Abstract
Background
Giant pandas (Ailuropoda melanoleuca) are the obligate host of the parasitic roundworm Baylisascaris schroederi. The infection of giant pandas with B. schroederi is very common. At present, little is known about the mechanism of immune interaction between B. schroederi and the host. As an important component of innate immunity, the NOD-like receptor 3 (NLRP3) inflammasome plays an important role in host immune response and the occurrence and development of infectious diseases.
Methods
We analyzed the regulation of NLRP3 inflammasome activation in monocyte-derived macrophages (MDMs) by the recombinant B. schroederi migratory larvae cysteine protease inhibitor rBsCPI-1, knowing from a previous study that the CPI-1 is highly expressed in B. schroederi migratory larvae. We first determined the effects of rBsCPI-1 and excretory–secretory products of B. schroederi migratory larvae on cell proliferation using the CCK-8 and LDH release assays. We then analyzed NLRP3 inflammasome activation, pyroptosis and pro-inflammatory cytokine release by quantitative-PCR, western blotting and enzyme-linked immunosorbent assay. The signaling pathway of rBsCPI-1 to activate NLRP3 inflammasomes was analyzed in activation and inhibition experiments. Finally, the effects of rBsCPI-1 on inflammasome activation in mice immunized with rBsCPI-1 were analyzed.
Results
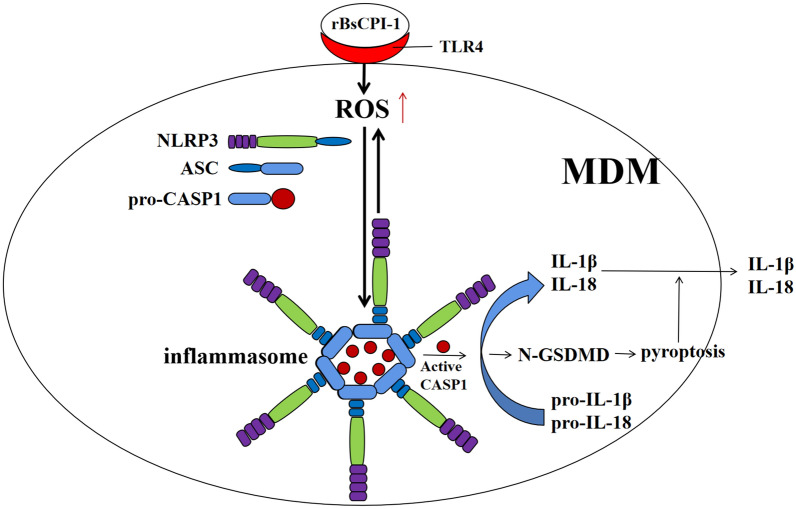
The activation and inhibition experiments revealed that rBsCPI-1 induced inflammasome activation through the TLR4–ROS–NLRP3 signaling pathway, with reactive oxygen species (ROS) not only functioning as an activator of the NLRP3 inflammasome, but also an activation product of the NLRP3 inflammasome. rBsCPI-1 promoted the activation and assembly of the NLRP3 inflammasome, which further converted the pro-inflammatory cytokines interleukin (IL)-1β and IL-18 into mature active forms. At the same time, caspase-1 cleaved gasdermin D to trigger cell pyroptosis. The results of animal immunization experiments further confirmed that rBsCPI-1 could induce the activation of the NLRP3 inflammasome.
Conclusions
rBsCPI-1 activates the inflammasome through the TLR4–ROS–NLRP3 signaling pathway and further induces the pyroptosis of MDMs and release of pro-inflammatory factors IL-1β and IL-18, thus promoting the occurrence and development of the inflammatory response in the host.
Graphical abstract
Keywords: Baylisascaris schroederi, Cysteine protease inhibitors, Inflammasome, ROS, Pyroptosis
Background
Baylisascariasis is a common and serious parasitic disease of giant pandas (Ailuropoda melanoleuca) caused by infection with adults or migratory larvae of the parasitic roundworm Baylisascaris schroederi. The adult roundworm usually resides in the intestines of giant pandas and can cause intestinal inflammation, intestinal blockage and even lethal intestinal rupture. The migratory larvae can migrate to various organs of giant pandas and induce visceral larva migrans (VLM), causing severe intestinal damage, helminthic hepatitis, pneumonia and acute pancreatitis, all of which have a great impact on the survival and health of giant pandas [1–4].
The structure and antigen components of parasites are more complex than those of bacteria and viruses and, consequently, it is extremely difficult to elucidate the complete immune interaction mechanism between parasites and hosts. Current research focuses on analyzing whether a specific protein in the excretory–secretory products plays an immunoregulatory role. Experimental data have shown that cysteine protease inhibitors (CPIs) play an important role in the regulation of host immune responses [5, 6]. The CPI secreted by worms may have a dual function of both regulating intrinsic proteases and inhibiting immune-related host’s proteases [7–10]. In a previous study, we cloned, expressed and purified recombinant B. schroederi migratory larvae CPI (rBsCPI-1), which was highly expressed in B. schroederi migratory larvae, and analyzed its regulation of the function of peripheral blood mononuclear-derived macrophages (MDMs). We found that rBsCPI-1 can exert a dual regulatory role on mouse immune cells through the recognition of Toll-like receptor 4 (TLR4) (unpublished results).
Inflammation is an important response of the host immune system to modulate defense mechanisms and control microbial invasion. However, prolonged or unrelieved inflammation increases the risk of inflammatory disease development and, therefore, precise control of the inflammatory response is essential for limiting pathogen infection without causing damage to the host. The inflammasome is a multi-protein complex which is an important component of the innate immune system. Activation of the inflammasome is initiated by pattern recognition receptors sensing pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs) and upregulating NOD-like receptor 3 (NLRP3) expression [11]. Intracellular NLRP3 can recruit apoptosis-associated speck-like protein-containing CARD (ASC) and caspase-1 precursor to form the NLRP3 inflammasome, thereby inducing the maturation and secretion of interleukin (IL)-1β and IL-18 [12]. Previous studies have shown that infection with Schistosoma mansoni, Toxoplasma gondii and Trypanosoma cruzi can activate the NLRP3 inflammasome in host cells [13–15]. Plasmodium vivax and Toxoplasma gondii have also been shown to activate various inflammasomes [16, 17].
Although research on the inflammasome has become a hot spot, there have been no relevant studies on the relationship between B. schroederi infection and inflammasome activation. It also remains to be explored whether rBsCPI-1 regulates the occurrence and development of inflammatory responses by activating the NLRP3 inflammasome. The findings of the study reported here will provide insights into the pathogenesis of baylisascariasis and also help in the conservation of the giant panda species.
Methods
Experimental animals and cells
Specific pathogen-free female BALB/c mice (n = 40 in total), aged approximately 6–8 weeks, were purchased from Chengdu Dashuo Laboratory Animal Co., Ltd. (Chengdu, China) The animal study protocol was reviewed and approved by the Animal Care and Use Committee of Sichuan Agricultural University (no.: SYXK 2019-189). All animal procedures used in this study were performed in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, Bethesda, MD, USA) and recommendations of the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines (http://www.nc3rs.org.uk/arrive-guidelines). All applicable institutional and national guidelines for the care and use of animals were followed.
Peripheral blood mononuclear cells (PBMCs) were separated using the Mouse Peripheral Blood Mononuclear Cell Separation Solution Kit (TBD, China) as previously described [18]. The PBMCs (1 × 106/ml) were cultured at 37 °C and 5% CO2 in RPMI 1640 complete medium (HyClone Laboratories LLC, Logan, UT, USA) supplemented with 2 mM l-glutamine, 50 ng/ml M-CSF (Solarbio Science & Technology Co., Ltd., Beijing, China), and 10% fetal bovine serum (Sigma-Aldrich, St. Louis, MO, USA). The medium was changed every 2 days until monocytes differentiated and proliferated sufficiently. Approximately 1 week of culture was needed to obtain MDMs [19].
Protein preparation
The excretory–secretory products of B. schroederi migratory larvae (BsES) were prepared as previously described [18]. The pET-32a-BsCPI-1 positive expression bacterial solution was expressed and purified as reported previously [18]. The protein concentrations of the prepared BsES and rBsCPI-1 were determined by the bicinchoninic acid (BCA) assay (Takarabio, Beijing, China). The contaminated endotoxin was removed by the ToxOut™ High Capacity Endotoxin Removal Kit (GenScript Biotech, China).
Cell proliferation assay
Approximately 100 µl MDM suspension (1 × 106/ml) was treated with concanavalin A (ConA: 10 µg/ml) alone or in combination with pET-32a, different concentrations of rBsCPI-1 (0, 5, 10, 15, 30, 50 µg/ml) or different concentrations of BsES (0, 1, 5, 10, 20, 30 µg/ml), in the same volume for 12 h. Next, 10 µl Cell Counting Kit 8 (CCK-8; Meilunbio, Dalian, China) solution was added to each well and incubated for 4 h before harvesting; the absorbance values were measured at 450 nm.
Lactate dehydrogenase cytotoxicity assay
Approximately 100 µl MDM suspension (1 × 106/m) was treated with phosphate-buffered saline (PBS), pET-32a, rBsCPI-1 or BsES for 12 h, following which the supernatant was collected and 150 µl of lactate dehydrogenase (LDH) release reagent was added to the supernatant at a 10× dilution in PBS. Following mixing, the solution was incubated at 37 °C and 5% CO2 for 1 h, then 120 µl of supernatant was collected and the absorbance values measured at 490 nm
Quantitative PCR analysis of inflammasome activation
Approximately 100 µl MDM suspension (1 × 106/ml) was treated respectively with PBS, pET-32a, rBsCPI-1, BsES and Nigericin sodium salt, a NLRP3 activator (10 μM; MedChemExpress, Monmouth Junction, NJ, USA). Quantitative PCR (qPCR) was used to analyze the transcription level of the NLRP3, AIM2, ASC, caspase-1, caspase-11, IL-1β and IL-18 genes using the primers shown in Table 1. Total RNA was extracted from MDMs using the Total RNA Extraction Kit (Solarbio). Complementary DNA (cDNA) was synthesized from total RNA using the Prime Script First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Waltham, MA, USA), and qPCR reactions were performed using Roche LightCycler 96 real-time PCR cycler (Roche, Basel, Switzerland). Amplifications were conducted in a 20-μl reaction volume containing 10 μl of TB Green Premix Ex Taq™ (Tli RNase H Plus; TaKaRa, Kusatsu, Japan), 2 μl of cDNA template (50 ng), 0.8 μl each of forward and reverse primer (10 μM) and 6.4 μl of ddH2O. The PCR amplification program consisted of 95 °C for 10 min; 40 cycles of 95 °C for 5 s, 60 °C for 30 s; and 95 °C for 5 s, 60 °C for 60 s, 95 °C for 1 s. Transcription levels of the target genes were normalized by subtracting the expression level of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and then calculating the relative expression using the 2−∆∆Ct method.
Table 1.
Primers of the detected genes
| Genes | Primers | Sequence | Accession no. |
|---|---|---|---|
| Nlrp1α | Forward | 5ʹ-ACATCCACATACTGCTCAC-3ʹ | NM_001004142 |
| Reverse | 5ʹ-ATCTTCACACCACCATCAC-3ʹ | ||
| Nlrp3 | Forward | 5ʹ-AGACCTCCAAGACCACTAC-3ʹ | BC116175 |
| Reverse | 5ʹ-ACATAGCAGCGAAGAACTC-3ʹ | ||
| Nlrp6 | Forward | 5ʹ-GGTGAAGGAGAGGAATGC-3ʹ | NM_133946 |
| Reverse | 5ʹ-GGATGAACAGTAGGCGATT-3ʹ | ||
| Nlrc4 | Forward | 5ʹ-GCTCAGTCCTCAGAACCT-3ʹ | NM_001033367 |
| Reverse | 5ʹ-GCCACCAACTTCATCTCTT-3ʹ | ||
| Aim2 | Forward | 5ʹ-CAGTTCCTCAGTTGTGGTT-3ʹ | NM_001013779 |
| Reverse | 5ʹ-GTCTAATCTTGTCTCCTTCCT-3ʹ | ||
| Asc | Forward | 5ʹ-CACAGGCAAGCACTCATT-3ʹ | NM_023258 |
| Reverse | 5’-CAGGTCAGGTTCCAGGAT-3’ | ||
| Caspase-1 | Forward | 5ʹ-GACATCCTTCATCCTCAGAA-3ʹ | NM_009807 |
| Reverse | 5ʹ-CTCCAGCAGCAACTTCAT-3ʹ | ||
| Caspase-11 | Forward | 5ʹ-CCATAGTGCTGCTCATCC-3ʹ | NM_175362 |
| Reverse | 5ʹ-CGCTGCTCCTCTACAATC-3ʹ | ||
| IL-1b | Forward | 5ʹ-CTTCAGGCAGGCAGTATC-3ʹ | NM_008361 |
| Reverse | 5ʹ-CAGCAGGTTATCATCATCATC-3ʹ | ||
| IL-18 | Forward | 5ʹ-ACTCTTGCGTCAACTTCA-3ʹ | BC024384 |
| Reverse | 5ʹ-GTCCTCTTACTTCACTGTCT-3ʹ | ||
| GAPDH | Forward | 5ʹ-TCTCCTGCGACTTCAACA-3ʹ | GU214026 |
| Reverse | 5ʹ-TGTAGCCGTATTCATTGTCA-3ʹ |
Western blotting analysis of inflammasome activation
Approximately 100 µl MDM suspension (1 × 106/ml) was treated with PBS, pET-32a, rBsCPI-1, BsES and Nigericin sodium salt, respectively. The cells were collected, and the MDM proteins were prepared as mentioned previously [18]. A total of 100 µg MDM proteins was separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and the separated proteins were blotted onto nitrocellulose filter membranes. The membranes were then blocked with 5% skim milk for 2 h at room temperature, following which they were incubated with Anti-ASC, Anti-cleaved N-terminal GSDMD, Anti-IL-1 beta and Anti-IL-18 (1:1000; diluted in 5% skim milk; ABclonal, Wuhan, China) at 4 °C overnight. After three washes, the membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibody (1:5000; diluted in 5% skim milk; ABclonal) for 2 h at room temperature. After washing, the membranes were exposed using ultrasensitive ECL chemiluminescence reagent (Meilunbio). The bands were quantified using densitometry and analyzed with Image J software.
Approximately 100 µl MDM suspension (1 × 106/ml) was pre-incubated with 1 mM N-acetylcysteine (NAC; a reactive oxygen species [ROS] inhibitor; MedChemExpress) for 4 h, following which rBsCPI-1 was added and the solution incubated for 12 h. The cells were then collected, and the MDM proteins were prepared and western blotting carried out as described above. Anti-NLRP3 (ABclonal) was diluted 1:1000 in 5% skim milk.
Approximately 100 µl MDM suspension (1 × 106/ml) was pre-incubated with 10 µM MCC950 Sodium (a NLRP3 inhibitor; MedChemExpress) for 4 h, following which rBsCPI-1 was added and the solution incubated for 12 h. The cells were then collected, and MDM proteins were prepared and western blotting was carried out as mentioned above. Anti-caspase1, Anti-GSDMD, Anti-cleaved N-terminal GSDMD, Anti-IL-1 beta and Anti-IL-18 (ABclonal) were each diluted 1:1000 in 5% skim milk.
Enzyme-linked immunosorbent assay for cytokine expression
Approximately 100 µL MDM suspension (1 × 106/ml) was treated with PBS, pET-32a, rBsCPI-1, BsES and Nigericin sodium salt, respectively. Cell culture supernatant were collected by centrifugation for 15 min at 1000 g, following which 100 μl of standard and sample was added to each well, and the wells incubated for 2 h at 37 °C. After incubation, the liquid was removed from each well, 100 μl of biotin antibody (1×) was added and the wells incubated for 1 h at 37 °C, followed by three washes. HRP-avidin (100 μl; 1×) was added to each well and the wells incubated for 1 h at 37 °C. After washing, 100 μl of TMB Substrate was added to each well and the wells incubated for 30 min. Finally, 50 μl of Stop Solution was added to each well and the absorbance values were measured at 450 nm.
ROS assay
The TLR-4 on MDMs were pre-activated or pre-blocked with lipopolysaccharide (LPS; a TLR4-specific activator; Sigma-Aldrich) or 7E3 (a TLR-4-blocking antibody; Abcam, Cambridge, UK) for 24 h, and then rBsCPI-1 was added and incubated for 12 h. The fluorogenic dye 2′-7′dichlorofluorescin diacetate (DCFH-DA; Solarbio) was diluted 1:1000 with serum-free medium to a final concentration 10 μM/l. MDMs were incubated with DCFH-DA at 37 °C, 5% CO2 for 20 min, then washed three times with serum-free cell culture medium to sufficiently removed DCFH-DA that did not enter the cells. The cells were then collected and detected by measurement fluorescence at 488 nm excitation and 525 nm emission wavelengths.
MDMs were pre-incubated with 10 µM Nigericin or MCC950 sodium for 4 h. rBsCPI-1 was then added and the solution incubated for 12 h. ROS analysis experiments were then carried out.
In vivo experiment
A total of 18 mice were divided into three groups of six mice each, and each group of six mice injected via the intraperitoneal route with 50 µg rBsCPI-1, an equal volume of PBS or pET-32a, for a total of three times at 7-day intervals. The mice were then sacrificed 7 days after the last immunization, and MDMs were collected. The relative expression of the NLRP3, AIM2, ASC, caspase-1, caspase-11, IL-1β and IL-18 genes were measured using the same qPCR instrumentation and protocols as described above.
Statistical analysis
All data are expressed as mean ± standard deviation (SD). Statistical analysis was done using GraphPad Prism 5 software (GraphPad Software, San Diego, CA, USA. Image J software was used to quantify the protein band intensity. Differences between groups were assessed by one-way analysis of variance in SPSS version 26.0 software (SPSS IBM Corp., Armonk, NY, USA. P < 0.05 was considered to indicate statistical significance.
Results
rBsCPI-1 affected cell proliferation
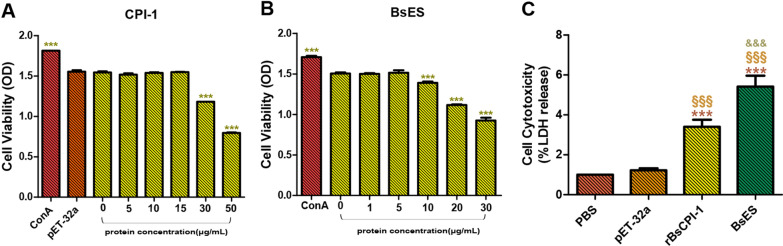
In order to analyze the effect of rBsCPI-1 on cell proliferation and determine the optimal reaction concentration of rBsCPI-1 and BsES co-incubated with MDMs, we performed cell proliferation experiments using the CCK-8 kit. As shown in Fig. 1a, b, the proliferation ability of MDMs gradually decreased with increasing concentrations of rBsCPI-1 and BsES until the rBsCPI-1 concentration reached 30 μg/ml (P < 0.001) and the BsES concentration reached 10 μg/ml (P < 0.001), at which levels the proliferation of MDMs was significantly inhibited. Therefore, in order to exclude the effect of cell proliferation on subsequent experiments, we selected 15 μg/ml rBsCPI-1 and 5 μg/ml BsES as the optimal reaction concentrations for co-incubation with MDMs.
Fig. 1.
rBsCPI-1 and BsES affect the proliferation of monocyte-derived macrophages (MDMs).A, B Proliferation was measured by the Cell Counting Kit 8 (CCK-8) assay after the cells were stimulated with ConA alone or combined with rBsCPI-1 (A) and BsES (B). The optical density (OD) values at 450 nm were considered to be the cell proliferation index. The data from 3 independent experiments were analyzed and expressed as the mean ± standard deviation (SD). Asterisks indicate a significant difference compared with the 0 μg/ml group at *P < 0.05, **P < 0.01, ***P < 0.001. c The cell cytotoxicity assay was used to assess the release level of LDH at OD490. Significant differences versus the PBS group are indicated by ***P < 0.001; versus the pET-32a (pET-32a) group by §§§P < 0.001; and versus the rBsCPI-1 group by &&&P < 0.001. BsES, Excretory–secretory products of Baylisacaris schroederi migratory larvae; ConA, Concanavalin A; LDH, lactate dehydrogenase; PBS, phosphate-buffered saline; rBsCPI-1, recombinant B. schroederi migratory larvae cysteine protease inhibitor
At the same time, we analyzed the cell cytotoxicity of rBsCPI-1 and BsES on MDMs by detecting the release of LDH. The results showed that the release of LDH was significantly increased in the rBsCPI-1 and BsES group compared with the control and pET-32a group (P < 0.001). Also, there was also difference between the rBsCPI-1 and the BsES group (P < 0.001) (Fig. 1c). We therefore concluded that rBsCPI-1 and BsES have a cell cytotoxic effect on MDMs.
rBsCPI-1 and BsES activated the NLRP3 inflammasome
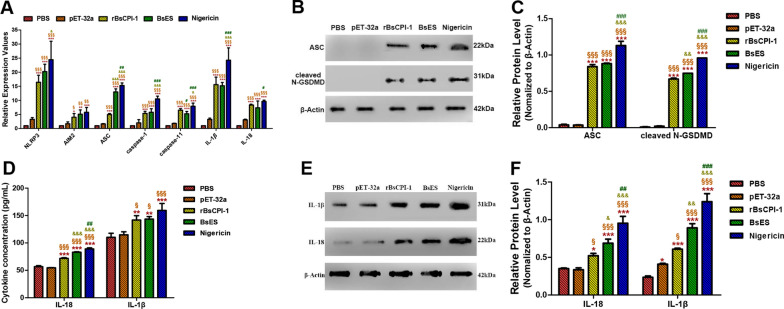
The activation of the NLRP3 inflammasome in MDMs under the effects of PBS, pET-32a, rBsCPI-1, BsES and Nigericin was analyzed by qPCR. The experimental results showed that pET-32a did not cause inflammasome activation compared with the control group, while BsES caused significant increases in the transcript levels of the NLRP3, AIM2, ASC, caspase-1, caspase-11, IL-1β and IL-18 genes. Meanwhile, rBsCPI-1 caused a significant increase in the transcript levels of these genes compared to the levels in the control group and a significant decrease compared to those in the Nigericin-positive group. With the exception of ASC, there was no significant difference between BsES and rBsCPI-1 in inducing a significant increase in the transcription levels of the other genes (Fig. 2a).
Fig. 2.
rBsCPI-1 and BsES induced inflammasome activation. A Quantitative PCR analysis of the transcription levels of the NOD-like receptor 3 (NLRP3) inflammasome-related genes in MDMs. B, C A representative western blot band of ASC and cleaved N-terminal gasdermin D (GSDMD) are shown in B, and a graph of the quantified band density is shown in C. D The enzyme-linked immunosorbent assay (ELISA) detected the secreted IL-1β and IL-18 in the cell supernatant. E, F A representative western blotting band of retained IL-1β and IL-18 in the cells are shown in E, and the graph of the quantified band density is shown in F. Data are shown as mean ± SD of 3 replicates per group. Significant differences versus the control group are indicated by *P < 0.05, **P < 0.01, ***P < 0.001; versus pET-32a group by §P < 0.05, §§P < 0.01, §§§P < 0.001; versus the rBsCPI-1 group by &P < 0.05, &&P < 0.01, &&&P < 0.001; and versus the BsES group by #P < 0.05, ##P < 0.01, ###P < 0.001. GSDMD, Gasdermin D; IL, interleukin
Western blotting results showed that ASC was significantly activated by rBsCPI-1, BsES and Nigericin compared with the control group (P < 0.001). Meanwhile, rBsCPI-1, BsES and Nigericin promoted gasdermin D (GSDMD) cleavage in response to canonical and non-canonical inflammasome activators (Fig. 2b, c).
At the same time, we detected the level of secreted (in the supernatant) and retained (in the cell) cytokines using the enzyme-linked immunosorbent assay (ELISA) (Fig. 2d) and western blotting (Fig. 2e, f). The results showed that the level of IL-1β and IL-18 secreted and retained were all significantly higher in the rBsCPI-1, BsES, and Nigericin groups compared with the control and pET-32a groups. Also, the levels of cytokines in the Nigericin-positive group were significantly higher than those in the rBsCPI-1 and BsES groups. Based on these results, we drew a preliminary conclusion that rBsCPI-1 and BsES can induce the activation of inflammasomes in MDMs.
TLR4 induced ROS production
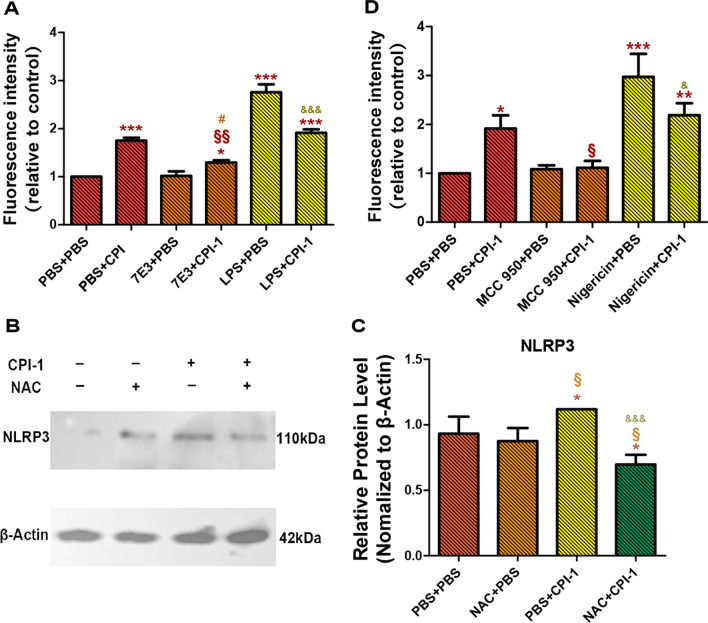
We pre-incubated MDMs with TLR4 activator (LPS) and TLR4 inhibitor (7E3) for 24 h, then added rBsCPI-1 for stimulation. The fluorescence intensity of intracellular DCFH-DA was detected to analyze the expression of ROS. The experimental results showed that rBsCPI-1 induced a high expression of ROS and that there was no significant difference in ROS expression between the 7E3 + PBS groups and the control group (P > 0.05). The expression of ROS in the LPS + PBS and LPS + CPI-1 groups (P < 0.001) were significantly higher than that in the control group, and the expression of ROS in the LPS + CPI-1 group was significantly lower than that in the LPS + PBS group (P < 0.001) (Fig. 3a).
Fig. 3.
rBsCPI-1 affects NLRP3 activation through the TLR4-ROS-NLRP3 pathway. A The fluorescence intensity of intracellular fluorogenic dye 2′-7′dichlorofluorescin diacetate (DCFH-DA) was detected at 488 nm excitation and 525 nm emission wavelengths. The data presented are representative of 3 independent experiments. Significant differences versus the control group (PBS + PBS) are indicated by *P < 0.05, ***P < 0.001; for the LPS + CPI-1 group versus the LPS + PBS group by &&&P < 0.001; for the 7E3 + CPI-1 group versus the 7E3 + PBS group by #P < 0.05; and for the LPS + CPI-1 group versus the LPS + PBS group by &&P < 0.01. B, C A representative western blot is shown in B, and the graph of the quantified band density is shown in C. C *P < 0.05; versus the NAC + PBS group by §P < 0.05; versus PBS + CPI-1 group by &&&P < 0.001. D Fluorescence intensity of intracellular DCFH-DA. Significant differences versus the control group are indicated by *P < 0.05, **P < 0.01, ***P < 0.001; for the MCC 950 + CPI-1 group or Nigericin + CPI-1 group versus the PBS + CPI-1 group by §P < 0.05; for the Nigericin + CPI-1 group versus the Nigericin + PBS group by &P < 0.05. 7E3, TLR-4-blocking antibody; LPS, lipopolysaccharide (TLR4 activator); MCC 950, MCC950 Sodium (a NLRP3 inhibitor); NAC, N-acetylcysteine (a ROS inhibitor); ROS, reactive oxygen species; TLR4, Toll-like receptor 4
ROS induced NLRP3 activation
We pre-incubated MDMs with the ROS inhibitor (NAC) for 4 h and then added rBsCPI-1 for stimulation. NLRP3 activation was assessed by western blotting. The results showed that under the action of rBsCPI-1, the expression of NLRP3 in MDMs was significantly higher than that in the control group (P < 0.05). The addition of rBsCPI-1 after pre-incubation with NAC could induce an decrease in the expression of NLRP3 compared with the control (P < 0.05) and PBS + CPI-1 group (P < 0.001) groups (Fig. 3b, c).
NLRP3 activation induced ROS production
We pre-incubated MDMs with NLRP3 activator (Nigericin) and NLRP3 inhibitor (MCC950) for 4 h and then added rBsCPI-1 for stimulation. The fluorescence intensity of the intracellular DCFH-DA was detected to analyze the expression of ROS. The results showed that rBsCPI-1 induced a high expression of ROS (P < 0.05), but at a lower level than that induced by Nigericin. The expression of ROS in the Nigericin + CPI-1 group was significantly lower than that in the Nigericin + PBS group (P < 0.05). On the other hand, there was no significant difference in ROS expression when the MCC 950 + PBS and MCC 950 + CPI-1 groups were compared with the control group, while the expression in the MCC950 + CPI-1 group was significantly lower than that in the PBS + CPI-1 group (P < 0.05) (Fig. 3d).
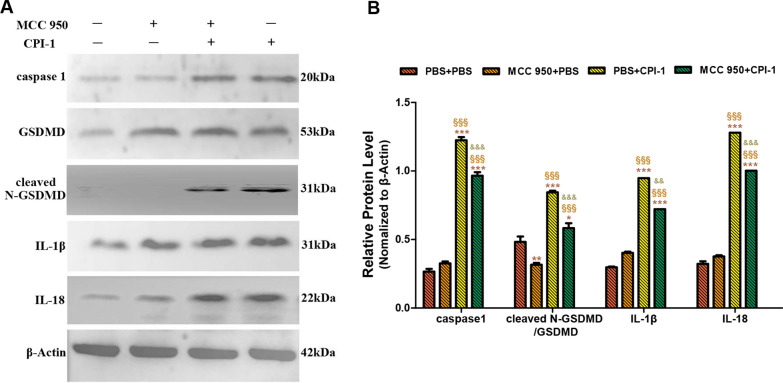
rBsCPI-1 induced cell pyroptosis
The expression of pyroptosis-related proteins was detected by western blotting. The experimental results showed that under the action of rBsCPI-1, the expression levels of the pyroptosis-related proteins caspase-1, cleaved N-terminal GSDMD, IL-1β and IL-18 were significantly higher than those in the control group (P < 0.001). At the same time, when MDMs were pre-incubated with the NLRP3 inhibitor MCC950, the expression levels of caspase-1, IL-1β and IL-18 were not significantly different from those in the control group. The expression levels of caspase-1, cleaved N-terminal GSDMD, IL-1β and IL-18 in the MCC950 + CPI-1 group were significantly higher than those in the control group, and significantly lower than those in the PBS + CPI-1 group (Fig. 4).
Fig. 4.
The activation of NLRP3 inflammasome-induced pyroptosis. a b A representative western blotting is shown in A, and the graph of the quantified band density is shown in B. Data are shown as the mean ± SD of 3 replicates per group. Significant differences versus the control group are indicated by *P < 0.05, **P < 0.01, ***P < 0.001; versus the MCC 950 + PBS group by §P < 0.05; versus the PBS + CPI-1 group by &P < 0.05, &&&P < 0.001
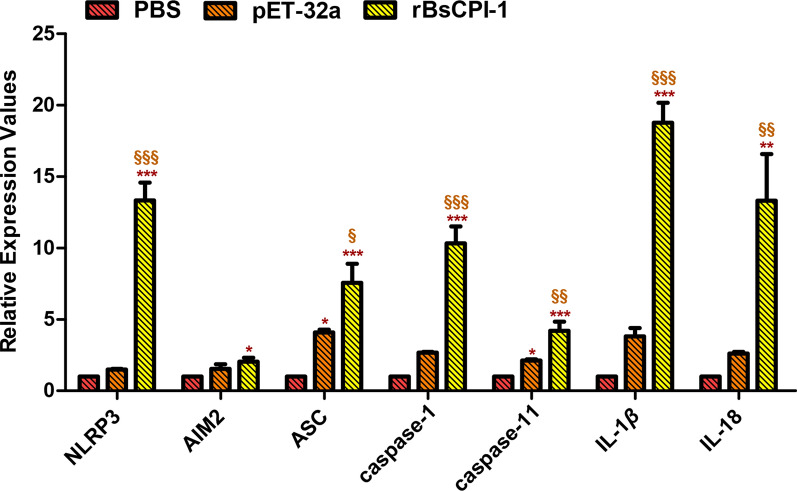
In vivo experiment
We immunized mice by intraperitoneal injection with rBsCPI-1 for the in vivo experiments and extracted MDMs at 7 days after the last immunization. The qPCR results showed that rBsCPI-1 immunization induced the transcription levels of the NLRP3, AIM2, ASC, caspase-1, caspase-11, IL-1β and IL-18 compared to those in the control and pET-32a group (Fig. 5). Therefore, we preliminarily drew the conclusion that rBsCPI-1 induces NLRP3 inflammasome activation in MDMs.
Fig. 5.
Analysis of the activation of the NLRP3 inflammasome in vivo. Data are shown as the mean ± SD of 3 replicates per group. Significant differences versus the control group are indicated by *P < 0.05, **P < 0.01, ***P < 0.001; significant differences versus the pET-32a group are indicated by §P < 0.05, §§P < 0.01, §§§P < 0.001
Discussion
Inflammation is a protective process that limits microbial infection. Innate immune cells recognize PAMPs or DAMPs through pattern recognition receptors, including membrane-bound TLRs and cytoplasmic NLRs, to regulate the occurrence and development of inflammatory responses.
Studies have shown that NLRs play a key role in inducing inflammatory responses to invading parasites during infection [20]. Different members of the NLR family respond to different stimuli; for example, ligand recognition by NLR family members such as NLRP1, NLRP3, NLRP6 and NLRC4 leads to inflammasome activation. Inflammasomes are an important part of the innate immune system and can detect microbial pathogens and activate inflammatory cytokines [21, 22]. Neospora caninum infection induces NLRP3 inflammasome activation [23]. NLRP1, NLRP3 and AIM2 inflammasomes are involved in the immune response during Toxoplasma gondii infection [24]. The expression of NLPR3 was found to be higher in the peritoneal macrophages of Trichinella spiralis-infected mice than in uninfected mice [25], a finding that was consistent with previous studies [26, 27]. The NLRP3 inflammasome is activated in response to Leishmania infection, and is an important host response for limiting parasite replication in vitro and in vivo [28]. A recent study showed that AIM2 can amplify signals of infection and trigger atypical NLRP3 activation [29], and another study showed that the inflammasome adaptor ASC is required for their assembly [30]. Once the inflammasome complex is assembled, pro-caspase-1/-11 can be cleaved into active caspase-1/-11, which further converts the pro-inflammatory cytokines IL-1β and IL-18 into mature active forms. Caspase-1 cleavage of GSDMD triggers cell pyroptosis [31–34]. Pyroptosis is characterized by continuous swelling of the cells until the rupture of the cell membrane, resulting in the release of cell contents and the activation of inflammatory reactions [34–37].
It is well known that parasite excretory–secretory (ES) proteins function as bioactive molecules that directly interact with the host immune system. In previous study, we successfully predicted and annotated the secretory proteome by using the whole-genome and transcriptome data of B. schroederi [38]. At the same time, we found that many of the previously reported proteases and protease inhibitors involved in the parasite–host interaction were also abundant in the secretory proteome of B. schroederi. We then cloned and expressed the CPI that is highly expressed in migratory larvae of B. schroederi (rBsCPI-1) and proved that rBsCPI-1 is a component of the ES proteins. We also found that rBsCPI-1 antigen was also recognized by serum from mouse infected with B. schroederi (unpublished findings). Therefore, in the present study we focused on analyzing whether ES protein and rBsCPI-1 have regulatory effects on the activation of inflammasomes.
After co-incubating PBS (negative control), pET-32a, rBsCPI-1, BsES and Nigericin (positive control) with MDMs, we were able to detect activation of inflammasomes. The qPCR, western blotting and ELISA results showed that both rBsCPI-1 and BsES can significantly induce the high expression of NLRP3, caspase-1/-11 and ASC and further promote cleavage of the N-terminal of GSDMD while, at the same time, promoting the production and secretion of IL-1β and IL-18, thereby regulating the inflammatory response. We then analyzed the signaling pathway by which rBsCPI-1 regulates the NLRP3 inflammasome activation. Studies have shown that NLRP3 activation requires immune cells to be exposed to priming stimuli, such as TLRs or cytokine receptors. After initiation, NLRP3 is activated by certain stimuli, such as ion flux, ROS and lysosomal damage [39].
ROS is involved in the regulation of many important cellular events, including proliferation, differentiation, immune response, cell growth and cell survival [40, 41]. ROS production has been identified as a key factor in NLRP3 inflammasome activation in parasite infections such as those by Plasmodium falciparum [42], Trypanosoma cruzi [43] and Toxoplasma gondii [44]. However, ROS-induced activation of NLRP3 inflammasome remains controversial [45–48]. TLR4 plays a key role in mediating ROS production [49]. Our previous experiments have confirmed that rBsCPI-1 can play an immunoregulatory role by being recognized by TLR4 on the surface of MDMs. Therefore, we speculated that rBsCPI-1 activates the NLRP3 inflammasome by binding to TLR4 and then inducing the high expression of intracellular ROS. To verify this speculation, we pre-blocked MDMs with TLR4 blocking antibody for 24 h, then incubated rBsCPI-1 with MDMs for 12 h; changes in ROS expression were detected in MDMs. The experimental results showed that the ability of rBsCPI-1 to induce ROS production decreased significantly when the TLR4 was blocked. On the other hand, we pre-incubated MDMs with a ROS inhibitor (NAC) for 4 h, and then co-incubated MDMs with rBsCPI-1. Western blotting results showed that the expression level of NLRP3 activated by rBsCPI-1 was significantly reduced with ROS inhibition, and even significantly lower than that in the control and NAC + PBS groups. We speculate that the reason for this may be that rBsCPI-1, as a CPI, negatively impacted the inflammatory caspases when ROS was inhibited, thereby further inhibiting the activation of the inflammasome. This led us to make a preliminary suggestion that rBsCPI-1 induces MDMs to generate high levels of ROS after binding to TLR4 on the surface of MDMs, and that ROS acts as an activator of NLRP3, thereby inducing the activation of NLRP3. In brief, rBsCPI-1 induces inflammasome assembly via the TLR4–ROS–NLRP3 pathway.
Studies have shown that the NLRP3 receptor as well as ASC and caspase-1/-11 are critical for the secretion of active factors that ultimately induce the production of ROS [50]. Therefore, ROS not only plays a key role in the activation of the inflammasome, but it is also a product of its own activation [51]. We pre-incubated MDMs with NLRP3 inhibitor (MCC950) for 4 h and then co-incubated them with rBsCPI-1. Changes in the expression level of ROS were detected. The level of ROS production in the MCC950 + CPI-1 group was significantly lower than that in the PBS + CPI-1 group. Therefore, our experimental results proved that ROS is both an NLRP3 activator and, to a certain extent, an NLRP3 activation product. We also detected the activation of caspase-1, IL-1β, IL-18 and pyroptosis marker cleaved N-terminal GSDMS induced by rBsCPI-1 after MDMs were pre-incubated with MCC950 Sodium. The experimental results showed that the activation levels of caspase-1, IL-1β, IL-18 and cleaved N-terminal GSDMS in the MCC950 + CPI-1 group were significantly lower than those in the PBS + CPI-1 group, but significantly higher than those in the control group. Therefore, our results preliminarily confirm that rBsCPI-1 induces pyroptosis by activating the NLRP3 inflammasome. We also performed recombinant protein immunization mice experiments, and the experimental results showed that rBsCPI-1 significantly induced the activation of NLRP3, caspase-1/-11, IL-1β and IL-18, thereby promoting the assembly of the NLRP3 inflammasome, which in turn is involved in the regulation of inflammatory response; these results are consistent with those of the in vitro experiments.
Conclusions
We analyzed the effect of rBsCPI-1 in a mice model through in vitro and in vivo experiments and arrived at the following conclusions. rBsCPI-1 induces the activation of inflammasomes through the TLR4–ROS–NLRP3 signaling pathway. On the other hand, activation of the NLRP3 inflammasome further induces ROS expression. Inflammasomes induce cell pyroptosis and promote the production and secretion of the pro-inflammatory cytokines IL-1β and IL-18, thereby regulating the occurrence and development of inflammatory responses.
Acknowledgements
The authors are extremely grateful to the teachers and colleagues at the Public Laboratory of Sichuan Province, Sichuan Agricultural University for kindly allowing us to conduct the protein purification experiments and use their fluorescence microscope in their laboratories. We would also like to thank the native English-speaking scientists of Elixigen Company (Huntington Beach, California) for editing our manuscript.
Abbreviations
- B. schroederi
Baylisascaris schroederi
- CPI
Cysteine protease inhibitors
- NLRP3
Nucleotide-binding oligomerization domain, leucine-rich repeat and pyrin domain-containing 3
- MDMs
Monocyte derived macrophages
- ES
Excretory-secretory antigen
- TLR4
Toll-like receptor 4
- ROS
Reactive oxygen species
- IL-1β
Interleukin-1 beta
- IL-18
Interleukin-18
- GSDMD
Gasdermin D
- PRRs
Pattern recognition receptors
- PAMPs
Pathogen-associated molecular patterns
- DAMPs
Damage-associated molecular patterns
- ASC
Apoptosis-associated speck-like protein containing CARD
- AIM2
Absent in melanoma 2
- LDH
Lactate dehydrogenase
- NAC
N-Acetylcysteine
- DCFH-DA
2,7-Dichlorodi-hydrofluorescein diacetate
- LPS
Lipopolysaccharide
- NOD
Cytoplasmic nucleotide oligomerization domain
- NLR
NOD-like receptor
- NLRC4
Nucleotide-binding oligomerization domain, leucine-rich repeat and caspase recruitment domain-containing 4
Author contributions
XJY participated in the design of the study, feeding of the experimental animals, conducting the experiments and statistical analysis and writing of the manuscript. XL and XJY participated in the feeding of the experimental animals and performed the experiments. GXB, XY, HR and XJ contributed to sample collection and performed the experiments. YGY participated in the design of the study. GXB, XY and PXR helped in study design. All authors read and approved the final manuscript.
Funding
This work was supported by a Grant from the Research Fund (Project No. CPF2017-24) for the Chengdu Research of Giant Panda Breeding, and also supported by a Grant from the Special funding for postdoctoral research projects in Sichuan Province (Project No. 2122999018).
Availability of data and materials
The datasets used or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The animal study protocol was reviewed and approved by the Animal Care and Use Committee of Sichuan Agricultural University (no. SYXK 2019-189). All animal procedures used in this study were performed in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, Bethesda, MD, USA) and recommendations of the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines (http://www.nc3rs.org.uk/arrive-guidelines). All applicable institutional and national guidelines for the care and use of animals were followed.
Consent for publish
Not applicable.
Competing interests
The authors declare that they do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jingyun Xu, Email: xujingyun621@126.com.
Xiaobin Gu, Email: guxiaobin198225@126.com.
Yue Xie, Email: xyue1985@126.com.
Ran He, Email: ranhe1991@hotmail.com.
Jing Xu, Email: xujing90ss@gmail.com.
Lang Xiong, Email: xionglang1121@outlook.com.
Xuerong Peng, Email: pxuerong@126.com.
Guangyou Yang, Email: guangyou1963@126.com.
References
- 1.Peng Z, Zhang C, Shen M, Bao H, Hou Z, He S, et al. Baylisascaris schroederi Infection in Giant Pandas (Ailuropoda melanoleuca) in Foping National Nature Reserve, China. J Wildl Dis. 2017;53:854–858. doi: 10.7589/2016-08-190. [DOI] [PubMed] [Google Scholar]
- 2.Qin Z, Liu S, Bai M, Geng Y, Miller DL, Zhao R, et al. First report of fatal baylisascariasis-induced acute pancreatitis in a giant panda. Parasitol Int. 2021;84:102380. doi: 10.1016/j.parint.2021.102380. [DOI] [PubMed] [Google Scholar]
- 3.Zhao GH, Xu MJ, Zhu XQ. Identification and characterization of microRNAs in Baylisascaris schroederi of the giant panda. Parasit Vectors. 2013;6:216. doi: 10.1186/1756-3305-6-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou X, Yu H, Wang N, Xie Y, Liang YN, Li DS, et al. Molecular diagnosis of Baylisascaris schroederi infections in giant panda (Ailuropoda melanoleuca) feces using PCR. J Wildl Dis. 2013;49:1052–1055. doi: 10.7589/2012-07-175. [DOI] [PubMed] [Google Scholar]
- 5.Sun Y, Liu G, Li Z, Chen Y, Liu Y, Liu B, et al. Modulation of dendritic cell function and immune response by cysteine protease inhibitor from murine nematode parasite Heligmosomoides polygyrus. Immunology. 2013;138:370–381. doi: 10.1111/imm.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dainichi T, Maekawa Y, Ishii K, Zhang T, Nashed BF, Sakai T, et al. Nippocystatin, a cysteine protease inhibitor from Nippostrongylus brasiliensis, inhibits antigen processing and modulates antigen-specific immune response. Infect Immun. 2001;69:7380–7386. doi: 10.1128/IAI.69.12.7380-7386.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Serveau C, Lalmanach G, Juliano MA, Scharfstein J, Juliano L, Gauthier F. Investigation of the substrate specificity of cruzipain, the major cysteine proteinase of Trypanosoma cruzi, through the use of cystatin-derived substrates and inhibitors. Biochem J. 1996;313:951–956. doi: 10.1042/bj3130951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Irvine JW, Coombs GH, North MJ. Cystatin-like cysteine proteinase inhibitors of parasitic protozoa. FEMS Microbiol Lett. 1992;75:67–72. doi: 10.1111/j.1574-6968.1992.tb05395.x. [DOI] [PubMed] [Google Scholar]
- 9.Smith BO, Picken NC, Westrop GD, Bromek K, Mottram JC, Coombs GH. The structure of Leishmania mexicana ICP provides evidence for convergent evolution of cysteine peptidase inhibitors. J Biol Chem. 2006;281:5821–5828. doi: 10.1074/jbc.M510868200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mishra M, Singh V, Tellis MB, Joshi RS, Pandey KC, Singh S. Cyclic peptide engineered from phytocystatin inhibitory hairpin loop as an effective modulator of falcipains and potent antimalarial. J Biomol Struct Dyn. 2020;9:1–13. doi: 10.1080/07391102.2020.1848629. [DOI] [PubMed] [Google Scholar]
- 11.Pei G, Dorhoi A. NOD-Like receptors: guards of cellular homeostasis perturbation during infection. Int J Mol Sci. 2021;22:6714. doi: 10.3390/ijms22136714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Celias DP, Motrán CC, Cervi L. Helminths turning on the NLRP3 inflammasome: pros and cons. Trends Parasitol. 2020;36:87–90. doi: 10.1016/j.pt.2019.10.012. [DOI] [PubMed] [Google Scholar]
- 13.Chen TTW, Cheng PC, Chang KC, Cao JP, Feng JL, Chen CC, et al. Activation of the NLRP3 and AIM2 inflammasomes in a mouse model of Schistosoma mansoni infection. J Helminthol. 2019;94:e72. doi: 10.1017/S0022149X19000622. [DOI] [PubMed] [Google Scholar]
- 14.Chu JQ, Gao FF, Wu W, Li C, Pan Z, Sun J, et al. Expression profiles of NOD-like receptors and regulation of NLRP3 inflammasome activation in Toxoplasma gondii-infected human small intestinal epithelial cells. Parasit Vectors. 2021;14:153. doi: 10.1186/s13071-021-04666-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matteucci KC, Pereira GJS, Weinlich R, Bortoluci KR. Frontline science: autophagy is a cell autonomous effector mechanism mediated by NLRP3 to control Trypanosoma cruzi infection. J Leukoc Biol. 2019;106:531–540. doi: 10.1002/JLB.HI1118-461R. [DOI] [PubMed] [Google Scholar]
- 16.Santos MLS, Reis EC, Bricher PN, Sousa TN, Brito CFA, Lacerda MVG, et al. Contribution of inflammasome genetics in Plasmodium vivax malaria. Infect Genet Evol. 2016;40:162–166. doi: 10.1016/j.meegid.2016.02.038. [DOI] [PubMed] [Google Scholar]
- 17.Gorfu G, Cirelli KM, Melo MB, Mayer-Barber K, Crown D, Koller BH, et al. Dual role for inflammasome sensors NLRP1 and NLRP3 in murine resistance to Toxoplasma gondii. MBio. 2014;5:e01117-13. doi: 10.1128/mBio.01117-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu JY, Gu XB, Xie Y, He R, Xu J, Xiong L, et al. Regulatory effects of a novel cysteine protease inhibitor in Baylisascaris schroederi migratory larvae on mice immune cells. Parasit Vectors. 2022;15:121. doi: 10.1186/s13071-022-05240-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin X, Kruth HS. Culture of macrophage colony-stimulating factor differentiated human monocyte-derived macrophages. J Vis Exp. 2016;112:54244. doi: 10.3791/54244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Carvalho RVH, Zamboni DS. Infammasome activation in response to intracellular protozoan parasites. Trends Parasitol. 2020;36:459–472. doi: 10.1016/j.pt.2020.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013;13:397–411. doi: 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lamkanfi M, Mueller JL, Vitari AC, Misaghi S, Fedorova A, Deshayes K, et al. Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. J Cell Biol. 2009;187:61–70. doi: 10.1083/jcb.200903124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang XC, Gong PT, Zhang X, Li S, Lu XY, Zhao CY, et al. NLRP3 inflammasome participates in host response to Neospora caninum infection. Front Immunol. 2018;9:1791. doi: 10.3389/fimmu.2018.01791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y, Zhu J, Cao Y, Shen J, Yu L. Insight into inflammasome signaling: implications for Toxoplasma gondii infection. Front Immunol. 2020;11:583193. doi: 10.3389/fimmu.2020.583193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guan F, Jiang W, Bai Y, Hou X, Jiang C, Zhang C, et al. Purinergic P2X7 receptor mediates the elimination of Trichinella spiralis by activating NF-κB/NLRP3/IL-1β pathway in macrophages. Infect Immun. 2021;89:e00683-20. doi: 10.1128/IAI.00683-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin X, Yang Y, Ding J, Liu X, Shi H, Luo X, et al. Nod-like receptor pyrin domain containing 3 plays a key role in the development of Th2 cell-mediated host defenses against Trichinella spiralis infection. Vet Parasitol. 2021;297:109159. doi: 10.1016/j.vetpar.2020.109159. [DOI] [PubMed] [Google Scholar]
- 27.Jin X, Bai X, Yang Y, Ding J, Shi H, Fu B, et al. NLRP3 played a role in Trichinella spiralis-triggered Th2 and regulatory T cells response. Vet Res. 2020;51:107. doi: 10.1186/s13567-020-00829-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lima-Junior DS, Costa DL, Carregaro V, Cunha LD, Silva ALN, Mineo TWP, et al. Inflammasome-derived IL-1b production induces nitric oxidemediated resistance to Leishmania. Nat Med. 2013;19:909–915. doi: 10.1038/nm.3221. [DOI] [PubMed] [Google Scholar]
- 29.Cunha LD, Silva ALN, Ribeiro JM, Mascarenhas DPA, Quirino GSF, Santos LL, et al. AIM2 engages active but unprocessed Caspase-1 to induce noncanonical activation of the NLRP3 inflammasome. Cell Rep. 2017;20:794–805. doi: 10.1016/j.celrep.2017.06.086. [DOI] [PubMed] [Google Scholar]
- 30.Tschopp J, Schroder K. NLRP3 inflammasome activation: the convergence of multiple signalling pathways on ROS production? Nat Rev Immunol. 2010;10:210. doi: 10.1038/nri2725. [DOI] [PubMed] [Google Scholar]
- 31.Jackson JA, Friberg IM, Little S, Bradley JE. Review series on helminths, immune modulation and the hygiene hypothesis: immunity against helminths and immunological phenomena in modern human populations: coevolutionary legacies? Immunology. 2009;126:18–27. doi: 10.1111/j.1365-2567.2008.03010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lamkanfi M, Dixit VM. Inflammasomes and their roles in health and disease. Annu Rev Cell Dev Biol. 2012;28:137–161. doi: 10.1146/annurev-cellbio-101011-155745. [DOI] [PubMed] [Google Scholar]
- 33.Shi J, Gao W, Shao F. Pyroptosis: Gasdermin-mediated programmed necrotic cell death. Trends Biochem Sci. 2017;42:245–254. doi: 10.1016/j.tibs.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 34.Man SM, Karki R, Kanneganti TD. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol Rev. 2017;277:61–75. doi: 10.1111/imr.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Case CL, Kohler LJ, Lima JB, Strowig T, de Zoete MR, Flavell RA, et al. Caspase-11 stimulates rapid flagellin-independent pyroptosis in response to legionella pneumophila. Proc Natl Acad Sci USA. 2013;110:1851–1856. doi: 10.1073/pnas.1211521110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 37.Malik A, Kanneganti TD. Inflammasome activation and assembly at a glance. J Cell Sci. 2017;130:3955–3963. doi: 10.1242/jcs.207365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xie Y, Wang S, Wu S, Gao S, Meng Q, Wang C, et al. Genome of the giant panda roundworm illuminates its host shift and parasitic adaptation. Genomics Proteomics Bioinform. 2021 doi: 10.1016/j.gpb.2021.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kelley N, Jeltema D, Duan Y, He Y. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci. 2019;20:3328. doi: 10.3390/ijms20133328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamada J, Yoshimura S, Yamakawa H, Sawada M, Nakagawa M, Hara S, et al. Cell permeable ROS scavengers, Tiron and Tempol, rescue PC12 cell death caused by pyrogallol or hypoxia/reoxygenation. Neurosci Res. 2003;45:1–8. doi: 10.1016/S0168-0102(02)00196-7. [DOI] [PubMed] [Google Scholar]
- 41.Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24:981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jaramillo M, Godbout M, Olivier M. Hemozoin induces macrophage chemokine expression through oxidative stress-dependent and -independent mechanisms. J Immunol. 2005;174:475–484. doi: 10.4049/jimmunol.174.1.475. [DOI] [PubMed] [Google Scholar]
- 43.Silva GK, Costa RS, Silveira TN, Caetano BC, Horta CV, Gutierrez FR, et al. Apoptosis-associated speck-like protein containing a caspase recruitment domain infammasomes mediate IL-1beta response and host resistance to Trypanosoma cruzi infection. J Immunol. 2013;191:3373–3383. doi: 10.4049/jimmunol.1203293. [DOI] [PubMed] [Google Scholar]
- 44.Gov L, Schneider CA, Lima TS, Pandori W, Lodoen MB. NLRP3 and potassium efux drive rapid IL-1beta release from primary human monocytes during Toxoplasma gondii infection. J Immunol. 2017;199:2855–2864. doi: 10.4049/jimmunol.1700245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ritter M, Gross O, Kays S, Ruland J, Nimmerjahn F, Saijo S, et al. Schistosoma mansoni triggers Dectin-2, which activates the Nlrp3 inflammasome and alters adaptive immune responses. Proc Natl Acad Sci USA. 2010;107:20459–20464. doi: 10.1073/pnas.1010337107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zamboni DS, Lima-Junior DS. Infammasomes in host response to protozoan parasites. Immunol Rev. 2015;265:156–171. doi: 10.1111/imr.12291. [DOI] [PubMed] [Google Scholar]
- 47.Gupta AK, Ghosh K, Palit S, Barua J, Das PK, Ukil A. Leishmania donovani inhibits infammasome-dependent macrophage activation by exploiting the negative regulatory proteins A20 and UCP2. FASEB J. 2017;31:5087–5101. doi: 10.1096/fj.201700407R. [DOI] [PubMed] [Google Scholar]
- 48.Li L, Wang XC, Gong PT, Zhang N, Zhang X, Li S, et al. ROS-mediated NLRP3 infammasome activation participates in the response against Neospora caninum infection. Parasit Vectors. 2020;13:449. doi: 10.1186/s13071-020-04331-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Asehnoune K, Strassheim D, Mitra S, Kim JY, Abraham E. Involvement of reactive oxygen species in Toll-like receptor 4-dependent activation of NF-kappa B. J Immunol. 2004;172:2522–2529. doi: 10.4049/jimmunol.172.4.2522. [DOI] [PubMed] [Google Scholar]
- 50.Ty MC, Zuniga M, Götz A, Kayal S, Sahu PK, Mohanty A, et al. Malaria inflammation by xanthine oxidase-produced reactive oxygen species. EMBO Mol Med. 2019;1:1–15. doi: 10.15252/emmm.201809903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martinon F. Signaling by ROS drives inflammasome activation. Eur J Immunol. 2010;40:616–619. doi: 10.1002/eji.200940168. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used or analysed during the current study are available from the corresponding author on reasonable request.