Abstract
The current study coupled fabric phase sorptive extraction (FPSE) with ultraperformance liquid chromatography method with electrospray ionization and tandem mass detection (UPLC-ESI-MS/MS) for fast and sensitive determination of tadalafil (TAD) in a bioequivalence study. Fabric phase sorptive extraction allowed direct extraction of TAD from the sample matrix with improved selectivity, repeatability, and recoveries. A sol–gel Carbowax 20 M (CX-20 M) coated FPSE membrane revealed the best extraction efficiency for TAD because of its strong affinity for analytes via intermolecular interactions, high mass transfer rate to FPSE membrane, and high permeability. An automated multiple reaction monitoring (MRM) optimizer was employed for the best selection of the precursor and product ions, ion breakdown profile, the fine adjustment of the fragmentor voltages for each precursor ions, and the collision energies for the product ions. The chromatographic separation was conducted using a mobile phase A: 5.0 mM ammonium acetate with 0.1 % formic acid in water and mobile phase B: formic acid (0.1%) in acetonitrile in ratio (55:45, v/v) through isocratic elution mode on an Agilent EclipsePlus C18 (50 × 2.1 mm, 1.8 μm) column and the flow rate was adjusted at 0.4 mL min−1. The total run time per sample was 1.0 min. The method was validated by FDA standards for bioanalytical method validation over a concentration range of 0.1–100 ng mL−1 with a correlation coefficient of 0.9993 and the lower limit of quantitation (LLOQ) was 0.1 ng mL−1 in rat plasma. Intra- and inter-assay precision (%RSD) were lower than 4.1% and accuracy (%RE) was within 2.4%. The developed FPSE-UPLC-ESI-MS/MS method was effectively used in a randomized, two-way, single-dose, crossover study to compare the bioequivalence of two TAD formulations from different companies in male rats and verified to be bioequivalent.
Keywords: Fabric phase sorptive extraction, Tadalafil, Sildenafil, Automated MRM Optimization, UPLC-MS/MS, Bioequivalence study
1. Introduction
Erectile dysfunction (ED) is the male's inability to acquire and keep an adequate erection of the penis to allow acceptable sexual intercourse (Thomas, 2002). ED represents a common problem affecting over 150 million men and is firmly linked with cardiovascular disease especially among older men (Travison et al., 2007). Tadalafil (TAD) is a potent and selective phosphodiesterase enzyme inhibitor type-5 (PDE5), the predominant isozyme that metabolizes cyclic guanosine monophosphate (cGMP) in the penis corpus cavernosum (Brock et al., 2002, Padma-Nathan, 2003). Its inhibition causes a rise in levels of the intracellular cGMP and thus boosts the nitric oxide–cGMP cascade, this results in augmented smooth muscle relaxation and hence, prolongation of the erection (Kuan and Brock, 2002). TAD also exhibits a higher selectivity for PDE5 compared to the first authorized PDE5 inhibitor, sildenafil (SLD). In comparison to SLD, it has shown efficacy and safety as an oral ED treatment as it is 1000 times selective for PDE5 while SLD is 41 times (Bischoff, 2004). Tadalafil also has a prolonged half-life (17.5 h) attributed to delayed hepatic clearance, low volume of distribution, and high bioavailability (80%). TAD's pharmacokinetic features allow for a longer duration of action (12–36 h) with once-daily dosage, making it easier to reclaim sexual erection (Washington and Shindel, 2010). Because of these pharmacological characteristics, there has been an interest in creating a novel formulation with TAD for ED therapy (Kim et al., 2018). Thus, there is a need to develop rapid and sensitive bioanalytical method targeting quantitation of TAD in bioavailability and bioequivalence studies.
Thus far, several bioanalytical methods for quantifying TAD as a single analyte or in combination with other PDE5 inhibitors in different biological samples are currently available in the literature. Diverse bioanalytical techniques were employed for the determination of TAD involving; fluorometry (Abu El-Enin et al., 2016), gas-chromatography with mass spectrometry (GC–MS) (Strano-Rossi et al., 2010, Nikolaou et al., 2011), high performance liquid chromatography with ultraviolet detection (HPLC-UV) (Cheng and Chou, 2005, Shakya et al., 2007), HPLC with fluorescence detection (Farthing et al., 2010), liquid chromatography with mass spectrometry (LC-MS) (Tanaka et al., 2020), liquid chromatography with tandem mass spectrometry (LC-MS/MS)(Ramakrishna et al., 2004, Rust et al., 2012, Unceta et al., 2012, Lee et al., 2013, Ma et al., 2013, Bhadoriya et al., 2018, de Souza Madeira et al., 2021) and ultraperformance liquid chromatography with tandem mass spectrometry (UPLC-MS/MS)(Proença et al., 2013, Kim et al., 2017). Although a variety of analytical methods were available, many of them lacked the sensitivity and selectivity required to quantify trace amounts of TAD in biological samples (Cheng and Chou, 2005, Shakya et al., 2007, Abu El-Enin et al., 2016). Some of these methods needed a rather long run time of more than 5 min, making them unsuitable for high-throughput TAD determination (Farthing et al., 2010, Tanaka et al., 2020).
Traditional sample preparation techniques for measuring TAD in biological samples involves protein precipitation, liquid–liquid extraction (LLE) and solid phase extraction (SPE). Although, protein precipitation is a simple technique, it is not sufficient to remove all interfering plasma components. LLE necessitates complex and time-consuming sample preparation procedures, large amounts of organic solvents that are incompatible with green chemistry standards. Additionally, SPE suffered from various problems as clogging and batch-to-batch variations. These drawbacks affect the application of these techniques in routine analysis of TAD in biological samples (Ramakrishna et al., 2004, Rust et al., 2012, Lee et al., 2013, Ma et al., 2013, Bhadoriya et al., 2018). The current trend in sample preparation in biological samples is the adoption of novel sample preparation procedures to overcome these shortcomings in analysis of TAD.
Recently, fabric phase sorptive extraction (FPSE) was introduced as a new innovation of sorbent-based sorptive microextraction that integrates the advanced features of sol–gel derived sorbents with the flexibility and permeability of fabric substrates to produce a device for simple, rapid, and reproducible sample preparation workflow. This technique provides several advantages including large surface area with the ability to directly separate and preconcentrate the analytes even in complex biological matrices making it best suited for routine analysis. Moreover, FPSE reduces the number of sample preparation steps, lowering the risk of analyte loss and improve extraction robustness (Kabir et al., 2013). A cellulose cotton or polyester fabric substrate is working via the anticipated chemistry in the FPSE membrane, resulting in a durable and adaptable microextraction device with high permeability, quick extraction equilibrium, and excellent chemical stability without significant loss of extraction performance. Furthermore, FPSE can use the hydrophobicity features of the fabric substrates to adjust the selectivity of the FPSE media (Locatelli et al., 2021).
Sol-gel coating technology, utilized to produce FPSE microextraction membrane, is a simple, highly efficient, and reproducible chemical coating method that gives coating uniformity with variable thickness (Samanidou et al., 2016b). In FPSE, the porous fabric substrate employed to retain sol–gel sorbents resembles the SPE extraction process, resulting in quicker mass transfer and high extraction recovery. Because of the flexibility of FPSE media, it may be inserted directly into the sample container, eliminating the requirement for numerous transfer processes, that could result in analyte loss (Alampanos et al., 2020). To adjust the polarity of the extraction sorbent, a wide range of commercially available organic polymers, macromolecules, and organically modified sol–gel precursors with various polarities, may be employed in the sol solution scheme (Mazaraki et al., 2021).
The new automated optimizer tools have been introduced for MRM optimization that greatly increase method sensitivity, enhance method reproducibility, and lowers the time required for MRM method development, especially for multi-analyte MRM techniques that need the optimization of more than two MRMs for several analytes (Mastovska et al., 2017). The MRM optimizer tool offers a great degree of versatility for automating the optimization of MRM conditions such as the selection of the best precursor and product ions, fine optimization of fragmentor voltage and collision energy (CE) values. Hence, the MRM optimizer tool was employed to assist the MRM optimization procedures in the developed UPLC-MS/MS methodology.
The current work was intended to use FPSE combined with UPLC-MS/MS to develop a rapid, sensitive, and reliable method for the quantitation of TAD in rat plasma. FPSE has
been assessed for the first time for the extraction of TAD and SLD, the internal standard (IS), from plasma samples. Various FPSE media were evaluated for the most efficient performance criteria. The method was validated in agreement with FDA guidelines for bioanalytical method validation U.S. Department of Health and Human Services, 2018 then employed to study bioequivalence of TAD in rats.
2. Experimental
2.1. Chemicals and reagents
TAD reference standard (99.0%) was gifted from SAJA Pharmaceuticals company (Jeddah, Saudi Arabia) and SLD reference standard (97.5%) purchased from Acros Organics (Geel, Belgium). Sigma Aldrich (Seelze, Germany) provided sol–gel active polymers of polyethylene glycol Carbowax-20 M (CX 20 M), polydimethylsiloxane (PDMS), polydimethyldiphenylsiloxane (PDMDPS), polytetrahydrofuran (PTHF), ammonium acetate, and formic acid. Substrates for FPSE media were provided by Jo-Ann Fabric (Miami, FL, USA). A Millipore water filtration system (Bedford, MA, USA) was utilized to get ultra-pure water. Pharmaceutical formulations were bought from the local pharmacy; Cials®20 mg tablets (Eli Lilly Company, Indianapolis, IN, USA) and Snafi®20 mg tablets (Spimaco, Riyadh, Saudi Arabia). Sprague Dawley male rats (190–220 g) supplied from Animal house, Taibah University (AlMadinah AlMunawarah, Saudi Arabia).
2.2. Preparation of standard and quality control samples
TAD and SLD stock solutions were made by dissolving the accurately weighed quantities in methanol at a concentration of 1 mg mL−1 and were stored at 4 °C in light protected flasks. The calibration standards for TAD were prepared by serial dilution with pooled blank plasma from rats to obtain final concentrations of 0.1, 0.5, 2, 10, 20, and 100 ng mL−1. Quality control (QC) samples were made in the same manner in blank rat plasma at lower limit of quantitation (LLQC; 0.1 ng mL−1), low (LQC; 0.5 ng mL−1), middle (MQC; 10 ng mL−1), and high (HQC; 100 ng mL−1) concentrations. The calibration standards and quality control samples were kept at − 30 ◦C in sealed, light-resistant vials. Before being assayed, all rat plasma samples were defrosted immediately at ambient temperature.
2.3. FPSE sample preparation protocol
Prior to analyte extraction, the FPSE membrane were conditioned by soaking it in 2 mL of methanol for 2 min. The FPSE was held with tweezers to stay away from direct contact. The FPSE membrane was then rinsed 2/3 times in ultra-pure water to eliminate any remaining organic solvents, making it ready for the extraction. A 100-μL aliquot of plasma was placed into a screw-capped glass vial (5-mL) containing cleaned FPSE membrane, followed by 10 μL of the IS solution (SLD; 500 ng mL−1) and 500-μL of ultra-pure water. In the vial, a clean teflon-coated magnetic stirrer was placed. To achieve appropriate analyte diffusion, the magnetic stirrer was set at the medium level for 20 min. The FPSE membrane was then placed in a clean vial with 500-μL methanol and stirred for 10 min for back extraction of the analytes. The eluate was dried under a nitrogen stream and reconstituted with 100-μL methanol before injection of 5-μL into the UPLC–ESI-MS/MS system.
2.4. UPLC-ESI-MS/MS conditions
The UPLC-ESI-MS/MS analysis was performed on an Agilent UPLC system (Agilent, CA, United States) comprised of a 1260 Infinity II quaternary pump with an integrated degassing unit and a column thermostat and a 1260 Infinity II auto-sampler (Agilent Technologies) and NG CASTORE XS iQ nitrogen generator (LNI Swissgas, Switzerland). The chromatographic separation was run on an EclipsePlus C18 RRHD (50 × 2.1 mm, 1.8 μm) column (Agilent) operating in reversed phase mode at a flowrate of 0.4 mL min−1 using a mobile phase A: 5.0 mM ammonium acetate with 0.1 % formic acid in water and mobile phase B: formic acid (0.1%) in acetonitrile in ratio (55:45, v/v). The column was held at 40 °C and equilibrated with the mobile phase. An Ultivo triple quadrupole mass spectrometer was connected to the UPLC system (Agilent Technologies). TAD was measured by positive electrospray ionization (ESI) in the MRM mode. Mass Hunter Quantitative Data Analysis software was used to process the data. The TAD and IS mass parameters were optimized through Mass Hunter optimizer software using on column mode with the standard solutions at a concentration of 100 ng mL−1 in the same mobile phase. The quantifier MRM transitions were selected based on the most intense fragment ion. The measurement was achieved using MRM mode with the transition’s m/z 390.2 → 268.0 for TAD and 475.2 → 283.0 for SLD. The dwell time was adjusted to 25 ms for each MRM transition. The ESI Jet Stream source parameters were tuned to provide the strongest MRM signals. They were: gas temperature, 300 °C; gas flow, 10 L/min; nebulizer gas, 15 psi; capillary voltage, 4000 V; fragmentor voltage, 135 V for TAD and 170 for SLD (IS); collision energy (CE) 10 V for TAD and 45 V for SLD (IS).
2.5. Validation of analytical method
The developed methodology was validated in compliance with the FDA guidance for bioanalytical method validation in the United States (U.S. Department of Health and Human Services) (U.S. Department of Health and Human Services, 2018).
To evaluate method selectivity, blank rat plasma samples from six separate rats were spiked with TAD and IS at the lower limit of quantitation (LLOQ) level to see if interfering elements were present at the analytes' retention times. The peak areas of the co-eluting substances have to be less than 20% of the TAD response at the LLOQ level.
The method's linearity was assessed by constructing the calibration curve (n = 5) for TAD using six different concentration levels covering a concentration range of 0.1–100 ng mL−1, which corresponded to the expected concentration range after a single oral dosage of TAD. The calibration curve was built by the least squares' linear regression of the peak area ratio of TAD to IS vs the analyte concentrations. The deviations of the re-calculated concentrations from the spiked levels were limited to 15% of the expected value (only 20% for the LLOQ). The method's sensitivity was assessed by calculating the LLOQ. LLOQ was calculated using five replicates in three consecutive runs as the lowest the calibration curve level with less than 20% precision and accuracy.
The intra-day and inter-day's precision and accuracy were calculated by five replicates analysis of TAD QC samples at four concentration levels: LLOQ (0.5 ng mL−1), low (LQC; 0.5 ng mL−1), middle (MQC; 10 ng mL−1), and high (HQC; 100 ng mL−1) on three successive working days. The precision was determined as a percentage of the relative standard deviations (RSD%), and the accuracy as the relative error (RE%) between the calculated and real levels. The RSD percent for the tested samples should be within 15%, and the accuracy should be less than 15%, while the LLOQ should be less than 20%.
To expand the upper quantitation limit with appropriate precision and accuracy, the dilution integrity was performed. It was estimated for five replicates by diluting ultrahigh quality control sample (UHQC) (500 ng mL−1) to 100 ng mL−1 (5-times) and 10 ng mL−1 (50-times) using blank rat plasma. The diluted samples were processed and measured by the developed FPSE-UPLC-ESI-MS/MS method and compared with the real level. The acceptable values for analysis of TAD samples for the accuracy (RE%) and precision (RSD%) were within 15%.
The stabilities of TAD were evaluated by making three sets of QC samples at three QC levels LQC, MQC, and HQC (0.5 ng mL−1, 10 ng mL−1 and 100 ng mL−1) and was treated under a variety of storage and handling situations. Bench-top stability was determined by allowing triplicates of frozen QC samples to defrost at room temperature for 6 h on the bench-top. Whereas the long-term stability was tested by keeping at − 30 °C for 30 days then permitting to equilibrate with room temperature. The freeze–thaw stability was evaluated after three freeze–thaw cycles (freezing for 24 h at − 30 °C, then defrosting at ambient temperature). The processed sample stability was evaluated by reanalyzing the QC samples after storage in an autosampler at room temperature for 24 h. The results were determined by comparison of the peak areas of the samples to newly prepared QC samples with the same level. The samples were judged stable if results were less than 15% of RE.
2.6. Application to a bioequivalence study
The developed FPSE-UPLC-ESI-MS/MS method was utilized to determine plasma level of TAD in a bioequivalence study of test and reference formulations in a randomized, two-way, crossover study after oral single dose administration to Sprague Dawley male rats. As stated in FDA regulations for bioequivalence and bioavailability studies, a pilot study could be used to test method applicability in real cases. The test and reference tablets were Cials®20 mg TAD tablets from Eli Lilly Company (Indianapolis, USA) and Snafi®20 mg TAD tablets from Spimaco (Riyadh, Saudi Arabia), respectively. The study was performed as per U.S. Department of Health and Human Services, Food and Drug Administration guidelines for bioavailability and bioequivalence Studies (Studies). The Ethic Committee of college of pharmacy, Taibah University approved the study protocol (Approval number: COPTU-REC-17–20210705). A total number of 20 Sprague Dawley male rats (190–220 g), as a model animal, from the animal house of Taibah University were used in the study. Animals were maintained separately in cages with wire-mesh made of galvanized steel for one week prior the rat experiments for acclimation in a room with regulated lighting and a controlled room temperature (25 °C). Sick, wounded, and abnormal animals were excluded. The rats were separated into two groups (n = 10): Group A- given Cials®20 mg TAD tablets, whereas Group B given Snafi®20 mg TAD tablets. The two test and reference tablets were crushed, suspended and provided by oral gavage to rats in 5 mL of water in a dose of 1 mg/kg as stated in previous report (Lee et al., 2013). Blood samples were taken from retro-orbital plexuses (0.3 mL) before (0 h) and after at 0.5, 1, 2, 4, 6, 8, 12, and 24 h in heparinized tubes, then centrifuged at 15,000 rpm for 5 min. The supernatant plasma was gathered and kept in deep-freezer (−30 °C) till analysis. Before analysis, the frozen samples were defrosted and left to equilibrate at ambient temperature then diluted 5 times with blank rat plasma for fitting within the calibration range. TAD pharmacokinetic parameters were assessed using a non-compartmental analysis model by a moment analysis program from the plasma concentration vs time curve. Area under the curve (AUC), Area under the first moment curve (AUMC), Mean Residence Time (MRT), terminal phase half-life (T1/2), clearance (CL), maximum concentration (Cmax) and the first occurrence of Cmax (Tmax) were the assessed pharmacokinetic parameters.
3. Results and discussion
3.1. Method development of UPLC-ESI-MS/MS with automated MRM optimization
The UPLC-MS/MS is the gold standard for quantitative determination in a variety of applications, particularly when the analyte of interest to be measured in a complex biological matrix because of its selectivity, sensitivity, and analysis speed. To achieve the best results for the target analytes, it is necessary to make fine optimization for the developed UPLC-MS/MS. SLD was selected as the I.S. in this study because it has comparable chemical characteristics to TAD.
The optimization of MRM is an essential part in UPLC-MS/MS method development. A newly introduced MassHunter Optimizer program provided a flexible tool for automating the optimization of MRM settings, such as the choice of precursor and product ions as well as the fine tuning of collision energies and fragmentor voltages (Higton, 2001, Mastovska et al., 2017). Under ESI conditions, TAD and the I.S. have superior sensitivity in positive ion detection mode compared to the negative ion detection mode attributed to the presence of a basic amine functional group in TAD and SLD structures (Kim et al., 2017). To enhance ionization in the positive ion mode, a low-pH mobile phase containing 0.1% formic acid was used.
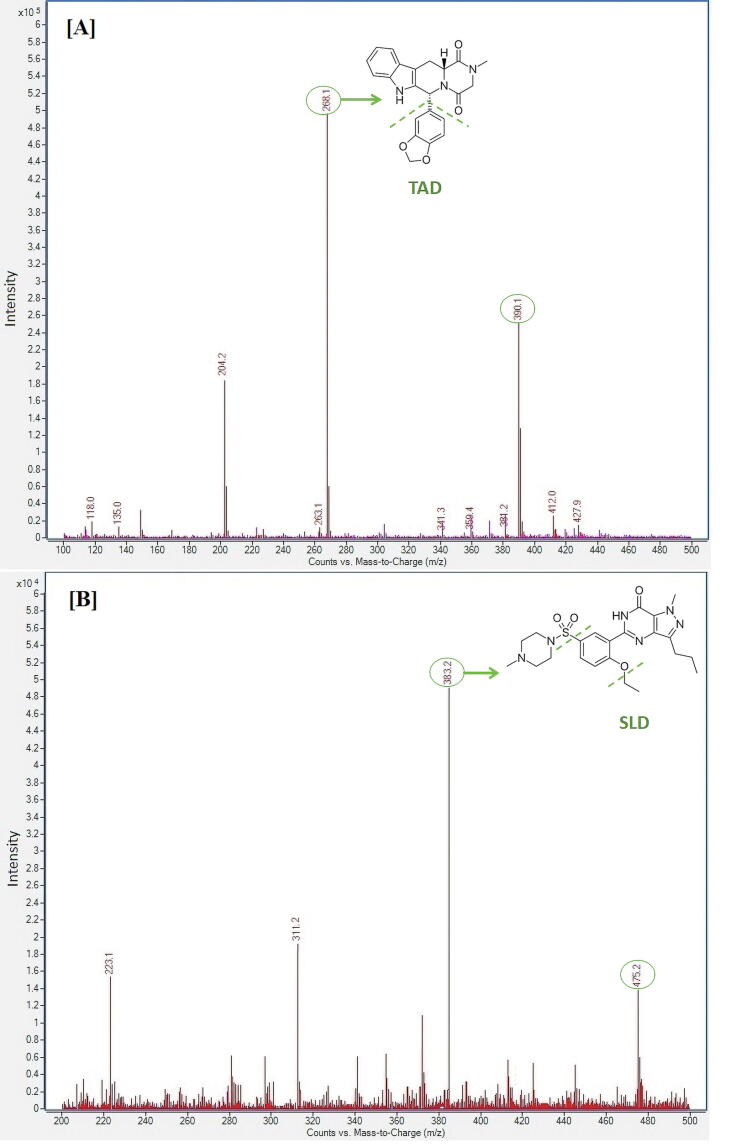
The precursor ions were optimized for TAD and SLD using the optimizer program for positive ions, various adducts and charge states for the most abundant precursor ions. Low abundant precursor ions and ions with m/z ≤ 100 were excluded from consideration. The monitored m/z values were determined in selected ion monitoring (SIM) scan mode based on the entered formula, compound structure, mobile phase content and ionization mode. The obtained spectra revealed prominent protonated molecular ions [M + H]+ at m/z 390.2 and 475.2 for TAD and SLD, respectively (Fig. 1 [A] and [B]). Product ions were chosen for TAD and SLD using the MRM optimizer program as the most abundant ions in a composite product ion scan spectrum acquired for the given precursor ion at different collision energies. The optimizer settings were adjusted for the two most abundant ions. For TAD, the most abundant product ions were found at m/z 268.0 and 204.0 while for SLD the most abundant peaks were at m/z 283.0 and 311.2. The collision-induced dissociation mass spectra showed extremely stable and strong product ions at m/z 268.0 corresponding to the loss of benzodioxole fraction from TAD and at m/z 283.0 corresponding to loss of (4-methylpiperazin-1-yl) sulfonyl and ethyl moieties from SLD (Fig. 1).
Fig. 1.
Collision-induced dissociation mass spectrum of (A) TAD and (B) SLD (I.S.) in positive ion electrospray ionization mode. The MRM transitions was m/z 390.2 → 268 for TAD and 475.2 → 283 for SLD.
Additionally, the fragmentor voltage and collision energy of Q1 and Q3 were appropriately tuned for TAD and SLD using the optimizer program to meet the necessary specificity and sensitivity of the developed UPLC-ESI-MS/MS method as seen in Fig. S1 for TAD and Fig. S2 for SLD. The system firstly adjusts the coarse fragmentor voltage, which produce the maximum ion intensity of the selected precursor ions, and then refines the fragmentor voltage by stepping in smaller increments to provide the best response. The tested fragmentor voltage range was from 100 to 200 V. The maximum ion intensity was obtained at 135 V for TAD m/z 390.2 and at 170 V for SLD m/z 475.2. Thus, it was chosen as the optimum MRM settings.
The CE optimization entails the acquisition of the precursor ion to product ion transition at variable CEs keeping capillary voltage at 4000 V. The CEs were varied across the selected CE range 0 to 100 V for the two selected transitions: 390.2 → 268.0 and 390.2 → 204.0 for TAD while 475.2 → 283 and 475.2 → 311.2 for SLD. The highest abundance for TAD was seen for transition 390.2 → 268.0 at 10 V for TAD while the highest abundance for SLD was found for transition 475.2 → 283.0 at 45 V. Therefore, it was selected as the optimum MRM settings. The dwell time was also adjusted for the best detector response and a dwell time ≤ 25 ms is advised for the mentioned MRM transitions.
Afterward, UPLC conditions were optimized for the best resolution, peak shape and analytical response in a short retention time to suite the high-throughput ability in clinical studies. At first, we investigated several C18 reversed-phase columns including Kromasil (150 mm × 4.6 mm,1.8 μm), Hypersil BDS C18 column (150 × 4.6 mm,3 µm), and EclipsePlus C18 RRHD (50 × 2.1 mm,1.8 μm). The best resolution, peak shape, and analysis time for TAD and SLD was obtained using EclipsePlus C18 RRHD (50 × 2.1 mm,1.8 μm). The effect of mobile phase composition peak separation on the UPLC and analysis time was studied of both TAD and IS. Several organic solvents were investigated involving methanol, ACN and ethanol. It was observed that acetonitrile afforded better resolution, sensitivity, and short analysis time (less than 1.0 min) for both TAD and IS, as well as the advantage of lower backpressure on the column. Methanol and ethanol were not sufficient for complete separation of the investigated drugs. Therefore, ACN was chosen for subsequent work. Additionally, it was discovered that both TAD and IS needed ammonium acetate in the buffer to achieve effective ionization and fragmentation in the MRM detection. Hence the effect of ammonium acetate concentration was tested in the range 1–20 mM. The best detector response for TAD and IS was seen at 5 mM ammonium acetate for 55% buffer ratio in the mobile phase. In addition, adding formic acid to acetonitrile and ammonium acetate buffer was necessary to produce efficient ionization and provide sharp peaks. Hence formic acid 0.01–1% was tested in the mobile phase and 0.1% formic acid was the best. Under the optimum UPLC conditions, TAD and IS were eluted quickly with less than 1.0 min that is suitable for high-throughput assays in clinical studies.
3.2. Optimization of FPSE extraction protocol
As the developed UPLC-ESI-MS/MS was intended to improve high-throughput effectiveness in clinical exams, the FPSE was chosen as a sample preparation approach One of the most significant advantages of using FPSE is its ability to extract target analytes directly from biological samples by simple immersion in the extraction fluid without previous sample preprocessing (Locatelli et al., 2021). However, it was necessary to optimize the FPSE extraction procedures from plasma samples for several FPSE parameters including the sorbent type, stirring time, and back-extraction solvent type volume.
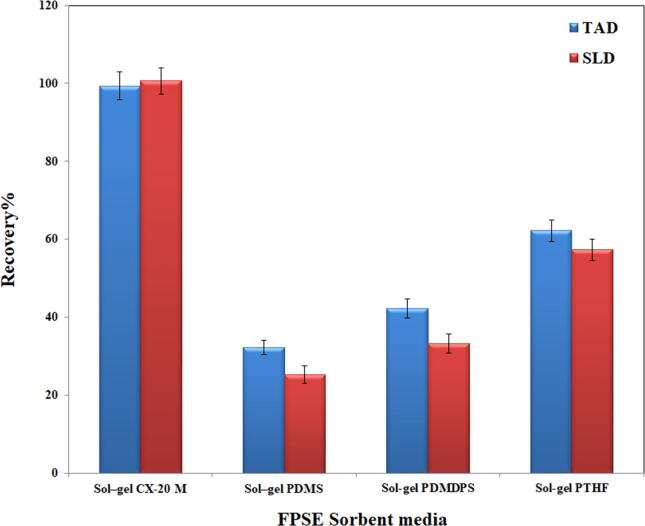
In this work, four different FPSE sorbent media, via the sol–gel coating process on hydrophilic cellulose fabric substrate, were evaluated in the current methodology for plasma extraction of TAD. These FPSE includes sol–gel silica Carbowax® 20 M with 8.63 mg/cm2 sorbent loading, sol–gel polydimethylsiloxane (sol–gel PDMS) with a sorbent loading of 4.56 mg/cm2, sol–gel polydimethyldiphenylsiloxane (sol–gel PDMDPS), with a sorbent loading of 1.93 mg/cm2 sol–gel polytetrahydrofuran (sol–gel PTHF), with a sorbent loading of 3.96 mg/cm2. All these FPSE media are recommended for medium and less polar aromatic compounds. Preparation of these FPSE media were according to previous reports (Roldán-Pijuán et al., 2015, Samanidou et al., 2016a, Samanidou et al., 2016b). Fig. 2 shows recovery% of TAD and SLD (I.S) using the investigated FPSE sorbent media. The sol–gel CX 20 M was shown to be the best FPSE sorbent membrane for TAD and SLD extraction because of its strong affinity for analytes via intermolecular interactions, high mass transfer rate to FPSE membrane, and high permeability. Stirring duration was also optimized in the 5–30-minute range, and the extraction equilibrium was detected after 20 min of stirring, with no additional improvement after that period. The back extraction solvent was tested using methanol, acetonitrile, ethanol, and methanol: acetonitrile (1:1). The maximum extraction recovery was observed for methanol. It was discovered that preliminary proteins precipitation is not needed during the back extraction since methanol not only extract analytes from the FPSE membrane, but it also accomplishes protein precipitation. The volume of back extracting solvent is an essential parameter that requires to be optimized to enhance the extraction recovery. Different volumes of methanol in the range 0.1–1 mL were tested. The recovery% increased till 500-µL of methanol and then reached to a plateau.
Fig. 2.
Effect of different FPSE sorbent media on the extraction recoveries of TAD and SLD (I.S.).
To investigate the performance of the selected FPSE membrane, the FPSE extraction recoveries and matrix effect were tested using sol–gel CX 20 M at the three concentration levels (0.5 ng mL−1, 10 ng mL−1 and 100 ng mL−1). The test was performed by comparison of the peak area response of plasma samples spiked before FPSE extraction at five replicates to unextracted analytes of equivalent concentration and was represented as recovery %. Results were shown in Table 1. It was observed to be within 97.95–99.36% for TAD and 98.12–100.6% for IS indicating good extraction recovery with the adopted FPSE sample preparation method. The matrix effect was evaluated by comparison of the peak area ratio of plasma samples spiked with TAD and SLD after FPSE extraction to that of pure standard of equivalent concentrations of TAD and SLD and was expressed as IS normalized matrix factors (MF) (Table 1). The obtained MF was 0.943–0.998 for TAD and 0.957–0.990 for IS. The IS-normalized matrix factors were ranged from 0.985 to 1.008. These findings reveal that the extraction method gives satisfactory extraction efficacy without substantial interference from coeluted plasma matrix substances with TAD and IS peaks.
Table 1.
Extraction recovery and matrix effect of TAD and IS in rat plasma samples using sol–gel CX 20 M at the three concentration levels and assay by the developed FPSE-UPLC-ESI- MS/MS method.
| QC samples |
Recovery* (%) |
Matrix factor* |
|||
|---|---|---|---|---|---|
| TAD | IS | TAD | IS | IS-normalized | |
| LQC (0.5 ng mL -1) | 97.95 | 98.12 | 0.943 | 0.957 | 0.985 |
| MQC (10 ng mL -1) | 98.76 | 99.55 | 0.972 | 0.980 | 0.992 |
| HQC (100 ng mL -1) | 99.36 | 100.6 | 0.998 | 0.990 | 1.008 |
*Average of five determinations.
3.3. Validation of the developed UPLC-ESI-MS/MS method
Validation experiments were carried out in accordance with US-FDA requirements for bioanalytical method validation to ensure that the proposed FPSE-UPLC-ESI-MS/MS method is valid for quantitation of TAD in the plasma samples for bioequivalence studies.
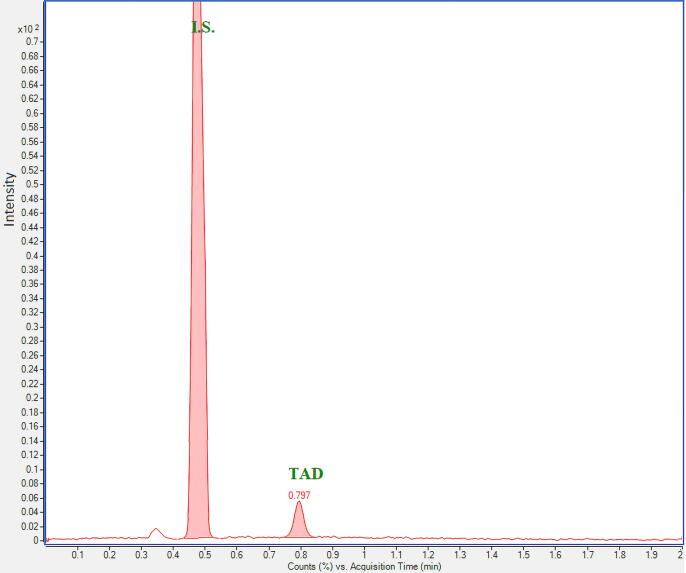
Selectivity was tested using blank rat plasma samples spiked with TAD at LLOQ level and IS plasma samples. A typical MRM chromatogram of TAD at 0.1 ng mL−1 and IS (50 ng mL−1) spiked in blank rat plasma is displayed in Fig. 3. It is obvious that no interfering peaks from endogenous substances were identified at the retention times of TAD (0.797 min) and IS (0.476 min) in rat plasma samples from six separate sources. The co-eluting components did not exceed 5% of the TAD peak area at LLOQ.
Fig. 3.
MRM chromatogram of blank rat plasma spiked with TAD at LLOQ (0.1 ng mL−1) and the I.S. (50 ng mL−1) (transition 390.2 → 268 for TAD and m/z 475.2 → 283 for IS).
The calibration graph for TAD showed good linearity across the tested concentration range 0.1–100 ng mL−1 with a determination coefficient (r2) of 0.9986 and correlation coefficient (r) of 0.9993 (Table 2). The noted deviation for the re-calculated concentrations from the spiked samples was within ± 10% of the expected value. The method's sensitivity was evaluated using five replicates and the LLOQ of TAD in rat plasma was 0.1 ng mL−1 with RSD% and RE% of 4.2% and − 2.54 %, respectively. The slope of the calibration graph was 3.154 (±0.16) reflecting the sensitivity of the developed UPLC-ESI-MS/MS method. The developed method was compared to reported methods for its sensitivity using LLOQ concentration. It was noticed to be 5–100 times more sensitive compared to HPLC-UV methods (Cheng and Chou, 2005, Shakya et al., 2007), 1000 times than HPLC-fluorescence method (Farthing et al., 2010), 20–25 times GC–MS methods (Strano-Rossi et al., 2010, Nikolaou et al., 2011), and 5–20 times than LC-MS/MS (Ramakrishna et al., 2004, Rust et al., 2012, Unceta et al., 2012, Lee et al., 2013, Ma et al., 2013, Proença et al., 2013, Kim et al., 2017, Bhadoriya et al., 2018, de Souza Madeira et al., 2021).
Table 2.
The linear regression analyses for the calibration curve data and sensitivity of TAD in rat plasma analyzed by the developed FPSE-UPLC-ESI-MS/MS method.
| Validation parameter | TAD regression data |
|---|---|
| Linearity and rangea, b | |
| Calibration range (ng mL−1) | 0.1–100 |
| Calibration equation | y = 3.154 X + 0.0024 |
| Slope (±SD) | 3.154 (±0.16) |
| Intercept (±SD) | 0.0024 (±0.0001) |
| Determination coefficient (r2) | 0.9986 |
| Correlation coefficient (r) | 0.9993 |
| Sensitivity | |
| LLOQ (ng mL−1) | 0.1 |
a Peak area ratio of the TAD/SLD (I.S.) against concentrations (ng mL−1).
b Data presented as mean (n = 5) ± SD.
The intra-day and inter-day's precision and accuracy for TAD analysis in rat plasma were evaluated for the concentration levels LLOQ, LQC, MQC, and HQC (Table 3). The intra-day precision, expressed as RSD%, was varied from 1.64 to 4.08% while inter-day's precision was ranged from 2.06 to 5.15%. On the other hand, the intra-day accuracy, as RE% was within − 1.2–1.44% and the inter-day's accuracy was between − 2.5–1.84%. the calculated and true concentrations. Accordingly, the accuracy and precision of the developed FPSE-UPLC-ESI-MS/MS method proved excellent reproducibility in clinical studies.
Table 3.
Precision and accuracy for assay of TAD quality control samples in rat plasma using the developed FPSE-UPLC-ESI-MS/MS method.
| QC sample | Intra-day (n = 5) | Inter-day(n = 3) | ||||
|---|---|---|---|---|---|---|
|
Calculated conc.(ng mL -1) (Mean ± SD) |
RSD (%) |
RE (%) |
Calculated conc.(ng mL -1) (Mean ± SD) |
RSD (%) |
RE (%) |
|
| LLOQ (0.1 ng mL -1) LQC (0.5 ng mL -1) MQC (10 ng mL -1) HQC (100 ng mL -1) |
0.098 ± 0.004 0.495 ± 0.015 10.120 ± 0.215 101.44 ± 1.66 |
4.08 3.03 2.15 1.64 |
−1.2 − 1.0 1.2 1.44 |
0.097 ± 0.005 0.488 ± 0.020 9.950 ± 0.240 101.84 ± 2.10 |
5.15 4.10 2.41 2.06 |
−2.5 − 2.4 − 0.5 1.84 |
The upper concentration limit was extended to UHQC (500 ng mL−1) then the samples was diluted with blank rat plasma by 5–fold and 50–folds (Table 4). The accuracy (RE%) for dilution integrity of 5- and 50-times dilution was found to be − 1.0 and 1.6 whereas the precision (RSD%) results was 2.63 % and 2.56%, respectively. Hence, the upper concentration limit could be extended to 500 ng mL−1 with acceptable accuracy and precision.
Table 4.
Dilution integrity of TAD with blank rat plasma and assay using the developed FPSE-UPLC-ESI-MS/MS method.
| QC sample | Dilution times |
Actual conc. (ng mL -1) |
Calculated conc* ± SD (ng mL -1) |
Accuracy (RE%) |
Precision (RSD%) |
|---|---|---|---|---|---|
| UHQC (500 ng mL -1) |
5 50 |
100 10 |
100.30 ± 2.64 9.75 ± 0.25 |
− 1.0 1.6 |
2.63 2.56 |
*Average of five determinations.
The stabilities of TAD in rat plasma were estimated under a diversity of storing and processing conditions and the results of stability tests are summarized in Table 5. The results of the benchtop stability for the TAD were ranged from − 1.6 to − 0.1% as RE% with precision (RSD%) of 1.85–2.52%, whereas the long-term stability results were varied from − 2.6 to 1.1% with RSD% of 2.04–3.70%. The freeze–thaw stability was found to be − 3.8–0.25% with RSD% of 2.05–4.57% while the processed sample stability showed − 2.0–0.5% with RSD% of 1.83–2.25%. The performed stability experiments demonstrate no apparent degradation results given that the RE% and RSD% for all samples were within 15%. Accordingly, TAD was stable in rat plasma under the storage and processing situations and was suitable for the bioequivalence study.
Table 5.
Stability results for TAD in rat plasma under a variety of storage conditions and assay using the developed FPSE-UPLC-ESI-MS/MS method.
|
Stability tests |
Actual conc. (ng mL -1) |
Calculated conc.(ng mL -1) (Mean ± SD) |
RSD% | RE% |
|---|---|---|---|---|
| Benchtop stability | 0.5 10 100 |
0.492 ± 0.012 9.98 ± 0.251 99.90 ± 1.850 |
2.44 2.52 1.85 |
−1.60 − 0.19 − 0.10 |
| Long term stability | 0.5 10 100 |
0.487 ± 0.018 10.11 ± 0.219 99.46 ± 1.015 |
3.70 2.17 2.04 |
−2.60 1.10 − 0.54 |
| Freeze–thaw stability | 0.5 10 100 |
0.481 ± 0.022 9.85 ± 0.263 100.22 ± 2.054 |
4.57 2.67 2.05 |
−3.8 − 1.54 0.22 |
| Processed sample stability | 0.5 10 100 |
0.490 ± 0.011 10.05 ± 0.195 99.48 ± 1.824 |
2.25 1.94 1.83 |
−2.0 0.50 − 0.52 |
a Average of five determinations.
3.4. Application to bioequivalence studies
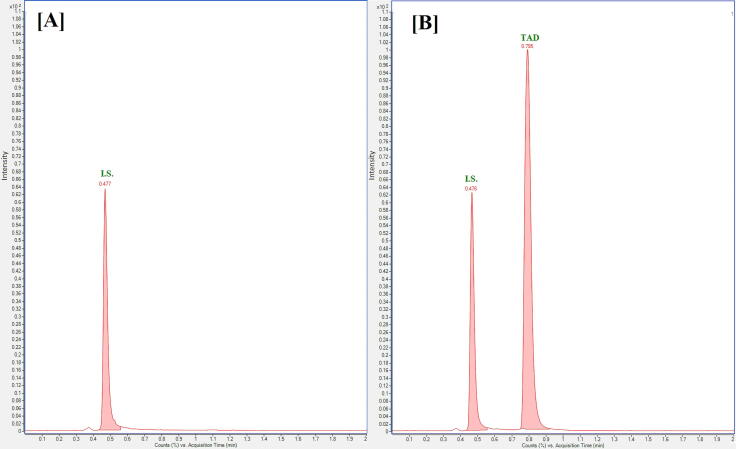
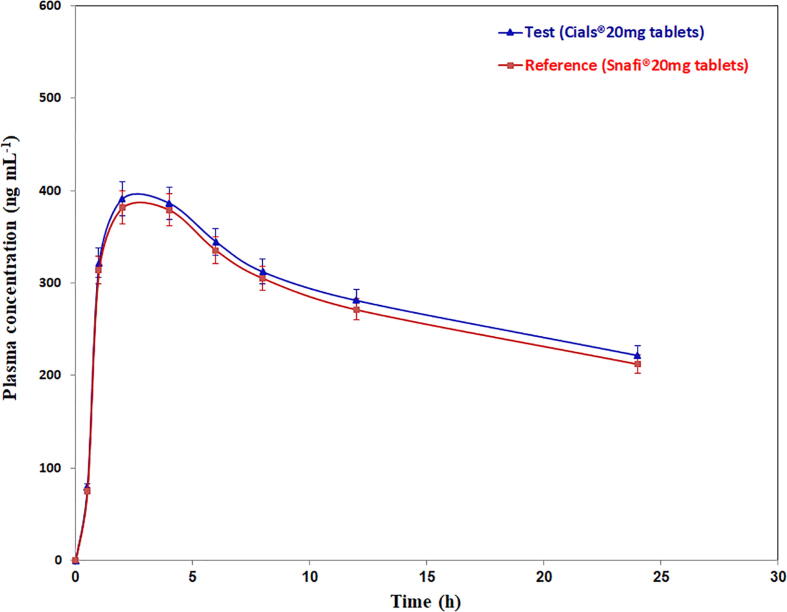
Although the primary goal of a bioequivalence study is to ensure the effectiveness of the test formulation, the aim of this work was to guarantee that the developed FPSE-UPLC-ESI-MS/MS method applicable in a real clinical bioequivalence study. The developed methodology was employed to study bioequivalence of two commercial tablets including TAD in a pilot study after a single oral dose of a test and a reference tablet to male rats. Cials®20 mg TAD tablets and Snafi®20 mg TAD tablets were used as test and reference formulations. The bioequivalence study was constructed in a randomized, two-way, crossover design that minimizes the variances in the experimental model (Guidance for Industry: Bioavailability and Bioequivalence Guidance for Industry, 2004). The blood samples were gathered at 0, 0.5, 1, 2, 4, 6, 8, 12, and 24 h of test and reference tablets administration and then analyzed by the developed FPSE-UPLC-ESI-MS/MS method. MRM chromatograms of rat plasma collected before (0 h) and after 2 h from Cials®20 mg TAD administration, diluted 5 times with blank rat plasma and spiked with IS are seen in Fig. 4. The mean plasma concentration–time profile of TAD attained for test and reference tablets in the bioequivalence study after single oral dose administration is exhibited in Fig. 5. The main pharmacokinetic parameters of the test and reference formulations were assessed using non-compartmental analysis model using moment analysis program as displayed in Table 6. It was discovered that TAD was absorbed into the systemic circulation with a peak concentration (Cmax) of 396.45 and 381.4 ng mL−1 for the test and reference formulations in about 2.0 h. The terminal phase half-life of TAD (T1/2) was 30.0 and 28.64 h for the test and reference formulations. The method proved sufficient sensitivity to quantify plasma levels of TAD up to 24 h. While the data were compared to previous studies, there were no significant differences in any pharmacokinetic parameter (Ramakrishna et al., 2004, Ma et al., 2013, Bhadoriya et al., 2018, Shao et al., 2022). The formulations were compared in 90% confidence interval and were within the acceptance criterion of 80%–125%. These findings verify the bioequivalence of the test and reference formulations in terms of rate and extent of absorption. Consequently, the developed FPSE-UPLC-ESI-MS/MS method proved to be effective for clinical and bioequivalence studies of TAD.
Fig. 4.
MRM chromatograms of rat plasma collected before (0 h) (A), after 2 h (B) from TAD administration (Cials®20 mg tablets), diluted 5 times with blank rat plasma and spiked with (50 ng mL−1) from IS and analyzed by the developed FPSE-UPLC-ESI-MS/MS method (transition 390.2 → 268 for TAD and m/z 475.2 → 283 for IS).
Fig. 5.
Plasma concentration–time profile of TAD in a bioequivalence study after oral administration of reference and test tablets to male rats. Data are represented as mean ± S.D. (n = 10).
Table 6.
Pharmacokinetic parameters of TAD in rat plasma for reference and test formulations after oral administration using the developed FPSE-UPLC-ESI-MS/MS method.
| Pharmacokinetic parameter | Mean (n = 10) ± SD | |
|---|---|---|
| Test (Cials®20 mg tablets) |
Reference (Snafi®20 mg tablets) |
|
| Cmax, ng mL−1 Tmax, h AUC0-t, ng.h mL−1 AUC0-∞, ng.h mL−1 AUMC0-t, ng.h mL−1 AUMC0-∞, ng.h mL−1 MRT, h T1/2, h CL, mL hr-1 |
391.5 ± 20.8 2.0 ± 0.5 6851.7 ± 351.6 16345.1 ± 831.5 75156.9 ± 3712.0 713546.6 ± 31215.7 10.97 ± 0.6 30.0 ± 1.2 145.9 ± 7.9 |
381.4 ± 18.6 2.0 ± 0.5 6636.3 ± 345.3 15328 ± 763.7 72448.4 ± 3596.9 640860.2 ± 29578.3 10.92 ± 0.6 28.64 ± 1.1 150.7 ± 8.2 |
4. Conclusion
An efficient, fast, sensitive, and reproducible FPSE-UPLC-ESI-MS/MS method was developed and validated for the quantitation of TAD in a bioequivalence study. FPSE provided a significant simplification of the TAD sample preparation protocol by direct extraction from plasma, removal of protein precipitation step, and the use of little amounts of organic solvent. It also offers better selectivity, repeatability, and recoveries in comparison with previous approaches. The automated MRM optimizer provided significantly worth advantages in method sensitivity and decreased the time required for MRM optimizations. Compared to the reported methods in the literature, this method is 5–100 times more sensitive with a short run time of 1.0 min that suits the high-throughput ability in clinical studies. The developed method was successfully employed in a bioequivalence study of TAD after a single oral dose in rats with a great criterion.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors express great thanks to King Abdulaziz City for Science and Technology (KACST), Saudi Arabia for funding this work (project no. 13-NAN86-05). Also, the authors extend their appreciation to Taibah University Science and Technology Unit for its supervision and support.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jsps.2022.06.001.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Abu El-Enin M.A.B., Al-Ghaffar Hammouda M.-S., El-Sherbiny D.T., El-Wasseef D.R., El-Ashry S.M. Validated spectrofluorimetric method for determination of two phosphodiesterase inhibitors tadalafil and vardenafil in pharmaceutical preparations and spiked human plasma. Luminescence. 2016;31(1):173–178. doi: 10.1002/bio.2941. [DOI] [PubMed] [Google Scholar]
- Alampanos V., Kabir A., Furton K.G., Roje Ž., Vrček I.V., Samanidou V. Fabric phase sorptive extraction combined with high-performance-liquid chromatography-photodiode array analysis for the determination of seven parabens in human breast tissues: Application to cancerous and non-cancerous samples. J. Chromatogr. A. 2020;1630:461530. doi: 10.1016/j.chroma.2020.461530. [DOI] [PubMed] [Google Scholar]
- Bhadoriya A., Dasandi B., Parmar D., Shah P.A., Shrivastav P.S. Quantitation of tadalafil in human plasma using a sensitive and rapid LC-MS/MS method for a bioequivalence study. J. Pharm. Anal. 2018;8(4):271–276. doi: 10.1016/j.jpha.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff E. Potency, selectivity, and consequences of nonselectivity of PDE inhibition. Int. J. Impot. Res. 2004;16(Suppl 1):S11–S14. doi: 10.1038/sj.ijir.3901208. [DOI] [PubMed] [Google Scholar]
- Brock G.B., McMahon C.G., Chen K.K., et al. Efficacy and safety of tadalafil for the treatment of erectile dysfunction: results of integrated analyses. J. Urol. 2002;168:1332–1336. doi: 10.1097/01.ju.0000028041.27703.da. [DOI] [PubMed] [Google Scholar]
- Cheng C.-L., Chou C.-H. Determination of tadalafil in small volumes of plasma by high-performance liquid chromatography with UV detection. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2005;822(1-2):278–284. doi: 10.1016/j.jchromb.2005.06.017. [DOI] [PubMed] [Google Scholar]
- de Souza Madeira C.R., Millan Fachi M., Concentino V., de Fátima Cobre A., Leal Badaró Trindade A.C., Gonçalves A.G., Pontarolo R. A validated LC-MS/MS assay for the quantification of phosphodiesterase-5 inhibitors in human plasma. J. Chromatogr. B. 2021;1179:122829. doi: 10.1016/j.jchromb.2021.122829. [DOI] [PubMed] [Google Scholar]
- Farthing C.A., Farthing D.E., Koka S., Larus T., Fakhry I., Xi L., Kukreja R.C., Sica D., Gehr T.W.B. A simple and sensitive HPLC fluorescence method for determination of tadalafil in mouse plasma. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2010;878(28):2891–2895. doi: 10.1016/j.jchromb.2010.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidance for Industry Bioavailability and Bioequivalence Studies, ICH E6 (R2), Good Clinical Practice, U.S. Department of Health and Human Services, Food and Drug Administration, Centre for Drug Evaluation and Research (CDER), Centre for Biologics Evaluation and Research. (CBER) 2004 https://www.fda.gov/files/drugs/published/Guidance-for-Industry-Bioavailability-and-Bioequivalence-Studies-for-Orally-Administered-Drug-Products---General-Considerations.PDF [Google Scholar]
- Higton D.M. A rapid, automated approach to optimisation of multiple reaction monitoring conditions for quantitative bioanalytical mass spectrometry. Rapid Commun. Mass Spectrom. 2001;15(20):1922–1930. doi: 10.1002/rcm.454. [DOI] [PubMed] [Google Scholar]
- Kabir A., Furton K.G., Malik A. Innovations in sol-gel microextraction phases for solvent-free sample preparation in analytical chemistry. TrAC, Trends Anal. Chem. 2013;45:197–218. doi: 10.1016/j.trac.2012.11.014. [DOI] [Google Scholar]
- Kim J.-S., Kim M.-S., Baek I.-H. Enhanced Bioavailability of Tadalafil after Intranasal Administration in Beagle Dogs. Pharmaceutics. 2018;10:187–193. doi: 10.3390/pharmaceutics10040187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K.-Y., Nam M., Kwon H.-J., Kim K.-H., Kang S.-H., Kim S.-I., Kim C.-W., Cho S.-H. Validated UPLC-MS/MS method for the determination of tadalafil in human plasma and its application to a pharmacokinetic study. Transl. Clin. Pharmacol. 2017;25(1):21. doi: 10.12793/tcp.2017.25.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuan J., Brock G. Selective phosphodiesterase type 5 inhibition using tadalafil for the treatment of erectile dysfunction. Expert Opin. Invest. Drugs. 2002;11(11):1605–1613. doi: 10.1517/13543784.11.11.1605. [DOI] [PubMed] [Google Scholar]
- Lee J.H., Oh J.-H., Lee Y.-J. Simple and sensitive liquid chromatography-tandem mass spectrometry methods for quantification of tadalafil in rat plasma: application to pharmacokinetic study in rats. Arch. Pharm. Res. 2013;36(4):457–463. doi: 10.1007/s12272-013-0046-1. [DOI] [PubMed] [Google Scholar]
- Locatelli M., Tartaglia A., Ulusoy H.I., Ulusoy S., Savini F., Rossi S., Santavenere F., Merone G.M., Bassotti E., D’Ovidio C., Rosato E., Furton K.G., Kabir A. Fabric-Phase Sorptive Membrane Array As a Noninvasive In Vivo Sampling Device For Human Exposure To Different Compounds. Anal. Chem. 2021;93(4):1957–1961. doi: 10.1021/acs.analchem.0c0466310.1021/acs.analchem.0c04663.s001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma B., Shang X., Zhang Q.i., Li J., Liu Y., Cao X., Xu Q. Rapid analysis of tadalafil in human blood plasma and seminal plasma by liquid chromatography/tandem mass spectrometry. J. Pharm. Biomed. Anal. 2013;77:149–157. doi: 10.1016/j.jpba.2013.01.019. [DOI] [PubMed] [Google Scholar]
- Mastovska, K., Zulkoski, J., Zweigenbaum, J., 2017. Triggered MRM LC/MS/MS Method Development – Practical Considerations for MRM Optimization Using Agilent MassHunter Optimizer Software. Agilent Technologies, Agilent Technologies. Publication number 5991-7195EN.
- Mazaraki K., Kabir A., Furton K.G., Fytianos K., Samanidou V.F., Zacharis C.K. Fast fabric phase sorptive extraction of selected β-blockers from human serum and urine followed by UHPLC-ESI-MS/MS analysis. J. Pharm. Biomed. Anal. 2021;199:114053. doi: 10.1016/j.jpba.2021.114053. [DOI] [PubMed] [Google Scholar]
- Nikolaou P., Papoutsis I., Athanaselis S., Alevisopoulos G., Khraiwesh A., Pistos C., Spiliopoulou C. Development and validation of a GC/MS method for the determination of tadalafil in whole blood. J. Pharm. Biomed. Anal. 2011;56(3):577–581. doi: 10.1016/j.jpba.2011.05.036. [DOI] [PubMed] [Google Scholar]
- Padma-Nathan H. Efficacy and tolerability of tadalafil, a novel phosphodiesterase 5 inhibitor, in treatment of erectile dysfunction. Am. J. Cardiol. 2003;92(9):19–25. doi: 10.1016/s0002-9149(03)00828-2. [DOI] [PubMed] [Google Scholar]
- Proença P., Mustra C., Marcos M., Franco J.M., Corte-Real F., Vieira D.N. Validated UPLC-MS/MS assay for the determination of synthetic phosphodiesterase type-5 inhibitors in postmortem blood samples. J. Forensic Leg. Med. 2013;20(6):655–658. doi: 10.1016/j.jflm.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Ramakrishna N.V.S., Vishwottam K.N., Puran S., Koteshwara M., Manoj S., Santosh M., Chidambara J., Wishu S., Sumatha B. Quantitation of tadalafil in human plasma by liquid chromatography-tandem mass spectrometry with electrospray ionization. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2004;809(2):243–249. doi: 10.1016/j.jchromb.2004.06.026. [DOI] [PubMed] [Google Scholar]
- Roldán-Pijuán M., Lucena R., Cárdenas S., Valcárcel M., Kabir A., Furton K.G. Stir fabric phase sorptive extraction for the determination of triazine herbicides in environmental waters by liquid chromatography. J. Chromatogr. A. 2015;1376:35–45. doi: 10.1016/j.chroma.2014.12.027. [DOI] [PubMed] [Google Scholar]
- Rust K.Y., Wilkens H., Kaiser R., Bregel D., Wilske J., Kraemer T. Detection and validated quantification of the phosphodiesterase type 5 inhibitors sildenafil, vardenafil, tadalafil, and 2 of their metabolites in human blood plasma by LC-MS/MS–application to forensic and therapeutic drug monitoring cases. Ther. Drug Monit. 2012;34(6):729–735. doi: 10.1097/FTD.0b013e31827318b8. [DOI] [PubMed] [Google Scholar]
- Samanidou V., Kaltzi I., Kabir A., Furton K.G. Simplifying sample preparation using fabric phase sorptive extraction technique for the determination of benzodiazepines in blood serum by high-performance liquid chromatography. Biomed. Chromatogr. 2016;30(6):829–836. doi: 10.1002/bmc.3615. [DOI] [PubMed] [Google Scholar]
- Samanidou V., Kehagia M., Kabir A., Furton K.G. Matrix molecularly imprinted mesoporous sol-gel sorbent for efficient solid-phase extraction of chloramphenicol from milk. Anal. Chim. Acta. 2016;914:62–74. doi: 10.1016/j.aca.2016.02.003. [DOI] [PubMed] [Google Scholar]
- Shakya A.K., Abu-awwad A.N.A., Arafat T.A., Melhim M. Validated liquid chromatographic-ultraviolet method for the quantitation of tadalafil in human plasma using liquid-liquid extraction. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007;852(1-2):403–408. doi: 10.1016/j.jchromb.2007.01.049. [DOI] [PubMed] [Google Scholar]
- Shao R., Yang D.-D., Ruan Z.-R., Chen J.-L., Hu Y., Jiang B.o., Lou H.-G. Pharmacokinetic and Bioequivalence Evaluation of 2 Tadalafil Tablets in Healthy Male Chinese Subjects Under Fasting and Fed Conditions. Clin. Pharmacol. Drug Dev. 2022;11(2):165–172. doi: 10.1002/cpdd.1007. [DOI] [PubMed] [Google Scholar]
- Strano-Rossi S., Anzillotti L., de la Torre X., Botrè F. A gas chromatography/mass spectrometry method for the determination of sildenafil, vardenafil and tadalafil and their metabolites in human urine. Rapid Commun. Mass Spectrom. 2010;24(11):1697–1706. doi: 10.1002/rcm.4568. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Uchida S., Hakamata A., et al. Simultaneous LC-MS analysis of plasma concentrations of sildenafil, tadalafil, bosentan, ambrisentan, and macitentan in patients with pulmonary arterial hypertension. Pharmazie. 2020;75:236–239. doi: 10.1691/ph.2020.0021. [DOI] [PubMed] [Google Scholar]
- Thomas J.A. Pharmacological aspects of erectile dysfunction. Jpn. J. Pharmacol. 2002;89(2):101–112. doi: 10.1254/jjp.89.101. [DOI] [PubMed] [Google Scholar]
- Travison T.G., Shabsigh R., Araujo A.B., Kupelian V., O’Donnell A.B., McKinlay J.B. The natural progression and remission of erectile dysfunction: results from the Massachusetts Male Aging Study. J. Urol. 2007;177(1):241–246. doi: 10.1016/j.juro.2006.08.108. [DOI] [PubMed] [Google Scholar]
- Unceta N., Echeazarra L., Montaña M., Sallés J., Gómez-Caballero A., Goicolea M.A., Barrio R.J. Validation of an LC-ESI-MS/MS method for the quantitation of phosphodiesterase-5 inhibitors and their main metabolites in rat serum and brain tissue samples. J. Pharm. Biomed. Anal. 2012;70:529–533. doi: 10.1016/j.jpba.2012.04.030. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services Food and Drug Administration. 2018 https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf [Google Scholar]
- Washington S.L., 3rd, Shindel A.W. A once-daily dose of tadalafil for erectile dysfunction: compliance and efficacy. Drug Des Devel Ther. 2010;4:159–171. doi: 10.2147/dddt.s9067. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.