of PHPT, particularly in reoperative situations after “unsuccessful parathyroidectomy.” It is important to escape the temptation to remain committed to the referred diagnosis of the patient and start the assessment anew to confirm the diagnosis.23 This may require repeat biochemical evaluation over longer periods of time. Convincing biochemical diagnosis must be the solid ground on which the next decision-making step occurs. The American Association of Endocrine Surgeons released evidence-based recommendations in 2016 to assist clinicians with the optimal treatment of patients with PHPT and discuss the indications for parathyroidectomy.24 This is relevant both at initial and in reoperative situations. Even when discrete and noncontroversial indications, such as age of <50 years, osteoporosis, or nephrolithiasis, exist, these do not supersede the need to question whether clear diagnosis has been assured.
Situations where discordant or unexpected results are encountered should serve as an opportunity to pause and reconsider the diagnosis before proceeding. As exemplified in this case report, several aspects of the workup made us question the diagnosis of PHPT and ultimately recommend genetic testing. The patient had multiple suspicious factors for FHH, including her young age of presentation, strong family history of hypercalcemia (including known CASR VUS), discordant 24-hour urine calcium results, assessment of “multigland disease” at initial surgery, and atypical biochemical pattern with normal serum calcium, mildly elevated ionized calcium, and normal PTH levels. While she did have a normal serum calcium and PTH 6 weeks after surgery, repeat laboratory tests 8 months later demonstrated mild hypercalcemia with a normal PTH level–which is consistent with FHH. We posit that the transient normal calcium shortly after surgery was likely related to the fact she had a subtotal parathyroidectomy. Extrapolating the data of parathyroidectomy in infants with neonatal severe hyperparathyroidism, which is a severe form of FHH only seen in newborn infants, the treatment after failure of medical management is parathyroidectomy.25,26 Parathyroidectomy will never “cure” these infants who have CASR sequence variations; however, it will temporarily shift their calcium levels closer to the normal range. This is not a permanent phenomenon and often they require repeated future parathyroidectomy operations to maintain the status quo and prevent symptomatic hypercalcemia.25,26
A family history of hypercalcemia, regardless of a confirmed FHH diagnosis, has historically been and continues to be among the strongest predictors of underlying genetic or hereditary disorders. A patient’s young age at presentation is more typical of FHH because hypercalcemia is likely to be discovered in childhood or early adulthood. MEN1 is also a strong consideration, and either case requires genetic testing. In contrast, PHPT most often develops in late adulthood and in postmenopausal women.7 Unfortunately, the patient could not provide us with records of prior calcium levels to confirm long-standing hypercalcemia, which would have provided even stronger evidence to suggest FHH prior to genetic testing. Although unusual, low bone density, nephrolithiasis, and pancreatitis can occur with FHH, and it is unclear whether the relationship is causal or coincidental.1,3 The pathology findings were not convincing of true multigland hyperplasia: 1 normal parathyroid was observed, 1 “abnormal” gland had a normal weight of <60 mg, and the remaining 2 “abnormal” glands weighed <80 mg, which were only marginally enlarged.27 Referral for genetic testing early in the time course of the clinical evaluation can be tremendously helpful. Certainly, such referral prior to a first parathyroid surgery can avoid unnecessary procedures in the patient or their family members.
This patient’s overall clinical picture, complemented by her family’s pedigree, is most consistent with FHH and with the recognition that the CASR variant harbored in family members should be newly viewed as pathogenic. In the outreach to genetic counselors and genetic testing companies that accompanied her management after referral to our center, new knowledge was gained about the nuances of interpreting CASR sequence variation results. Specifically, this patient stimulated greater appreciation of the process of determining which sequence variations are benign, pathogenic, or VUS. The latter designation is often confusing to clinicians who may wonder what more exactly needs to be known for VUS to be reclassified from unknown significance to pathogenic or potentially pathogenic. The Invitae Corporation outlines their particular classification system (https://www.invitae.com/static/data/WhitePaper_Variant-Classification-Method.pdf) that adheres to recommendations from the American College of Medical Genetics and Genomics.28,29 Each genetic testing company publishes their unique adaptation of the American College of Medical Genetics and Genomics recommendations, and physicians can inquire about the details related to a specific patient or sequence variation directly with the company.
In this patient’s case, Invitae shared that the CASR VUS in exon 3 c.392C>A (p.Ala110Asp) achieved 1.5 of 5 points needed to be reclassified as a pathogenic sequence variation (4 points for likely pathogenic), based on the Sherloc 5-tier classification system.28, 29, 30 One point was awarded because CASR c.392C>A is a sequence variation absent from the general population. One-half point was awarded because Invitae proprietary algorithms predict that the area of the CaSR protein affected by this sequence variation is likely disruptive to function. Furthermore, the scientific advisory boards of each genetic testing company, including Invitae, can reclassify a sequence variation if clinical information can be provided directly from treating physicians and genetic counselors. Case reports in peer-reviewed published literature can also contribute to this process because they are queried periodically for new knowledge about sequence variations not submitted directly to companies. The collective effort of the medical community to share relevant clinical information about patients with FHH and CASR sequence variations has the potential to inform reclassification decisions and, thus, future patient care. Movement from the status of FHH diagnostic ambiguity to greater clarity and understanding is profoundly valuable to both patients, who seek closure on their health condition, and clinicians, who seek to advise the best course of treatment.
In summary, beyond emphasizing salient features of FHH and diagnostic challenges in line with what has previously been published, this case provides evidence of a novel missense sequence variation of the CASR causing FHH. Reporting newly discovered sequence variations with the context of a family’s medical history is important because it allows recognition and wider awareness of new pathologic CASR variants. This expands the registry of already known sequence variations and their associated clinical pathology. Genetic testing is more accessible and cost-effective now than ever before in contemporary approaches to personalized medicine. Genetic testing for CASR sequence variations should be embraced as a routine and complementary tool for clinical diagnosis of FHH, especially in nonclassical clinical presentations and as early as possible in the appropriate course of workup. Knowledge from such dedicated genetic evaluation potentially changes both medical and surgical managements.
Disclosure
The authors have no multiplicity of interest to disclose.
References
- 1.Lee J.Y., Shoback D.M. Familial hypocalciuric hypercalcemia and related disorders. Best Pract Res Clin Endocrinol Metab. 2018;32(5):609–619. doi: 10.1016/j.beem.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hovden S., Rejnmark L., Ladefoged S.A., Nissen P.H. AP2S1 and GNA11 mutations - not a common cause of familial hypocalciuric hypercalcemia. Eur J Endocrinol. 2017;176(2):177–185. doi: 10.1530/EJE-16-0842. [DOI] [PubMed] [Google Scholar]
- 3.Vargas-Poussou R., Mansour-Hendili L., Baron S., et al. Familial hypocalciuric hypercalcemia types 1 and 3 and primary hyperparathyroidism: similarities and differences. J Clin Endocrinol Metab. 2016;101(5):2185–2195. doi: 10.1210/jc.2015-3442. [DOI] [PubMed] [Google Scholar]
- 4.Christensen S.E., Nissen P.H., Vestergaard P., Mosekilde L. Familial hypocalciuric hypercalcaemia: a review. Curr Opin Endocrinol Diabetes Obes. 2011;18(6):359–370. doi: 10.1097/MED.0b013e32834c3c7c. [DOI] [PubMed] [Google Scholar]
- 5.Marx S.J., Spiegel A.M., Levine M.A., et al. Familial hypocalciuric hypercalcemia: the relation to primary parathyroid hyperplasia. N Engl J Med. 1982;307(7):416–426. doi: 10.1056/NEJM198208123070707. [DOI] [PubMed] [Google Scholar]
- 6.Marx S.J., Stock J.L., Attie M.F., et al. Familial hypocalciuric hypercalcemia: recognition among patients referred after unsuccessful parathyroid exploration. Ann Intern Med. 1980;92(3):351–356. doi: 10.7326/0003-4819-92-3-351. [DOI] [PubMed] [Google Scholar]
- 7.Bilezikian J.P. Primary hyperparathyroidism. J Clin Endocrinol Metab. 2018;103(11):3993–4004. doi: 10.1210/jc.2018-01225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Applewhite M.K., White M.G., Tseng J., et al. Normohormonal primary hyperparathyroidism is a distinct form of primary hyperparathyroidism. Surgery. 2017;161(1):62–69. doi: 10.1016/j.surg.2016.03.038. [DOI] [PubMed] [Google Scholar]
- 9.Jackson C.E., Boonstra C.E. Hereditary hypercalcemia and parathyroid hyperplasia without definite hyperparathyroidism. J Lab Clin Med. 1966;68:883. [Google Scholar]
- 10.Marx S.J., Goltzman D. Evolution of our understanding of the hyperparathyroid syndromes: a historical perspective. J Bone Miner Res. 2019;34(1):22–37. doi: 10.1002/jbmr.3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pollak M.R., Brown E.M., Chou Y.H., et al. Mutations in the human Ca(2+)-sensing receptor gene cause familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Cell. 1993;75(7):1297–1303. doi: 10.1016/0092-8674(93)90617-y. [DOI] [PubMed] [Google Scholar]
- 12.Eastell R., Brandi M.L., Costa A.G., D'Amour P., Shoback D.M., Thakker R.V. Diagnosis of asymptomatic primary hyperparathyroidism: proceedings of the Fourth International Workshop. J Clin Endocrinol Metab. 2014;99(10):3570–3579. doi: 10.1210/jc.2014-1414. [DOI] [PubMed] [Google Scholar]
- 13.Bhangu J.S., Selberherr A., Brammen L., Scheuba C., Riss P. Efficacy of calcium excretion and calcium/creatinine clearance ratio in the differential diagnosis of familial hypocalciuric hypercalcemia and primary hyperparathyroidism. Head Neck. 2019;41(5):1372–1378. doi: 10.1002/hed.25568. [DOI] [PubMed] [Google Scholar]
- 14.Christensen S.E., Nissen P.H., Vestergaard P., Heickendorff L., Brixen K., Mosekilde L. Discriminative power of three indices of renal calcium excretion for the distinction between familial hypocalciuric hypercalcaemia and primary hyperparathyroidism: a follow-up study on methods. Clin Endocrinol (Oxf) 2008;69(5):713–720. doi: 10.1111/j.1365-2265.2008.03259.x. [DOI] [PubMed] [Google Scholar]
- 15.Arshad M.F., McAllister J., Merchant A., et al. Urinary calcium indices in primary hyperparathyroidism (PHPT) and familial hypocalciuric hypercalcaemia (FHH): which test performs best? Postgrad Med J. 2021;97(1151):577–582. doi: 10.1136/postgradmedj-2020-137718. [DOI] [PubMed] [Google Scholar]
- 16.Li S.R., McCoy K.L., Levitt H.E., Kelley M.L., Carty S.E., Yip L. Is routine 24-hour urine calcium measurement useful during the evaluation of primary hyperparathyroidism? Surgery. 2022;171(1):17–22. doi: 10.1016/j.surg.2021.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore EC, Berber E, Jin J, Krishnamurthy V, Shin J, Siperstein A. Calcium creatinine clearance ratio is not helpful in differentiating primary hyperparathyroidism from familial herpercalcemic hypocalciuria: a study of 1000 patients. Preprint. Posted online October 5, 2018. Endocr Pract. https://doi.org/10.4158/EP-2018-0350 [DOI] [PubMed]
- 18.Bertocchio J.P., Tafflet M., Koumakis E., et al. Pro-FHH: a risk equation to facilitate the diagnosis of parathyroid-related hypercalcemia. J Clin Endocrinol Metab. 2018;103(7):2534–2542. doi: 10.1210/jc.2017-02773. [DOI] [PubMed] [Google Scholar]
- 19.Wallace L.B., Parikh R.T., Ross L.V., et al. The phenotype of primary hyperparathyroidism with normal parathyroid hormone levels: how low can parathyroid hormone go? Surgery. 2011;150(6):1102–1112. doi: 10.1016/j.surg.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 20.Kurian R., Madegowda Chandrashekar G., Antony M.A., Chandra L., Kant R. Severe symptomatic hypercalcemia in a patient with familial hypocalciuric hypercalcemia. Cureus. 2021;13(11):e20057. doi: 10.7759/cureus.20057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veldeman L., Robbrecht S., Breckpot J., Weynand B., Decallonne B. A case of a heterozygous inactivating CASR variant with adult-onset symptomatic hypercalcemia requiring extensive surgery. Calcif Tissue Int. 2020;107(1):104–108. doi: 10.1007/s00223-020-00693-4. [DOI] [PubMed] [Google Scholar]
- 22.Marstrand S.D., Tofteng C.L., Jarløv A., Borgwardt L., Schwarz P. Concomitant familial hypocalciuric hypercalcemia and single parathyroid adenoma: a case report. J Med Case Rep. 2021;15(1):471. doi: 10.1186/s13256-021-03051-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shin J.J., Milas M., Mitchell J., Berber E., Ross L., Siperstein A. Impact of localization studies and clinical scenario in patients with hyperparathyroidism being evaluated for reoperative neck surgery. Arch Surg. 2011;146(12):1397–1403. doi: 10.1001/archsurg.2011.837. [DOI] [PubMed] [Google Scholar]
- 24.Wilhelm S.M., Wang T.S., Ruan D.T., et al. The American Association of Endocrine Surgeons guidelines for definitive management of primary hyperparathyroidism. JAMA Surg. 2016;151(10):959–968. doi: 10.1001/jamasurg.2016.2310. [DOI] [PubMed] [Google Scholar]
- 25.Murphy H., Patrick J., Báez-Irizarry E., et al. Neonatal severe hyperparathyroidism caused by homozygous mutation in CASR: a rare cause of life-threatening hypercalcemia. Eur J Med Genet. 2016;59(4):227–231. doi: 10.1016/j.ejmg.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Al-Shanafey S., Al-Hosaini R., Al-Ashwal A., Al-Rabeeah A. Surgical management of severe neonatal hyperparathyroidism: one center's experience. J Pediatr Surg. 2010;45(4):714–717. doi: 10.1016/j.jpedsurg.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 27.Yao K., Singer F.R., Roth S.I., Sassoon A., Ye C., Giuliano A.E. Weight of normal parathyroid glands in patients with parathyroid adenomas. J Clin Endocrinol Metab. 2004;89(7):3208–3213. doi: 10.1210/jc.2003-031184. [DOI] [PubMed] [Google Scholar]
- 28.Richards C.S., Bale S., Bellissimo D.B., et al. ACMG recommendations for standards for interpretation and reporting of sequence variations: revisions 2007. Genet Med. 2008;10(4):294–300. doi: 10.1097/GIM.0b013e31816b5cae. [DOI] [PubMed] [Google Scholar]
- 29.Duzkale H., Shen J., McLaughlin H., et al. A systematic approach to assessing the clinical significance of genetic variants. Clin Genet. 2013;84(5):453–463. doi: 10.1111/cge.12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nykamp K., Anderson M., Powers M., et al. Sherloc: a comprehensive refinement of the ACMG-AMP variant classification criteria. Genet Med. 2017;19(10):1105–1117. doi: 10.1038/gim.2017.37. [DOI] [PMC free article] [PubMed] [Google Scholar]