Abstract
This study assesses the association between COVID-19 mRNA booster immunization compared with vaccination with the primary mRNA vaccination series alone and odds of hospitalization for COVID-19.
Vaccination with a booster dose of COVID-19 mRNA vaccine has been associated with decreased risk of developing severe COVID-19 compared with no vaccination.1,2 However, among individuals already fully vaccinated with the primary series of an mRNA vaccine, less is known about how much protection is added by a booster and how long that protection lasts. We assessed the association between COVID-19 mRNA booster immunization compared with vaccination with the primary mRNA vaccination series alone and the odds of hospitalization for COVID-19.
Methods
This study included adults who received either 2 or 3 doses of an mRNA COVID-19 vaccine and were hospitalized between October 1, 2021, and July 26, 2022, within the Providence health care network in 6 western US states. This study was approved by the Providence institutional review board, with a waiver of informed consent.
We performed a case-control study using methods previously described.3 Cases were individuals admitted to the hospital for COVID-19, which was defined as having a final coded diagnosis of COVID-19 or a positive nucleic acid amplification test (NAAT) for symptomatic SARS-CoV-2 and receiving treatment with remdesivir or dexamethasone. Each case was matched with 4 controls who were admitted to the hospital nonelectively for reasons other than COVID-19 within 3 days in the same geographic region as cases and received their second dose of COVID-19 vaccine (ie, completed their primary series) within 7 days of the case. We collected demographics, comorbidities, COVID-19 vaccination data, and history of prior COVID-19 (positive SARS-CoV-2 NAAT in the presence of symptoms) from electronic medical records. We determined if hospitalization occurred when the Omicron variant accounted for more than 50% of community COVID-19 cases based on US Centers for Disease Control and Prevention regional estimates.
We used multivariable conditional logistic regression to identify factors associated with hospitalization for COVID-19. We calculated the odds of hospitalization for COVID-19 among individuals who received 3 vs 2 doses of mRNA vaccine by time since receiving a booster dose. R version 4.1.2 (R Foundation for Statistical Computing) was used for the statistical analysis, with a 2-sided P < .05 defining statistical significance.
Results
There were 3062 cases (mean age, 70.8 [SD, 15.4] years; 52.6% were men; and 34.7% were boosted) and 12 248 matched controls (mean age, 67.1 [SD, 18.2] years; 46.7% were men; and 49.3% were boosted) included (Table). Factors associated with an increased odds of hospitalization for COVID-19 included age of 70 years or older, male sex, cognitive disease, chronic obstructive pulmonary disease, diabetes, immunodeficiency, obesity, rheumatologic disease, transplant, and BNT162b2 (Pfizer-BioNTech) vaccine.
Table. Characteristics of Cases and Controls and the Results of the Multivariable Analysis for the Outcome of Hospitalization for COVID-19.
| Cases (n = 3062) |
Matched controls (n = 12 248) |
Multivariable analysisa | ||
|---|---|---|---|---|
| Adjusted OR (95% CI) |
P value | |||
| Age, mean (SD), y | 70.8 (15.4) | 67.1 (18.2) | ||
| Age group, No. (%) | ||||
| ≤69 y | 1216 (39.7) | 5873 (48.0) | 1 [Reference] | |
| 70-79 y | 883 (28.8) | 3005 (24.5) | 1.47 (1.31-1.64) | <.001 |
| ≥80 y | 963 (31.5) | 3370 (27.5) | 1.57 (1.38-1.78) | <.001 |
| Sex, No. (%) | ||||
| Female | 1452 (47.4) | 6523 (53.3) | 1 [Reference] | <.001 |
| Male | 1610 (52.6) | 5725 (46.7) | 1.32 (1.21-1.44) | |
| Race and ethnicity, No. (%) | ||||
| African American/Black | 117 (3.8) | 509 (4.2) | 0.94 (0.76-1.17) | .57 |
| Asian | 157 (5.1) | 763 (6.2) | 0.84 (0.70-1.01) | .07 |
| Hispanic | 440 (14.4) | 1584 (12.9) | 1.13 (0.99-1.29) | .06 |
| White | 2092 (68.3) | 8459 (69.1) | 1 [Reference]b | |
| Otherc | 256 (8.4) | 933 (7.6) | 1 [Reference]b | |
| Comorbidities, No. (%) | ||||
| Hypertension | 2298 (75.0) | 8695 (71.0) | 0.97 (0.87-1.08) | .60 |
| Diabetes | 1270 (41.5) | 4031 (32.9) | 1.35 (1.23-1.48) | <.001 |
| Chronic kidney disease | 1027 (33.5) | 3577 (29.2) | 0.95 (0.86-1.05) | .33 |
| Congestive heart failure | 998 (32.6) | 3651 (29.8) | 0.89 (0.80-0.98) | .02 |
| Chronic obstructive pulmonary disease | 789 (25.8) | 2102 (17.2) | 1.75 (1.57-1.94) | <.001 |
| Body mass index ≥35d | 588 (19.2) | 1860 (15.2) | 1.42 (1.27-1.59) | <.001 |
| Alcohol or drug dependence | 576 (18.8) | 2898 (23.7) | 0.71 (0.62-0.81) | <.001 |
| Cancer | 568 (18.5) | 2231 (18.2) | 0.92 (0.83-1.03) | .14 |
| Cognitive disease | 533 (17.4) | 1683 (13.7) | 1.15 (1.02-1.30) | .02 |
| Immunodeficiency | 334 (10.9) | 547 (4.5) | 2.68 (2.26-3.18) | <.001 |
| Current smoker | 246 (8.0) | 1252 (10.2) | 0.93 (0.77-1.11) | .41 |
| Rheumatologic disease | 243 (7.9) | 733 (6.0) | 1.18 (1.00-1.40) | .04 |
| Transplant | 123 (4.0) | 183 (1.5) | 2.04 (1.54-2.69) | <.001 |
| Hospitalization during period with >50% Omicron prevalence among COVID-19 cases in the community, No. (%)e | 2470 (80.7) | 9906 (80.9) | 0.39 (0.16-0.96) | .04 |
| Time between second mRNA vaccine dose and hospital admission, median (IQR), d | 305 (27-394) | 305 (27-394) | ||
| Time between second mRNA vaccine dose and booster, median (IQR), d | 231 (199-259) | 235 (205-260) | ||
| Received same brand of vaccine for primary series with or without a booster | ||||
| mRNA-1273 (Moderna) | 1297 (42.4) | 5361 (43.8) | 1 [Reference] | <.001 |
| BNT162b2 (Pfizer-BioNTech) | 1625 (53.1) | 6132 (50.1) | 1.17 (1.08-1.27) | |
| Received both the BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna) vaccines | 140 (4.6) | 755 (6.2) | ||
| Received mRNA booster | 1064 (34.7) | 6036 (49.3) | 0.41 (0.37-0.46) | <.001 |
| Prior COVID-19 | 112 (3.7) | 777 (6.3) | 0.44 (0.36-0.55) | <.001 |
Abbreviation: OR, odds ratio.
Controlled for the following factors: age, sex, race, alcohol or drug dependence, cancer, cognitive disease, congestive heart failure, chronic kidney disease, chronic obstructive pulmonary disease, diabetes, hypertension, immunodeficiency, obesity, rheumatologic disease, smoking status, transplant, hospitalization during period with greater than 50% Omicron prevalence among COVID-19 cases in the community, brand of COVID-19 mRNA vaccine, receipt of mRNA booster, and prior COVID-19.
Referent category for race included White race and Other race combined.
American Indian/Alaska Native, Native Hawaiian/Other Pacific Islander, or unknown race and ethnicity.
Calculated as weight in kilograms divided by height in meters squared.
Based on US Centers for Disease Control and Prevention regional estimates (https://covid.cdc.gov/covid-data-tracker/#variant-proportions).
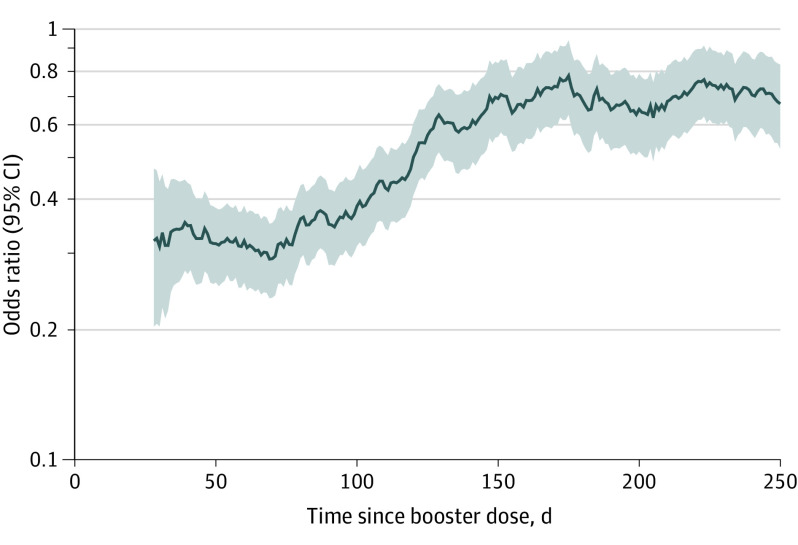
In the multivariable analysis, booster vaccination was associated with decreased odds of hospitalization for COVID-19 (34.7% of cases vs 49.3% of matched controls; adjusted OR, 0.41 [95% CI, 0.37-0.46]). The odds of hospitalization varied based on time since booster: less than 50 days (adjusted OR, 0.24 [95% CI, 0.18-0.30]), 50 to 100 days (adjusted OR, 0.24 [95% CI, 0.20-0.29]), 101 to 150 days (adjusted OR, 0.47 [95% CI, 0.38-0.58]), and longer than 150 days (adjusted OR, 0.72 [95% CI, 0.61-0.84]). The Figure displays the odds of hospitalization for COVID-19 based on time from the booster dose.
Figure. Odds of Hospitalization for COVID-19 After 3 vs 2 Doses of mRNA COVID-19 Vaccine by Time Since Booster Dose.
The shaded areas indicate the 95% CIs. The 30-day rolling average is depicted.
Discussion
In a large US population, mRNA boosters were associated with decreased odds of hospitalization compared with the mRNA vaccine primary series alone, with the magnitude of the association attenuated with more time since the booster dose.
Studies comparing COVID-19 rates among boosted individuals vs unvaccinated individuals have found 55% to 99% lower odds of COVID-19 among those who are boosted.1,2,4 By matching cases with controls based on the date of second mRNA dose, this study was able to measure the added benefit of a booster dose to the primary series. This study’s findings are similar to the hazard ratio of 0.48 for hospitalization for COVID-19 associated with boosters that was found in a study with shorter follow-up.5 Because the 2-dose primary series reduces long-term risk for hospitalization,3 even if the magnitude of the association attenuated over time after 3 vs 2 vaccine doses, the overall risk for hospitalization among vaccinated individuals remains low.
Limitations include possible booster vaccination at outside facilities; however, Providence hospitals routinely capture outside vaccination data upon admission. Some cases or controls may have been misclassified. The predominant SARS-CoV-2 variant changed over the course of the study, with 81% of participants hospitalized when Omicron predominated, and these findings may not apply to future variants.
Section Editors: Jody W. Zylke, MD, Deputy Editor; Kristin Walter, MD, Senior Editor.
References
- 1.Accorsi EK, Britton A, Shang N, et al. Effectiveness of homologous and heterologous Covid-19 boosters against Omicron. N Engl J Med. 2022;386(25):2433-2435. doi: 10.1056/NEJMc2203165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrews N, Stowe J, Kirsebom F, et al. Effectiveness of COVID-19 booster vaccines against COVID-19–related symptoms, hospitalization and death in England. Nat Med. 2022;28(4):831-837. doi: 10.1038/s41591-022-01699-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wright BJ, Tideman S, Diaz GA, French T, Parsons GT, Robicsek A. Comparative vaccine effectiveness against severe COVID-19 over time in US hospital administrative data: a case-control study. Lancet Respir Med. 2022;10(6):557-565. doi: 10.1016/S2213-2600(22)00042-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tartof SY, Slezak JM, Puzniak L, et al. Durability of BNT162b2 vaccine against hospital and emergency department admissions due to the Omicron and Delta variants in a large health system in the USA: a test-negative case-control study. Lancet Respir Med. 2022;10(7):689-699. doi: 10.1016/S2213-2600(22)00101-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butt AA, Talisa VB, Shaikh OS, Omer SB, Mayr FB. Relative vaccine effectiveness of a SARS-CoV-2 mRNA vaccine booster dose against the Omicron variant. Clin Infect Dis. 2022;ciac328. doi: 10.1093/cid/ciac328 [DOI] [PMC free article] [PubMed] [Google Scholar]