Abstract
Gefitinib is a tyrosine kinase inhibitor (TKI) of the epidermal growth factor receptor (EGFR), used for the treatment of advanced or metastatic non-small cell lung cancer. Recently, studies proved that Gefitinib-induced cardiotoxicity through induction of oxidative stress leads to cardiac hypertrophy. The current study was conducted to understand the mechanisms underlying gefitinib-induced cardiac hypertrophy through studying the roles of angiotensin II (AngII), oxidative stress, and mitogen-activated protein kinase (MAPK) pathway. Male Wistar albino rats were treated with valsartan, gefitinib, or both for four weeks. Blood samples were collected for AngII and cardiac markers measurement, and hearts were harvested for histological study and biochemical analysis. Gefitinib caused histological changes in the cardiac tissues and increased levels of cardiac hypertrophy markers, AngII and its receptors. Blocking of AngII type 1 receptor (AT1R) via valsartan protected hearts and normalized cardiac markers, AngII levels, and the expression of its receptors during gefitinib treatment. valsartan attenuated gefitinib-induced NADPH oxidase and oxidative stress leading to down-regulation of JNK/p38-MAPK pathway. Collectively, AT1R blockade adjusted AngII-induced NADPH oxidase and JNK/p38-MAPK leading to attenuation of gefitinib-induced cardiac hypertrophy. This study found a pivotal role of AngII/AT1R signaling in gefitinib-induced cardiac hypertrophy, which may provide novel approaches in the management of EGFRIs-induced cardiotoxicity.
Keywords: Gefitinib, angiotensin II, Cardiac hypertrophy, NADPH oxidase, MAPK
1. Introduction
Receptor tyrosine kinases (RTKs) play an essential role in the regulation of a wide range of cellular processes including cell growth, differentiation, motility, survival and metabolism governing cancer cell growth (Lemmon and Schlessinger 2010). Over the past two decades, dysregulated RTKs were involved in the progression of cancer which became a target for cancer treatment by developing tyrosine kinase inhibitors (TKIs). Currently, more than 100 TKIs are in late stages of clinical development with promising clinical outcomes (Pottier et al., 2020). TKIs were sorted based on their targeted pathways EGFR, vascular endothelial growth factor receptor (VEGFR), and anaplastic lymphoma tyrosine kinase (Pottier et al., 2020). Despite the clinical benefits of TKIs, cancer management with TKIs causes toxicity and organ dysfunction that reduce the quality of life and commitment of therapy (Tseng et al., 2020). Among these toxicities, cardiotoxicity is one of the major complications that are related to TKIs therapy, which is varying in the degree of frequency and severity between TKIs including hypertension, cardiac hypertrophy, and myocardial infarction (Chaar et al., 2018).
Gefitinib is, a small tyrosine kinase inhibitor, used for treatment of advanced or metastatic non-small cell lung cancer (NSCLC) in adult patients with over-expressed epidermal EGFR (Kazandjian et al., 2016). Gefitinib binds to ATP-binding site causing downregulation of EGFR leading to deactivation of the related down signaling pathways and inhibition of tumor pathogenesis in lung cancer (Seshacharyulu et al., 2012, Zhao et al., 2016). In 2015, gefitinib has been approved by The United States Food and Drug Administration (FDA) as the first-line therapy of patients with metastatic NSCLC (Kazandjian et al., 2016). Although gefitinib provides a significant advance in cancer treatment and survival rate, cardiovascular complications have been indicated during treatment include arterial fibrillation, electrocardiogram changes, and myocarditis (Zaborowska-Szmit et al., 2020). Recently, in-vitro and in-vivo studies have found a correlation between gefitinib and induction of cardiac hypertrophy (Korashy et al., 2016). It was examined through the evaluation of cardiac hypertrophy markers and histological changes in cardiomyocyte cell line (H9c2) and rodent models (Korashy et al., 2016). On the molecular levels, various mechanistic pathways were in-volved in gefitinib-induced cardiac hypertrophy causing oxidative stress and apoptosis (Korashy et al., 2016). Still, further investigation is needed to understand the mechanism underlying gefitinib-induced cardiac hypertrophy, and protective therapy approach is required to reduce its cardiovascular toxicity.
Renin angiotensin II system (RAS) is one of the major factors that contribute to the pathogenesis of hypertension and cardiac hypertrophy (Ahmad et al., 2009, Kurdi and Booz, 2011). These actions are mainly induced through the overproduction of angiotensin II (AngII) leading to in-crease in vascular tone and blood intravascular volume (Cowan and Young, 2009, Kurdi and Booz, 2011). AngII exerts its actions through binding to its type 1 receptor (AT1R) causing hypertension that deleteriously affects heart function leading to left ventricular hypertrophy and fibrosis (Cowan and Young, 2009, Kurdi and Booz, 2011). Other evidence supports that the presence of local RAS in the cardiac tissues including all the components of RAS for synthesis and the release of AngII causing cardiac hypertrophy independently of blood pressure (Mazzolai et al., 1998, Piratello et al., 2010). Previous studies showed a correlation be-tween activation of AT1R and NADPH oxidase in the production of reactive oxygen species (ROS), which are implicated in the pathogenesis of hypertension and cardiac hypertrophy (Nguyen Dinh Cat et al., 2013, Chen et al., 2020). Growing evidence shows that the activation of AngII/AT1R down signaling pathways is mostly associated with the induction of NADPH oxidase-derived ROS (Nguyen Dinh Cat et al., 2013, Chen et al., 2020). ROS formation induces phosphorylation of p38 and JNK and activation of mitogen-activated protein kinase (MAPK) signaling pathway leading to induction of cardiac hypertrophy as recently approved (Chen et al., 2020, Liu et al., 2021). However, AT1R blockade attenuates various pathological pathways associated with AngII/AT1R signaling and manages AngII-induced cardiac hypertrophy (Kawai et al., 2017). Treatment with selective AT1R blockers (ARBs) provides cardioprotection through an increased antioxidant capacity and regulation of MAPK pathway in the cardiomyocyte leading to reduce the cardiovascular mortality and morbidity rates (Ararat and Brozovich, 2009, Munger, 2011, Streicher et al., 2010).
Therefore, this study was conducted to identify the role of RAS in gefitinib-induced cardiac hypertrophy and investigate if AT1R blockade via valsartan can attenuate cardiac hypertrophy induced by gefitinib via assessment of cardiac enzymes include creatine kinase-MB (CK-MB), N-terminal pro-B-type natriuretic peptide (NT-proBNP), and cardiac troponin I (cTnI).
2. Materials and methods
2.1. Animal
Male Wistar albino rats weighing 180–200 g (n = 32) were obtained from the Animal Care Center, College of Pharmacy, King Saud University (Riyadh, Saudi Arabia). Rats were fed with a standard chow pellet diet and had free access to water under controlled conditions (25 °C and a 12 h light/dark cycle). All animal experiments described in this study complied with the National Institutes of Health guidelines for the Care and Use of Laboratory Animals and were approved by the local institutional research ethics committee of King Saud University on March 4, 2021 (KSU-SE-21-22).
2.2. Treatment
Thirty-two rats were divided into four groups (n = 8 rats/group) and treated for continuous 28 days as shown in Table 1. The first group received saline vehicle by intraperitoneal (i.p.) injection for 28 days as a control group. The second group was treated with valsartan (Ark Pharm, USA) (30 mg/kg/day, i.p.) for 28 days. The third group was treated with vehicle for 7 days and then received gefitinib (Ark Pharm, USA) (30 mg/kg/day, o.p.) for 21 days. The last group was treated with valsartan (30 mg/kg/day) for 7 days and then received both valsartan and gefitinib (30 mg/kg/day) for 21 days. Gefitinib was prepared daily before administration in corn oil (0.5 ml) (Zhang et al., 2017), whereas rat nontreated with gefitinib were received 0.5 ml corn oil orally as a vehicle. Valsartan was dissolved in saline by adding 0.1 M NaOH to obtain a clear solution and the PH was normalized using 0.1 M HCl (Jiao et al., 2012). The dose of gefitinib (Korashy et al., 2016, Alhoshani et al., 2020), and valsartan (Alhazzani et al., 2021, Ulutas et al., 2021) were selected based on previous published articles.
Table 1.
Type of treatment and duration.
| Group | Type of treatment and Duration | |
|---|---|---|
| Control | Vehicle for 28 days | |
| Valsartan | Valsartan (30 mg/kg/day, i.p.) for 28 days | |
| Gefitinib | Vehicle for 7 days | Gefitinib (30 mg/kg/day, o.p.) for 21 days |
| GEF + VAL | Valsartan (30 mg/kg) for 7 days | Valsartan (30 mg/kg) + Gefitinib (30 mg/kg) for 21 days |
On day 28th, rats were anesthetized using ketamine/xylazine mixture and then the blood and hearts were harvested for further studies. Serum was separated for measurement of cardiac enzymes, while plasma was collected for AngII measurement. Hearts were washed with ice-cold phosphate buffer saline (PBS) and weighed for HW/BW ratio as cardiac hypertrophy index, and then fixed in 4% formaldehyde solution for histopathology studies. Other hearts were snap-frozen by direct immersion in liquid nitrogen and stored at −80 °C until analysis using western blot analysis and biochemical assays.
2.3. Cardiac histology
The immersed hearts into formaldehyde were kept at room temperature for at least 48 hrs and then embedded in a paraffin block. The left ventricles of the fixed hearts were sectioned at 3 µm thickness and deparaffinized and hydrated with xylene and alcohol for staining with hematoxylin and eosin (H&E). The ventricular wall thickness was taken from five different regions of the heart of each group. For the TUNEL assay and according to the manufacturer’s instructions, paraffin-embedded cardiac sections were stained using the in situ BrdU-Red DNA fragmentation (Abcam, USA). The deparaffinized heart sections were incubated with Br-dUTP for 1 hr at 37 °C followed by 30 min incubation with anti-BrdU-Red antibodies at room temperature (25 °C). Percentage of apoptotic cells was obtained via number of apoptotic cells divided by total number cells then multiplied by 100. The prepared slides were evaluated by a specialized histopathologist to identify the structural changes and apoptosis in the cardiac tissues in all groups.
2.4. Assessment of cardiac enzymes and AngII levels by ELISA
At the time of analysis, the frozen serum and plasma samples were thawed at room temperature (25 °C). Commercial ELISA kits were used to measure serum protein levels of CK-MB, NT-proBNP, cTnI (G-biosciences, USA), and plasma levels of AngII (ABclonal Technology, USA) as per manufacturer's protocol.
2.5. Detection of apoptosis by flow cytometry
Rat cardiomyocyte H9c2 cells (ATCC®, USA) were seeded in 12-well plates at a density of 105 cells/well and cultured at 37 °C in 5% CO2. Cells were treated for 24 hrs with vehicle (DMSO), valsartan (10 µm), gefitinib (10 µm), or GEF + VAL to determine the apoptotic rate using annexin V staining (Korashy et al., 2016, Tian et al., 2018). In valsartan groups, cells were incubated for 1 hr with valsartan (10 µm) followed by 24 hrs incubation with DMSO or gefitinib, respectively. After incubation, cardiomyocytes from all treated groups were washed with PBS and then centrifuged at 300 × g for 5 min. Cells were resuspended with 0.5 ml PBS and stained with annexin V-FITC/Propidium Iodide (PI) for analysis using Cytomics FC500 Flow Cytometer (Beckman Coulter, Inc., USA).
2.6. Detection of cardiomyocytes with reactive oxygen species (ROS) by Muse cell Analyzer
The detection of cardiomyocytes undergoing oxidative stress was performed using Muse Oxidative Stress Kit (Millipore, USA). According to the manufacturer’s protocol, treated cells were incubated with dihydroethidium (DHE), which is a cell mem-brane-permeable redox-sensitive probe that reacted with superoxide anions and formed DNA-binding fluorophore. The percentage of cells with reactive oxygen species (ROS + ) or without reactive oxygen species (ROS-) were quantified using Muse Cell Analyzer (Millipore, USA).
2.7. Western blot analysis
A small portion of the heart was cut into small pieces and then homogenized using Potter Elvehjem homogenizer in a mixture of RIPA buffer and protease/phosphatase inhibitor cocktail. The homogenate was centrifuged at 14,000 × g for 10 min and then protein concentration was measured via Direct Detect® Spectrometer (Millipore, USA). At the time of analysis, the supernatant was added to 2x Laemmli sample loading buffer containing β-Mercaptoethanol with total protein 40 µg. The mixture was resolved on 10% SDS-PAGE gels and transferred to PVDF membranes via a Trans-Blot Turbo Transfer System. Then, the membrane was blocked using 5% non-fat milk in Tris Buffered Saline (TBS) with 0.1% TWEEN 20 at room temperature for 1 h. The membrane was washed using TBST and incubated at 4 °C overnight into anti-AT1R, AT2R, NOX2, NOX4 (Abcam, USA), phospho-p38, total p38, phospho-JNK, total JNK primary antibody (Cell Signaling Technology, USA) or GAPDH primary antibody (ABclonal Technology, USA) as a housekeeping protein. All the primary antibodies were diluted at a ratio 1:1000. After incubation with the primary anti-body, the membrane was washed and incubated with secondary goat anti-rabbit or goat anti-mouse IgG for 1 hr at room temperature (25 °C). After washing with TBST, bands were visualized by Immobilon Western Chemiluminescent HRP Substrate (Millipore, USA) using a Bio-Rad gel-imaging system (Bio-Rad, USA). Image J software was used to quantify the data.
2.8. Measurement of oxidative stress markers in hearts
Cardiac tissues were homogenized in ice-cold phosphate buffer and then centrifuged using refrigerated centrifuge and Direct Detect® Spectrometer was used to determine the protein concentration in the collected supernatant. Each sample was aliquoted for measurement of glutathione (GSH), malondialdehyde (MDA), and catalase (CAT) levels to assess the effect of gefitinib and valsartan on the cardiac oxidative stress.
Sedlak and Lindsay’s method was used to measure GSH concentration in the cardiac tissues as described before (Imam et al., 2018). Samples and standards were mixed with dithiobis(2-nitrobenzoic acid) (DTNB) and then the absorbance of the mixture was taken within 5 min at 412 nm.
MDA levels were measured as an indicator of lipid peroxidation (LPO) (Imam et al., 2018). As described before, the collected supernatant was mixed with trichloroacetic acid (TCA) and thiobarbituric acid (TBA) and heated for 1 hr at 90 ± 5 °C in water bath. All samples were kept in ice cold water for 10 min and then centrifuged at 8000 × g for 10 min. The supernatant was separated, and the absorbance was taken at 650 nm (Imam et al., 2018).
Cardiac CAT activity was evaluated using Claibone method through incubation of the supernatant with hydrogen peroxide (H2O2) (Imam et al., 2018). CAT-dependent H2O2 consumption was measured through observing the reduction of H2O2 absorbance each minute at 240 nm. It was expressed as nm of H2O2 consumed/min/mg protein (Imam et al., 2018).
2.9. Statistical analysis
The statistical comparisons between groups were performed via an analysis of variance (ANOVA) approach followed by the Tukey–Kramer multiple comparison test using GraphPad prism 9. Data were expressed as mean ± SEM and P value < 0.05 was considered statistically significant.
3. Results
3.1. Effect of gefitinib and valsartan on heart weight to body weight (HW/BW) ratio and cardiac hypertrophy markers
HW/BW ratio index was calculated to assess the hypertrophic response to gefitinib and valsartan treatment as an indicator of cardiac hypertrophy. Gefitinib treatment increased HW/BW ratio significantly as compared with control group, which showed that gefitinib treatment might induce cardiac hypertrophy (Fig. 1A). In contrast, GEF + VAL group possessed a lower HW/BW ratio than the untreated gefitinib group (Fig. 1A). In control and valsartan groups, no significant difference in HW/BW ratio was noticed among the study (Fig. 1A). For further investigation, serum levels of CK-MB, NT-proBNP, and cTnI were measured to evaluate the cardiac hypertrophy induced by gefitinib and the role of valsartan in preventing this action. As expected, gefitinib significantly increased CK-MB, NT-proBNP, and cTnI levels in serum. Valsartan attenuated the induction of cardiac hypertrophy markers in GEF + VAL group (Fig. 1B-D). These results approved that gefitinib causes cardiac hypertrophy and can be prevented by blocking AT1R. Moreover, histological examinations and RAS were examined for further study of AT1R blockade in prevention of gefitinib-induced cardiac hypertrophy.
Fig. 1.
HW/BW index and serum cardiac hypertrophy markers after treatment with gefitinib (30 mg/kg) and/or valsartan (30 mg/kg). (A) Effect of gefitinib and/or valsartan on heart weight to body weight ratio index. (B, C, D) Effect of gefitinib and/or valsartan on serum levels of c-TnI, TN-proBNP, and CK-MB, respectively. Data are expressed as the mean ± SEM; n = 6. *P < 0.05, **P < 0.01, ****P < 0.0001. GEF, gefitinib; VAL, valsartan.
3.2. Histological changes during gefitinib and valsartan treatment in cardiac tissues
Gefitinib caused cardiac hypertrophy characterized by marked increased thickness of the myocardium (Fig. 2C). The ventricular thickness was measured at x4 magnification, and it found high thickness with constriction and congestion in the gefitinib group (Fig. 2G, I). In GEF + VAL, normal thickness of the myocardium was shown in (Fig. 2D, H, I), whereas valsartan treatment prevented the induced cardiac injuries caused by gefitinib. Normal thickness of the ventricular wall was noticed in control and valsartan groups (Fig. 2A-B, E-F, I). These results found that histological changes occurred only in the gefitinib group, which was blocked through administration of valsartan with gefitinib as shown in (Fig. 2C, G, I) and (Fig. 2D, H, I), respectively.
Fig. 2.
Histological changes and ventricular thickness in H&E-stained sections of the heart after treatment with gefitinib (30 mg/kg) and/or valsartan (30 mg/kg). (A, E, I) Sections of the heart obtained from the control group showed a normal thickness of the ventricular wall and myocardial fibers. (B, F, I) Sections of the heart obtained from the valsartan group showed a normal thickness of the ventricular myocardium. (C, G, I) Sections of the heart obtained from the gefitinib group showed the presence of cardiac hypertrophy characterized by a marked increase in thickness of the myocardium with constriction and congestion. (D, H, I) Sections of the heart obtained from the GEF + VAL group showing normal thickness of the myocardial wall; x4 & x400 magnification. (I) Ventricular thickness (µm) values are expressed as the mean ± SEM. ****P < 0.0001. GEF, gefitinib; VAL, valsartan.
3.3. Effect of gefitinib and valsartan on apoptosis
The result of TUNEL assay showed that gefitinib treatment was able to induce apoptosis, which showed a high number of apoptotic nuclei in cardiomyocytes and endothelial cells (Fig. 3A-D). The percentage of apoptotic cells to the total number cells was significantly increased (∼55%) in the gefitinib group as compared to the control group (∼3%) (Fig. 3E). Valsartan co-treatment attenuated the induction of apoptosis by gefitinib in the GEF + VAL group. The results showed decrease in the number of apoptotic nuclei and percentage of apoptotic cells (∼13%) in the GEF + VAL group as compared to the gefitinib group (Fig. 3C-E). In the control and valsartan groups, results showed a few numbers of apoptotic nuclei and ratio of apoptosis (Fig. 3A, B, E).
Fig. 3.
Number of apoptotic cells after treatment with gefitinib (30 mg/kg) and/or valsartan (30 mg/kg). (A-D) Photomicrographs of paraffin embedded cardiac sections stained by TUNEL assay for apoptosis, (red arrow) apoptotic nuclei (A) control showing a few numbers of apoptotic nuclei, (B) treated with valsartan also revealing a few numbers, (C) treated with gefitinib posting heavy incidence of apoptotic shrunken nuclei of cardiomyocytes and endothelial cells, (D) treated with GEF + VAL displaying lesser number; x100 magnification. (E) Percentage of apoptotic cells was obtained via number of apoptotic cells divided by total number cells then multiplied by 100. Data are expressed as the mean ± SEM. ****P < 0.0001. GEF, gefitinib; VAL, valsartan.
3.4. Effect of gefitinib and valsartan on AngII levels and the expression of its cardiac receptors
Plasma levels of AngII were significantly increased during gefitinib treatment (Fig. 4A). Induction of AngII might lead to increase in the expression of its receptors, whereas AT1R and AT2R were highly expressed in the cardiac tissues as shown in western blot data (Fig. 4B-D). In contrast, blocking AT1R by valsartan during gefitinib treatment attenuated the induction of plasma AngII levels, AT1R, and AT2R expression (Fig. 4A-B). In control and valsartan groups, results showed normal levels of AngII and its cardiac receptors expression (Fig. 4). Induction of RAS system in gefitinib-induced cardiac hypertrophy might be related to the pathogenesis of the disease. However, AT1R overactivation through gefitinib-induced AngII was studied to find its roles in the activation of down signaling pathways causing cardiomyocyte hypertrophy.
Fig. 4.
Plasma levels of AngII and AT1R/AT2R expression in the heart after treatment with gefitinib (30 mg/kg) and/or valsartan (30 mg/kg). (A) Measurement of plasma AngII levels in all groups showing induction of AngII during gefitinib treatment, which was modified by valsartan (n = 6). (B-D) Protein expression of AT1R and AT2R were highly expressed in gefitinib group as compared to the control and the GEF + VAL groups (B) Representative data from western blot analysis of AT1R, AT2R, and GAPDH protein expression. (C) Quantification of AT1R protein levels. (D) Quantification of AT2R protein levels. Data are expressed as the mean ± SEM; n = 6 in AngII measurement, and only one of the five representative experiments of western blot analysis is shown (n = 5). *P < 0.05, **P < 0.01, ****P < 0.0001. GEF, gefitinib; VAL, valsartan.
3.5. Effect of gefitinib and valsartan on apoptosis and oxidative stress in rat cardiomyocyte
In vitro studies on rat cardiomyocyte H9c2 cells were performed to study the effect of gefitinib and valsartan on apoptosis and oxidative stress. The obtained results showed that gefitinib increased the early apoptotic/apoptotic cardiomyocytes but co-treatment with valsartan reversed the effect mediated by gefitinib (Fig. 5A-C). In oxidative stress assessment, gefitinib markedly decreased the percentage of cardiomyocytes with ROS- and increased the percentage of cardiomyocytes with ROS + as compared with vehicle-treated cells (Fig. 5D-F). In contrast, concomitant treatment of valsartan with gefitinib increased the percentage of cardiomyocytes with ROS- and reduced the percentage of cardiomyocytes with ROS + as compared with gefitinib alone treated cells (Fig. 5D-F).
Fig. 5.
Detection of apoptosis and oxidative stress after treatment with gefitinib (30 mg/kg) and/or valsartan (30 mg/kg). (A-C) the percentage of early apoptotic (B) and apoptotic (C) H9c2 cells (n = 3). (D-F) the percentage of H9c2 cells with ROS- (E) and ROS+ (F) (n = 3). (G-I) measurement of oxidative stress markers in the heart. Cardiac GSH (G), CAT (H) and MDA (I) levels (n = 6). Data are expressed as the mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001. GEF, gefitinib; VAL, valsartan.
To confirm the role of AT1R blockade in the prevention of gefitinib-mediated oxidative stress, cardiac oxidative stress was evaluated through measurement of GSH, MDA, and CAT enzyme levels in cardiac homogenates. Gefitinib decreased the antioxidants (GSH & CAT) levels and induced the oxidant (MDA) levels in the heart (Fig. 5G-I). In contrast, GSH, MDA and CAT levels were corrected to the normal levels with valsartan treatment as shown in (Fig. 5G-I). Results demonstrated that gefitinib disturbed the balance between the antioxidants and oxidants, which might be related to the excessive production of ROS in the heart.
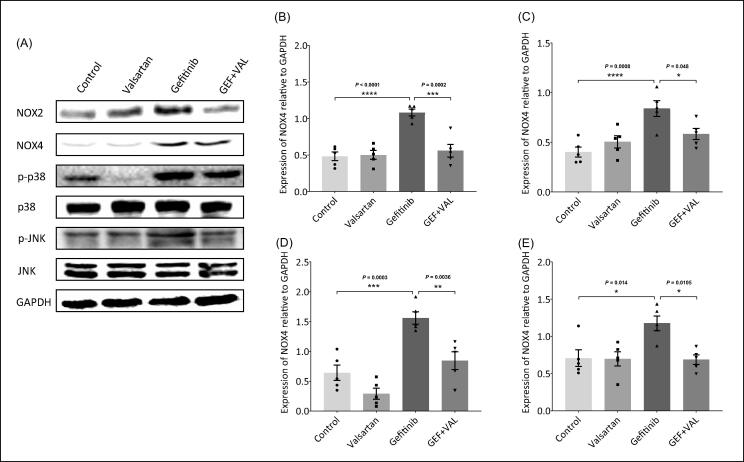
3.6. Effect of gefitinib and valsartan on NADPH oxidase expression
For further investigation of the effect of gefitinib and valsartan in the induction of cardiac oxidative stress. This study assessed the expression of NADPH oxidase, which is the main source of ROS in the cardiac tissues, through protein levels measurement of its cardiac subunits (NOX2 & NOX4). Gefitinib increased the protein expression of NOX2 and NOX4 as compared with control group, but the co-treatment with valsartan reversed the effect mediated by gefitinib in GEF + VAL group (Fig. 6A-C). Results showed that gefitinib induced NADPH oxidase leading to ROS formation and oxidative stress in the heart.
Fig. 6.
NOX2, NOX4 and JNK/p38 MAPK protein expression in the heart after treatment with gefitinib (30 mg/kg) and/or valsartan (30 mg/kg). (A-E) Protein expression of cardiac NOX2, NOX4, phospho-p38 (p-p38), and phospho-JNK (p-JNK) were highly expressed in gefitinib group as compared to the control and the GEF + VAL groups. (A) Representative data from western blot analysis of NOX2, NOX4, p-p38, p38, p-JNK, JNK, and GAPDH protein expression. (B) Quantification of NOX2 protein levels. (C) Quantification of NOX4 protein levels. (D) Quantification of p-p38 protein levels. (E) Quantification of p-JNK protein levels. Data are expressed as the mean ± SEM; only one of the five representative experiments is shown (n = 5). *P < 0.05, ***P < 0.001, ****P < 0.0001. GEF, gefitinib; VAL, valsartan.
3.7. Effect of gefitinib and valsartan on JNK/p38-MAPK expression
NADPH oxidase-mediated ROS formation might activate MAPK pathway in cardiac hypertrophy induced by gefitinib. As expected, gefitinib increased the protein levels of the phosphorylated JNK/p38-MAPK as compared with control group (Fig. 6A, D, E). AT1R blockade via valsartan downregulated the expression of the phosphorylated JNK/p38-MAPK in GEF + VAL group (Fig. 6A, D, E). These results revealed that gefitinib induced MAPK pathway via ROS formation mediated by activation of AngII/AT1R/NOX in the cardiomyocyte.
4. Discussion
Management of cardiotoxicity associated with EGFRIs therapy is a clinical challenge in cancer patients that requires a multifaceted approach for diagnosis and treatment. EGFRI-associated cardiac adverse events (CAE) vary widely, depending on each specific drug, duration of treatment and comorbid health problems. CAE induced by EGFRIs ranges from QT prolongation to heart failure (Morissette et al., 2015). Still, the correlation between RAS and induction of cardiovascular complications in EGFRIs therapy is not fully understood. In the current study, our results demonstrate that 21 days of gefitinib treatment caused cardiac hypertrophy as approved by the elevation of cardiac enzymes (CK-MB, NT-proBNP, and cTnI) and the histological changes in the heart. Furthermore, the finding results revealed that an increase in plasma AngII levels, cardiac AT1R/AT2R expression, oxidative stress, NADPH oxidase and JNK/p38-MAPK activation during gefitinib treatment. In contrast, AT1R blockade by valsartan managed the associated cardiac complications on the histological, enzymatic, and cellular levels, whereas the dysregulation of RAS, oxidant/antioxidant balance, NADPH oxidase and JNK/p38-MAPK was modified in the cardiac tissues.
Gefitinib has been reported in induction of cardiovascular complications in many cases (Zaborowska-Szmit et al., 2020). One of the most vascular complications of gefitinib is induction of acute coronary syndrome, which was noticed in a study of twenty patients who received gefitinib for two weeks (Kanazawa et al., 2005). Thereafter, a diabetic and hypertensive female patient had lung adenocarcinoma and then diagnosed with acute coronary syndrome after two months of gefitinib treatment course (Yamaguchi et al., 2005). However, antiplatelet drugs were recommended with gefitinib treatment to avoid the incidence of acute coronary syndrome (Zaborowska-Szmit et al., 2020). Cardiotoxicity induced by gefitinib has been reported in several cases including myocarditis and myocardial infarction (Truell et al., 2005, Lynch et al., 2011). In 2005, a case of a 71-year-old male was reported with fulminant myocarditis after a week of gefitinib treatment (Morissette et al., 2015). In addition, recurrent myocardial infarction was reported in a patient treated with gefitinib for metastatic carcinoid tumor (Lynch et al., 2011). Thereafter, in-vitro and in-vivo studies were conducted to understand the mechanisms underlying gefitinib-induced QT prolongation and cardiotoxicity (Korashy et al., 2016, Jie et al., 2021). Studies approved the ability of gefitinib to cause cardiac hypertrophy in cardiomyocyte cell line (H9c2) and Wistar albino rats through induction of oxidative stress and apoptosis (Zaborowska-Szmit et al., 2020). The findings from the present study were consistent with previous studies in showing the capability of gefitinib to induce cardiac hypertrophy as approved by histological studies and measurement of serum cardiac enzymes. Furthermore, current study validated gefitinib-induced oxidative stress via in-vitro and in-vivo studies, which found an elevation in ROS formation and disruption of oxidant/antioxidant balance in the cardiomyocytes and the cardiac tissues, respectively.
AngII is a potent cardiac hypertrophic agent that has been involved in the pathogenesis of cardiac hypertrophy in various pathological conditions such as diabetes, hypertension and anticancer-mediated cardiotoxicity (Ahmad et al., 2009, Masuda et al., 2012, Pinter et al., 2018). Our findings demonstrated that RAS system might be involved in the induction of cardiac hypertrophy induced by gefitinib. As expected, it was found that 21 days of gefitinib treatment increased plasma AngII levels and its cardiac AT1R/AT2R expression. Although the AT1R induces the majority of AngII effects, effects of AT2R upon binding to AngII are still insufficiently understood. Studies showed that AT2R attenuated AT1R-mediated pathways such as proliferation and apoptosis (Dasgupta and Zhang 2011). In contrast, other study demonstrated that AT2R can mediate cardiomyocyte hypertrophy with no effects on AT1R-induced hypertrophy in cardiomyocytes treated with AngII (D’Amore et al., 2005). As described in the previous studies, AT2R expression is usually low during health condition, and increased in pathological situation as a tissue response to damage (Barker et al., 2006). In the current study, AT1R blockade via valsartan during gefitinib treatment might contribute to prevent the induced cardiac injury leading to return AT2R receptor to the normal expression. Still, the exact mechanisms of gefitinib-induced RAS including AT2R and downregulation of AT2R after AT1R blockade in gefitinib-induced cardiotoxicity are not clear and need further investigation. AT1R is the main participant in activation of NADPH oxidase provoking superoxide production and imbalance between oxidants/antioxidants causing oxidative stress in cardiovascular diseases (Hingtgen et al., 2006). Previous studies found that AT1R activation upon binding to AngII stimulated NOX2-containing NADPH oxidase leading to induction of ROS-induced cardiac hypertrophy (Hingtgen et al., 2006). NOX2 and NOX4 are the predominant subunits of NADPH oxidase found in the heart (Sirker et al., 2007). In heart diseases, it was found that upregulation of NADPH oxidase induces ROS formation through superoxide production in the cardiomyocytes causing cell injury and hypertrophy (Sirker et al., 2007, Dikalov and Nazarewicz, 2013). Our results found that NOX2 and NOX4 were highly expressed in the cardiac tissues and ROS production was increased in the cardiomyocytes in gefitinib-mediated oxidative stress, which might be promoted by the induced AT1R. In the current study, activation of AT1R-mediated NADPH oxidase inducing ROS formation is consistent with previous studies that demonstrated the role of ROS in AngII-induced cardiac hypertrophy (Wen et al., 2019). AT1R is redox dependent receptor causing overproduction of ROS and might result in overactivation of AT1R-mediated cardiomyocyte signaling pathways such as MAPK (Dikalov and Nazarewicz 2013). As shown in a previous study, overproduction of ROS induced by NADPH oxidase led to activation of JNK/p38-MAPK pathway that plays a pivotal role in cardiomyocyte hypertrophy and apoptosis in a rat model of pancreatitis (Wen et al., 2019). Moreover, another research study showed the role of AngII-induced ROS in activation JNK/p38-MAPK pathway resulted in hypertrophic responses in rat neonatal cardiomyocytes (Nishida et al., 2005). Consistent with previous studies, our findings demonstrated that gefitinib stimulated AngII/AT1R-mediated ROS formation and JNK/p38-MAPK pathway causing cardiac hypertrophy. For further investigation, the present study was also conducted to identify the role of AT1R blockade in the prevention of consequences that associated with RAS activation and providing cardiac protection in cardiotoxicity induced by gefitinib.
AT1R blockers (ARBs) are classified chemically into bi-phenyl tetrazole analogs (azilsartan, candesartan, irbesartan, losartan, olmesartan and valsartan) or non-biphenyl tetrazole analogs (eprosartan and telmisartan) (Singh et al., 2018). ARBs provide a cardioprotective role in various pathological conditions through downregulation of AngII-mediated pathways causing oxidative stress, inflammation and apoptosis in the cardiomyocyte (Munger, 2011, Escobales et al., 2019). Valsartan is a potent AT1R blocker that showed a beneficial effect in the prevention of cardiotoxicity and cardiovascular diseases (Goyal et al., 2011, Akolkar et al., 2015, Zhang et al., 2016). The current study found that valsartan downregulated the induced AngII receptors, whereas AT1R blockade corrected the histological changes and cardiac enzymes in gefitinib treatment. Valsartan attenuated AngII-mediated NADPH oxidase leading to regulation of ROS formation, MDA, GSH and CAT levels in the cardiomyocyte during cardiac hypertrophy induced by gefitinib. Moreover, AT1R blockade by valsartan downregulated JNK/p38-MAPK through inhibition of AT1R-mediated NADPH oxidase and ROS formation. These findings correspond with previous study demonstrated that valsartan corrected AT1R-induced NOX/ROS/MAPK pathway leading to the prevention of cardiomyocyte hypertrophy (Cheng et al., 2021). To our knowledge, there is no prospective study showed the link between the cardiotoxicity of gefitinib and RAS system. Still, further investigation is needed to identify the factors that involved in induction of AngII formation and expression of AT1R and AT2R in gefitinib-induced cardiac hypertrophy.
5. Conclusions
In the current study, our results showed that gefitinib-induced cardiac hypertrophy through overactivation of AngII/AT1R leading to NOX-mediated ROS formation resulted in activation of JNK/p38-MAPK signaling pathway in the cardiomyocyte. AT1R blockade by valsartan attenuated these effects provided cardioprotection during gefitinib treatment (Fig. 7). However, our study demonstrated that AngII and its receptors might be a potential target in the management of gefitinib-induced cardiotoxicity.
Fig. 7.
Graphical summary. Gefitinib induced RAS system mediating cardiac oxidative stress and activation of JNK/p38 MAPK pathway leading to cardiac hypertrophy.
All authors declare that they have no conflict of interest.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group no (RG-1441-386).
Footnotes
Peer review under responsibility of King Saud University.
References
- Ahmad S., Cesana F., Lamperti E., Gavras H., Yu J. Attenuation of angiotensin II-induced hypertension and cardiac hypertrophy in transgenic mice overexpressing a type 1 receptor mutant. Am. J. Hypertens. 2009;22(12):1320–1325. doi: 10.1038/ajh.2009.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akolkar G., Bhullar N., Bews H., Shaikh B., Premecz S., Bordun K.A., Cheung D.Y., Goyal V., Sharma A.K., Garber P., Singal P.K., Jassal D.S. The role of renin angiotensin system antagonists in the prevention of doxorubicin and trastuzumab induced cardiotoxicity. Cardiovasc Ultrasound. 2015;13:18. doi: 10.1186/s12947-015-0011-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhazzani K., Alotaibi M.R., Alotaibi F.N., Aljerian K., As Sobeai H.M., Alhoshani A.R., Alanazi A.Z., Alanazi W.A., Alswayyed M. Protective effect of valsartan against doxorubicin-induced cardiotoxicity: Histopathology and metabolomics in vivo study. J. Biochem. Mol. Toxicol. 2021;35(9) doi: 10.1002/jbt.22842. [DOI] [PubMed] [Google Scholar]
- Alhoshani A., Alanazi F.E., Alotaibi M.R., Attwa M.W., Kadi A.A., Aldhfyan A., Akhtar S., Hourani S., Agouni A., Zeidan A., Korashy H.M. EGFR Inhibitor Gefitinib Induces Cardiotoxicity through the Modulation of Cardiac PTEN/Akt/FoxO3a Pathway and Reactive Metabolites Formation. In Vivo and in Vitro Rat Studies. Chem. Res. Toxicol. 2020;33(7):1719–1728. doi: 10.1021/acs.chemrestox.0c0000510.1021/acs.chemrestox.0c00005.s001. [DOI] [PubMed] [Google Scholar]
- Ararat E., Brozovich F.V. Losartan decreases p42/44 MAPK signaling and preserves LZ+ MYPT1 expression. PLoS ONE. 2009;4(4):e5144. doi: 10.1371/journal.pone.0005144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker T.A., Massett M.P., Korshunov V.A., Mohan A.M., Kennedy A.J., Berk B.C. Angiotensin II type 2 receptor expression after vascular injury: differing effects of angiotensin-converting enzyme inhibition and angiotensin receptor blockade. Hypertension. 2006;48(5):942–949. doi: 10.1161/01.HYP.0000241061.51003.b7. [DOI] [PubMed] [Google Scholar]
- Chaar M., Kamta J., Ait-Oudhia S. Mechanisms, monitoring, and management of tyrosine kinase inhibitors-associated cardiovascular toxicities. Onco Targets Ther. 2018;11:6227–6237. doi: 10.2147/OTT.S170138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Ge Z., Huang S., Zhou L., Zhai C., Chen Y., Hu Q., Cao W., Weng Y., Li Y. Delphinidin attenuates pathological cardiac hypertrophy via the AMPK/NOX/MAPK signaling pathway. Aging (Albany NY). 2020;12:5362–5383. doi: 10.18632/aging.102956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng D., Tu W., Chen L., Wang H., Wang Q., Liu H., Zhu N., Fang W., Yu Q. MSCs enhances the protective effects of valsartan on attenuating the doxorubicin-induced myocardial injury via AngII/NOX/ROS/MAPK signaling pathway. Aging (Albany NY). 2021;13:22556–22570. doi: 10.18632/aging.203569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan B.R., Young A.A. Left ventricular hypertrophy and renin-angiotensin system blockade. Curr. Hypertens. Rep. 2009;11:167–172. doi: 10.1007/s11906-009-0030-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amore A., Black M.J., Thomas W.G. The angiotensin II type 2 receptor causes constitutive growth of cardiomyocytes and does not antagonize angiotensin II type 1 receptor-mediated hypertrophy. Hypertension. 2005;46(6):1347–1354. doi: 10.1161/01.HYP.0000193504.51489.cf. [DOI] [PubMed] [Google Scholar]
- Dasgupta C., Zhang L. Angiotensin II receptors and drug discovery in cardiovascular disease. Drug Discov Today. 2011;16(1-2):22–34. doi: 10.1016/j.drudis.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikalov S.I., Nazarewicz R.R. Angiotensin II-induced production of mitochondrial reactive oxygen species: potential mechanisms and relevance for cardiovascular disease. Antioxid. Redox Signal. 2013;19(10):1085–1094. doi: 10.1089/ars.2012.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobales N., Nuñez R.E., Javadov S. Mitochondrial angiotensin receptors and cardioprotective pathways. Am J Physiol Heart Circ Physiol. 2019;316(6):H1426–H1438. doi: 10.1152/ajpheart.00772.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal S., Bharti S., Sahoo K.C., Sharma A.K., Arya D.S. Valsartan, an angiotensin II receptor blocker, attenuates cardiac dysfunction and oxidative stress in isoproterenol-induced cardiotoxicity. Cardiovasc. Toxicol. 2011;11(2):148–156. doi: 10.1007/s12012-011-9108-0. [DOI] [PubMed] [Google Scholar]
- Hingtgen S.D., Tian X., Yang J., Dunlay S.M., Peek A.S., Wu Y., Sharma R.V., Engelhardt J.F., Davisson R.L. Nox2-containing NADPH oxidase and Akt activation play a key role in angiotensin II-induced cardiomyocyte hypertrophy. Physiol. Genomics. 2006;26(3):180–191. doi: 10.1152/physiolgenomics.00029.2005. [DOI] [PubMed] [Google Scholar]
- Imam F., Al-Harbi N.O., Al-Harbi M.M., Ansari M.A., Al-Asmari A.F., Ansari M.N., Al-Anazi W.A., Bahashwan S., Almutairi M.M., Alshammari M., Khan M.R., Alsaad A.M., Alotaibi M.R. Apremilast prevent doxorubicin-induced apoptosis and inflammation in heart through inhibition of oxidative stress mediated activation of NF-κB signaling pathways. Pharmacol. Rep. 2018;70(5):993–1000. doi: 10.1016/j.pharep.2018.03.009. [DOI] [PubMed] [Google Scholar]
- Jiao K.-L., Li Y.-G., Zhang P.-P., Chen R.-H., Yu Y.i. Effects of valsartan on ventricular arrhythmia induced by programmed electrical stimulation in rats with myocardial infarction. J. Cell Mol. Med. 2012;16(6):1342–1351. doi: 10.1111/j.1582-4934.2011.01502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jie L.-J., Li Y.-D., Zhang H.-Q., Mao L., Xie H.-B., Zhou F.-G., Zhou T.-L., Xie D., Lin J.-L., Li G.-Y., Cai B.-N., Zhang Y.-H., Wang Y. Mechanisms of gefitinib-induced QT prolongation. Eur. J. Pharmacol. 2021;910:174441. doi: 10.1016/j.ejphar.2021.174441. [DOI] [PubMed] [Google Scholar]
- Kanazawa S., Yamaguchi K., Kinoshita Y., Muramatsu M., Komiyama Y., Nomura S. Gefitinib affects functions of platelets and blood vessels via changes in prostanoids balance. Clin. Appl. Thromb. Hemost. 2005;11(4):429–434. doi: 10.1177/107602960501100409. [DOI] [PubMed] [Google Scholar]
- Kawai T., Forrester S.J., O'Brien S., Baggett A., Rizzo V., Eguchi S. AT1 receptor signaling pathways in the cardiovascular system. Pharmacol. Res. 2017;125:4–13. doi: 10.1016/j.phrs.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazandjian D., Blumenthal G.M., Yuan W., He K., Keegan P., Pazdur R. FDA Approval of Gefitinib for the Treatment of Patients with Metastatic EGFR Mutation-Positive Non-Small Cell Lung Cancer. Clin. Cancer Res. 2016;22:1307–1312. doi: 10.1158/1078-0432.CCR-15-2266. [DOI] [PubMed] [Google Scholar]
- Korashy H.M., Attafi I.M., Ansari M.A., Assiri M.A., Belali O.M., Ahmad S.F., Al-Alallah I.A., Anazi F.E., Alhaider A.A. Molecular mechanisms of cardiotoxicity of gefitinib in vivo and in vitro rat cardiomyocyte: Role of apoptosis and oxidative stress. Toxicol. Lett. 2016;252:50–61. doi: 10.1016/j.toxlet.2016.04.011. [DOI] [PubMed] [Google Scholar]
- Kurdi M., Booz G.W. New take on the role of angiotensin II in cardiac hypertrophy and fibrosis. Hypertension. 2011;57(6):1034–1038. doi: 10.1161/HYPERTENSIONAHA.111.172700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon M.A., Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141(7):1117–1134. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F., Fan L.M., Geng L., Li J.M. p47phox-Dependent Oxidant Signalling through ASK1, MKK3/6 and MAPKs in Angiotensin II-Induced Cardiac Hypertrophy and Apoptosis. Antioxidants. 2021;10:1363. doi: 10.3390/antiox10091363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch D.R., Kickler T.S., Rade J.J. Recurrent myocardial infarction associated with gefitinib therapy. J. Thromb. Thrombolysis. 2011;32(1):120–124. doi: 10.1007/s11239-010-0539-4. [DOI] [PubMed] [Google Scholar]
- Masuda T., Muto S., Fujisawa G., Iwazu Y., Kimura M., Kobayashi T., Nonaka-Sarukawa M., Sasaki N., Watanabe Y., Shinohara M., Murakami T., Shimada K., Kobayashi E., Kusano E. Heart angiotensin II-induced cardiomyocyte hypertrophy suppresses coronary angiogenesis and progresses diabetic cardiomyopathy. Am J Physiol Heart Circ Physiol. 2012;302(9):H1871–H1883. doi: 10.1152/ajpheart.00663.2011. [DOI] [PubMed] [Google Scholar]
- Mazzolai L., Nussberger Jürg, Aubert J.-F., Brunner D.B., Gabbiani G., Brunner H.R., Pedrazzini T. Blood pressure-independent cardiac hypertrophy induced by locally activated renin-angiotensin system. Hypertension. 1998;31(6):1324–1330. doi: 10.1161/01.hyp.31.6.1324. [DOI] [PubMed] [Google Scholar]
- Morissette P., Regan H.K., Fitzgerald K., Bernasconi S., Gerenser P., Travis J., Fanelli P., Sannajust F., Regan C.P. QT interval correction assessment in the anesthetized guinea pig. J. Pharmacol. Toxicol. Methods. 2015;75:52–61. doi: 10.1016/j.vascn.2015.05.007. [DOI] [PubMed] [Google Scholar]
- Munger M.A. Use of Angiotensin receptor blockers in cardiovascular protection: current evidence and future directions. P T. 2011;36:22–40. [PMC free article] [PubMed] [Google Scholar]
- Nguyen Dinh Cat A., Montezano A.C., Burger D., Touyz R.M. Angiotensin II, NADPH oxidase, and redox signaling in the vasculature. Antioxid. Redox Signal. 2013;19(10):1110–1120. doi: 10.1089/ars.2012.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida M., Tanabe S., Maruyama Y., Mangmool S., Urayama K., Nagamatsu Y., Takagahara S., Turner J.H., Kozasa T., Kobayashi H., Sato Y., Kawanishi T., Inoue R., Nagao T., Kurose H. G alpha 12/13- and reactive oxygen species-dependent activation of c-Jun NH2-terminal kinase and p38 mitogen-activated protein kinase by angiotensin receptor stimulation in rat neonatal cardiomyocytes. J. Biol. Chem. 2005;280:18434–18441. doi: 10.1074/jbc.M409710200. [DOI] [PubMed] [Google Scholar]
- Pinter M., Kwanten W.J., Jain R.K. Renin-Angiotensin System Inhibitors to Mitigate Cancer Treatment-Related Adverse Events. Clin. Cancer Res. 2018;24:3803–3812. doi: 10.1158/1078-0432.CCR-18-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piratello A.C., Moraes-Silva I., Paulini J., Souza P.R., Sirvente R., Salemi V., Flues K., Moreira E.D., Mostarda C., Cunha T., Casarini D.E., Irigoyen M.C. Renin angiotensin system and cardiac hypertrophy after sinoaortic denervation in rats. Clinics (Sao Paulo). 2010;65(12):1345–1350. doi: 10.1590/S1807-59322010001200019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pottier C., Fresnais M., Gilon M., Jérusalem G., Longuespée R., Sounni N.E. Tyrosine Kinase Inhibitors in Cancer: Breakthrough and Challenges of Targeted Therapy. Cancers. 2020;12:731. doi: 10.3390/cancers12030731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshacharyulu P., Ponnusamy M.P., Haridas D., Jain M., Ganti A.K., Batra S.K. Targeting the EGFR signaling pathway in cancer therapy. Expert Opin Ther Targets. 2012;16(1):15–31. doi: 10.1517/14728222.2011.648617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K.D., Unal H., Desnoyer R., Karnik S.S. Divergent Spatiotemporal Interaction of Angiotensin Receptor Blocking Drugs with Angiotensin Type 1 Receptor. J. Chem. Inf. Model. 2018;58(1):182–193. doi: 10.1021/acs.jcim.7b0042410.1021/acs.jcim.7b00424.s001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirker A., Zhang M., Murdoch C., Shah A.M. Involvement of NADPH oxidases in cardiac remodelling and heart failure. Am. J. Nephrol. 2007;27:649–660. doi: 10.1159/000109148. [DOI] [PubMed] [Google Scholar]
- Streicher J.M., Ren S., Herschman H., Wang Y. MAPK-activated protein kinase-2 in cardiac hypertrophy and cyclooxygenase-2 regulation in heart. Circ. Res. 2010;106(8):1434–1443. doi: 10.1161/CIRCRESAHA.109.213199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H., Yu D., Hu Y., Zhang P., Yang Y., Hu Q., Li M. Angiotensin II upregulates cyclophilin A by enhancing ROS production in rat cardiomyocytes. Mol. Med. Rep. 2018;18:4349–4355. doi: 10.3892/mmr.2018.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truell J.S., Fishbein M.C., Figlin R. Myocarditis temporally related to the use of gefitinib (Iressa) Arch. Pathol. Lab. Med. 2005;129:1044–1046. doi: 10.5858/2005-129-1044-MTRTTU. [DOI] [PubMed] [Google Scholar]
- Tseng L.C., Chen K.H., Wang C.L., Weng L.C. Effects of tyrosine kinase inhibitor therapy on skin toxicity and skin-related quality of life in patients with lung cancer: An observational study. Medicine. 2020;99 doi: 10.1097/MD.0000000000020510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulutas Z., Ermis N., Ozhan O., Parlakpinar H., Vardi N., Ates B., Colak C. The Protective Effects of Compound 21 and Valsartan in Isoproterenol-Induced Myocardial Injury in Rats. Cardiovasc. Toxicol. 2021;21(1):17–28. doi: 10.1007/s12012-020-09590-6. [DOI] [PubMed] [Google Scholar]
- Wen Y.i., Liu R., Lin N., Luo H., Tang J., Huang Q., Sun H., Tang L. NADPH Oxidase Hyperactivity Contributes to Cardiac Dysfunction and Apoptosis in Rats with Severe Experimental Pancreatitis through ROS-Mediated MAPK Signaling Pathway. Oxid Med Cell Longev. 2019;2019:1–18. doi: 10.1155/2019/4578175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K., Kanazawa S., Kinoshita Y., Muramatsu M., Nomura S. Acute myocardial infarction with lung cancer during treatment with gefitinib: the possibility of gefitinib-induced thrombosis. Pathophysiol. Haemost. Thromb. 2005;34:48–50. doi: 10.1159/000088548. [DOI] [PubMed] [Google Scholar]
- Zaborowska-Szmit M., Krzakowski M., Kowalski D.M., Szmit S. Cardiovascular Complications of Systemic Therapy in Non-Small-Cell Lung Cancer. J Clin Med. 2020;9:1268. doi: 10.3390/jcm9051268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M.J., Gu Y., Wang H., Zhu P.F., Liu X.Y., Wu J. Valsartan attenuates cardiac and renal hypertrophy in rats with experimental cardiorenal syndrome possibly through down-regulating galectin-3 signaling. Eur. Rev. Med. Pharmacol. Sci. 2016;20:345–354. [PubMed] [Google Scholar]
- Zhang Q., Li R., Chen X., Lee S.B., Pan J., Xiong D., Hu J., Miller M.S., Szabo E., Lubet R.A., Wang Y., You M. Effect of weekly or daily dosing regimen of Gefitinib in mouse models of lung cancer. Oncotarget. 2017;8(42):72447–72456. doi: 10.18632/oncotarget.19785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z.Q., Yu Z.Y., Li J., Ouyang X.N. Gefitinib induces lung cancer cell autophagy and apoptosis via blockade of the PI3K/AKT/mTOR pathway. Oncol Lett. 2016;12:63–68. doi: 10.3892/ol.2016.4606. [DOI] [PMC free article] [PubMed] [Google Scholar]