Abstract
Mitochondria are double-membraned cytoplasmic organelles that are responsible for the production of energy in eukaryotic cells. The process is completed through oxidative phosphorylation (OXPHOS) by the respiratory chain (RC) in mitochondria. Thousands of mitochondria may be present in each cell, depending on the function of that cell. Primary mitochondria disorder (PMD) is a clinically heterogeneous disease associated with germline mutations in mitochondrial DNA (mtDNA) and/or nuclear DNA (nDNA) genes, and impairs mitochondrial structure and function. Mitochondrial dysfunction can be detected in early childhood and may be severe, progressive and often multi-systemic, involving a wide range of organs. Understanding epigenetic factors and pathways mutations can help pave the way for developing an effective cure. However, the lack of information about the disease (including age of onset, symptoms, clinical phenotype, morbidity and mortality), the limits of current preclinical models and the wide range of phenotypic presentations hamper the development of effective medicines. Although new therapeutic approaches have been introduced with encouraging preclinical and clinical outcomes, there is no definitive cure for PMD. This review highlights recent advances, particularly in children, in terms of etiology, pathophysiology, clinical diagnosis, molecular pathways and epigenetic alterations. Current therapeutic approaches, future advances and proposed new therapeutic plans will also be discussed.
Keywords: Mitochondrial diseases, Epigenetic alterations, Gene editing, Fetal gene therapy, Nanomedicine
1. Introduction
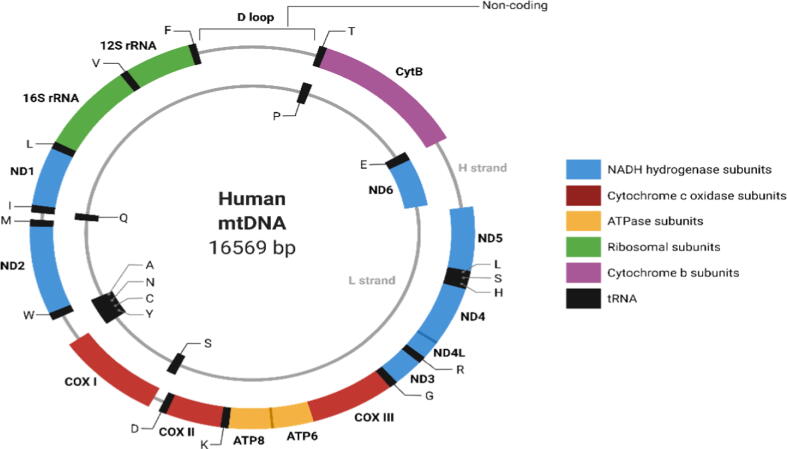
Mitochondria are double-membraned cytoplasmic organelles known for their energy production in eukaryotic cells. They serve as energy factories that produce the energy required for the body to function through metabolic processes, in particular, oxidative phosphorylation (OXPHOS) (Alahmad et al., 2019, La Morgia et al., 2020). Other mitochondrial functions are homeostatic regulations, apoptotic cell death signaling, and implications for innate immunity (Chan, 2012, Green et al., 2014, Mills et al., 2017). The mitochondria contain several copies of their own 16.569 DNA building blocks (base pairs), a closed circular genome, which varies from 100 to 10,000 copies/cell depending on the cellular energy demand (Alahmad et al., 2019), as shown in Fig. 1. Eukaryotic cells carry two types of genomes, the mitochondrial DNA (mtDNA) and the nuclear DNA (nDNA) (Lane et al., 2013). Both genomes control the oxidative phosphorylation process and encode structural mitochondrial proteins. It was reported that the mitochondria contain approximately 1500 types of proteins, 13 of which are encoded by the mtDNA while the rest are encoded by nDNA (Wang et al., 2021). In addition, the mtDNA contains 37 genes, which encode 22 mitochondrial-transfer RNAs (mt-tRNAs), 2 mitochondrial-ribosomal RNAs (mt-rRNA) and 13 OXPHOS protein subunits (Alahmad et al., 2019).
Fig. 1.
Human mitochondrial DNA. The human mitochondrial genome is organized as double-stranded a light (inner) and a heavy (outer) circular molecule containing 37 genes. 13 of these genes encode one polypeptide subunit, which is involved in the regulation of respiratory chain (RC), while the remaining 24 are necessary for RNA translation mechanism, 2 for making molecules called ribosomal RNAs (rRNAs) and 22 for transfer RNAs (tRNAs). Created with Biorender.com.
When mitochondria start to lose their oxidative phosphorylation function, which is triggered by mutations in mtDNA and nDNA genes, it will lead to mitochondrial diseases (Gorman et al., 2016). Such disorders are chronic, heterogeneous, genetically inherited, and can affect an individual at any age and in any body organ, including nerves, the brain and other major organs. It was estimated that one in every 5,000 newborns (Pérez-Albert et al., 2018, Rahman, 2020) and one in 8,000 adults has a mitochondrial genetic disease (Watson et al., 2020), with the mtDNA mutation responsible for 15 to 30% of pediatric cases and more than 50% of adult cases (Tan et al., 2020). Depending on the type of mitochondrial disease, severity of the symptoms, and organ affected, a person can live a near-normal life or suffer from a drastic change in their health that could lead to mortality. Mitochondrial diseases are governed mainly by mutations in the mtDNA and nDNA genomes that represent approximately 75% to 95% of all cases (Wang et al., 2021). Additionally, a secondary mitochondrial dysfunction can occur due to other diseases, such as Alzheimer's, diabetes, and cancer, which are not caused by a genetic-related factor.
This review highlights the recent advancements in mitochondrial diseases in their etiology, pathophysiology, clinical diagnosis, molecular pathways and epigenetic alterations. Additionally, the current therapeutic approaches and future advances in the treatment of mitochondrial diseases are also discussed.
2. Types of mitochondrial diseases
Mitochondrial diseases are genetic disorders that may affect single or multiple organs. Mitochondrial diseases can be grouped into syndromic and non-syndromic (Finsterer, 2012). Syndromic mitochondrial disorders usually include various organs or tissues with recurrent patterns of signs and symptoms, which can frequently affect some major organs, such as the skeletal muscles, the nervous system, the auditory system, the ophthalmic system, heart, gastrointestinal tract, kidneys, and lungs (Finsterer and Scorza, 2017, Muraresku et al., 2018). Mitochondrial diseases could also be categorized based on mutations affecting the mitochondrial metabolic pathways into primary and secondary. The former type is due to mutations in the genes, mtDNA or nDNA, responsible for OXPHOS, or the genes encoding for proteins that interfere with the OXPHOS pathway.
On the other hand, secondary mitochondrial disorders may involve genetic, non-OXPHOS genes, and environmental factors (Niyazov et al., 2016). The prevalence of primary mitochondrial diseases was estimated as 1 in every 5000 newborns (Pérez-Albert et al., 2018). In childhood, mitochondrial disorders are not defined by specific symptoms; instead, a group of genetic, clinical, and biochemical manifestations are used to diagnose such diseases (Gorman et al., 2016). Several children who suffer from mitochondrial diseases display non-classical multi-organ diseases, where organs with high energy demands such as muscles, kidneys, pancreas, and the brain are highly affected (Rahman, 2020, van de Loo et al., 2020). Diagnosis and differentiation between primary and secondary mitochondrial disorders are a challenge for physicians due to the clinical manifestations' closeness. In addition, mitochondrial diseases can decline rapidly or continue for an extended period with recurrent episodes of aggravation in response to stressors like infections (Muraresku et al., 2018). Fig. 2 demonstrates the clinical features of mitochondrial diseases.
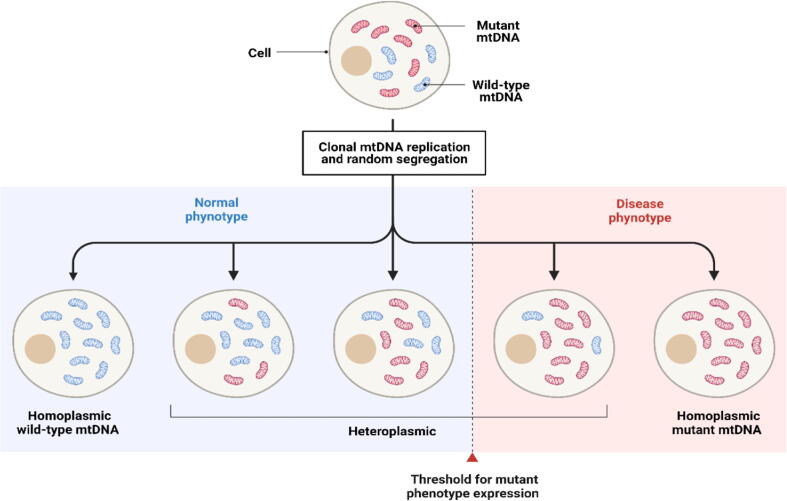
Fig. 2.
Schematic presentation of homoplasmic and heteroplasmic mitochondrial DNA. A single cell may obtain wild type copies of mtDNA (homoplasmy) or a mixture of mutant and wild-type mtDNA (heteroplasmy)). The proportion of mutant mtDNA copies determines the penetrance and severity of phenotype expression, and the cell will be affected if it exceeds a specific limit (threshold). Abbreviations: mtDNA, mitochondrial DNA. Created with Biorender.com.
Neurologic manifestations are among the most severe consequences of mitochondrial diseases that can result in several conditions, such as Leigh syndrome, Epilepsia partialis continua, occipital stroke-like episodes, axonal sensorimotor neuropathy, myoclonus, and ataxia (Martikainen and Chinnery, 2015, Muraresku et al., 2018, Riquin et al., 2020). In addition, neuropsychological disorders were also recorded, such as impaired consciousness, hallucinations, reasoning, confusional state, depression, autism and unsteadiness (Finsterer, 2006, Koene et al., 2013). In the case of the ophthalmic system, different regions of the eye could be affected, which are expressed in the form of progressive ptosis and external ophthalmoplegia, visual loss (retro-chiasmal), pigmentary retinopathy and bilateral optic atrophy (Al-Enezi et al., 2008, Yu-Wai-Man and Newman, 2017, Lock et al., 2021). In infants and adolescents, these neuro-ophthalmologic symptoms may be progressive and lead to serious eye complications with decreased vision efficacy (Muraresku et al., 2018).
Hearing loss at both ears (i.e. bilateral) is usually associated with mitochondrial dysfunctions, particularly in mitochondrial encephalomyopathy, lactic acidosis and stroke like episodes or MELAS (Chinnery et al., 2000, Di Stadio et al., 2018). Metabolically active ear parts, such as the stria vascularis and the outer hair cells, are the most affected. In children with bilateral hearing loss, mitochondrial dysfunctions could be the leading cause, or other neurological disorders can also be involved (Vandana et al., 2016). The heart is another vital organ that might deteriorate by primary mitochondrial dysfunction. Lack of functions in the myocardium cells is characterized by deformed muscle structure and functions (Meyer et al., 2013). Several syndromes have been reported in children with primary mitochondrial diseases, for instance, mitochondrial cardiomyopathy, Wolff-Parkinson-White, and Kearns-Sayre syndromes. Individuals with mitochondrial cardiomyopathy often display heart hypertrophy and left ventricular noncompaction. Individuals with MELAS are most likely to develop arrhythmia, including Wolff-Parkinson-White and ventricular pre-excitation (Ng and Turnbull, 2016). The disease severity ranges from asymptomatic to life-threatening tachyarrhythmia and heart failure and could eventually lead to cardiac death (Debray et al., 2007, Meyer et al., 2013).
The gastrointestinal tract is also affected by primary mitochondrial disorders, in which numerous manifestations will cause permanent and untreatable disability due to the difficulty of early diagnosis (Rose et al., 2017). These manifestations include gastroesophageal sphincter dysfunction, dysphagia, hepatopathy, pancreatitis, intestinal pseudo-obstruction and gastroparesis (Finsterer and Frank, 2017). (Moreover, patients diagnosed with Crohn's disease commonly suffer from mitochondrial neurogastrointestinal encephalopathy Patel et al., 2019, Tawk et al., 2020).
The kidneys are among the organs that are likely to fail due to mitochondrial disorders due to the high energy consumption in this organ (Finsterer and Scorza, 2017, Govers et al., 2021). Kidney diseases resulting from mitochondrial dysfunctions can be categorized into primary and secondary. In the first category, several diseases have been reported, such as acute or chronic kidney failure, nephrotic syndrome, renal tubular acidosis, tubulointerstitial nephritis, and nephrocalcinosis (Finsterer and Frank, 2016). Renal insufficiency in the second category includes either organ other than the kidneys, for example, the heart and pancreas, or other diseases such as rhabdomyolysis (Yokoyama et al., 2016). A previous study has reported a significant relation between mitochondrial dysfunctions, chronic kidney diseases and sarcopenia (Takemura et al., 2020).
Children with mitochondrial diseases have frequently suffered from several physicals, neuropsychological disorders, behavior and speech disorders, high morbidity, and recurrent episodes of anxiety and depression (van de Loo et al., 2020). Due to the impaired mitochondrial functions, affected children are usually unable to participate in daily activities, which could aggravate psychiatric disorders (Lindenschot et al., 2018, Riquin et al., 2020). Caregivers and parents might also suffer from stress that can exacerbate hospitalization and the need for clinical intervention (Morava et al., 2010, Sofou, 2013).
3. Etiology and pathophysiology of mitochondrial diseases
The human mitochondria double-strand DNA, i.e. mtDNA, comprises 37 genes (16569 bp in a circular shape) encoding 13 proteins (7 subunits of respiratory chain complex I, 3 subunits of complex IV, 2 subunits of complex V and 1 subunit of complex III), 22 tRNAs, and 2 rRNAs. Each mitochondrion has multiple copies of mtDNA (2–10 copies), while the number of mitochondria in each cell can vary. The inheritance of mtDNA is maternally transmitted, in contrast to the nDNA, which has a biparental transmission pattern (Sato and Sato, 2013, An et al., 2020). Additionally, there are 1,500 identified genes presented in the nDNA, which are essential for the mitochondrial structure and function (Li et al., 2020).
The mutation rate of the mtDNA is considered higher (greater than10 times) than the nDNA. This could link to the missing protection of the histone complex and the high sensitivity of mtDNA to oxidative stress with less efficient mtDNA repair mechanisms, such as the base excision repair pathway (Gredilla, 2010, Alexeyev et al., 2013). In addition, the DNA polymerase (DNA polymerase γ) activity in the mitochondria has a relatively low fidelity rate which can rise the mutation during the mitochondrial DNA replication (Song et al., 2005). The mutation in the mitochondria may affect the whole mtDNA in the cell (homoplasmy) or partially (heteroplasmy), as shown in Fig. 2. Consequently, the severity of the mitochondrial diseases is influenced by both phenomena (Tuppen et al., 2010, Moggio et al., 2014). In addition to the primary mitochondrial function, i.e. generating energy, the mitochondria have other roles in calcium metabolism, innate metabolism, cell death, stem cell maintenance, autophagy, inflammation and senescence (Smith et al., 2012). Therefore, mutation at the mtDNA or nDNA levels can have a variety of pathogenesis outcomes.
The nuclear genome is essential for mitochondrial function, with more than 1,000 genes involved. The mutations of greater than 280 genes have been reported to cause mitochondrial disorders, where the defect may be inherited as autosomal dominant (8 genes), recessive (262 genes) or X- linked pattern (14 genes) (Tuppen et al., 2010, Frazier et al., 2019, Stenton and Prokisch, 2020). Historically, the first nDNA mutation (autosomal recessive) was associated with human mitochondrial disease at the SDHA gene, which is involved in encoding a structural subunit of complex II, as reported in 1995 by Bourgeron et al. (Bourgeron et al., 1995). On the other hand, the first X-linked recessive mutations identified in 2007 at the NDUFA1 gene were p.Gly8Arg and p.Arg37Se (Fernandez-Moreira et al., 2007). The prevalence of nDNA mutation triggering mitochondrial diseases is estimated to be 1 in 35,000 (Gorman et al., 2015). Leigh syndrome is a common neurodegenerative mitochondrial disorder with a 1 in 40,000, which can be caused by approximately 90 gene mutations, including the mtDNA and nDNA genomes (Gerards et al., 2016, Rahman et al., 2017). An example of this is the mutation in the SURF1 nDNA gene, which is linked with Cytochrome c oxidase deficiency (Wedatilake et al., 2013).
The prevalence of mtDNA mutation causing mitochondrial disease is estimated to be 1 in 5,000 (Schon et al., 2021). The main two types of mutation are large deletion and point mutations, mostly maternal transmitted with some de novo mutations have also been reported (Ruiz-Pesini et al., 2007). The first large-scale mtDNA deletion was previously reported in 1988 by Massimo et al., who observed a large deletion in the mtDNA ranging from 2.0 to 7.0 kb that caused Kearns-Sayre syndrome (Zeviani et al., 1988). The most common large-scale mtDNA deletion resulted from the omission of 4977 bp located between the locus 8470 and 13447, which involved the encoding of 15 genes from ATPase8 gene to ND5 gene (Holt et al., 1989, Shoffner et al., 1989, Yusoff et al., 2019). In contrast, point mutation can involve an insertion, deletion or substitution, affecting either the respiratory chain coding genes or the RNA coding genes (mt-rRNA and mt-tRNA). Mutations in 2 mt-rRNA may directly affect the ribosome's function on the other 13 proteins synthesized by the mt-DNA. An example is 847C > U (m.1494C > T) and 908A > G (m.1555A > G), which are linked to hearing impairment (Guan et al., 2006, Bindu and Reddy, 2008). Furthermore, m.3243A > G mutation is one of the most common mutations in the tRNAs genes, with a heterogeneous effect on the muscles and the nervous system, causing mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes (MELAS) (Pickett et al., 2018).
4. Clinical features of mitochondrial diseases
The childhood-onset of mitochondrial diseases have mainly resulted from recessive nDNA or mutations in mtDNA that exist at high levels of mtDNA heteroplasmy (Taylor and Turnbull, 2005). As mitochondrial diseases have diverse phenotypes and usually cause multi-organs dysfunction, the clinical features and diagnosis are relatively complicated (Alston et al., 2017). Moreover, the tenuous link between the observed clinical phenotype and the genotype in mitochondrial disease patients complicates the accurate diagnosis (Stenton and Prokisch, 2020).
The pediatric-onset of mitochondrial disorders have several clinical features that are regularly observed, such as fatigue, vomiting, failure to thrive, encephalopathy, seizures, hypotonia, exercise intolerance and dysautonomia, as illustrated in Fig. 3 (Kanungo et al., 2018). However, most of these clinical features are not specific to mitochondrial diseases, and confirmation analysis should be performed (i.e. molecular genetic screening analysis). The congenital disabilities in children with mitochondrial dysfunctions are frequently associated with different complications, as in the case of renal disease and proximal tubulopathy that occurs due to the mutations in RMND1 (O'Toole, 2014), RRM2B (Stojanovic et al., 2013) genes, respectively.
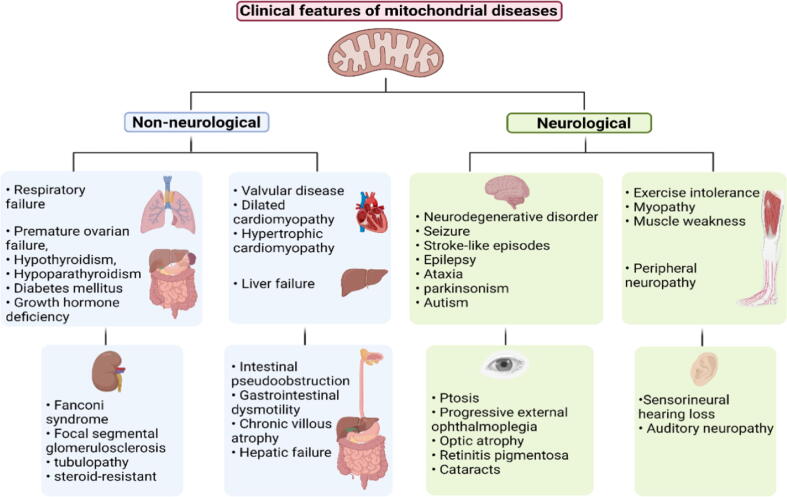
Fig. 3.
Schematic presentation of clinical features of mitochondrial diseases. The clinical features of mitochondrial diseases vary between patients and have non-neurological or neurological characteristics, commonly involving two or more organ systems causing dysfunction of any organ or tissue. Created with Biorender.com.
In addition to previously mentioned clinical features of mitochondrial diseases, hypertrophic cardiomyopathy is frequently observed in children with mitochondrial disorders, and it is associated with MTO1 or AARS2 mutations (Baruffini et al., 2013, Euro et al., 2015). Moreover, excessive hair growth (hypertrichosis) and dysmorphic disorder are common clinical manifestations in children with mitochondrial diseases that are caused by mutations in SURF1 (Baertling et al., 2013) and FBXL4 (Ballout et al., 2019), respectively. The modifications in SUCLA2, SUCLG1, MT-RNR1 (m.1555A > G), MT-TL1 (m.3243A > G), RMND1 and RRM2B in children can lead to sensorineural hearing loss (Rahman, 2020). The typical clinical features of pediatric mitochondrial diseases are summarized in Fig. 4.
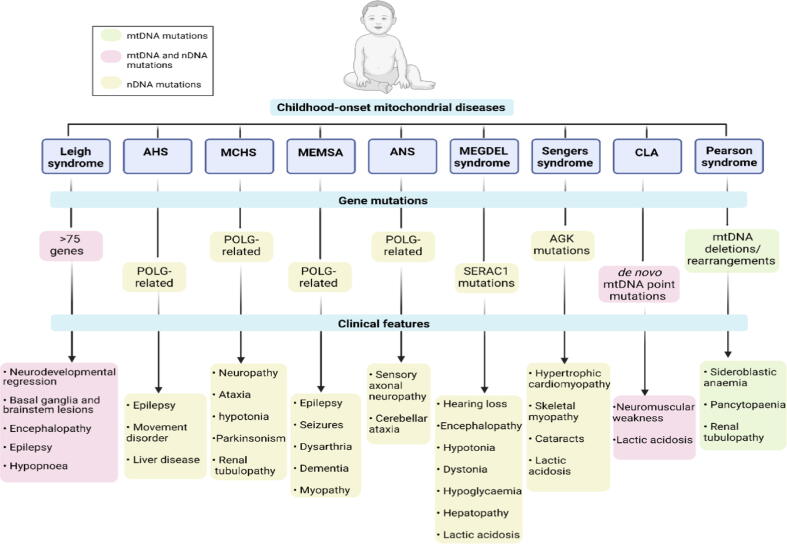
Fig. 4.
Clinical presentation of childhood-onset mitochondrial diseases. Genotypic and phenotypic features of mitochondrial diseases in children. Abbreviations: ASH, Alpers–Huttenlocher syndrome; myopathy sensory ataxia; ANS, Ataxia neuropathy spectrum; CLA, Congenital lactic acidosis. Created with Biorender.com.
5. Clinical diagnosis of mitochondrial diseases
Mitochondrial diseases can be evaluated through different diagnostic testing, such as blood, urine, molecular genetics and tissue biopsy analyses. To confirm the mitochondrial disease's diagnosis, several conventional biomarkers could be used, which are specific enzymes, anaerobic glucose metabolism and products or metabolic intermediates (i.e., alanine, lactate, creatine kinase, pyruvate, deoxyuridine, thymidine, acylcarnitines and organic acids) (Boenzi and Diodato, 2018). It is beneficial to conduct metabolic screening analysis in urine and blood samples to detect mitochondrial dysfunctions in their early stages. Different diagnostic biochemical screening tests might also be used that include complete blood count, urine organic acid analysis, urine amino acid analysis, hormone screening, hemoglobin A1C, comprehensive chemistry panel, blood lactate and pyruvate, creatine kinase, ammonia and carnitine, acylcarnitine and lipoprotein profiles (Muraresku et al., 2018). Additional biochemical screening tests can be performed when needed to confirm the mitochondrial disorder.
One of the most valuable biomarkers in the diagnosis of mitochondrial disease is the metabolic fingerprints of OXPHOS deficiency (Finsterer and Zarrouk-Mahjoub, 2018). For instance, it has been demonstrated that the fibroblast growth factor 21 (FGF21) level is a potential biomarker for muscle-manifesting mitochondrial disease (Finsterer and Zarrouk-Mahjoub, 2018). The elevated growth-differentiation factor 15 (GDF15) has been detected in blood samples collected from mitochondrial dysfunction patients and is considered another potential biomarker (Yatsuga et al., 2015). The measurement of abnormal quantities of organic acids in urine may be used as a diagnostic tool to detect several mitochondrial disorders in children (Yatsuga et al., 2015), such as methylmalonic aciduria (caused by mutations in SUCLA2 and SUCLG1) (Landsverk et al., 2014), and 3-methylglutaconic aciduria (caused by mutations in TAZ, TMEM70 and SERAC1) (Tort et al., 2013).
Molecular genetic testing is an essential diagnostic tool that helps identify the molecular etiology that caused the mitochondrial dysfunction; hence, it could improve therapeutic outcomes. The first-line molecular diagnostic test of mitochondrial dysfunction is the whole-exome sequencing that includes mtDNA sequencing in proband, parental or family members (Lieber et al., 2013, McCormick et al., 2013). This is a crucial test to identify de novo dominant mutations in the affected individuals and increase the interpretation accuracy of variant pathogenicity. The next-generation sequencing (NGS) of mtDNA and its content in the diseased tissues may be considered the most successful approach to identifying the primary mitochondrial diseases. Biotin and thiamine responsive basal ganglia disease (SLC19A3) and riboflavin transport deficiencies (SLC52A2 or SLC52A3) were reported to have phenotypic overlap with mitochondrial dysfunctions (Ferreira et al., 2017).
In addition to biochemical screening analysis and molecular genetic testing, tissue analysis is another important test to diagnose mitochondrial diseases, particularly in symptomatic tissues that have mtDNA mutations or mitochondrial dysfunctions (Dimmock and Lawlor, 2017). The mitochondrial enzymes and functions can be evaluated through a skin biopsy (Newell et al., 2019). This tissue screening might include the measurement of integrated mitochondrial OXPHOS and the analysis of enzymatic activity of the electron transport chain (ETC) complex (Germain et al., 2019). Biopsy from skeletal muscles can be performed to understand the degree of mitochondrial dysfunctions or confirm the dysfunction as an alternative approach to a failed blood genetic analysis (Ahmed et al., 2018). Further clinical diagnostic testing may be conducted on high energy demanding tissues, i.e. kidneys or muscle, such as ETC complexes I-IV enzyme activity analyses (Parikh et al., 2015).
6. Epigenetic alterations in mitochondrial diseases
The significant differences between the mtDNA and the nDNA are that there are several copies of mtDNA that lack histone. The histone contains several cationic amino acids, such as lysine and arginine, which will cause the DNA chromosome to compact; hence, its absence in the mtDNA could be more vulnerable to epigenetic alterations (Chinnery et al., 2012). Epigenetic variation is a change that may occur in the genetic expression, which is not heritable (Sharma et al., 2019). Several mtDNA variabilities are also inheritable (D’Aquila et al., 2015).
The primary epigenetic regulation of the mitochondria can occur in the mtDNA methylation, non-coding RNAs (ncRNA) modification, and posttranslational modification (Sharma et al., 2019). DNA methylation is the process in which a methyl group from S-adenosyl-methionine binds to the DNA nucleotide, mainly adenine (A) and cytosine (C), by the support of DNA methyltransferases (DNMTs) (Maresca et al., 2015). This process of DNA methylation is essential for the stability of the whole genome (Jin and Robertson, 2013). Regarding the mtDNA methylation, it was reported by Rebelo et al. that the mtDNA might be methylated in different sites, suggesting that this methylation is caused by DNMTs, which rely on the occupied level of proteins around the mtDNA (Rebelo et al., 2009). It was reported that the level of DNA methylation could also be observed in cancerous cells (Horvath, 2013). However, low levels of mtDNA methylation could be less observed in pediatrics, as the methylated area in the mtDNA declined upon ageing (Heyn et al., 2012, Horvath, 2013).
The communication between mitochondria and the nucleus plays a critical role in cellular homeostasis. The nucleus controls the mitochondrion's gene expression and post-translation process; however, the nuclear gene expression and protein activity are mediated by the mitochondria through signal transport from mitochondria to cytosol. This crosstalk is controlled by several signals, such as microRNA (miRNA), a subclass of ncRNA (Cavalcante et al., 2020). The transcription of miRNA, which is non-coding RNA, occurs in the nucleus as primary miRNA to be transformed to precursor miRNA by Drosha and then to its mature form by Dicer at the cytoplasm. The primary function of miRNA is to inhibit the translation of mRNA via destabilizing its binding to the 3′ untranslated region of mRNA (Chipman and Pasquinelli, 2019). miRNA can play a vital role in mitochondrial function. For instance, several miRNAs such as miR-138, miR-1291, miR-150, and miR-199a-3p can cause a change in the regulation of the expression of some glycolytic enzymes’ glucose transporters. This explains the role of miRNA in controlling glucose uptake by the mitochondrion (Chow et al., 2010, Srinivasan and Das, 2015). In addition, lipid metabolism could be affected by miRNA. It was reported that miR-33a/b might control the metabolism by targeting ATP binding cassette subfamily A member 1 (ABCA1), a cholesterol transporter (Rayner et al., 2011). In addition, miR-24, miR-126, and miR-143 can also regulate the mitochondrial lipid metabolism by inhibiting Apolipoprotein L6 (APOL6) (Ye et al., 2013).
Additionally, the production of reactive oxygen species (ROS) from the mitochondria could trigger the expression of hypoxia-inducible factor 1 (HIF-1) (Weinberg et al., 2015). The ncRNA has a vital role in regulating gene expression and controlling the mitochondria. For example, miRNA from the nucleus might control the mitochondrial gene expression, which depends on the adenosine triphosphate (ATP) produced by the mitochondria (Duarte et al., 2014).
7. Current and future treatments of mitochondrial diseases
There is no cure or an FDA (i.e. Food and Drug Administration)-approved therapy currently available for mitochondrial diseases owing to the different genes and phenotypes associated with the cause of such disorders. Nevertheless, few symptomatic treatments have been proven by clinical trials as palliative therapies in the last decade. A mitochondrial cocktail, i.e. a combination of vitamins, cofactors, nutrients and antioxidants, may alleviate symptoms, limit disease progression, and overcome mitochondrial toxins. This symptom-based management aims to enhance mitochondrial function by supporting the electron transport chain and treating mitochondrial dysfunction's consequences (Parikh et al., 2009, Pfeffer et al., 2012).
7.1. Nutritional supplement and exercise
Poor diet and extreme malnutrition lead to secondary mitochondrial dysfunctions, while overeating increases ROS formation and generates toxic metabolites (Wortmann et al., 2009). Therefore, specific dietary restrictions have been shown to ameliorate mitochondrial health in patients with mitochondrial disorders; thus, evaluating individuals' nutritional necessity and deficiencies are significantly needed (Morava et al., 2006). A High-carbohydrate diet has been reported to increase oxidative stress, which can be metabolically challenging for those with impaired oxidative phosphorylation (El-Hattab et al., 2012, Munnich et al., 2012). On the other hand, the ketogenic diet (high-fat diet) has been beneficial for patients with pyruvate dehydrogenase deficiency but not helpful in the case of pyruvate carboxylase deficiency and treating fatty acid oxidation disorders (Bough et al., 2006).
Mitochondrial diseases affecting the respiratory chain could be treated with agents known to enhance the electron transport and substrate supply and bypass its components. Several phenotypes of mitochondrial disorders resulting from the biosynthetic defects of Coenzyme Q10 (CoQ10) were treated with CoQ10 supplementation and found to decrease the elevated levels of lactate in post-exercise and increase oxygen consumption (Rötig et al., 2000, Di Giovanni et al., 2001). Other food supplements, including vitamins and amino acids, may be used as redox agents and intracellular buffering for ATP (Parikh et al., 2009). Other symptoms relevant to mitochondrial diseases, including stroke-like episodes, myopathy, diabetes, and lactic acidosis associated with nitric oxide (NO) depletion, could be treated with NO natural precursors, for example, arginine and citrulline, to restore the production of NO (El-Hattab et al., 2012, Pfeffer et al., 2012, Almannai and El-Hattab, 2021).
Physical therapy and exercise have been found to elevate mitochondrial health and decrease the burden of dysfunctional mitochondria. Patients with mitochondrial disorders are exercise-intolerant due to low maximal oxygen uptake, but a gradual endurance exercise could help overcome such difficulty and enhance mitochondria enzymic activity (Parikh et al., 2009). In addition, physical therapy leads to mitochondrial biogenesis induced by the high expression of the master transcription regulator PGC-1α. Accumulating evidence was demonstrated that exercise elevates mitochondrial ROS, which triggers the organelle biogenesis pathway by the high expression of the master transcription regulator PGC-1α leading to increased mitochondrial quantity and quality (Taivassalo et al., 2001, Kang and Li Ji, 2012). In addition, physical training can improve OXPHOS, respiratory capacity and electron flow, reducing ROS production (Holloway, 2017, Memme et al., 2021).
7.2. Pharmacological agents
Analogues of CoQ, for instance, idebenone, mitoquinone and short-chain CoQ10 with improved pharmaceutical and pharmacological properties were developed to boost the electron transport chain of mitochondria and evaluated clinically for their therapeutic efficacy. These natural and synthetic quinones demonstrated potential antioxidant activities against toxic metabolites from the defected mitochondria and accumulated ROS (Suárez-Rivero et al., 2021). For example, a study by Klopstock et al. showed remarkable success in treating the visual acuity of a large group of patients using idebenone (Klopstock et al., 2011). Many applications in clinical trials, such as Leber’s Hereditary Optic Neuropathy (LHON; NLM, 2013), Parkinson’s disease (NLM, 2018), and MELAS syndrome (NLM, 2016), have assured the safety of idebenone and its efficacy, even at higher doses. It has passed phase III evaluation (Suárez-Rivero et al., 2021). Other CoQ analogues with diverse side-chain displayed unique biological activities and enhanced pharmacological properties, such as bioavailability, mitochondrial accumulation and antioxidant effect. Kagan et al. found that CoQ analogues with shorter isoprenoid side chains have more antioxidant potential (Kagan et al., 1990). In addition, antioxidants, such as lipoic acid and N-acetyl-cysteine were used to decrease ROS-induced toxicity and accumulated ROS from the defected mitochondria.
7.3. Gene editing technology
Other than the controversial Mitochondrial Replacement Therapy (MRT) utilized to prevent the inherited mitochondrial DNA (mtDNA) mutations (Klopstock et al., 2016),genome-editing therapy can be considered the ultimate treatment strategy to which mitochondrial diseases patients hold their hopes out. Despite being the prominent approach for gene editing, the Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)/Cas9 technology falls behind in mitochondrial diseases due to the encountered difficulties in delivering the Cas9 nuclease and a guide RNA (gRNA) along with a homologous repair template coincidentally to mitochondria for editing to take place (Gammage et al., 2018a, Gammage et al., 2018b). Alternatively, restriction endonucleases have been developed as an effective tool for gene editing owing primarily to their ability to form linear fragments of mtDNA after selective cleavage of the mutant mtDNA while leaving the wild type intact. Elimination of the mtDNA fragments pursues rapidly by the exonucleolytic activity of enzymatic machinery, including the mitochondrial polymerase gamma (POLG) (Nissanka et al., 2018), the mitochondrial replicative DNA helicase Twinkle (TWNK), and the mitochondrial genome maintenance exonuclease 1(MGME1) (Peeva et al., 2018), leading to an increased population of the wild type mtDNA. However, the indispensable use of gRNAs constrains the application of such restriction endonucleases.
This limitation has steered the research toward developing programmable nucleases that can induce specific elimination of the mutant mtDNA. These nucleases include the mitochondrial-targeted- zinc finger nucleases (mtZFN) delivered by an adeno-associated virus (AAV; Gammage et al., 2018a, Gammage et al., 2018b), and transcription activator-like effector nucleases (mitoTALENs) (Bacman et al., 2018), both corrected the mtDNA heteroplasmy through inducing a specific elimination of the mutant mtDNA in a mouse model. Nonetheless, these programmable nucleases cannot cause particular nucleotide changes in mtDNA nor be applied to homoplasmic mtDNA mutations due to the potential harmful destruction of all mtDNA copies (Stewart and Chinnery, 2015).
A safer gene therapy reliant on base editing was recently developed by Mok et al. (Mok et al., 2020). They successfully engineered an interbacterial cytidine deaminase toxin, called DddA, to split into non-toxic halves that could be activated upon contact with mtDNA. Such base editors consist of a catalytically inactive Cas9 protein conjugated to a bacterial deaminase and a single guide RNA (sgRNA) to facilitate the single-nucleotide conversion via deamination reaction. DddA- base editors may catalyze the programmable transformation of C•G-to-T•A with high target specificity enabling the precise manipulation of human mtDNA without inducing double-strand cleavage. Collectively, these promising tools exhibit preclinical evidence for a robust shift in mtDNA heteroplasmy. However, their translation into clinics is still pending, owing to the lack of efficient delivery of nucleic acids to mitochondria (Gammage et al., 2018a, Gammage et al., 2018b).
7.4. Gene therapy
Until now, there are no successful therapies available for mitochondrial diseases. Treatment is primarily symptomatic and does not significantly change disease progression (Tashiro et al., 2018). One of the proposed alternative therapeutic approaches is gene therapy, which involves transferring normal genetic materials to targeted cells to correct congenital disabilities and, hence, prevent or even treat diseases (David and Peebles, 2008).
LHON, caused by abnormalities in the mitochondrial encoded MT-ND4 gene, is the only primary mitochondrial disease currently undergoing active clinical gene therapy trials (Slone and Huang, 2020). Nearly 70% of patients with LHON carry pathogenic variants in the mtDNA gene encoding subunit 4 of complex IV (MT-ND4) (Tashiro et al., 2018). Multiple trials are currently investigating the treatment of LHON using AAV and MT-ND4 coding sequences that have been modified to carry a mitochondrial targeting sequence (Slone and Huang, 2020). This helps restore ATP production deficiencies in mutant ND4 cells by re-expressing the ND4 gene (Tashiro et al., 2018). In 2011, Yang et al. performed the world's first LHON gene therapy study by injecting 0.05 mL of AAV2-ND4 into the vitreous cavity of one eye for nine patients. After this treatment, systemic examinations and visual function checks were carried out over 36 months to determine the safety and efficiency of gene therapy (Yang et al., 2016, Zhang et al., 2017). One patient received an AAV2-ND4 injection in their second eye a year after gene therapy (Yang et al., 2016). After the 36-month follow-up period, no serious adverse events in the eye or the rest of the body were found in any nine patients (Yang et al., 2016, Zhang et al., 2017). Several measurements were used, including best-corrected visual acuity (BCVA), which showed that six of the nine patients improved their vision compared to before treatment. Meanwhile, visual field tests showed seven of the nine patients showed progress compared to baseline measurements. In terms of retinal nerve fiber layer thickness and Visual Evoked Potential (VEP), all patients showed improvements compared to baseline measurements recorded before treatment (Zhang et al., 2017). Another study was performed in 2014, in which five patients with LHON who had the m.11778G > A mutation were treated with a unilateral intravitreal injection of AAV2-P1ND4-v2. Three individuals showed no tangible improvement in visual acuity, while two cases showed higher visual acuity. There were no substantial side effects (Feuer et al., 2016). Another study was done by Yu et al., which showed that using AAV containing mitochondrial targeting sequence (MTS) to deliver the human ND4 into the eyes of rodents helped to prevent visual loss and atrophy of the optic nerve induced by the mutant ND4 allele (Yu et al., 2012). These studies have shown that gene therapy as a potential treatment for LHON may be a promising avenue of exploration for future mitochondrial disease treatments.
In-utero gene therapy (IUGT) or fetal gene therapy is a sub-branch of gene therapy that hopes to prevent early, irreversible and fatal pathogenic changes in inherited genetic diseases (Rashnonejad et al., 2019). Potential genetic therapeutic agents can be delivered to the fetus either into the umbilical vein in the womb or via direct injection into the fetal organs. Both approaches assume that treating fetal life could prevent or mitigate irreversible pathological mutations linked with rare diseases and enhance clinical outcomes compared to postnatal treatments (Bottani et al., 2020). The advantages of IUGT may overcome limitations in postnatal gene therapy, including small fetal size, access to a large number of proliferative stem/progenitor cells in different organs and minimizing disease complexities before birth by treating irreversible pathological changes (Peranteau and Flake, 2020). Although fetal gene therapy might be beneficial for early-onset primary mitochondrial dysfunction diseases, it is still in its early stages.
One of the earliest mutations in mitochondria before birth was found in SURF1-associated Leigh syndrome. This gene is responsible for the OXPHOS process, and evidence suggests that a mutation in the SURF1 function may lead to metabolic impairments in neural progenitor cells (NPCs), where the glycolytic state cannot be switched to OXPHOS metabolism, resulting in abnormal proliferation and insufficient support for neuronal morphogenesis (Inak et al., 2019). Similar neuronal defects were detected in the SURF1 knocked-down pig model (Quadalti et al., 2018), suggesting that OXPHOS defects may impair the NPC cellular metabolism in early development, triggering neurological phenotypes. This example supports the importance of prenatal intervention for infants as a crucial treatment strategy for mitochondrial diseases (Bottani et al., 2020).
Due to the lack of PMD-specific treatments, fetal therapeutic approaches and curative strategies proposed for other genetic diseases should be considered (Bottani et al., 2020). For instance, recent research showed that introducing AAV serotype-9 (AAV9)-EGFP to spinal muscular atrophy (SMA) mouse embryos through IU-intracerebroventricular injections led to overexpression of EGFP proteins in different parts of the CNS, producing more transduced neural stem cells. Mouse fetuses received a single IU-intracerebroventricular injection of a single-stranded (ss) or self-complementary (SC) AAV9-SMA therapeutic vectors, leading to an improved muscle pathology and motor neuron survival (Rashnonejad et al., 2019). Learning from such examples sheds light on fetal therapy, yet, more work remains in this area. Ethical issues and parental and fetal safety must be considered before clinical trials with fetal gene therapy can be initiated in human pregnancies (Bottani et al., 2020).
7.5. Mitochondrial-targeted nanomedicine
Nano-medical interventions for clinical trial innovation may assist in accelerating the clinical translation of a successful gene editing therapy to overcome mitochondrial diseases. In support of this proposal, lipid-based nanoparticles (NPs) have been used to deliver two COVID-19 vaccines accelerating their clinical trials and, consequently, their approval by the FDA (Alsudir et al., 2021). The application of nanotechnology represents a breakthrough in battling the COVID-19 pandemic in its early stages. Similarly, tailoring mitochondrial-targeting NPs for the selective delivery of nucleic acids could accelerate the translation of gene editing therapy into clinics and promote the development of a successful personalized nanomedicine. Hyperpolarization and hydrophobicity of the mitochondrial membrane might contribute to tailoring the mitochondrial-targeting NPs by moderating the degree of hydrophobicity and charge density of the NPs and governing their size and shape. Developing a library of NPs with various such properties can help discover the optimal/ideal mitochondrial-targeting nanoplatforms.
There are two developed mitochondrial targeting routes; passive and active. The passive route is based upon the negative potential of the mitochondrial membrane that permits the design/fabrication of positively charged NPs to increase cellular uptake. Contrarily, the active route involves decorating the NP surface with mitochondrial specific ligands such as delocalized lipophilic cations (DLCs), including triphenylphosphonium (TPP+) (Neuzil et al., 2013), tetramethylrhodamine-5-isothiocyanate (Zhang et al., 2018), cationic heterocycles (Hickey et al., 2008), and dequalinium (DQA) (Mallick et al., 2019). The efficiency of these DLCs in delivering large cargoes is highly dependent on the size and charge density of the conjugates (Lu et al., 2016). Other targeting ligands include mitochondrial peptides (Horton et al., 2008, Chen et al., 2013, Jean et al., 2016, Wisnovsky et al., 2016), vitamin E analogue α-tocopheryl (Cheng et al., 2013), 7-amino coumarin (Lee et al., 2018), hypericin & glycyrrhetinic acid (Han et al., 2018), aptamers such as cytochrome c (Cyt c) (Mo et al., 2014, Chen et al., 2015) that could bind cardiolipin confined to the mitochondrial inner membrane, and the “MitoLigands” (Xiao et al., 2021) (SeSe-TPP and miniCPM3 consisting of two D- and L- alternating hydrophobic naphthylalanine amino acids and three positively charged arginines (Arg)). For nuclear targeting, the most reported ligands include nuclear localization signal sequences (NLSs) (Cheng et al., 2017) such as SV40 T antigen (Lu et al., 2021), adenoviruses containing dsDNA covalently linked to a terminal protein (TP) (Al-Wassiti et al., 2021), transactivator of transcription (TAT) peptide (Sun et al., 2020), and KRRRR (Cheng et al., 2019) that plays a leading role in nucleocytoplasmic trafficking.
Significant efforts have been made to develop mitochondrial targeting NPs, including polymeric, lipid, organic, or inorganic NPs, summarized in Table 1. Nevertheless, none of these NPs has been utilized to deliver the genetic materials required for gene therapy. Thereby, there is an urgent need to investigate the optimal mitochondrial-targeting nanoplatforms to stabilize and provide the therapeutic gene in a simple, safe and affordable manner.
Table 1.
A summary of mitochondrial-targeting NPs along with their targeting ligands.
| Mitochondrial- Targeting NPs | Targeting route | Targeting ligand | Ref. |
|---|---|---|---|
| Poly-L-lysine self-assembling NPs | Active | Aptamer Cyt c | (Chen et al., 2017a, Chen et al., 2017b) |
| Poly (amidoamine) (PAMAM) dendrimer NPs | Active | TPP+ | (Biswas et al., 2012) |
| Poly (D, L-lactic-co-glycolic acid)-block-poly ethylene glycol (PLGA-PEG NPs) | Active | TPP+ | (Marrache and Dhar, 2012, Marrache et al., 2013) |
| Poly (ε-caprolactone) (PCL) NPs | Active | TPP+ | (Cho et al., 2015) |
| Poly (ethylene oxide)-block-poly (propylene oxide)-block-poly (ethylene oxide), PEO-PPO-PEO NPs | Active | TPP+ | (Wang et al., 2020) |
| PEG- Polydopamine (PDA) NPs | Active | TPP+ | (Li et al., 2017) |
| Hyaluronic acid-D-α-tocopherol succinate (HA-TS) NPs | Active | TPP+ | (Lee and Cho, 2019) |
| Glycol chitosan | Active | DQA | (Mallick et al., 2019) |
| PEG2000- 1, 2-Distearoyl-sn-glycero-3 phosphoethanolamine (DSPE)- Liposomes | Active | DQA | (Li et al., 2013) |
| Polypyrrole-silica (Py-SiO2) hybrid NPs | Active | TPP+ | (Xu et al., 2020) |
| SiO2-PDA-Fe3O4 | Active | TPP+ | (Guo et al., 2016) |
| Gold-Platinum bimetallic (Au-Pt) NPs | Active | TPP+ | (Song et al., 2017) |
| Au nanostars | Active | TPP+ | (Chen et al., 2017a, Chen et al., 2017b) |
| Ceria (CeO2) NPs | Active | TPP+ | (Kwon et al., 2016) |
| Zirconia (ZrO2) NPs | Active | TPP+ | (Chen et al., 2018) |
| Nitrogen-doped graphene quantum dots | Active | TPP+ | (Guo et al., 2017) |
7.6. Future directions
Several treatments show a great promise for primary mitochondrial disorders, yet, only a few of them have undergone controlled clinical trials or remain inconclusive (Garone and Viscomi, 2018). Currently, one medicine, in particular, idebenone, offers enough scientific evidence for treating mitochondrial dysfunctions, in addition to its ability to treat acute vision loss in LHON (Klopstock et al., 2011, Rudolph et al., 2013, Singh et al., 2021, Tinker et al., 2021). However, there is continuous interest in developing potential therapeutic alternatives for mitochondrial diseases. Significant progress has been made in the fundamental understanding of mitochondrial biology and the ability to detect genetic abnormalities in most patients (Russell et al., 2020). The use of gene therapy to correct heteroplasmic mtDNA defects has been investigated for more than 25 years and is close to becoming a reality in its application clinically (Murphy and Hartley, 2018).
Meanwhile, the ability to conduct meaningful clinical trials to validate new treatments increases. The rarity of primary mitochondrial disorders has impacted the conduction of successful clinical trials, design and funding (Augustine et al., 2013). However, significant breakthroughs in developing new therapeutic approaches will continue in the coming decade through further advances in gene therapy and screening assays to improve mitochondrial function (Garone and Viscomi, 2018). Alongside the innovative approaches to clinical trial design, the development of new technologies and the creation of virtual controls will herald a new era, particularly in personalized medicine, for patients with mitochondrial diseases (Garone and Viscomi, 2018).
8. Conclusion
Mitochondrial diseases affecting newborns and the elderly can be distinguished by their clinical, biochemical and genetic complexities. Recently, advanced technology has enhanced the understanding of mitochondrial disorders' manifestations, diagnosis, prognosis, management and prevention, and determined the underlying genetic mutations of the complex clinical phenotypes associated with such dysfunctions. Several essential mitochondrial genes have been identified, where they could be targeted to improve mitochondrial function. Although no medicine has yet been developed specifically to target the mitochondrion, palliative therapy is usually considered instead. More viable cures should be evaluated through more precise medical strategies that target an individual's genetic variations. Developing new therapeutic approaches and the increasing number of clinical trials could hold a great future in treating mitochondrial diseases.
Funding
This work was supported by the National Industrial Development and Logistics Program (NIDLP) through the Health Initiative and the Technology Leader Program Initiative, project numbers 20-0103 and 20-0051.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ahmed S.T., Craven L., Russell O.M., Turnbull D.M., Vincent A.E. Diagnosis and Treatment of Mitochondrial Myopathies. Neurotherapeutics : J. Am. Soc. Exp. NeuroTherapeut. 2018;15(4):943–953. doi: 10.1007/s13311-018-00674-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Enezi M., Al-Saleh H., Nasser M. Mitochondrial disorders with significant ophthalmic manifestations. Middle East Afr. J. Ophthalmol. 2008;15:81–86. doi: 10.4103/0974-9233.51998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Wassiti H.A., Thomas D.R., Wagstaff K.M., Fabb S.A., Jans D.A., Johnston A.P., Pouton C.W. Adenovirus Terminal Protein Contains a Bipartite Nuclear Localisation Signal Essential for Its Import into the Nucleus. Int. J. Mol. Sci. 2021;22(7):3310. doi: 10.3390/ijms22073310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alahmad A., Muhammad H., Pyle A., Albash B., McFarland R., Taylor R. Mitochondrial disorders in the Arab Middle East population: the impact of next generation sequencing on the genetic diagnosis. J. Biochem. Clin. Genet. 2019:54–64. doi: 10.24911/JBCGenetics/183-1548325196. [DOI] [Google Scholar]
- Alexeyev M., Shokolenko I., Wilson G., et al. The maintenance of mitochondrial DNA integrity–critical analysis and update. Cold Spring Harbor Perspect. Biol. 2013;5 doi: 10.1101/cshperspect.a012641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almannai M., El-Hattab A.W. Nitric Oxide Deficiency in Mitochondrial Disorders: The Utility of Arginine and Citrulline. Front. Mol. Neurosci. 2021;14 doi: 10.3389/fnmol.2021.682780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alston C.L., Rocha M.C., Lax N.Z., et al. The genetics and pathology of mitochondrial disease. J. Pathol. 2017;241:236–250. doi: 10.1002/path.4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsudir S.A., Almalik A., Alhasan A.H. Catalogue of self-targeting nano-medical inventions to accelerate clinical trials. Biomater. Sci. 2021;9(11):3898–3910. doi: 10.1039/d1bm00235j. [DOI] [PubMed] [Google Scholar]
- An P., Wei L.-L., Zhao S., Sverdlov D.Y., Vaid K.A., Miyamoto M., Kuramitsu K., Lai M., Popov Y.V. Hepatocyte mitochondria-derived danger signals directly activate hepatic stellate cells and drive progression of liver fibrosis. Nat. Commun. 2020;11(1) doi: 10.1038/s41467-020-16092-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine E.F., Adams H.R., Mink J.W. Clinical trials in rare disease: challenges and opportunities. J. Child Neurol. 2013;28(9):1142–1150. doi: 10.1177/0883073813495959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacman S.R., Kauppila J.H.K., Pereira C.V., Nissanka N., Miranda M., Pinto M., Williams S.L., Larsson N.-G., Stewart J.B., Moraes C.T. MitoTALEN reduces mutant mtDNA load and restores tRNA(Ala) levels in a mouse model of heteroplasmic mtDNA mutation. Nat. Med. 2018;24(11):1696–1700. doi: 10.1038/s41591-018-0166-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baertling F., Mayatepek E., Distelmaier F. Hypertrichosis in presymptomatic mitochondrial disease. J. Inherit. Metab. Dis. 2013;36(6):1081–1082. doi: 10.1007/s10545-013-9593-3. [DOI] [PubMed] [Google Scholar]
- Ballout R.A., Al Alam C., Bonnen P.E., Huemer M., El-Hattab A.W., Shbarou R. FBXL4-Related Mitochondrial DNA Depletion Syndrome 13 (MTDPS13): A Case Report With a Comprehensive Mutation Review. Front Genet. 2019;10 doi: 10.3389/fgene.2019.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baruffini E., Dallabona C., Invernizzi F., Yarham J.W., Melchionda L., Blakely E.L., Lamantea E., Donnini C., Santra S., Vijayaraghavan S., Roper H.P., Burlina A., Kopajtich R., Walther A., Strom T.M., Haack T.B., Prokisch H., Taylor R.W., Ferrero I., Zeviani M., Ghezzi D. MTO1 mutations are associated with hypertrophic cardiomyopathy and lactic acidosis and cause respiratory chain deficiency in humans and yeast. Hum. Mutat. 2013;34(11):1501–1509. doi: 10.1002/humu.22393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindu L.H., Reddy P.P. Genetics of aminoglycoside-induced and prelingual non-syndromic mitochondrial hearing impairment: a review. Int. J. Audiol. 2008;47:702–707. doi: 10.1080/14992020802215862. [DOI] [PubMed] [Google Scholar]
- Biswas S., Dodwadkar N.S., Piroyan A., Torchilin V.P. Surface conjugation of triphenylphosphonium to target poly(amidoamine) dendrimers to mitochondria. Biomaterials. 2012;33(18):4773–4782. doi: 10.1016/j.biomaterials.2012.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boenzi S., Diodato D. Biomarkers for mitochondrial energy metabolism diseases. Essays Biochem. 2018;62:443–454. doi: 10.1042/ebc20170111. [DOI] [PubMed] [Google Scholar]
- Bottani E., Lamperti C., Prigione A., Tiranti V., Persico N., Brunetti D. Therapeutic approaches to treat mitochondrial diseases:“one-size-fits-all” and “precision medicine” strategies. Pharmaceutics. 2020;12(11):1083. doi: 10.3390/pharmaceutics12111083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bough K.J., Wetherington J., Hassel B., Pare J.F., Gawryluk J.W., Greene J.G., Shaw R., Smith Y., Geiger J.D., Dingledine R.J. Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann. Neurol.: Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 2006;60(2):223–235. doi: 10.1002/ana.20899. [DOI] [PubMed] [Google Scholar]
- Bourgeron T., Rustin P., Chretien D., Birch-Machin M., Bourgeois M., Viegas-Péquignot E., Munnich A., Rötig A. Mutation of a nuclear succinate dehydrogenase gene results in mitochondrial respiratory chain deficiency. Nat. Genet. 1995;11(2):144–149. doi: 10.1038/ng1095-144. [DOI] [PubMed] [Google Scholar]
- Cavalcante G.C., Magalhães L., Ribeiro-dos-Santos Â., Vidal A.F. Mitochondrial Epigenetics: Non-Coding RNAs as a Novel Layer of Complexity. Int. J. Mol. Sci. 2020;21(5):1838. doi: 10.3390/ijms21051838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan D.C. Fusion and fission: interlinked processes critical for mitochondrial health. Annu. Rev. Genet. 2012;46(1):265–287. doi: 10.1146/annurev-genet-110410-132529. [DOI] [PubMed] [Google Scholar]
- Chen H., Tian J., Liu D., et al. Dual aptamer modified dendrigraft poly-l-lysine nanoparticles for overcoming multi-drug resistance through mitochondrial targeting. J. Mater. Chem. B. 2017;5:972–979. doi: 10.1039/c6tb02714h. [DOI] [PubMed] [Google Scholar]
- Chen S., Lei Q., Qiu W.-X., Liu L.-H., Zheng D.-W., Fan J.-X., Rong L., Sun Y.-X., Zhang X.-Z. Mitochondria-targeting “Nanoheater” for enhanced photothermal/chemo-therapy. Biomaterials. 2017;117:92–104. doi: 10.1016/j.biomaterials.2016.11.056. [DOI] [PubMed] [Google Scholar]
- Chen T.-T., Tian X., Liu C.-L., Ge J., Chu X., Li Y. Fluorescence activation imaging of cytochrome c released from mitochondria using aptameric nanosensor. J. Am. Chem. Soc. 2015;137(2):982–989. doi: 10.1021/ja511988w. [DOI] [PubMed] [Google Scholar]
- Chen W.-H., Chen J.-X., Cheng H., Chen C.-S., Yang J., Xu X.-D., Wang Y., Zhuo R.-X., Zhang X.-Z. A new anti-cancer strategy of damaging mitochondria by pro-apoptotic peptide functionalized gold nanoparticles. Chem. Commun. (Camb.) 2013;49(57):6403. doi: 10.1039/c3cc43283a. [DOI] [PubMed] [Google Scholar]
- Chen X., Fu C., Wang Y., Wu Q., Meng X., Xu K.e. Mitochondria-targeting nanoparticles for enhanced microwave ablation of cancer. Nanoscale. 2018;10(33):15677–15685. doi: 10.1039/c8nr03927e. [DOI] [PubMed] [Google Scholar]
- Cheng G., Zielonka J., McAllister D.M., Mackinnon A.C., Joseph J., Dwinell M.B., Kalyanaraman B. Mitochondria-targeted vitamin E analogs inhibit breast cancer cell energy metabolism and promote cell death. BMC cancer. 2013;13(1) doi: 10.1186/1471-2407-13-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Sun C., Liu R., Yang J., Dai J., Zhai T., Lou X., Xia F. A Multifunctional Peptide-Conjugated AIEgen for Efficient and Sequential Targeted Gene Delivery into the Nucleus. Angew. Chem. Int. Ed. Engl. 2019;58(15):5049–5053. doi: 10.1002/anie.201901527. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Sun C., Ou X., Liu B., Lou X., Xia F. Dual-targeted peptide-conjugated multifunctional fluorescent probe with AIEgen for efficient nucleus-specific imaging and long-term tracing of cancer cells. Chem. Sci. 2017;8(6):4571–4578. doi: 10.1039/c7sc00402h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnery P.F., Elliott H.R., Hudson G., Samuels D.C., Relton C.L. Epigenetics, epidemiology and mitochondrial DNA diseases. Int. J. Epidemiol. 2012;41(1):177–187. doi: 10.1093/ije/dyr232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnery P.F., Johnson M.A., Wardell T.M., et al. The epidemiology of pathogenic mitochondrial DNA mutations. Ann. Neurol. 2000;48:188–193. [PubMed] [Google Scholar]
- Chipman L.B., Pasquinelli A.E. miRNA Targeting: Growing beyond the Seed. Trends Genet. 2019;35:215–222. doi: 10.1016/j.tig.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho D.Y., Cho H., Kwon K., Yu M., Lee E., Huh K.M., Lee D.H., Kang H.C. Triphenylphosphonium-conjugated poly (ε-caprolactone)-based self-assembled nanostructures as nanosized drugs and drug delivery carriers for mitochondria-targeting synergistic anticancer drug delivery. Adv. Funct. Mater. 2015;25(34):5479–5491. doi: 10.1002/adfm.201501422. [DOI] [Google Scholar]
- Chow T.-F., Mankaruos M., Scorilas A., Youssef Y., Girgis A., Mossad S., Metias S., Rofael Y., Honey R.J., Stewart R., Pace K.T., Yousef G.M. The miR-17-92 Cluster is Over Expressed in and Has an Oncogenic Effect on Renal Cell Carcinoma. J. Urol. 2010;183(2):743–751. doi: 10.1016/j.juro.2009.09.086. [DOI] [PubMed] [Google Scholar]
- D’Aquila P., Bellizzi D., Passarino G. Mitochondria in health, aging and diseases: the epigenetic perspective. Biogerontology. 2015;16(5):569–585. doi: 10.1007/s10522-015-9562-3. [DOI] [PubMed] [Google Scholar]
- David A.L., Peebles D. Gene therapy for the fetus: is there a future? Best Practice Res. Clin. Obstet. Gynaecol. 2008;22(1):203–218. doi: 10.1016/j.bpobgyn.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Debray F.-G., Lambert M., Chevalier I., Robitaille Y., Decarie J.-C., Shoubridge E.A., Robinson B.H., Mitchell G.A. Long-term outcome and clinical spectrum of 73 pediatric patients with mitochondrial diseases. Pediatrics. 2007;119(4):722–733. doi: 10.1542/peds.2006-1866. [DOI] [PubMed] [Google Scholar]
- Di Giovanni S., Mirabella M., Spinazzola A., Crociani P., Silvestri G., Broccolini A., Tonali P., Di Mauro S., Servidei S. Coenzyme Q10 reverses pathological phenotype and reduces apoptosis in familial CoQ10 deficiency. Neurology. 2001;57(3):515–518. doi: 10.1212/wnl.57.3.515. [DOI] [PubMed] [Google Scholar]
- Di Stadio A., Pegoraro V., Giaretta L., Dipietro L., Marozzo R., Angelini C. Hearing impairment in MELAS: new prospective in clinical use of microRNA, a systematic review. Orphanet J. Rare Dis. 2018;13(1) doi: 10.1186/s13023-018-0770-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmock D.P., Lawlor M.W. Presentation and Diagnostic Evaluation of Mitochondrial Disease. Pediatr. Clin. North Am. 2017;64(1):161–171. doi: 10.1016/j.pcl.2016.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte F.V., Palmeira C.M., Rolo A.P. The Role of microRNAs in Mitochondria: Small Players Acting Wide. Genes (Basel). 2014;5:865–886. doi: 10.3390/genes5040865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hattab A.W., Hsu J.W., Emrick L.T., Wong L.-J., Craigen W.J., Jahoor F., Scaglia F. Restoration of impaired nitric oxide production in MELAS syndrome with citrulline and arginine supplementation. Mol. Genet. Metab. 2012;105(4):607–614. doi: 10.1016/j.ymgme.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euro L., Konovalova S., Asin-Cayuela J., Tulinius Már, Griffin H., Horvath R., Taylor R.W., Chinnery P.F., Schara U., Thorburn D.R., Suomalainen A., Chihade J., Tyynismaa H. Structural modeling of tissue-specific mitochondrial alanyl-tRNA synthetase (AARS2) defects predicts differential effects on aminoacylation. Front. Genet. 2015;6 doi: 10.3389/fgene.2015.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Moreira D., Ugalde C., Smeets R., Rodenburg R.J.T., Lopez-Laso E., Ruiz-Falco M.L., Briones P., Martin M.A., Smeitink J.A.M., Arenas J. X-linked NDUFA1 gene mutations associated with mitochondrial encephalomyopathy. Ann. Neurol. 2007;61(1):73–83. doi: 10.1002/ana.21036. [DOI] [PubMed] [Google Scholar]
- Ferreira C.R., Whitehead M.T., Leon E. Biotin-thiamine responsive basal ganglia disease: Identification of a pyruvate peak on brain spectroscopy, novel mutation in SLC19A3, and calculation of prevalence based on allele frequencies from aggregated next-generation sequencing data. Am. J. Medical Genet. Part A. 2017;173(6):1502–1513. doi: 10.1002/ajmg.a.38189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuer W.J., Schiffman J.C., Davis J.L., Porciatti V., Gonzalez P., Koilkonda R.D., Yuan H., Lalwani A., Lam B.L., Guy J. Gene Therapy for Leber Hereditary Optic Neuropathy. Ophthalmology. 2016;123(3):558–570. doi: 10.1016/j.ophtha.2015.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finsterer J. Central nervous system manifestations of mitochondrial disorders. Acta Neurol. Scand. 2006;114(4):217–238. doi: 10.1111/j.1600-0404.2006.00671.x. [DOI] [PubMed] [Google Scholar]
- Finsterer J. Inherited mitochondrial disorders. Adv. Exp. Med. Biol. 2012;942:187–213. doi: 10.1007/978-94-007-2869-1_8. [DOI] [PubMed] [Google Scholar]
- Finsterer J., Frank M. Prevalence of neoplasms in definite and probable mitochondrial disorders. Mitochondrion. 2016;29:31–34. doi: 10.1016/j.mito.2016.05.002. [DOI] [PubMed] [Google Scholar]
- Finsterer J., Frank M. Gastrointestinal manifestations of mitochondrial disorders: a systematic review. Therapeut. Adv. Gastroenterol. 2017;10(1):142–154. doi: 10.1177/1756283X16666806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finsterer J., Scorza F.A. Renal manifestations of primary mitochondrial disorders. Biomed. Reports. 2017;6:487–494. doi: 10.3892/br.2017.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finsterer J., Zarrouk-Mahjoub S. Biomarkers for Detecting Mitochondrial Disorders. J. Clin. Med. 2018;7(2):16. doi: 10.3390/jcm7020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier A.E., Thorburn D.R., Compton A.G. Mitochondrial energy generation disorders: genes, mechanisms, and clues to pathology. J. Biol. Chem. 2019;294(14):5386–5395. doi: 10.1074/jbc.R117.809194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammage P.A., Moraes C.T., Minczuk M. Mitochondrial Genome Engineering: The Revolution May Not Be CRISPR-Ized. Trends Genet. : TIG. 2018;34(2):101–110. doi: 10.1016/j.tig.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammage P.A., Viscomi C., Simard M.-L., Costa A.S.H., Gaude E., Powell C.A., Van Haute L., McCann B.J., Rebelo-Guiomar P., Cerutti R., Zhang L., Rebar E.J., Zeviani M., Frezza C., Stewart J.B., Minczuk M. Genome editing in mitochondria corrects a pathogenic mtDNA mutation in vivo. Nat. Med. 2018;24(11):1691–1695. doi: 10.1038/s41591-018-0165-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garone C., Viscomi C. Towards a therapy for mitochondrial disease: an update. Biochem. Soc. Trans. 2018;46:1247–1261. doi: 10.1042/BST20180134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerards M., Sallevelt S.C.E.H., Smeets H.J.M. Leigh syndrome: Resolving the clinical and genetic heterogeneity paves the way for treatment options. Mol. Genet. Metab. 2016;117(3):300–312. doi: 10.1016/j.ymgme.2015.12.004. [DOI] [PubMed] [Google Scholar]
- Germain N., Dessein A.-F., Vienne J.-C., Dobbelaere D., Mention K., Joncquel M., Dekiouk S., Laine W., Kluza J., Marchetti P. First-line Screening of OXPHOS Deficiencies Using Microscale Oxygraphy in Human Skin Fibroblasts: A Preliminary Study. Int. J. Med. Sci. 2019;16(7):931–938. doi: 10.7150/ijms.32413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman G.S., Chinnery P.F., DiMauro S., Hirano M., Koga Y., McFarland R., Suomalainen A., Thorburn D.R., Zeviani M., Turnbull D.M. Mitochondrial diseases. Mitochondrial Diseases. Nature Rev.. Dis. Primers. 2016;2(1) doi: 10.1038/nrdp.2016.80. [DOI] [PubMed] [Google Scholar]
- Gorman G.S., Schaefer A.M., Ng Y., et al. Prevalence of nuclear and mitochondrial DNA mutations related to adult mitochondrial disease. Ann. Neurol. 2015;77:753–759. doi: 10.1002/ana.24362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govers L.P., Toka H.R., Hariri A., Walsh S.B., Bockenhauer D. Mitochondrial DNA mutations in renal disease: an overview. Pediatric Nephrol. (Berlin, Germany). 2021;36(1):9–17. doi: 10.1007/s00467-019-04404-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gredilla R. DNA damage and base excision repair in mitochondria and their role in aging. J. Aging Res. 2010;2011:1–9. doi: 10.4061/2011/257093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D.R., Galluzzi L., Kroemer G. Cell biology. Metabolic control of cell death. Science (New York, N.Y.). 2014;345:1250256. doi: 10.1126/science.1250256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan M.-X., Yan Q., Li X., Bykhovskaya Y., Gallo-Teran J., Hajek P., Umeda N., Zhao H., Garrido G., Mengesha E., Suzuki T., Castillo I.D., Peters J.L., Li R., Qian Y., Wang X., Ballana E., Shohat M., Lu J., Estivill X., Watanabe K., Fischel-Ghodsian N. Mutation in TRMU related to transfer RNA modification modulates the phenotypic expression of the deafness-associated mitochondrial 12S ribosomal RNA mutations. Am. J. Hum Genet. 2006;79(2):291–302. doi: 10.1086/506389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M., Xiang H.-J., Wang Y., Zhang Q.-L., An L., Yang S.-P., Ma Y., Wang Y., Liu J.-G. Ruthenium nitrosyl functionalized graphene quantum dots as an efficient nanoplatform for NIR-light-controlled and mitochondria-targeted delivery of nitric oxide combined with photothermal therapy. Chem. Commun. (Camb.) 2017;53(22):3253–3256. doi: 10.1039/c7cc00670e. [DOI] [PubMed] [Google Scholar]
- Guo R., Peng H., Tian Y.e., Shen S., Yang W. Mitochondria-Targeting Magnetic Composite Nanoparticles for Enhanced Phototherapy of Cancer. Small (Weinheim an der Bergstrasse, Germany). 2016;12(33):4541–4552. doi: 10.1002/smll.201601094. [DOI] [PubMed] [Google Scholar]
- Han C., Zhang C., Ma T., Zhang C., Luo J., Xu X., Zhao H., Chen Y., Kong L. Hypericin-functionalized graphene oxide for enhanced mitochondria-targeting and synergistic anticancer effect. Acta Biomater. 2018;77:268–281. doi: 10.1016/j.actbio.2018.07.018. [DOI] [PubMed] [Google Scholar]
- Heyn H., Li N., Ferreira H.J., Moran S., Pisano D.G., Gomez A., Diez J., Sanchez-Mut J.V., Setien F., Carmona F.J., Puca A.A., Sayols S., Pujana M.A., Serra-Musach J., Iglesias-Platas I., Formiga F., Fernandez A.F., Fraga M.F., Heath S.C., Valencia A., Gut I.G., Wang J., Esteller M. Distinct DNA methylomes of newborns and centenarians. Proc. Natl. Acad. Sci. 2012;109(26):10522–10527. doi: 10.1073/pnas.1120658109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey J.L., Ruhayel R.A., Barnard P.J., Baker M.V., Berners-Price S.J., Filipovska A. Mitochondria-targeted chemotherapeutics: the rational design of gold(I) N-heterocyclic carbene complexes that are selectively toxic to cancer cells and target protein selenols in preference to thiols. J. Am. Chem. Soc. 2008;130(38):12570–12571. doi: 10.1021/ja804027j. [DOI] [PubMed] [Google Scholar]
- Holloway G.P. Nutrition and Training Influences on the Regulation of Mitochondrial Adenosine Diphosphate Sensitivity and Bioenergetics. Sports Med. (Auckland, NZ). 2017;47(S1):13–21. doi: 10.1007/s40279-017-0693-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt I.J., Harding A.E., Cooper J.M., Schapira A.H.V., Toscano A., Clark J.B., Morgan-Hughes J.A. Mitochondrial myopathies: clinical and biochemical features of 30 patients with major deletions of muscle mitochondrial DNA. Ann. Neurol. 1989;26(6):699–708. doi: 10.1002/ana.410260603. [DOI] [PubMed] [Google Scholar]
- Horton K.L., Stewart K.M., Fonseca S.B., Guo Q., Kelley S.O. Mitochondria-Penetrating Peptides. Chem. Biol. 2008;15(4):375–382. doi: 10.1016/j.chembiol.2008.03.015. [DOI] [PubMed] [Google Scholar]
- Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14(10):R115. doi: 10.1186/gb-2013-14-10-r115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inak G., Rybak-Wolf A., Lisowski P., et al. SURF1 mutations causative of Leigh syndrome impair human neurogenesis. bioRxiv. 2019:551390. doi: 10.1101/551390. [DOI] [Google Scholar]
- Jean S.R., Ahmed M., Lei E.K., Wisnovsky S.P., Kelley S.O. Peptide-Mediated Delivery of Chemical Probes and Therapeutics to Mitochondria. Acc. Chem. Res. 2016;49(9):1893–1902. doi: 10.1021/acs.accounts.6b00277. [DOI] [PubMed] [Google Scholar]
- Jin B., Robertson K.D. DNA methyltransferases, DNA damage repair, and cancer. Adv. Exp. Med. Biol. 2013;754:3–29. doi: 10.1007/978-1-4419-9967-2_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan V.E., Serbinova E.A., Koynova G.M., Kitanova S.A., Tyurin V.A., Stoytchev T.S., Quinn P.J., Packer L. Antioxidant action of ubiquinol homologues with different isoprenoid chain length in biomembranes. Free Radical Biol. Med. 1990;9(2):117–126. doi: 10.1016/0891-5849(90)90114-x. [DOI] [PubMed] [Google Scholar]
- Kang C., Li Ji L.i. Role of PGC-1α signaling in skeletal muscle health and disease. Ann. N. Y. Acad. Sci. 2012;1271(1):110–117. doi: 10.1111/j.1749-6632.2012.06738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanungo S., Morton J., Neelakantan M., Ching K., Saeedian J., Goldstein A. Mitochondrial disorders. Mitochondrial Disorders. Ann. Transl. Med. 2018;6(24):475. doi: 10.21037/atm.2018.12.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopstock T., Klopstock B., Prokisch H. Mitochondrial replacement approaches: challenges for clinical implementation. Genome Med. 2016;8:126. doi: 10.1186/s13073-016-0380-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopstock T., Yu-Wai-Man P., Dimitriadis K., et al. A randomized placebo-controlled trial of idebenone in Leber’s hereditary optic neuropathy. Brain. 2011;134:2677–2686. doi: 10.1093/brain/awr170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koene S., Jansen M., Verhaak C.M., De Vrueh R.LA., De Groot I.JM., Smeitink J.AM. Towards the harmonization of outcome measures in children with mitochondrial disorders. Dev. Med. Child Neurol. 2013;55:698–706. doi: 10.1111/dmcn.12119. [DOI] [PubMed] [Google Scholar]
- Kwon H.J., Cha M.-Y., Kim D., Kim D.K., Soh M., Shin K., Hyeon T., Mook-Jung I. Mitochondria-Targeting Ceria Nanoparticles as Antioxidants for Alzheimer's Disease. ACS Nano. 2016;10(2):2860–2870. doi: 10.1021/acsnano.5b08045. [DOI] [PubMed] [Google Scholar]
- La Morgia C., Maresca A., Caporali L., Valentino M.L., Carelli V. Mitochondrial diseases in adults. J. Intern. Med. 2020;287(6):592–608. doi: 10.1111/joim.13064. [DOI] [PubMed] [Google Scholar]
- Landsverk M.L., Zhang V.W., Wong L.-J., Andersson H.C. A SUCLG1 mutation in a patient with mitochondrial DNA depletion and congenital anomalies. Mol. Genet. Metab. Rep. 2014;1:451–454. doi: 10.1016/j.ymgmr.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane N., Martin W.F., Raven J.A., Allen J.F. Energy, genes and evolution: introduction to an evolutionary synthesis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013;368(1622):20120253. doi: 10.1098/rstb.2012.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.H., Kim K.Y., Jin H., Baek Y.E., Choi Y., Jung S.H., Lee S.S., Bae J., Jung J.H. Self-Assembled Coumarin Nanoparticle in Aqueous Solution as Selective Mitochondrial-Targeting Drug Delivery System. ACS Appl. Mater. Interfaces. 2018;10(4):3380–3391. doi: 10.1021/acsami.7b17711. [DOI] [PubMed] [Google Scholar]
- Lee S.Y., Cho H.-J. Mitochondria Targeting and Destabilizing Hyaluronic Acid Derivative-Based Nanoparticles for the Delivery of Lapatinib to Triple-Negative Breast Cancer. Biomacromolecules. 2019;20(2):835–845. doi: 10.1021/acs.biomac.8b01449. [DOI] [PubMed] [Google Scholar]
- Li H., Slone J., Huang T. The role of mitochondrial-related nuclear genes in age-related common disease. Mitochondrion. 2020;53:38–47. doi: 10.1016/j.mito.2020.04.012. [DOI] [PubMed] [Google Scholar]
- Li N., Zhang C.-X., Wang X.-X., Zhang L., Ma X.u., Zhou J., Ju R.-J., Li X.-Y., Zhao W.-Y., Lu W.-L. Development of targeting lonidamine liposomes that circumvent drug-resistant cancer by acting on mitochondrial signaling pathways. Biomaterials. 2013;34(13):3366–3380. doi: 10.1016/j.biomaterials.2013.01.055. [DOI] [PubMed] [Google Scholar]
- Li W.-Q., Wang Z., Hao S., He H., Wan Y., Zhu C., Sun L.-P., Cheng G., Zheng S.-Y. Mitochondria-Targeting Polydopamine Nanoparticles To Deliver Doxorubicin for Overcoming Drug Resistance. ACS Appl. Mater. Interfaces. 2017;9(20):16793–16802. doi: 10.1021/acsami.7b01540. [DOI] [PubMed] [Google Scholar]
- Lieber D.S., Calvo S.E., Shanahan K., Slate N.G., Liu S., Hershman S.G., Gold N.B., Chapman B.A., Thorburn D.R., Berry G.T., Schmahmann J.D., Borowsky M.L., Mueller D.M., Sims K.B., Mootha V.K. Targeted exome sequencing of suspected mitochondrial disorders. Neurology. 2013;80(19):1762–1770. doi: 10.1212/WNL.0b013e3182918c40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenschot M., de Groot I.J.M., Koene S., Satink T., Steultjens E.M.J., Nijhuis-van der Sanden M.W.G. Everyday Activities for Children with Mitochondrial Disorder: A Retrospective Chart Review. Occupational Therapy Int. 2018;2018:1–8. doi: 10.1155/2018/5716947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock J.H., Irani N.K., Newman N.J. Neuro-ophthalmic manifestations of mitochondrial disorders and their management. Taiwan J. Ophthalmol. 2021;11:39–52. doi: 10.4103/tjo.tjo_68_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J., Wu T., Zhang B., Liu S., Song W., Qiao J., Ruan H. Types of nuclear localization signals and mechanisms of protein import into the nucleus. Cell Commun. Signaling : CCS. 2021;19(1) doi: 10.1186/s12964-021-00741-y10.21203/rs.3.rs-967554/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P., Bruno B.J., Rabenau M., Lim C.S. Delivery of drugs and macromolecules to the mitochondria for cancer therapy. J. Controlled Release : Off. J. Controll. Release Soc. 2016;240:38–51. doi: 10.1016/j.jconrel.2015.10.023. [DOI] [PubMed] [Google Scholar]
- Mallick S., Song S.J., Bae Y., Choi J.S. Self-assembled nanoparticles composed of glycol chitosan-dequalinium for mitochondria-targeted drug delivery. Int. J. Biol. Macromol. 2019;132:451–460. doi: 10.1016/j.ijbiomac.2019.03.215. [DOI] [PubMed] [Google Scholar]
- Maresca A., Zaffagnini M., Caporali L., et al. DNA methyltransferase 1 mutations and mitochondrial pathology: is mtDNA methylated? Front. Genet. 2015;6 doi: 10.3389/fgene.2015.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrache S., Dhar S. Engineering of blended nanoparticle platform for delivery of mitochondria-acting therapeutics. PNAS. 2012;109(40):16288–16293. doi: 10.1073/pnas.1210096109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrache S., Tundup S., Harn D.A., Dhar S. Ex vivo programming of dendritic cells by mitochondria-targeted nanoparticles to produce interferon-gamma for cancer immunotherapy. ACS Nano. 2013;7(8):7392–7402. doi: 10.1021/nn403158n. [DOI] [PubMed] [Google Scholar]
- Martikainen M.H., Chinnery P.F. Mitochondrial disease: mimics and chameleons. Practical neurology. 2015;15(6):424–435. doi: 10.1136/practneurol-2015-001191. [DOI] [PubMed] [Google Scholar]
- McCormick E., Place E., Falk M.J. Molecular genetic testing for mitochondrial disease: from one generation to the next. Neurotherapeutics : J. Am. Soc. Exp. NeuroTherapeut. 2013;10(2):251–261. doi: 10.1007/s13311-012-0174-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memme J.M., Erlich A.T., Phukan G., Hood D.A. Exercise and mitochondrial health. J. Physiol. 2021;599(3):803–817. doi: 10.1113/JP278853. [DOI] [PubMed] [Google Scholar]
- Meyer, J. N., M. C. Leung, J. P. Rooney, et al., 2013. Mitochondria as a target of environmental toxicants. Toxicol. Sci. : Off. J. Soc. Toxicol. 134, 1–17. 10.1093/toxsci/kft102. [DOI] [PMC free article] [PubMed]
- Mills E.L., Kelly B., O'Neill L.A.J. Mitochondria are the powerhouses of immunity. Nat. Immunol. 2017;18(5):488–498. doi: 10.1038/ni.3704. [DOI] [PubMed] [Google Scholar]
- Mo R., Jiang T., Gu Z. Enhanced anticancer efficacy by ATP-mediated liposomal drug delivery. Angew. Chem. Int. Ed. Engl. 2014;53(23):5815–5820. doi: 10.1002/anie.201400268. [DOI] [PubMed] [Google Scholar]
- Moggio M., Colombo I., Peverelli L., et al. Mitochondrial disease heterogeneity: a prognostic challenge. Acta Myologica : Myopathies Cardiomyopathies : Off. J. Mediterranean Soc. Myol. 2014;33:86–93. [PMC free article] [PubMed] [Google Scholar]
- Mok B.Y., de Moraes M.H., Zeng J., Bosch D.E., Kotrys A.V., Raguram A., Hsu F., Radey M.C., Peterson S.B., Mootha V.K., Mougous J.D., Liu D.R. A bacterial cytidine deaminase toxin enables CRISPR-free mitochondrial base editing. Nature. 2020;583(7817):631–637. doi: 10.1038/s41586-020-2477-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morava E., Gardeitchik T., Kozicz T., de Boer L., Koene S., de Vries M.C., McFarland R., Roobol T., Rodenburg R.J.T., Verhaak C.M. Depressive behaviour in children diagnosed with a mitochondrial disorder. Mitochondrion. 2010;10(5):528–533. doi: 10.1016/j.mito.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Morava E., Rodenburg R., van Essen H.Z., De Vries M., Smeitink J. Dietary intervention and oxidative phosphorylation capacity. J. Inherit. Metab. Dis. 2006;29(4):589. doi: 10.1007/s10545-006-0227-x. [DOI] [PubMed] [Google Scholar]
- Munnich A., Rötig A., Rio M. In: Inborn Metabolic Diseases: Diagnosis and Treatment. Saudubray J.-.-M., van den Berghe G., Walter J.H., editors. Berlin, Heidelberg, Springer, Berlin; Heidelberg: 2012. Defects of the Respiratory Chain; pp. 223–238. [Google Scholar]
- Muraresku C.C., McCormick E.M., Falk M.J. Mitochondrial Disease: Advances in clinical diagnosis, management, therapeutic development, and preventative strategies. Current Genetic Med. Reports. 2018;6(2):62–72. doi: 10.1007/s40142-018-0138-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M.P., Hartley R.C. Mitochondria as a therapeutic target for common pathologies. Nat. Rev. Drug Discovery. 2018;17(12):865–886. doi: 10.1038/nrd.2018.174. [DOI] [PubMed] [Google Scholar]
- Neuzil J., Dong L.-F., Rohlena J., Truksa J., Ralph S.J. Classification of mitocans, anti-cancer drugs acting on mitochondria. Mitochondrion. 2013;13(3):199–208. doi: 10.1016/j.mito.2012.07.112. [DOI] [PubMed] [Google Scholar]
- Newell C., Khan A., Sinasac D., Shoffner J., Friederich M.W., Van Hove J.L.K., Hume S., Shearer J., Sosova I. Hybrid gel electrophoresis using skin fibroblasts to aid in diagnosing mitochondrial disease. Neurology. Genetics. 2019;5(3):e336. doi: 10.1212/NXG.0000000000000336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng Y.S., Turnbull D.M. Mitochondrial disease: genetics and management. J. Neurol. 2016;263(1):179–191. doi: 10.1007/s00415-015-7884-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissanka N., Bacman S.R., Plastini M.J., Moraes C.T. The mitochondrial DNA polymerase gamma degrades linear DNA fragments precluding the formation of deletions. Nat. Commun. 2018;9(1) doi: 10.1038/s41467-018-04895-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyazov D.M., Kahler S.G., Frye R.E. Primary Mitochondrial Disease and Secondary Mitochondrial Dysfunction: Importance of Distinction for Diagnosis and Treatment. Mol. Syndromol. 2016;7:122–137. doi: 10.1159/000446586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NLM, 2013. Study to Assess Efficacy, Safety and Tolerability of Idebenone in the Treatment of Leber’s Hereditary Optic Neuropathy, National Library of Medicine, Available online: https://ClinicalTrials.gov/show/NCT00747487, accessed on 5 April 2022.
- NLM, 2016. Study of Idebenone in the Treatment of Mitochondrial Encephalopathy Lactic Acidosis & Stroke-like Episodes (MELAS), National Library of Medicine, Available online: https://ClinicalTrials.gov/show/NCT00887562 (accessed on 5 April 2022).
- NLM, 2018. Idebenone Treatment of Early Parkinson's Diseasesymptoms (ITEP), National Library of Medicine, Available online: https://ClinicalTrials.gov/show/NCT00747487 (accessed on 5 April 2022).
- O'Toole J.F. Renal manifestations of genetic mitochondrial disease. Int. J. Nephrol. Renovascular Dis. 2014;7:57–67. doi: 10.2147/ijnrd.s37887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh S., Goldstein A., Koenig M.K., Scaglia F., Enns G.M., Saneto R., Anselm I., Cohen B.H., Falk M.J., Greene C., Gropman A.L., Haas R., Hirano M., Morgan P., Sims K., Tarnopolsky M., Van Hove J.L.K., Wolfe L., DiMauro S. Diagnosis and management of mitochondrial disease: a consensus statement from the Mitochondrial Medicine Society. Genetics Med. : Off. J. Am. College Medical Genet. 2015;17(9):689–701. doi: 10.1038/gim.2014.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh S., Saneto R., Falk M.J., Anselm I., Cohen B.H., Haas R. A modern approach to the treatment of mitochondrial disease. Current Treatment Opt. Neurol. 2009;11(6):414–430. doi: 10.1007/s11940-009-0046-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R., Coulter L.L., Rimmer J., Parkes M., Chinnery P.F., Swift O. Mitochondrial neurogastrointestinal encephalopathy: a clinicopathological mimic of Crohn's disease. BMC Gastroenterol. 2019;19(1) doi: 10.1186/s12876-018-0925-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeva V., Blei D., Trombly G., Corsi S., Szukszto M.J., Rebelo-Guiomar P., Gammage P.A., Kudin A.P., Becker C., Altmüller J., Minczuk M., Zsurka G., Kunz W.S. Linear mitochondrial DNA is rapidly degraded by components of the replication machinery. Nat. Commun. 2018;9(1) doi: 10.1038/s41467-018-04131-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peranteau W.H., Flake A.W. The future of in utero gene therapy. Molecular Diagnosis Therapy. 2020;24(2):135–142. doi: 10.1007/s40291-020-00445-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Albert P., de Lucas Collantes C., Fernández-García M., et al. Mitochondrial Disease in Children: The Nephrologist's Perspective. JIMD Reports. 2018;42:61–70. doi: 10.1007/8904_2017_78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer G., Majamaa K., Turnbull D.M., et al. Treatment for mitochondrial disorders. Cochrane Database Systematic Rev. 2012 doi: 10.1002/14651858.CD004426.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett S.J., Grady J.P., Ng Y.S., et al. Phenotypic heterogeneity in m.3243A>G mitochondrial disease: The role of nuclear factors. Ann. Clin. Transl. Neurol. 2018;5:333–345. doi: 10.1002/acn3.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadalti C., Brunetti D., Lagutina I., Duchi R., Perota A., Lazzari G., Cerutti R., Di Meo I., Johnson M., Bottani E., Crociara P., Corona C., Grifoni S., Tiranti V., Fernandez-Vizarra E., Robinson A.J., Viscomi C., Casalone C., Zeviani M., Galli C. SURF1 knockout cloned pigs: early onset of a severe lethal phenotype. Biochimica et Biophysica Acta (BBA)-Molecular Basis Disease. 2018;1864(6):2131–2142. doi: 10.1016/j.bbadis.2018.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman J., Noronha A., Thiele I., Rahman S. Leigh map: A novel computational diagnostic resource for mitochondrial disease. Ann. Neurol. 2017;81(1):9–16. doi: 10.1002/ana.24835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman S. Mitochondrial disease in children. J. Intern. Med. 2020;287(6):609–633. doi: 10.1111/joim.13054. [DOI] [PubMed] [Google Scholar]
- Rashnonejad A., Amini Chermahini G., Gündüz C., Onay H., Aykut A., Durmaz B., Baka M., Su Q., Gao G., Özkınay F. Fetal gene therapy using a single injection of recombinant AAV9 rescued SMA phenotype in mice. Mol. Ther. 2019;27(12):2123–2133. doi: 10.1016/j.ymthe.2019.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner K.J., Esau C.C., Hussain F.N., McDaniel A.L., Marshall S.M., van Gils J.M., Ray T.D., Sheedy F.J., Goedeke L., Liu X., Khatsenko O.G., Kaimal V., Lees C.J., Fernandez-Hernando C., Fisher E.A., Temel R.E., Moore K.J. Inhibition of miR-33a/b in non-human primates raises plasma HDL and lowers VLDL triglycerides. Nature. 2011;478(7369):404–407. doi: 10.1038/nature10486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebelo A.P., Williams S.L., Moraes C.T. In vivo methylation of mtDNA reveals the dynamics of protein–mtDNA interactions. Nucleic Acids Res. 2009;37:6701–6715. doi: 10.1093/nar/gkp727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riquin E., Duverger P., Cariou C., Barth M., Prouteau C., Van Bogaert P., Bonneau D., Roy A. Neuropsychological and Psychiatric Features of Children and Adolescents Affected With Mitochondrial Diseases: A Systematic Review. Front. Psychiatry. 2020;11 doi: 10.3389/fpsyt.2020.00747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose S., Bennuri S.C., Murray K.F., Buie T., Winter H., Frye R.E., Hashimoto K. Mitochondrial dysfunction in the gastrointestinal mucosa of children with autism: A blinded case-control study. PLoS ONE. 2017;12(10):e0186377. doi: 10.1371/journal.pone.0186377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rötig A., Appelkvist E.-L., Geromel V., Chretien D., Kadhom N., Edery P., Lebideau M., Dallner G., Munnich A., Ernster L., Rustin P. Quinone-responsive multiple respiratory-chain dysfunction due to widespread coenzyme Q10 deficiency. The Lancet. 2000;356(9227):391–395. doi: 10.1016/S0140-6736(00)02531-9. [DOI] [PubMed] [Google Scholar]
- Rudolph G., Dimitriadis K., Büchner B., Heck S., Al-Tamami J., Seidensticker F., Rummey C., Leinonen M., Meier T., Klopstock T. Effects of idebenone on color vision in patients with leber hereditary optic neuropathy. J. Neuroophthalmol. 2013;33(1):30–36. doi: 10.1097/WNO.0b013e318272c643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Pesini E., Lott M.T., Procaccio V., Poole J.C., Brandon M.C., Mishmar D., Yi C., Kreuziger J., Baldi P., Wallace D.C. An enhanced MITOMAP with a global mtDNA mutational phylogeny. Nucleic Acids Res. 2007;35:D823–D828. doi: 10.1093/nar/gkl927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell O.M., Gorman G.S., Lightowlers R.N., Turnbull D.M. Mitochondrial diseases: hope for the future. Cell. 2020;181(1):168–188. doi: 10.1016/j.cell.2020.02.051. [DOI] [PubMed] [Google Scholar]
- Sato M., Sato K. Maternal inheritance of mitochondrial DNA by diverse mechanisms to eliminate paternal mitochondrial DNA. Biochimica et Biophysica Acta (BBA) – Mol. Cell Res. 2013;1833(8):1979–1984. doi: 10.1016/j.bbamcr.2013.03.010. [DOI] [PubMed] [Google Scholar]
- Schon, K. R., R. Horvath, W. Wei, et al., 2021. Use of whole genome sequencing to determine genetic basis of suspected mitochondrial disorders: cohort study. BMJ. 375, e066288–e066288. 10.1136/bmj-2021-066288. [DOI] [PMC free article] [PubMed]
- Sharma N., Pasala M.S., Prakash A. Mitochondrial DNA: Epigenetics and environment. Environ. Mol. Mutagen. 2019;60(8):668–682. doi: 10.1002/em.22319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoffner J.M., Lott M.T., Voljavec A.S., Soueidan S.A., Costigan D.A., Wallace D.C. Spontaneous Kearns-Sayre/chronic external ophthalmoplegia plus syndrome associated with a mitochondrial DNA deletion: a slip-replication model and metabolic therapy. PNAS. 1989;86(20):7952–7956. doi: 10.1073/pnas.86.20.7952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A., Faccenda D., Campanella M. Pharmacological advances in mitochondrial therapy. EBioMedicine. 2021;65:103244. doi: 10.1016/j.ebiom.2021.103244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slone J., Huang T. The special considerations of gene therapy for mitochondrial diseases. npj Genomic Med. 2020;5:1–7. doi: 10.1038/s41525-020-0116-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R.A.J., Hartley R.C., Cochemé H.M., Murphy M.P. Mitochondrial pharmacology. Trends Pharmacol. Sci. 2012;33(6):341–352. doi: 10.1016/j.tips.2012.03.010. [DOI] [PubMed] [Google Scholar]
- Sofou K. Mitochondrial disease: a challenge for the caregiver, the family, and society. J. Child Neurol. 2013;28(5):663–667. doi: 10.1177/0883073813481622. [DOI] [PubMed] [Google Scholar]
- Song S., Pursell Z.F., Copeland W.C., Longley M.J., Kunkel T.A., Mathews C.K. DNA precursor asymmetries in mammalian tissue mitochondria and possible contribution to mutagenesis through reduced replication fidelity. PNAS. 2005;102(14):4990–4995. doi: 10.1073/pnas.0500253102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y., Shi Q., Zhu C., Luo Y., Lu Q., Li H.e., Ye R., Du D., Lin Y. Mitochondrial-targeted multifunctional mesoporous Au@Pt nanoparticles for dual-mode photodynamic and photothermal therapy of cancers. Nanoscale. 2017;9(41):15813–15824. doi: 10.1039/c7nr04881e. [DOI] [PubMed] [Google Scholar]
- Srinivasan H., Das S. Mitochondrial miRNA (MitomiR): a new player in cardiovascular health. Can. J. Physiol. Pharmacol. 2015;93(10):855–861. doi: 10.1139/cjpp-2014-0500. [DOI] [PubMed] [Google Scholar]
- Stenton S.L., Prokisch H. Genetics of mitochondrial diseases: Identifying mutations to help diagnosis. EBioMedicine. 2020;56:102784. doi: 10.1016/j.ebiom.2020.102784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J.B., Chinnery P.F. The dynamics of mitochondrial DNA heteroplasmy: implications for human health and disease. Nat. Rev. Genet. 2015;16(9):530–542. doi: 10.1038/nrg3966. [DOI] [PubMed] [Google Scholar]
- Stojanović V., Mayr J.A., Sperl W., Barišić N., Doronjski A., Milak G. Infantile peripheral neuropathy, deafness, and proximal tubulopathy associated with a novel mutation of the RRM2B gene: case study. Croatian Medical J. 2013;54(6):579–584. doi: 10.3325/cmj.2013.54.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suárez-Rivero J.M., Pastor-Maldonado C.J., Povea-Cabello S., Álvarez-Córdoba M., Villalón-García I., Munuera-Cabeza M., Suárez-Carrillo A., Talaverón-Rey M., Sánchez-Alcázar J.A. Coenzyme Q(10) Analogues: Benefits and Challenges for Therapeutics. Antioxidants (Basel, Switzerland). 2021;10(2):236. doi: 10.3390/antiox10020236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Liang Y., Hao N.a., Fu X., He B., Han S., Cao J., Ma Q., Xu W., Sun Y. Novel polymeric micelles as enzyme-sensitive nuclear-targeted dual-functional drug delivery vehicles for enhanced 9-nitro-20(S)-camptothecin delivery and antitumor efficacy. Nanoscale. 2020;12(9):5380–5396. doi: 10.1039/c9nr10574c. [DOI] [PubMed] [Google Scholar]
- Taivassalo T., Shoubridge E.A., Chen J., Kennaway N.G., DiMauro S., Arnold D.L., Haller R.G. Aerobic conditioning in patients with mitochondrial myopathies: physiological, biochemical, and genetic effects. Ann. Neurol. 2001;50(2):133–141. doi: 10.1002/ana.1050. [DOI] [PubMed] [Google Scholar]
- Takemura K., Nishi H., Inagi R. Mitochondrial Dysfunction in Kidney Disease and Uremic Sarcopenia. Front. Physiol. 2020;11 doi: 10.3389/fphys.2020.565023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J., Wagner M., Stenton S.L., Strom T.M., Wortmann S.B., Prokisch H., Meitinger T., Oexle K., Klopstock T. Lifetime risk of autosomal recessive mitochondrial disorders calculated from genetic databases. EBioMedicine. 2020;54:102730. doi: 10.1016/j.ebiom.2020.102730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro, R., Onoue, N., Shinozaki, T., 2018. Mitochondrial Cardiomyopathy. Current Perspectives on Cardiomyopathies, IntechOpen.
- Tawk A., Hussein Kamarreddine M., Dagher M., Abboud G., Chams M., Ghandour-Hajj F., Khoury M., Farhat S. Clinicopathology and Diagnosis Delay in a 40-Year-Old with Mitochondrial Neurogastrointestinal Encephalomyopathy (MNGIE) Case Reports Gastroenterol. 2020;14(1):124–130. doi: 10.1159/000506187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R.W., Turnbull D.M. Mitochondrial DNA mutations in human disease. Nat. Rev. Genet. 2005;6(5):389–402. doi: 10.1038/nrg1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinker R.J., Lim A.Z., Stefanetti R.J., McFarland R. Current and emerging clinical treatment in mitochondrial disease. Mol. Diagnosis Therapy. 2021;25(2):181–206. doi: 10.1007/s40291-020-00510-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tort F., García-Silva M.T., Ferrer-Cortès X., Navarro-Sastre A., Garcia-Villoria J., Coll M.J., Vidal E., Jiménez-Almazán J., Dopazo J., Briones P., Elpeleg O., Ribes A. Exome sequencing identifies a new mutation in SERAC1 in a patient with 3-methylglutaconic aciduria. Mol. Genet. Metab. 2013;110(1-2):73–77. doi: 10.1016/j.ymgme.2013.04.021. [DOI] [PubMed] [Google Scholar]
- Tuppen H.A.L., Blakely E.L., Turnbull D.M., Taylor R.W. Mitochondrial DNA mutations and human disease. Biochim. Biophysica Acta (BBA) - Bioenergetics. 2010;1797(2):113–128. doi: 10.1016/j.bbabio.2009.09.005. [DOI] [PubMed] [Google Scholar]
- van de Loo K.F.E., Custers J.A.E., Koene S., Klein I.-L., Janssen M.C.H., Smeitink J.A.M., Verhaak C.M. Psychological functioning in children suspected for mitochondrial disease: the need for care. Orphanet J. Rare Dis. 2020;15(1) doi: 10.1186/s13023-020-1342-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandana V.P., Bindu P.S., Sonam K., Govindaraj P., Taly A.B., Gayathri N., Chiplunkar S., Govindaraju C., Arvinda H.R., Nagappa M., Sinha S., Thangaraj K. Audiological manifestations in mitochondrial encephalomyopathy lactic acidosis and stroke like episodes (MELAS) syndrome. Clin. Neurol. Neurosurg. 2016;148:17–21. doi: 10.1016/j.clineuro.2016.04.024. [DOI] [PubMed] [Google Scholar]
- Wang H.e., Zhang F., Wen H., Shi W., Huang Q., Huang Y., Xie J., Li P., Chen J., Qin L., Zhou Y.i. Tumor- and mitochondria-targeted nanoparticles eradicate drug resistant lung cancer through mitochondrial pathway of apoptosis. J. Nanobiotechnol. 2020;18(1) doi: 10.1186/s12951-019-0562-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Song J., Chuai Y., Chen F.u., Song C., Shu M., Wang Y., Li Y., Zhai X., Han S., Yao S., Shen K., Shang W., Zhang L. The mining and construction of a knowledge base for gene-disease association in mitochondrial diseases. Sci. Rep. 2021;11(1) doi: 10.1038/s41598-021-03249-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson E., Davis R., Sue C.M. New diagnostic pathways for mitochondrial disease. J. Transl. Genet. Genomics. 2020;4:188–202. doi: 10.20517/jtgg.2020.31. [DOI] [Google Scholar]
- Wedatilake Y., Brown R.M., McFarland R., Yaplito-Lee J., Morris A.A.M., Champion M., Jardine P.E., Clarke A., Thorburn D.R., Taylor R.W., Land J.M., Forrest K., Dobbie A., Simmons L., Aasheim E.T., Ketteridge D., Hanrahan D., Chakrapani A., Brown G.K., Rahman S. SURF1 deficiency: a multi-centre natural history study. Orphanet J. Rare Dis. 2013;8(1) doi: 10.1186/1750-1172-8-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg S., Sena L., Chandel N. Mitochondria in the regulation of innate and adaptive immunity. Immunity. 2015;42(3):406–417. doi: 10.1016/j.immuni.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisnovsky S., Lei E., Jean S., Kelley S. Mitochondrial Chemical Biology: New Probes Elucidate the Secrets of the Powerhouse of the Cell. Cell Chem. Biol. 2016;23(8):917–927. doi: 10.1016/j.chembiol.2016.06.012. [DOI] [PubMed] [Google Scholar]
- Wortmann S.B., Zweers-van Essen H., Rodenburg R.J.T., van den Heuvel L.P., de Vries M.C., Rasmussen-Conrad E., Smeitink J.A.M., Morava E. Mitochondrial energy production correlates with the age-related BMI. Pediatr. Res. 2009;65(1):103–108. doi: 10.1203/PDR.0b013e31818d1c8a. [DOI] [PubMed] [Google Scholar]
- Xiao Q., Du W., Dong X., Du S., Ong S.Y., Tang G., Zhang C., Yang F., Li L., Gao L., Yao S.Q. Cell-Penetrating Mitochondrion-Targeting Ligands for the Universal Delivery of Small Molecules, Proteins and Nanomaterials. Chem. (Weinheim an der Bergstrasse, Germany). 2021;27(47):12207–12214. doi: 10.1002/chem.202101989. [DOI] [PubMed] [Google Scholar]
- Xu J., Shamul J.G., Wang H., Lin J., Agarwal P., Sun M., Lu X., Tkaczuk K.H.R., He X. Targeted Heating of Mitochondria Greatly Augments Nanoparticle-Mediated Cancer Chemotherapy. Adv. Healthcare Mater. 2020;9(14):2000181. doi: 10.1002/adhm.v9.1410.1002/adhm.202000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S., Ma S.-q., Wan X., He H., Pei H., Zhao M.-J., Chen C., Wang D.-W., Dong X.-Y., Yuan J.-J., Li B. Long-term outcomes of gene therapy for the treatment of Leber's hereditary optic neuropathy. EBioMedicine. 2016;10:258–268. doi: 10.1016/j.ebiom.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsuga S., Fujita Y., Ishii A., Fukumoto Y., Arahata H., Kakuma T., Kojima T., Ito M., Tanaka M., Saiki R., Koga Y. Growth differentiation factor 15 as a useful biomarker for mitochondrial disorders. Ann. Neurol. 2015;78(5):814–823. doi: 10.1002/ana.24506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q., Zhao X.u., Xu K., Li Q., Cheng J., Gao Y., Du J., Shi H., Zhou L. Polymorphisms in lipid metabolism related miRNA binding sites and risk of metabolic syndrome. Gene. 2013;528(2):132–138. doi: 10.1016/j.gene.2013.07.036. [DOI] [PubMed] [Google Scholar]
- Yokoyama J., Yamaguchi H., Shigeto H., Uchiumi T., Murai H., Kira J.-I. [A case of rhabdomyolysis after status epilepticus without stroke-like episodes in mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes] Rinsho shinkeigaku = Clin. Neurol. 2016;56(3):204–207. doi: 10.5692/clinicalneurol.cn-000834. [DOI] [PubMed] [Google Scholar]
- Yu-Wai-Man P., Newman N.J. Inherited eye-related disorders due to mitochondrial dysfunction. Hum. Mol. Genet. 2017;26:R12–R20. doi: 10.1093/hmg/ddx182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Koilkonda R.D., Chou T.-H., Porciatti V., Ozdemir S.S., Chiodo V., Boye S.L., Boye S.E., Hauswirth W.W., Lewin A.S., Guy J. Gene delivery to mitochondria by targeting modified adenoassociated virus suppresses Leber’s hereditary optic neuropathy in a mouse model. Proc. Natl. Acad. Sci. 2012;109(20) doi: 10.1073/pnas.1119577109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusoff A.A.M., Abdullah W.S.W., Khair S., et al. A comprehensive overview of mitochondrial DNA 4977-bp deletion in cancer studies. Oncol. Rev. 2019;13:409. doi: 10.4081/oncol.2019.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeviani M., Moraes C.T., DiMauro S., Nakase H., Bonilla E., Schon E.A., Rowland L.P. Deletions of mitochondrial DNA in Kearns-Sayre syndrome. Neurology. 1988;38(9):1339. doi: 10.1212/wnl.38.9.1339. [DOI] [PubMed] [Google Scholar]
- Zhang D., Wen L., Huang R.u., Wang H., Hu X., Xing D.a. Mitochondrial specific photodynamic therapy by rare-earth nanoparticles mediated near-infrared graphene quantum dots. Biomaterials. 2018;153:14–26. doi: 10.1016/j.biomaterials.2017.10.034. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Tian Z., Yuan J., Liu C., Liu H., Ma S.Q., Li B. The progress of gene therapy for Leber's optic hereditary neuropathy. Curr. Gene Ther. 2017;17(4) doi: 10.2174/1566523218666171129204926. [DOI] [PMC free article] [PubMed] [Google Scholar]