Abstract
Allovahlkampfia spelaea (A. spelaea) is a free-living amoeba, proved to cause Acanthamoeba-like keratitis with quite difficult treatment. This study aimed to evaluate the amoebicidal effect of Allium cepa (A. cepa) on A. spelaea trophozoites and cysts both in vitro and in vivo using Chinchilla rabbits as an experimental model of this type of keratitis. Chemical constituents of the aqueous extract of A. cepa were identified using Liquid Chromatography-mass Spectrometry (LC-MS). In vitro, A. cepa showed a significant inhibitory effect on trophozoites and cysts compared to the reference drug, chlorhexidine (CHX) as well as the non-treated control (P < 0.05) with statistically different effectiveness in terms of treatment durations and concentrations. No cytotoxic effect of A. cepa on corneal cell line was found even at high concentrations (32 mg/ml) using agar diffusion method. The in vivo results confirmed the efficacy of A. cepa where the extract enhanced keratitis healing with complete resolution of corneal ulcers in 80% of the infected animals by day 14 (post infection)pi compared to 70% recovery with CHX after 20 treatment days. The therapeutic effect was also approved at histological, immune-histochemical, and parasitological levels. Our findings support the potential use of A. cepa as an effective agent against A. spelaea keratitis.
Keywords: Allovahlkampfia, Allium cepa, Keratitis, Amoeba
1. Introduction
Amoebic keratitis is a disease that could eventually lead to loss of vision. Genus Acanthamoeba is the most common cause of this disease and is usually associated with contact lens wearing or eye trauma (Henriquez and Khan, 2009, Lorenzo-Morales et al., 2013, Randag et al., 2019). Yet, this capability might not be limited to this genus. An increasing number of reports raised awareness of the existence of other free-living amoebae (FLA) causing keratitis, alone and with co-infection with Acanthamoeba or Candida, such as Hartmanella and the heterolobosean Vahlkampfia (Abedkhojasteh et al., 2013, Alexandrakis et al., 1998, Arnalich-Montiel et al., 2013, Kinnear, 2003, Lorenzo-Morales et al., 2007, Niyyati et al., 2010).
The genus, Allovahlkampfia has been formalized in 2009 to place a new heterolobosean that did not fit elsewhere (Walochnik and Mulec, 2009) and is currently organized, does not have flagellates or amebo-flagellates but all members produce un-operculated cysts. They are variable in size and vary significantly in rRNA sequence, with inserts exist in some (Geisen et al., 2015). Some Allovahlkampfia spp. have been found in animal intestinal tracts or associated with microbial infection (Ozkoc et al., 2008). Some studies have revealed Allovahlkampfia existence in human activity-related places (De Obeso Fernadez Del Valle and Maciver, 2017, Tyml et al., 2016). Reyes-Batlle and colleagues (2019) documented the first report on the isolation of Allovahlkampfia genus from dishcloths in the Spanish territory (Reyes-Batlle et al., 2019).
Allovahlkampfia spelaea (A. spelaea), in 2009, was identified as a new FLA species inhabiting caves (Walochnik and Mulec, 2009). In the form of a vegetative trophozoite and a cyst, A. spelaea exists in nature and propagates by forming cysts with single cell wall and single nucleus which can survive for many years in the environment (Walochnik and Mulec, 2009). It has been molecularly confirmed as a causative agent of amoebic human keratitis (Tolba et al., 2016) and acts as a vehicle of other pathogenic microbes (Mohamed and Huseein, 2016), making it worthy of study.
Diagnosis of amoebic keratitis is challenging, and the available treatments are long-lasting and not fully-effective against all strains. A combination of cationic antiseptics (polyhexamethylene biguanide), which inhibit the membrane, and aromatic diamidines (propamidine isethionate), which prevent DNA synthesis, are usually used to treat eye infections with the potentially pathogenic amoeba (Lorenzo-Morales et al., 2015). Chlorhexidine (CHX), alone or in combination with propamidine isethionate, has also been applied (Henriquez and Khan, 2009, Sifaoui et al., 2020). However, treatment for amoeba keratitis remains problematic and has not been largely successful, partially because the infection is frequently well-advanced before diagnosis in addition to the low sensitivity of both trophozoites and cysts to the available anti-amoebic drugs (Ozpinar et al., 2020, Shahbaz et al., 2020, Siddiqui et al., 2012). These facts make research for new effective agents necessary. One area of interest is the use of plant sources that constitute a promising line to find new alternatives as recommended by WHO (2002) to be used more effectively in the healthcare systems. Several medicinal plants extracts have proven to be at least partially growth inhibitors to FLA as Acanthamoeba (Anwar et al., 2020, Degerli et al., 2012, Derda et al., 2009, El-Sayed et al., 2012, Goze et al., 2009, Hadaś et al., 2017, Malatyali et al., 2012, Ortillés et al., 2017, Polat et al., 2008, Polat et al., 2007, Ródio et al., 2008, Tepe et al., 2012).
Allium cepa L. (A. cepa) is a popular folk remedy that has been cultivated since ancient times and is highly valued for its therapeutic properties (Kyung, 2012). It has exerted beneficial properties against different parasitic diseases such as schistosomiasis, cryptosporidiosis, and trichinellosis (Upadhyay, 2016). Moreover, it has already been proven to be highly leishmanicidal and trypanocidal (Krstin et al., 2018). Additionally, its anti-cancer, anti-microbial, anti-diabetic anti-oxidant, immune-protective, and other biological potentials have been scientifically confirmed (Abdel-Maksoud and El-Amin, 2011, Jeje et al., 2021, Sidhu et al., 2019, Teshika et al., 2019).
In vitro testing for amoebicidal effects of pharmaceutical agents and natural products on trophozoites and cysts is imperative to evaluate new drugs and may assist the management of a patient's treatment regimen (Anwar et al., 2020, Elder et al., 1994). Meanwhile, in vivo studies are used to explore the impact of these agents on the pattern of host immune response towards amoebic infection and providing knowledge about the pathogenesis and cellular differentiation processes. They hold the key to enhanced management. Unfortunately, these studies are still not sufficient and need additional investigations (Lorenzo-Morales et al., 2015).
This study was designed to test the in vitro effectiveness of A. cepa on the growth of A. sepaea trophozoites and cysts and its cytotoxic potentials on corneal cells. A rabbit model of keratitis was also used to explore, in vivo, the impact of A. cepa on A. spelaea infection using clinical, histological, immunohistochemical, and parasitological assays.
2. Materials and methods
2.1. Chemicals
All chemicals used in the present study were high analytical grade products purchased from Sigma (Sigma-Aldrich, Saint Louis, USA).
2.2. Plant materials and extraction procedures
Bulbs of A. cepa (500 g) were collected from a local market in Assiut Governorate, Assiut, Egypt in March 2018. The plant verification was done in the Department of Pharmacognosy, Faculty of Pharmacy, Assiut University, Egypt. Voucher specimens were deposited in the Herbarium of the same department (No. 6–2018). Freshly peeled bulbs of A. cepa (250 g) were blended with water (100 ml). Fresh juice was obtained by squeezing and filtering the resultant slurry through a sterile fine cloth and the filtrate was then lyophilized (Sokmen et al., 1999). The yield (24.5 g) was frozen (−20 °C) until used. The extract was re-dissolved in its solvent before each experiment.
2.3. Liquid Chromatography-Mass Spectrometry (LC-MS) studies
The chemical constituents of the aqueous extract were determined using LC-MS. LC-MS analysis was performed using 6530 Q-TOF LC/MS (Agilent Technologies) equipped with an autosampler (G7129A), a Quat. Pump (G7104C), and A Zorbax RP-18 column from Agilent Technologies (G7116A), 2.7 μ, (150 × 3 mm i.d.) was used for the analysis. Mobile phases were water (100%) as phase A and methanol (100%) as phase B, 0–2 min, isocratic 99% A, 2–16 min, linear gradient 99–94% A; 16–25 min, linear gradient 6–99% B; 25–30 min, isocratic 99% B. Solvents were delivered at a total flow rate of 0.4 ml/min. The MS spectra were acquired using ESI in both positive and negative ionization modes with a capillary voltage of 4000 V. The mass spectra were recorded in the m/z range of 100 to 2500 m/z. The gas temperature and drying gas flow were 320 OC and 10 L·/min, respectively. The skimmer and fragmentator voltages were set at 65 and 130 V, respectively and collision energy was 10 V. The nebulization pressure was 40 psig. A 4 μl volume of the extracts were injected onto the analytical column for analysis. The mass fragmentations were identified by using spectrum database for organic compounds in SDBS application.
2.4. Evaluation of microbial contamination and endotoxin production
Total aerobic microbial count and total combined yeasts/moulds count were used for quantitative enumeration of mesophilic bacteria or fungi that may grow under aerobic conditions in Allium cepum extract using the pour plating technique (EDQM Council of Europe 2014). The bacterial endotoxin test was performed by the limulus amoebacyte lysate assay (gel-clot technique) as reported by Hussaini and Hassanali (1987) (Hussaini and Hassanali, 1987).
2.5. Amoeba
This study has been performed with A. spelaea isolated from a case of human keratitis. The species identification of this isolate was based on cyst morphology and the molecular approach previously done by our team (strain KS1; GenBank accession number EU696948.1) (Tolba et al., 2016). Culturing of corneal scrapings, trials of axenic culture to obtain a significant number of parasites with a minimum of bacteria and medium preparation have been previously described in detail (Tolba et al., 2016). Cysts were collected from prolonged incubation of trophozoites on NNA-E. coli plates for 6 days using phosphate-buffered saline (PBS) containing 0.01NHCL for 15 min, washed 3 times in PBS by centrifugation at 600xg for 4 min. Cysts were then suspended in 10 ml Trypticase Soy Broth with Yeast Extract (TSY) and kept in 25 cm2 Falcon culture flasks. Trophozoites were obtained by suspending the collected cysts in sterile Page’s saline. They were left to excyst in culture flask and the supernatant was discarded and replaced. The flask was chilled on ice for 3 min and shacked to collect trophozoites. Counting was done using a hemocytometer and the number was adjusted to have 1 × 105 trophozoites/cysts/ml.
2.6. In vitro anti amoebic activity
The plant extract was solubilized with 1% Tween 20 at a concentration of 0.016% and sterile water to a concentration of 32 mg/ml and was adjusted by serial dilution. It was tested at final concentrations of (0.03, 0.06, 0.125, 0.5, 1, 2, 4, 8, 16, and 32 mg/ml. For the assessment of in vitro amoebicidal activity, 100 μl of the calibrated trophozoite/cyst suspension (containing 1x104 parasite) was inoculated into each well of a 96-well plate, and then the plate was left for 20 min without disturbance to allow the amoebae adherence onto the wells' surface. Then, the PBS solution was removed; and 100 μl of each concentration of the extract was added into the wells. The plate was sealed and incubated at 30 °C for different incubation periods (24, 36, and 72 h). The same procedure was applied to control wells containing only trophozoite/cyst suspension and sterile distilled water as non-treated control. The effect of Tween was excluded by using a control well containing the parasite plus Tween-20 at a concentration of 0.016%. In addition, controls containing only the parasite in PBS, plus 0.02% chlorhexidine gluconate (prepared from a solution 20% in H2O CHX, C-9394; Sigma) as a reference drug control were submitted to the same procedure. All the tests were repeated three times. Following the incubation periods, the viability was determined by the Eosin/trypan blue dye exclusion method and compared with that observed in the control cultures. Briefly, from each test and control wells, separately, 0.1 ml was transferred into 0.1 ml of 1% stain (Eosin/trypan). Unstained (viable) and stained (nonviable) trophozoites or cysts were enumerated in the hemocytometer 10 min later after the stain addition (Fig. 1, Fig. 2). Cells viability was assessed microscopically at × 400 using the following formula: (L÷ (L + D) × 100 where L is the number of live trophozoites/cysts (unstained), and D the number of dead trophozoites/cysts with 3 counts of 100 amoebas being examined each time, and repeated twice. For cultures containing no viable cysts, the results obtained were confirmed via inoculation onto NNA-E. coli plate, at 30 °C for an additional 72 h, and examined to detect any viable trophozoites or cysts (Degerli et al., 2012).
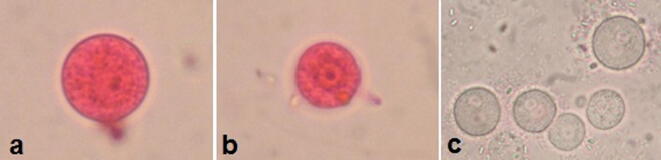
Fig. 1.
Light microscopy of non-viable cysts (a) and trophozoites (b) of A. spelaea stained with trypan blue stain (X1000).
Fig. 2.
Light microscopy of non-viable (a and b) and viable (c) cysts of A. spelaea, stained with Eosin stain (X1000).
2.7. Cytotoxicity on corneal cells
The cytotoxicity of A. cepa was tested in vitro by the agar diffusion method, according to the international standards ISO 7405: 1997 (ISO - ISO, n.d., ISO, 2008). Briefly, commercially availablecorneal cells (ATCC PCS-700-010) were cultured in 0.25% trypsin solution (Gibco, Germany) were seeded in Petri dishes (35 mm diameter), (Nunc, Wiesbadan, Germany) with a density of 1 × l06 cells/petri dish then sub-cultured once a week. When the confluent cell layer is formed, the medium was discarded and replaced by a medium with 1.5% agarose (FMC BioProducts, Rockland, ME, USA) and stained with neutral red after solidification of the agarose. Cells were protected from light to prevent their damage from the photo-activation of the stain. For each Allium concentration, four replications were carried out and four additional dishes were done using DMEM and absolute phenol as the negative and the positive controls respectively. The plates were incubated for 24 h at 37 °C in a 5% CO2 incubator, and then the cell lysis was observed by an inverted microscope and scored. One score was given for each sample, and the median value for the parallel samples was calculated for the lysis zone (Özan et al., 2013, Polat et al., 2007).
2.8. In vivo anti amoebic activity
2.8.1. Ethical considerations
The animal studies reported herein were performed in strict accordance with the ethical animal guidelines and regulations set by the Animal Care Committee of the Faculty of Medicine, of Assiut University, Egypt and in compliance with the internationally accepted principles for laboratory animal use and care (ARRIVE guidelines) (McGrath and Lilley, 2015). Ethical approval was granted by the Animal Care Committee of the Faculty of Medicine, of Assiut University, Egypt (No. 42/2017). All procedures were performed under adequate anesthesia, and every possible effort was made to minimize animal suffering and to reduce the number of used animals.
2.8.2. Animals and amoeba-induced keratitis
2.8.2.1. Amoeba
Based upon the results of the current in vitro study, the in vivo assay of A. cepa was done using 1 × 105 trophozoites/ml as the infective suspension.
2.8.2.2. Animals
In total, thirty-three apparently healthy adult female Chinchilla rabbits (5 to 6 weeks of age) weighing 1500–2000 g, collected from the animal house of the Faculty of Medicine, Assiut University, Assiut, Egypt, were utilized for this study. Animals were housed and tested following the institutional ethical committee guidelines as stated above. Rabbits were maintained under standard laboratory conditions and 12:12 light/dark cycles with free access to food and water ad-libitum. They were allowed to acclimatize for one week to the housing conditions before conduction of the experiments. All corneas were examined before experimentation to exclude any pre-existing abnormalities.
2.8.2.3. Animal anesthesia and local disinfection
Animals were anaesthetized using an intra-peritoneal injection of a mixture of ketamine hydrochloride (75 mg/ml) and medetomidine hydrochloride (1 mg/kg) in saline. Complete sedation was assured by pinching rabbit toes. Ethanol 70% was used to disinfect the eyelids and the region surrounding the eye using a cotton tip swab.
2.8.2.4. Induction of amoebic keratitis
Three rabbits were left uninfected serving as negative controls. The remaining animals (30 rabbits) were subjected to induction of keratitis as described by Larkin and Easty, 1990, Meiyu and Xinyi, 2010 (Larkin and Easty, 1990, Meiyu and Xinyi, 2010). In each rabbit, under anesthesia, the corneas of both eyes were scratched three times vertically and three times horizontally with a sterile 27-gauge syringe needle and then 10 ul of stimulating suspension of amoeba trophozoites were instilled onto one of the scratched corneas. Animals were kept under anesthesia for 30 min to allow attachment of trophozoites. To confirm the infection, 3 days post-inoculation, the eyes of the animals were monitored by slit-lamp examination. Additionally, microscopic examination of 10% potassium hydroxide wet mounts of corneal scrapings of each of the infected animals was performed to observe the presence of A. spelaea trophozoites and/or cysts.
Intra-peritoneal Peroxicam (Feldene) at a dose of (1 mg/kg) was administered every 24 h for 3 days to relieve pain. The infected rabbits’ weight was measured every day to avoid unnoticed weight loss which can indicate animal suffering and, in this case, Peroxicam treatment was continued until animal recovery.
2.8.3. Study groups and therapy
The rabbits were divided randomly into 4 main groups.
Group 1 (G1): (n = 10) Infected rabbits that received, A. cepa solution, at a concentration of 32.00 mg/ml.
Group 2 (G2): (n = 10) Infected rabbits treated with a solution of CHX as a reference drug at a concentration of 0.02% (drug control).
Group 3 (G3): (n = 10), the infected non-treated control group that received 0.05% ethanol in PBS (positive control).
Group 4 (G4): (n = 3) the non-infected non-treated group that served as the negative control.
All the reagents were prepared and administered as eye drops 3 days after inoculation and continued for 28 days. During the first week, therapeutic agents were applied eight times per day, and for the last 3 weeks, they were applied only three times per day. Then the role of A. cepa on Allovahlkampfia keratitis (AK) was evaluated by clinical, histo-pathological, and parasitological assays.
2.8.4. Evaluation of treatment
2.8.4.1. Clinical evaluation (examination and scoring of rabbit eyes)
The clinical score of keratitis was evaluated daily by comparing the severity of keratitis in animals with infected non-treated corneas versus treated animals throughout the observation period. Amoebic keratitis and corneal opacity were inspected and graded using a slit lamp (SL-D7, Topcon Corp., Tokyo, Japan) following the grading scheme of van Klink et al., 1993, Meiyu and Xinyi, 2010 (van Klink et al., 1993, Meiyu and Xinyi, 2010). Briefly, grading was done according to a 4-point rating scale: 1 (if ≤ 25% cornea was involved), 2 (if > 25% but ≤ 50%), 3 (if > 50% but < 75%), and 4 (if ≥ 75%). Higher clinical scores were related to a worse clinical status and healthy corneas were given a score of zero in each category.
2.8.4.2. Histopathological evaluation of rabbits' corneal tissue
The animals of different groups were anesthetized and sacrificed at the end of the observation period (on 28th day pi) under the experimental protocol described above. The hemi- corneas of each animal were fixed in 10% neutral formalin for 48 h. After dehydration in ascending concentrations of alcohols, samples were embedded in paraffin, sliced (4 μm thick) and stained with hematoxylin and eosin (H&E), Masson trichrome, and PAS stains according to standard methods and examined using Olympus light microscope (Olympus Corp, Shinjuku, Tokyo, Japan).
2.8.4.3. Immunohistochemistry
Immunohistochemical staining was performed using the avidin–biotin immunoperoxidase method. Briefly; 4 µm thick sections, taken from the previously prepared formalin-fixed paraffin-embedded blocks, were deparaffinized and rehydrated through descending graded ethanol series down to distilled water. Endogenous peroxidase activity was blocked using 3% hydrogen peroxide treatment for 7 min. For corneal antigenic epitopes retrieval, the sections were microwaved in citrate buffer; pH 6 for a total of 12 min. Rabbit polyclonal anti-human antibodies against T cell CD3, B cell CD20, and macrophage CD68 (Thermo Scientific) were used. Incubation with all the primary antibodies was carried out in a moist chamber for 1 h at room temperature. Secondary staining kits were used according to the manufacturer's instructions (Thermo scientific corporation Fremont, CA, USA). Counterstaining was done with hematoxylin and examined by Olympus light microscopy. Tonsil sections were used as positive control while negative controls were obtained by omission of the primary antibody.
2.8.4.4. Parasitological evaluation
2.8.4.4.1. A. Spelaea growth after treatment (AGAT)
For detection of amoeba, (on 28th day pi), hemi-corneas of all groups were homogenized, separately; each in 1 ml Page’s amoeba saline with a glass grinder and 100 µl of undiluted homogenate was added to each well of 25-well Petri dishes containing NNA-E. coli. Then they were incubated at 30 °C for 14 days and amoebic growth was observed daily by the inverted microscopy. If no growth was seen on day 14, the culture was considered negative (Meiyu and Xinyi, 2010).
2.9. Statistical analysis
Categorical variables were described by number and percentage (N, %), while continuous variables were described by the mean and standard deviation (Mean, SD). Chi-square test was used to compare between categorical variables while comparison between continuous variables was done by t-test and ANOVA (parametric tests), Mann Whitney U, and Kruskal-Wallis H (non-parametric tests). Continuous variables were tested for normal distribution using the Kolmogorov Smirnov test and Q-Q Plots. A two-tailed p < 0.05 was considered statistically significant. All analyses were performed with the IBM SPSS 20.0 software (IBM Corp, Armonk, NY, USA) (“IBM Corp. Released 2020. IBM SPSS Statistics for Windows, Version 27.0. Armonk, NY: IBM Corp,” n.d.).
3. Results
3.1. Liquid Chromatography-Mass Spectrometry (LC-MS) studies
The analysis of the aqueous extract of A. cepa by LC-MS revealed 120 compounds; the major compounds are listed in Table 1.
Table 1.
Major identified compounds from A. cepa aqueous extract by LC-MS.
| Peak No. | RT (min) | Area percentage % | Mass (m/z) | Molecular formula | Proposed compound name | Ion species |
|---|---|---|---|---|---|---|
| 1 | 1.936 | 100 | 203.0531 | C6H12O6 | L-Sorbose | (M + K)+ |
| 2 | 4.284 | 15.57 | 527.1595 | C18H32 O16 | Viscutin 1 | (M + Na)+ |
| 3 | 4.988 | 13.93 | 215.0166 | C6H10O8 | Galactaric acid | (M + Na)+ [–H2O] |
| 4 | 5.693 | 12.02 | 217.0822 | C8H14N2O6 | L-threonyl-L-aspartic acid | (M + H) + [–H2O] |
| 5 | 6.397 | 13.65 | 705.1835 | C24H42O21 | Mannan | (M + K)+ |
| 6 | 7.571 | 10.99 | 182.0813 | C9H13NO4 | 9-hydroxy-7E-Nonene- 3,5-diynoic acid |
(M + H) + [–H2O] |
| 7 | 10.506 | 10.85 | 851.2655 | C30H52O26 | Verbascose | (M + Na)+ |
| 8 | 12.15 | 4.51 | 851.2655 | C10H13N5O4 | Vidarabine | (M + H)+ |
| 9 | 13.911 | 20.61 | 279.101 | C10H18N2O5S | Met Glu | (M + H)+ |
| 10 | 16.729 | 3.19 | 1175.3702 | C42H72O36 | Maltoheptaose | (M + Na)+ |
| 11 | 18.842 | 1.57 | 102.1279 | C6H14O | 4-Methyl-1-pentanol | (M + NH4) + [–H2O] |
| 12 | 20.134 | 4.16 | 102.1279 | C60H102O51 | 1,4-b-D-Mannan | (M + NH4) + [–H2O] |
| 13 | 20.604 | 5.03 | 416.1101 | C14H23N3O8S | gamma-L-Glutamyl-S-(2-carboxy-1-propyl)cysteinylglycine | (M + Na)+ |
| 14 | 22.482 | 3.93 | 313.0831 | C11H18N2O5S | N-gamma-Glutamyl-S-(1-propenyl)cysteine | (M + Na)+ |
| 15 | 23.069 | 2.59 | 295.1292 | C14H18N2O5 | Glutamyl-phenylalanine | (M + H)+ |
| 16 | 23.891 | 3.95 | 345.1528 | C14H23N3O7 | Glu Pro Thr | M+ |
| 17 | 30.466 | 17.35 | 353.2671 | C19H38O4 | MG(16:0/0:0/0:0) | (M + Na)+ |
| 18 | 31.758 | 12.08 | 381.2984 | C21H39N3O3 | Cannabisativine | M+ |
| 19 | 33.284 | 23.48 | 675.6768 | C44H85NO3 | Cer(d18:1/26:1(17Z)) | (M + NH4) + [–H2O] |
3.2. Evaluation of microbial contamination and endotoxin production
Total aerobic microbial count and total combined yeasts/moulds count were negative for the tested Allium extract. Tested Allium extract was endotoxin free.
3.3. In vitro anti amoebic activity
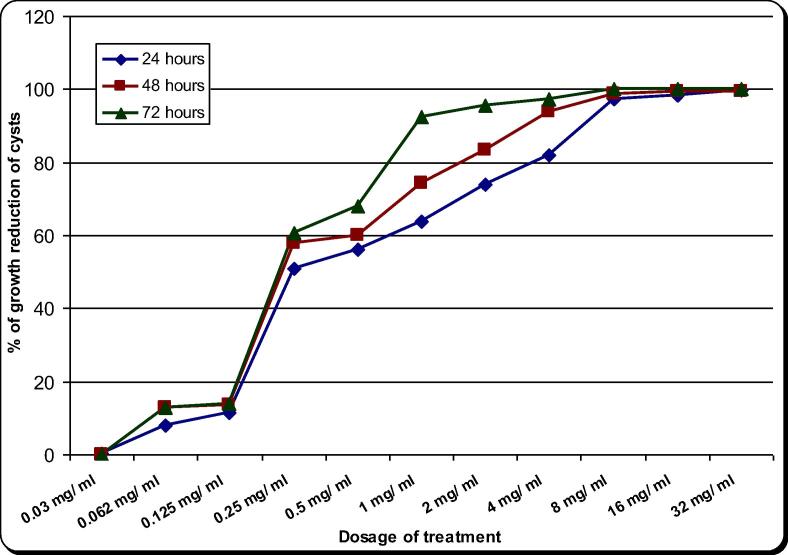
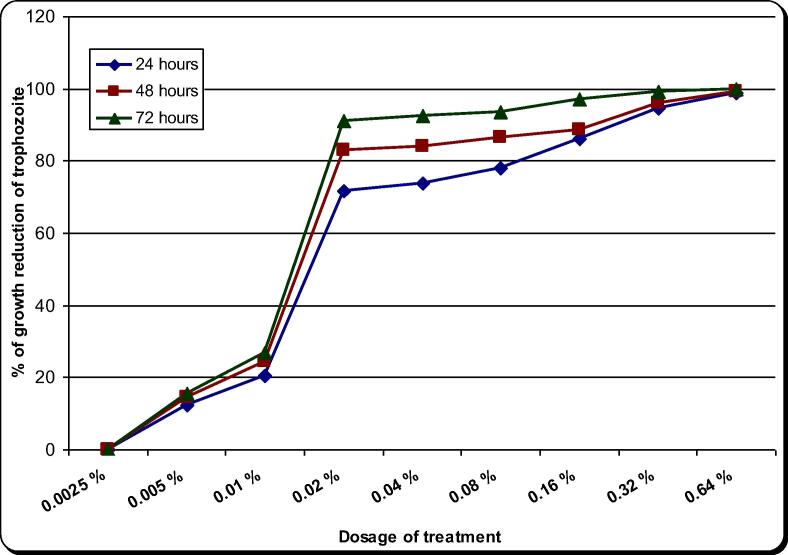
The effect of the aqueous extract of A. cepa on A. spelaea trophozoites and cysts is presented in Table 2, Table 3 and Fig. 3, Fig. 4 and S1 and S2 figures. On the other hand CHX (the drug control) effect was presented in Table 4, Table 5 and Fig. 5, Fig. 6 and S3 and S4 figures.The natural extract exhibited remarkable activity potential against trophozoites and cysts. The minimal inhibitory concentration (MIC) of A. cepa on trophozoites (100% growth reduction) was 4.00 mg/ml after 72 h and 8.00 mg/ml after 48 h. On the other hand, its MIC on cysts was 8.00 mg/ml after 72 h. The concentrations 1.00 and 2.00 mg/ml showed growth reduction of trophozoites by 69–98.7% in all incubation periods. Meanwhile growth reduction of cysts at concentrations of 1.00, 2.00 and 4.00 mg/ml was 61.7–97.3% in all incubation periods. In comparison, CHX 0.02% (drug control) showed inhibitory effect on the growth of trophozoites by 71.7–91% and cysts by 55.00–81.00% in all incubation periods. On the other hand, the majority of the trophozoites and cysts achieved to remain viable in the case of non-treated control. A. cepa extract showed a significant inhibitory effect both on trophozoites and cysts as compared to the drug control (chlorhexidine) as well as non-treated control (P < 0.05). Moreover, the effectiveness of the extract against the trophozoites and cysts was found statistically different in terms of experimental durations in higher concentrations (P < 0.05). However, at concentrations of 16 and 32 mg/ml, the extract gave almost the same effect on trophozoites and cysts with no significant difference.
Table 2.
Effect of Allium aqueous extract on the in vitro growth of A. spelaea trophozoites for different incubation periods.
| Dose (mg/ml) | 24 h |
48 h |
72 h |
P-value |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | ||
| 32 mg/ml | 99.67 ± 0.582,3 | 100.00 ± 0.002,3 | 100.00 ± 0.002,3 | 0.422 |
| 16 mg/ml | 99.67 ± 0.582,3 | 100.00 ± 0.002,3 | 100.00 ± 0.002,3 | 0.422 |
| 8 mg/ml | 99.00 ± 1.002,3 | 100.00 ± 0.002,3 | 100.00 ± 0.002,3 | 0.125 |
| 4 mg/ml | 93.00 ± 2.652,3 | 99.33 ± 1.152,3 | 100.00 ± 0.002,3 | 0.0041 |
| 2 mg/ml | 85.33 ± 0.582,3 | 97.33 ± 1.532,3 | 98.67 ± 1.532,3 | 0.0001 |
| 1 mg/ml | 69.00 ± 1.003 | 82.00 ± 2.653 | 84.00 ± 1.002,3 | 0.0001 |
| 0.5 mg/ml | 63.67 ± 4.933 | 60.00 ± 0.002,3 | 71.67 ± 3.792,3 | 0.0191 |
| 0.25 mg/ml | 55.67 ± 1.152,3 | 58.00 ± 2.002,3 | 63.00 ± 3.612,3 | 0.0281 |
| 0.125 mg/ml | 24.00 ± 1.002,3 | 13.67 ± 1.532,3 | 22.33 ± 2.892,3 | 0.0011 |
| 0.062 mg/ ml | 11.67 ± 0.582,3 | 13.00 ± 0.002,3 | 14.33 ± 2.312,3 | 0.137 |
| 0.03 mg/ml | 0.33 ± 0.582 | 0.00 ± 0.002,3 | 0.00 ± 0.002,3 | 0.422 |
| Drug control (CHX 0.02%) | 71.67 ± 1.53 | 83.00 ± 2.65 | 91.00 ± 1.00 | 0.0001 |
| Non-treated control | 0.67 ± 0.58 | 2.33 ± 0.58 | 4.00 ± 1.00 | 0.0051 |
| 1% tween 20 | 0.67 ± 0.58 | 2.67 ± 1.15 | 5.67 ± 1.15 | 0.0031 |
Significant difference in comparison between different times at the same dose.
Significant difference in comparison with drug control in the same time interval.
Significant difference in comparison with non-treated control in the same time interval.
Table 3.
Effect of Allium aqueous extract on the in vitro growth of A. spelaea cysts for different incubation periods.
| Dose (mg/ml) | 24 h |
48 h |
72 h |
P-value1 |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | ||
| 32 mg/ml | 99.67 ± 0.582,3 | 99.33 ± 1.152,3 | 100.00 ± 0.002,3 | 0.579 |
| 16 mg/ml | 98.33 ± 0.582,3 | 99.33 ± 0.582,3 | 100.00 ± 0.002,3 | 0.0141 |
| 8 mg/ml | 97.33 ± 1.532,3 | 98.67 ± 0.582,3 | 100.00 ± 0.002,3 | 0.0371 |
| 4 mg/ml | 82.00 ± 2.002,3 | 94.00 ± 4.002,3 | 97.33 ± 1.532,3 | 0.0011 |
| 2 mg/ml | 74.00 ± 5.292,3 | 83.33 ± 4.162,3 | 95.67 ± 0.582,3 | 0.0011 |
| 1 mg/ml | 64.00 ± 5.293 | 74.33 ± 3.792,3 | 92.33 ± 2.522,3 | 0.0001 |
| 0.5 mg/ml | 56.33 ± 1.153 | 60.00 ± 0.003 | 68.00 ± 2.652,3 | 0.0001 |
| 0.25 mg/ml | 51.00 ± 0.002,3 | 58.00 ± 2.003 | 60.67 ± 4.042,3 | 0.0101 |
| 0.125 mg/ml | 11.67 ± 0.582,3 | 13.67 ± 1.532,3 | 14.00 ± 0.002,3 | 0.0461 |
| 0.062 mg/ml | 8.00 ± 1.002,3 | 13.00 ± 0.002,3 | 13.00 ± 0.002,3 | 0.0001 |
| 0.03 mg/ml | 0.33 ± 0.582,3 | 0.00 ± 0.002,3 | 0.00 ± 0.002,3 | 0.422 |
| Drug control (CHX 0.02%) | 56.67 ± 3.06 | 61.67 ± 1.53 | 81.00 ± 1.00 | 0.0001 |
| Non-treated control | 2.67 ± 0.58 | 3.67 ± 0.58 | 6.33 ± 1.53 | 0.0101 |
| 1% tween 20 | 3.33 ± 1.53 | 5.00 ± 1.73 | 6.00 ± 1.00 | 0.155 |
Significant difference in comparison between different times at the same dose.
Significant difference in comparison with drug control in the same time interval.
Significant difference in comparison with non-treated control in the same time interval.
Fig. 3.
Effect of Allium aqueous extract on the in vitro growth of A. spelaea trophozoites for different incubation periods.
Fig. 4.
Effect of Allium aqueous extract on the in vitro growth of A. spelaea cysts for different incubation periods.
Table 4.
Effect of chlorhexidine (Drug control) on the in vitro growth of A. spelaea trophozoites for different incubation periods.
| Dose (%) | 24 h |
48 h |
72 h |
P-value1 |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | ||
| 0.64% | 98.67 ± 0.582 | 99.33 ± 0.582 | 100.00 ± 0.002 | 0.0371 |
| 0.32% | 94.67 ± 1.532 | 96.00 ± 1.002 | 99.33 ± 1.152 | 0.0101 |
| 0.16% | 86.00 ± 1.002 | 88.67 ± 0.582 | 97.00 ± 1.002 | 0.0001 |
| 0.08% | 78.00 ± 3.612 | 86.33 ± 0.582 | 93.67 ± 0.582 | 0.0001 |
| 0.04% | 73.67 ± 0.582 | 84.00 ± 1.732 | 92.33 ± 1.152 | 0.0001 |
| 0.02% | 71.67 ± 1.532 | 83.00 ± 2.652 | 91.00 ± 1.002 | 0.0001 |
| 0.01% | 20.33 ± 0.582 | 24.33 ± 2.522 | 27.00 ± 1.002 | 0.0061 |
| 0.005% | 12.33 ± 0.582 | 14.33 ± 2.522 | 15.67 ± 1.152 | 0.115 |
| 0.0025% | 0.00 ± 0.00 | 0.00 ± 0.002 | 0.00 ± 0.002 | – |
| Non-treated control | 0.67 ± 0.58 | 2.33 ± 0.58 | 4.00 ± 1.00 | 0.0051 |
Table 5.
Effect of chlorhexidine (Drug control) on the in vitro growth of A. spelaea cysts for different incubation periods.
| Dose (%) | 24 h |
48 h |
72 h |
P-value1 |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | ||
| 0.64% | 98.00 ± 1.002 | 99.33 ± 0.582 | 100.00 ± 0.002 | 0.0271 |
| 0.32% | 79.00 ± 1.002 | 83.33 ± 0.582 | 87.33 ± 2.082 | 0.0011 |
| 0.16% | 76.67 ± 0.582 | 78.33 ± 0.582 | 85.67 ± 1.532 | 0.0001 |
| 0.08% | 73.67 ± 0.582 | 75.67 ± 0.582 | 84.33 ± 1.152 | 0.0001 |
| 0.04% | 61.00 ± 1.002 | 65.33 ± 0.582 | 83.67 ± 0.582 | 0.0001 |
| 0.02% | 54.00 ± 1.002 | 62.33 ± 2.082 | 80.67 ± 1.152 | 0.0001 |
| 0.01% | 15.67 ± 0.582 | 18.00 ± 1.002 | 48.67 ± 3.212 | 0.0001 |
| 0.005% | 8.00 ± 1.002 | 7.67 ± 0.582 | 8.67 ± 0.58 | 0.317 |
| 0.0025% | 0.00 ± 0.002 | 0.00 ± 0.002 | 0.00 ± 0.002 | – |
| Non-treated control | 2.67 ± 0.58 | 3.67 ± 0.58 | 6.33 ± 1.53 | 0.0101 |
Fig. 5.
Effect of chlorhexidine (Drug control) on the in vitro growth of A. spelaea trophozoites for different incubation periods.
Fig. 6.
Effect of chlorhexidine (Drug control) on the in vitro growth of A. spelaea cysts for different incubation periods.
3.4. Cytotoxic potentials of A. cepa on corneal cells
A. cepa extracts at the five different concentrations used in this study were not found cytotoxic on human corneal cell lines even at a concentration of 32 mg/ml.
3.5. In vivo anti amoebic activity
3.5.1. Confirmation of infection
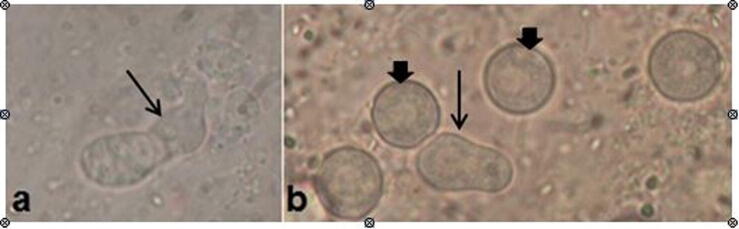
The Chinchilla rabbits were found susceptible to in vivo corneal infection with A. spelaea. AK was successfully established in all the experimentally infected groups (30 rabbits). Meanwhile, typical trophozoites and cysts were observed on 10% potassium hydroxide wet mounts of corneal scrapings of these eyes 3 days pi (Fig. 7).
Fig. 7.
Trophozoites (thin arrows) and cysts (thick arrows) of A. spelaea (a&b) were observed on 10% potassium hydroxide wet mounts of corneal scrapings (infected untreated group) (X1000).
3.5.2. Clinical evaluation
Keratitis was significantly more severe in the infected untreated group compared to the other two treatment groups receiving either A. cepa or CHX eye drops. There were no differences in clinical scores between the two treatment groups during the observation period in 80% of cases (70% showed complete resolution and 10% showed mild infection). Only 20% of eyes showed moderate infection in the CHX treated animals compared to a mild degree of infection in the A. cepa treated group (Table 6).
Table 6.
Effect of Allium cepa (32.00 mg/ml) on A. keratitis at the end of the experiment (28th-day pi) compared to controls.
| Group1 | Group2 | Goup3 | ||||
|---|---|---|---|---|---|---|
| Total number of infected eyes/% | 10 | 100% | 10 | 100% | 10 | 100% |
| Absence of clinical signs$ | 8 | 80% | 7 | 70% | 0 | 0% |
| Mild infection* | 2 | 20% | 1 | 10% | 0 | 0% |
| Moderate infection** | 0 | 0% | 2 | 20% | 1 | 10% |
| Severe infection*** | 0 | 0% | 0 | 0% | 9 | 90% |
| Endophthalmitis | 0 | 0% | 0 | 0% | 4 | 40% |
| Dead rabbits | 0 | 0% | 0 | 0% | 2 | 20% |
| Isolation of A. spaelae (AGAT)# | 1 | 10% | 3 | 30% | 10 | 100% |
Total score = 0.
Total score ≤ 5.
Total score = 6 to 10.
Total score ≥ 11.
Allovahlcamfia spelaea growth after treatment.
Meanwhile, the infected untreated animals had a more severe and prolonged course of infection than the infected treated animals, and the severity of corneal involvement was significantly greater (p < 0.001). In contrast, the CHX treated animals displayed a mild to moderate clinical infection during the initial onset of the disease, yet the disease cleared within 20 days after treatment in 70% of rabbits. Administration of the natural extract (A. cepa) resulted in a much milder clinical course of A. spelaea keratitis (AK) and a rapid acceleration of the resolution of corneal disease (80%) (Table 6).
At the end of the observation period (28th day pi), 100% (n: 10) of infected untreated animals still demonstrated evidence of corneal disease, compared to a 10% and 30% incidence of infection in A. cepa and CHX treated animals respectively (Table 6).
3.5.2.1. Clinical characterization of A. spelaea keratitis (AK)
-
•
In the infected non treated rabbits
The rabbit corneas in this group developed severe AK (100%) after exposure to the viable Allovahlkampfia trophozoites. On the 3rd day pi, mild keratitis was observed with redness of the conjunctiva and swollen eyelids. On day 5 pi, obvious corneal ulcers were noticed. On day 7 pi, the ulcer enlarged with severe conjunctiva congestion, and the corneal stroma were moderately to severely opaque because of edema and infiltration (Figs. 8, and 9c). Neovascularization was visible in the more severely infected animals with severe congestion. On the 14th day pi, the ulcer destroyed most of the cornea forming descemetocele in 3 rabbits (3/10) (Fig. 8). Clinically, the infection worsened causing the death of two rabbits after the 15th day pi due to severe endophthalmitis (Fig. 10f, i). On the 28th day pi, the stroma of only three infected corneas became less infiltrated and started to form scars and pannus (Fig. 10c).
-
•
In the infected rabbits treated with A. cepa
Fig. 8.
Two representative cases of severe Allovahlkampfia keratitis in infected untreated rabbits at different times pi showing; definite corneal ulcers stained with fluorescein staining (arrowheads), corneal opacity due to stromal infiltration and edema (thick arrows), keratomalacia (curved arrow), and descemetocele (thin long arrows). (N) The right eye of normal control.
Fig. 9.
Three representative cases of Allovahlkampfia keratitis, 28th day pi: (a)A. cepa treated eye (G1) showing mild infection, (b) CHX. treated eye (G2) showing moderate infection and (c) Infected untreated eye (G3) with severe infection. G3 showed the highest clinical scores of corneal edema, and opacity/infiltration, complicated with keratomalacia.
Fig. 10.
Nine representative cases of Allovahlkampfia keratitis at 28th day post-inoculation: (a, b)A. cepa treated group with complete resolution (a) and mild infection (b). (g, e, h) CHX treated group, with complete resolution (g) and moderate infection (e, h), and (c, f and i) infected untreated group with severe infection showing the highest clinical scores of epithelial defects (c), corneal edema, and opacity/infiltration, complicated with keratomalacia and endophthalmitis (f and i). (d) The normal cornea of the negative control rabbit.
Like the infected non-treated group, all eyes exposed to infection with the same number of trophozoites developed mild AK on the 3rd day pi. An ulcer was noticeable on day 5 pi. However, the corneas started to clear 7 days after treatment. By 10th day pi, the epithelial ulcers healed, stromal infiltration and edema diminished, and the keratitis was completely resolved in 80% of the infected animals at 14th day pi. By the 28th day after infection, all signs of keratitis had disappeared, although mild scarring of the cornea developed in two rabbits with mild infection and corneal opacity was noticed due to edema and infiltration (20% of eyes) (Figs. 9a, 10a,b, 11 and 12a, b).
-
•
In the infected rabbits treated with CHX
Fig. 11.

A representative case of A. spelaea keratitis (AK) treated with Allium cepa. (a) Rabbit’s eye stained with fluorescein staining showing an ulcer (5 days pi). (b) Rabbit’s eye with opaque cornea due to infiltration and edema “focal keratitis” (curved arrow) (10 days pi). (c) Rabbit’s eye with normal cornea with mild congestion of the conjunctiva (14–28 days pi).
Fig. 12.
Four representative cases of Allovahlkampfia keratitis at different times pi of (a and b)A. cepa treated eyes with mild infection (G1) showing mild edema and infiltration 5 days pi. At 10th to 14th days pi, the inflammation diminished and complete resolution was observed at 21st-28th days pi.(c and d) Two representative cases of CHX. treated eyes with moderate infection (G2) showing, an ulcer stained with fluorescent dye at 5th day pi. Edema and opacity, scars and pannus formation were observed till the end of the observation period.
Comparable to the A. cepa treated group, the corneas of this group showed complete resolution of AK in 70% of eyes within 20 days pi. However, one case showed mild corneal opacity due to stromal edema, in addition to two cases that showed moderate infection with scars and pannus formation (20%) till the end of the observation period (Fig. 9b, Fig. 10g, e, h and Fig. 12c, d).
-
•
In non-infected non-treated rabbits (negative control rabbits)
The corneas of the negative control rabbits remained clear and transparent during the experimental period, the sclera was white, and the iris had normal vascularity (Fig. 10d). Abrasion alone did not produce any signs of corneal damage after 5 days of the procedure.
3.5.3. Histo-pathological evaluation
The histopathological features of the corneas from the various groups mirrored the clinical observations. Microscopic examination of the sections from non-infected non treated control group (normal rabbits) showed that the epithelium and the stroma were well-defined, and the stromal fibres were well arranged. Inflammatory cell infiltrates were absent (Fig. 13a and Fig. 14a).
Fig. 13.
Photomicrographs of the corneal sections from rabbits challenged with A. spelaea(a) normal cornea; the epithelium and stroma are well-defined with no inflammatory cell infiltrate. (b- e) Corneas from the infected untreated group showing, epithelial ulceration with numerous acute and chronic inflammatory cell infiltrate in the corneal stroma (b), conjunctivilization of the corneal epithelium (c), neovascularization (d), abscess formation (arrows) (e), and multiple cysts of A. spelaea (arrows) in the anterior segment of the corneal stroma (f). (a-e) stained with hematoxylin and eosin stain (H& E X400, (f) stained with periodic acid Schiff stain (PAS X1000).
Fig. 14.
Photomicrographs of the corneal sections stained with Masson's trichrome stain (x400). Noninfected control group showing normal cornea (a). Infected untreated control group showing neovascularization and unorganized collagen fibers which replaced the regular stromal fibers (b&c). Treatment groups; G1 and G2 respectively; showing less vascularization and more organized collagen fibers of the stroma (d& e). (f) Scar healing is evident in severe keratitis cases of the infected untreated control group.
Histo-pathological examination of corneal sections of infected rabbits with A. spelaea without treatment (infected untreated control group) revealed features of acute and chronic inflammation mainly in the anterior corneal segment. The corneal epithelium was unorganized and showed focal epithelial ulceration (Fig. 13b), metaplastic changes in the form of conjunctivalization, and destruction of Bowman's layer (Fig. 13c). The stroma, mainly in the anterior two thirds, showed infiltration by excess inflammatory cells in the form of polymorph nuclear leucocytes (PMNLs), pus cells, with focal aggregation, lymphocytes, and histiocytes associated with the irregularity of collagen fibers (Fig. 13d). There was also associated neovascularization and edema detected in the corneal stroma (Fig. 13b-e and Fig. 14b&c). Cysts of A. spelaea were detected in some cases, with slight chronic inflammatory cell infiltrate and little tissue necrosis, throughout the corneal stroma using PAS stain (Fig. 13f). A complication in the form of abscess formation was detected in one case (Fig. 13e).
Histo-pathological examination of corneal sections from treated groups either by the CHX, or A. cepa showed noticeable improvement compared with the infected non-treated group. The group treated with the standard drug revealed normal corneal epithelium with intact Bowman's layer. The stroma was formed of parallel bundles of collagenous fibers with no inflammatory cell infiltrate. Meanwhile, the group treated with the natural element showed re-epithelization of the cornea with the formation of stromal collagenous fibres (Fig. 14d, e and Fig. 15).
Fig. 15.
Photomicrographs of the cornea stained with H & E (x400). (a & b) showing initiation of healing with neovascularisation and few inflammatory cell infiltrates in the treatment groups G1& G2 respectively. (c&d) showing complete healing with re-epithelialization of the corneal epithelium and the absence of inflammatory cell infiltrate in the corneal stroma in the treatment groups (G1&G2 respectively).
3.5.3.1. Immunophenotyping
The inflammatory cells in the corneal stroma of the infected untreated group were found to be CD3 positive T lymphocytes (Fig. 16a) and CD68 positive histiocytes (Fig. 16b) and were found negative for CD20 lymphocytes (B cell marker), suggesting a predominance of T lymphocytes with macrophages.
Fig. 16.
Photomicrographs of the cornea stained immunohistochemically (x400). (a) CD3 positive lymphocytes in the corneal stroma. (b) CD68 positive histiocytes in the corneal stroma.
3.5.4. Parasitological evaluation
3.5.4.1. A. spelaea growth after treatment (AGAT):
On day 28 pi, from the eyes of infected untreated rabbits, typical trophozoites and cysts of A. spelaea were successfully isolated and grown on the lawn of E. coli in non-nutrient agar plates within 72 h. On the other hand, in the infected treated groups scored zero, no amoebic growth was observed for all sub-cultured corneas and the cultures were considered negative after 14 days of incubation. However, other grades showed culture positivity of 10%, and 30% of Allium cepa and CHX. treated groups respectively with a statistically significant difference between the treated and the infected non-treated control groups (p ≤ 0.05) (Table 6 and Fig. 17).
Fig. 17.

A typical cyst (a) and trophozoite (b) of A. spelaea, isolated from corneal tissues of the infected untreated group at 28th day pi, after culture on NNA-E. coli (X1000).
4. Discussion
A. spelaea is one of the FLA that was identified for the first time in 2009 as a causative agent of keratitis (Tolba et al., 2016). Amoebic keratitis is a serious ocular infection with an increased number of difficulty-controlled cases. The potential problems in control mainly come from the difficulty of laboratory diagnosis and the increasing level of resistance of both trophozoites and cysts to the commonly used anti-amoebic drugs (Chopra et al., 2020, Degerli et al., 2012).
The currently used treatment of this type of keratitis involves a combination of some amoebicidal drugs, yet, still with an un-avoided severe decline in the final visual acuity, with high opportunistic secondary ocular complications that worsen the prognosis. This severely compromising pathology paves the mandatory need for a new effective drug. Plants are known as a potential source of many bioactive natural derivatives for medicinal applications which may be the solution of this problem (Elsheikha et al., 2020, Malatyali et al., 2012, Reyes-Batlle et al., 2020).
In this study, Allium cepa was evaluated for its in vitro amoebicidal effect against Allovahlkampfia. It showed significant time and dose-dependent amoebicidal effects on both trophozoites and cysts, compared to the drug control (chlorhexidine) and non-treated control. As expected, and in line with the previous studies, the cysts were found to be more resistant to the effect of the extract than the trophozoites. A. cepa is one of the most effective medicinal plants in the treatment of many parasitic diseases. The study of Hussian et al. 2017 (Hussian et al., 2017), showed a significant inhibitory effect of its ethanol extract against the in vitro growth and motility of Entamoeba gingivalis compared to the reference drug, metronidazole.
As amoebic keratitis is still a challenge to ophthalmologists and since it requires a long period of treatment(Papa et al., 2020, Polat et al., 2012), the therapeutic effect of A. cepa was assessed in this study as a treating agent compared to chlorhexidine, the standard therapeutic agent usually used by ophthalmologists.
The in-vivo studies provide an advantage over in vitro testing in ophthalmological research because of preservation of ocular tissue integration and functionality. The schedule of the pattern and duration of treatment was designed according to Polat et al. (2012) (Polat et al., 2012). So, the treatment doses were chosen based on the best results obtained from our in vitro assay and the cytotoxicity results. Therefore, the mentioned amoebicidal concentrations were selected.
Our results demonstrated that Allium cepa had the highest cure rate at a concentration of 32 mg/ml. The mechanisms by which the consumption of some plant extracts can affect the parasite viability and mobility, both in vitro and in vivo, might be associated with an enhanced host immune response towards the parasites (Mantawy et al., 2011). Many plants have been reported to have some compounds that are directly active against parasites. These active compounds are usually secondary metabolites that have been associated with many defensive mechanisms. Saponin, tannins, alkaloids, non-protein amino acids, and other polyphenols, glycolides and liginin are all examples of secondary metabolites which could be considered responsible for the anti-parasitic action. In this regard, onion contains sulphuric compounds that have been associated with anti-parasitic action through their effect on the host immune response as they contain immune-modulator fractions, which affect the course of the disease and shift the cytokine pattern from Th2-lymphocytes to Th1-lymphocytes mediated immune responses which are, in turn, responsible for the host immune resistance (Elkadery et al., 2019, Krstin et al., 2018, Mantawy et al., 2011). This was evident in our study by the positive inflammatory cells for T-cell marker CD3.
In addition, Allium cepa is known to prevent or attenuate the decline in tissue anti-oxidant enzymes. Hence, this extract could provide some cellular protection against the reactive oxygen species occurring due to infection (Mantawy et al., 2011). Further studies are required to understand the detailed mechanisms involved in the amoebicidal actions of the extract, its pharmacokinetics and pharmacodynamics parameters, and its ocular toxicity in animal models of amoebic keratitis (Lorenzo-Morales et al., 2015).
The histopathological changes observed in this study may be due to the amoebic degradation of chemokines, cytokines, the complement pathway, antibodies, and macrophages (Sun et al., 2013, Zorzi et al., 2019). The chronic inflammatory cells infiltration and the tissue necrosis observed, all indicate that the cysts consumed their required nutrients from the corneal stroma. As estimated by Sun et al. (2013), the slight inflammatory response and little tissue necrosis are usually correlated with the presence of abundant cysts in the development stage, indicating that they get their nutrients from the corneal stroma. Once the stroma becomes necrosed and no longer supplying enough nutrition, they transfer to a healthier position. This may explain why the parasites were found few or were not showing up in the necrosed sections (Sun et al., 2013).
Histopathology sections of the corneas of the group treated with Allium cepa revealed an improved histopathological pattern compatible to a large extent with that of the drug control and both induced neovascularization, which is an indication of tissue healing and that is believed to be associated with lymphocytic infiltration that was observed by the positive inflammatory cells for T-cell marker in this group. The continuance of inflammatory response in the convalescence stage could be due to cyst antigenicity that may cause persistent scleral and corneal inflammation even with the absence of active amoebic infection (Sun et al., 2013).
Immunohistochemical evaluation of the immune response in amoebic keratitis has its value because many features of this infection usually show an unusual response of the host to the pathogen (Larkin and Easty, 1991). The predominance of T-lymphocytes, with macrophages, observed in our immunohistochemistry results is in agreement with that of Larkin and Easty (1991) who estimated no B-lymphocytes while some T-lymphocytes were observed. These T cells could be sensitized either to microbial antigens, altered structures of host cellular and/or basement membrane, or both. In both animal and human diseases, the cause of the absence of B-lymphocytes in the inflammatory cell infiltrate is still uncertain. Further studies are required to explain more comprehensively the host immune response to our pathogen. As regards the growth of the parasite after treatment, all the sub-cultured corneas scored 0, showed no amoebic growth, and the cultures were considered negative. Whereas Yang reported that the cysts of Acanthamoeba were still present up to 31 months after anti-amoeba treatment (Yang et al., 2001). This may be explained by the efficient lethality of A. Cepa extract or, probably, the less resistance of our pathogen. Increased awareness about the pathogenesis and cellular differentiation processes in A. spelaea infection is the key for better diagnosis and development of actual therapeutic measures.
Nevertheless, this study was faced with some challenges, the most important of which is the lack of a completely identical study to determine the regimen of treatment and evaluating the results, as the most studies are evaluating the natural products against Acanthamoeba, not the newly emerged Allovahlkampfia. Moreover, the theranostic approaches, which combine both therapeutic and diagnostic methods, have the potential to overcome conventional diagnostic and therapeutic limitations associated with the management of resistant diseases such as Acanthamoeba infections. However, it requires development of smart materials for improved laboratory testing. Recent technologies such as nanomaterials have already shown promising theranostic applications in non-communicable diseases and these can provide a breakthrough against Acanthamoeba infections.
5. Conclusion
The present study provides the first evidence that Allium cepa has an in vitro and in vivo amoebicidal effect against Allovahlkampfia trophozoites and cysts. Meanwhile, Allium cepa did not show cytotoxicity on the corneal cells even at high concentrations. Our findings could assist in guiding and developing effective therapeutic approaches against the challenging amoebic keratitis. Further studies should be carried out to explain the detailed mechanisms involved in this amoebicidal activity.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
We acknowledge the Medical Research Center at the Faculty of Medicine, Assiut University, Assiut, Egypt, for providing the needed research equipment. We would like also to thank workers, technicians and staff working in the animal house of the Faculty of Medicine, Assiut University, Assiut, Egypt.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical consideration
The animal studies reported herein were performed in strict accordance with the ethical animal guidelines and regulations set by the Animal Care Committee of the Faculty of Medicine, of Assiut University, Egypt and in accordance with the internationally accepted principles for laboratory animal use and care. Ethical approval was granted by the Animal Care Committee of the Faculty of Medicine, of Assiut University, Egypt (No. 42/2017). All procedures were performed under adequate anesthesia, and every possible effort was made to minimize animal suffering and to reduce the number of used animals.
Author Contributions
Data curation: Hanan E.M. Eldeek, Haiam Mohamed Mahmoud Farrag, Enas Abd El Hameed Mahmoud Huseein and Heba E.M. El-Deek.
Formal analysis: Hanan E.M. Eldeek, Enas Abd El Hameed Mahmoud Huseein, Mohammed Essa Marghany Tolba, Heba E.M. El-Deek2, Haiam Mohamed Mahmoud Farrag.
Investigation: Hanan E.M. Eldeek, Enas Abd El Hameed Mahmoud Huseein, Mohammed Essa Marghany Tolba, Heba E.M. El-Deek, Marwa Omar Ali, Soad A. H. Bayoumi, Ebtisam Shawky Ahmed Hassanin , Samia S. Alkhalil, Haiam Mohamed Mahmoud Farrag.
Methodology: Hanan E.M. Eldeek1*, Enas Abd El Hameed Mahmoud Huseein1, Heba E.M. El-Deek2, Marwa Omar Ali3 and Soad A. H. Bayoumi, Haiam Mohamed Mahmoud Farrag.
Supervision: Mohammed Essa Marghany Tolba1, Zedan Z. Ibraheim
Writing – original draft: Enas Abd El Hameed Mahmoud Huseein, Haiam Mohamed Mahmoud Farrag, Heba E.M. El-Deek2 and Soad A. H. Bayoumi.
Writing – review & editing: Hanan E.M. Eldeek, Mohammed Essa Marghany Tolba1, Ebtisam Shawky Ahmed Hassanin, Samia S. Alkhalil.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jsps.2022.06.005.
Appendix A. Supplementary dmaterial
The following are the Supplementary data to this article:
References
- Abdel-Maksoud G., El-Amin A.R. A review on the materials used during the mummification processes in ancient Egypt. Mediterr. Archaeol. Archaeom. 2011;11:129–150. [Google Scholar]
- Abedkhojasteh H., Niyyati M., Rahimi F., Hei-Dari M., Farnia S., Rezaeian M. First Report of Hartmannella keratitis in a Cosmetic Soft Contact Lens Wearer in Iran. Iran. J. Parasitol. 2013;8:481. [PMC free article] [PubMed] [Google Scholar]
- Alexandrakis G., Miller D., Huang A.J.W. Amebic Keratitis Due to Vahlkampfia Infection Following Corneal Trauma. Arch. Ophthalmol. 1998;116:950–951. [PubMed] [Google Scholar]
- Anwar, Areeba, Ting, E.L.S., Anwar, Ayaz, Ain, N. ul, Faizi, S., Shah, M.R., Khan, N.A., Siddiqui, R., 2020. Antiamoebic activity of plant-based natural products and their conjugated silver nanoparticles against Acanthamoeba castellanii (ATCC 50492). AMB Express 10, 1–10. 10.1186/S13568-020-0960-9/FIGURES/7. [DOI] [PMC free article] [PubMed]
- Arnalich-Montiel F., Lorenzo-Morales J., Irigoyen C., Morcillo-Laiz R., López-Vélez R., Muñoz-Negrete F., Piñero J.E., Valladares B. Co-isolation of Vahlkampfia and acanthamoeba in acanthamoeba-like keratitis in a Spanish population. Cornea. 2013;32:608–614. doi: 10.1097/ICO.0B013E31825697E6. [DOI] [PubMed] [Google Scholar]
- Chopra, R., Pá, P., Mulholland, P.J., Hau, S.C., 2020. In Vivo Confocal Microscopy Morphologic Features and Cyst Density in Acanthamoeba Keratitis. 10.1016/j.ajo.2020.03.048. [DOI] [PubMed]
- De Obeso Fernadez Del Valle, A., Maciver, S.K., 2017. Allovahlkampfia minuta nov. sp., (Acrasidae, Heterolobosea, Excavata) a New Soil Amoeba at the Boundary of the Acrasid Cellular Slime Moulds. https://www.ejournals.eu/Acta-Protozoologica/ 2017, 181–189. 10.4467/16890027AP.17.016.7497. [DOI]
- Degerli S., Berk S., Malatyali E., Tepe B. Screening of the in vitro amoebicidal activities of Pastinaca armenea (Fisch. & C.A.Mey.) and Inula oculus-christi (L.) on Acanthamoeba castellanii cysts and trophozoites. Parasitol. Res. 2012;110:565–570. doi: 10.1007/S00436-011-2524-Z. [DOI] [PubMed] [Google Scholar]
- Derda M., Hadaś E., Thiem B. Plant extracts as natural amoebicidal agents. Parasitol. Res. 2009;104(3):705–708. doi: 10.1007/s00436-008-1277-9. [DOI] [PubMed] [Google Scholar]
- El-Sayed N.M., Ismail K.A., Ahmed S.-E.-G., Hetta M.H. In vitro amoebicidal activity of ethanol extracts of Arachis hypogaea L., Curcuma longa L. and Pancratium maritimum L. on Acanthamoeba castellanii cysts. Parasitol. Res. 2012;110(5):1985–1992. doi: 10.1007/s00436-011-2727-3. [DOI] [PubMed] [Google Scholar]
- Elder M.J., Kilvingtonj S., Dart K.G. A clinicopathologic study of in vitro sensitivity testing and Acanthamoeba keratitis. Invest. Ophthalmol. Vis. Sci. 1994;35:1059–1064. [PubMed] [Google Scholar]
- Elkadery A.A.S., Elsherif E.A., Ezz Eldin H.M., Fahmy I.A.F., Mohammad O.S. Efficient therapeutic effect of Nigella sativa aqueous extract and chitosan nanoparticles against experimentally induced Acanthamoeba keratitis. Parasitol. Res. 2019;118(8):2443–2454. doi: 10.1007/s00436-019-06359-x. [DOI] [PubMed] [Google Scholar]
- Elsheikha H.M., Siddiqui R., Khan N.A. Drug discovery against acanthamoeba infections: Present knowledge and unmet needs. Pathogens. 2020;9:1–17. doi: 10.3390/pathogens9050405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisen S., Bonkowski M., Zhang J., De Jonckheere J.F. Heterogeneity in the genus Allovahlkampfia and the description of the new genus Parafumarolamoeba (Vahlkampfiidae; Heterolobosea) Eur. J. Protistol. 2015;51(4):335–349. doi: 10.1016/j.ejop.2015.05.003. [DOI] [PubMed] [Google Scholar]
- Goze I., Alim A., Dag S., Tepe B., Polat Z.A. In vitro amoebicidal activity of Salvia staminea and Salvia caespitosa on Acanthamoeba castellanii and their cytotoxic potentials on corneal cells. J. Ocul. Pharmacol. Ther. 2009;25(4):293–298. doi: 10.1089/jop.2008.0132. [DOI] [PubMed] [Google Scholar]
- Hadaś E., Derda M., Cholewiński M. Evaluation of the effectiveness of tea tree oil in treatment of Acanthamoeba infection. Parasitol. Res. 2017;116(3):997–1001. doi: 10.1007/s00436-017-5377-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriquez, F.L., Khan, N., 2009. Khan NA: Acanthamoeba: Biology and Pathogenesis. Parasites Vectors 2009 21 2, 1–2. 10.1186/1756-3305-2-16. [DOI]
- Hussaini S.N., Hassanali H.T. Limulus amoebocyte lysate assay of endotoxin: A method for visual detection of the positive gel reaction. J. Med. Microbiol. 1987;24(1):89–90. doi: 10.1099/00222615-24-1-89. [DOI] [PubMed] [Google Scholar]
- Hussian, R.S.H., Jabuk, S.I.A., Al-Khafaji, M.S.A., AL-Mamoori, S.O.H., Al Mahmory, A.M., Amran, Z., Al-Hindi, Z.S., 2017. In-Vitro the Anti-Protozoal activity of Onions extract (Allium cepa) and metronidazole in Entamoebagingivalis which cultured in Tysgm-9 medium. J. Glob. Pharma Technol. 9, 149–152.
- IBM Corp. Released 2020. IBM SPSS Statistics for Windows, Version 27.0. Armonk, NY: IBM Corp, n.d.
- ISO Evaluation of biocompatibility of medical devices used in dentistry - ISO 7405. Am. Natl. Stand. Dent. 2008;2008:42. [Google Scholar]
- ISO - ISO 10993-5:2009 - Biological evaluation of medical devices — Part 5: Tests for in vitro cytotoxicity [WWW Document], n.d. URL https://www.iso.org/standard/36406.html (accessed 12.11.21).
- Jeje S.O., Akpan E.E., Kunle-Alabi O.T., Akindele O.O., Raji Y. Protective role of Allium cepa Linn (onion) juice on maternal dexamethasone induced alterations in reproductive functions of female offspring of Wistar rats. Curr. Res. Physiol. 2021;4:145–154. doi: 10.1016/J.CRPHYS.2021.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnear F.B. Cytopathogenicity of acanthamoeba, vahlkampfia and hartmannella: quantative & qualitative in vitro studies on keratocytes. J. Infect. 2003;46:228–237. doi: 10.1053/JINF.2002.1116. [DOI] [PubMed] [Google Scholar]
- van Klink F., Alizadeh H., He Y., Mellon J.A., Silvany R.E., McCulley J.P., Niederkorn J.Y. The role of contact lenses, trauma, and Langerhans cells in a Chinese hamster model of Acanthamoeba keratitis. Invest. Ophthalmol. Vis. Sci. 1993;34:1937–1944. [PubMed] [Google Scholar]
- Krstin, S., Sobeh, M., Braun, M., Wink, M., 2018. Anti-Parasitic Activities of Allium sativum and Allium cepa against Trypanosoma b. brucei and Leishmania tarentolae. Med. (Basel, Switzerland) 5, 37. 10.3390/MEDICINES5020037. [DOI] [PMC free article] [PubMed]
- Kyung K.H. Antimicrobial properties of allium species. Curr. Opin. Biotechnol. 2012;23(2):142–147. doi: 10.1016/j.copbio.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Larkin D.F., Easty D.L. Experimental Acanthamoeba keratitis: II. Immunohistochemical evaluation. Br. J. Ophthalmol. 1991;75(7):421–424. doi: 10.1136/bjo.75.7.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin D.F., Easty D.L. Experimental Acanthamoeba keratitis: I. Preliminary findings. Br. J. Ophthalmol. 1990;74(9):551–555. doi: 10.1136/bjo.74.9.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo-Morales J., Khan N.A., Walochnik J. An update on Acanthamoeba keratitis: diagnosis, pathogenesis and treatment. Parasite. 2015;22:10. doi: 10.1051/parasite/2015010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo-Morales J., Martín-Navarro C.M., López-Arencibia A., Arnalich-Montiel F., Piñero J.E., Valladares B. Acanthamoeba keratitis: an emerging disease gathering importance worldwide? Trends Parasitol. 2013;29(4):181–187. doi: 10.1016/j.pt.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Lorenzo-Morales J., Martínez-Carretero E., Batista N., Álvarez-Marín J., Bahaya Y., Walochnik J., Valladares B. Early diagnosis of amoebic keratitis due to a mixed infection with Acanthamoeba and Hartmannella. Parasitol. Res. 2007;102(1):167–169. doi: 10.1007/s00436-007-0754-x. [DOI] [PubMed] [Google Scholar]
- Malatyali E., Tepe B., Degerli S., Berk S., Akpulat H.A. In vitro amoebicidal activity of four Peucedanum species on Acanthamoeba castellanii cysts and trophozoites. Parasitol. Res. 2012;110(1):167–174. doi: 10.1007/s00436-011-2466-5. [DOI] [PubMed] [Google Scholar]
- Mantawy M.M., Ali H.F., Rizk M.Z. Efeitos Terapêuticos Do Allium Sativum E Allium Cepa Na Infecção Experimental Pelo Schistosoma Mansoni. Rev. Inst. Med. Trop. Sao Paulo. 2011;53:155–163. doi: 10.1590/S0036-46652011000300007. [DOI] [PubMed] [Google Scholar]
- McGrath J.C., Lilley E. Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br. J. Pharmacol. 2015;172(13):3189–3193. doi: 10.1111/bph.12955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiyu R., Xinyi W. Evaluation of three different methods to establish animal models of Acanthamoeba keratitis. Yonsei Med. J. 2010;51:121–127. doi: 10.3349/YMJ.2010.51.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed M.E., Huseein E.A. Allovahlkampfia spelaea is a Potential Environmental Host for Pathogenic Bacteria. J. Bacteriol. Parasitol. 2016;07(01) doi: 10.4172/2155-959710.4172/2155-9597.1000255. [DOI] [Google Scholar]
- Niyyati M., Lorenzo-Morales J., Rezaie S., Rahimi F., Martín-Navarro C.M., Mohebali M., Maghsood A.H., Farnia S., Valladares B., Rezaeian M. First report of a mixed infection due to Acanthamoeba genotype T3 and Vahlkampfia in a cosmetic soft contact lens wearer in Iran. Exp. Parasitol. 2010;126(1):89–90. doi: 10.1016/j.exppara.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Ortillés Á., Belloc J., Rubio E., Fernández M.T., Benito M., Cristóbal J.Á., Calvo B., Goñi P. In-vitro development of an effective treatment for Acanthamoeba keratitis. Int. J. Antimicrob. Agents. 2017;50(3):325–333. doi: 10.1016/j.ijantimicag.2017.03.033. [DOI] [PubMed] [Google Scholar]
- Özan F., Özdem M., Özan Ü., Şençimen M., Polat Z.A. Evaluation of cytotoxic effect of garlic on human gingival fibroblasts: A preliminary study. Gulhane Med. J. 2013;55:276–280. doi: 10.5455/GULHANE.28177. [DOI] [Google Scholar]
- Ozkoc S., Tuncay S., Delibas S.B., Akisu C., Ozbek Z., Durak I., Walochnik J. Identification of Acanthamoeba genotype T4 and Paravahlkampfia sp. from two clinical samples. J. Med. Microbiol. 2008;57:392–396. doi: 10.1099/jmm.0.47650-0. [DOI] [PubMed] [Google Scholar]
- Ozpinar N., Ozpinar H., Bakay B.B., Tunc T. Amoebicidal activity of benzothiazole on Acanthamoeba castellanii cysts and trophozoites and its cytotoxic potentials. Acta Trop. 2020;203:105322. doi: 10.1016/j.actatropica.2019.105322. [DOI] [PubMed] [Google Scholar]
- Papa V., Rama P., Radford C., Minassian D.C., Dart J.K.G. Acanthamoeba keratitis therapy: time to cure and visual outcome analysis for different antiamoebic therapies in 227 cases. Br. J. Ophthalmol. 2020;104(4):575–581. doi: 10.1136/bjophthalmol-2019-314485. [DOI] [PubMed] [Google Scholar]
- Polat Z.A., Obwaller A., Vural A., Walochnik J. Efficacy of miltefosine for topical treatment of Acanthamoeba keratitis in Syrian hamsters. Parasitol. Res. 2012;110(2):515–520. doi: 10.1007/s00436-011-2515-0. [DOI] [PubMed] [Google Scholar]
- Polat Z.A., Vural A., Ozan F., Tepe B., Özcelik S., Cetin A. In vitro evaluation of the amoebicidal activity of garlic (Allium sativum) extract on Acanthamoeba castellanii and its cytotoxic potential on corneal cells. J. Ocul. Pharmacol. Ther. 2008;24(1):8–14. doi: 10.1089/jop.2007.0035. [DOI] [PubMed] [Google Scholar]
- Polat Z.A., Vural A., Tepe B., Cetin A. In vitro amoebicidal activity of four Allium species on Acanthamoeba castellanii and their cytotoxic potentials on corneal cells. Parasitol. Res. 2007;101(2):397–402. doi: 10.1007/s00436-007-0487-x. [DOI] [PubMed] [Google Scholar]
- Randag A.C., van Rooij J., van Goor A.T., Verkerk S., Wisse R.P.L., Saelens I.E.Y., Stoutenbeek R., van Dooren B.T.H., Cheng Y.Y.Y., Eggink C.A., Oldenburg C.E. The rising incidence of Acanthamoeba keratitis: A 7-year nationwide survey and clinical assessment of risk factors and functional outcomes. PLoS ONE. 2019;14(9):e0222092. doi: 10.1371/journal.pone.0222092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-Batlle M., Freijo M.B., López-Arencibia A., Lorenzo-Morales J., McNaughton-Smith G., Piñero J.E., Abad-Grillo T. Identification of N-acyl quinolin-2(1H)-ones as new selective agents against clinical isolates of Acanthamoeba keratitis. Bioorg. Chem. 2020;99:103791. doi: 10.1016/j.bioorg.2020.103791. [DOI] [PubMed] [Google Scholar]
- Reyes-Batlle M., Hernández-Piñero I., Rizo-Liendo A., López-Arencibia A., Sifaoui I., Bethencourt-Estrella C.J., Chiboub O., Valladares B., Piñero J.E., Lorenzo-Morales J. Isolation and molecular identification of free-living amoebae from dishcloths in Tenerife, Canary Islands. Spain. Parasitol. Res. 2019;118(3):927–933. doi: 10.1007/s00436-018-06193-7. [DOI] [PubMed] [Google Scholar]
- Ródio C., da Rocha Vianna D., Kowalski K.P., Panatieri L.F., von Poser G., Rott M.B. In vitro evaluation of the amebicidal activity of Pterocaulon polystachyum (Asteraceae) against trophozoites of Acanthamoeba castellanii. Parasitol. Res. 2008;104(1):191–194. doi: 10.1007/s00436-008-1186-y. [DOI] [PubMed] [Google Scholar]
- Shahbaz, M.S., Anwar, Ayaz, Saad, S.M., Kanwal, Anwar, Areeba, Khan, K.M., Siddiqui, R., Khan, N.A., 2020. Antiamoebic activity of 3-aryl-6,7-dimethoxyquinazolin-4(3H)-one library against Acanthamoeba castellanii. Parasitol. Res. 119, 2327–2335. 10.1007/S00436-020-06710-7/FIGURES/5f. [DOI] [PubMed]
- Siddiqui R. Antibacterial and Anti-Acanthamoebic Properties of Catha Edulis (Khat) J. Bacteriol. Parasitol. 2012;03(07) doi: 10.4172/2155-959710.4172/2155-9597.1000152. [DOI] [Google Scholar]
- Sidhu J.S., Ali M., Al-Rashdan A., Ahmed N. Onion (Allium cepa L.) is potentially a good source of important antioxidants. J. Food Sci. Technol. 2019;56(4):1811–1819. doi: 10.1007/s13197-019-03625-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sifaoui, I., Yanes, E.C., Reyes-Batlle, M., Rodríguez-Expósito, R.L., Piñero, J.E., Lorenzo-Morales, J., 2020. Combined Amoebicidal Effect of Atorvastatin and Commercial Eye Drops against Acanthamoeba castellanii Neff: In Vitro Assay Based on Mixture Design. 10.3390/pathogens9030219. [DOI] [PMC free article] [PubMed]
- Sokmen A., Jones B.M., Erturk M. The in vitro antibacterial activity of Turkish medicinal plants. J. Ethnopharmacol. 1999;67(1):79–86. doi: 10.1016/S0378-8741(98)00189-5. [DOI] [PubMed] [Google Scholar]
- Sun Y., Hong J., Zhang P., Peng R., Xiao G. Pathological characteristics of the different stages of Acanthamoeba keratitis. Histopathology. 2013;63(6):862–868. doi: 10.1111/his.12237. [DOI] [PubMed] [Google Scholar]
- Tepe B., Malatyali E., Degerli S., Berk S. In vitro amoebicidal activities of Teucrium polium and T. chamaedrys on Acanthamoeba castellanii trophozoites and cysts. Parasitol. Res. 2012;110(5):1773–1778. doi: 10.1007/s00436-011-2698-4. [DOI] [PubMed] [Google Scholar]
- Teshika J.D., Zakariyyah A.M., Zaynab T., Zengin G., Rengasamy K.RR., Pandian S.K., Fawzi M.M. Traditional and modern uses of onion bulb (Allium cepa L.): a systematic review. Crit. Rev. Food Sci. Nutr. 2019;59(sup1):S39–S70. doi: 10.1080/10408398.2018.1499074. [DOI] [PubMed] [Google Scholar]
- Tolba M.E.M., Huseein E.A.M., Farrag H.M.M., Mohamed H.E.D., Kobayashi S., Suzuki J., Ali T.A.M., Sugano S., Bhattacharya A. Allovahlkampfia spelaea Causing Keratitis in Humans. PLoS Negl. Trop. Dis. 2016;10(7):e0004841. doi: 10.1371/journal.pntd.0004841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyml T., Skulinová K., Kavan J., Ditrich O., Kostka M., Dyková I. Heterolobosean amoebae from Arctic and Antarctic extremes: 18 novel strains of Allovahlkampfia, Vahlkampfia and Naegleria. Eur. J. Protistol. 2016;56:119–133. doi: 10.1016/J.EJOP.2016.08.003. [DOI] [PubMed] [Google Scholar]
- Upadhyay R.K. Nutraceutical, pharmaceutical and therapeutic uses of Allium cepa: A review. Int. J. Green Pharm. 2016;10:S46–S64. [Google Scholar]
- Walochnik, J., Mulec, J., 2009. Free-living Amoebae in Carbonate Precipitating Microhabitats of Karst Caves and a New Vahlkampfiid Amoeba, Allovahlkampfia spelaea gen. nov., sp. nov. https://www.ejournals.eu/Acta-Protozoologica/ 2009, 25–33.
- Yang Y.F., Matheson M., Dart J.K.G., Cree I.A. Persistence of acanthamoeba antigen following acanthamoeba keratitis. Br. J. Ophthalmol. 2001;85:277–280. doi: 10.1136/BJO.85.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorzi G.K., Schuh R.S., Maschio V.J., Brazil N.T., Rott M.B., Teixeira H.F. Box Behnken design of siRNA-loaded liposomes for the treatment of a murine model of ocular keratitis caused by Acanthamoeba. Colloids Surf. B. Biointerfaces. 2019;173:725–732. doi: 10.1016/J.COLSURFB.2018.10.044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.