Abstract
Background
The main aim of this study was to investigate the cost-effectiveness of Ribociclib in the treatment of patients with breast cancer by assessing the published evidence.
Method
A systematic review of the published literature was conducted to identify the economic evaluations/cost-effectiveness study of Ribociclib. In this study, several databases were inspected, including PubMed, NHS Economic Evaluation, Cochran, and Scopus. Studies were eligible if they assessed the cost-effectiveness of Ribociclib and reported incremental cost-effectiveness ratio (ICER). The study was performed and conducted following the PRISMA reporting guidelines.
Results
Of 70 studies identified, 8 articles meet our inclusion criteria. The cost-effectiveness threshold varied from $24,144.18 in Spain to $198,000/QALY in the USA. Moreover, the result demonstrated that the mean ICER varied across different countries $1,863.47/QALY in Spain and $813,132/QALY in the USA.
Conclusion
Among all CDK4/6 inhibitors medications, current evidence indicated that the use of Ribociclib for HER2- negative breast cancer management was beneficial and considered to be cost-effective. Future research is needed to investigate the role of Ribociclib in long-term treatment.
Keywords: Cost-effectiveness, Economics evaluation, Breast cancer, Ribociclib, CDK4/6 inhibitors, Systematic review
1. Background
Breast cancer is a worldwide health issue that affects women in different age group (Diaby et al., 2015). Globally, there were 1.67 million women newly diagnosed with breast cancer, with more than 25% incident cases and leading cause of mortality among female cancer (15% of all female cancer deaths) (Ferlay et al., 2015). According to the diagnostic index breast cancer costs per patient within one year for stander disease stages 0, I/II, III, and IV were $71,909, $97,066, $159,442, and $182,655, respectively (Blumen et al., 2016). Multiple treatment options were found to treat advanced breast cancer (ABC) in women with HR-positive and HER2-negative. Endocrine therapy with selective estrogen receptor modulators, steroidal aromatase inhibitors, and estrogen receptor down regulators remains one of the most current standards of care to treat women with HR + HER2– ABC (Paterson et al., 1990, Howell et al., 2002). Misregulation in cyclin-dependent kinases (CDK)4/6 activation plays a critical role in the sequence of breast cancer patients by leading cells to divide uncontrollably. Moreover, CDK4/6 inhibitors are effective drugs that contain Ribociclib, Palbociclib, and Abemaciclib have overturned the management of women patients with breast cancer who have metastasized, but these benefits correlated with higher cost (Niu et al., 2019).
With the limited healthcare budget and the availability of many treatment alternatives which vary in cost and efficacy, the decision-makers under pressure to select the optimal medications. Applied health economic evaluation tools such as cost-effectiveness to determine the value of the medication that will help to assess the value of the medication and used to support in decision-making process.
Ribociclib is a medication that works selectively by inhibiting CDK4 and CDK6. In March 2017, Ribociclib was approved by the US FDA for advanced or metastatic stage breast cancer with human epidermal growth factor receptor 2 (HER2)-negative and hormone receptor (HR)-positive (Tripathy et al., 2017). Ribociclib is indicated only when combined with aromatase inhibitor medications as an endocrine-based therapy to treat advanced breast cancer (USFDA, 2017). Ribociclib is available as an oral 200 mg Film-coated tablet form taken for 21 days followed by seven days off treatment (USFDA, 2017). The most common adverse events (AE) related to Ribociclib are mild to moderate severity in general which are neutropenia, nausea, fatigue and grade 1/2 QTc prolongation when the patients taking a high dose of Ribociclib (Tripathy et al., 2017).
Clinical effectiveness of Ribociclib has been demonstrated in several studies, but its value in terms of cost-effectiveness is still questionable, so that's why we are interested to do such a systematic review to assess the value of this medication in clinical practice. Recently, there was an increase in the number of studies that assesses the cost-effectiveness of this drug to assess whether the Ribociclib is cost-effective at its current price which is critical these days especially with increases in health care spending that put significant pressure on health care budget. Accordingly, the purpose of this systematic review is to investigate the value of Ribociclib as an add-on treatment for HER2 negative breast cancer patients by systematically synthesize recently published economics studies.
2. Method
2.1. Study design
A systematic review was developed according to the PICO Model and the search results were performed using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram. The study was registered in PROSPERO with registration number CRD42021238314.
2.2. Search strategy
Studies that compared the cost-effectiveness of Ribociclib versus other medications were considered eligible. In this study, several databases were inspected, including PubMed, NHS Economic Evaluation, Cochran, and Scopus. The search approach that we follow in this study was identified and addressed with the following search terms: ((Cost-effectiveness) OR (Cost-utility) OR (Cost) OR (Economics) OR (Economics evaluation) OR (ICER) OR (QALY)) AND ((Ribociclib) OR (Kisqali) OR (D1/CDK4) OR (CDK6)) in title or abstract.
2.3. Main outcome
The primary outcomes regarding the use of Ribociclib were life-years gained (LYG), quality-adjusted life-year (QALY), and the incremental cost-effectiveness ratio (ICER).
2.4. Eligibility criteria
Two authors assessed the articles independently according to inclusion and exclusion criteria. Studies were included if (1) utilized full Ribociclib economic evaluations; (2) focused on breast cancer; (3) published in the English language; (4) reported the main outcomes (QALY, LYG, and ICER). Studies were excluded if (1) built as case reports studies; (2) not had full-text articles; and (3) discussed other cancers; (4) did not report the main outcomes.
2.5. Data extraction
After we selected the studies for this systematic review, two authors extracted the study characteristics and the relevant information independently, including (name of the first author with the year of publication, year of study, country, treatment, competitor(s), population, cycle, time horizon, analysis, health effect(s), perspective, source of clinical evidence, source of utility, sensitivity analysis, discounting, study funding, model type, costs reported in the studies, currency price and the year of cost, cost of the drug, Total QALY, ICER, threshold (per QALY), results).
3. Results
3.1. Search results
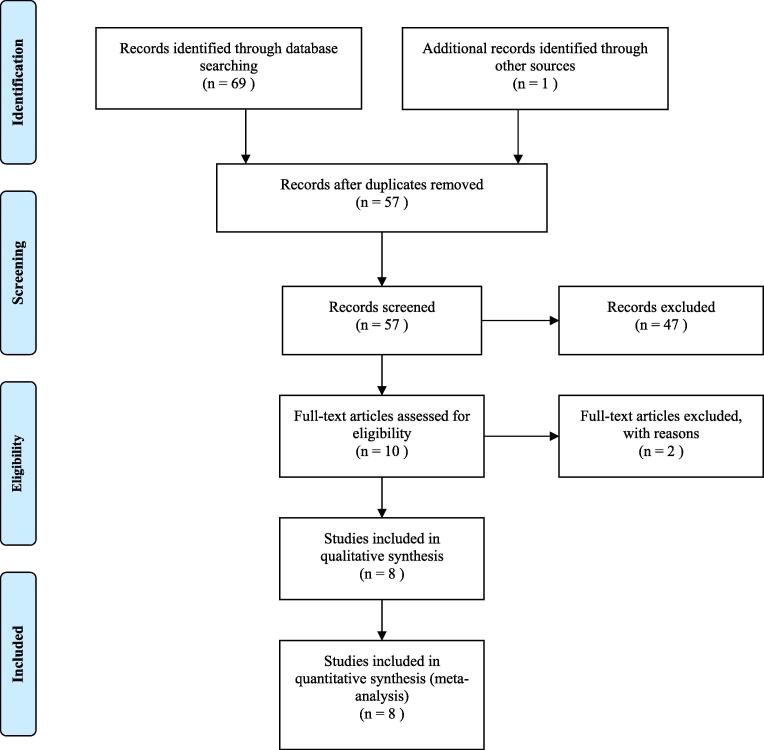
The primary search appears to have a number of 70 studies from all databases. After that, 13 duplicate articles were removed. According to titles and/or abstracts 49 articles were excluded. Two studies were excluded, because the authors as they calculated ICER based on progression-free survival. In this review, eight studies met our eligibility criteria and were included in the study. Search results and criteria used for selecting of the studies were demonstrated using the PRISMA flow diagram in Fig. 1.
Fig. 1.
Process of the systematic literature search, according to the preferred reporting items for systematic review and meta-analyses.
3.2. Study characteristics
Table 1 summarized the description of each study analyzed in the review. The eight studies selected for our inclusion criteria were published between 2018 and 2021. Four research studies were carried out in the United States (USA) (Mistry et al., 2018, Zhang and Long, 2019, Yang et al., 2020, Huang et al., 2021), two were conducted in China(Wan et al., 2019, Huang et al., 2021), one was in the United Kingdom (UK)(Suri et al., 2019), one was in Singapore(Loke et al., 2021), and one in Spain(Galve-Calvo et al., 2018), and Benji city (Wan et al., 2019). All studies used 600 mg/day as a dose of Ribociclib for a 4 weeks cycle for treating advanced breast cancer women with HER2- negative. The treatment regimen with Ribociclib was different which varied from Letrozole, Fulvestrant, and other endocrine therapies. Moreover, the comparators in studies were Palbociclib plus Letrozole, Letrozole alone, placebo plus Fulvestrant, and endocrine therapy alone.
Table 1.
Description of each study analyzed in the review.
| Author/ year of publication (ref.) | Year of study | Country | Treatment | Comparator(s) | Population | Cycle | Time horizon |
|---|---|---|---|---|---|---|---|
| Jiangping Yang et al, 2020.[11] | 2018 | USA. | RIB (600 mg/day) + FUL. | Placebo + FUL. | Women with HR+/HER2- ABC. | 4 weeks. | 10 years. |
| Rohit Mistry et al, 2018.[12] | 2018 | USA. | RIB (600 mg/day) + LET. | PAL + LET and LET alone. | Women with HR+/HER2- ABC or MBC. | 4 weeks. | 40-year. |
| Bingnan Zhang et al, 2019.[13] | 2018 | USA. | RIB (600 mg/day) + LET. | LET alone. | Women with HR+/HER2- ABC or MBC. | 4 weeks. | Lifetime. |
| Gaurav Suri et al, 2019.[14] | 2019 | UK. | RIB (600 mg/day) + LET. | PAL + LET. | Women with HR+/HER2- ABC. | 4 weeks. | 40 years. |
| Lydia Loke et al, 2020.[15] | 2020 | Singapore. | RIB (600 mg/day) + ET. | ET alone. | Women with HR+/HER2- ABC. | 4 weeks. | 10 years. |
| Elena galve-Calvo et al, 2018.[16] | 2018 | Spain. | RIB (600 mg/day) + LET. | PAL + LET. | Women with HR+/HER2- ABC or MBC. | 4 weeks. | 15 years. |
| XiaoMin Wan et al, 2019.[17] | 2018 | China and Beijing city. | RIB (600 mg/day) + LET. | LET alone. | Women with HR+/HER2- ABC. | 4 weeks. | Lifetime. |
| Xiaoting Huang, 2020.[18] | 2021 | USA, China. | RIB (600 mg/day) + ET. | ET alone. | Women with HR+/HER2- ABC. | 4 weeks. | Lifetime. |
| Author/ year of publication (ref.) | Analysis | Health effect(s) | Perspective | Source of clinical evidence | Source of utility–Population | Sensitivity analysis | Discounting |
|---|---|---|---|---|---|---|---|
| Jiangping Yang et al, 2020.[11] | CEA. | QALYs, LYs. | Payer. | MONALEESA-3 and PALOMA3 trials. | PF: 0.715 PD: 0.443 EQ-5D–UK. |
DSA. | 3% |
| Rohit Mistry et al, 2018.[12] | CEA. | QALYs, LYs. | Payer. | MONALEESA-2, PALOMA-1 and PALOMA-2 trails. | PF: 0.837 PD: 0.443 EQ-5D-5L–UK. |
DSA, PSA. | 3% |
| Bingnan Zhang et al, 2019.[13] | CEA. | QALYs. | Payer. | MONALEESA-2, PALOMA-1 and PALOMA-2 trails. | PF: 0.8345 PD: 0.5050 EQ-5D–UK. |
DSA. | 3% |
| Gaurav Suri et al, 2019.[14] | CEA. | QALYs, LYs. | Payer. | MONALEESA-2, PALOMA-1 and PALOMA-2 trails. | PF: 0.8345 PD: 0.5050 EQ-5D-5L–UK. |
DSA, PSA. | – |
| Lydia Loke et al, 2020.[15] | CEA. | QALYs, LYs. | Payer. | MONALESSA-7 trail. | PF: 0.73 PD: 0.64 SG–Singapore, EQ-5D–Canada. |
DSA, PSA. | 3% |
| Elena galve-Calvo et al, 2018.[16] | CEA. | QALYs, LYs. | Payer. | MONALEESA-2 and PALOMA-2 trails | PF: 0.8345 PD: 0.5050 EQ-5D-5L–UK. |
DSA, PSA. | 3% |
| XiaoMin Wan et al, 2019.[17] | CEA. | QALYs, LYs. | Payer. | MONALEESA-2 trial. | PF: 0.7 PD: 0.58 Visual analog scale and SG. |
PSA. | 3% |
| Xiaoting Huang, 2020.[18] | CEA. | QALYs, LYs. | Payer. | MONALEESA-7 trail. | PF: 0.85 PD: 0.52 EQ-5D–Japan, EQ-5D–UK |
DSA, PSA. | 3% |
| Author/ year of publication (ref.) | Funding | Model type | Costs reported in the studies | Currency price, year | Cost of the drug |
|---|---|---|---|---|---|
| Jiangping Yang et al, 2020.[11] | – | Markov model. | Drug, serious AEs (grades 3–4), monitoring, best supportive care and end-of-life care costs. | US dollars,2020. | $13,835. |
| Rohit Mistry et al, 2018.[12] | Novartis. | Partitioned survival model. | Drug, AEs, monitoring, disease management and Health-state (PFS, PD) costs. | US dollars, 2016. | $10,950. |
| Bingnan Zhang et al, 2019.[13] | – | Markov cohort model. | Drug and AEs costs. | US dollars, 2016. | $13,140. |
| Gaurav Suri et al, 2019.[14] | – | Partitioned survival model. | Drug, AEs, monitoring and health state specific disease monitoring costs. | UK pound sterling, 2016. | £58,358. |
| Lydia Loke et al, 2020.[15] | – | Partitioned survival model. | Drug, AEs, administration, pharmacy preparation, medical consultations, CT scans, laboratory tests and end-of-life care costs. | Singapore dollar (SGD), 2018. | SGD $2929. |
| Elena galve-Calvo et al, 2018.[16] | – | Cohort-based partitioned survival model. | Drug, monitoring and administration costs. | Spanish euros, 2017. | €4,831.34. |
| XiaoMin Wan et al, 2019.[17] | Health and Family Planning Commission of Hunan Province. | Discrete event simulation (DES) model. | Drug, AEs and administration costs. | US dollars, 2015. | $830, $1320. |
| Xiaoting Huang, 2020.[18] | Fujian Medical University. | Markov model. | Drug, serious AEs, monitoring and best supportive care costs. | US dollars, 2019. | $13114. |
RIB = Ribociclib; LET = Letrozole; ET = Endocrine therapies; FUL = Fulvestrant; PAL = Palbociclib; ABC = Advanced breast cancer; MBC = Metastatic breast cancer; CEA = Cost-effectiveness analysis; QALYs = Quality-adjusted life years; LYs = Life years; PFS = Progression-free survival; PD = Progressed-disease; PF = Progression-free; EQ-5D-5L = EuroQol 5-dimension 5-level; SG = Standard gamble; DSA = Deterministic sensitivity analysis; PSA = Probabilistic sensitivity analysis.
3.3. Efficacy data
The efficacy data were mainly obtained from different clinical trials, such as (PALOMA-1, PALOMA-2, MONALEESA-2, MONALEESA-3, and MONALEESA-7) trials.
3.4. Cost data
All studies reported the costs as direct medical costs from a payer perspective with a 3% discount rate. Five studies(Mistry et al., 2018, Wan et al., 2019, Zhang and Long, 2019, Yang et al., 2020, Huang et al., 2021) used US dollars as a currency price and were reported in different years between 2015 and 2020 and three studies(Galve-Calvo et al., 2018, Suri et al., 2019, Loke et al., 2021) used different currencies including UK pound sterling, and Singapore dollar (SGD), Spanish euros. The monthly cost of Ribociclib 600 mg/day was ranging between $830 in China and Beijing city (Wan et al., 2019) to $81,272.28 (£58,358) in the UK (Suri et al., 2019).
3.5. Cost-effectiveness results
The value of health effect used for economic evaluation in most studies was quality-adjusted life-year (QALY) and life-years (LYs). The source of utility for most studies was conducted by using EQ-5D [(Galve-Calvo et al., 2018, Mistry et al., 2018, Suri et al., 2019, Yang et al., 2020, Huang et al., 2021, Loke et al., 2021)] All studies were performed a sensitivity analysis to ensure the toughness of their results in which five studies used both deterministic sensitivity analysis and probabilistic sensitivity analysis to interpret the result (Galve-Calvo et al., 2018, Mistry et al., 2018, Suri et al., 2019, Huang et al., 2021, Loke et al., 2021), two studies rely on only deterministic sensitivity analysis (Zhang and Long, 2019, Yang et al., 2020), and one study relies on probabilistic sensitivity analysis study alone(Huang et al., 2021). Furthermore, we found different variables can affect the sensitivity analysis across different studies.
Table 2 summarized the economic evaluation state in all studies included (funding, model type, costs reported in the studies, currency price and the year of cost, cost of the drug, total QALY, ICER, threshold (per QALY), and results). The majority of studies were not funded by any sources except three studies (Mistry et al., 2018, Wan et al., 2019, Huang et al., 2021) funded by professional institutions(Wan et al., 2019, Huang et al., 2021) and private industry(Mistry et al., 2018). The results were varied according to data analysis models which were analyzed based on the partitioned survival model in four studies(Galve-Calvo et al., 2018, Mistry et al., 2018, Suri et al., 2019, Loke et al., 2021), the Markov model in three studies (Zhang and Long, 2019, Yang et al., 2020, Huang et al., 2021), and the Discrete event simulation (DES) model in one study (Wan et al., 2019). The cost-effectiveness threshold varied from $24,144.18/QALY (€20,000/QALY) in Spain (Galve-Calvo et al., 2018) to $198,000/QALY in the USA (Mistry et al., 2018). Moreover, the result demonstrated that the mean ICER varied across different countries $1,863.47/QALY (€1,543.62/QALY) in Spain (Galve-Calvo et al., 2018) and $813,132/QALY in the USA (Yang et al., 2020) with total QALYs between 2.17 and 6.37, respectively. Two studies showed ICER as dominant in the USA (Mistry et al., 2018) by -$323,116.279/QALY and in the UK (Suri et al., 2019) by -$44,991.1/QALY (-£323,05.34/QALY). According to the finding of the studies, the use of Ribociclib is considered cost-effective in the USA (Mistry et al., 2018), UK (Suri et al., 2019), Spain (Galve-Calvo et al., 2018), and China and Beijing city (Wan et al., 2019). However, Other studies in the USA (Zhang and Long, 2019, Huang et al., 2021), Singapore (Loke et al., 2021), and China (Huang et al., 2021) demonstrated that the Ribociclib is considered not cost-effective, because the comparators and model types were varied.
Table 2.
Economic evaluation of the studies.
| Author/ year of publication (ref.) | Total QALY | ICER | Threshold (per QALY) | Result |
|---|---|---|---|---|
| Jiangping Yang et al, 2020.[11] | 2.17 QALYs. | $813,132/QALY. | $150,000/QALY. | Not CE. |
| Rohit Mistry et al, 2018.[12] | 3.07 QALYs, 2.99 QALYs. | $210,369/QALY, Dominant. | Above $198,000/QALY. | CE. |
| Bingnan Zhang et al, 2019.[13] | 2.94 QALYs. | $440,000/QALY. | $100,000/QALY. | Not CE. |
| Gaurav Suri et al, 2019.[14] | 3.296 QALYs. | Dominant. | £30 000/QALY. | CE. |
| Lydia Loke et al, 2020.[15] | 3.4386 QALYs. | SGD $197,667/QALY. | Below SGD $198,000/QALY. | Not CE. |
| Elena galve-Calvo et al, 2018.[16] | 3.313 QALYs. | €1,543.62/QALY. | €20,000 to €30,000/QALY. | CE. |
| XiaoMin Wan et al, 2019.[17] | 2.293 QALYs. | $24,126/QALY, $53,071/QALY. | $24,360/QALY, $53,384/QALY. | CE. |
| Xiaoting Huang, 2020.[18] | 6.37 QALYs. | $61,454.96/QALY. | $150,000/QALY. | Not CE. |
3.6. Quality assessment
To ensure the quality of studies, two authors evaluated the studies independently by using Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist and resolved any disagreement by the third author. According to Table 3, studies with at least 20 items of 24 items from the CHEERS checklist were included in this systematic review (Husereau et al., 2013).
Table 3.
CHEERS checklist results.
| Author/ year of publication (ref.) | CHEERS checklist results (%) |
|---|---|
| Jiangping Yang et al, 2020.[11] | 23 (95.83%) |
| Rohit Mistry et al, 2018.[12] | 22 (91.67%) |
| Bingnan Zhang et al, 2019.[13] | 22 (91.67%) |
| Gaurav Suri et al, 2019.[14] | 20 (83.33%) |
| Lydia Loke et al, 2020.[15] | 24 (100%) |
| Elena galve-Calvo et al, 2018.[16] | 22 (91.67%) |
| XiaoMin Wan et al, 2019.[17] | 23 (95.83%) |
| Xiaoting Huang, 2020.[18] | 24 (100%) |
4. Discussion
4.1. Main findings
The cost of cancer treatment is still raising over the years and it becomes important to conduct economic evaluation tools such as cost-effectiveness to evaluate the value of the new intervention to provide an evidence-based that can help health administrators to make the decision regarding the new medication. This systematic review includes evidence from eight cost-effectiveness studies of Ribociclib as an add-on therapy to standard regimens, such as (Fulvestrant, Letrozole, and other endocrine therapies). These endocrine therapies are considered as a first-line treatment option to treat advanced breast cancer women with HER2- negative. However, the use of CDK 4/6 inhibitors, such as (Ribociclib) is considered as add-on therapy when the first-line treatments failed to get an adequate response. In this study, we performed a systematic review analysis to collect comprehensive data to evaluate the value of Ribociclib in the treatment of HER2- negative Breast Cancer across different settings. All studies reported the direct costs only, and used QALY as the main outcome. Four studies (Galve-Calvo et al., 2018, Mistry et al., 2018, Suri et al., 2019, Wan et al., 2019) considered Ribociclib as cost-effective and the other studies (Zhang and Long, 2019, Yang et al., 2020, Huang et al., 2021, Loke et al., 2021) considered Ribociclib as not cost-effective. This indicates that the medication can be cost effective in one country but not in others leading to various approval, pricing and reimbursement decision across countries. The reported ICER were varied across different studies, this mainly because of heterogeneity in the parameters used in the economic model and because of the variation between health care systems, population characteristics, baseline risk factors, variation in the models and threshold used. Moreover, the difference in the threshold between the countries may affect the final results. Therefore, generalized is often an issue with economic evaluation and caution should be taken when interpreteing the result of cost-effectiveness analysis because of these different variables and what is relevant for one country may be not relevant or transferable to other settings (Barbieri et al., 2010).
4.2. Efficacy versus effectiveness inputs
Studies collected the outcome data (ie. health-related quality of life data) using different measurements, populations, and sources. QALY is a country-specific and should be obtained using local data of the country of interest. Different utility data from different countries have been studied before and found that there are variations in the utility value that should be taken into consideration. The variation in QALY is often due to differences in the method used, population and cultural dissimilarities. However, in some countries these data are often not available so the best practice is to transferability of utilities from another country with appropriate adjustment as transferring these data without adjustment can result in inaccurate CEA results which may lead to a wrong decision (Knies et al., 2010, Ferreira and Ferreira, 2014).
The efficacy data were obtained from different evidence and clinical trials. No study used real-world evidence to estimate the cost-effectiveness which is the optimal way to assess the value of the medication. We recognize the benefit and risk of using efficacy data extracted from RCT studies; however, it is the best available efficacy data for the analysis when the real world data is not available which is often the case with the new medication. Using the real-world data in conducting future economic evaluations would give more insight into the value of the medication in real practice which may change the cost-effectiveness results.
All eight studies included in our systematic review indicate that the medication have better health outcomes in women with HER2- negative breast cancer.
4.3. Adverse events from Ribociclib use
All studies considered the adverse effect cost based on clinical trials. Studies assigned a serious adverse effect based on Common Terminology Criteria for Adverse Events (Grade 3 and above), such as (Severe neutropenia, QT prolongation, hepatic dysfunction, etc).
4.4. Perspective and cost estimation
All eight studies based their evaluations on only a payer perspective which is commonly used in practice as the payer is mainly interested in the results of these studies. However, previous studies demonstrated that using broader perspective such as societal perspectives might alter cost-utility analyses. Therefore, future studies to estimate the societal perspective are needed. All costs in the studies were converted to the study year by measuring the consumer price index. Only direct medical costs were considered for all studies to align with a payer perspective. The cost of the drug was varied significantly from one country to another ranging between $830 in China and Beijing city (Wan et al., 2019) to $81,272.28 (£58,358) in the UK (Suri et al., 2019). Also, we observed variation in the cost resources of direct medical costs some of them were derived from local health systems, expert panels, third-party payers, and previously published literature. On the other hand, the study in Spain (Galve-Calvo et al., 2018) used list ex-factory prices for drug costs only and the cost was higher than Spain’s NHS. Even though in Spain they considered the higher cost of the drug, they concluded that the Ribociclib was cost-effective. These variations in cost estimates should be taken into account when comparing the ICER across different studies.
4.5. Outcome measures
QALYs are well confirmed around the world, and the application of this approach keeps up to expand internationally to measure the health outcomes of cancer patients. Outcomes in model-based studies were different in studies and reported costs, QALYs, were variable because of the difference in competitors. Also, the cost-effectiveness studies(Galve-Calvo et al., 2018, Mistry et al., 2018, Suri et al., 2019, Wan et al., 2019) concerned with Ribociclib have been carried out in China and Beijing city, USA, and some have been conducted in other European countries.
4.6. Quality of the studies
Majority of studies included in this systematic review scored high in CHEERS checklist, and considered high-quality studies.
4.7. Strengths/limitations
This is the first systematic review that evaluates the cost-effectiveness of Ribociclib in HER2- negative breast cancer as far as we know. Our systematic review was performed using Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Also, our study was registered on the PROSPERO site. We use CHEERS to assess quality for all including studies. Nevertheless, we identified some limitations in cost-effectiveness studies. First, as far as we know all studies that were included in this systematic review for the economic appraisal of Ribociclib had observed only direct medical costs; even though, indirect costs take an important role in economic evaluation studies. Second, one study didn’t justify the discount rate, and one study was funded by pharmaceutical companies.
In addition, our study could carry some limitations worth addressing. This study excludes studies that are non-English languages although they could provide highly valuable information and results. Although, we used the most common database PubMed, NHS Economic Evaluation, Cochran, and Scopus. However, using more databases that include studies might add helpful data to this systematic review. Moreover, this search was focus only on published studies and exclude gray literature and conference abstracts which may have different results. Costs included in those studies were not comprehensive as only direct medical cost were included and indirect cost such as loss of productivity were not included, so the full value of the medication may not be captured which could have a large impact on CE results. Majority of studies were based on efficacy evidence from RCT, this may not reflect the actual RWE and effectiveness in daily practice which may influence the result of future CE studies. Thus, the cost-effectiveness evidence should be re-evaluated over time as more evidence becomes available. All these limitations affect generalizability of this review and should be interpreted with caution.
5. Conclusion
According to our investigation among all CDK4/6 inhibitors medications, current evidence indicates that the use of Ribociclib appears to be clinically beneficial and considered to be cost-effectiveness intervention for HER2- negative breast cancer management. Uncertainties remain about long-term effects, thus future research is needed to investigate the role of Ribociclib using real-world data to provide more insight about their actual value in clinical practice.
Funding
The author(s) received no specific funding for this work.
Availability of data and material
The original contributions presented in the study are diincluded in the article, further inquiries can be directed to the corresponding author.
Authors' contributions
BB, WA, and MA design the study. MA and WA collect the data. BB analyzed the data, BB, WA and MA contributed to the interpretation of the results. MA and WA draft the article, BB edit and proof read the manuscript. All authors contributed to the article and approved the submitted version.
Code availability
N/A.
Ethics approval
N/A.
Consent to participate
N/A.
Consent for publication
N/A.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors acknowledge financial support from Researchers Supporting Project number (RSP-2021/76) King Saud University, Riyadh, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jsps.2022.06.002.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References:
- Barbieri M., Drummond M., Rutten F., Cook J., Glick H.A., Lis J., Reed S.D., Sculpher M., Severens J.L. What do international pharmacoeconomic guidelines say about economic data transferability? Value in Health. 2010;13(8):1028–1037. doi: 10.1111/j.1524-4733.2010.00771.x. [DOI] [PubMed] [Google Scholar]
- Blumen H., Fitch K., Polkus V. Comparison of treatment costs for breast cancer, by tumor stage and type of service. Am. Health Drug Benefits. 2016;9:23. [PMC free article] [PubMed] [Google Scholar]
- Diaby V., Tawk R., Sanogo V., Xiao H., Montero A.J. A review of systematic reviews of the cost-effectiveness of hormone therapy, chemotherapy, and targeted therapy for breast cancer. Breast Cancer Res. Treat. 2015;151(1):27–40. doi: 10.1007/s10549-015-3383-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlay J., Soerjomataram I., Dikshit R., Eser S., Mathers C., Rebelo M., Parkin D.M., Forman D., Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- Ferreira, L., Ferreira, P., 2014. Health state values and country-specific value sets. Encyclopedia of Quality of Life and Well-Being Research. Dordrecht, Netherlands: Springer, pp. 2744–2749.
- Galve-Calvo E., González-Haba E., Gostkorzewicz J., et al. Cost-effectiveness analysis of ribociclib versus palbociclib in the first-line treatment of HR+/HER2− advanced or metastatic breast cancer in Spain. ClinicoEconomics Outcomes Res.: CEOR. 2018;10:773. doi: 10.2147/CEOR.S178934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell A., Robertson J.F.R., Quaresma Albano J., Aschermannova A., Mauriac L., Kleeberg U.R., Vergote I., Erikstein B., Webster A., Morris C. Fulvestrant, formerly ICI 182,780, is as effective as anastrozole in postmenopausal women with advanced breast cancer progressing after prior endocrine treatment. J. Clin. Oncol. 2002;20(16):3396–3403. doi: 10.1200/JCO.2002.10.057. [DOI] [PubMed] [Google Scholar]
- Huang X., Lin S., Rao X., Zeng D., Wang H., Weng X., Huang P. First-line Treatment with Ribociclib plus Endocrine Therapy for Premenopausal Women with Hormone-receptor-positive Advanced Breast Cancer: A Cost-effectiveness Analysis. Clinical Breast Cancer. 2021;21(4):e479–e488. doi: 10.1016/j.clbc.2021.01.019. [DOI] [PubMed] [Google Scholar]
- Husereau D., Drummond M., Petrou S., Carswell C., Moher D., Greenberg D., Augustovski F., Briggs A.H., Mauskopf J., Loder E. Consolidated health economic evaluation reporting standards (CHEERS) statement. Eur. J. Health Econ. 2013;14(3):367–372. doi: 10.1007/s10198-013-0471-6. [DOI] [PubMed] [Google Scholar]
- Knies S., Evers S.M.A.A., Candel M.J.J.M., Severens J.L., Ament A.J.H.A. Utilities of the EQ-5D: transferable or not? Transparency Transferability. 2010;27(9):767–779. doi: 10.2165/11314120-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Loke L., Lee S.-C., Pearce F., Ng K., Aziz M.I.A. Cost-effectiveness of ribociclib as initial treatment for premenopausal women with advanced breast cancer in Singapore. Cancer Reports. 2021;4(1) doi: 10.1002/cnr2.v4.110.1002/cnr2.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistry R., May J.R., Suri G., Young K., Brixner D., Oderda G., Biskupiak J., Tang D., Bhattacharyya S., Mishra D., Bhattacharyya D., Dalal A.A. Cost-effectiveness of ribociclib plus letrozole versus palbociclib plus letrozole and letrozole monotherapy in the first-line treatment of postmenopausal women with HR+/HER2-advanced or metastatic breast cancer: a US payer perspective. J. Managed Care Specialty Pharmacy. 2018;24(6):514–523. doi: 10.18553/jmcp.2018.24.6.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu Y., Xu J., Sun T. Cyclin-dependent kinases 4/6 inhibitors in breast cancer: current status, resistance, and combination strategies. J. Cancer. 2019;10(22):5504–5517. doi: 10.7150/jca.32628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson A., Hanson J., Pritchard K., et al. Comparison of antiestrogen and progestogen therapy for initial treatment and consequences of their combination for second-line treatment of recurrent breast cancer. Seminars Oncol. 1990 [PubMed] [Google Scholar]
- Suri G., Chandiwana D., Lee A., Mistry R. Cost-effectiveness analysis of ribociclib plus letrozole versus palbociclib plus letrozole in the United Kingdom. J. Health Econ. Outcomes Res. 2019;6(2):20–31. doi: 10.36469/9725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathy D., Bardia A., Sellers W.R. Ribociclib (LEE011): mechanism of action and clinical impact of this selective cyclin-dependent kinase 4/6 inhibitor in various solid tumors. Clin. Cancer Res. 2017;23(13):3251–3262. doi: 10.1158/1078-0432.CCR-16-3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USFDA. 2017. Ribociclib (Kisqali). Retrieved 14 February 2021, from https://www.fda.gov/drugs/resources-information-approved-drugs/ribociclib-kisqali.
- Wan X., Zhang Y., Ma J., et al. Ribociclib in hormone-receptor-positive advanced breast cancer: Establishing a value-based cost in China. The Breast. 2019;43:1–6. doi: 10.1016/j.breast.2018.10.004. [DOI] [PubMed] [Google Scholar]
- Yang J., Han J., Tian M., et al. Cost-Effectiveness of Ribociclib for Hormone Receptor-Positive HER2-Negative Advanced Breast Cancer. Cancer Manage. Res. 2020;12:12905. doi: 10.2147/CMAR.S284556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Long E.F. Cost-effectiveness analysis of palbociclib or ribociclib in the treatment of advanced hormone receptor-positive, HER2-negative breast cancer. Breast Cancer Res. Treat. 2019;175(3):775–779. doi: 10.1007/s10549-019-05190-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are diincluded in the article, further inquiries can be directed to the corresponding author.