Abstract
The wound healing process is usually susceptible to different bacterial infections due to the complex physiological environment, which significantly impairs wound healing. The topical application of antibiotics is not desirable for wound healing because the excessive use of antibiotics might cause bacteria to develop resistance and even the production of super bacteria, posing significant harm to human well-being. Wound dressings based on adhesive, biocompatible, and multi-functional hydrogels with natural antibacterial agents have been widely recognized as effective wound treatments. Hydrogels, which are three-dimensional (3D) polymer networks cross-linked through physical interactions or covalent bonds, are promising for topical antibacterial applications because of their excellent adhesion, antibacterial properties, and biocompatibility. To further improve the healing performance of hydrogels, various modification methods have been developed with superior biocompatibility, antibacterial activity, mechanical properties, and wound repair capabilities. This review summarizes hundreds of typical studies on various ingredients, preparation methods, antibacterial mechanisms, and internal antibacterial factors to understand adhesive hydrogels with natural antibacterial agents for wound dressings. Additionally, we provide prospects for adhesive and antibacterial hydrogels in biomedical applications and clinical research.
Keywords: Adhesive hydrogels, Antibacterial agents, Wound dressings, Wound healing
Graphical abstract
1. Introduction
Secondary injuries and wound infections account for a substantial proportion of human deaths. In contrast, wound protection and targeted treatments can help avoid secondary injuries and resist pathogen invasion [[1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12]]. Among the wound treatments, dressing is a relatively simple and safe treatment, so it has been widely studied and used [[13], [14], [15], [16], [17], [18], [19]]. Due to the fragility and tissue fluid exudation of the wound, the dressing should have favorable mechanical properties and absorption. In addition, the wound needs a barrier that prevents physical interference and pathogen invasion for proper healing, so applicable tissue adhesion and antibacterial properties are necessary properties of the dressing and also the basic requirements that should be met [[20], [21], [22], [23]]. Therefore, designing a wound dressing with biocompatibility and antibacterial performance has recently become a hot topic in modern medicine [10,[24], [25], [26], [27]]. Among the recently developed dressings, hydrogels with great adhesion, permeability, antibacterial properties, and biocompatibility are considered the most suitable wound dressings [9,13,24,28,29]. Hydrogel is a kind of hydrophilic gel with a 3D network structure that can rapidly expand and hold a large volume of water in a swelling state without dissolving [30]. First, by taking advantage of their porous structure and appropriate swelling ratio, hydrogels allow the presence of oxygen and can absorb exudates, providing a relatively humid environment for wound healing [[31], [32], [33]]. Second, hydrogels with improved adhesiveness, which can be achieved by chemical modification and introduction of functional structures and groups, such as catechol, hydrogen bonds and amide bonds, can promote gas exchange and inhibit the proliferation of anaerobic bacteria [27,[34], [35], [36], [37], [38], [39], [40], [41]]. Unlike the traditional wound dressings (such as gauze, and cotton), hydrogel dressings loaded with bioactive molecules exhibit ideal biological activity. The bioactive molecules can be slowly released from the hydrogel matrix, which can promote the wound healing process [[42], [43], [44]].
The ideal wound dressings should have multiple forms of interaction with wound surfaces, such as adsorption of exudate and gas exchange, to create a biocompatible environment for wound healing [45,46]. Hydrogels have become increasingly attractive due to their superior permeability and biocompatibility. In addition, the mechanical properties of the hydrogels play a vital role in wound healing. On the one hand, good mechanical strength can ensure the stable realization of the internal functions. On the other hand, appropriate adhesion between the wound dressing and tissue is essential to avoid the easy dropping of dressings and secondary injuries [13,[47], [48], [49]]. Furthermore, the hydrogels can withstand the mechanical deformations from body movements, maintaining stable connections between wounds and themselves. Therefore, it is necessary to develop suitable adhesive hydrogels. Under normal physiological conditions, tissue injury initiates the acute wound healing process, but when the underlying pathological mechanism or bacterial invasion interferes with the healing process, it is interrupted, hindering the wound healing process [[50], [51], [52], [53]]. Therefore, pathogen isolation is essential for wound treatment.
Adhesion is the premise for hydrogel dressings to function normally in the human body, and an antibacterial property is a key for hydrogel dressings to promote wound healing [[54], [55], [56]]. Hydrogel wound dressings with antibacterial properties must be properly attached to biological tissues to ensure smooth wound healing. Therefore, research and development of antibacterial agents loaded hydrogels with high adhesion, biocompatibility, and good mechanical properties could effectively solve this problem (Fig. 1) [34,35,38,40,51,52,[57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81]]. This review aims to elaborate on various adhesion types and mechanisms and antibacterial factors and mechanisms, which would benefit researchers in understanding the development of hydrogel wound dressings with adhesion and antibacterial functions for biomedical applications.
Fig. 1.
The development of antibacterial adhesive hydrogels over the past few years [34,35,38,40,51,52,[57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81]].
2. Adhesive hydrogels
The adhesive property of hydrogel wound dressings is an indispensable aspect that has drawn the attention of researchers [46,54,83]. An ideal adhesive hydrogel wound dressing requires good adhesion to human tissues and can avoid secondary damage. Only under this premise can hydrogel dressings work stably in the human body. Adhesion generally refers to bonding the same or different material surfaces, while bio-adhesion refers to any combination of surfaces of living organisms [79]. Bio-adhesion in pharmacies is generally used to describe the adhesion between polymers (both synthetic and natural) and soft tissues (such as membranes of the gastrointestinal tract, mouth, and skin). Several mechanisms have been investigated to explain the adhesion process [83,84], as listed in Table 1. The mechanism is a detailed description of a reaction or phenomenon, which explains the adhesion results and phenomena by analyzing the morphology and structure of the adhesion process. According to the adhesion mechanism, adhesive hydrogel wound dressings can be divided into physical and chemical effects and other mechanisms, as discussed in this section. The advantages and disadvantages of these adhesives are listed in Table 2.
Table 1.
Comparisons of different adhesive mechanisms for hydrogels.
| Hydrogel | Adhesion type | Features | Adhesion strength (kPa) | Ref. |
|---|---|---|---|---|
| QCS/PAM | PDA | Strong and repeatable tissue adhesion, good tensile property (1154%), antibacterial ability | 27.2 | [84] |
| PAA/CS | TA | Skin affinity, highly stretchable, self-recovery capacity, antifatigue property | 20 | [79] |
| PVA/Graphene | PDA | Good tensile strength (1.174 MPa), excellent elongation (331%), recyclable, antibacterial ability | 1.735 | [81] |
| CS/Gelatin | DA | Sustained drug release, anti-infection capacity, antibacterial ability | >20 | [86] |
| OHA | DA | Tissue adhesion, self-healing property | 15.2 | [49] |
| Gelatin | DA | No cytotoxicity, tunability | 43 | [34] |
| Gelatin | TA | Self-healing property | 36 | [78] |
| PAA | TA | Repeatable, conductive, self-solidified, antibacterial ability | 25 | [87] |
| PEG | Amidation | Antibacterial ability, controllable gelation time | 20 | [27] |
| Gelatin | Amidation | Antibacterial ability, biocompatibility | 4.8–56 | [88] |
| TPU/PAAm | Hydrogen bonding | Self-healing property, compliance | 9.46 | [89] |
| PLS/CS/RSF | Imine linkage | Biodegradability, biocompatibility, low immunogenicity | 70 | [90] |
| NB/CMC/CMC | Imine linkage | Biocompatibility, biodegradability, antibacterial ability | 56.85–97.65 | [43] |
Table 2.
Advantages and disadvantages of adhesive.
| Adhesion mechanism | Advantages | Disadvantages |
|---|---|---|
| Catechol-Quinone Balance | Durable adhesiveness: hemostasis (seal to stop bleeding, chitosan); antibacterial (EPL, chitosan); antioxidant | Toxic oxidant; not easy to store |
| Hydrogen Bond | Reversible adhesion and not affected by the surface chemistry | Limited adhesion |
| Amide Bond | Injectable; in situ glue; hemostasis | Limited adhesion |
| Imine Bond | Injectable; in situ glue; hemostasis | Limited adhesion |
| Topology | Wet tissue adhesion: act on any wet material when triggered by environmental stimuli | Environmental irritation may be harmful |
| Static Electricity | Wet adhesion; hemostasis (BIL) | Positive charge may affect cells |
2.1. Physical interactions induced adhesion
The basic principle of physical adhesion is the surface bonding between two substances through physical interactions [48,49]. Physical adhesion includes catechol-quinone adhesion and hydrogen bonds adhesion and both of them are reversible. The catechol-quinone adhesion is usually durable and stable; the hydrogen bonding adhesion can be strong or weak, depending on the electronegativity of certain atoms in the molecule [27,38,43]. We introduce their applications in this section by comparing and discussing them based on specific research.
2.1.1. Adhesive hydrogels based on catechol-quinone balance
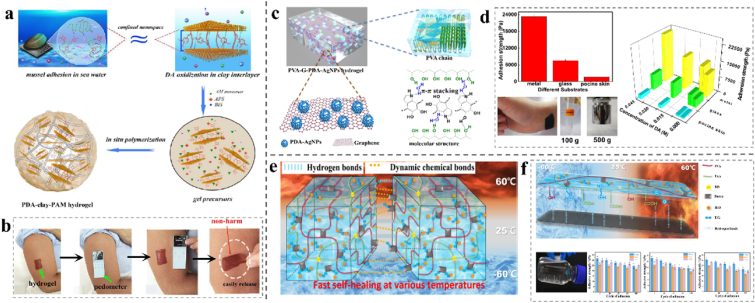
Dopamine (DA) contains numerous catechol groups, recognized as a vital factor for rapid and robust wet tissue adhesion [85,86]. Nanomechanical tests illustrated that mussel foot proteins with lysine cations can “clean” salts and hydrophobic residues on the substrate surfaces, which can destroy the barrier of the hydration layer and provide suitable conditions for catechol adhesion [38]. In addition, it has been documented that when DA approaches cationic lysine or is incorporated with polyampholytic peptides, dehydration would increase [82], which would offer DA stronger underwater cohesion. The results showed that the adhesive hydrogel wound dressings had excellent waterproofing performance. Typically, DA is grafted onto the main chain to improve the adhesiveness of the hydrogel. Gowda A. H.J. prepared a gelatin-dopamine conjugate (Gel-DA) hydrogel via chemical cross-linking using sodium periodate. The adhesion property is related to the DA content, and there is a maximum value at which the 46% substitution formula has a better adhesion property of approximately 43 kPa than the other formulations. No cytotoxicity was observed [34]. Therefore, the biosafety of this hydrogel as a wound dressing is outstanding. Pham et al. reported gelatin polyethylene glycol-dopamine (AgNPs-gel-PEG-DA) hydrogels, which improved their adhesiveness by grafting catechol groups onto the main gelatin chain. Simultaneously, hydrogels have excellent antibacterial properties against gram-positive and gram-negative bacteria [[90], [91]]. This type of hydrogel simultaneously has good adhesion and antibacterial effects and is a type of hydrogel wound dressing with promising applications. Han et al. developed a PDA-clay-polyacrylamide (PAM) hydrogel with strong adhesion and toughness using a two-step procedure. As shown in Fig. 2a and b, DA is inserted into clay nanosheets and partially oxidized between the layers, giving rise to free catechol groups in the PDA-inserted clay nanosheets. Acrylamide monomers are then added and polymerized in situ to form a hydrogel, presenting repeatable and durable adhesion. It can adhere directly to the human skin without triggering an inflammatory response and was easily removed without causing any harm. This free-standing, adhesive, tough, and biocompatible hydrogel may be more suitable for surgical applications in comparison to adhesives that involve gelation and additional agents in situ [38]. Chen et al. added PDA-intercalated silicate nanosheets to a chitosan/gelatin hydrogel, and the hydrogel with optimal ratio of DA exhibits good bonding properties to various substrates, and the bonding strength to pigskin can reach more than 20 kPa [86]; thus, it can be used as an extensive hydrogel binding dressing. A series of multi-functional chitosan-based double-crosslinked hydrogels were prepared by quaternized chitosan (QCS) and polyacrylamide (PAM), using PDA as a novel connecting bridge in Chen's research to improve the repeatability of adhesion. The best mechanical properties of the hydrogels can be obtained when the components of QCS = 12% and DA = 0.4 wt%, which pertains to strength and repeatability of tissue adhesion (27.2 kPa) and suitable tensile property (1154%). The obtained hydrogel killed 99% of Staphylococcus aureus (S. aureus) and Escherichia coli (E. coli) cells [85]. These characteristics indicate that it is a repeatable adhesive hydrogel with superior antibacterial properties. Furthermore, as shown in Fig. 2c and d, a dynamic supramolecule hydrogel composed of polyvinyl alcohol (PVA) graphene (G) PDA AgNPs with biocompatible, conductive, and self-adhesive layered network structures was prepared. The tensile strength reached 1.174 MPa, and the elongation was 331% [81]. Biocompatibility determines whether the hydrogel can be applied to wounds. Reactions between different materials are mainly manifested in the changes in the physical and chemical properties of the materials.
Fig. 2.
(a) Design strategy for the preparation of PDA-clay-PAM hydrogel. (b) A piece of hydrogel directly adhered to a human arm and a pedometer was attached to the hydrogel, then the pedometer was detached from the arm after a movement and the hydrogel was easily peeled off from the skin without causing any harm or allergies and no residue was observed. Reproduced with permission [38]. Copyright 2017, American Chemical Society. (c) Schematic diagram of the preparation of multifunctional PVA-G-PDA-AgNPs composite hydrogel. (d) Adhesive strength of the PVA-G-PDA-AgNPs hydrogels on various substrates and the effect of concentration of DA on adhesion strength to different substrates. Reproduced with permission [88]. Copyright 2020, American Chemical Society. (e) Mechanisms of self-healing behavior of oxidizing polyacrylic acid-polyvinyl alcohol-borax. f) Mechanisms of the reversible adhesion and repeatable adhesive strength values of O-PAA-PVA-B to multiple substrates. Reproduced with permission [95]. Copyright 2020, American Chemical Society.
Tannic acid (TA) is a kind of natural, water-soluble plant polyphenol. It is widely used in the biomedical field owing to its antioxidant properties, good adhesion, and low cost. TA contains three catechol groups and provides multiple active sites for surface coating. Furthermore, TA can form a persistent phenol-quinone equilibrium in hydrogels, thus achieving a long-term and repeatable adhesion effect [78]. Therefore, it has attracted the attention of many researchers for its application in adhesion hydrogel wound dressings [35,40,48,79,80]. In various methods, coating TA onto materials is considered as an effective approach [78,80,83]. Hydrogels with self-healing and adhesion properties can be obtained by adjusting the amount of sodium periodate. Based on clam chemical principles, Du et al. developed a new hydrogel dressing consisting of polyethylene glycol diacrylate/quaternized chitosan/tannic acid (PEGDA/QCS/TA). It has good antibacterial activity against S. aureus and E. coli and excellent dry/wet adhesion ability against various substrates [35], making it suitable for wound dressings in dry/wet conditions.
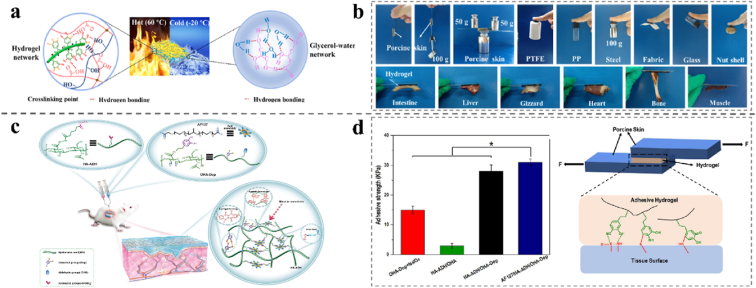
Additionally, a succession of plant-inspired adhesive hydrogels was developed based on Ag/tannic acid-cellulose nanofibers (Ag/TA-CNF) induced reversible quinone/catechol reactions. The dynamic redox system, generating catechol groups inside the hydrogel constantly endows the hydrogels with repeatable adhesion. In addition, the hydrogel sustains high adhesion after storage at extreme temperatures for 30 days. This study may inspire researchers to find new approaches for developing reusable and long-lasting adhesive hydrogels based on dynamic catechol chemistry (Fig. 3a and b) [92]. Jia et al. prepared a conductive and self-solidified adhesive antibacterial biosensor hydrogel by in situ reductions of AgNPs chelated with TA and catalyzed by nano-enzymes. In other words, nano-enzymes retain rich phenolic hydroxyl groups and maintain the dynamic equilibrium of phenol-quinone, which makes hydrogels self-adhesive and can repeatedly adhere to different surfaces for a long time [87].
Fig. 3.
(a) Covalent and non-covalent bonds in the hydrogel network and the strong hydrogen bonds between water and glycerol firmly locked moisture in the hydrogel. (b) The adhesion of the hydrogel to various materials. Reproduced with permission [97]. Copyright 2021, Elsevier. (c) Illustration of the preparation of the injectable and multi cross-linked double-network AF127/HA-ADH/OHA-Dop hydrogel and the application in full-thickness skin wound healing. (d) Average lap shear strength of different hydrogels to porcine skin tissues (n = 3) and schematic illustration of the hydrogel's adhesion to biological tissues involving covalent bonds to different nucleophiles and the generation of hydrogen bonds. Reproduced with permission [49]. Copyright 2020, American Chemical Society.
2.1.2. Adhesive hydrogels based on hydrogen bonds
Hydrogels have been widely studied because of their excellent biocompatibility as 3D network polymers with high water content. However, owing to their poor mechanical properties, the applications of hydrogels are limited [46,93]. Although traditional chemically crosslinked hydrogels have good mechanical properties, the irreversible destruction of chemical bonds renders the gel network unrecoverable, limiting the practical application of hydrogels [25,28]. Therefore, improving the mechanical properties of hydrogels and crosslinking by reversible physical actions are two important directions of hydrogel research. Hydrogen bond has developed since it was first proposed. It refers to the interaction between adjacent hydrogen atoms and the bond between electronegative atoms with lone pair electrons in another molecule. The tissue adhesiveness of the hydrogels was derived from the hydrogen bonds formed between the hydroxyl groups in the polymer chains and the amino groups of the tissue [44,94]. The quadruple hydrogen bond system can form hydrogen bonds with a strong dimerization index. The synthesis of quadruple hydrogen bond system units is facile, and raw materials are easy to obtain.
Many alcohols and polysaccharides contain rich hydroxyl groups, and the large number of hydrogen bonds between the molecules give the hydrogel repeatable adhesion properties. Therefore, they are often used to improve the adhesion of hydrogels. In 2021, Mao et al. developed a series of starch-based gel-point adhesive hydrogels with viscoelastic properties. The tissue adhesion of starch hydrogels was studied and the results showed great adhesiveness on porcine skin [44]. Based on this, Su et al. proposed a reversible adhesive hydrogel consisted of polyvinyl alcohol, polyacrylic acid, ethylene glycol, borax, and water. The typical mechanism of hydrogen bond interactions in the hydrogels is demonstrated in Fig. 2e and f. This organ gel can repeatedly adhere to diversified substrates, including glass, rubber, and metal, containing abundant and active adhesive functional groups with an adhesive strength greater than 18 kPa [95]. Novel 3D-bayrice-like microspheres with dynamic non-covalent bonds were constructed by an in situ self-assembly process to realize a rigid, adhesive, fatigue-resistant, hydrogel with waxy corn starch/polyvinyl alcohol double-crosslinked framework. The hydrogel can successfully adhere to both soft and hard substrates, such as skin, plastic, gauze, glass, and metal [94]. Han et al. prepared a gelatin-based hydrogel by simply mixing 2, 3, 4-trihydroxy benzaldehyde (THB) and combining a small number of iron ions with a gelatin solution through a Schiff base reaction. It exhibited strong adhesion to various materials due to the formation of hydrogen bonding, and pyrogallol partly imparted an intense antibacterial activity to the hydrogel [96]. Besides, Yu et al. constructed a novel supramolecular poly(N-isopropylacrylamide) hybrid hydrogel dressing that combined adhesion with thermal contraction to achieve noninvasive wound closure. The hydrogel is composed of quaternized chitosan-graft-β-cyclodextrin, adenine, and polypyrrole nanotubes via host-guest interaction and hydrogen bonds. This skin wound dressing, demonstrates thermal contraction of 47% remaining in the area after 2 h at 37 °C and tissue adhesion of 5.74 kPa and photothermal/intrinsic antibacterial activity (higher than 99% killing ratio within 5 min after irradiation), achieving contractive and protective characteristics while promoting healing [97].
2.2. Chemical interactions induced adhesion
Chemical adhesion is the surface bonding between two substances based on chemical reactions that produce new chemical bonds or structures, including amide bonds and imine bonds [27,98,99]. Both amide and imine bonds are covalent bonds with good stability suitable for alkaline and neutral environments. The following section provides a specific introduction of these adhesive hydrogels.
2.2.1. Adhesive hydrogels based on amide bonds
Amidation refers to the reaction in which an amide group replaces a hydrogen atom on the amino group. Injectable hydrogels initiated by amidation reactions have the inherent ability to covalently bind to the interface of biological tissues and are superior to other chemically crosslinked hydrogels and currently developed tissue adhesives [90,[100], [101], [102]]. A Chinese medicine (Borax) was proposed to regulate the gelation time in the amidation trigger system. Deprotonation of the amino group can be accelerated in the initial alkaline buffering environment provided by borax. Using the tissue adhesion model of polyethylene glycol-lysozyme (PEG-LZM), the gelation rate can be adjusted as the concentration of borax changes, retaining the same physical properties. The adhesion strength of the PEG-LZM/Borax hydrogels on pig skin was measured by stretching and the result was approximately 20 kPa. Additionally, the biological activity of borax can improve its antibacterial ability [27]. It is an adhesive hydrogel dressing with an adjustable gel time and antibacterial properties, which may be used for minimally invasive, gastric perforation wound closure. Khalil et al. developed an antibacterial and bio-adhesive hydrogel with ciprofloxacin (CPX) micelles incorporated into the GelCORE formulation. The CPX-loaded GelCORE hydrogels exhibited high adhesion to the corneal tissue, making them suitable for the closure of eye injuries [103]. Hou et al. developed a nano-titanium dioxide (N TiO2) mixed with a polyurethane/polyacrylamide (PU/PAA) multifunctional hydrogel that has excellent adhesion and self-healing properties and can closely adhere to any 3D curved wound surface, which may be applied to joint wounds [89].
2.2.2. Adhesive hydrogels based on imine bonds
The difference between an amide bond and an imine bond is that an amide bond has only one carbonyl group attached to its nitrogen atom; while, an imine has two attached carboxyl groups, making it structurally distinguishable [101]. Wang et al. designed a chitosan/aldehyde-chitosan (CS/ACS) hydrogel with an adjustable double-layer structure with adhesion, self-healing, and antibacterial properties in the different layers. After cross-linking with phytic acid (PA), the mechanical properties of the CS/ACS/PA hydrogels were enhanced. A dynamic covalent imine linkage formed by amino and aldehyde groups endowed the hydrogel with self-healing, antibacterial and adhesive properties, making it a potential candidate for biomedical applications [104]. Regenerated silk fibroin (RSF) and polylysine-modified chitosan (PLS-CS) were used as the raw materials, and a composite biological adhesive (PLS-CS/RSF) was prepared by crosslinking with calcium ions. PLS-CS/RSF shows superior adhesive properties to various substrates, with an adhesion strength of up to 70 kPa. Bacteriostatic rates of E. coli and S. aureus were 100%. This hydrogel can inhibit the secretion of inflammatory factor, tumor necrosis factor, stimulating vascular factor, and α-smooth muscle actin, as well as release vascular growth factor and promote angiogenesis during the process of wound healing, which are suitable characteristics for full thickness wound closure [90]. Jia et al. prepared a series of hydrogels using poly (3-dimethyl (methacryloyloxyethyl) ammonium propane sulfonate) -co-poly (methacryloyl amidoadenine) (PDMAPS-co-PMA-Ade) and CS. Hydrogel wound dressing can efficiently adhere to the skin surrounding the wound and facilitate antibacterial therapy and wound healing [101]. Ma et al. a photoresponsive chitosan hydrogel (NB-CMC/CMC hydrogel) based on in situ imines crosslinking was prepared as an emergency wound dressing. The o-nitrobenzene in modified carboxymethyl chitosan (CMCS) is transformed into o-nitrobenzaldehyde under ultraviolet irradiation and crosslinked with the amino groups on the tissue surface so that LBA has excellent tissue adhesive and antibacterial properties [43]. Tang et al. designed a biocompatible hydrogel composed of polysaccharides and polyacrylamide, with good antibacterial activity and tissue adhesion. The alginate/chitosan hydrogels were covalently cross-linked with the tissue and improved adhesion to the tissue surface. The addition of polyacrylamide improved the mechanical properties of the hydrogels [105]. Du et al. developed a new chitosan-based hydrogel adhesive synthesized using caffeic acid-modified chitosan as the primary raw material. The interfacial adhesive force makes the adhesives adhere strongly to the tissue surface, and the cohesive force maintains the stability of the adhesives by withstanding the mechanical forces applied by the surrounding tissues [106].
2.3. Other adhesive mechanisms
2.3.1. Adhesive hydrogels based on topological adhesion
Among the reported preparations of hydrogels with adhesive properties, direct bonding between the two polymer networks is the most widely used [55]. However, this method requires modification of the corresponding functional groups on the hydrogel polymer molecular chain, and the resulting adhesion is usually weak. Topologically bonding hydrogels have attracted considerable attention because of their excellent adhesion properties. Topological adhesion refers to the diffusion of a polymer chain into a pre-existing elastomer and in situ crosslinking to form topological entanglement, resulting in the bonding of the two polymers [55]. The first polymer network is formed when a composite polymer material is prepared via topological adhesion. Then, the precursor of the second network (including the monomer, crosslinking agent, and initiator) is diffused into the former and crosslinked in situ.
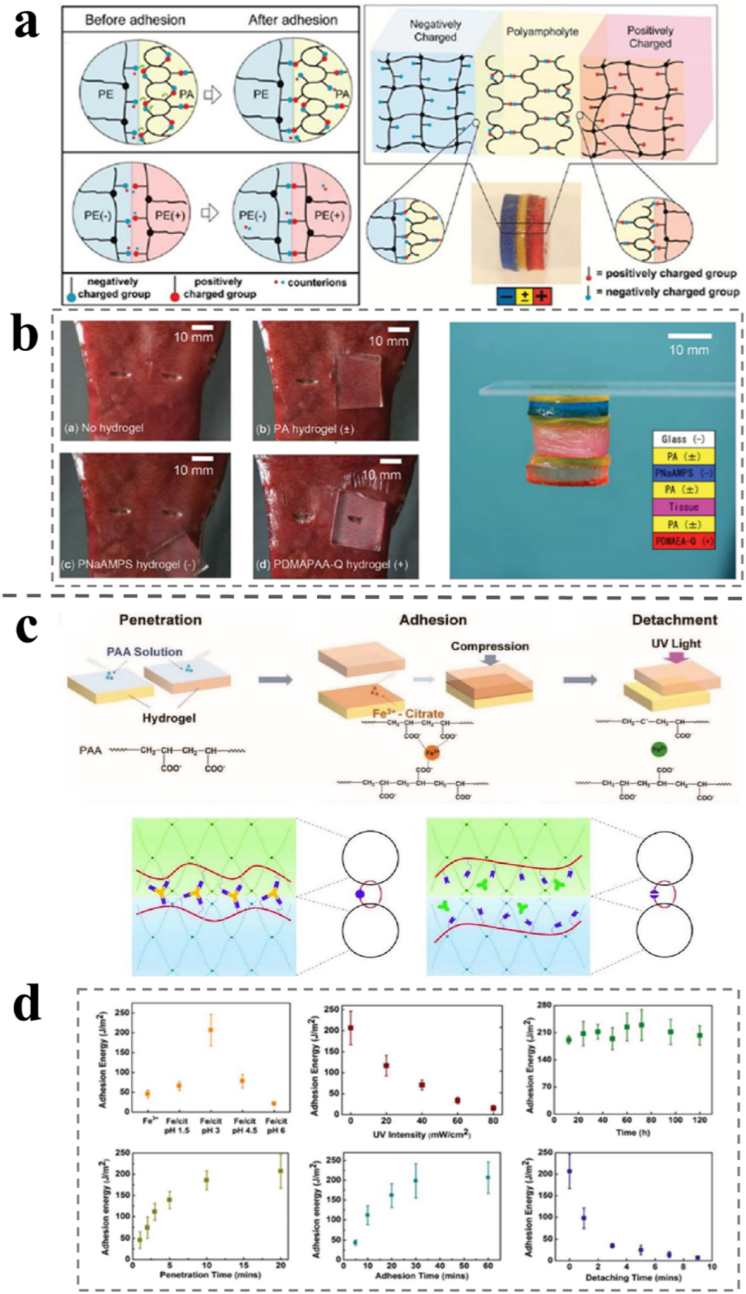
The topological adhesion process requires a higher composition of hydrogels, and the bond is relatively firm. Yu et al. used this method to prepare hydrogel-lubricated medical catheters [99]. First, the hydrophobic substrate was pre-treated with an organic solvent containing a 10 wt% hydrophobic initiator to swell it. Then, it was immersed in the pre-gel solution containing the monomer, hydrophilic initiator, and crosslinking agents before the final monomer crosslinking. The hydrophobic initiator connects the hydrogel network to the base polymer chain as a grafting agent. Simultaneously, it can also reflect excess oxygen and prevent it from inhibiting polymerization. The thickness of the hydrogel coating prepared in this manner was approximately 5–25 μm, the binding was firm, and the coating was uniform. In this case, another polymer chain must be diffused into two base polymer networks. The third complex network was obtained by in situ crosslinking, which was topologically entangled with the other two networks. The third polymer network sutured two polymer networks together, acting as molecular sutures. Gao et al. reported a photoresponsive reversible hydrogel bonding method, as shown in Fig. 4c and d [107]. An aqueous solution containing a polyacrylic acid (PAA) molecular chain is painted between the two hydrogel layer of polyacrylamide (PAM). After a certain time, the PAA chain diffused into the PAM hydrogel to a certain depth. The two hydrogels detach easily when the bonded hydrogel is irradiated with ultraviolet light, the reduction of Fe3+ to Fe2+ cannot coordinate with the carboxyl group, and the PAA network is de-crosslinked to complete the debonding. The adhesion energies of PAM hydrogels effected by UV light and natural light demonstrates that the separation can be incurred as required by certain irritation and avoided unnecessary loss of adhesion.
Fig. 4.
(a) Schematic diagram of self-adjustable and photo-reversible topological adhesion of a PA hydrogel to a charged hydrogel. (b) Adhesion behaviors of hydrogels with different charges to the surface of a piece of pork liver tissue and adhesion of the PA hydrogel to biological tissues and hydrogels. Reproduced with permission [107]. Copyright 2019, Wiley-VCH. (c) Schematic diagram of the adhesion mechanism of amphoteric polyelectrolyte (PA) hydrogels. (d) Adhesion energies at different pH, UV intensities, time, penetration time, adhesion time, and detaching time. Reproduced with permission [110]. Copyright 2015, Wiley-VCH.
2.3.2. Adhesive hydrogels based on static electricity
Adjacent cationic amino acids and aromatic compounds can induce protein adhesion in the negative lipid bilayer via electrostatic interactions. Bacteria are ubiquitous in the natural environment and can attach to almost any surface through self-regulation of the extracellular polymer matrix (EPM), regardless of the physical and chemical properties of the material surface [64,108]. EPM redistributes the surface charge groups to provide sufficient interaction sites for adsorption according to substrate properties [109]. Duan et al. prepared an intelligent bilayer hydrogel by electrostatic adhesion of a chitosan (CS) hydrogel and cellulose/carboxymethyl cellulose (C/CMC) according to the above principles of hydrogel [110]. At the interface of the two-layer hydrogel, close adhesion was formed between the NH3+ of the CS layer and the -COO- of the C/CMC layer by strong electrostatic attraction. The adhesion force between CS and C/CMC hydrogels measured by the lap shear test was approximately 20 kPa, which is much higher than that between two different hydrogels (two CS layers or two C/CMC layers). It can be used to prepare multilayer hydrogels with various functions.
Recently, it has been reported that adjacent cationic amino acids and aromatics can form a protein adhesive in a negative electrical lipid bilayer through electrostatic interactions [111,112]. Therefore, it is possible to prepare adhesive hydrogels with negatively charged surfaces by constructing a cationic-aromatic sequence. Fan et al. prepared a series of alternating cation-aryl copolymerization hydrogels by conventional free-radical polymerization in one pot (poly(cation-adj-π)) [113]. Quaternary ammonium salt cations in the gel can remove salt ions from the surface of the substrate, and the hydrophobic aryl structure can destroy the surface hydration layer. Simultaneously, a region with a low dielectric constant is provided to enhance electrostatic interactions. Thus, adhesion between the poly(cation-adj-π) hydrogel and the substrate underwater is realized. The adjacent cationic aryl structure prevents water molecules and salt ions from entering the interface and enhances hydrogel adhesion. However, control hydrogels, which do not contain aryl groups, and cations are not adjacent to each other and cannot destroy the hydration layer on the substrate surface. Hence, the electrostatic interaction at the interface is easily destroyed, resulting in weak adhesion. The adjacent sequence of cationic aryl groups in the polymer plays a decisive role in adhesion. Polymer synthesis is simple and easy to control, which emphasizes the importance of the monomer sequence on material properties. It references the study of electrostatic interactions under high ionic strength and has guiding significance for the development of adhesion to the physiological environment. Inspired by nature, Roy et al. prepared a self-adjustable adhesive polyampholyte (PA) hydrogel for adhesion to tissues, as shown in Fig. 4a and b [114]. The neutral PA with equally positive and negative charges is randomly distributed on the polymer network of the hydrogel. Dynamic ionic bonds within and between the molecular chains can be formed at a high charge density between the opposite charges. The dynamic ion bonds on the surface of PA hydrogels produce adsorption sites by forming polarization-induced ion bond complexes, thus forming surfaces with opposite charges or polarities, which can be adsorbed on physiological surfaces. When the dual electric layers of the neutral PA hydrogel are close to the polyelectrolyte (PE) or protein, PA is polarized by the polar material. Consequently, the charge is redistributed to achieve adhesion to the polar surface [115]. In addition, the living tissue adhesion test results showed that both the PA hydrogel and the positive PE hydrogel had good adhesion to biological tissues. Additionally, this adjustable mechanism also gives discern in comprehending the biological adhesion.
2.3.3. Adhesive hydrogels based on multi-mechanisms
Some known hydrogels have multiple adhesion mechanisms, in most of which the molecules contain a variety of tissue-reactive functional groups. Yang et al. simulated the catechol groups in mussel proteins with oxidized hyaluronic acid-dopamine (OHA-DA). They prepared an aldehyde-terminated Pluronic F127/Adipic acid dihydrazide-modified HA/OHA-DA(AF127/HA-ADH/OHA-DA) hydrogel with robust adhesion properties, capable of accelerating full-thickness skin wound healing (Fig. 3c and d) [49], which can be applied to wound dressings that require strong adhesion. Li et al. prepared transparent in situ hydrogels with superb compressibility, biodegradability, self-recovery, biocompatibility, and antibacterial activity by oxidizing hydroxyethyl starch (O-HES) and modified carboxymethyl chitosan (M-CMCs) and the Schiff base reaction for wound healing promotion. The adhesion property of this hydrogel is enhanced by cooperating hydrogen and imine bonds. The M-CMCS/O-HES hydrogel demonstrated high adhesion strength (14.07 ± 1.15 kPa) and superior wound healing effects of full-thickness skin defects [116]. PVA injectable hydrogels were prepared by dynamic covalent bonding and ion interactions on a 4-carboxyphenylboric acid bridge using chitosan-modified metronidazole microcapsules (CS@MTZ) as the crosslinking agent. This hydrogel has good adhesion under wet conditions by expelling the water boundary layer on the tooth, so it may have promising application in tooth wounds [117]. Liang et al. developed a series of adhesive antibacterial self-healing hydrogels by dual-dynamic-bond cross-linking among quaternized chitosan (QCS), protocatechualdehyde (PA) and ferric iron (Fe). Catechol and aldehyde groups have been demonstrated as tissue-reactive functional groups with strong tissue adhesiveness in PAs. The smart hydrogel exhibits multifunctional adhesiveness, good biocompatibility, antibacterial activity and injectability, and excellent wound healing effectiveness [118].
Hence, the development of adhesive hydrogels as wound dressings is growing fast and each of the designs differs in adhesion strength; however, significant challenges still exist in this field. According to the statistics, a lot of adhesive hydrogels as wound dressings have already been clinically tested and exhibited good application prospects. The combination of various adhesion mechanisms can be further optimized to achieve the desired adhesion properties. Furthermore, the fast forward of biomedical engineering, such as 3D-bioprinting and stem cell incorporation, are believed to lead to a new generation of hydrogel-based wound dressings with high adhesion ability in the near future.
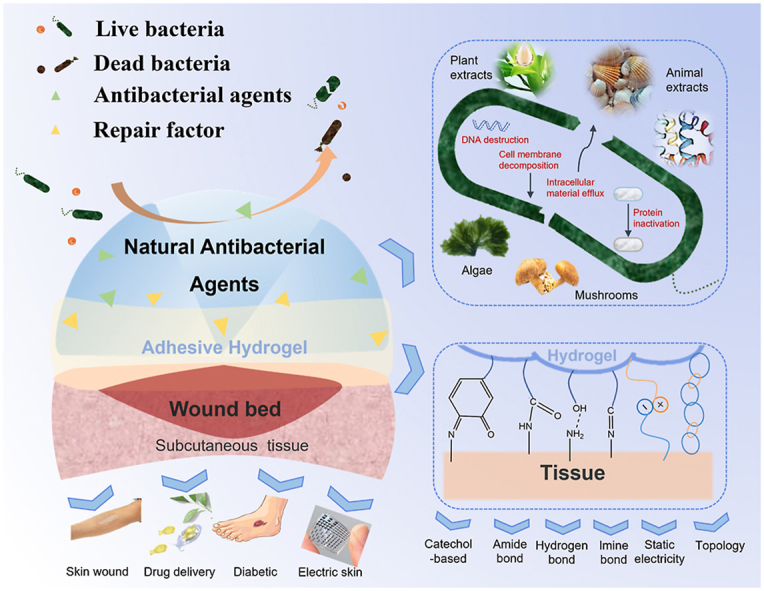
3. Natural antibacterial agents-incorporated hydrogels
Clinically, wound infections can cause wound suppuration, prolong wound healing time, and even lead to sepsis in serious cases, endangering life and health [119]. Thus, wound dressings should have antibacterial and bacteriostatic functions. Various antibacterial agents have undergone rapid development, to meet the needs of wounds regarding biocompatibility, disinfection efficiency and mechanical properties of wound dressing. In the history of medicine, Hippocrates was first recorded using Ag powder for ulcer healing [120]. Gold has been well known since the Roman times [66], and it has been proved to have vigorous antibacterial activity against a wide range of bacteria [121]. The antibacterial properties of copper and some metal oxides (such as ZnO) have also been discovered [135,137]. When constructed at nanoscale, antibacterial metal and metal oxides can adsorb microorganisms better, making their antibacterial properties more effective due to the increase in specific surface area and active sites [60]. Furthermore, inorganic antibacterial agents can improve antibacterial performance and maintain relatively long-term activity, reducing drug resistance. The antibacterial mechanism of metal ions and metal oxides is that their nanoparticles can destroy cell membranes or cause harmful changes in organelles [122]. Unfortunately, the biocompatibility of inorganic antibacterial agents cannot meet the requirements of human implantation and need to be improved using the synergistic effect of natural antibacterial agents.
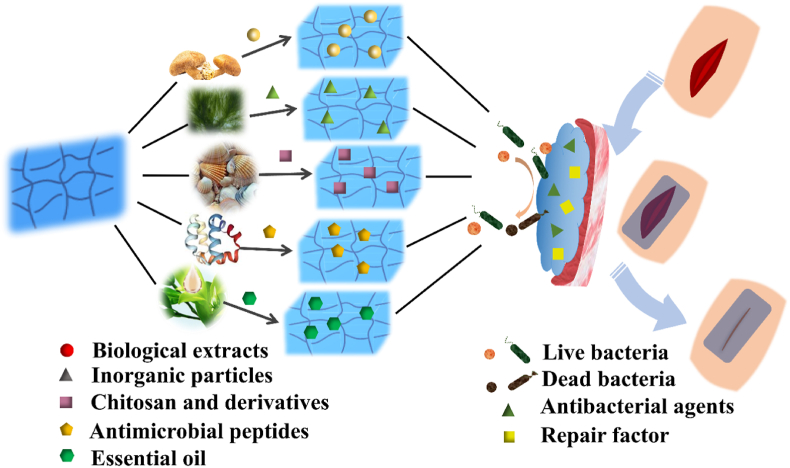
Antibiotics are classified as secondary metabolites originated from microorganisms, as well as chemically synthesized or semi-synthesized analogous compounds, which could suppress the growth and survival of other microorganisms [123]. Antibiotics are recognized as useful therapeutic agents in the application of treating human infectious diseases, but their overuse stimulated the generation of bacterial resistance [124]. Therefore, based on the need for biocompatibility and availability, the development and utilization of more natural antibacterial extracts has become imminent [119,125]. Natural antibacterial agents have been widely studied because of their excellent biocompatibility and availability. These agents are obtained from natural sources including animals, plants, bacteria, algae and fungi [126]. The main antibacterial compounds of plants are present in plant-derived essential oils [127,128]. A large amount of antibacterial agents of animal sources is represented by antibacterial peptides [129]. Some polysaccharides (such as Chitosan) and lipids derived from animals have also shown antibacterial properties. Other bioactive compounds include algae and mushroom sources which can combat bacterial invasion in their natural environment. The development of novel antibacterial agents with multiple applicability, such as in biomedical devices and pharmaceutical application, is promising [130]. The antibacterial mechanism of natural agents relies upon the positively charged organic molecules and negatively charged microbial cell membranes being combined, leading to the rupture of the bacterial biofilm, which causes the outflow or inactivation of proteins and other components that cause bacterial death [125,131].
This section introduces the preparation methods and antibacterial mechanisms of hydrogels with natural antibacterial agents as wound dressings, as well as the synergistic effects of these antibacterial agents in promoting wound healing (Fig. 5). Details of the different antibacterial factors are presented in Table 3. Up until now, the preparation methods of hydrogels mainly consist of physical cross-linking and chemical cross-linking as shown in Table 4.
Fig. 5.
Schematic illustration of natural antibacterial agents that impart superior broad-spectrum antibacterial activity to the hydrogel wound dressings.
Table 3.
Introduction and comparison of various natural antibacterial factors.
| Classification | Antibacterial mechanism | Advantages | Disadvantages |
|---|---|---|---|
| Silver-zeolite | Metal ions contact sterilization and catalytic sterilization. | Strong affinity that can work both in the presence and absence of oxygen. | High cost |
| Silver-phosphate | Slow-release action and photocatalytic action. | Strong adsorption function, large specific surface area, nontoxicity, stable chemical properties. | High cost |
| Silver-soluble glass | Slow release of sterilization. | High chemical activity, long-term sustained-release antibacterial activity. | Easy to change color, high cost. |
| Titanium dioxide | Photocatalytic antibacterial activity. | Stable chemical properties, broad spectrum of sterilization, excellent acid and alkali resistance, nontoxicity, rich raw material sources. | Difficult to sedimentation, not easy to recover, not selective of sterilization. |
| Zinc oxide class | Photocatalysis, metal ion dissolution and active oxygen antibacterial. | High activity, rapid antibacterial, high safety, low cost, biocompatibility, controllable morphology. | Only under ultraviolet light with sterilization, degradation of organic matter, self-purification ability, no selectivity. |
| Quaternary ammonium salt | Adsorption, penetration, film breaking. | Low cost, fast antibacterial speed. | Poor durability, large effective drug dose, easy to induce drug resistance. |
| Halide amine | Contact with sterilization or release of oxidizing halogen cation. | Strong stability, broad spectrum of sterilization, high efficiency, easy degradation and low toxicity. | Cannot be directly deposited on the surface of matrix. |
| Chitosan | Destroys the cell wall of bacteria and hinders their free movement. | Excellent biocompatibility, low cost, nontoxicity. | Poor water solubility |
| Antimicrobial peptides | Disrupt bacterial membranes and inactivate nucleic acids and cytoplasmic proteins. | High efficiency, broad-spectrum of sterilization, high killing potency, minimum risk for drug resistance. | Poor proteolytic stability, high toxicity toward mammalian cells. |
| Plant extracts | Rupture of the bacterial biofilm by electrostatic interaction | Controllable release of antibacterial agents, prolonged effects, positive effects on growth factors and collagen deposition. | Poor durability |
| Algae and mushroom extracts | Product targeted secondary metabolites | Broad-spectrum of sterilization, excellent antibacterial activity. | Poor anti-fungi activity, limited extraction solvent. |
Table 4.
Preparation method of hydrogels with natural antibacterial agents.
| Preparation | methods | Mechanisms | Advantages | Disadvantages |
|---|---|---|---|---|
| Ionic Interaction | Actional interaction between metal-ligand interaction or oppositely charged groups | Superior self-healing ability, fatigue resistance, ionic conductivity, environmental response | Complex preparation process and poor mechanical properties | |
| Physical | Hydrogen Bond | Oxhydryl-hydrogen atom interaction | Self-recovery and self-repair properties | Unstable in aqueous environments |
| Cross-Linking | Crystallization | Freeze-thaw process | Soft, flexible, and variable pore-size hydrogels | Instability |
| Hydrophobic Interaction | Cross-linking molecules with hydrophobic moieties in a water-soluble polymer solution | Strong and stable | Poor ductility | |
| Protein-protein Interaction | Non-covalent bond interactions | Excellent cell infiltration capacity | Not resistant to high temperature | |
| Chemical | Conjugation Reaction | Michael addition reaction, Diels–Alder addition reaction, and Schiff's base reaction | Outstanding transparency, biodegradability, biocompatibility, and self-recovery | Instability |
| Cross-Linking | Free Radical Polymerization | Cross-linking under the action of heat or light for covalent bond formation | Antibacterial property, high strain sensitivity, repeatable self-adhesion, and stretchability | Uncontrollable rate of polymerization |
| Enzymatic Reaction | Enzyme driven catalysis | Reversible stiffening/softening capability, adjustable mechanical and biochemical properties | Strict reaction conditions |
3.1. Natural antibacterial agents-loaded hydrogels
Widely used natural antibacterial agents are under long-term exploration, including chitosan, antibacterial peptides, and other biological extracts [52,72,132]. Owing to their extensive sources, low toxicity, biocompatibility, and other advantages [133], natural agents offer a promising option to improve antibacterial materials and solve the problem of antibiotic resistance [119]. However, their antibacterial persistence is a major limitation to their application.
3.1.1. Chitosan-loaded antibacterial hydrogels
Ideally, wound dressings should minimize pain and inconvenience for patients and treat wounds at a reasonable cost [23]. Chitosan is the most widely studied physical wound dressing, owing to its advantages of being easy film forming, nontoxic, antibacterial, biodegradable, and biocompatible [51,52,[57], [58], [59], [60], [61], [62]]. Relevant studies have shown that the binding generated between the negatively charged bacterial cell membranes and the positively charged chitosan molecules cause the break of bacterial biofilm, allowing proteins and other components to flow out, and resulting in the inactivation and death of the bacteria [59,60,134]. At low concentrations, the combination between positively charged chitosan and the negatively charged bacterial surface can lead to the aggregation of bacteria. In contrast, at higher concentrations, many positive charges can merge with the surface charge of bacteria to maintain the bacteria in a suspended state [134].
Chitosan has many outstanding biological characteristics, as follows: (1) as a natural biopolymer, it is biodegradable, and the degradation products are low-toxic; (2) it can bind to mammalian and microbial cells; (3) it can promote the regeneration of connective tissue and accelerate wound healing; (4) it can facilitate the generation of osteoblasts and accelerate bone regeneration; and (5) it has hemostasis, antibacterial, and anti-tumor activities [59,60,134]. Chitosan and its derivatives have attracted considerable attention due to their effects in accelerating wound healing. Hence, chitosan-based materials have promising applications and prospects in wound dressing [135]. Chitosan can only dissolve under acidic conditions and play an antibacterial role, significantly restricting its application in a biological environment due to its poor water solubility [134]. However, the antibacterial properties of chitosan are weak, and chitosan derivatives have difficulty meeting the complex antibacterial requirements in clinical practice [136]. Therefore, combining chitosan with other agents is necessary to enhance its antibacterial properties.
Due to the durable and stable antibacterial properties of metals, many researchers have mixed metals with chitosan to prepare wound dressings with durable antibacterial properties and good biocompatibility [59,134]. In particular, Zhang et al. prepared a new hydrogel membrane from CMCS via water emulsion copolymerization, which exhibited good thermal stability, swelling properties, and biodegradability. The hydrogel showed superior inhibition of E. coli and S. aureus [137]. Using silver nitrate, gelatin, and CMCS as the raw materials, hydrogels containing different amounts of silver were prepared by radiation reduction and crosslinking at room temperature. When gelatin/carboxymethyl chitosan is formed, Ag+ is reduced in situ to AgNPs. The hydrogel has an excellent bacteriostatic effect on E. coli, and with an increase in silver nitrate content, its bacteriostatic ability is significantly enhanced [57]. An antibacterial CMCS/ZnO nanocomposite hydrogel was successfully prepared by the in situ formation of ZnO nanorods from crosslinked CMCS. The sterilization rate of the hydrogel for 6 h in E. coli was 99% [58].
Furthermore, Wang et al. reported a double-syringe injection device that could be used to continue the production of CMCS-Zn supramolecular hydrogel fibers. CMCS exhibits blue fluorescence when in contact with zinc ions. The fluorescence become weak after the release of zinc ions, which can be used as a visual indicator of the antibacterial effect [61]. A simple method was proposed for preparing polyoxometalate (POM) hydrogels with adhesion, conductivity, self-healing, and antibacterial properties. Moreover, a double-network hydrogel, chitosan-silicotungstic acid (CS/SiW), was prepared by polymerizing polyacrylamide (PAM) [138]. Multifunctional chitosan/carboxymethyl chitosan/silver nanoparticle (CS/CMCS/AgNPs) polyelectrolyte composite physical hydrogels were constructed using α-hydroxyl in situ photoreduction of CMCS and the sol-gel transformation method of semi-dissolved acidification [59]. A simple method for preparing an antibacterial nanocomposite hydrogel to accelerate wound healing was explored. After chitosan is quaternized and cross-linked with polyacrylamide, AgNPs are formed in situ to endow an antibacterial hydrogel with high synergism and low cytotoxicity [62].
Synergistic antibacterial effects of chitosan and organic matter have also been reported. Methylacrylamide dopamine (MADA) and acrylic acid (AA) was copolymerized with ethyl methacrylate (2-dimethylamino) (DMAEMA) and QCS and quaternized chitosan to form an interpenetrating network, where a type of contact antibacterial hydrogel AA-co-MADA-co-DMAEMA (AMD)was proposed, as shown in Fig. 6e and f. The antibacterial activity of the hydrogel is enhanced after the active catechol groups of MADA exposes gram-positive bacteria to the hydrogel. The suspensions of the AMD hydrogel were clearer compared to the blank group. The antibacterial effects are further promoted owing to QCS addition (AMD-QCS). The suspensions of AMD-QCS hydrogel were the clearest, demonstrating the best antibacterial effect. The AMD-QCS hydrogel is endowed with superb antibacterial ability by the effective and significant broad-spectrum inhibition of QCS and DMAEMA [52]. Citral is a monoterpene aldehyde that can inhibit the growth of various pathogens by affecting the mitochondrial structure. Citral was reacted with polycationic CS and polyanionic carboxymethyl (CMC) to synthesize polyelectrolyte composite hydrogel microspheres [60]. A novel antibacterial membrane (HA-CC-GS) was developed by the intermolecular covalent bonding of hyaluronic acid, carboxylated CS, and gentamicin, which have good hygroscopic properties, suitable water vapor permeability rates (WVPR), excellent mechanical properties, and significant resistance to enzymatic hydrolysis [136]. The hydrogel can potentially be applied to wounds in the internal environment.
Fig. 6.
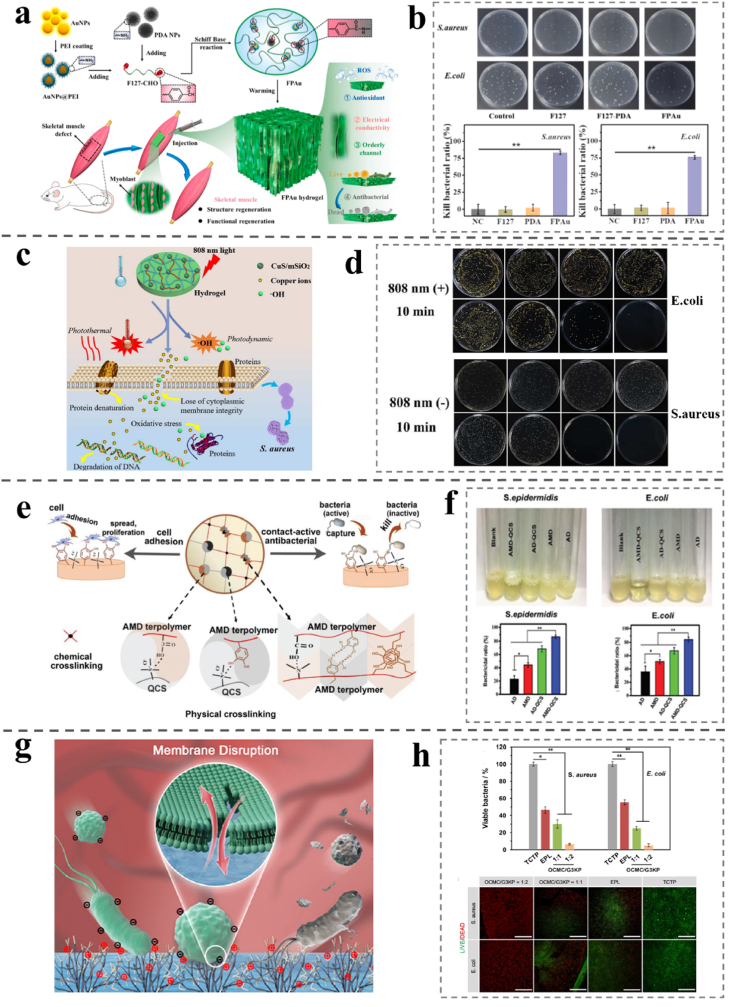
(a) Schematic illustration of the FPAu hydrogels application for the fabrication and skeletal muscle regeneration. (b) Antibacterial activity of FPAu hydrogels. Reproduced with permission [144]. Copyright 2021, Elsevier. (c) Schematic illustration of the bacteria killing processes with the hybrid hydrogel under 808 nm NIR light irradiation. (d) Antibacterial activity of the copper loaded hybrid hydrogel. Reproduced with permission [145]. Copyright 2018, The Royal Society of Chemistry. (e) The structure and function of the cell-affinitive, recoverable, ultra-tough, and contact-antibacterial AMD-QCS hydrogel. (f) S. epidermidis and E. coli suspensions and OD measurement of the growth of S. epidermidis and E. coli that were cultured with different specimens for 24 h. OD: optical density. Reproduced with permission [52]. Copyright 2019, Wiley-VCH. (g) Schematic illustration of the antibacterial mechanism of positively charged dendritic polylysine. (h) Quantitative result of the bactericidal efficacy against E. coli and S. aureus and LIVE/DEAD fluorescence images of E. coli and S. aureus incubated with OCMC/G3KP hydrogels with different dendrimer concentrations. Scale bar, 0 μm (∗P < 0.05, ∗∗P < 0.01, and n = 5). Reproduced with permission [74]. Copyright 2020, American Chemical Society.
3.1.2. Peptides-loaded antibacterial hydrogels
Antibacterial peptides (AMPs) have broad-spectrum antibacterial properties and exhibit superior antibacterial properties against multidrug-resistant bacteria [[63], [64], [65], [66], [67], [68], [69], [70],139]. Many active peptides are cationic amphiphilic peptides containing many cationic arginine, lysine, or residues, which can destroy bacterial membranes and cytoplasmic proteins and inactivate nucleic acids, resulting in bacterial inactivation and death [23,140]. Milk protein retains rich bioactive peptides, which are usually not active in the mother protein containing numerous active sites to realize antibacterial activities, angiotensin, converting enzyme inhibition, immunomodulation, mineral binding, and antioxidant functions [140].
Scientists have found that forming a composite of a self-assembling peptides and a natural polymers can improve biocompatibility [131,139,140]. Zhou et al. designed and synthesized a 2-(methacryloyloxy) ethyl 2-(trimethylammonium) ethyl phosphate (MPC) copolymers P(MPC-co-GMA) hydrogels for wound dressings with intrinsic antibacterial and anti-fouling performance. The P(MPC-co-GMA) hydrogel showed a favorable cytocompatibility with L929 cells and RBCs. The killing rate of bacteria were almost 100%. This polyphosphorylcholine hydrogel with inherent excellent antibacterial adhesion behavior and drug release ability (Fig. 7a and b) [141]. Based on natural self-assemble proteins, hydrophobic naphthyl branched-chain (Nap) acetyl groups and hydrophilic peptide amphiphilic peptides are composed of the main chain constructed for antibiotic polymyxin B slow-release supramolecular hydrogels. Under neutral pH, amphiphilic peptides can negatively or positively charge polymyxin B to form an electrostatic attraction through intermolecular hydrogen bond forces between the peptide skeleton and the hydrophobic Nap branched chain of p-stacking. In amphiphilic peptide self-assembly supramolecular hydrogels, the electrostatic attraction function can guarantee the continuous effect of polymyxin B in the supramolecular hydrogel. Thus, the growth of E. coli was effectively inhibited [67]. Thus the antibacterial durability of the hydrogel wound dressing was greatly improved.
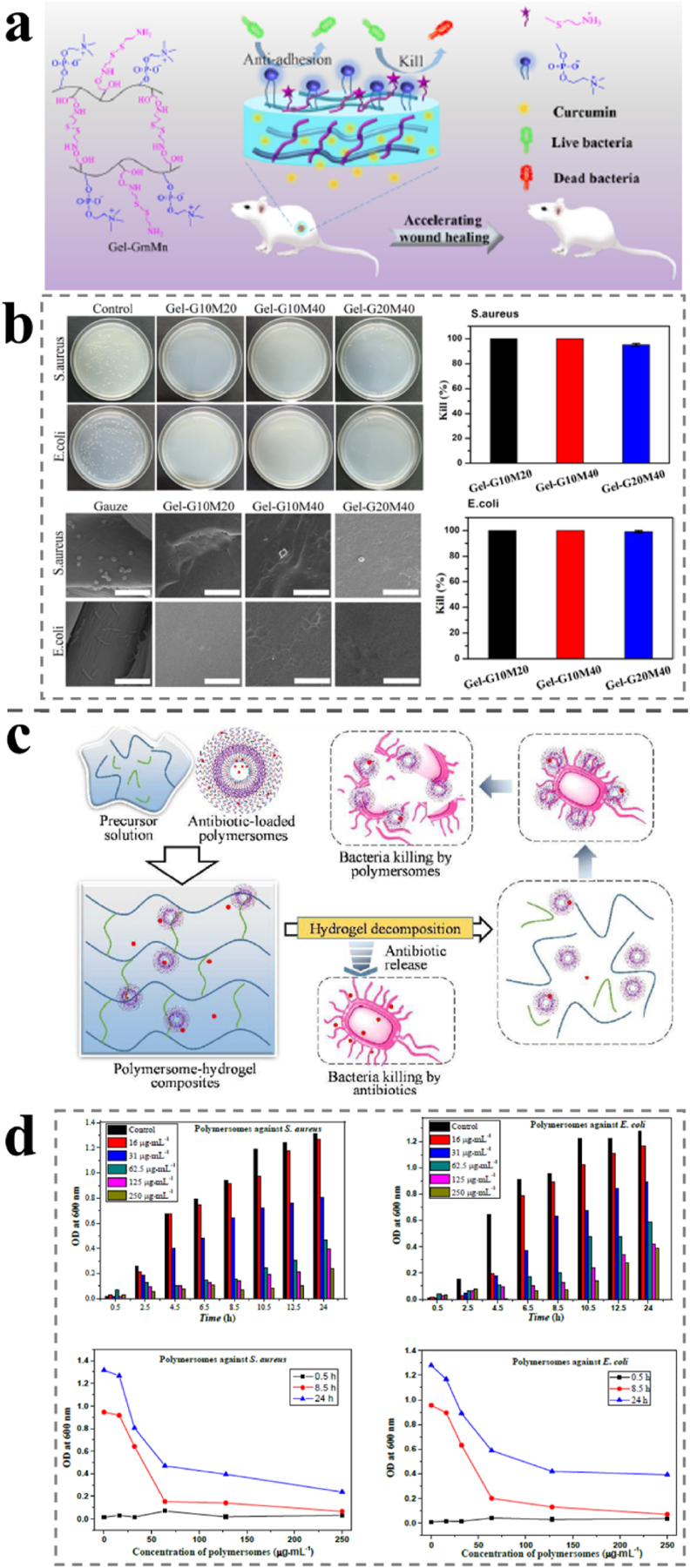
Fig. 7.
(a) Illustration of the accelerated wound healing by the antibacterial and non-fouling hydrogel loading curcumin as an anti-inflammatory drug. (b) Antibacterial activity of hydrogels against S. aureus and E. coli and SEM images of the surfaces of the gauze (as a control) and the hydrogels after contact with bacterial suspension. The scale bar is 5 μm. Reproduced with permission [144]. Copyright 2020, American Chemical Society. (c) Illustration of the antibiotic-loaded polymer-hydrogel composites capable of quick and long-term antibacterial activities. (d) Antibacterial activity in terms of OD value against S. aureus and E. coli in the presence of polymersomes at different concentrations. OD: optical density. Reproduced with permission [146]. Copyright 2018, The Royal Society of Chemistry.
Additionally, self-assembled peptide hydrogels are a kind of new hemostatic material, through self-assembly to form a hydrogel, because of their intrinsic biocompatibility and biodegradability, and the majority of researchers can design them, and a rational molecular design can make the peptide and its hydrogel reflect biological signals [135]. However, self-assembled peptide hydrogels have poor hydrolytic stability and high toxicity to mammalian cells; therefore, it is essential to enhance the stability, prolong the storage time, and control the release of self-assembled peptides for application in wound dressings [23]. In contrast, a modified cotton composite hydrogel wound dressing was prepared by impregnation with alginate from antibacterial lactobacilli and CMCS. In addition, it has antibacterial activity against S. aureus with no cytotoxicity to fibroblasts [142]. Hong et al. presented a polymersome-hydrogel composed of antibiotics and antibacterial peptides. The hydrogels are self-assembled from poly (ε-caprolactone) -block-poly (lysine-stat-phenylalanine) [PCL-b-P(Lys-stat-Phe)], which exhibits continuous antibacterial activity (Fig. 7c and d). This polymersome-hydrogel has demonstrated prospective application in injectable antibacterial wound dressings [143].
3.1.3. Other biological extracts-loaded antibacterial hydrogels
Natural antibacterial substances are recognized as promising substitute agents for addressing the shortages of synthetic antibiotics. The attractive biological properties, including antibacterial ones displayed in numerous naturally derived compounds endow them to be used as therapeutic agents [[71], [72], [73], [74], [75], [76], [77]]. The melanin extracted from the Mytilus edulis shell can promote the healing of wounds infected by drug-resistant bacteria in vivo under the wavelength of 808 nm near-infrared laser irradiation, which proves that it has a good photothermal transformation effect and antibacterial activity [146]. Zuo et al. studied the inhibitory effects of four pentadiene flavonoids from mulberry root skin on methicillin-resistant S. aureus (MRSA) and their synergistic effect with 11 types of conventional antibacterial agents. Antibacterial activity and synergistic effects assessed by broth microdilution, checkerboard dilution, and time-kill curve assays. This study is the first to reveal the synergistic effect of 1–4 pentadiene flavones with 11 antibacterial agents against MRSA and the reversal of resistance to aminoglycosides, particularly amikacin. These results may be helpful to put forward the application of new antibacterial agents and synergists against MRSA infections [147]. Zhu et al. described a fast and high efficiency antibacterial effects and inherent hemostatic ability of OCMC/G3KP hydrogels. The antibacterial experiment shows that almost complete killing of the gram-negative or gram-positive bacteria on the surface of both the 25% OCMC/G3KP = 1:2 and 1:1 hydrogel. The OCMC/G3KP hydrogel showed intrinsic broad-spectrum inhibition of both gram-negative and gram-positive bacteria dispense with introducing other exogenous antibiotics (Fig. 6g and h) [70].
In addition, the essential oil components of the serpentine bark were studied. The contents of linoleic acid and other acids were the highest. The antibacterial zones of S. aureus and Proteus were 31 mm and 38 mm, respectively. The bacteriostatic activity follows the principle of dose-dependence [148]. Using gas chromatography/mass spectrometry (GC/MS) for six kinds of pear plant volatile oil chemical composition analysis, the essential ingredient for 2, 4-glutaraldehyde, leaf alcohol, and nonyl aldehyde was determined. Antiviral experiments show that the volatile oil of Pyrus has broad-spectrum antibacterial activity, with the highest antibacterial activity and a minimum inhibitory concentration (MIC) of 2.5 μM/mL against S. aureus [149]. Mango leaves Cabo of methanol extract of phenol were studied for SAII3 and S. aureus antibacterial effects, and the hydrogels containing Cabo phenol (the original concentration of 130 mg/mL). The results showed that the prepared hydrogel system has a prominent effect in treating drug-resistant S. aureus [150].
3.2. Synergistic antibacterial agents- loaded hydrogels
Due to the overuse of antibiotic overuse in previous years, many bacterial strains have developed resistance to drugs, making many antibacterial drugs less effective. For example, bacteria spontaneously secrete an enzyme that works against the drug to render it ineffective when it enters the bacteria to suppress its metabolism [151,152]. According to the investigation, the mechanism of drug resistance by bacteria mainly includes antibiotics flowing out of bacterial cells via enzymatic modification and degradation of antibiotic molecules or effluent pumps and changes in antibiotic targets that prevent the antibiotic from binding, resulting in loss of activity [119]. These different mechanisms suggest that multifarious approaches need to be adopted to combat bacterial resistance, including using both antibiotics and non-antibiotics to make them work in concert. Therefore, existing methods and strategies urgently require new improvements. A series of “double-safety antibacterial measures” has been proposed. The method of combining drugs with antibacterial agents synergistically can cut down the dosage to reduce the toxicity to human bodies. Several studies have been conducted on the synergistic antibacterial effects of varies antibacterial agents.
Metal nanoparticles are considered an attractive alternative for enhancing the antibacterial effects of antibiotics because of their outstanding antibacterial activity when combined with a wide variety of antibiotics [125]. Among the research, an earlier study explored whether two antibacterial agents combined have a synergistic antibacterial effect. For instance, Ge et al. prepared a nanocomposite hydrogel using ultrasmall gold@polymine nanoparticles and poly doping nanoparticles as materials via double crosslinking of the F127–CHO network [144]. As shown in Fig. 6a and b, the AuNPs are modified by PEI and can effectively crosslink with F127–CHO to improve their antibacterial activities. The antibacterial activity of Au shows that the growth of S. aureus and E. coli are significantly inhibited, with a killing rate of 75% after exposure to the hydrogel for 2 h. Photothermal therapy (PPTT) is a non-invasive therapy based on the production of thermal energy from photon energy, which can increase cellular temperature. Zinc ions and cationic antibacterial agents can inhibit volatile expiratory compounds (VSC) caused by bacterial action in the oral cavity. Zinc ion antibacterial agents and two cationic antibacterial agents were tested in vitro. These combinations exhibited synergistic inhibitory effects [153]. The antibacterial activities of a series of silver-N-heterocyclic carbenes (Ag–NHCs) prepared in the past decade have been studied. The antibacterial activities of S. aureus and E. coli were assessed. The results show that NHCs show excellent inhibition of S. aureus and E. coli, and a slight difference in the structure of NHC ligands resulting in significant changes in the activity. The degree of synergism provided by AgNPs and cinnamaldehyde, a representative essential oil (cinnamaldehyde) was assessed. The presence of cinnamaldehyde has significantly improved the activity of nanoparticles. Electron and atomic force microscopy also showed extensive damage to the bacterial cell envelope in the presence of these two agents [154]. AgNPs were synthesized by a green method using plant extracts of aircraft nymphs as reducing and capping agents. The particles are doped with sodium alginate to form thin films. Plants provide an excellent platform for NP synthesis. At a very low concentration (approximately 25 μM/L), the membrane has inhibited the whole strain [155]. Human β-defensins (HBDs), skin lysozyme, and cathelicidin LL-37 are factors that endow the skin with antibacterial properties. The isolated and synergistic activities of HBDS, lysozyme, and LL-37 against E. coli and S. aureus were studied in neutral and acidic environments. Under neutral and acidic pH conditions, HBD-1, -2, -3, lysozyme, and LL-37 showed dose-dependent inhibition of E. coli and S. aureus [131]. Different and typical antibacterial hydrogels are compared and summarized in Table 5.
Table 5.
Comparisons of different antibacterial hydrogels.
| Antibacterial agent | Polymer | Features | Antibacterial activity | Ref. |
|---|---|---|---|---|
| Pterostilbene/Gentamicin | – | Antibacterial ability | S. aureus, E. coli, P. aeruginosa | [72] |
| Natural melanin | – | Excellent photothermal conversion effects, biocompatible, conglutinant | S. aureus, E. coli | [146] |
| Polymyxin B. | Amphiphilic peptide | Sustained releasing, good biocompatibility | E. coli | [67] |
| Ag/Biological extract | Alginate | Controllable releasing, thermal | S. aureus, E. coli | [155] |
| Ag/Curcumin | Bacterial cellulose | High cytocompatibility, antioxidant activity |
S. aureus, P. aeruginosa, C. auris |
[120] |
| AuNPs | F127–CHO | Antioxidant activity, antibacterial ability, electroconductive activity | S. aureus, E. coli | [125] |
| AuNRs | CS | Antibacterial ability, low cytotoxicity | S. aureus, S. epidermidis, E. coli | [129] |
| AuNPs/Graphitic | CS/PVA | Antibacterial ability, biocompatibility | S. aureus, E. coli | [130] |
| Cu-MOP | – | Outstanding biocompatibility, antibacterial ability | S. aureus, E. coli | [132] |
| CuNPs | GelMA | Antibacterial ability | S. aureus, E. coli | [131] |
| CuSNPs | MPS | Antibacterial ability, tissue regeneration capability | S. aureus, E. coli | [134] |
| ZnONPs | CMCS | High swelling behavior | S. aureus, E. coli | [58] |
| AgNPs | CS/PAA | High synergism, low cytotoxicity, without-contacting antibacterial ability | S. aureus, E. coli | [62] |
| AgNPs | CS-CMCS | Self-healing, high biocompatibility, high swelling behavior | S. aureus, P. aeruginosa | [59] |
| AgNPs | Gelatin | Excellent antibacterial ability | E. coli | [57] |
| CMCS/Gentamicin | HA | Hydroscopicity, well vapor permeability rate, enzymatic resistance | S. aureus, P. aeruginosa | [136] |
| 4-HPA | PLL/Aga | Injectable, recoverable, good biocompatibility, stability | S. aureus, E. coli | [102] |
| Citral/CS | CMC | Antifungal ability, antioxidant | E. coli, S. aureus, B. subtilis | [60] |
| CMCS | CMCS | Good thermal stability, swelling property, biodegradability | S. aureus, E. coli | [137] |
| ZnNPs | CMCS | Injectable, fluorescent color indication | S. aureus, E. coli | [61] |
| CS | SiW/PAM | Adhesive, conductive, self-healing | E. coli | [138] |
| QCS | DMAEMA | Ultra-tough, recoverable, cell-affinitive | S. aureus, E. coli | [52] |
| AMP | Alginate | Recoverable, good biocompatibility | A. baumannii, E. coli, P. aeruginosa, S. aureus | [140] |
| AMP | CMCS/Alginate | Superior biocompatibility, no cytotoxicity | S. aureus | [142] |
| AMP | – | Highly selective, effective, safe, well-tolerated, self-assembling | E. coli, P. aeruginosa | [139] |
| AMP/Penicillin | PCL-b-P-(Lys-stat-Phe) | Injectable, quick and long-acting antibacterial ability | S. aureus, E. coli | [143] |
The disadvantages of synergistic antibacterial agents-loaded hydrogels are the strict requirements for the preparation method, the addition of a variety of antibacterial agents may have antagonistic effects and combined shortcomings, that may cause adverse effects on the wound. The variety of plant extracts is wide, and the antibacterial properties of some of them are worth the affirmation. Natural antibacterial agents derived from animal sources are mainly polypeptides and some macromolecular substances such as chitosan, whose antibacterial effect is known. In addition, a few antibacterial agents are derived from microorganisms; however further development is required in this area for validation. The application of natural antibacterial agents should be further improved to use their antibacterial properties to their full potential. In the case of synergistic application, further research into the characteristics of plentiful antibacterial agents, exploration of the appropriate synergistic effects, and reduction of the side effects of non-natural antibacterial agents in the synergistic application should be the focus areas of future efforts.
4. Applications of natural antibacterial agents-loaded adhesive hydrogels
Although traditional wound dressings have a protective effect on the wound surface, they cannot meet the current requirements of wound dressings in terms of water and air permeability, antibacterial properties, and biocompatibility. Biomedical hydrogel dressings are a new type of wound dressing with a 3D network structure and strong water absorption ability [9,12,14]. The substantial water absorption property of hydrogels has inspired many researchers to study hydrogels as wound dressings [156]. In 1962, Dr. Winter first proposed the new discovery that a moisturizing environment for wound healing is helpful in animal experiments [12]. As a new biomedical dressing, hydrogel dressing has broad application prospects [12,19,157].
Each year millions of people suffer from skin wounds that cannot heal. Chronic inflammation, ischemia, and hypoxia are key factors in diabetic wound healing, leading to amputation unless properly treated [50]. Hydrogel dressings that are antibacterial, moderately adhesive, and permeable can modulate and possibly ameliorate these processes, thereby promoting wound healing [14]. Wearable strain sensors (WSS) have been widely applied in wearable devices with high sensitivity and high signal-to-noise ratio, such as soft robot and human health monitoring [[157], [158], [159]]. Wearable devices with fast preparation speed, adjustable sensitivity, and large volume have been increasingly attractive in the healthcare field [160]. Electronic hydrogel skin with high adhesion and antibacterial properties can help monitor wound healing. Hydrogel composites have become promising biological wound monitoring sensors as they are flexible, low-cost, and high-quality conductive polymer composites [161,162]. The research status of functional biomedical hydrogel dressings and application prospects is discussed in this section (Fig. 8).
Fig. 8.
Compositions and applications of antibacterial adhesive hydrogels.
4.1. Applied to skin wounds
Wound dressings are often used during medical procedures to help absorb and remove exudates from the wound and protect the injured area from the external environment. A certain degree of adhesion is also needed to prevent it from falling off or causing secondary injury to the wound [163]. Polymer hydrogels are new wound dressings for pyrosis and other skin injuries; they have good biocompatibility, moderate adhesion, and air permeability and can absorb wound exudate and accelerate wound healing [164,165]. With the increasing demand for medical dressings, the preparation of wound dressings using polymer-micellar adhesive hydrogel complexes alone cannot meet the need for clinical application. Adding drugs with antibacterial, hemostatic, or healing effects to dressings to form composite hydrogel dressings with therapeutic functions has become an important development direction in this field. Wang et al. introduced dopamine into the ε-poly lysine system (EPL) system to obtain a pure protein derivative (PPD) polymer, similar to natural mussel filament proteins. They prepared an injectable hydrogel (PPD-E) for in situ catalysis of Horseradish Peroxidase (HRP) [64]. In this hydrogel, EPL, an inherent antibacterial material, exhibits inherent antibacterial properties without the need to add exogenous antibiotics. EPL exhibits broad-spectrum antibacterial activity. At the same time, it also shows wet adhesion, faster wound healing, hemostasis, and skin regeneration properties than other hydrogels, owing to the synergistic effect of lysine and catechol. Xi et al. prepared a compound hydrogel based on a natural antibiotic (aloe-emodin) and carbon dots [56]. The hydrogel first utilizes the heat generated by near-infrared light and reactive oxygen species to rapidly inhibit the growth of bacteria. After the light was stopped, it continued to release aloe-emodin to achieve a long-term antibacterial effect. According to the in vivo experimental results, alternation of the two mechanisms have superior antibacterial activity S. aureus. Han et al. synthesized nanogels with long-lasting antibacterial activity and adhesion by the free radical polymerization of styrene, polycaprolactone hydroxyethyl methacrylate, and polyhexamethyl guanidine methacrylate monomers [166]. The antibacterial components of nanogels can destroy cell membranes and cause cell lysis. The macromolecular chain of the guanidine hydrochloride segment and the hydrophobic polycaprolactone chain can prevent bacterial adhesion. In addition, cotton fabric grafted with nanogels has strong hydrophobicity and a high antibacterial rate, which can effectively prevent bacterial adhesion. After 50 cleaning cycles, the antibacterial effect of the cotton fabric against S. aureus and E. coli remained as high as 86%. The micellar-hydrogel complex prepared by Patel et al. comprised two different diblock polypeptide micelle systems. The amino groups on the surface of the curcumin-loaded poly (L-lysine)-B-poly (phenylalanine) (PLL-B-PPA) micelles reacted with Genipin to form a micellar-hydrogel network structure [167]. PLL-B-PPA micelles carrying amphotericin B are physically embedded in interconnected networks. The experimental results showed that the blank micellar hydrogel had no harmful effects on wound healing. In contrast, the application of the drug had a positive effect on all stages of wound repair and healing, leading to increased wound contraction, granulation formation, epithelial reformation, and minimal inflammatory response. Moreover, Yuan et al. designed a creative hydrogel with double cross-linked structure using the catechol-Fe3+ chelation bond and a Schiff base. The unique double cross-linked structure of the hydrogel results in enhanced adhesive strength (19.3 kPa) and better self-healing property, which makes the hydrogel suitable for dynamic wounds. While the excellent shape adaptability (97.1 ± 1.3% of recovery) makes the hydrogel suitable for wounds with irregular shapes. The hydrogel exhibits not only good biodegradability during the wound healing process but also superior inherent antibacterial activity (100% killing ratio) and hemostatic property [168]. This double cross-linked multifunctional hydrogel is a promising candidate for a dynamic burn wound dressing.
As the number of patients with diabetes worldwide increases, so does the need to care for and treat closely related chronic wounds [169]. There is much research on wound dressings but few for chronic diabetic wounds [11]. Due to the damage to immune function and blood vessels, diabetic wounds can easily develop chronic inflammation, coupled with long-term bacterial infections, resulting in long-term treatment. Adding antibiotics, anti-inflammatory drugs, and growth factors to dressings is the primary way to eliminate chronic inflammation and promote proliferation. However, these drugs are prone to inactivation, drug resistance, and other toxic side effects. Therefore, adhesive hydrogel wound dressings containing natural antibacterial agents have attracted widespread attention. For instance, Chen et al. designed a series of self-healing, adhesive and antibacterial hydrogels based on gelatin methacrylate (GelMA), adenine acrylate (AA), and CuCl2 to promote diabetic wound healing. These hydrogels exhibit efficient self-healing properties, remarkable fatigue resistance, and good adhesive properties through covalent bonding, coordination complexation of Cu2+ and carboxyl groups and hydrogen bonding. The GelMA/AA/Cu1.0 hydrogel (containing 1.0 mg/mL Cu2+) with well-balanced biocompatibility and antibacterial properties significantly promoted the healing process in a full-thickness skin diabetic wound model [170]. Wang et al. developed an injectable, antibacterial, self-healing polypeptide-based FHE hydrogel (F127/OHA-EPL). The internal stimuli-responsive adipose-derived mesenchymal stem cell-released exosomes (AMSC-exo) synergistically enhanced chronic wound healing and skin regeneration. In addition, the FHE@exo hydrogel exhibited promoted healing effects compared to exosomes or FHE hydrogel alone [171]. To further improve the regeneration process, Chen et al. prepared a hydrogel by synchronously loading an angiogenic drug and desferrioxamine (DFO) through coordinative crosslinking of multi-arm thiolated polyethylene glycol (SH-PEG) with silver nitrate (AgNO3). A hydrogel with resistance to mechanical irritation and antibacterial and angiogenic effects was obtained. The effects of antibacterial and repairing diabetic skin wounds of the hydrogel are excellent in vivo studies [172]. Furthermore, Liang et al. constructed a hydrogel dressing based on the double dynamic bond of the Schiff-base and phenylboronate ester which acts as a pH/glucose dual-responsive dressing that releases metformin, exhibits enhanced adhesion and promotes self-healing, its easy-removable and has antibacterial, antioxidant, and conductive properties while aiding hemostasis. They achieved this by using multifunctional phenylboronic acid and benzaldehyde bifunctional polyethylene glycol-co-poly (glycerol sebacic acid)/dihydrocaffeic acid and L-arginine co-grafted chitosan (PEGS-PBA-BA/CS-DA-LAG, denoted as PC) [173]. This work provided a localized, dual-response drug release strategy for the treatment of type II diabetic feet.
4.2. Applied to scar-inhibition
The scar formation and hair follicle regeneration are closely related in the healing process of skin burn wounds [174]. Excessive pathological fibroblasts at the scar site severely hinder the migration of hair follicle stem cells to the damaged site [175]. Therefore, it is of great clinical significance to develop wound dressings that inhibit scar formation and promote hair follicle regeneration. Several studies have explored various ways to solve the problem of scarring. For instance, Gao et al. designed a bilayered nanofibrous membrane (PQCQS) by electrospinning poly(ε-caprolactone) (PCL)/quaternized silicone (PQS, outer layer) and polyvinyl alcohol/collagen/quaternized chitosan (PCQC, inner layer) to be used as wound dressings for damaged skin. These membranes exhibit excellent hydrophilicity, outstanding thermal stability, comparable mechanical properties with human skin, efficient hemostatic performance, and cytocompatibility. The PQCQS membranes can keep a good balance between antibacterial activity and cell proliferation. Additionally, the PQCQS membranes significantly expedit the wound healing process and inhibit good scar hyperplasia in a rabbit ear full-thickness wound defect model [176]. Likewise, Zhang et al. designed nano composites, Cur-Fe-mesoporous silica nanoparticles (Cur-Fe-SiO2, MFSC) to achieve synergistic activity in both inhibiting scar hyperplasia and promoting hair follicle regeneration, by the released bioactive components such as SiO32−, Fe3+, and Fe-Cur chelates. He et al. prepared nanocapsules (NCs) with a sophisticated structure featuring PFD at their core and CeO2 in their shell using the layer-by-layer method; these NCs are referred to as PFD/CeO2 NCs. Cellular experiments verifies that PFD/CeO2 NCs have good biocompatibility, satisfactory cellular uptake, and favorable ROS-scavenging capacity. To be applied directly to the wound, PFD/CeO2 NCs were then adhered to plasma-etched polylactic acid (PLA) fiber membranes to prepare a new wound dressing. Animal experiments further demonstrate that the dressing accelerates the epithelialization of the wound, reduces the levels of ROS and TGF, improves the arrangement and proportion of collagen fibers, and finally, achieves satisfactory wound-repairing and anti-scarring effects [177]. Similarly, Shan et al. investigated the effect of a silk fibroin/gelatin (SF/GT) electrospun nanofibrous dressing loaded with astragaloside IV (AS) on deep partial-thickness burn wounds. This dressing accelerates wound healing and inhibites scar formation in vivo by stimulating wound closure. The AS-loaded SF/GT nanofibrous dressing also promotes cell adhesion and proliferation with good biocompatibility in contrast to the blank control [178]. Moreover, Jin et al. Modified bacterial cellulose (BC), a good candidate for wound dressing, with a stripe pattern by a simple static culture method using patterned PDMS templates in order to investigate the inhibition of cicatricial contractions. In vivo experiments of the injury model demonstrate that pBC effectively inhibits inflammatory response, reduces accumulation of fibroblasts, and significantly decreases the scar contraction compared to the control and standard groups, indicating its good hypertrophic scar inhibition effect. Additionally, it was found that if the width of the stripes matches the size of the cell, the pBC has better anti-scar effect, which can also be extended to other dressings [179]. It is of great clinical significance to design wound dressing materials with combined excellent wound healing properties and superior capability to suppress hypertrophic scar formation.
4.3. Applied to electronic skin
Electronic skin is a new wearable, flexible bionic tactile sensor with a unique structure and good machinability. Its spatial resolution can reach the millimeter level, close to that of human skin. It can detect the hardness, temperature, surface morphology, and quality of a tested object through contact [159,180]. After the electronic skin is attached to the human skin, it can monitor the pulse, heartbeat, temperature, and other physiological indicators in real-time to reflect the changes in human health data and realize the diagnosis of diseases [23,158]. Conductive hydrogels are considered good candidates for electronic skin because they are similar to natural soft tissue/extracellular matrix, have good biocompatibility, electrical conductivity, high adhesion, and mechanical strength [14]. In addition, drug-loaded electronic skin can shorten the wound healing time and repair itself by slowly releasing antibacterial and anti-inflammatory drugs [163]. Gan et al. synthesized polyacrylamide/chitosan hydrogels by UV photopolymerization, in which chitosan was used as a template to adsorb and fix the conductive polymer polypyrrole onto the hydrogels [181]. The hydrogel can be applied in controlled-release systems for drugs with an adjusted release rate by stimulating voltage. In oxidation polymerization, free-anion drugs can be added to the polypyrrole skeleton as a dopant molecule. When polypyrrole is electrochemically reduced to a negative potential, the positive charge state of the polymer skeleton is changed, and the anionic drug is released to achieve controlled release of the anti-inflammatory drug dexamethasone. The effects of promoting wound healing and regeneration of the hydrogel were assessed via a full-thickness skin defect model in rats. More importantly, the hydrogel can be applied as an electronic skin to monitor human movement in real-time and as a stress sensor to detect loads. Further, Cheng et al. developed an intelligent artificial composite made of spider silk protein (spidroin) and programmable woven textile (i-SPT) with high tensile properties that possesses biochemical sensing abilities and air permeability as well as motion monitoring capabilities for wound management. High sensitivity for motion monitoring is endowed by the highly integrated microelectronic circuit on the i-SPT [182]. Similarly, Tian et al. prepared a multifunctional hydrogel for stress sensing and wound healing using a simple one-pot method and solution replacement method. This hydrogel is highly biocompatible, providing excellent antibacterial adhesion to aid the wound healing process, and good electrical conductivity to enhance sensitivity and stability [183]. Lei et al. designed and prepared supramolecular polyelectrolyte hydrogels with compression resilience and self-healing ability. The shape could be made arbitrary at room temperature by regulating the non-covalent bond interactions between the polymers [184]. Hydrogels are transparent and have skin-like mechanical properties and the sensory ability to sense temperature, strain, and pressure changes. It can be used as an intelligent biomimetic material for simulating a range of skin functions. For example, when it is attached to a plastic prosthetic finger, the prosthetic finger can sense external strain and temperature stimuli through capacitance and resistance signals, respectively, thus simulating the mechanical and temperature receptors of human skin and monitoring the bending and straightening motion of the finger according to the capacitance changes during deformation.
Based on the layered structure of the skin, Zhang et al. prepared a stretchable, conductive and super-wear-resistant hydrogel strain sensor with a double-layer structure by the surface in situ polymerization crosslinked by hydrophobic association [185]. The hydrogel can be used as a sensitive, wearable device to detect a wide range of human movements and small physiological signals by strain sensing. In addition, the outer layer of the adhesive provides the hydrogel with a solid ability to adhere to the skin and effects to block the electrical current of the hard conductive layer, thereby protecting the skin from electrical stimulation. A typical stretchable strain sensors was constructed based on multifunctional hydrogels prepared by the synergistic actions between polyacrylamide (PAM), biocompatible macromolecule sodium alginate (SA), and Ca2+. Moreover, Tang et al. reported a novel self-healing, antibacterial, and multifunctional wound dressing for sutureless wound closure and real-time monitoring of the healing parameters. The self-healing elastomer contains cetyltrimethylammonium bromide (CTAB) and has high mechanical toughness (35 MJ m(−3)), biocompatibility, and outstanding antibacterial activity (bactericidal rate reaches approximately 90% in 12 h), enabling the wound dressing to effectively inhibit bacterial growth and accelerate healing of the infected wound. The integrated sensor array within the multifunctional wound dressing can monitor the temperature, pH, and glucose level of the wound area in real-time, providing reliable and timely information of the condition of the wound. Ultimately, the reported multifunctional dressing would be of high value in managing the burden associated with wound healing via personalized monitoring and treatment approaches, digital and other targeted solutions for health care [186]. Furthermore, Dong et al. developed a multifunctional hydrogel dressing based on alginate and polycation for the treatment and monitoring of infected wounds. The hydrogel exhibits an excellent antibacterial property against both E. coli and S. aureus. The hydrogel shows multiple response modes of strain, pressure, temperature, and sensing stability in the further multifunctional sensory tests [187]. This work has provided the basis for the development of a smart hydrogel dressing to accelerate wound healing while also monitoring the healing process.
5. Conclusion and outlooks
Hydrogels can be made from water-soluble natural or synthetic polymers by modulating various experimental conditions such as pH, temperature, ionic strength, and ultraviolet radiation. Hydrogels are one of the most promising biological materials in antibacterial activity due to their 3D structure, high hydrophilicity, cell adhesion and biocompatibility. In this study, the research progress on antibacterial hydrogels in recent years was categorized according to different components, preparation methods, mechanisms, and antibacterial factors and was thoroughly reviewed. Adhesive hydrogels with natural antibacterial agents may enable broad applications. As a first example, in wound dressing and transdermal drug delivery, suitable adhesion between a hydrogel and the skin may be required for intimate contact without limiting the movement of the patient. The excellent antibacterial property and biocompatibility can inhibit infections from the external environment and ensure the basic vital signs of patients. Noninvasive, painless detach, more efficient, and anti-infection property is also required when the function of the hydrogel is completed. Compared with traditional pressure sensitive adhesive tape or bioinspired adhesive pad using micro-structures such as micro-suction cups, adhering a hydrogel to the skin may be more time-consuming. However, this strategy has advantages such as water-resistance, strong adhesion, painless detach, and wound healing benefits coming from the antibacterial hydrogel environment. It is hoped that the combined characteristics of adhesion and efficient antibacterial property will be developed using various chemical combinations to open the doors of innovation in medicine and engineering.
First, researchers must develop hydrogel dressings with precise and intelligent properties and well-regulated antibacterial activity. Since antibacterial requirements differ between patients and wound-healing stages, the adaptability of hydrogel dressings to microenvironmental changes and the controllability of an appropriate antibacterial response is required. In addition, antibacterial hydrogels are expected to shorten the wound healing duration in different wound environments, with their customizable antioxidant, anti-inflammatory and angiogenesis promoting properties, and other characteristics. Some hydrogels can be used as carriers for the controllable release of antibacterial agents, particularly natural antibacterial agents.
Finally, developing an intelligent wound-dressing system with real-time monitoring properties is necessary. In the case of state-of-the-art passive wound dressing treatments, it is still a challenge to achieve the optimal wound healing state and to control the dynamics and visualized monitoring of the wounds simultaneously. Combined with rapid developments in bioelectronic devices, a smart electronic comprehensive wound dressing promises to provide diagnosis at an early stage of infection through on-demand infection treatment and real-time monitoring. The integration and utilization of the adhesive, antibacterial, biocompatible, and mechanical properties of hydrogels to develop advanced wound dressings with comprehensive functions is a major challenge for researchers and medical staff to provide reliable wound treatments.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
H.Y., M.W. and L.L. contributed equally to this work. The work was supported in part by the National Natural Science Foundation of China (51973180), National Science Foundation for Young Scientists of Jiangsu Province (Grant No. BK20190903), Natural Science Foundation of the Jiangsu Higher Education Institutions of China (Grant No. 19KJB430043), Fundamental Research Funds for the Central Universities of China (20720220085), Yangzhou Social Development Project (YZ2020065), and Lvyangjinfeng Talent Program of Yangzhou. It is also supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2021R1A2C1008380), Nano Material Technology Development Program [NRF-2015M3A7B6027970].
Contributor Information
Liwei Lin, Email: lin-official@snu.ac.kr.
Dongan Wang, Email: dwang229@cityu.edu.hk.
Yuanzhe Piao, Email: parkat9@snu.ac.kr.
Data availability
Data will be made available on request.
References
- 1.Song J., Yuan C., Jiao T., Xing R., Yang M., Adams D.J., Yan X. Multifunctional antimicrobial biometallohydrogels based on amino acid coordinated self-assembly. Small. 2020;16(8) doi: 10.1002/smll.201907309. [DOI] [PubMed] [Google Scholar]
- 2.Lu Y., Wu Y., Liang J., Libera M.R., Sukhishvili S.A. Self-defensive antibacterial layer-by-layer hydrogel coatings with pH-triggered hydrophobicity. Biomaterials. 2015;45:64–71. doi: 10.1016/j.biomaterials.2014.12.048. [DOI] [PubMed] [Google Scholar]
- 3.Yuk H., Varela C.E., Nabzdyk C.S., Mao X., Padera R.F., Roche E.T., Zhao X. Dry double-sided tape for adhesion of wet tissues and devices. Nature. 2019;575(7781):169–174. doi: 10.1038/s41586-019-1710-5. [DOI] [PubMed] [Google Scholar]
- 4.Whiteley M., Diggle S.P., Greenberg E.P. Progress in and promise of bacterial quorum sensing research. Nature. 2017;551(7680):313–320. doi: 10.1038/nature24624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang H., Qadeer A., Mynarcik D., Chen W. Delivery of rosiglitazone from an injectable triple interpenetrating network hydrogel composed of naturally derived materials. Biomaterials. 2011;32(3):890–898. doi: 10.1016/j.biomaterials.2010.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saleh B., Dhaliwal H.K., Portillo-Lara R., Shirzaei Sani E., Abdi R., Amiji M.M., Annabi N. Local immunomodulation using an adhesive hydrogel loaded with miRNA-laden nanoparticles promotes wound healing. Small. 2019;15(36) doi: 10.1002/smll.201902232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie F., Jiang L., Xiao X., Lu Y., Liu R., Jiang W., Cai J. Quaternized polysaccharide-based cationic micelles as a macromolecular approach to eradicate multidrug-resistant bacterial infections while mitigating antimicrobial resistance. Small. 2022;18(12) doi: 10.1002/smll.202104885. [DOI] [PubMed] [Google Scholar]
- 8.Feng P., Luo Y., Ke C., Qiu H., Wang W., Zhu Y., Hou R., Xu L., Wu S. Chitosan-based functional materials for skin wound repair: mechanisms and applications. Front. Bioeng. Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.650598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang K., Han Q., Chen B., Zheng Y., Zhang K., Li Q., Wang J. Antimicrobial hydrogels: promising materials for medical application. Int. J. Nanomed. 2018;13:2217–2263. doi: 10.2147/IJN.S154748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan Z., Ye H., Wu D. Recent advances on polymeric hydrogels as wound dressings. APL Bioeng. 2021;5(1) doi: 10.1063/5.0038364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dumville J.C., Soares M.O., O'Meara S., Cullum N. Systematic review and mixed treatment comparison: dressings to heal diabetic foot ulcers. Diabetologia. 2012;55(7):1902–1910. doi: 10.1007/s00125-012-2558-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamoun E.A., Kenawy E.S., Chen X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017;8(3):217–233. doi: 10.1016/j.jare.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang X., Tan B., Wu Y., Zhang M., Liao J. A review on hydrogels with photothermal effect in wound healing and bone tissue engineering. Polymers. 2021;13(13):2100. doi: 10.3390/polym13132100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asadi N., Pazoki-Toroudi H., Del Bakhshayesh A.R., Akbarzadeh A., Davaran S., Annabi N. Multifunctional hydrogels for wound healing: special focus on biomacromolecular based hydrogels. Int. J. Biol. Macromol. 2021;170:728–750. doi: 10.1016/j.ijbiomac.2020.12.202. [DOI] [PubMed] [Google Scholar]
- 15.Lin H., Yin C., Mo A., Hong G. Applications of hydrogel with special physical properties in bone and cartilage regeneration. Materials. 2021;14(1):235. doi: 10.3390/ma14010235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park J.S., An S.J., Jeong S.I., Gwon H.J., Lim Y.M., Nho Y.C. Chestnut honey impregnated carboxymethyl cellulose hydrogel for diabetic ulcer healing. Polymers. 2017;9(7):248. doi: 10.3390/polym9070248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peng L., Chang L., Si M., Lin J., Wei Y., Wang S., Liu H., Han B., Jiang L. Hydrogel-coated dental device with adhesion-inhibiting and colony-suppressing properties. ACS Appl. Mater. Interfaces. 2020;12(8):9718–9725. doi: 10.1021/acsami.9b19873. [DOI] [PubMed] [Google Scholar]
- 18.Shi C., Wang C., Liu H., Li Q., Li R., Zhang Y., Liu Y., Shao Y., Wang J. Selection of appropriate wound dressing for various wounds. Front. Bioeng. Biotechnol. 2020;8:182. doi: 10.3389/fbioe.2020.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thoniyot P., Tan M.J., Karim A.A., Young D.J., Loh X.J. Nanoparticle-hydrogel composites: concept, design, and applications of these promising, multi-functional materials. Adv. Sci. 2015;2(1–2) doi: 10.1002/advs.201400010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pourshahrestani S., Zeimaran E., Kadri N.A., Mutlu N., Boccaccini A.R. Polymeric hydrogel systems as emerging biomaterial platforms to enable hemostasis and wound healing. Adv. Healthc. Mater. 2020;9(20) doi: 10.1002/adhm.202000905. [DOI] [PubMed] [Google Scholar]
- 21.Zawani M., Fauzi M.B. Injectable hydrogels for chronic skin wound management: a concise review. Biomedicines. 2021;9(5):527. doi: 10.3390/biomedicines9050527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang X., Xia L., Chen X., Chen Z., Wu F. Hydrogel-based phototherapy for fighting cancer and bacterial infection. Sci. China Mater. 2017;60(6):487–503. [Google Scholar]
- 23.Zhong Y., Xiao H., Seidi F., Jin Y. Natural polymerbased antimicrobial hydrogels without synthetic antibiotics as wound dressings. Biomacromolecules. 2020;21(8):2983–3006. doi: 10.1021/acs.biomac.0c00760. [DOI] [PubMed] [Google Scholar]
- 24.Alven S., Aderibigbe B.A. Chitosan and cellulose-based hydrogels for wound management. Int. J. Mol. Sci. 2020;21(24):9656. doi: 10.3390/ijms21249656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aderibigbe B.A., Buyana B. Alginate in wound dressings. Pharmaceutics. 2018;10(2):42. doi: 10.3390/pharmaceutics10020042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng B., Pei B., Wang Z., Hu Q. Advances in chitosan-based superabsorbent hydrogels. RSC Adv. 2017;7(67):42036–42046. [Google Scholar]
- 27.Tan H., Jin D., Sun J., Song J., Lu Y., Yin M., Chen X., Qu X., Liu C. Enlisting a traditional Chinese medicine to tune the gelation kinetics of a bioactive tissue adhesive for fast hemostasis or minimally invasive therapy. Bioact. Mater. 2021;6(3):905–917. doi: 10.1016/j.bioactmat.2020.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Catoira M.C., Fusaro L., Di Francesco D., Ramella M., Boccafoschi F. Overview of natural hydrogels for regenerative medicine applications. J. Mater. Sci. Mater. Med. 2019;30(10):115. doi: 10.1007/s10856-019-6318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun J., Tan H., Liu H., Jin D., Yin M., Lin H., Qu X., Liu C. A reduced polydopamine nanoparticle-coupled sprayable PEG hydrogel adhesive with anti-infection activity for rapid wound sealing. Biomater. Sci. 2020;8(24):6946–6956. doi: 10.1039/d0bm01213k. [DOI] [PubMed] [Google Scholar]
- 30.Fan L., Ge X., Qian Y., Wei M., Zhang Z., Yuan W.E., Ouyang Y. Advances in synthesis and applications of self-healing hydrogels. Front. Bioeng. Biotechnol. 2020;8:654. doi: 10.3389/fbioe.2020.00654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang L., Liu M., Zhang Y., Pei R. Recent progress of highly adhesive hydrogels as wound dressings. Biomacromolecules. 2020;21(10):3966–3983. doi: 10.1021/acs.biomac.0c01069. [DOI] [PubMed] [Google Scholar]
- 32.Dong Z., Sun Y., Chen Y., Liu Y., Tang C., Qu X. Injectable adhesive hydrogel through a microcapsule cross-link for periodontitis treatment. ACS Appl. Bio Mater. 2019;2(12):5985–5994. doi: 10.1021/acsabm.9b00912. [DOI] [PubMed] [Google Scholar]
- 33.Du X., Hou Y., Wu L., Li S., Yu A., Kong D., Wang L., Niu G. An anti-infective hydrogel adhesive with non-swelling and robust mechanical properties for sutureless wound closure. J. Mater. Chem. B. 2020;8(26):5682–5693. doi: 10.1039/d0tb00640h. [DOI] [PubMed] [Google Scholar]
- 34.Gowda A.H.J., Bu Y., Kudina O., Krishna K.V., Bohara R.A., Eglin D., Pandit A. Design of tunable gelatin-dopamine based bioadhesives. Int. J. Biol. Macromol. 2020;164:1384–1391. doi: 10.1016/j.ijbiomac.2020.07.195. [DOI] [PubMed] [Google Scholar]
- 35.Du X., Wu L., Yan H., Qu L., Wang L., Wang X., Ren S., Kong D., Wang L. Multifunctional hydrogel patch with toughness, tissue adhesiveness, and antibacterial activity for sutureless wound closure. ACS Biomater. Sci. Eng. 2019;5(5):2610–2620. doi: 10.1021/acsbiomaterials.9b00130. [DOI] [PubMed] [Google Scholar]
- 36.Gan D., Xing W., Jiang L., Fang J., Zhao C., Ren F., Fang L., Wang K., Lu X. Plant-inspired adhesive and tough hydrogel based on Ag-Lignin nanoparticles-triggered dynamic redox catechol chemistry. Nat. Commun. 2019;10(1):1487. doi: 10.1038/s41467-019-09351-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao L., Zhou Y., Peng J., Xu C., Xu Q., Xing M., Chang J. A novel dual-adhesive and bioactive hydrogel activated by bioglass for wound healing. NPG Asia Mater. 2019;11(1):1–11. [Google Scholar]
- 38.Han L., Lu X., Liu K., Wang K., Fang L., Weng L.T., Zhang H., Tang Y., Ren F., Zhao C., Sun G., Liang R., Li Z. Mussel-inspired adhesive and tough hydrogel based on nanoclay confined dopamine polymerization. ACS Nano. 2017;11(3):2561–2574. doi: 10.1021/acsnano.6b05318. [DOI] [PubMed] [Google Scholar]
- 39.He J., Shi M., Liang Y., Guo B. Conductive adhesive self-healing nanocomposite hydrogel wound dressing for photothermal therapy of infected full-thickness skin wounds. Chem. Eng. J. 2020;394 [Google Scholar]
- 40.Ke X., Dong Z., Tang S., Chu W., Zheng X., Zhen L., Chen X., Ding C., Luo J., Li J. A natural polymer based bioadhesive with self-healing behavior and improved antibacterial properties. Biomater. Sci. 2020;8(15):4346–4357. doi: 10.1039/d0bm00624f. [DOI] [PubMed] [Google Scholar]
- 41.Liu L., Xiang Y., Wang Z., Yang X., Yu X., Lu Y., Deng L., Cui W. Adhesive liposomes loaded onto an injectable, self-healing and antibacterial hydrogel for promoting bone reconstruction. NPG Asia Mater. 2019;11(1):1–18. [Google Scholar]
- 42.Pierau L., Versace D.L. Light and hydrogels: a new generation of antimicrobial materials. Materials. 2021;14(4):787. doi: 10.3390/ma14040787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma Y., Yao J., Liu Q., Han T., Zhao J., Ma X., Tong Y., Jin G., Qu K., Li B., Xu F. Liquid bandage harvests robust adhesive, hemostatic, and antibacterial performances as a first-aid tissue adhesive. Adv. Funct. Mater. 2020;30(39) [Google Scholar]
- 44.Mao Y., Li P., Yin J., Bai Y., Zhou H., Lin X., Yang H., Yang L. Starch-based adhesive hydrogel with gel-point viscoelastic behavior and its application in wound sealing and hemostasis. J. Mater. Sci. Technol. 2021;63:228–235. [Google Scholar]
- 45.Wahid F., Zhong C., Wang H.S., Hu X.H., Chu L.Q. Recent advances in antimicrobial hydrogels containing metal ions and metals/metal oxide nanoparticles. Polymers. 2017;9(12):636. doi: 10.3390/polym9120636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pinnaratip R., Bhuiyan M.S.A., Meyers K., Rajachar R.M., Lee B.P. Multifunctional biomedical adhesives. Adv. Healthc. Mater. 2019;8(11) doi: 10.1002/adhm.201801568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Turabee M.H., Thambi T., Lee D.S. Development of an injectable tissue adhesive hybrid hydrogel for growth factor-free tissue integration in advanced wound regeneration. ACS Appl. Bio Mater. 2019;2(6):2500–2510. doi: 10.1021/acsabm.9b00204. [DOI] [PubMed] [Google Scholar]
- 48.Wang Q., Zhang H., Pan X., Ma X., Cao S., Ni Y. Adhesive, transparent tannic acid@ sulfonated lignin-PAM ionic conductive hydrogel electrode with anti-UV, antibacterial and mild antioxidant function. Materials. 2019;12(24):4135. doi: 10.3390/ma12244135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang B., Song J., Jiang Y., Li M., Wei J., Qin J., Peng W., Lasaosa F.L., He Y., Mao H., Yang J., Gu Z. Injectable adhesive self-healing multicross-linked double-network hydrogel facilitates full-thickness skin wound healing. ACS Appl. Mater. Interfaces. 2020;12(52):57782–57797. doi: 10.1021/acsami.0c18948. [DOI] [PubMed] [Google Scholar]
- 50.Gao D., Zhang Y., Bowers D.T., Liu W., Ma M. Functional hydrogels for diabetic wound management. APL Bioeng. 2021;5(3) doi: 10.1063/5.0046682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ding F., Nie Z., Deng H., Xiao L., Du Y., Shi X. Antibacterial hydrogel coating by electrophoretic co-deposition of chitosan/alkynyl chitosan. Carbohydr. Polym. 2013;98(2):1547–1552. doi: 10.1016/j.carbpol.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 52.Gan D., Xu T., Xing W., Ge X., Fang L., Wang K., Ren F., Lu X. Mussel-inspired contact-active antibacterial hydrogel with high cell affinity, toughness, and recoverability. Adv. Funct. Mater. 2019;29(1) [Google Scholar]
- 53.Guo Z., Wang Y., Tan T., Ji Y., Hu J., Zhang Y. Antimicrobial d-peptide hydrogels. ACS Biomater. Sci. Eng. 2021;7(4):1703–1712. doi: 10.1021/acsbiomaterials.1c00187. [DOI] [PubMed] [Google Scholar]
- 54.Xie C., Wang X., He H., Ding Y., Lu X. Mussel-inspired hydrogels for self-adhesive bioelectronics. Adv. Funct. Mater. 2020;30(25):2676–2687. [Google Scholar]
- 55.Yang J., Bai R., Chen B., Suo Z. Hydrogel adhesion: a supramolecular synergy of chemistry, topology, and mechanics. Adv. Funct. Mater. 2019;30(2) [Google Scholar]
- 56.Xi J., Wu Q., Xu Z., Wang Y., Zhu B., Fan L., Gao L. Aloe-emodin/carbon nanoparticle hybrid gels with light-induced and long-term antibacterial activity. ACS Biomater. Sci. Eng. 2018;4(12):4391–4400. doi: 10.1021/acsbiomaterials.8b00972. [DOI] [PubMed] [Google Scholar]
- 57.Zhou Y., Zhao Y., Wang L., Xu L., Zhai M., Wei S. Radiation synthesis and characterization of nanosilver/gelatin/carboxymethyl chitosan hydrogel. Radiat. Phys. Chem. 2012;81(5):553–560. [Google Scholar]
- 58.Wahid F., Yin J., Xue D., Xue H., Lu Y., Zhong C., Chu L. Synthesis and characterization of antibacterial carboxymethyl chitosan/ZnO nanocomposite hydrogels. Int. J. Biol. Macromol. 2016;88:273–279. doi: 10.1016/j.ijbiomac.2016.03.044. [DOI] [PubMed] [Google Scholar]
- 59.Yang J., Chen Y., Zhao L., Feng Z., Peng K., Wei A., Wang Y., Tong Z., Cheng B. Preparation of a chitosan/carboxymethyl chitosan/AgNPs polyelectrolyte composite physical hydrogel with self-healing ability, antibacterial properties, and good biosafety simultaneously, and its application as a wound dressing. Compos. B Eng. 2020;197 [Google Scholar]
- 60.Ma H., Zhao Y., Lu Z., Xing R., Yao X., Jin Z., Wang Y., Yu F. Citral-loaded chitosan/carboxymethyl cellulose copolymer hydrogel microspheres with improved antimicrobial effects for plant protection. Int. J. Biol. Macromol. 2020;164:986–993. doi: 10.1016/j.ijbiomac.2020.07.164. [DOI] [PubMed] [Google Scholar]
- 61.Wang Y., Zhou Y., Li X., Huang J., Wahid F., Zhong C., Chu L. Continuous production of antibacterial carboxymethyl chitosan-zinc supramolecular hydrogel fiber using a double-syringe injection device. Int. J. Biol. Macromol. 2020;156:252–261. doi: 10.1016/j.ijbiomac.2020.04.073. [DOI] [PubMed] [Google Scholar]
- 62.Xiao X., Zhu Y., Liao J., Wang T., Sun W., Tong Z. High-efficient and synergetic antibacterial nanocomposite hydrogel with quaternized chitosan/Ag nanoparticles prepared by one-pot UV photochemical synthesis. Biopolymers. 2020;111(6) doi: 10.1002/bip.23354. [DOI] [PubMed] [Google Scholar]
- 63.Duplantier A.J., van Hoek M.L. The human cathelicidin antimicrobial peptide LL-37 as a potential treatment for polymicrobial infected wounds. Front. Immunol. 2013;4:143. doi: 10.3389/fimmu.2013.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang R., Li J., Chen W., Xu T., Yun S., Xu Z., Xu Z., Sato T., Chi B., Xu H. A biomimetic mussel-inspired ε-poly-l-lysine hydrogel with robust tissue-anchor and anti-infection capacity. Adv. Funct. Mater. 2017;27(8) [Google Scholar]
- 65.Wan Y., Liu L., Yuan S., Sun J., Li Z. pH-Responsive peptide supramolecular hydrogels with antibacterial activity. Langmuir. 2017;33(13):3234–3240. doi: 10.1021/acs.langmuir.6b03986. [DOI] [PubMed] [Google Scholar]
- 66.Arampatzioglou A., Papazoglou D., Konstantinidis T., Chrysanthopoulou A., Mitsios A., Angelidou I., Maroulakou I., Ritis K., Skendros P. Clarithromycin enhances the antibacterial activity and wound healing capacity in type 2 diabetes mellitus by increasing LL-37 load on neutrophil extracellular traps. Front. Immunol. 2018;9:2064. doi: 10.3389/fimmu.2018.02064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu L., Shen Q., Huang L., Xu X., He H. Charge-mediated co-assembly of amphiphilic peptide and antibiotics into supramolecular hydrogel with antibacterial activity. Front. Bioeng. Biotechnol. 2020;8 doi: 10.3389/fbioe.2020.629452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rodrigues B., Morais T.P., Zaini P.A., Campos C.S., Almeida-Souza H.O., Dandekar A.M., Nascimento R., Goulart L.R. Antimicrobial activity of Epsilon-poly-L-lysine against phytopathogenic bacteria. Sci. Rep. 2020;10(1) doi: 10.1038/s41598-020-68262-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Feng X., Zhang X., Li S., Zheng Y., Shi X., Li F., Guo S., Yang J. Preparation of aminated fish scale collagen and oxidized sodium alginate hybrid hydrogel for enhanced full-thickness wound healing. Int. J. Biol. Macromol. 2020;164:626–637. doi: 10.1016/j.ijbiomac.2020.07.058. [DOI] [PubMed] [Google Scholar]
- 70.Zhu H., Mei X., He Y., Mao H., Tang W., Liu R., Yang J., Luo K., Gu Z., Zhou L. Fast and high strength soft tissue bioadhesives based on a peptide dendrimer with antimicrobial properties and hemostatic ability. ACS Appl. Mater. Interfaces. 2020;12(4):4241–4253. doi: 10.1021/acsami.9b18720. [DOI] [PubMed] [Google Scholar]
- 71.Sarhan W.A., Azzazy H.M., El-Sherbiny I.M. Honey/chitosan nanofiber wound dressing enriched with allium sativum and cleome droserifolia: enhanced antimicrobial and wound healing activity. ACS Appl. Mater. Interfaces. 2016;8(10):6379–6390. doi: 10.1021/acsami.6b00739. [DOI] [PubMed] [Google Scholar]
- 72.Lee W.X., Basri D.F., Ghazali A.R. Bactericidal effect of pterostilbene alone and in combination with gentamicin against human pathogenic bacteria. Molecules. 2017;22(3):463. doi: 10.3390/molecules22030463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tenci M., Rossi S., Aguzzi C., Carazo E., Sandri G., Bonferoni M.C., Grisoli P., Viseras C., Caramella C.M., Ferrari F. Carvacrol/clay hybrids loaded into in situ gelling films. Int. J. Pharm. 2017;531(2):676–688. doi: 10.1016/j.ijpharm.2017.06.024. [DOI] [PubMed] [Google Scholar]
- 74.Alzagameem A., Klein S.E., Bergs M., Do X.T., Korte I., Dohlen S., Huwe C., Kreyenschmidt J., Kamm B., Larkins M., Schulze M. Antimicrobial activity of lignin and lignin-derived cellulose and chitosan composites against selected pathogenic and spoilage microorganisms. Polymers. 2019;11(4):670. doi: 10.3390/polym11040670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang W., Fortunati E., Bertoglio F., Owczarek J.S., Bruni G., Kozanecki M., Kenny J.M., Torre L., Visai L., Puglia D. Polyvinyl alcohol/chitosan hydrogels with enhanced antioxidant and antibacterial properties induced by lignin nanoparticles. Carbohydr. Polym. 2018;181:275–284. doi: 10.1016/j.carbpol.2017.10.084. [DOI] [PubMed] [Google Scholar]
- 76.Zhao M., Cheng J., Guo B., Duan J., Che C. Momilactone and related diterpenoids as potential agricultural chemicals. J. Agric. Food Chem. 2018;66(30):7859–7872. doi: 10.1021/acs.jafc.8b02602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rong L., Shen X., Wang B., Mao Z., Feng X., Sui X. Antibacterial thyme oil-loaded organo-hydrogels utilizing cellulose acetoacetate as reactive polymer emulsifier. Int. J. Biol. Macromol. 2020;147:18–23. doi: 10.1016/j.ijbiomac.2020.01.052. [DOI] [PubMed] [Google Scholar]
- 78.Zhao Q., Mu S., Long Y., Zhou J., Chen W., Astruc D., Gaidau C., Gu H. Tannin-tethered gelatin hydrogels with considerable self-healing and adhesive performances. Macromol. Mater. Eng. 2019;304(4) [Google Scholar]
- 79.Cui C., Shao C., Meng L., Yang J. High-strength, self-adhesive, and strain-sensitive chitosan/poly(acrylic acid) double-network nanocomposite hydrogels fabricated by salt-soaking strategy for flexible sensors. ACS Appl. Mater. Interfaces. 2019;11(42):39228–39237. doi: 10.1021/acsami.9b15817. [DOI] [PubMed] [Google Scholar]
- 80.Hao S., Shao C., Meng L., Cui C., Xu F., Yang J. Tannic acid-silver dual catalysis induced rapid polymerization of conductive hydrogel sensors with excellent stretchability, self-adhesion, and strain-sensitivity properties. ACS Appl. Mater. Interfaces. 2020;12(50):56509–56521. doi: 10.1021/acsami.0c18250. [DOI] [PubMed] [Google Scholar]
- 81.Fan L., Xie J., Zheng Y., Wei D., Yao D., Zhang J., Zhang T. Antibacterial, Self-adhesive, recyclable, and tough conductive composite hydrogels for ultrasensitive strain sensing. ACS Appl. Mater. Interfaces. 2020;12(19):22225–22236. doi: 10.1021/acsami.0c06091. [DOI] [PubMed] [Google Scholar]
- 82.Zhang S., Hou J., Yuan Q., Xin P., Cheng H., Gu Z., Wu J. Arginine derivatives assist dopamine-hyaluronic acid hybrid hydrogels to have enhanced antioxidant activity for wound healing. Chem. Eng. J. 2020;392 [Google Scholar]
- 83.Zhu W., Chuah Y.J., Wang D.A. Bioadhesives for internal medical applications: a review. Acta Biomater. 2018;74:1–16. doi: 10.1016/j.actbio.2018.04.034. [DOI] [PubMed] [Google Scholar]
- 84.Zhang Y., Chen Q., Dai Z., Dai Y., Xia F., Zhang X. Nanocomposite adhesive hydrogels: from design to application. J. Mater. Chem. B. 2021;9(3):585–593. doi: 10.1039/d0tb02000a. [DOI] [PubMed] [Google Scholar]
- 85.Chen Y., Wang Q., Li D., Mensah A., Qiu Y., Ke H., Wei Q. Mussel-inspired double cross-linked hydrogels with desirable mechanical properties, strong tissue-adhesiveness, self-healing properties and antibacterial properties. Mater. Sci. Eng. C Mater. Biol. Appl. 2021;120 doi: 10.1016/j.msec.2020.111690. [DOI] [PubMed] [Google Scholar]
- 86.Chen Y., Qiu Y., Wang Q., Li D., Hussain T., Ke H., Wei Q. Mussel-inspired sandwich-like nanofibers/hydrogel composite with super adhesive, sustained drug release and anti-infection capacity. Chem. Eng. J. 2020;399 [Google Scholar]
- 87.Jia Z., Lv X., Hou Y., Wang K., Ren F., Xu D., Wang Q., Fan K., Xie C., Lu X. Mussel-inspired nanozyme catalyzed conductive and self-setting hydrogel for adhesive and antibacterial bioelectronics. Bioact. Mater. 2021;6(9):2676–2687. doi: 10.1016/j.bioactmat.2021.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Khalil I.A., Saleh B., Ibrahim D.M., Jumelle C., Yung A., Dana R., Annabi N. Ciprofloxacin-loaded bioadhesive hydrogels for ocular applications. Biomater. Sci. 2020;8(18):5196–5209. doi: 10.1039/d0bm00935k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hou Y., Song Y., Sun X., Jiang Y., He M., Li Y., Chen X., Zhang L. Multifunctional composite hydrogel bolus with combined self-healing, antibacterial and adhesive functions for radiotherapy. J. Mater. Chem. B. 2020;8(13):2627–2635. doi: 10.1039/c9tb02967b. [DOI] [PubMed] [Google Scholar]
- 90.Wang R., Zhu J., Jiang G., Sun Y., Ruan L., Li P., Cui H. Forward wound closure with regenerated silk fibroin and polylysine-modified chitosan composite bioadhesives as dressings. ACS Appl. Bio Mater. 2020;3(11):7941–7951. doi: 10.1021/acsabm.0c01064. [DOI] [PubMed] [Google Scholar]
- 91.Pham T.N., Jiang Y.S., Su C.F., Jan J.S. In situ formation of silver nanoparticles-contained gelatin-PEG-dopamine hydrogels via enzymatic cross-linking reaction for improved antibacterial activities. Int. J. Biol. Macromol. 2020;146:1050–1059. doi: 10.1016/j.ijbiomac.2019.09.230. [DOI] [PubMed] [Google Scholar]
- 92.Chen Y., Zhang Y., Mensaha A., Li D., Wang Q., Wei Q. A plant-inspired long-lasting adhesive bilayer nanocomposite hydrogel based on redox-active Ag/Tannic acid-cellulose nanofibers. Carbohydr. Polym. 2021;255 doi: 10.1016/j.carbpol.2020.117508. [DOI] [PubMed] [Google Scholar]
- 93.Zhao X., Liang Y., Huang Y., He J., Han Y., Guo B. Physical double-network hydrogel adhesives with rapid shape adaptability, fast self-healing, antioxidant and NIR/pH stimulus-responsiveness for multidrug-resistant bacterial infection and removable wound dressing. Adv. Funct. Mater. 2020;30(17) [Google Scholar]
- 94.Qin Y., Qiu C., Hu Y., Ge S., Wang J., Jin Z. In situ self-assembly of nanoparticles into waxberry-like starch microspheres enhanced the mechanical strength, fatigue resistance, and adhesiveness of hydrogels. ACS Appl. Mater. Interfaces. 2020;12(41):46609–46620. doi: 10.1021/acsami.0c10327. [DOI] [PubMed] [Google Scholar]
- 95.Su X., Wang H., Tian Z., Duan X., Chai Z., Feng Y., Wang Y., Fan Y., Huang J. A solvent co-cross-linked organogel with fast self-healing capability and reversible adhesiveness at extreme temperatures. ACS Appl. Mater. Interfaces. 2020;12(26):29757–29766. doi: 10.1021/acsami.0c04933. [DOI] [PubMed] [Google Scholar]
- 96.Han N., Xu Z., Cui C., Li Y., Zhang D., Xiao M., Fan C., Wu T., Yang J., Liu W. A Fe(3+)-crosslinked pyrogallol-tethered gelatin adhesive hydrogel with antibacterial activity for wound healing. Biomater. Sci. 2020;8(11):3164–3172. doi: 10.1039/d0bm00188k. [DOI] [PubMed] [Google Scholar]
- 97.Yu R., Li M., Li Z., Pan G., Liang Y., Guo B. Supramolecular thermo-contracting adhesive hydrogel with self-removability simultaneously enhancing noninvasive wound closure and MRSA-infected wound healing. Adv. Healthc. Mater. 2022;11(13) doi: 10.1002/adhm.202102749. [DOI] [PubMed] [Google Scholar]
- 98.Wang R., Zhu J., Jiang G., Sun Y., Ruan L., Li P., Cui H. Forward wound closure with regenerated silk fibroin and polylysine-modified chitosan composite bioadhesives as dressings. ACS Appl. Bio Mater. 2020;3(11):7941–7951. doi: 10.1021/acsabm.0c01064. [DOI] [PubMed] [Google Scholar]
- 99.Yu Y., Yuk H., Parada G.A., Wu Y., Liu X., Nabzdyk C.S., Youcef-Toumi K., Zang J., Zhao X. Multifunctional "Hydrogel Skins" on diverse polymers with arbitrary shapes. Adv. Mater. 2019;31(7) doi: 10.1002/adma.201807101. [DOI] [PubMed] [Google Scholar]
- 100.Zheng Z., Bian S., Li Z., Zhang Z., Liu Y., Zhai X., Pan H., Zhao X. Catechol modified quaternized chitosan enhanced wet adhesive and antibacterial properties of injectable thermo-sensitive hydrogel for wound healing. Carbohydr. Polym. 2020;249 doi: 10.1016/j.carbpol.2020.116826. [DOI] [PubMed] [Google Scholar]
- 101.Yan J., Ji Y., Huang M., Li T., Liu Y., Lü S., Liu M. Nucleobase-inspired self-adhesive and inherently antibacterial hydrogel for wound dressing. ACS Mater. Lett. 2020;2(11):1375–1380. [Google Scholar]
- 102.Lei K., Wang K., Sun Y., Zheng Z., Wang X. Rapid-fabricated and recoverable dual-network hydrogel with inherently anti-bacterial abilities for potential adhesive dressings. Adv. Funct. Mater. 2020;31(6) [Google Scholar]
- 103.Khalil I.A., Saleh B., Ibrahim D.M., Jumelle C., Yung A., Dana R., Annabi N. Ciprofloxacin-loaded bioadhesive hydrogels for ocular applications. Biomater. Sci. 2020;8(18):5196–5209. doi: 10.1039/d0bm00935k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang Y., Garcia C.R., Ding Z., Gabrilska R., Rumbaugh K.P., Wu J., Liu Q., Li W. Adhesive, self-healing, and antibacterial chitosan hydrogels with tunable two-layer structures. ACS Sustain. Chem. Eng. 2020;8(49):18006–18014. [Google Scholar]
- 105.Tang X., Gu X., Wang Y., Chen X., Ling J., Yang Y. Stable antibacterial polysaccharide-based hydrogels as tissue adhesives for wound healing. RSC Adv. 2020;10(29):17280–17287. doi: 10.1039/d0ra02017f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Du X., Liu Y., Yan H., Rafique M., Li S., Shan X., Wu L., Qiao M., Kong D., Wang L. Anti-infective and pro-coagulant chitosan-based hydrogel tissue adhesive for sutureless wound closure. Biomacromolecules. 2020;21(3):1243–1253. doi: 10.1021/acs.biomac.9b01707. [DOI] [PubMed] [Google Scholar]
- 107.Gao Y., Wu K., Suo Z. Photodetachable adhesion. Adv. Mater. 2019;31(6) doi: 10.1002/adma.201806948. [DOI] [PubMed] [Google Scholar]
- 108.Renner L.D., Weibel D.B. Physicochemical regulation of biofilm formation. MRS Bull. 2011;36(5):347–355. doi: 10.1557/mrs.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Czaczyk K., Myszka K. Biosynthesis of extracellular polymeric substances (EPS) and its role in microbial biofilm formation. Pol. J. Environ. Stud. 2007;16(6):799–806. [Google Scholar]
- 110.Duan J., Liang X., Zhu K., Guo J., Zhang L. Bilayer hydrogel actuators with tight interfacial adhesion fully constructed from natural polysaccharides. Soft Matter. 2017;13(2):345–354. doi: 10.1039/c6sm02089e. [DOI] [PubMed] [Google Scholar]
- 111.Zhang W., Crocker E., McLaughlin S., Smith S.O. Binding of peptides with basic and aromatic residues to bilayer membranes. J. Biol. Chem. 2003;278(24):21459–21466. doi: 10.1074/jbc.M301652200. [DOI] [PubMed] [Google Scholar]
- 112.McLaughlin S., Wang J., Gambhir A., Murray D. PIP(2) and proteins: interactions, organization, and information flow. Annu. Rev. Biophys. Biomol. Struct. 2002;31:151–175. doi: 10.1146/annurev.biophys.31.082901.134259. [DOI] [PubMed] [Google Scholar]
- 113.Fan H., Wang J., Tao Z., Huang J., Rao P., Kurokawa T., Gong J.P. Adjacent cationic-aromatic sequences yield strong electrostatic adhesion of hydrogels in seawater. Nat. Commun. 2019;10(1):1–8. doi: 10.1038/s41467-019-13171-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Roy C.K., Guo H.L., Sun T.L., Ihsan A.B., Kurokawa T., Takahata M., Nonoyama T., Nakajima T., Gong J.P. Self-adjustable adhesion of polyampholyte hydrogels. Adv. Mater. 2015;27(45):7344–7348. doi: 10.1002/adma.201504059. [DOI] [PubMed] [Google Scholar]
- 115.Dobrynin A.V., Colby R.H., Rubinstein M. Polyampholytes. J. Polym. Sci. Part B-Polym. Phys. 2004;42(19):3513–3538. [Google Scholar]
- 116.Li J., Yu F., Chen G., Liu J., Li X., Cheng B., Mo X., Chen C., Pan J. Moist-retaining, self-recoverable, bioadhesive, and transparent in situ forming hydrogels to accelerate wound healing. ACS Appl. Mater. Interfaces. 2020;12(2):2023–2038. doi: 10.1021/acsami.9b17180. [DOI] [PubMed] [Google Scholar]
- 117.Dong Z., Sun Y., Chen Y., Liu Y., Tang C., Qu X. Injectable adhesive hydrogel through a microcapsule cross-link for periodontitis treatment. ACS Appl. Bio Mater. 2019;2(12):5985–5994. doi: 10.1021/acsabm.9b00912. [DOI] [PubMed] [Google Scholar]
- 118.Liang Y., Li Z., Huang Y., Yu R., Guo B. Dual-dynamic-bond cross-linked antibacterial adhesive hydrogel sealants with on-demand removability for post-wound-closure and infected wound healing. ACS Nano. 2021;15(4):7078–7093. doi: 10.1021/acsnano.1c00204. [DOI] [PubMed] [Google Scholar]
- 119.Allahverdiyev A.M., Kon K.V., Abamor E.S., Bagirova M., Rafailovich M. Coping with antibiotic resistance: combining nanoparticles with antibiotics and other antimicrobial agents. Expert Rev. Anti Infect. Ther. 2011;9(11):1035–1052. doi: 10.1586/eri.11.121. [DOI] [PubMed] [Google Scholar]
- 120.Gupta A., Briffa S.M., Swingler S., Gibson H., Kannappan V., Adamus G., Kowalczuk M., Martin C., Radecka I. Synthesis of silver nanoparticles using curcumin-cyclodextrins loaded into bacterial cellulose-based hydrogels for wound dressing applications. Biomacromolecules. 2020;21(5):1802–1811. doi: 10.1021/acs.biomac.9b01724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nirmala G.A., Pandian K. Antibacterial efficacy of aminoglycosidic antibiotics protected gold nanoparticles——a brief study. Colloid. Surf. Physicochem. Eng. Asp. 2007;297(1–3):63–70. [Google Scholar]
- 122.Roland S., Jolivalt C., Cresteil T., Eloy L., Bouhours P., Hequet A., Mansuy V., Vanucci C., Paris J.M. Investigation of a series of silver-N-heterocyclic carbenes as antibacterial agents: activity, synergistic effects, and cytotoxicity. Chemistry. 2011;17(5):1442–1446. doi: 10.1002/chem.201002812. [DOI] [PubMed] [Google Scholar]
- 123.Demain A.L., Sanchez S. Microbial drug discovery: 80 Years of progress. J. Antibiot. 2009;62(1):5–16. doi: 10.1038/ja.2008.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nisha A.R. Antibiotic residues-A global health hazard. Vet. World. 2018;1:375. [Google Scholar]
- 125.Leon-Buitimea A., Garza-Cardenas C.R., Garza-Cervantes J.A., Lerma-Escalera J.A., Morones-Ramirez J.R. The demand for new antibiotics: antimicrobial peptides, nanoparticles, and combinatorial therapies as future strategies in antibacterial agent design. Front. Microbiol. 2020;11:1669. doi: 10.3389/fmicb.2020.01669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gyawali R., Ibrahim S.A. Natural products as antimicrobial agents. Food Control. 2014;46:412–429. [Google Scholar]
- 127.Pisoschi A.M., Pop A., Georgescu C., Turcus V., Olah N.K., Mathe E. An overview of natural antimicrobials role in food. Eur. J. Med. Chem. 2018;143:922–935. doi: 10.1016/j.ejmech.2017.11.095. [DOI] [PubMed] [Google Scholar]
- 128.Tiwari B.K., Valdramidis V.P., O'Donnell C.P., Muthukumarappan K., Bourke P., Cullen P.J. Application of natural antimicrobials for food preservation. J. Agric. Food Chem. 2009;57(14):5987–6000. doi: 10.1021/jf900668n. [DOI] [PubMed] [Google Scholar]
- 129.Hoskin D.W., Ramamoorthy A. Studies on anticancer activities of antimicrobial peptides. Biochim. Biophys. Acta. 2008;1778(2):357–375. doi: 10.1016/j.bbamem.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shannon E., Abu-Ghannam N. Antibacterial derivatives of marine algae: an overview of pharmacological mechanisms and applications. Mar. Drugs. 2016;14(4):81. doi: 10.3390/md14040081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chen X., Niyonsaba F., Ushio H., Okuda D., Nagaoka I., Ikeda S., Okumura K., Ogawa H. Synergistic effect of antibacterial agents human beta-defensins, cathelicidin LL-37 and lysozyme against Staphylococcus aureus and Escherichia coli. J. Dermatol. Sci. 2005;40(2):123–132. doi: 10.1016/j.jdermsci.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 132.Gunes O.C., Ziylan Albayrak A. Antibacterial polypeptide nisin containing cotton modified hydrogel composite wound dressings. Polym. Bull. 2020;78(11):6409–6428. [Google Scholar]
- 133.Zhang W., Xiao P., Lin L., Guo F., Wang Q., Piao Y., Diao G. Study of a water-soluble supramolecular complex of curcumin and β-cyclodextrin polymer with electrochemical property and potential anti-cancer activity. Chin. Chem. Lett. 2021;33(8):4043–4047. [Google Scholar]
- 134.Moeini A., Pedram P., Makvandi P., Malinconico M., Gomez d'Ayala G. Wound healing and antimicrobial effect of active secondary metabolites in chitosan-based wound dressings: a review. Carbohydr. Polym. 2020;233 doi: 10.1016/j.carbpol.2020.115839. [DOI] [PubMed] [Google Scholar]
- 135.Selvam C., Jordan B.C., Prakash S., Mutisya D., Thilagavathi R. Pterocarpan scaffold: a natural lead molecule with diverse pharmacological properties. Eur. J. Med. Chem. 2017;128:219–236. doi: 10.1016/j.ejmech.2017.01.023. [DOI] [PubMed] [Google Scholar]
- 136.Huang S., Chen H., Deng Y., You X., Fang Q., Lin M. Preparation of novel stable microbicidal hydrogel films as potential wound dressing. Polym. Degrad. Stabil. 2020;181 [Google Scholar]
- 137.Zhang M., Yang M., Woo M.W., Li Y., Han W., Dang X. High-mechanical strength carboxymethyl chitosan-based hydrogel film for antibacterial wound dressing. Carbohydr. Polym. 2021;256 doi: 10.1016/j.carbpol.2020.117590. [DOI] [PubMed] [Google Scholar]
- 138.Wei X., Ma K., Cheng Y., Sun L., Chen D., Zhao X., Lu H., Song B., Yang K., Jia P. Adhesive, conductive, self-healing, and antibacterial hydrogel based on chitosan–polyoxometalate complexes for wearable strain sensor. ACS Appl. Polym. Mater. 2020;2(7):2541–2549. [Google Scholar]
- 139.Azoulay Z., Aibinder P., Gancz A., Moran-Gilad J., Navon-Venezia S., Rapaport H. Assembly of cationic and amphiphilic beta-sheet FKF tripeptide confers antibacterial activity. Acta Biomater. 2021;125:231–241. doi: 10.1016/j.actbio.2021.02.015. [DOI] [PubMed] [Google Scholar]
- 140.Um J., Manguy J., Anes J., Jacquier J.C., Hurley D., Dillon E.T., Wynne K., Fanning S., O'Sullivan M., Shields D.C. Enriching antimicrobial peptides from milk hydrolysates using pectin/alginate food-gels. Food Chem. 2021;352 doi: 10.1016/j.foodchem.2021.129220. [DOI] [PubMed] [Google Scholar]
- 141.Zhou L., Lei D., Wang Q., Luo X., Chen Y. Biocompatible polyphosphorylcholine hydrogels with inherent antibacterial and nonfouling behavior effectively promote skin wound healing. ACS Appl. Bio Mater. 2020;3(8):5357–5366. doi: 10.1021/acsabm.0c00666. [DOI] [PubMed] [Google Scholar]
- 142.Gunes O.C., Ziylan Albayrak A. Antibacterial polypeptide nisin containing cotton modified hydrogel composite wound dressings. Polym. Bull. 2020;78(11):6409–6428. [Google Scholar]
- 143.Hong Y., Xi Y., Zhang J., Wang D., Zhang H., Yan N., He S., Du J. Polymersome-hydrogel composites with combined quick and long-term antibacterial activities. J. Mater. Chem. B. 2018;6(39):6311–6321. doi: 10.1039/c8tb01608a. [DOI] [PubMed] [Google Scholar]
- 144.Ge J., Li Y., Wang M., Gao C., Yang S., Lei B. Engineering conductive antioxidative antibacterial nanocomposite hydrogel scaffolds with oriented channels promotes structure-functional skeletal muscle regeneration. Chem. Eng. J. 2021;425 [Google Scholar]
- 145.Li M., Liu X., Tan L., Cui Z., Yang X., Li Z., Zheng Y., Yeung Kelvin W.K., Chu P.K., Wu S. Noninvasive rapid bacteria-killing and acceleration of wound healing through photothermal/photodynamic/copper ion synergistic action of a hybrid hydrogel. Biomater. Sci. 2018;6(8):2110–2121. doi: 10.1039/c8bm00499d. [DOI] [PubMed] [Google Scholar]
- 146.Liu Y., Ma W., Wei Y., Xu Y. Photothermal effect-based cytotoxic ability of melanin from mytilus edulis shells to heal wounds infected with drug-resistant bacteria in vivo. Biomed. Environ. Sci. 2020;33(7):471–483. doi: 10.3967/bes2020.052. [DOI] [PubMed] [Google Scholar]
- 147.Zuo G.Y., Yang C.X., Han J., Li Y.Q., Wang G.C. Synergism of prenylflavonoids from morus alba root bark against clinical MRSA isolates. Phytomedicine. 2018;39:93–99. doi: 10.1016/j.phymed.2017.12.023. [DOI] [PubMed] [Google Scholar]
- 148.Noreen R., Intisar A., Ghaffar A., Jabeen F., Abid M.A., Din M.I., Irfan M., Faiz F., Sattar T. Constituents of volatile oil from bark of clerodendrum serratum (L.) and its antibacterial activity. J. Essent. Oil Bear. Plant. 2018;21(1):198–205. [Google Scholar]
- 149.Fan Y., Feng H., Liu L., Zhang Y., Xin X., Gao D. Chemical components and antibacterial activity of the essential oil of six pyrrosia species. Chem. Biodivers. 2020;17(10) doi: 10.1002/cbdv.202000526. [DOI] [PubMed] [Google Scholar]
- 150.Chirayath R.B., Viswanathan A., Jayakumar R., Biswas R., Vijayachandran L.S. Development of mangifera indica leaf extract incorporated carbopol hydrogel and its antibacterial efficacy against Staphylococcus aureus. Colloid. Surf. Biointer. 2019;178:377–384. doi: 10.1016/j.colsurfb.2019.03.034. [DOI] [PubMed] [Google Scholar]
- 151.Betts J.W., Wareham D.W., Haswell S.J., Kelly S.M. Antifungal synergy of theaflavin and epicatechin combinations against Candida albicans. J. Microbiol. Biotechnol. 2013;23(9):1322–1326. doi: 10.4014/jmb.1303.03010. [DOI] [PubMed] [Google Scholar]
- 152.Zuo G.Y., Zhang X.J., Han J., Li Y.Q., Wang G.C. In vitro synergism of magnolol and honokiol in combination with antibacterial agents against clinical isolates of methicillin-resistant Staphylococcus aureus (MRSA) BMC Compl. Alternative Med. 2015;15:425. doi: 10.1186/s12906-015-0938-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Young A., Jonski G., Rolla G. Combined effect of zinc ions and cationic antibacterial agents on intraoral volatile sulphur compounds (VSC) Int. Dent. J. 2003;53(4):237–242. doi: 10.1111/j.1875-595x.2003.tb00751.x. [DOI] [PubMed] [Google Scholar]
- 154.Ghosh I.N., Patil S.D., Sharma T.K., Srivastava S.K., Pathania R., Navani N.K. Synergistic action of cinnamaldehyde with silver nanoparticles against spore-forming bacteria: a case for judicious use of silver nanoparticles for antibacterial applications. Int. J. Nanomed. 2013;8:4721–4731. doi: 10.2147/IJN.S49649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gudimalla A., Jose J., Varghese R.J., Thomas S. Green synthesis of silver nanoparticles using nymphae odorata extract incorporated films and antimicrobial activity. J. Polym. Environ. 2020;29(5):1412–1423. [Google Scholar]
- 156.Zhang H., Cheng J., Ao Q. Preparation of alginate-based biomaterials and their applications in biomedicine. Mar. Drugs. 2021;19(5):264. doi: 10.3390/md19050264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lin L., Fang Y., Liao Y., Chen G., Gao C., Zhu P. 3D Printing and digital processing techniques in dentistry: a review of literature. Adv. Eng. Mater. 2019;21(6) [Google Scholar]
- 158.Lin L., Choi Y., Chen T., Kim H., Lee K.S., Kang J., Lyu L., Gao J., Piao Y. Superhydrophobic and wearable TPU based nanofiber strain sensor with outstanding sensitivity for high-quality body motion monitoring. Chem. Eng. J. 2021;419 [Google Scholar]
- 159.Lin L., Wang L., Li B., Luo J., Huang X., Gao Q., Xue H., Gao J. Dual conductive network enabled superhydrophobic and high performance strain sensors with outstanding electro-thermal performance and extremely high gauge factors. Chem. Eng. J. 2020;385 [Google Scholar]
- 160.Wang X., Li M., Fang Q., Zhao W., Lou D., Hu Y., Chen J., Wang X., Tan W. Flexible electrical stimulation device with chitosan-Vaseline(R) dressing accelerates wound healing in diabetes. Bioact. Mater. 2021;6(1):230–243. doi: 10.1016/j.bioactmat.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhang W., Lin L., Zhang L., Choi Y., Cho Y., Chen T., Gao J., Yao H., Piao Y. An in situ self-assembly dual conductive shell nanofiber strain sensor with superior sensitivity and antibacterial property. Adv. Mater. Interfac. 2021;9(4) [Google Scholar]
- 162.Zhang W., Piao S., Lin L., Yin Y., Guo J., Jiang Z., Cho Y., Li R., Gao J., Pang H., Piao Y. Wearable and antibacterial HPMC-anchored conductive polymer composite strain sensor with high gauge factors under small strains. Chem. Eng. J. 2022;435 [Google Scholar]
- 163.Ambekar R.S., Kandasubramanian B. Advancements in nanofibers for wound dressing: a review. Eur. Polym. J. 2019;117:304–336. [Google Scholar]
- 164.Francesko A., P P., Tzanov T. Hydrogel dressings for advanced wound management. Curr. Med. Chem. 2018;25(41):5782–5797. doi: 10.2174/0929867324666170920161246. [DOI] [PubMed] [Google Scholar]
- 165.Dabiri G D.E., Phillips T. Choosing a wound dressing based on common wound characteristics. Adv. Wound Care. 2016;5(1):32–41. doi: 10.1089/wound.2014.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Han H., Zhu J., Wu D., Li F., Wang X., Yu J., Qin X. Guanidine nanogels: inherent guanidine nanogels with durable antibacterial and bacterially antiadhesive properties. Adv. Funct. Mater. 2019;29(12) [Google Scholar]
- 167.Patel M., Nakaji-Hirabayashi T., Matsumura K. Effect of dual-drugreleasing micelle-hydrogel composite on wound healing in vivo in full-thickness excision wound rat model. J. Biomed. Mater. Res. 2019;107(5):1094–1106. doi: 10.1002/jbm.a.36639. [DOI] [PubMed] [Google Scholar]
- 168.Yuan Y., Shen S., Fan D. A physicochemical double cross-linked multifunctional hydrogel for dynamic burn wound healing: shape adaptability, injectable self-healing property and enhanced adhesion. Biomaterials. 2021;276 doi: 10.1016/j.biomaterials.2021.120838. [DOI] [PubMed] [Google Scholar]
- 169.Hilton J.R., Williams D.T., Beuker B., Miller D.R., Harding K.G. Wound dressings in diabetic foot disease. Clin. Infect. Dis. 2004;39:S100–S103. doi: 10.1086/383270. [DOI] [PubMed] [Google Scholar]
- 170.Chen J., He J., Yang Y., Qiao L., Hu J., Zhang J., Guo B. Antibacterial adhesive self-healing hydrogels to promote diabetic wound healing. Acta Biomater. 2022;146:119–130. doi: 10.1016/j.actbio.2022.04.041. [DOI] [PubMed] [Google Scholar]
- 171.Wang C., Wang M., Xu T., Zhang X., Lin C., Gao W., Xu H., Lei B., Mao C. Engineering bioactive self-healing antibacterial exosomes hydrogel for promoting chronic diabetic wound healing and complete skin regeneration. Theranostics. 2019;9(1):65–76. doi: 10.7150/thno.29766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chen H., Cheng R., Zhao X., Zhang Y., Tam A., Yan Y., Shen H., Zhang Y.S., Qi J., Feng Y., Liu L., Pan G., Cui W., Deng L. An injectable self-healing coordinative hydrogel with antibacterial and angiogenic properties for diabetic skin wound repair. NPG Asia Mater. 2019;11(1):1–12. [Google Scholar]
- 173.Liang Y., Li M., Yang Y., Qiao L., Xu H., Guo B. pH/Glucose dual responsive metformin release hydrogel dressings with adhesion and self-healing via dual-dynamic bonding for athletic diabetic foot wound healing. ACS Nano. 2022;16(2):3194–3207. doi: 10.1021/acsnano.1c11040. [DOI] [PubMed] [Google Scholar]
- 174.Zhang N., Gao T., Wang Y., Liu J., Zhang J., Yao R., Wu F. Modulating cationicity of chitosan hydrogel to prevent hypertrophic scar formation during wound healing. Int. J. Biol. Macromol. 2020;154:835–843. doi: 10.1016/j.ijbiomac.2020.03.161. [DOI] [PubMed] [Google Scholar]
- 175.Zhang Z., Zhang Y., Li W., Ma L., Wang E., Xing M., Zhou Y., Huan Z., Guo F., Chang J. Curcumin/Fe-SiO2 nano composites with multi-synergistic effects for scar inhibition and hair follicle regeneration during burn wound healing. Appl. Mater. Today. 2021;23 [Google Scholar]
- 176.Gao Z., Su C., Wang C., Zhang Y., Wang C., Yan H., Hou G. Antibacterial and hemostatic bilayered electrospun nanofibrous wound dressings based on quaternized silicone and quaternized chitosan for wound healing. Eur. Polym. J. 2021;159 [Google Scholar]
- 177.He J., Meng X., Meng C., Zhao J., Chen Y., Zhang Z., Zhang Y. Layer-by-layer pirfenidone/cerium oxide nanocapsule dressing promotes wound repair and prevents scar formation. Molecules. 2022;27(6):1830. doi: 10.3390/molecules27061830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Shan Y., Peng L., Liu X., Chen X., Xiong J., Gao J. Silk fibroin/gelatin electrospun nanofibrous dressing functionalized with astragaloside IV induces healing and anti-scar effects on burn wound. Int. J. Pharm. 2015;479(2):291–301. doi: 10.1016/j.ijpharm.2014.12.067. [DOI] [PubMed] [Google Scholar]
- 179.Jin M., Chen W., Li Z., Zhang Y., Zhang M., Chen S. Patterned bacterial cellulose wound dressing for hypertrophic scar inhibition behavior. Cellulose. 2018;25(11):6705–6717. [Google Scholar]
- 180.Xi Loh E.Y., Fauzi M.B., Ng M.H., Ng P.Y., Ng S.F., Ariffin H., Mohd Amin M.C.I. Cellular and molecular interaction of human dermal fibroblasts with bacterial nanocellulose composite hydrogel for tissue regeneration. ACS Appl. Mater. Interfaces. 2018;10(46):39532–39543. doi: 10.1021/acsami.8b16645. [DOI] [PubMed] [Google Scholar]
- 181.Gan D., Han L., Wang M., Xing W., Xu T., Zhang H., Wang K., Fang L., Lu X. Conductive and tough hydrogels based on biopolymer molecular templates for controlling in situ formation of polypyrrole nanorods. ACS Appl. Mater. Interfaces. 2018;10(42):36218–36228. doi: 10.1021/acsami.8b10280. [DOI] [PubMed] [Google Scholar]
- 182.Cheng C., Qiu Y., Tang S., Lin B., Guo M., Gao B., He B. Artificial spider silk based programmable woven textile for efficient wound management. Adv. Funct. Mater. 2021;32(6) [Google Scholar]
- 183.Tian S., Wang M., Wang X., Wang L., Yang D., Nie J., Ma G. Smart hydrogel sensors with antifreezing, antifouling properties for wound healing. ACS Biomater. Sci. Eng. 2022;8(5):1867–1877. doi: 10.1021/acsbiomaterials.1c01599. [DOI] [PubMed] [Google Scholar]
- 184.Lei Z., Wu P. A supramolecular biomimetic skin combining a wide spectrum of mechanical properties and multiple sensory capabilities. Nat. Commun. 2018;9(1):1134. doi: 10.1038/s41467-018-03456-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zhang Q., Liu X., Duan L., Gao G. Ultra-stretchable wearable strain sensors based on skin-inspired adhesive, tough and conductive hydrogels. Chem. Eng. J. 2019;365:10–19. [Google Scholar]
- 186.Tang N., Zhang R., Zheng Y., Wang J., Khatib M., Jiang X., Jiang X., Zhou C., Omar R., Saliba W., Wu W., Yuan M., Cui D., Haick H. Highly efficient self-healing multifunctional dressing with antibacterial activity for sutureless wound closure and infected wound monitoring. Adv. Mater. 2022;34(3) doi: 10.1002/adma.202106842. [DOI] [PubMed] [Google Scholar]
- 187.Dong H., Wang L., Du L., Wang X., Li Q., Wang X., Zhang J., Nie J., Ma G. Smart polycationic hydrogel dressing for dynamic wound healing. Small. 2022;18(25) doi: 10.1002/smll.202201620. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.