Abstract
Background
Establishing the subgroup analysis of the fallopian tubes (tubes) is a commonly undertaken diagnostic investigation for women with subfertility. This is usually achieved by flushing contrast medium through the tubes and visualising patency on radiographs, ultrasonography or laparoscopy. Many women were noted to conceive in the first three to six months after tubal flushing, raising the possibility that tubal flushing could also be a treatment for infertility. There has been debate about which contrast medium should be used (water‐soluble or oil‐soluble media) as this may influence pregnancy rates. An important adverse event during tubal flushing is intravasation (backflow of contrast medium into the blood or lymphatic vessels),which could lead to embolism although it is asymptomatic in most cases.
Objectives
To evaluate the effectiveness and safety of tubal flushing with oil‐soluble contrast media (OSCM) and water‐soluble contrast media (WSCM) on subsequent fertility outcomes in women with subfertility.
Search methods
We searched the Cochrane Gynaecology and Fertility Group Specialised Register of controlled trials, MEDLINE, Embase, CENTRAL, PsycINFO, reference lists of identified articles and trial registries. The most recent search was conducted in April 2020.
Selection criteria
Randomised controlled trials (RCTs) comparing tubal flushing with OSCM, WSCM with each other or with no treatment, in women with subfertility.
Data collection and analysis
Two review authors independently selected the trials, assessed risk of bias and extracted data. We contacted study authors for additional information. The overall quality of the evidence was assessed using GRADE methods.
Main results
Fifteen trials involving 3864 women were included in this systematic review. Overall, the quality of evidence varied from very low to moderate: the main limitations were risk of bias, heterogeneity and imprecision.
OSCM versus no treatment
Four studies (506 women) were included in this comparison.
Tubal flushing with OSCM may increase the odds of live birth (odds ratio (OR) 3.27, 95% confidence interval (CI) 1.57 to 6.85, 3 RCTs, 204 women, I2 = 0, low‐quality evidence). This suggests that if the chance of live birth following no treatment is assumed to be 11%, the chance following tubal flushing with OSCM would be between 16% and 46%.
Tubal flushing with OSCM may increase in the odds of clinical pregnancy (OR 3.54, 95% CI 2.08 to 6.02, 4 RCTs, 506 women, I2 = 18%, low‐quality evidence). This suggests that if the chance of clinical pregnancy following no treatment is assumed to be 9%, the chance following tubal flushing with OSCM would be between 17% and 37%.
No study measured intravasation or other adverse events such as infection, haemorrhage and congenital abnormalities.
WSCM versus no treatment
Only one study (334 women) was included in this comparison.
We are uncertain whether tubal flushing with WSCM increase live birth compared to no treatment (OR 1.13, 95% CI 0.67 to 1.91, 1 RCT, 334 women, low‐quality evidence). This suggests that if the chance of live birth following no treatment is assumed to be 21%, the chance following tubal flushing with WSCM would be between 15% and 33%.
We are uncertain whether tubal flushing with WSCM increases clinical pregnancy compared to no treatment (OR 1.14, 95% CI 0.71 to 1.84, 1 RCT, 334 women, low‐quality evidence). This suggests that if the chance of clinical pregnancy following no treatment is assumed to be 27%, the chance following tubal flushing with WSCM would be between 29% and 40%.
One case with pelvic infection was reported in the WSCM group and no case with infection in the no treatment group in a one study (334 women). Meta‐analysis was not performed due to the rare events.
No study measured intravasation or other adverse events such as infection, haemorrhage and congenital abnormalities.
OSCM versus WSCM
Six studies (2598 women) were included in this comparison.
Three studies reported live birth, including two with higher live birth in the OSCM group (OR 1.64, 95% CI 1.27 to 2.11, 1119 women; OR 3.45, 95% CI 1.97 to 6.03, 398 women); and one with insufficient evidence of a difference between groups (OR 0.92, 95% CI 0.60 to 1.40, 533 women). Given the substantial heterogeneity observed (I2 = 86%), meta‐analysis was not performed.
Tubal flushing with OSCM probably increased in the odds of intravasation (asymptomatic) compared to tubal flushing with WSCM (OR 5.00, 95% CI 2.25 to 11.12, 4 RCTs, 1912 women, I2 = 0, moderate‐quality evidence). This suggests that if the chance of intravasation following tubal flushing with WSCM is assumed to be 1%, the chance following tubal flushing with OSCM would be between 2% and 9%.
Tubal flushing with OSCM may increase the odds of clinical pregnancy (OR 1.42, 95% CI 1.10 to 1.85, 6 RCTs, 2598 women, I2 = 41%, low‐quality evidence). This suggests that if the chance of clinical pregnancy following tubal flushing with WSCM is assumed to be 26%, the chance following tubal flushing with OSCM would be between 28% and 39%.
We are uncertain whether tubal flushing with OSCM decreases the odds of infection (OR 0.22, 95% CI 0.04 to 1.22, 2 RCTs, 662 women, I2 = 0, very low‐quality evidence) or haemorrhage (OR 0.65, 95% CI 0.40 to 1.06, 2 RCTs, 662 women, I2 = 0, very low‐quality evidence).
Three neonates with congenital abnormalities were reported in the OSCM group while no congenital abnormality was reported in the WSCM group in one study (1119 women). No meta‐analysis was performed due to the rare events.
Authors' conclusions
The evidence suggests that compared to no treatment, tubal flushing with OSCM may increase the chance of live birth and clinical pregnancy, while it is uncertain whether tubal flushing with WSCM improves those outcomes. Compared to tubal flushing with WSCM, OSCM may improve clinical pregnancy while meta‐analysis was impossible for live birth due to heterogeneity. Evidence also suggests that OSCM is associated with an increased risk of asymptomatic intravasation. Overall, adverse events, especially long‐term adverse events, are poorly reported across studies.
Plain language summary
Tubal flushing for subfertility
Review question
Cochrane authors reviewed the evidence about the effect of using different contrast media during the flushing of fallopian tubes in women with subfertility.
Background
Blocked fallopian tubes means that sperm cannot reach the egg in the tube. Establishing whether the tubes are open (patent) is important and requires contrast media (dye) to be pushed through the tubes either at the time of an x‐ray (hysterosalpingogram), during ultrasound (hysterosalpingo‐contrast‐sonography) or during keyhole operation (laparoscopy). It has been reported that many women conceive in the first three to six months following tubal flushing although it is unclear why this occurs. There has been debate about whether oil‐soluble contrast medium (OSCM) or water‐soluble contrast medium (WSCM) should be used, as this may influence live birth. An important adverse event during the procedure is the backflow of contrast medium into the blood or lymphatic vessels, which is called intravasation and is generally asymptomatic.
Study characteristics
The evidence was current to April 2020. We included randomised controlled trials (RCTs) looking at the effect of flushing the tubes with OSCM and WSCM with each other or with no treatment in women with subfertility. Such women were those who had not been able to conceive after at least six months of unprotected sexual intercourse. We also looked at the rates of adverse events, including intravasation, infection and bleeding.
Key results
Fifteen trials involving 3864 women were included in this systematic review. Compared to no treatment, tubal flushing with OSCM may increase the chance of live birth and clinical pregnancy. This suggests that if the chance of live birth following no treatment is assumed to be 11%, the chance following tubal flushing with OSCM would be between 16% and 46%. We are uncertain whether tubal flushing with WSCM increases live birth or clinical pregnancy compared to no treatment. This suggests that if the chance of live birth following no treatment is assumed to be 21%, the chance following tubal flushing with WSCM would be between 15% and 33%. In the comparison between OSCM versus WSCM, the data were not sufficiently similar to combine in a meta‐analysis. Tubal flushing with OSCM may increase the chance of clinical pregnancy. With regards to adverse events, tubal flushing with OSCM probably increased in the chance of intravasation (asymptomatic) compared to tubal flushing with WSCM. This suggests that if the chance of intravasation following tubal flushing with WSCM is assumed to be 1%, the chance following tubal flushing with OSCM would be between 2% and 9%. Evidence on other adverse events was poorly reported and inconclusive.
Quality of the evidence
The overall quality of the evidence was very low to moderate for all comparisons. The main limitations were imprecision, risk of bias and inconsistency. There were too few studies to evaluate the risk of publication bias.
Summary of findings
Background
Description of the condition
Subfertility, also called infertility, is estimated to affect 186 million people globally (Inhorn 2015), The prevalence of infertility is at least 12% (Datta 2016; Zhou 2018), Common causes of subfertility include male factor subfertility, ovulation dysfunction, damage or blockage of the fallopian tubes, with a large proportion still unexplained (Evers 2002; Farquhar 2019).
Fallopian tubes play an important role in gamete transport, fertilisation and early embryo development and transport (Lyons 2006). Dysfunction of the Fallopian tubes is a major cause of infertility and accounts for up to 30% of couples with subfertility (Evers 2002). Therefore, establishing the patency of the fallopian tubes is a commonly undertaken diagnostic investigation for women with subfertility and it constitutes an essential part of the fertility work‐up, as recommended in the clinical guidelines (ASRM 2015; NICE 2013).
Description of the intervention
Tubal patency testing is usually achieved by flushing contrast medium through the fallopian tubes and visualising patency on radiographs (hysterosalpingography, HSG), ultrasonography (hysterosalpingo‐contrast sonography, or HyCoSy) or laparoscopy with dye testing. These were introduced into clinical practice as diagnostic testing approaches. However, it has been noted that many women conceive in the first three to six months after tubal flushing, which has raised the possibility that tubal flushing could also be a treatment for subfertility. There has been debate about which contrast medium should be used (water‐soluble contrast medium (WSCM) or oil‐soluble contrast media (OSCM)) as this may influence fertility outcomes.
One of the earlier descriptions of a possible beneficial therapeutic effect of OSCM came from a radiologist (Gillespie 1965). Gillespie had changed practice from OSCM to WSCM for safety reasons. A decreased pregnancy rate from 41% to 27% over the following 12 months prompted a change back to the use of oily media, and the pregnancy rate rose again to 44%. Other non‐randomised controlled studies (Acton 1988; Barwin 1971; DeCherney 1980; Mackey 1971; Yaegashi 1987) supported the hypothesis of the fertility‐enhancing effect of OSCM.
Traditionally, HSGs were performed with OSCM. Their use was gradually replaced by WSCM for a number of reasons; (i) WSCM permits better imaging of the tubal mucosal folds and ampullary rugae (internal architecture of the fallopian tubes) than OSCM (Soules 1982); (ii) OSCM have a high viscosity, which results in slow filling of the fallopian tubes often necessitating an inconveniently late film after 24 hours; (iii) OSCM reabsorption is slow, leading to prolonged persistence of OSCM within the pelvic cavity; (iv) if there is accumulation of OSCM within a blocked fallopian tube a chronic inflammatory reaction, called a lipo‐granuloma, may occur; this has not been reported in women with patent fallopian tubes and is not known to have long‐term consequences (Acton 1988); (v) the potential consequences of intravasation (backflow of contrast medium into the blood or lymphatic vessels) of OSCM into the pelvic blood vessels and lymphatics are allergic reactions or anaphylaxis (Lindequist 1991); and (vi) WSCM are generally cheaper than OSCM.
On the other hand, irrespective of subsequent pregnancy rates, OSCM offer some advantages over WSCM; (i) the slow filling of the fallopian tubes owing to the higher viscosity of OSCM can necessitate a 'late' film but some authorities regard the 24‐hour film as an advantage because of the additional information this gives, mainly in the evaluation of adhesions after slow peritoneal spillage (Bateman 1987); and (ii) less pain has been reported with OSCM than with WSCM, probably because of less chemical irritation of the peritoneum (Soules 1982).
In current clinical practice, OSCM is mainly used during HSG and WSCM is used in all types of tubal patency testing procedures, including HSG, HyCoSy and laparoscopy. Laparoscopy with dye testing has been widely accepted as the gold standard to evaluate fallopian tube patency (Saunders 2011) and it is recommended for women with potential comorbidities so that tubal and other pelvic pathology can be assessed at the same time (NICE 2013). More recently, transvaginal hydrolaparoscopy has been applied in clinics as an alternative and safe method to evaluate tubal patency in an outpatient setting (Coenders‐Tros 2016). HSG has a longer history, but it is worth noting that all radiopaque contrast media used for HSG at present contain iodine and therefore HSG is not suitable for women who are sensitive to iodine (Lim 2011), and women are exposed to pelvic radiation during HSG procedures. HyCoSy is also becoming popular due to the potential advantages including comprehensive evaluation, methodologically simple, cost‐effective, and time‐efficient (Lim 2011).
One of the most important adverse events of tubal flushing is intravasation. It is mostly asymptomatic and occurs in 1% to 7% of the cases (Roest 2020; Dusak 2013). However, in very rare circumstances it could lead to life‐threatening condition such as embolism (Chalazonitis 2009). The safety concerns about thyroid function of mother and child is based on the effects of iodinated contrast media (So 2017; Satoh 2015) and therefore are only applicable to contrast media during HSG, not other procedures.
How the intervention might work
There are a number of explanations behind the theory of improving fertility outcomes after flushing of the fallopian tubes as summarised in Figure 1. The main potential mechanisms include the following.
1.

Main potential mechanisms of tubal flushing on subsequent fertility outcomes.
This figure illustrates the potential effects of tubal flushing on the Fallopian tubes, the endometrium and the peritoneum. (Developed by Rui Wang and reproduced here with permission)
(i) The fallopian tubes: mechanical flushing out of debris or mucus plugs from the fallopian tubes, therefore unblocking undamaged tubes (Gillespie 1965). Such debris may not necessarily block the fallopian tube but may hinder conception or embryo transport along the fallopian tube. In addition, contrast media could also enhance ciliary activity (Soules 1982).
Histological examination of resected 'obstructed' tubal segments often fails to confirm luminal occlusion (Grant 1971), but amorphous matter has been found within tubal sections (Sulak 1987) and its presence confirmed at falloposcopy (Kerin 1991). Histology of this tissue, obtained by hydrotubating the tube at falloposcopy, has revealed casts of the tube comprised of aggregates of histiocytic‐like cells from the mucosal stroma.
Observational studies (Capitanio 1991; Novy 1988; Thurmond 1990) have reported a high tubal patency and pregnancy rate after selective transcervical fallopian tube catheterisation under fluoroscopic or hysteroscopic control in women with previously diagnosed proximal tubal obstruction on HSG with a WSCM or dye laparoscopy. This might be attributable to the 'flushing out' of isthmic plugs. This hypothesis is also supported by a recent study where women with moderate to severe pain during HSG were found to have a higher ongoing pregnancy rate, especially in those undergoing HSG with OSCM (van Welie 2019). The increased intrauterine pressure during tubal flushing could potentially cause pain as well as flushing out pregnancy‐hindering debris or mucus plugs (van Welie 2019).
(ii) The endometrium: increasing endometrial receptivity via immunobiological effect on the endometrium (Sawatari 1993; Yun 2004). It is possible that endometrial leukocyte populations may be altered and there is increasing evidence that uterine natural killer cells play an important role in the successful development of early pregnancy (Fukui 1999).
(iii) The peritoneum: modulation of peritoneal macrophages (Johnson 1992). OSCM have been shown to alter interleukin and prostaglandin production by peritoneal macrophages (Sawatari 1993) and to modulate peritoneal macrophage activity amongst rats during phagocytosis of sperm (Mikulska 1994). More recent evidence reveals the modulation effect of OSCM on dendritic cell and regulatory T cell profiles in the peritoneal cavity, which may contribute to the improvement of fertility outcomes (Izumi 2017).
Why it is important to do this review
The first systematic review in this field was published in 1994 (Watson 1994). The original Cochrane Review (Vandekerckhove 1996) first published in 1996, was an expansion and update of that review. There have since been four further updates (Johnson 2002; Johnson 2005a; Johnson 2007; Mohiyiddeen 2015).
Tubal flushing is a relatively low‐cost minimally‐invasive investigation which is routinely undertaken during initial assessment of infertile couples. However, the effects of tubal flushing with different contrast media on subsequent fertility outcomes remain uncertain in all previous versions. With emerging evidence on this topic over the past five years, it is important to revisit the evidence from trials and update this Cochrane Review.
Objectives
To evaluate the effectiveness and safety of tubal flushing with oil‐soluble contrast media (OSCM) and water‐soluble contrast media (WSCM) on subsequent fertility outcomes in women with subfertility.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) were included. Non‐randomised studies and quasi‐randomised studies were excluded.
Types of participants
Women with subfertility, defined as inability to achieve pregnancy after at least six months of regular unprotected intercourse.
Types of interventions
Tubal flushing with OSCM, WSCM or both. Tubal flushing procedure could be performed in the following approaches.
Tubal flushing by means of hysterosalpingography (HSG)
Tubal flushing at the time of laparoscopy
Tubal flushing at the time of hysterosalpingo contrast sonography (HyCoSy)
Control groups could receive placebo, no treatment or an alternative type of tubal flushing.
Types of outcome measures
Primary outcomes
Primary effectiveness outcome: live birth or ongoing pregnancy per woman. Ongoing pregnancy was only used when live birth was not reported.
Primary safety outcome: intravasation.
Secondary outcomes
Clinical pregnancy per woman
Miscarriage per woman
Ectopic pregnancy per woman
Procedural pain (as a continuous outcome). Pain as dichotomous outcome was also included.
Adverse events (infection, haemorrhage)
Long‐term complications
Search methods for identification of studies
We searched for all published and unpublished RCTs of tubal flushing for women with subfertility, without language restriction and in consultation with the Gynaecology and Fertility Group Information Specialist. The most recent search was conducted in April 2020.
Electronic searches
We searched the following electronic databases, trial registers, and websites;
Cochrane Gynaecology and Fertility Group (CGFG) Specialised Register of Controlled Trials (PROCITE platform; searched 20 April 2020) (Appendix 1)
CENTRAL, via the Cochrane Central Register of Studies Online (CRSO) (Web platform; searched 20 April 2020) (Appendix 2). CENTRAL contains records from CINAHL and the trial registries; clinicaltrials.gov and the World Health Organisation International Trials Registry Platform search portal
MEDLINE (OVID platform; searched from 1946 to 20 April 2020) (Appendix 3)
Embase (OVID platform; searched from 1980 to 20 April 2020) (Appendix 4)
PsycINFO (OVID platform; searched from 1806 to 20 April 2020) (Appendix 5).
We combined the MEDLINE search with the Cochrane highly sensitive search strategy for identifying randomised trials, which appears in Chapter 6 of the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2011). We combined Embase search with trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN) (Scottish Intercollegiate Network www.sign.ac.uk/what-we-do/methodology/search-filters/).
Other electronic sources of trials
Trials registers were searched for ongoing and registered trials:
National Research Register (NRR) (www2.le.ac.uk/library/find/databases/n/nationalresearchregister);
Current Controlled Trials (http://www.controlled‐trials.com);
NHS Centre for Reviews and Dissemination (www.crd.york.ac.uk/CRDWeb/);
US National Institutes of Health (NHI) Clinical Trials Register (www.clinicaltrials.gov);
Epistemonikos database (www.epistemonikos.org).
We searched for any trials with the following keywords
Hysterosalpingogram, HSG or salpingogram
Lipiodol or ethiodol
Water‐soluble contrast media, WSCM, oil‐soluble contrast media or OSCM
Tubal flushing
Searching other resources
In this update, we checked the citation lists of included trials, eligible studies and relevant review articles. We contacted the first or corresponding authors of trials eligible for inclusion to ascertain if they were aware of any ongoing or unpublished trials. We also contacted experts in the field to obtain additional studies.
Data collection and analysis
Selection of studies
After an initial screen of titles and abstracts retrieved by the search, we retrieved the full texts of all potentially eligible studies. Two review authors (from RW, KC and BWM) independently selected the trials for inclusion. Differences of opinion were resolved by consensus after consultation with the other review author (LM and AW).
Data extraction and management
Two of the review authors (RW and KC) independently extracted data, and differences of opinion were resolved by consensus. We sought additional information on trial methodology or actual original trial data from the corresponding authors of trials which appeared to meet the eligibility criteria if aspects of methodology were unclear, or if data were in a form unsuitable for meta‐analysis.
Assessment of risk of bias in included studies
Two review authors (RW and KC) independently assessed the included studies using the Cochrane 'Risk of bias' assessment tool (Higgins 2011) to assess the following seven domains: selection (random sequence generation and allocation concealment); performance (blinding of participants and personnel); detection (blinding of outcome assessors); attrition (incomplete outcome data); reporting (selective reporting); and other bias. Judgements were assigned as high, low or unclear risk of bias for each domain (Higgins 2011). Disagreements were resolved by discussion with a third review author (LM). The conclusions were presented in the 'Risk of Bias' tables and incorporated into the interpretation of review findings by means of sensitivity analyses (Sensitivity analysis). For performance and detection bias, we differentiated subjective outcomes (e.g. pain) and objective outcomes (e.g. all fertility outcomes, intravasation).
Measures of treatment effect
For dichotomous data the numbers of events in the control and intervention groups of each study were used to calculate Mantel‐Haenszel odds ratios (ORs) and 95% confidence intervals (95% CIs).
For continuous data (for example procedural pain), mean differences (MDs) and 95% CIs were calculated.
Unit of analysis issues
The primary analysis was per woman randomised. Miscarriage and ectopic pregnancy were analysed per pregnancy.
Dealing with missing data
The data were analysed on an intention‐to‐treat basis as far as possible and attempts were made to obtain missing data from the original investigators. Where these were unobtainable, imputation of individual values was undertaken for dichotomous outcomes. For instance, live births were assumed not to have occurred in participants with unreported outcomes.
Assessment of heterogeneity
Statistical heterogeneity between the results of different studies was examined by checking the results of Chi2 tests and the I2 statistic. If the I2 was > 50% and Chi2 P value < 0.05, indicating substantial heterogeneity. If I2 was > 80%, then the data were not pooled in a meta‐analysis.
If statistical heterogeneity was present, although the results were pooled, reasons for the heterogeneity were sought and the meta‐analysis results interpreted cautiously.
As part of the heterogeneity assessment we carried out a priori defined subgroup analysis.
Assessment of reporting biases
In view of the difficulty in detecting and correcting for publication bias and other reporting biases, we aimed to minimise their potential impact by ensuring a comprehensive search for eligible studies. If there were 10 or more studies in an analysis, we planned to use a funnel plot to explore the possibility of small‐study effects (a tendency for estimates of the intervention effect to be more beneficial in smaller studies).
Data synthesis
If the studies were sufficiently similar, we combined the data using a fixed‐effect model in the following comparisons.
Tubal flushing with OSCM versus No treatment.
Tubal flushing with WSCM versus No treatment.
Tubal flushing with OSCM versus WSCM.
Tubal flushing with OSCM and WSCM versus WSCM alone.
An increase in the odds of a particular outcome (which may be beneficial, for example in the case of live birth; or detrimental, for example in the case of a complication) was displayed graphically in the meta‐analyses to the right of the centre‐line and a decrease in the odds of an outcome was displayed graphically to the left of the centre line.
Subgroup analysis and investigation of heterogeneity
Subgroup analysis was performed to determine whether findings differed in studies performed mainly for diagnostic reasons as opposed to studies performed mainly for therapeutic reasons. The subgroup analysis was performed for the primary effectiveness outcome.
If we detected significant heterogeneity (defined as P < 0.05 in the Chi2 heterogeneity test), we explored possible explanations in sensitivity analyses. We used a random‐effects model if significant heterogeneity was present.
Sensitivity analysis
A priori, we planned the following sensitivity analyses for the primary effectiveness outcome:
a) restricting the analysis to studies at low risk of bias;
b) using alternative imputation methods (analysing only the available data, i.e. ignoring the missing data);
c) using risk ratios instead of odds ratios;
d) using a random‐effects model instead of a fixed‐effect model.
Overall quality of the body of evidence: 'Summary of findings' tables
We evaluated the overall quality of evidence by assessing five domains: study limitations (risk of bias), inconsistency, imprecision, indirectness and publication bias for each outcome. We presented the overall quality of evidence as high, moderate, low or very low in 'Summary of findings' tables by using GRADEpro tool. We included the following outcomes in the 'Summary of findings' tables: live birth, clinical pregnancy, intravasation, infection, haemorrhage and long‐term complications. We only presented the 'Summary of findings' table for tubal flushing with OSCM versus no treatment, tubal flushing with WSCM versus no treatment, and tubal flushing with OSCM versus WSCM. We did not present 'Summary of findings' tables for tubal flushing with OSCM and WSCM versus WSCM alone, given that the combination approach of tubal flushing is seldom used in clinical practice.
Results
Description of studies
Results of the search
In the April 2020 search, 343 articles were screened for title and abstract. After excluding irrelevant articles, 13 full‐text reports were further assessed. Finally, two studies (three reports) (Dreyer 2017; Johnson 2019) met our inclusion criteria, four articles were excluded (Reilly 2019; van Rijswijk 2018a; van Rijswijk 2018b; Pham 2017) and six studies are ongoing (Cai 2019; Hassan 2015; Legro 2018; Mijatovic 2018; Rosielle 2019; Zhang 2020). The updated review includes 15 trials involving 3864 women.
See Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies .
See Figure 2 for details of the screening and selection process.
2.
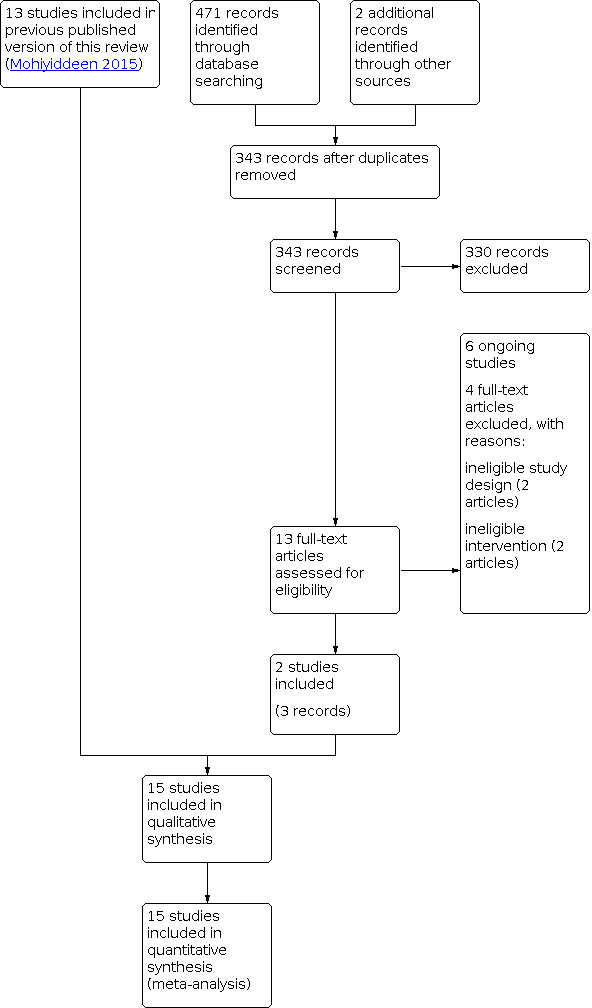
Study flow diagram.
Included studies
Types of studies
The 15 included studies were all parallel group randomised controlled trials (RCTs).
Eight trials were conducted primarily for therapeutic reasons (Al‐Fadhli 2006;Dreyer 2017; Johnson 2004; Letterie 1990; Lindborg 2009; Nugent 2002; Steiner 2003; Johnson 2019);
Seven trials were conducted primarily for diagnostic reasons (Alper 1986; De Boer 1988; Lindequist 1994; Ogata 1993; Rasmussen 1991; Spring 2000; Yang 1989).
Types of interventions
Four trials including 506 participants assessed tubal flushing with oil‐soluble contrast media (OSCM) versus no treatment (Johnson 2004; Nugent 2002; Ogata 1993; Johnson 2019). Johnson 2019 compared immediate tubal flushing with OSCM versus delayed tubal flushing with OSCM (3‐month delay). Data within the first three months were included and the trial was considered as tubal flushing with OSCM versus no treatment.
One trial including 334 participants assessed tubal flushing with : water‐soluble contrast media (WSCM) versus no treatment (Lindborg 2009).
Six trials including 2598 participants assessed tubal flushing with OSCM versus WSCM (Alper 1986; De Boer 1988; Dreyer 2017; Lindequist 1994; Rasmussen 1991; Spring 2000).
Five trials including 686 participants assessed tubal flushing with compared OSCM and WSCM versus WSCM alone (Al‐Fadhli 2006; Letterie 1990; Spring 2000; Steiner 2003; Yang 1989).
The included studies and their methodological details are summarised in the table Characteristics of included studies.
Types of participants
Women or couples with infertility were considered eligible for inclusion and all trials included at least women with unexplained infertility. Fifteen trials involving 3864 women were included in this systematic review; the number of participants in each study ranged from 12 (Johnson 2019) to 1119 (Dreyer 2017). The duration of infertility was at least six months in all but three trials where duration of infertility was not specified (Al‐Fadhli 2006; Ogata 1993; Yang 1989).
The mean age or age range was not stated in three trials (Ogata 1993; Rasmussen 1991; Johnson 2019), and the exclusion criteria were not stated in four trial comparisons (Johnson 2004; Letterie 1990; Spring 2000; Spring 2000). The remaining trials based their exclusion criteria on iodine allergy (Al‐Fadhli 2006; Dreyer 2017; Johnson 2019), bilateral tubal blockage (Alper 1986; Lindborg 2009; Ogata 1993), previous infertility surgery (De Boer 1988), male factor infertility, suspected anovulation (Lindborg 2009), technical difficulties with the hysterosalpingogram (HSG) (Lindequist 1994; Rasmussen 1991), and causes of infertility other than unexplained (Nugent 2002).
Primary outcomes
Our primary effectiveness outcome was a composite of live birth and ongoing pregnancy. Seven studies reported live birth (Dreyer 2017; Johnson 2004; Lindborg 2009; Nugent 2002; Rasmussen 1991; Spring 2000; Johnson 2019) and the other studies without reporting live birth also did not report ongoing pregnancy.
Our primary safety outcomes was intravasation. Four studies reported this outcome (Alper 1986; Dreyer 2017; Lindequist 1994; Rasmussen 1991).
Secondary outcomes
All 15 studies reported clinical pregnancy.
Seven studies reported miscarriage (Dreyer 2017; Johnson 2004; Lindequist 1994; Lindborg 2009; Nugent 2002; Spring 2000; Johnson 2019).
Four studies reported ectopic pregnancy (Johnson 2004; Lindequist 1994; Lindborg 2009; Spring 2000).
Two studies reported procedural pain as a continuous outcome (Alper 1986; Dreyer 2017) and two reported it as a dichotomous outcome (Lindequist 1994; Rasmussen 1991).
Two studies reported short‐term adverse events (Alper 1986; Lindequist 1994).
One study reported neonatal congenital anomalies (Dreyer 2017). No trials reported other long‐term complications.
For all fertility outcomes, seven studies had a follow‐up of six months (Al‐Fadhli 2006; Alper 1986; De Boer 1988; Dreyer 2017; Johnson 2004; Lindborg 2009; Nugent 2002). Two had a follow‐up of nine months (Lindequist 1994; Rasmussen 1991), and two had a 12‐month follow‐up (Letterie 1990; Spring 2000). The other studies had a follow‐up of three (Johnson 2019), four (Ogata 1993), eight (Yang 1989), or 18 months (Steiner 2003), respectively. All pregnancies within the follow‐up period and subsequent live births resulting from these pregnancies were reported.
Excluded studies
In this 2020 update, four articles were excluded as they referred to irrelevant interventions (Reilly 2019; van Rijswijk 2018a) or study design (Pham 2017; van Rijswijk 2018b). In the previous version of this Cochrane Review, 10 studies were excluded from the review: one was not truly randomised with the use of alternate assignment (Schwabe 1983), five were non‐randomised comparative studies of HSG with OSCM versus WSCM (Acton 1988; Barwin 1971; DeCherney 1980; Gillespie 1965; Yaegashi 1987), one was a three‐way non‐randomised comparative study of HSG with OSCM versus WSCM versus no treatment (Mackey 1971), and one did not report pregnancy outcomes (Wolf 1989). Another was a recent observational study of pregnancy rates in women undergoing HSG with OSCM (Court 2014). See Characteristics of excluded studies.
Risk of bias in included studies
See Characteristics of included studies; Figure 3; Figure 4.
3.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
4.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
Sequence generation
Nine trials were rated as at low risk of bias in this domain as they used computer‐generated lists or random number tables (Al‐Fadhli 2006; Alper 1986; Dreyer 2017; Johnson 2004; Letterie 1990; Lindborg 2009; Nugent 2002; Spring 2000; Steiner 2003). The method of sequence generation was not adequately described in five studies, which were rated as at unclear risk of bias (De Boer 1988; Lindequist 1994; Ogata 1993; Rasmussen 1991; Yang 1989).
Allocation concealment
Adequate concealment of assigned treatment prior to allocation was reported in five trials (Dreyer 2017; Johnson 2004; Johnson 2019; Lindborg 2009; Nugent 2002) which were rated as at low risk of bias in this domain. Nine studies did not clearly report an adequate method of allocation concealment and were rated as at unclear risk (Al‐Fadhli 2006; Alper 1986; De Boer 1988; Letterie 1990; Lindequist 1994; Ogata 1993; Rasmussen 1991; Spring 2000; Yang 1989). Allocation sequence was not concealed in one study, which was rated as at high risk (Steiner 2003).
Blinding
Only one trial (Yang 1989) stated that it was double‐blinded, though it was not specifically stated who was blinded. Five trials stated that blinding was not performed (Dreyer 2017; Johnson 2004; Johnson 2019; Lindborg 2009; Nugent 2002; Steiner 2003) and the other trials did not state whether blinding was used, although participant blinding and outcome assessors blinding would have been possible in trials where different contrast media were compared. We evaluated subjective outcomes (e.g. pain) and objective outcomes (e.g. all fertility outcomes and intravasation) separately when assessing risk of performance and detection bias. Objective outcomes such as fertility outcomes are unlikely to be affected due to performance or detection bias arising from non‐blinding. Therefore all studies were rated as at low risk of bias for performance and detection bias for objective outcomes. For subjective outcomes such as pain, non‐blinding is likely to introduce bias for both performance and detection bias, and therefore six non‐blinded trials (Dreyer 2017; Johnson 2004; Johnson 2019; Lindborg 2009; Nugent 2002; Steiner 2003) were rated as at high risk of bias in these two domains while the other trials were rated as unclear risk of bias in these two domains.
Incomplete outcome data
Randomisation was undertaken some time in advance of the tubal flushing procedure itself (at referral and at scheduling) in three trials (Lindequist 1994; Ogata 1993; Rasmussen 1991) and subsequently a number of participants were withdrawn before they underwent the HSG because they had conceived, changed their mind about undergoing the procedure or participating in the trial, or were subsequently found not to fulfil the criteria for the trial. Randomisation immediately before the procedure was more appropriate.
Withdrawals and losses to follow up after HSG varied from 0% (Nugent 2002; Yang 1989), 1% (Dreyer 2017; Spring 2000), 3% (Johnson 2004), 5% (Steiner 2003), 9% (Rasmussen 1991), 11% (Al‐Fadhli 2006), 11% (Lindborg 2009), 19% (Alper 1986), 21% (Lindequist 1994), 28% (Letterie 1990) and 37% (Ogata 1993) of participants who underwent the procedure; this was unclear for one trial (De Boer 1988). The highest withdrawal rate of 37% (Ogata 1993) was due to the fact that women underwent the HSG (or not) before any results of their other investigations were known, and only women with proof of ovulation in all four cycles of follow up were retained in the analysis. Incompleteness or loss to follow‐up accounted for approximately one half of the withdrawals in the other trials.
Other than in the trials where all randomised participants were analysed, it was impossible to recalculate the treatment effect based on the originally randomised groups (using the intention‐to‐treat principle). It was not obvious that the intention‐to‐treat principle was the best approach for analysis given the poor design (randomisation before eligibility established) of some of the trials. However, it is generally recommended to minimise bias in the design, conduct and analysis of RCTs of effectiveness. Only three trials (Dreyer 2017; Johnson 2004; Nugent 2002;) performed an intention‐to‐treat analysis. Only one trial (Alper 1986) specified outcome details for participants withdrawn from each randomised group. Recalculation of the odds ratio (OR) including these participants had little effect on the conclusions of this trial (OR 1.31, 95% CI 0.51 to 3.04 for all participants versus OR 1.31, 95% CI 0.56 to 3.09 after exclusion).
Selective reporting
The protocol of two studies (Dreyer 2017; Johnson 2019) were available and all prespecified outcomes were reported and therefore they were scored at low risk of bias in this domain. The study protocols were not available for all the other studies, including one study with retrospective registration (Lindborg 2009), and all these studies were scored at unclear risk of reporting bias.
Other potential sources of bias
One study was scored at high risk of 'other bias' given the imbalanced age distribution between WSCM and OSCM (Spring 2000). In another study, the authors planned to include women quote: "with a history of 12 months infertility and known endometriosis" in the protocol, but in the trial they also included one woman with quote: "a previous successful pregnancy following lipiodol" (Johnson 2019). Given its very small sample size (n = 12), the inclusion of this single participant is likely to introduce bias with results in favour of the group to which she was assigned. We found no other potential sources of within‐study bias in the included studies.
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings 1. Tubal flushing with oil‐soluble contrast media (OSCM) versus no intervention.
| Tubal flushing with oil‐soluble contrast media (OSCM) versus no intervention | ||||||
| Population: women with subfertility Intervention: tubal flushing with OSCM versus No intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No intervention | OSCM | |||||
| Live birth | 111 per 1000 | 290 per 1000 (164 to 461) | OR 3.27 (1.57 to 6.85) | 204 (3 studies) |
⊕⊕⊝⊝ low1 | |
| Clinical pregnancy | 88 per 1000 | 323 per 1000 (167 to 367) |
OR 3.54 (2.08 to 6.02) |
506 (4 studies) |
⊕⊕⊝⊝ low1 | |
| Intravasation | No study reported this outcome | |||||
| Infection | No study reported this outcome | |||||
| Haemorrhage | No study reported this outcome | |||||
| Long‐term complications | No study reported this outcome | |||||
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1Downgraded two levels due to very serious concerns on imprecision: optimal information size is not met and wide confidence intervals fail to exclude important benefit or important harm.
Summary of findings 2. Tubal flushing with water‐soluble contrast media (WSCM) versus no intervention.
| Tubal flushing with water‐soluble contrast media (WSCM) versus no intervention | ||||||
| Population: women with subfertility Intervention: tubal flushing with WSCM versus No intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No intervention | WSCM | |||||
| Live birth | 205 per 1000 | 225 per 1000 (147 to 330) | OR 1.13 (0.67 to 1.91) | 334 (1 study) | ⊕⊕⊝⊝ low1 | |
| Clinical pregnancy | 265 per 1000 | 291 per 1000 (204 to 399) |
OR 1.14 (0.71 to 1.84) |
334 (1 study) | ⊕⊕⊝⊝ low1 | |
| Intravasation | No study reported this outcome | |||||
| Infection | See comment | Not estimable | 334 (1 study) |
‐ | Rare events (WSCM: 1; No treatment: 0) were reported in one study and meta‐analysis was not performed. | |
| Haemorrhage | No study reported this outcome | |||||
| Long‐term complications | No study reported this outcome | |||||
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1 Downgraded two level due to concerns on imprecision.
Summary of findings 3. Tubal flushing with oil‐soluble contrast media (OSCM) versus water‐soluble contrast media (WSCM).
| Tubal flushing with oil‐soluble contrast media (OSCM) versus water‐soluble contrast media (WSCM) versus no intervention | ||||||
| Population: women with subfertility Intervention: tubal flushing with OSCM versus WSCM | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| WSCM | OSCM | |||||
| Live birth | See comments | See comments | Not estimable | 2035 (3 studies) |
See comment | Two studies showed benefit for OSCM, the other showed insufficient evidence of a difference. High heterogeneity (I‐squared 86%) were observed and therefore no meta‐analysis was performed. The only study with low risk of bias showed OSCM versus WSCM (OR 1.64, 95% CI 1.27 to 2.11). |
| Clinical pregnancy | 257 per 1000 | 33 per 1000 (276 to 390) |
OR 1.42 (1.10 to 1.85) |
2598 (6 studies) |
⊕⊕⊝⊝ low1,2 | |
| Intravasation | 8 per 1000 | 41 per 1000 (19 to 86) |
OR 5.00 (2.25 to 11.12) |
1912 (4 studies) |
⊕⊕⊕⊝ moderate1 | |
| Infection | 37 per 1,000 | 8 per 1,000 (2 to 44) |
OR 0.22 (0.04 to 1.22) |
662 (2 studies) |
⊕⊝⊝⊝ very low1,3 | |
| Haemorrhage | 154 per 1,000 | 106 per 1,000 (68 to 161) |
OR 0.65 (0.40 to 1.06) |
662 (2 studies) |
⊕⊝⊝⊝ very low1,3 | |
| Long‐term complications (congenital abnormalities) | See comment | Not estimable | 1119 (1 study) |
‐ | Rare events (OSCM: 3; WSCM 1) were reported in one study and meta‐analysis was not performed. | |
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1 Downgraded one level due to concerns on risk of bias. 2 Downgraded one level due to heterogeneity. 3 Downgraded two levels due to very serious concerns on imprecision.
(1) Tubal flushing with OSCM versus No treatment
Four studies compared tubal flushing between OSCM versus No treatment (Johnson 2004; Johnson 2019; Nugent 2002; Ogata 1993). Total participants included 506 women (range 12 to 302).
Primary outcomes
1.1 Live birth
Three studies reported live birth (Johnson 2004; Johnson 2019; Nugent 2002). Tubal flushing with OSCM may increase the odds of live birth (odds ratio (OR) 3.27, 95% confidence interval (CI) 1.57 to 6.85, 3 RCTs, 204 women, I2 = 0, low‐quality evidence). This suggests that if the chance of live birth following no treatment is assumed to be 11%, the chance following tubal flushing with OSCM would be between 16% and 46%. See Analysis 1.1; Figure 5.
1.1. Analysis.

Comparison 1: OSCM versus No treatment, Outcome 1: Live birth
5.

Forest plot of comparison: 1 OSCM versus No treatment, outcome: 1.1 Live birth.
Subgroup analysis
In all three trials, the intervention was intended primarily as a therapy (Johnson 2004; Johnson 2019; Nugent 2002) and therefore no subgroup analysis was performed.
Sensitivity analyses
Sensitivity analyses using risk ratio (RR 2.59, 95% CI 1.41 to 4.76, 3 RCTs, 204 women, I2 = 0%) or random‐effects model (OR 3.14, 95% CI 1.48 to 6.65, 3 RCTs, 204 women, I2 = 0%) showed results consistent with the primary analysis. The other sensitivity analyses were not possible.
1.2 Intravasation
This outcome was not reported.
Secondary outcomes
1.3 Clinical pregnancy
All four studies reported clinical pregnancy (Johnson 2004; Johnson 2019; Nugent 2002; Ogata 1993). Tubal flushing with OSCM may increase in the odds of clinical pregnancy (OR 3.54, 95% CI 2.08 to 6.02, 4 RCTs, 506 women, I2 = 18%, low‐quality evidence). This suggests that if the chance of clinical pregnancy following no treatment is assumed to be 9%, the chance following tubal flushing with OSCM would be between 17% and 37%. See Analysis 1.3; Figure 6 .
1.3. Analysis.

Comparison 1: OSCM versus No treatment, Outcome 3: Clinical Pregnancy
6.

Forest plot of comparison: 1 OSCM versus No treatment, outcome: 1.3 Clinical Pregnancy.
1.4 Miscarriage
Three studies reported miscarriage (Johnson 2004; Johnson 2019; Nugent 2002). We are uncertain whether tubal flushing with OSCM increase miscarriage per woman randomised or per pregnancy compared to no treatment (OR 1.68, 95% CI 0.46 to 6.16, 3 RCTs, 204 women, I2 = 0%, very low‐quality evidence; OR 0.81, 95% CI 0.16 to 4.10, 2 RCTs, 45 women, I2 = 0%, very low‐quality evidence). See Analysis 1.4; Analysis 1.5.
1.4. Analysis.

Comparison 1: OSCM versus No treatment, Outcome 4: Miscarriage per woman
1.5. Analysis.

Comparison 1: OSCM versus No treatment, Outcome 5: Miscarriage per pregnancy
1.5 Ectopic pregnancy
One study reported ectopic pregnancy per woman (Johnson 2004). We are uncertain whether tubal flushing with OSCM increases ectopic pregnancy per woman randomised compared to no treatment (OR 3.54, 95% CI 0.14 to 88.18, 1 RCT, 158 women, very low‐quality evidence). See Analysis 1.6.
1.6. Analysis.

Comparison 1: OSCM versus No treatment, Outcome 6: Ectopic pregnancy per woman
1.6 Procedural pain
This outcome was not reported.
1.7 Infection
This outcome was not reported.
1.8 Haemorrhage
This outcome was not reported.
1.9 Long‐term complications
This outcome was not reported.
(2) Tubal flushing with WSCM versus No treatment
Only one study made this comparison (Lindborg 2009): 334 women undergoing hysterosalpingo‐contrast‐sonography (HyCoSy) as a part of subfertility investigation were included.
Primary outcomes
2.1 Live birth
We are uncertain whether tubal flushing with WSCM increases live birth compared to no treatment (OR 1.13, 95% CI 0.67 to 1.91, 1 RCT, 334 women, low‐quality evidence). This suggests that if the chance of live birth following no treatment is assumed to be 21%, the chance following tubal flushing with WSCM would be between 15% and 33%. See Analysis 2.1; Figure 7.
2.1. Analysis.

Comparison 2: WSCM versus No treatment, Outcome 1: Live birth
7.

Forest plot of comparison: 2 WSCM versus No treatment, outcome: 2.1 Live birth.
Subgroup analysis
No subgroup analysis was performed given that one study was included (intended for therapy) in this comparison.
Sensitivity analyses
Using RR instead of OR did not affect the findings for this comparison (RR 1.10, 95% CI 0.73 to 1.66, 1 RCT, 334 women) and the other sensitivity analyses were not possible.
2.2 Intravasation
This outcome was not reported.
Secondary outcomes
2.3 Clinical pregnancy
We are uncertain whether tubal flushing with WSCM increases clinical pregnancy compared to no treatment (OR 1.14, 95% CI 0.71 to 1.84, 1 RCT, 334 women, low‐quality evidence). This suggests that if the chance of clinical pregnancy following no treatment is assumed to be 27%, the chance following tubal flushing with WSCM would be between 29% and 40%. See Analysis 2.3.
2.3. Analysis.

Comparison 2: WSCM versus No treatment, Outcome 3: Clinical Pregnancy
2.4 Miscarriage
We are uncertain whether tubal flushing with WSCM increases miscarriage per woman randomised or per pregnancy compared to no treatment (OR 1.12, 95% CI 0.42 to 2.97, 1 RCT, 334 women, low‐quality evidence; OR 1.01, 95% CI 0.35 to 2.90, 1 RCT, 93 women, low‐quality evidence). See Analysis 2.4; Analysis 2.5 .
2.4. Analysis.

Comparison 2: WSCM versus No treatment, Outcome 4: Miscarriage per woman
2.5. Analysis.

Comparison 2: WSCM versus No treatment, Outcome 5: Miscarriage per pregnancy
2.5 Ectopic pregnancy
We are uncertain whether tubal flushing with WSCM decreases ectopic pregnancy per woman randomised compared to no treatment (OR 0.99, 95% CI 0.06 to 15.93; 1 RCT, 334 women, very low‐quality evidence). See Analysis 2.6.
2.6. Analysis.

Comparison 2: WSCM versus No treatment, Outcome 6: Ectopic pregnancy per woman
2.6 Procedural pain
This outcome was not reported.
2.7 Infection
Lindborg 2009 reported one case with pelvic infection in the WSCM group and no case with infection in the no treatment group. Meta‐analysis was not performed. See Analysis 2.8.
2.8. Analysis.

Comparison 2: WSCM versus No treatment, Outcome 8: Infection
2.8 Haemorrhage
This outcome was not reported.
2.9 Long‐term complications
This outcome was not reported.
(3) Tubal flushing with OSCM versus WSCM
Six studies compared tubal flushing between OSCM versus WSCM (Alper 1986; De Boer 1988; Dreyer 2017; Lindequist 1994; Rasmussen 1991; Spring 2000). Total participants included 2598 women (range 29 to 1119).
Primary outcomes
3.1 Live birth
Three studies reported this outcome (Dreyer 2017; Rasmussen 1991;Spring 2000). Two studies reported higher live birth in the OSCM group (Dreyer 2017; Rasmussen 1991). One study with low risk of bias (Dreyer 2017) reported 214 of 557 women in the OSCM group (38%) compared to 155 of 562 women in the WSCM group (28%) had live births (OR 1.64, 95% CI 1.27 to 2.11). One study with unclear risk of bias (Rasmussen 1991) reported higher live birth in the oil‐group (OR 3.45, 95% CI 1.97 to 6.03). The third study with high risk of bias (Spring 2000) found insufficient evidence of a difference between the groups (OR 0.92, 95% CI 0.60 to 1.40). Substantial heterogeneity was observed (I2 = 86%) and meta‐analysis was not performed. See Analysis 3.1 and Figure 8.
3.1. Analysis.

Comparison 3: OSCM versus WSCM, Outcome 1: Live birth
8.

Forest plot of comparison: 3 OSCM versus WSCM, outcome: 3.1 Live birth.
Subgroup analysis
Two studies (Rasmussen 1991; Spring 2000) were conducted primarily for diagnostic reasons, while one (Dreyer 2017) was primarily for therapeutic reasons. The heterogeneity in the diagnostic subgroup was still high (I2 = 93%) and therefore meta‐analysis was not performed. Reasons for tubal flushing (diagnostic versus therapeutic) do not seem to be the source of heterogeneity. See Analysis 3.2.
3.2. Analysis.

Comparison 3: OSCM versus WSCM, Outcome 2: Live birth (subgroup analysis)
Substantial heterogeneity was observed (I2 = 86%) and was not reduced not reduced when using a different imputation approach (ignoring participants with missing outcome data), or using a different effect measure (risk ratio). These three included studies had different levels of risk of bias and different follow‐up time, which could have contributed to the heterogeneity.
Sensitivity analyses
Sensitivity analyses were not performed as meta‐analysis was not conducted for this outcome.
3.2 Intravasation
Four studies reported intravasation (Alper 1986; Dreyer 2017; Lindequist 1994; Rasmussen 1991). All cases of intravasation were asymptomatic. Tubal flushing with OSCM probably increased in the odds of intravasation compared to tubal flushing with WSCM (OR 5.00, 95% CI 2.25 to 11.12, 4 RCTs, 1912 women, I2 = 0, moderate‐quality evidence). This suggests that if the chance of intravasation following tubal flushing with WSCM is assumed to be 1%, the chance following tubal flushing with OSCM would be between 2% and 9%. See Analysis 3.3 and Figure 9 .
3.3. Analysis.

Comparison 3: OSCM versus WSCM, Outcome 3: Intravasation
9.

Forest plot of comparison: 3 OSCM versus WSCM, outcome: 3.3 Intravasation.
Secondary outcomes
3.3 Clinical pregnancy
Six studies reported clinical pregnancy (Alper 1986; De Boer 1988; Dreyer 2017; Lindequist 1994; Rasmussen 1991; Spring 2000). Results showed tubal flushing with OSCM may increase in the odds of clinical pregnancy (OR 1.42, 95% CI 1.10 to 1.85, 6 RCTs, 2598 women, I2 = 41%, low‐quality evidence). This suggests that if the chance of clinical pregnancy following tubal flushing with WSCM is assumed to be 26%, the chance following tubal flushing with OSCM would be between 28% and 39%. See Analysis 3.4 and Figure 10.
3.4. Analysis.

Comparison 3: OSCM versus WSCM, Outcome 4: Clinical pregnancy
10.

Forest plot of comparison: 3 OSCM versus WSCM, outcome: 3.4 Clinical pregnancy.
3.4 Miscarriage
Two studies reported miscarriage per woman (Dreyer 2017; Spring 2000). We are uncertain whether tubal flushing with OSCM decreases miscarriage per woman randomised (OR 0.83, 95% CI 0.56 to 1.24; 2 RCTs, 1652 women, I2 = 0%, very low‐quality evidence) or (OR 0.73, 95% CI 0.48 to 1.13, 2 RCTs, 603 women, I2 = 0, very low‐quality evidence). See Analysis 3.5; Analysis 3.6.
3.5. Analysis.

Comparison 3: OSCM versus WSCM, Outcome 5: Miscarriage per woman
3.6. Analysis.

Comparison 3: OSCM versus WSCM, Outcome 6: Miscarriage per pregnancy
3.5 Ectopic pregnancy
Two studies reported ectopic pregnancy per woman (Dreyer 2017; Spring 2000). We are uncertain whether tubal flushing with OSCM decreases ectopic pregnancy (OR 0.65, 95% CI 0.18 to 2.30, 2 RCTs, 1652 women, I2 = 0, very low‐quality evidence). See Analysis 3.7.
3.7. Analysis.

Comparison 3: OSCM versus WSCM, Outcome 7: Ectopic pregnancy per woman
3.6 Any procedural pain (dichotomous variable)
Two studies reported the incidence of any procedural pain (Lindequist 1994; Rasmussen 1991). Procedural pain was less frequently reported in the OSCM group (OR 0.13, 95% CI 0.08 to 0.22, 1 RCT, 417 women) in Rasmussen 1991, while pain was similar between groups (OR 1.05. 95% CI 0.64 to 1.73) in Lindequist 1994. Substantial heterogeneity was observed (I2 = 97%) and therefore meta‐analysis was not performed. See Analysis 3.8.
3.8. Analysis.

Comparison 3: OSCM versus WSCM, Outcome 8: Procedural pain (dichotomous variable)
3.7 Procedural pain (continuous variable)
Two studies reported procedural pain as a continuous variable (Alper 1986; Dreyer 2017). In Alper 1986, the mean pain was 2.9 (SD 0.9) and 3.2 (SD 1.6) in the OSCM and WSCM groups, respectively (Analysis 3.9). In Dreyer 2017, the median pain was 4.8 (interquartile range 3.0 to 6.4) and 5.0 (interquartile range 3.0 to 6.7) in the OSCM and WSCM groups, respectively. As the data in Dreyer 2017 showed a skewed distribution, they could not be converted to mean and SD and therefore meta‐analysis was not possible. Based on these two studies, the difference in pain between the groups appears small.
3.9. Analysis.

Comparison 3: OSCM versus WSCM, Outcome 9: Procedural pain (continuous variable)
3.9 Infection
Two studies reported infection (Lindequist 1994; Rasmussen 1991). We are uncertain whether tubal flushing with OSCM decreases the odds of infection (OR 0.22, 95% CI 0.04 to 1.22, 2 RCTs, 662 women, I2 = 0, very low‐quality evidence). This suggests that if the chance of infection following tubal flushing with WSCM is assumed to be 3.7%, the chance following tubal flushing with OSCM would be between 0.2% and 4.4%. See Analysis 3.10.
3.10. Analysis.

Comparison 3: OSCM versus WSCM, Outcome 10: Infection
3.10 Haemorrhage
Two studies reported haemorrhage (Lindequist 1994; Rasmussen 1991). We are uncertain whether tubal flushing with OSCM decreases the odds of haemorrhage (OR 0.65, 95% CI 0.40 to 1.06, 2 RCTs, 662 women, I2 = 0, very low‐quality evidence).This suggests that if the chance of haemorrhage following tubal flushing with WSCM is assumed to be 15%, the chance following tubal flushing with OSCM would be between 11% and 16%. See Analysis 3.11.
3.11. Analysis.

Comparison 3: OSCM versus WSCM, Outcome 11: Haemorrhage
3.11 Long‐term complications
Dreyer 2017 reported congenital abnormalities, in which three neonates with congenital abnormalities (skeletal dysplasia, oesophageal atresia or chromosomal mosaicism) were reported in the OSCM group while no congenital abnormalities were reported in the WSCM group. No meta‐analysis was performed.
(4) Tubal flushing with OSCM + WSCM versus WSCM alone
Five studies compared tubal flushing between OSCM and WSCM versus WSCM alone; (Al‐Fadhli 2006; Spring 2000; Steiner 2003; Letterie 1990; Yang 1989). Total participants included 686 women (range 29 ‐ 393).
Primary outcomes
4.1 Live birth
One study reported on live birth (Spring 2000). We are uncertain whether tubal flushing with OSCM + WSCM improves live birth compared to WSCM alone (OR 1.06, 95% CI 0.64 to 1.77, 1 RCT, 393 women, very low‐quality evidence). This suggests that if the chance of live birth following tubal flushing with WSCM is assumed to be 21%, the chance following tubal flushing with OSCM + WSCM would be between 14% and 32%. See Analysis 4.1; Figure 11.
4.1. Analysis.

Comparison 4: OSCM + WSCM versus WSCM, Outcome 1: Live birth
Subgroup analysis
No subgroup analysis was performed given that there was only one study in this comparison.
Sensitivity analyses
Using RR instead of OR did not affect the findings for this comparison and the other sensitivity analyses were not possible.
4.2 Intravasation
This outcome was not reported.
Secondary outcomes
4.3 Clinical pregnancy
All five studies reported clinical pregnancy (Al‐Fadhli 2006; Letterie 1990; Spring 2000; Steiner 2003; Yang 1989). We are uncertain whether tubal flushing with OSCM + WSCM improves clinical pregnancy compared to WSCM alone (OR 1.26, 95% CI 0.91 to 1.75, 5 RCTs, 686 women, I2 = 0, very low‐quality evidence).This suggests that if the chance of clinical pregnancy following tubal flushing with WSCM is assumed to be 32%, the chance following tubal flushing with OSCM + WSCM would be between 30% and 46%. See Analysis 4.3.
4.3. Analysis.

Comparison 4: OSCM + WSCM versus WSCM, Outcome 3: Clinical Pregnancy
4.4 Miscarriage
One study reported on miscarriage (Spring 2000). We are uncertain whether tubal flushing with OSCM + WSCM increases miscarriage per woman randomised or per pregnancy compared to WSCM alone (OR 1.19, 95% CI 0.61 to 2.35, 1 RCT, 393 women, very low‐quality evidence; OR 1.14, 95% CI 0.53 to 2.48, 1 RCT, 130 women, very low‐quality evidence). See Analysis 4.4; Analysis 4.5.
4.4. Analysis.

Comparison 4: OSCM + WSCM versus WSCM, Outcome 4: Miscarriage per woman
4.5. Analysis.

Comparison 4: OSCM + WSCM versus WSCM, Outcome 5: Miscarriage per pregnancy
4.5 Ectopic pregnancy
Two studies reported ectopic pregnancy per woman (Spring 2000; Letterie 1990). We are uncertain whether tubal flushing with OSCM + WSCM decreases ectopic pregnancy compared to WSCM alone (OR 0.48, 95% CI 0.05 to 4.38, 2 RCTs, 422 women, very low‐quality evidence). See Analysis 4.6.
4.6. Analysis.

Comparison 4: OSCM + WSCM versus WSCM, Outcome 6: Ectopic pregnancy per woman
4.6 Procedural pain
This outcome was not reported.
4.7 Infection
This outcome was not reported.
4.8 Haemorrhage
This outcome was not reported.
4.9 Long‐term complications
This outcome was not reported.
Discussion
Summary of main results
In this systematic review of 15 randomised controlled trials (RCTs) involving 3864 women with infertility, we compared tubal flushing with different contrast media, alone or in combination, with each other or no treatment. The evidence suggests that compared to no treatment, tubal flushing with OSCM may increase the chance of live birth and clinical pregnancy, while it is uncertain whether tubal flushing with water‐soluble contrast media (WSCM) improves those outcomes. Compared to tubal flushing with WSCM, oil‐soluble contrast media (OSCM) may improve clinical pregnancy while meta‐analysis was not performed for live birth due to heterogeneity. Evidence also suggests that OSCM is associated with an increased risk of intravasation. It is uncertain whether tubal flushing with OSCM + WSCM improves fertility outcomes compared to WSCM alone. Overall, adverse events, especially long‐term adverse events, were poorly reported across studies.
Overall completeness and applicability of evidence
The evidence was limited by the small number of included studies for several outcomes, including live birth. Among 15 included studies, only seven studies reported live birth (Dreyer 2017; Johnson 2004; Johnson 2019; Lindborg 2009;Nugent 2002; Rasmussen 1991; Spring 2000). These seven studies included 2721 women with infertility, accounting for approximately 70% of all included participants. The results on live birth were generally consistent with those on clinical pregnancy.
The adverse events were poorly reported. Pain was reported as a continuous outcome or a dichotomous outcome and therefore it is impossible to pool the data from these studies. Long‐term complications were only reported in one study (Dreyer 2017).
Tubal flushing with different contrast media during hysterosalpingogram (HSG), hysterosalpingo‐contrast sonography (HyCoSy) or laparoscopy is still mainly considered as a diagnostic tool in current practice. Our review provides emerging evidence supporting the use of tubal flushing with OSCM to improve subsequent fertility outcomes, but the comparison between OSCM and WSCM showed inconsistent results on live birth in different included studies. In addition, OSCM was only used during HSG in all included studies. Therefore it remained unclear whether the effects of OSCM remains similar in other settings such as HyCoSy or laparoscopy. It is worth noting that, in current clinical practice, all OSCMs used during HSG include iodine, which may impact on thyroid function for both the mother and the fetus (Roest 2020); however, none of the included studies reported thyroid function. As thyroid function is only applicable to contrast media containing iodine, it is not relevant to other contrast media such as those used during HyCoSy or laparoscopy and therefore was not used as an outcome of interest in the current review. However, it would be an important gap to fill before implementing OSCM during HSG as a therapeutic method into clinical practice.
Quality of the evidence
The overall quality of the evidence was low or very low except for one result with moderate‐quality evidence in one comparison. The main limitations were imprecision, risk of bias and heterogeneity. There were too few studies in any one comparison to evaluate the risk of publication bias. See Table 1; Table 2; Table 3.
The risk of bias in many of the primary studies was unclear or high for most domains. Only five studies described satisfactory methods of allocation concealment and most were unblinded. As noted above, this may not have unduly influenced findings for live birth and other pregnancy outcomes, but could influence the assessment of subjective outcomes such as pain.
Large heterogeneity was observed in live birth in the comparison between OSCM and WSCM. It could be partly explained by the different levels of risk of bias and different follow‐up times. However such heterogeneity was smaller when assessing clinical pregnancy in the same comparison and even smaller in other comparisons. Therefore, the findings between OSCM and WSCM need to be further confirmed in future high‐quality trials.
The source of funding was not stated in nine trials. In the remaining six, three were not industry supported (Dreyer 2017; Lindborg 2009; Spring 2000); in two studies it was stated only that products were supplied free of charge (Letterie 1990; Rasmussen 1991); one study was supported by both non‐industrial and industrial funding (Johnson 2004).
Potential biases in the review process
We conducted comprehensive searches without language restrictions. We also identified studies other than English (Ogata 1993), and six ongoing studies (Cai 2019; Hassan 2015; Legro 2018; Mijatovic 2018; Rosielle 2019; Zhang 2020). The review process is unlikely to result in potential biases.
Agreements and disagreements with other studies or reviews
Two meta‐analyses on this topic were published recently. One compared OSCM versus WSCM during HSG (Fang 2018), and the other one was a network meta‐analysis comparing all these tubal flushing strategies including both direct and indirect evidence and stratifying the pregnancy outcomes via different time points after randomisation (Wang 2019). Our results on the effectiveness of OSCM and WSCM are consistent with these meta‐analyses while outcomes in most comparisons were limited to six‐month follow‐up and data on outcomes beyond six months post randomisation are limited.
The evidence on WSCM versus no treatment in our review shows inconclusive results while the calculated odds ratio (OR) was in favour of WSCM. This is consistent with a recent large cohort study of over 4500 couples with unexplained infertility (Dreyer 2019) . In this study, the authors found that, within two years after first presentation at the fertility clinic, HSG improves ongoing pregnancy rate compared with no HSG, regardless of the contrast medium used (Dreyer 2019). Interestingly, when limiting to HSG with WSCM, the effect of tubal flushing became slightly smaller but still better than no HSG. This provides further insights into the effects of WSCM.
Authors' conclusions
Implications for practice.
The evidence suggests that compared to no treatment, tubal flushing with oil‐soluble contrast media (OSCM) may increase the chance of live birth and clinical pregnancy, while it is uncertain whether tubal flushing with : water‐soluble contrast media (WSCM) improves those outcomes. Compared to tubal flushing with WSCM, OSCM may improve clinical pregnancy while meta‐analysis was not performed for live birth due to heterogeneity. Evidence also suggests that OSCM is associated with an increased risk of intravasation. Overall, adverse events, especially long‐term adverse events, are poorly reported across studies.
Implications for research.
Further robust randomised controlled trials (RCTs) comparing OSCM versus WSCM, alone or in combination with each other or no treatment should be undertaken with live births as the primary outcome and comparative data on adverse events should also be reported. The effectiveness should also be evaluated in other causes of infertility. Further scientific research on the OSCM‐related improvement in fecundity may clarify its mechanism of working and explain some cases of hitherto 'unexplained' infertility. To investigate the potential advantages of tubal flushing with OSCM, an RCT comparing this approach with in vitro fertilisation (IVF) and intrauterine insemination for women with subfertility (either unexplained or with proven appropriately staged endometriosis) seems a logical next step.
What's new
| Date | Event | Description |
|---|---|---|
| 7 August 2020 | New search has been performed | The updated search was performed in April 2020 and two studies were added (Dreyer 2017; Johnson 2019). The conclusion in the comparison between oil‐soluble contrast media (OSCM) versus water‐soluble contrast media (WSCM) has changed. |
| 3 August 2020 | New citation required and conclusions have changed | The addition of two new studies and change to a review comparison has led to a change in the conclusions of this review. |
History
Protocol first published: Issue 2, 1996 Review first published: Issue 2, 1996
| Date | Event | Description |
|---|---|---|
| 16 April 2015 | New citation required but conclusions have not changed | Our conclusions have not changed with the addition of one new study. |
| 16 April 2015 | New search has been performed | One study added (Lindborg 2009); contact details updated; one new comparison added (water‐soluble contrast media versus no treatment); risk of bias tables updated; tables of characteristics of included studies updated; review adapted to new format; summary of findings table added. |
| 13 June 2008 | Amended | Converted to new review format. |
| 16 April 2007 | New citation required and conclusions have changed | Substantive amendment |
Notes
This review was previously known as 'Oil‐soluble versus water‐soluble media for assessing tubal patency with hysterosalpingography or laparoscopy in subfertile women'.
Acknowledgements
The authors acknowledge the contributions of these previous authors.
Patrick Vandekerckhove was the primary author of the original review, was involved in trial selection and data extraction of trials for the updated review and critically reviewed the updated review in 2007
Tasuku Harada and Richard Lilford were authors of the original review and commented on the updated review in 2007.
Ed Hughes was author of the original review, commented on the updated review in 2007, and 2015.
Anne Hardiman carried out the update in 2015.
We would like to acknowledge members of the editorial office in Auckland for assistance with updates.
The authors of the 2020 update thank Drs Ying Cheong, Vanessa Jordan and Vivienne Moore for providing peer review comment on the draft.
Appendices
Appendix 1. Cochrane Gynaecology and Fertility Group (CGFG) specialised register search strategy
PROCITE platform
Searched 20 April 2020
Keywords CONTAINS "fertility" or "subfertility" or "infertility" or "hysterosalpingogram" or "hysterosalpingography" or "laparoscopic chromopertubation" or "laparoscopy" or "Fallopian‐Tube‐Patency‐Tests" or "tubal flushing" or "tubal patency" or "flushing media" or Title CONTAINS "fertility" or "subfertility" or "infertility" or "hysterosalpingogram" or "hysterosalpingography" or "laparoscopic chromopertubation" or "laparoscopy" or "Fallopian‐Tube‐Patency‐Tests" or "tubal flushing" or "tubal patency" or "flushing media"
AND
Keywords CONTAINS "oil" or "oil‐soluble contrast" or "Water‐Soluble Contrast" or "Aqueous" or "lipiodol" or "lipiodol flushing" or "lipiodol‐pingyangmycin emulsion" or "Contrast‐Media" or "Flushing" or "tubal flushing" or Title CONTAINS "oil" or "oil‐soluble contrast" or "Water‐Soluble Contrast" or "Aqueous" or "lipiodol" or "lipiodol flushing" or "lipiodol‐pingyangmycin emulsion" or "Contrast‐Media" or "Flushing" or "tubal flushing"
(114 records)
Appendix 2. CENTRAL via the Cochrane Register of Studies Online (CRSO) search strategy
Web platform
Searched 20 April 2020
#1 MESH DESCRIPTOR Hysterosalpingography EXPLODE ALL TREES 125
#2 (hysterosalpingo* or salpingo*):TI,AB,KY 853
#3 (HyCoSy or HSG or HyFoSy):TI,AB,KY 231
#4 MESH DESCRIPTOR Laparoscopy EXPLODE ALL TREES 5457
#5 Laparoscop*:TI,AB,KY 18904
#6 MESH DESCRIPTOR Fallopian Tube Patency Tests EXPLODE ALL TREES 30
#7 (tub* adj3 (paten* or block*)):TI,AB,KY 347
#8 (subfertil* or infertil* or fertility or fertile):TI,AB,KY 10822
#9 endometriosis:TI,AB,KY 2136
#10 MESH DESCRIPTOR Infertility, Female EXPLODE ALL TREES 1364
#11 pregnancy:TI,AB,KY 48452
#12 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 72304
#13 (Soluble adj5 (oil* or water)):TI,AB,KY 81
#14 MESH DESCRIPTOR Contrast Media EXPLODE ALL TREES 3595
#15 MESH DESCRIPTOR Ethiodized Oil EXPLODE ALL TREES 72
#16 (tub* adj3 flush*):TI,AB,KY 21
#17 (pertubation or chromopertubat*):TI,AB,KY 40
#18 (contrast* adj5 (media or medium)):TI,AB,KY 4332
#19 (contrast* adj3 agent*):TI,AB,KY 1462
#20 lipiodol.tw:TI,AB,KY 0
#21 MESH DESCRIPTOR Iodipamide EXPLODE ALL TREES 20
#22 Iodipamide:TI,AB,KY 24
#23 MESH DESCRIPTOR Iodized Oil EXPLODE ALL TREES 187
#24 ethiodol:TI,AB,KY 2
#25 iotrolan:TI,AB,KY 39
#26 poppy:TI,AB,KY 58
#27 (OSCM or WSCM):TI,AB,KY 6
#28 (contrast adj3 material*):TI,AB,KY 533
#29 (aqueous adj5 contrast):TI,AB,KY 15
#30 (water adj5 contrast):TI,AB,KY 161
#31 (oil* adj5 contrast):TI,AB,KY 52
#32 #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 6117
#33 #12 AND #32 207
Appendix 3. MEDLINE search strategy
OVID platform
Searched from 1946 to 20 April 2020
1 exp Hysterosalpingography/ (4202) 2 (hysterosalpingo* or salpingo*).tw. (9441) 3 (HyCoSy or HSG or HyFoSy).tw. (1438) 4 Laparoscopy/ (84883) 5 laparoscop*.tw. (124251) 6 Fallopian Tube Patency Tests/ (662) 7 (tub* adj3 (paten* or block*)).tw. (3838) 8 (subfertil* or infertil* or fertility or fertile).tw. (140354) 9 endometriosis.tw. (22703) 10 Infertility, Female/ (28264) 11 pregnancy.tw. (375893) 12 or/1‐11 (646770) 13 (Soluble adj5 (oil* or water)).tw. (47490) 14 contrast media/ or ethiodized oil/ (86912) 15 (tub* adj3 flush*).tw. (186) 16 (pertubation or chromopertubat*).tw. (323) 17 (contrast* adj5 (media or medium)).tw. (26895) 18 (contrast* adj3 agent*).tw. (29538) 19 lipiodol.tw. (2716) 20 Iodipamide/ (742) 21 exp Iodized Oil/ (3935) 22 ethiodol.tw. (130) 23 iotrolan.tw. (200) 24 poppy.tw. (1067) 25 OSCM.tw. (20) 26 WSCM.tw. (22) 27 (contrast adj3 material*).tw. (8933) 28 (aqueous adj5 contrast).tw. (569) 29 (water adj5 contrast).tw. (4653) 30 (oil* adj5 contrast).tw. (934) 31 or/13‐30 (171663) 32 12 and 31 (2528) 33 randomized controlled trial.pt. (504013) 34 controlled clinical trial.pt. (93621) 35 randomized.ab. (476153) 36 randomised.ab. (95214) 37 placebo.tw. (212516) 38 clinical trials as topic.sh. (190790) 39 randomly.ab. (331201) 40 trial.ti. (216564) 41 (crossover or cross‐over or cross over).tw. (84229) 42 or/33‐41 (1346935) 43 exp animals/ not humans.sh. (4690983) 44 42 not 43 (1239668) 45 32 and 44 (162)
Appendix 4. Embase search strategy
OVID platform
Searched from 1980 to 20 April 2020
1 exp Hysterosalpingography/ (3729) 2 (hysterosalpingo* or salpingo*).tw. (13162) 3 (HyCoSy or HSG or HyFoSy).tw. (2195) 4 Laparoscopy/ (73489) 5 laparoscop*.tw. (197919) 6 exp tubal patency test/ (181) 7 (tub* adj3 (paten* or block*)).tw. (4639) 8 (subfertil* or infertil* or fertility or fertile).tw. (172854) 9 endometriosis.tw. (31648) 10 exp female infertility/ (41834) 11 pregnancy.tw. (451613) 12 or/1‐11 (822246) 13 (Soluble adj5 (oil* or water)).tw. (53602) 14 (tub* adj3 flush*).tw. (241) 15 (pertubation or chromopertubat*).tw. (459) 16 (contrast* adj5 (media or medium)).tw. (29109) 17 (contrast* adj3 agent*).tw. (38295) 18 lipiodol.tw. (4758) 19 ethiodol.tw. (259) 20 iotrolan.tw. (227) 21 (poppy or poppyseed).tw. (1242) 22 OSCM.tw. (26) 23 WSCM.tw. (25) 24 (contrast adj3 material*).tw. (10127) 25 (aqueous adj5 contrast).tw. (565) 26 (water adj5 contrast).tw. (4640) 27 (oil* adj5 contrast).tw. (1012) 28 exp adipiodone/ (291) 29 exp contrast medium/ (156361) 30 exp ethiodized oil/ (7370) 31 exp iodinated poppyseed oil/ (7370) 32 or/13‐31 (239065) 33 12 and 32 (4200) 34 Clinical Trial/ (962466) 35 Randomized Controlled Trial/ (595291) 36 exp randomization/ (86651) 37 Single Blind Procedure/ (38544) 38 Double Blind Procedure/ (168458) 39 Crossover Procedure/ (62718) 40 Placebo/ (335076) 41 Randomi?ed controlled trial$.tw. (225272) 42 Rct.tw. (36438) 43 random allocation.tw. (1994) 44 randomly.tw. (434667) 45 randomly allocated.tw. (34663) 46 allocated randomly.tw. (2518) 47 (allocated adj2 random).tw. (811) 48 Single blind$.tw. (24379) 49 Double blind$.tw. (200831) 50 ((treble or triple) adj blind$).tw. (1124) 51 placebo$.tw. (299940) 52 prospective study/ (592520) 53 or/34‐52 (2405044) 54 case study/ (67961) 55 case report.tw. (397511) 56 abstract report/ or letter/ (1090355) 57 or/54‐56 (1545507) 58 53 not 57 (2351354) 59 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) (5950807) 60 58 not 59 (2188200) 61 33 and 60 (517)
Appendix 5. PsycINFO search strategy
OVID platform
Searched from 1806 to 20 April 2020
1 exp Fertility Enhancement/ or exp Infertility/ (2226) 2 hysterosalpingog$.tw. (8) 3 HSG.tw. (31) 4 laparoscop$.tw. (495) 5 (tubal adj flush$).tw. (0) 6 (tub$ adj patency).tw. (2) 7 chromopertub$.tw. (0) 8 fertili$.tw. (10113) 9 or/1‐8 (12003) 10 oil$.tw. (4987) 11 Ethiodized Oil.tw. (0) 12 ethiodol.tw. (0) 13 iotrolan.tw. (0) 14 poppy.tw. (117) 15 Iodized Oil$.tw. (3) 16 IODIPAMIDE.tw. (0) 17 WATER.tw. (35347) 18 Contrast Medi$.tw. (140) 19 aqueous.tw. (691) 20 lipiodol.tw. (9) 21 OSCM.tw. (2) 22 WSCM.tw. (0) 23 or/10‐22 (40744) 24 9 and 23 (143)
Data and analyses
Comparison 1. OSCM versus No treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Live birth | 3 | 204 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.27 [1.57, 6.85] |
| 1.2 Intravasation | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.3 Clinical Pregnancy | 4 | 506 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.54 [2.08, 6.02] |
| 1.4 Miscarriage per woman | 3 | 204 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.68 [0.46, 6.16] |
| 1.5 Miscarriage per pregnancy | 2 | 45 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.16, 4.10] |
| 1.6 Ectopic pregnancy per woman | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.7 Procedural pain | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 1.8 Infection | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.9 Haemorrhage | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.10 Long‐term complications | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
1.2. Analysis.

Comparison 1: OSCM versus No treatment, Outcome 2: Intravasation
1.7. Analysis.

Comparison 1: OSCM versus No treatment, Outcome 7: Procedural pain
1.8. Analysis.

Comparison 1: OSCM versus No treatment, Outcome 8: Infection
1.9. Analysis.

Comparison 1: OSCM versus No treatment, Outcome 9: Haemorrhage
1.10. Analysis.

Comparison 1: OSCM versus No treatment, Outcome 10: Long‐term complications
Comparison 2. WSCM versus No treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Live birth | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.2 Intravasation | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 2.3 Clinical Pregnancy | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.4 Miscarriage per woman | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.5 Miscarriage per pregnancy | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.6 Ectopic pregnancy per woman | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.7 Procedural pain | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 2.8 Infection | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.9 Haemorrhage | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 2.10 Long‐term complications | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
2.2. Analysis.

Comparison 2: WSCM versus No treatment, Outcome 2: Intravasation
2.7. Analysis.

Comparison 2: WSCM versus No treatment, Outcome 7: Procedural pain
2.9. Analysis.

Comparison 2: WSCM versus No treatment, Outcome 9: Haemorrhage
2.10. Analysis.

Comparison 2: WSCM versus No treatment, Outcome 10: Long‐term complications
Comparison 3. OSCM versus WSCM.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Live birth | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.2 Live birth (subgroup analysis) | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.2.1 For diagnostic reasons | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.2.2 For therapeutic reasons | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.3 Intravasation | 4 | 1912 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.00 [2.25, 11.12] |
| 3.4 Clinical pregnancy | 6 | 2598 | Odds Ratio (M‐H, Random, 95% CI) | 1.42 [1.10, 1.85] |
| 3.5 Miscarriage per woman | 2 | 1652 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.56, 1.24] |
| 3.6 Miscarriage per pregnancy | 2 | 603 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.48, 1.13] |
| 3.7 Ectopic pregnancy per woman | 2 | 1652 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.18, 2.30] |
| 3.8 Procedural pain (dichotomous variable) | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.9 Procedural pain (continuous variable) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.10 Infection | 2 | 662 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.04, 1.22] |
| 3.11 Haemorrhage | 2 | 662 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.40, 1.06] |
| 3.12 Long‐term complications | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
3.12. Analysis.

Comparison 3: OSCM versus WSCM, Outcome 12: Long‐term complications
Comparison 4. OSCM + WSCM versus WSCM.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Live birth | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.2 Intravasation | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 4.3 Clinical Pregnancy | 5 | 686 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.91, 1.75] |
| 4.4 Miscarriage per woman | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.5 Miscarriage per pregnancy | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.6 Ectopic pregnancy per woman | 2 | 422 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.05, 4.38] |
| 4.7 Procedural pain | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 4.8 Infection | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 4.9 Haemorrhage | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 4.10 Long‐term complications | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
4.2. Analysis.

Comparison 4: OSCM + WSCM versus WSCM, Outcome 2: Intravasation
4.7. Analysis.

Comparison 4: OSCM + WSCM versus WSCM, Outcome 7: Procedural pain
4.8. Analysis.

Comparison 4: OSCM + WSCM versus WSCM, Outcome 8: Infection
4.9. Analysis.

Comparison 4: OSCM + WSCM versus WSCM, Outcome 9: Haemorrhage
4.10. Analysis.

Comparison 4: OSCM + WSCM versus WSCM, Outcome 10: Long‐term complications
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Al‐Fadhli 2006.
| Study characteristics | ||
| Methods | Method of randomisation: computer‐generated random table Allocation concealment: not mentioned Blinding: not mentioned Analysis: power calculation suggested a requirement for 27 women per contrast group and 39 recruited per group ITT analysis not performed but possible from the data Study setting: McGill University Health Center, Montreal, Quebec, Canada Duration of study: September 2002 to September 2004 Duration of follow‐up: 6 months Withdrawals 88 women recruited and randomised 1 woman in the Lipiodol group excluded (underwent an ovarian cystectomy during the same laparoscopy) 9 withdrawn after randomisation (4 lost to follow‐up; 5 had IVF immediately after laparoscopy with dye sufflation) 78 women analysed Source of funding not stated |
|
| Participants | Number of participants: 88 Mean age: 32 years (SD 0.6) WSCM; 31 years (SD 0.5) OSCM Inclusion criteria: infertile women, duration of infertility not mentioned Investigative work‐up: early follicular FSH < 10 IU/L, normal semen analysis (criteria not mentioned), ovulatory confirmation by mid‐luteal phase progesterone >25 mmol/L, patent fallopian tubes at HSG. Included women had normal laparoscopic findings or stage I‐II endometriosis Exclusion criteria: iodine allergy Breakdown by cause of infertility not specified Previous fertility treatments not specified |
|
| Interventions | Tubal flushing during laparoscopy; after sufflation with WSCM methylene blue dye, OSCM Lipiodol (ultra‐fluid; Guerbet/Ezem, Canada, Montreal, Quebec) versus WSCM saline 10 mL of contrast medium volume used Timing not specified with menstrual cycle Co‐interventions: excision of endometriosis during the laparoscopy was performed in 20 patients (11 WSCM + OSCM, 9 WSCM) Primarily intended as therapeutic procedure | |
| Outcomes | Pregnancy rate (method of diagnosis not specified) | |
| Notes | Unclear whether the assigned treatment was adequately concealed prior to allocation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | No mention |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Trials without blinding are unlikely to have performance bias for objective outcomes. |
| Blinding of participants and personnel (performance bias) Subjective outcome (pain) | Unclear risk | Unclear whether blinding was performed |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Fertility outcomes are objective outcomes and are unlikely to be affected by non‐blinding. |
| Blinding of outcome assessment (detection bias) Subjective outcome (pain) | Unclear risk | Unclear whether blinding was performed |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawals and losses to follow‐up totaled 11% |
| Selective reporting (reporting bias) | Unclear risk | No protocol or registration is available |
| Other bias | Low risk | No other potential bias identified |
Alper 1986.
| Study characteristics | ||
| Methods | Randomisation: random number table
Allocation concealment: not mentioned
Blinding: not mentioned Trial design: parallel group Analysis: power calculation not mentioned. ITT analysis not performed Study setting: single‐centre; Ottowa Civic Hospital, Ottowa, Canada Duration of trial: 8 months Duration of follow‐up: 6 months Withdrawals:13 (9.9%) withdrawn after HSG; 12 (9.2%) lost to follow‐up 131 women recruited and randomised 106 women analysed Source of funding not stated |
|
| Participants | No of women: 131 Mean age: mean age 29.3 years (SD 4.6) WSCM; 29.1 years (SD 2.9) OSCM Cause of infertility: Primary or secondary infertility for more than 12 months (mean or range of duration of pre‐existing infertility not stated, but duration and proportion of primary to secondary similar in two groups) Investigation work‐up: semen analysis, PCT, BBT and endometrial biopsy; diagnostic laparoscopy prior to HSG in most women Breakdown specified by cause for infertility Previous fertility treatments not specified Women with bilateral tubal blockage withdrawn after HSG; no other exclusions specified |
|
| Interventions | HSG with OSCM ethiodol (Savage Laboratories, Missouri City, USA) versus WSCM Renographin (ER Squibb & Sons, Princeton, USA) 10 mL to 20 mL of contrast volume used Timing: any day of menstrual cycle No co‐interventions Primarily intended as diagnostic procedure |
|
| Outcomes | Pregnancy (diagnosis based on urine hCG or serum beta‐hCG plus ultrasound, all the patients had pregnancies confirmed by ultrasound) Volume of contrast medium used Pain during HSG Intravasation |
|
| Notes | Unclear whether the assigned treatment was adequately concealed prior to allocation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table used |
| Allocation concealment (selection bias) | Unclear risk | No mention |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Trials without blinding are unlikely to have performance bias for objective outcomes. |
| Blinding of participants and personnel (performance bias) Subjective outcome (pain) | Unclear risk | Unclear whether blinding was performed |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Fertility outcomes are objective outcomes and are unlikely to be affected by non‐blinding. |
| Blinding of outcome assessment (detection bias) Subjective outcome (pain) | Unclear risk | Unclear whether blinding was performed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Follow‐up and withdrawals from the study totaled 19% |
| Selective reporting (reporting bias) | Unclear risk | No protocol or registration is available |
| Other bias | Low risk | No other potential bias identified |
De Boer 1988.
| Study characteristics | ||
| Methods | Randomisation: not stated Allocation concealment: not stated Blinding: not stated Analysis: not mentioned, ITT analysis not done Study setting: St Radboud University Hospital, Nijmegen, Holland Duration of trial: February 1985 to October 1986 Duration of follow‐up: 6 months Withdrawals: none Source of funding not stated |
|
| Participants | Number of participants: 175 Mean age: 29 years (19 to 44). Primary or secondary infertility for more than six months; mean infertility duration 37 months (SD 26.2) Investigation work‐up: normal PCT or sperm penetration test, or both, and BBT Breakdown by cause for infertility: unexplained only Previous fertility treatments not specified other than exclusion for women with previous infertility surgery |
|
| Interventions | HSG with OSCM ethiodol (Guerbet, France) versus WSCM iopamidol (Bracco, Italy) 10 mL of contrast medium volume used Timing: day 6 to 13 of menstrual cycle No co‐interventions Primarily intended as diagnostic procedure | |
| Outcomes | Pregnancy rate (diagnosis based on ultrasound, although ultrasound criteria not specified) Quality of visualisation of uterine cavity Quality of visualisation of ampullary tubal folds Time for contrast medium to disperse from pelvis |
|
| Notes | Unclear whether the assigned treatment was adequately concealed prior to allocation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method not mentioned |
| Allocation concealment (selection bias) | Unclear risk | No mention |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Trials without blinding are unlikely to have performance bias for objective outcomes. |
| Blinding of participants and personnel (performance bias) Subjective outcome (pain) | Unclear risk | Unclear whether blinding was performed |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Fertility outcomes are objective outcomes and are unlikely to be affected by non‐blinding. |
| Blinding of outcome assessment (detection bias) Subjective outcome (pain) | Unclear risk | Unclear whether blinding was performed |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Rates of loss to follow‐up and withdrawals from the study were unclear |
| Selective reporting (reporting bias) | Unclear risk | No protocol or registration is available |
| Other bias | Low risk | No other potential bias identified |
Dreyer 2017.
| Study characteristics | ||
| Methods | Method of randomisation: secured online randomisations program (ALEA, FormsVision) Allocation concealment: randomisation overseen by independent data manager Blinding: none Analysis: ITT principle Study setting: 27 hospitals in the Netherlands (4 academic, 12 teaching, 11 non‐teaching hospitals). Gynaecologists in these hospitals collaborated in a nationwide Dutch Consortium for Healthcare Evaluation and Research in Obstetrics and Gynaecology Duration of study: February 2012 to October 2014 Duration of follow‐up: 6 months Withdrawals: 3 in the OSCM group and 8 in the WSCM group Source of funding: VU University Medical Centre |
|
| Participants | Number of participants: 1119 (557 OSCM; 562 WSCM) Mean age: 32.8 years (OSCM); 33 years (WSCM) Inclusion criteria:
Exclusion criteria
Breakdown by cause of infertility not specified. Previous fertility treatments not specified. |
|
| Interventions | HSG with OSCM Lipiodol versus WSCM Telebrix hystero: infusion of 5 mL to 10 mL of contrast medium into the uterus with the use of a cervical vacuum cup, metal cannula (hysterophore) or balloon catheter. Four to five radiographs obtained during the infusion of contrast to evaluate the patency of both fallopian tubes were examined by a gynaecologist or radiologist. | |
| Outcomes |
Primary Outcome
Secondary Outcomes
|
|
| Notes | This trial was prospectively registered (the Netherlands Trial Register number, NTR3270) and the protocol was published as a supplementary material in Dreyer 2017 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was performed by the doctors or research nurses with the use of a secured online randomisation program (ALEA, FormsVision) with random block sizes of 2, 4, or 6, stratified according to hospital." |
| Allocation concealment (selection bias) | Low risk | Quote: "The secured online randomisation program was overseen by an independent data manager." |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Trials without blinding are unlikely to have performance bias for objective outcomes. |
| Blinding of participants and personnel (performance bias) Subjective outcome (pain) | High risk | Non‐blinding may affect the use of other interventions during follow‐up although the impact may be small |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "the trial was not blinded with respect to participants and caregivers". It is unlikely to affect objective outcomes. |
| Blinding of outcome assessment (detection bias) Subjective outcome (pain) | High risk | Non‐blinding is likely to affect subjective outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | OBCM: 3 lost to follow‐up; WBCM: 8 lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes in the protocol (Supplementary Material in Dreyer 2017) were reported except for quote: "coital frequency before and after HSG". The authors indicated that they removed this outcome in protocol version 1.2 (Supplementary Material in Dreyer 2017). As this change does not affect the outcome assessment of this review, we scored the reporting bias at low risk. |
| Other bias | Low risk | No other potential bias was detected |
Johnson 2004.
| Study characteristics | ||
| Methods | Randomisation: two computer‐generated random number sequences (A ‐ women with unexplained infertility; B ‐ women with endometriosis in the context of otherwise unexplained infertility) Allocation concealment: sequentially‐numbered sealed opaque envelopes Blinding: no blinding Trial design: parallel group Analysis: power calculation and ITT analysis done Study setting: single‐centre, University of Auckland Dept O&G with Fertility Plus, National Women's Hospital, Auckland, New Zealand Duration of trial: 3 years Duration of follow‐up: 6 months Withdrawals: none Two separate randomisation schedules were used for the endometriosis and unexplained infertility subpopulations Time of randomisation: on same cycle as HSG, usually several days before HSG Not blinded 158 women recruited and randomised No exclusions before HSG No withdrawals 2 protocol breaches 3 women lost to follow‐up 158 women analysed on ITT basis Duration of follow‐up: 6 months Single‐centre: University of Auckland Dept O&G with Fertility Plus, National Women's Hospital, Auckland, New Zealand Source of funding: The University of Auckland Research Committee and the Auckland Research Centre for Reproductive Medicine contributed seed funding. The Lipiodol was provided without charge by Guerbet and supplied free of charge initially by Aventis (New Zealand), later by Biotek (New Zealand). |
|
| Participants | No of women: 158 Mean age: 33.9 years (SD 2.9) for OSCM; 33.5 years (SD 3.8) for control Inclusion criteria: unexplained infertility (or endometriosis where fallopian tubes and ovaries unaffected by endometriotic disease) of duration >12 months, full investigation for the cause complete, age 18 to 39 years, biochemistry as below; confirmed bilateral tubal patency Cause of infertility: unexplained primary or secondary infertility (primary 54.8% OSCM, 60.0% no treatment) for more than 12 months (mean duration of pre‐existing infertility 54.8 months) Investigation work‐up: normal semen analysis by WHO criteria, early follicular FSH < 10 IU/L, ovulatory confirmation by serum progesterone > 25 mmol/L, normal fallopian tubes at laparoscopy and dye insufflation or HSG Breakdown by cause for infertility: pure unexplained 61%, endometriosis with normal fallopian tubes and ovaries 39%, all other causes for infertility excluded Previous fertility treatments: IVF 34%, IUI 44%, empirical clomiphene 60%, women with endometriosis having previous surgical treatment 60% |
|
| Interventions | HSG with OSCM Lipiodol versus no treatment 10 mL contrast medium volume used Timed after menses but prior to Day 12 Information sheet on fertile phase of the cycle given to both groups; no other co‐interventions Primarily intended as therapeutic procedure |
|
| Outcomes | Primary outcome: 1) clinical pregnancy (diagnosis based on positive pregnancy test and intrauterine gestation sac on ultrasound) 2) live birth Secondary outcome: 1) miscarriage 2) ectopic pregnancy 3) fetal death >20 weeks 4) termination 5) multiple pregnancy 6) adverse events |
|
| Notes | Assigned treatment was clearly adequately concealed prior to allocation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number sequences |
| Allocation concealment (selection bias) | Low risk | Allocation concealment: sequentially‐numbered sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Trials without blinding are unlikely to have performance bias for objective outcomes. |
| Blinding of participants and personnel (performance bias) Subjective outcome (pain) | High risk | Non‐blinding may affect the use of other interventions during follow‐up although the impact may be small |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Objective outcomes are unlikely to be affected by non‐blinding. |
| Blinding of outcome assessment (detection bias) Subjective outcome (pain) | High risk | Non‐blinding is likely to affect subjective outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up and withdrawals totaled 3% |
| Selective reporting (reporting bias) | Unclear risk | No protocol or registration is available |
| Other bias | Low risk | No other potential bias identified |
Johnson 2019.
| Study characteristics | ||
| Methods | Method of randomisation: a computer‐generated random number sequence (unknown to the executors of the assignment). Allocation concealment: securely maintained by storage in sealed, sequentially‐numbered opaque envelopes until the interventions were assigned Blinding: open‐label Analysis: ITT Study setting: a tertiary level fertility clinic in Auckland, New Zealand (Fertility Plus, Auckland District Health Board) Duration of study: 12 women were recruited and randomised between August 2005 and July 2006. Follow‐up continued for nine months from randomisation to April 2007 and outcomes of all pregnancies were ascertained in March 2008. Duration of follow‐up: 3 months (We only include data resulting from the first 3 months after randomisation, i.e. before the delayed HSG intervention) Withdrawals: 0 |
|
| Participants | Number of participants: 12 (6 OSCM; 6 no treatment) Mean age: detailed not reported, aged 18 to 39 Inclusion criteria
Exclusion criteria
Source of funding: not stated. |
|
| Interventions | Intervention: HSG with OSCM (Lipiodol). Endometrial samples were assessed for gene expression Comparator:no treatment The study compared an immediate HSG versus a delayed HSG. For both groups, only data resulting from the first 3 months after randomisation were included and therefore the two groups were considered as OSCN versus no treatment. Endometrial biopsy was performed in both groups. |
|
| Outcomes | Primary outcome: clinical pregnancy (positive pregnancy test with positive intrauterine gestation sac on ultrasound). Secondary outcomes:live birth, miscarriage and adverse events (unspecified) |
|
| Notes | Only pregnancy outcomes resulting from the first 3 months after randomisation were included. The protocol published as part of a MD thesis in 2007 (Johnson 2019). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:"Randomisation was performed using a computer‐generated random number sequence (unknown to the executors of the assignment). The randomisation was in a block of 12 but this was unknown to all, other than a statistician who generated the allocation sequence and the principal investigator (NJ)." |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation concealment was securely maintained by storage in sealed, sequentially‐numbered opaque envelopes until the interventions were assigned. Allocation was strictly maintained sequentially, all envelopes in the sequence being used." |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Trials without blinding are unlikely to have performance bias for objective outcomes. |
| Blinding of participants and personnel (performance bias) Subjective outcome (pain) | High risk | Non‐blinding may affect the use of other interventions during follow‐up although the impact may be small |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "There was also no blinding of the executor of the assignment, the clinician performing the lipiodol procedure, nor of the assessor of clinical outcomes at follow‐up." The pregnancy outcomes are objective outcomes and therefore they are unlikely to be affected by non‐blinding. |
| Blinding of outcome assessment (detection bias) Subjective outcome (pain) | High risk | Non‐blinding is likely to affect subjective outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes defined in the protocol were reported. |
| Other bias | High risk | The authors planned to include women "with a history of 12 months infertility and known endometriosis" in the protocol but in the trial they also included 1 woman with "a previous successful pregnancy following lipiodol". Given its small sample size, the inclusion of this single participant is likely to introduce bias towards the group she was assigned to. |
Letterie 1990.
| Study characteristics | ||
| Methods | Randomisation: random number scheme
Allocation concealment: no mention of this Blinding: no mention of this Trial design: parallel group Analysis: power calculation not mentioned; ITT analysis not feasible Study setting: single‐centre; Tripler Army Medical Centre, Honolulu, Hawaii, USA Duration of study: not mentioned Duration of follow‐up: 12 months Withdrawals: 11 withdrawn after randomisation (8 inadequate follow‐up and 3 quote: "inadequate coital exposure") Source of funding: not stated. |
|
| Participants | No of patients: 40 Mean age: 27 years (SD 3.5) OSCM; 25 years (SD 4.1) WSCM (not significant) Cause of infertility: unexplained infertility of mean duration 24 months (SD 14.5) OSCM; 28 months (SD 13.9) WSCM; inclusion criterion >12 months Inclusion criteria: ovulatory status as documented by biphasic basal body temperature with a 14‐day luteal phase; serum progesterone >3 ng/mL or in phase secretory endometrium on biopsy, or both; normal semen analysis; normal pelvic anatomy and bilateral patent tubes Exclusion criteria: iodine allergy; evidence of endometriosis, tubal disease or pelvic adhesions Breakdown by cause: not done Investigation work‐up: normal semen analysis; ovulatory confirmation based on BBT and serum progesterone or secretory phase, or both; normal prolactin, thyroxine and TSH; normal pelvis and bilateral tubal patency at laparoscopy Breakdown by cause for infertility: unexplained only Previous fertility treatments not specified Exclusions specified: where cause for infertility diagnosed; iodine allergy |
|
| Interventions | Tubal flushing during laparoscopy, after standard dye studies, with OSCM ethiodol (Savage Laboratories) versus WSCM Conray‐60 (Mallinckrodt Inc.) 20 mL contrast medium volume used Timing not specified with menstrual cycle No co‐interventions Primarily intended as therapeutic procedure | |
| Outcomes | Pregnancy (diagnostic criteria not specified) Ectopic pregnancy |
|
| Notes | Unclear whether the assigned treatment was adequately concealed prior to allocation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number scheme |
| Allocation concealment (selection bias) | Unclear risk | No mention |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Trials without blinding are unlikely to have performance bias for objective outcomes. |
| Blinding of participants and personnel (performance bias) Subjective outcome (pain) | Unclear risk | Unclear whether blinding was performed |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Fertility outcomes are objective outcomes and are unlikely to be affected by non‐blinding. |
| Blinding of outcome assessment (detection bias) Subjective outcome (pain) | Unclear risk | Unclear whether blinding was performed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Losses to follow‐up and withdrawals totaled 28% |
| Selective reporting (reporting bias) | Unclear risk | No protocol or registration is available |
| Other bias | Low risk | No other potential bias identified |
Lindborg 2009.
| Study characteristics | ||
| Methods | Randomisation: computer‐generated randomisation in blocks of 40 Allocation concealment: sealed‐opaque envelopes used Blinding: not done Analysis: ITT analysis done Trial design: parallel group Study setting: Reproductive unit at Sahlgrenska University, Gothenburg, Sweden Duration of study:December 2001 to May 2006 Duration of follow‐up: 6 months Withdrawals: clearly mentioned Source of funding: The study was supported by grants from University of Gothenburg/ Sahlgrenska University Hospital (LUA/ALF 7094) and from the Medical Society of Gothenburg. |
|
| Participants | Number of participants: 334 Mean age: 31.9 years Inclusions: at least 1 year of subfertility, already scheduled for HyCoSy Exclusions: > 40 years, severe male infertility, severe tubal pathology, suspected anovulation (menstrual period > 35 days) Breakdown for cause: 63% primary infertility, mean duration of infertility 2.1 years |
|
| Interventions | All received transvaginal scan prior to use of contrast medium (hydrosalpinx contraindication) Saline injected into uterine cavity to achieve distension, WSCM (Echovist, Bayer AG) instilled to evaluate tubal patency Maxiumum 15 mL contrast medium volume used Categorical statement made for each tube (patent, occluded, unclear) All received oral antibiotic post‐procedure Timing not specified with menstrual cycle No co‐interventions |
|
| Outcomes | Primary outcome: clinical pregnancy defined sonographically as visible fetal sac within 6 months Live birth Miscarriage Ectopic pregnancy |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes used |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Trials without blinding are unlikely to have performance bias for objective outcomes. |
| Blinding of participants and personnel (performance bias) Subjective outcome (pain) | High risk | Non‐blinding may affect the use of other interventions during follow‐up although the impact may be small |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Physicians were aware of allocation. Fertility outcomes are objective outcomes and are unlikely to be affected by non‐blinding. |
| Blinding of outcome assessment (detection bias) Subjective outcome (pain) | High risk | Non‐blinding is likely to affect subjective outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up; withdrawals totaled 11%; ITT analysis was performed |
| Selective reporting (reporting bias) | Unclear risk | No protocol is available. The trial was retrospectively registered (ISRCTN20715945) and therefore it is unclear whether the outcomes were selectively reported or not. |
| Other bias | Low risk | No other potential bias identified |
Lindequist 1994.
| Study characteristics | ||
| Methods | Method of randomisation: not stated Allocation concealment: not mentioned Blinding: not mentioned Analysis: no mention of power calculation, ITT analysis not performed nor possible Study design: parallel group Study setting: Odense University Hospital, Odense, Denmark Duration of study: September 1989 to April 1991 Duration of follow‐up: 20 to 39 months Withdrawals: 307 recruited and randomised, 60 patients excluded prior to HSG or lost to follow‐up, 5 withdrawn after HSG Source of funding: Schering, Copenhagen, Denmark (for the free delivery of iotrolan used in this study). |
|
| Participants | No of participants: 242 Mean age: 29.9 years OSCM (21 t0 43); 29.5 years WSCM (20 to 40) Inclusions: primary or secondary infertility for more than 12 months; secondary 48 (40%) OSCM, 42 (35%) WSCM Exclusion criteria: pregnant prior to HSG; HSG declined; technical difficulties leading to unsuccessful HSG; HSG not performed by authors; infertility < 12 months Mean duration of pre‐existing infertility 41 months OSCM, 40 months WSCM Breakdown by cause for infertility not specified Previous fertility treatments not specified |
|
| Interventions | HSG with OSCM Lipiodol (Laboratories Guerbet, France) versus HSG with WSCM Iotrolan 5 mL to 10 mL contrast medium volume used Timed between end of menses and Day 10 No co‐interventions Primarily intended as diagnostic procedure | |
| Outcomes | Pregnancy (method of diagnosis not specified, but data extracted from Danish Patient Database to complete information with respect to pregnancy) Image quality Pain Infection Haemorrhage |
|
| Notes | Unclear whether the assigned treatment was adequately concealed prior to allocation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention |
| Allocation concealment (selection bias) | Unclear risk | No mention |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Trials without blinding are unlikely to have performance bias for objective outcomes. |
| Blinding of participants and personnel (performance bias) Subjective outcome (pain) | Unclear risk | Unclear whether blinding was performed |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | All examinations and evaluations performed by authors but fertility outcomes are objective outcomes and are unlikely to be affected by non‐blinding. |
| Blinding of outcome assessment (detection bias) Subjective outcome (pain) | Unclear risk | Unclear whether blinding was performed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Losses to follow‐up and withdrawals totaled 21% |
| Selective reporting (reporting bias) | Unclear risk | No protocol or registration is available |
| Other bias | Low risk | No other potential bias identified |
Nugent 2002.
| Study characteristics | ||
| Methods | Method of randomisation: third party sealed envelopes with allocation inside Allocation concealment: adequate Blinding: not blinded Analysis: power calculation specified a requirement for 180 recruits but trial terminated early owing to slow recruitment rate and running out of time ITT analysis performed Study setting: Leeds General Infirmary and Princess Royal Hospital Hull, UK Duration of study: 10 months Duration of follow‐up: 6 months Withdrawals: none Source of funding: not stated. |
|
| Participants | Number of participants: 34 Mean age: 30.6 years (eligibility criterion < 36 years). Inclusion criteria: unexplained primary or secondary infertility (proportion of primary and secondary not stated) for more than 12 months (mean duration of pre‐existing infertility 49 months) Investigation work‐up: normal semen analysis by WHO criteria, ovulatory confirmation by serum progesterone or serial scanning, normal fallopian tubes at laparoscopy and dye insufflation or HSG Breakdown by cause for infertility: unexplained only, all other causes for infertility excluded Previous fertility treatments not specified |
|
| Interventions | HSG with OSCM Lipiodol versus no treatment 5.8 mL +/‐ 0.7 mL of contrast medium volume used Timing with menstrual cycle not specified Information sheet on fertile phase of the cycle given to both groups; no other co‐interventions Primarily intended as therapeutic procedure |
|
| Outcomes | Pregnancy rate (diagnosis based on positive pregnancy test)
Viable pregnancy (diagnosis based on fetal heart on ultrasound) Adverse events |
|
| Notes | Assigned treatment was clearly adequately concealed prior to allocation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random code in sealed‐numbered envelopes (provided by the author) |
| Allocation concealment (selection bias) | Low risk | Third party sealed envelope entry |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Trials without blinding are unlikely to have performance bias for objective outcomes. |
| Blinding of participants and personnel (performance bias) Subjective outcome (pain) | High risk | Non‐blinding may affect the use of other interventions during follow‐up although the impact may be small |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Fertility outcomes are objective outcomes and are unlikely to be affected by non‐blinding. |
| Blinding of outcome assessment (detection bias) Subjective outcome (pain) | High risk | Non‐blinding is likely to affect subjective outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants apparently included in analysis |
| Selective reporting (reporting bias) | Unclear risk | No protocol or registration is available |
| Other bias | Low risk | No other potential bias identified |
Ogata 1993.
| Study characteristics | ||
| Methods | Method of randomisation: not stated Allocation concealment: Blinding: not mentioned Analysis: no mention of power calculation. ITT analysis not done nor possible Study setting: University of Kyusyu, Fukuoka, Japan Study duration: November 1989 to February 1991 Duration of follow‐up: 4 months Withdrawals: 43/148 losses to follow‐up in the OSCM group and 69/154 in the WSCM group Source of funding: not stated. |
|
| Participants | Number of participants: 302 randomised (148 versus 154). Those who failed to complete the four ovulatory cycles of observation were excluded, so only 190 were included in analysis (105 versus 85) Mean age: not specified; said to be similar between the 2 groups Inclusion criteria: primary or secondary infertility (proportion not specified) having first visit to infertility clinic; duration of infertility not specified but said to be similar between the 2 groups Investigation work‐up: not specified, but rate of male infertility and PCT results said to be similar between the 2 groups Breakdown by cause for infertility not specified Previous fertility treatments not specified No exclusion criteria specified |
|
| Interventions | HSG with OSCM Lipiodol (Ultra‐Fluid) versus no HSG (the HSG was delayed for 4 months until after the analysis) Contrast medium volume not specified Timing with respect to menstrual cycle not specified No co‐interventions Primarily intended as diagnostic procedure | |
| Outcomes | Pregnancy (method of diagnosis not specified) | |
| Notes | Unclear whether the assigned treatment was adequately concealed prior to allocation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention of randomisation process |
| Allocation concealment (selection bias) | Unclear risk | No mention |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Trials without blinding are unlikely to have performance bias for objective outcomes. |
| Blinding of participants and personnel (performance bias) Subjective outcome (pain) | Unclear risk | Unclear whether blinding was performed |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Fertility outcomes are objective outcomes and are unlikely to be affected by non‐blinding. |
| Blinding of outcome assessment (detection bias) Subjective outcome (pain) | Unclear risk | Unclear whether blinding was performed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Losses to follow‐up and withdrawals totaled 37% (102/302) |
| Selective reporting (reporting bias) | Unclear risk | No protocol or registration is available |
| Other bias | Low risk | No other potential bias identified |
Rasmussen 1991.
| Study characteristics | ||
| Methods | Method of randomisation: not stated Allocation concealment: not stated Blinding: no mention Analysis: ITT analysis not done or not possible, no mention of power calculation Study setting: Odense University Hospital, Odense, Denmark Duration of study: 1985 to 1988 Duration of follow‐up: 9 months Withdrawals: 507 recruited and randomised, 78 excluded prior to HSG, 31 withdrawn after HSG, 14 lost to follow‐up (out of 207 in total) Source of funding: Nycomed AS, Oslo (free delivery of Omnipaque used in this study). |
|
| Participants | Number of participants: 398 Mean age: not stated Inclusion: primary or secondary infertility for more than 12 months (mean or range of duration of pre‐existing infertility not stated) Exclusion criteria: pregnant prior to HSG; HSG declined; technical difficulties leading to unsuccessful HSG; HSG not performed by authors Investigation work‐up: not stated Breakdown by cause for infertility not specified Previous fertility treatments not specified |
|
| Interventions | HSG with OSCM Lipiodol (Laboratories Guerbet, France) versus 3 types of WSCM: iohexol (Omnipaque 350, Nycomed, Oslo), Ioxaglate (Hexabrix 320, Laboratoire Guerbet, France), diatrizoate (Urografin, Schering, Berlin). As there were no outcome differences between the 3 groups using WSCM, they were combined in the analysis of results 5 mL to 10 mL contrast medium volume used Timing with menstrual cycle not specified No co‐interventions Primarily intended as diagnostic procedure | |
| Outcomes | Pregnancy (method of diagnosis not specified) Other outcomes of this trial (reported image quality, pain, infection, haemorrhage and intravasation) are reported in a separate publication (Lindequist 1991) |
|
| Notes | Unclear whether the assigned treatment was adequately concealed prior to allocation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | No mention |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Trials without blinding are unlikely to have performance bias for objective outcomes. |
| Blinding of participants and personnel (performance bias) Subjective outcome (pain) | Unclear risk | Unclear whether blinding was performed |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Fertility outcomes are objective outcomes and are unlikely to affected by non‐blinding. |
| Blinding of outcome assessment (detection bias) Subjective outcome (pain) | Unclear risk | Unclear whether blinding was performed |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Losses to follow‐up and withdrawals totaled 9% |
| Selective reporting (reporting bias) | Unclear risk | No protocol or registration is available |
| Other bias | Low risk | No other potential bias identified |
Spring 2000.
| Study characteristics | ||
| Methods | Method of randomisation: computer‐generated random numbers in blocks of 9 at each site Allocation concealment: adequate Blinding: not mentioned Analysis: power calculation suggested a requirement for 257 women per contrast group (achieved for 2 groups and recruitment abandoned for third group owing to difficulty recruiting) ITT analysis not performed Study setting: 10 centres coordinated by the Kaiser Permanente Medical Care Program Infertility Work Group, California, USA Duration of study: December 1993 to July 1996 Duration of follow‐up: 12 months Withdrawals: 673 recruited and randomised, 7 lost to follow‐up Source of funding: Supported in part by Kaiser Permanente Medical Care Program‐ Northern California Innovation Project grant no. 930198 |
|
| Participants | Number of participants: 666 Mean age: 29.3 years (SD 4.6) WSCM; 29.1 years (SD 2.9) OSCM Inclusion criteria: primary or secondary infertility (OSCM 35.0%, WSCM 37.1%, WSCM + OSCM 34.8% primary infertility). Mean duration of infertility: OSCM 3.13 years (SD 3.03), WSCM 3.15 years (SD 3.18), WSCM + OSCM 3.09 years (SD 3.61); eligibility criterion >12 months Investigation work‐up: not specified Breakdown by cause for infertility not specified Previous fertility treatments not specified Exclusion criteria: none |
|
| Interventions | HSG with OSCM ethiodol (Savage Laboratories, Melville, USA) versus WSCM diatrizoate and iodipamide (Bracco Diagnostics, New Brunswick, USA) versus both WSCM and OSCM Volume WSCM mean 9.4 mL (range 2 mL to 75 mL); OSCM mean 8.6 mL (range 1 mL to 55 mL); both‐ WSCM mean 8.2 mL (range 1 mL to 30 mL) and OSCM mean 6.mL (range 1 mL to 20 mL) Timing with menstrual cycle not specified Co‐interventions: artificial insemination performed in 25.3% OSCM; 24.6% WSCM; 24.8% WSCM + OSCM Primarily intended as diagnostic procedure | |
| Outcomes | Pregnancy (diagnostic criteria not specified)
Live birth Miscarriage Ectopic pregnancy |
|
| Notes | Assigned treatment was clearly adequately concealed prior to allocation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number scheme |
| Allocation concealment (selection bias) | Unclear risk | No mention |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Trials without blinding are unlikely to have performance bias for objective outcomes. |
| Blinding of participants and personnel (performance bias) Subjective outcome (pain) | Unclear risk | Unclear whether blinding was performed |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Same clinician provided patient details, carried out HSG and reported the results. Fertility outcomes are objective outcomes and are unlikely to be affected by non‐blinding. |
| Blinding of outcome assessment (detection bias) Subjective outcome (pain) | Unclear risk | Unclear whether blinding was performed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up and withdrawals totaled 1% |
| Selective reporting (reporting bias) | Unclear risk | No protocol or registration is available |
| Other bias | High risk | Groups unequal at baseline: younger women aged 20 to 24 more likely to be assigned to WSCM, women aged 35 to 39 more likely to be assigned to OSCM |
Steiner 2003.
| Study characteristics | ||
| Methods | Method of randomisation: computer‐generated random numbers Allocation concealment: not concealed Analysis: no power calculation, ITTanalysis not done Blinding: not done Study setting: University of Carolina, USA Duration of study: August 1996 to November 2000 Duration of follow‐up: 18 months Withdrawals: 698 recruited, 642 excluded, 3 lost to follow‐up Source of funding: not stated |
|
| Participants | Number of participants: 56 Mean age: 32.9 years (SD 3.4) WSCM; 32.6 years (SD 3.6) WSCM + OSCM Inclusion criteria: primary or secondary infertility (WSCM 57.5%, WSCM + OSCM 46.7% primary infertility) Mean duration of infertility: WSCM 2.9 years (SD 3.0), WSCM + OSCM 2.8 years (SD 2.3); eligibility criterion >12 months Exclusion criteria: iodine allergy, non‐patent tubes, refusal to participate Investigation work‐up: not specified Breakdown by cause for infertility specified but data for subpopulations could not be extracted Previous fertility treatments not specified |
|
| Interventions | HSG with WSCM Sinografin (Bracco Diagnostics, New Brunswick, USA) versus WSCM Sinografin + OSCM ethiodol (Savage Laboratories, Melville, USA) 5 mL to 10 mL contrast medium volume used Timing with menstrual cycle not specified Co‐interventions: ovulatory medication used in 61.5% WSCM; 53.3% WSCM + OSCM Primarily intended as therapeutic procedure |
|
| Outcomes | Pregnancy (self‐report or positive blood or urine pregnancy test) Time to conception |
|
| Notes | Allocation was not concealed from physicians; patients were informed of allocation after randomisation before treatment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated number scheme |
| Allocation concealment (selection bias) | High risk | Allocation sequence was not concealed |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Trials without blinding are unlikely to have performance bias for objective outcomes. |
| Blinding of participants and personnel (performance bias) Subjective outcome (pain) | High risk | Non‐blinding may affect the use of other interventions during follow‐up although the impact may be small |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Clinicians reporting the outcome were aware of the allocation. Fertility outcomes are objective outcomes and are unlikely to be affected by non‐blinding. |
| Blinding of outcome assessment (detection bias) Subjective outcome (pain) | High risk | Non‐blinding is likely to affect subjective outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up and withdrawals totaled 5% |
| Selective reporting (reporting bias) | Unclear risk | No protocol or registration is available |
| Other bias | Low risk | No other potential bias identified |
Yang 1989.
| Study characteristics | ||
| Methods | Method of randomisation: not stated Allocation concealment: unclear Blinding: double‐blind (but unclear who was blinded) Analysis: no mention of power calculation, ITT analysis not done Study setting: Mackay Memorial Hospital, Taipei, Japan Duration of study: October 1986 to March 1987 Duration of follow‐up: 8 months Withdrawals: none Source of funding: not stated |
|
| Participants | Number of participants: 109 Participant age: range 22 to 44 years; mean age WSCM 30.1 years, WSCM + OSCM 30.0 years Inclusion criteria: primary or secondary infertility for more than 12 months (mean or range of duration of pre‐existing infertility not stated) Exclusion criteria: not mentioned Investigative work‐up: not stated Breakdown specified by cause for infertility Previous fertility treatments not specified |
|
| Interventions | HSG with WSCM Telebrix Hystero (Laboratories Guerbet) versus WSCM Telebrix Htstero followed by OSCM Lipiodol Ultrafluide (Laboratories Guerbet) A volume of 10 mL WSCM and 5 mL OSCM were used Timing with menstrual cycle not specified No co‐interventions Primarily intended as diagnostic procedure | |
| Outcomes | Pregnancy (method of diagnosis not specified) | |
| Notes | Unclear whether the assigned treatment was adequately concealed prior to allocation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Trials without blinding are unlikely to have performance bias for objective outcomes. |
| Blinding of participants and personnel (performance bias) Subjective outcome (pain) | Unclear risk | Althought the authors stated "double blind", but it is unclear who was blinded. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Fertility outcomes are objective outcomes and are unlikely to affected by non‐blinding. |
| Blinding of outcome assessment (detection bias) Subjective outcome (pain) | Unclear risk | Althought the authors stated "double blind", but it is unclear who was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in analysis |
| Selective reporting (reporting bias) | Unclear risk | No protocol or registration is available |
| Other bias | Low risk | No other potential bias identified |
BBT: basal body temperature; CAT: computerised axial tomography; FSH: follicle stimulating hormone; hCG: human chorionic gonadotropin; HSG: hysterosalpingogram; HyCoSy: hysterosalpingo‐contrast sonography; ITT: intention‐to‐treat;IVF: in vitro fertilisation; OSCM: oil‐soluble contrast media; PCOS: polycystic ovary syndrome; PID: pelvic inflammatory disease; SD: standard deviation; VAS: visual analogue scale; WHO: World Health Organization; WSCM: water‐soluble contrast media.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Acton 1988 | Non‐randomised study comparing HSG with OSCM versus WSCM in 420 women |
| Barwin 1971 | Non‐randomised study comparing HSG with OSCM versus WSCM in 248 women |
| Court 2014 | Non‐randomised observational study looking at pregnancy rates in 100 patients undergoing HSG using OSCM |
| DeCherney 1980 | Non‐randomised study comparing HSG with OSCM versus WSCM in 339 women |
| Gillespie 1965 | Non‐randomised study comparing HSG with OSCM versus WSCM in 271 women |
| Mackey 1971 | Non‐randomised study of HSG with OSCM versus WSCM versus no treatment in 523 women. (Showed no therapeutic effect of HSG with WSCM (OR 0.87, 95% CI 0.47 to 1.59), but a significantly higher pregnancy rate after HSG with OSCM (OR 1.60, 95%CI 1.09 to 2.35)) |
| Perquin 2006 | Randomised controlled trial comparing HSG prior to laparoscopy and dye in 344 women |
| Pham 2017 | Ineligible study design. This is a cost‐effective analysis of an RCT. |
| Reilly 2019 | Ineligible intervention. This RCT compares Lipiodol bathing versus no intervention prior to IVF treatment. |
| Schwabe 1983 | Described as 'pseudo‐randomised' with alternate assignment (thus not a truly randomised trial and therefore excluded), studied HSG with OSCM versus WSCM in 198 women (121 analysed). (Showed no significant difference in the odds of pregnancy for OSCM versus WSCM (OR 2.00, 95% CI 0.74 to 5.45)) |
| van Rijswijk 2018a | Ineligible intervention. This is a protocol of an RCT comparing effectiveness of management guided by HyFoSy and by HSG. All women will undergo tubal testing by both HyFoSy and HSG in a randomised order during fertility work‐up. |
| van Rijswijk 2018b | Ineligible study design. This is a cost‐effective analysis of an RCT. |
| Wolf 1989 | Double‐blind RCT of HSG with iotrolan (WSCM) versus Iohexol versus diatrizoate assessing image quality and pain, but not pregnancy outcomes, in 60 women. A potential therapeutic effect on subsequent pregnancy outcomes could not be studied |
| Yaegashi 1987 | Non‐randomised study of HSG with OSCM versus WSCM in 224 women. The details of this study were confirmed after commissioning a translation from the original Japanese publication |
CI: confidence interval; HSG: hysterosalpingogram; HyFoSy: hysterosalpingo foam sonography; IVF: in vitro fertilisation; OSCM: oil‐soluble contrast media; OR: odds ratio; RCT: randomised controlled trial; WSCM: water‐soluble contrast media.
Characteristics of ongoing studies [ordered by study ID]
Cai 2019.
| Study name | ChiCTR1900025866 |
| Methods | RCT 1000 participants |
| Participants |
Inclusion criteria 1. Aged 20 to 39 years; female 2. Those who have normal sexual life and have not taken contraceptive measures to cohabit for more than one year without pregnancy 3. FSH level in the early follicular phase was less than 10 IU/L, progesterone level in the middle luteal phase was more than 25 mmol/L 4. Accord with the indication of hysterosalpingography 5. Understand and sign the informed consent Exclusion criteria 1. Contrast allergy is known 2. Fewer than 8 menstrual cycles per year, or no ovulation cycle induction is effective 3. Has received HSG or HyCoSy in the past year 4. Fallopian tube obstruction is known 5. Functional infertility of endometrium or ovary 6. Pelvic infection or organ lesions 7. Uterine bleeding (including menstruation) 8. The total number of motile sperm after husband's washing is less than 1 million/mL or that of non‐washing motile sperm is less than 10 million/mL 9. The patients with serious somatic diseases include tumours, cerebral infarction, brain trauma, encephalitis, liver and kidney failure, heart and lung failure, severe anaemia and other blood diseases |
| Interventions | Tubal flushing (HSG) with OSCM vs tubal flushing (HyCoSy) with WSCM |
| Outcomes | Clinical pregnancy, live birth, miscarriage, ectopic pregnancy, twin pregnancy, time to pregnancy |
| Starting date | 1 January 2020 |
| Contact information | The Third Affiliated Hospital of Guangzhou Medical University Mimi Zhou: 1139287171@qq.com; Mingjin Cai: mingjincai@163.com |
| Notes |
Hassan 2015.
| Study name | NCT02433418 |
| Methods | Open‐label RCT 300 participants |
| Participants |
Inclusion Criteria
Exclusion Citeria
|
| Interventions | Tubal flushing (HSG) with Urografin® (WSCM) vs no intervention |
| Outcomes | Primary outcome: pregnancy (defined as the presence of an intrauterine sac by vaginal ultrasound) |
| Starting date | May 2015 |
| Contact information | AbdelGany MA Hassan Cairo University Hospitals 00217801604 abdelgany2@gmail.com |
| Notes | The trial team was contacted but no response was received. |
Legro 2018.
| Study name | SHOW Trial (NCT03604549) |
| Methods | Triple‐blind (participant, care provider, investigator) RCT 55 participants |
| Participants |
Inclusion Criteria
Exclusion Criteria
|
| Interventions | Tubal flushing with OSCM (Lipiodol) versus tubal flushing with WSCM (saline) Tubal flushing in both groups will be performed after normal saline Sono HS |
| Outcomes | Ongoing pregnancy, procedural‐related pain assessed by visual analogue scale, live birth |
| Starting date | 11 January 2019 |
| Contact information | Richard Legro, M.D. Penn State Milton S. Hershey Medical Center Hershey, Pennsylvania, USA, 17033 |
| Notes |
Mijatovic 2018.
| Study name | H2Oil‐timing study (EUCTR2018‐004153‐24‐NL) |
| Methods | Open‐label RCT 554 participants |
| Participants |
Inclusion criteria In order to be eligible to participate in this study, a subject must meet all of the following criteria.
Exclusion criteria
|
| Interventions | Direct tubal flushing (HSG) with OSCM (Lipiodol) incorporated in the fertility work‐up versus delayed tubal flushing (HSG) with OSCM (Lipiodol) 6 months after fertility work‐up Pregnancy resulted from the first six months after randomisation will be eligible and the comparison will be considered as OSCM versus no treatment |
| Outcomes | ‐ Live birth ‐ Clinical pregnancy ‐ Ongoing pregnancy ‐ Miscarriage ‐ Ectopic pregnancy ‐ Multiple pregnancy ‐ Complications following HSG (infection, intravasation) ‐ Pregnancy outcomes (birth weight) ‐ Pregnancy complications ‐ Stillbirth ‐ Thyroid function of the woman (before and 1 month after HSG) ‐ Neonatal outcomes ‐ Additional fertility treatments (Intra‐uterine insemination, IVF, IVF/ICSI) ‐ Thyroid function of neonate (determined by heel prick) |
| Starting date | 30 July 2019 |
| Contact information | Dr. V. Mijatovic mijatovic@vumc.nl Amsterdam UMC ‐ location Vrije Universiteit Amsterdam |
| Notes |
Rosielle 2019.
| Study name | H2Olie2 (NL7925) |
| Methods | Open‐label RCT |
| Participants | Inclusion criteria: 1: with ovulation disorders (ovulation disorders will be defined as less than 8 menstrual cycles per year) or; 2: at high risk for tubal pathology (high risk for tubal pathology will be defined as a positive chlamydia infection, a pelvic inflammatory disease, known endometriosis, abdominal surgery (including tubectomy for ectopic pregnancy and appendectomy) and/or peritonitis in the medical history) or; 3: 39 years of age or over Exclusion criteria ‐ Iodinated contrast agent allergy ‐ Male subfertility defined as total motile sperm count < 3 x10^6 spermatozoa/mL ‐ Not willing or able to sign the consent form |
| Interventions | Tubal flushing (HSG) with OSCM versus tubal flushing (HSG) with WSCM |
| Outcomes |
|
| Starting date | August, 20, 2019 |
| Contact information | Kimmy Rosielle, k.rosielle@amsterdamumc.nl Phone: 020‐4444567 Amsterdam UMC |
| Notes |
Zhang 2020.
| Study name | ChiCTR2000031612 |
| Methods | Open‐label RCT 1040 participants |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions | Tubal flushing (HSG) with OSCM tubal flushing (HSG) with WSCM |
| Outcomes | Ongoing pregnancy, clinical pregnancy, live birth, miscarriage, ectopic pregnancy, procedural pain, thyroid function |
| Starting date | 5 April 2020 |
| Contact information | Jian Zhang, ipmch@foxmail.com International Peace Maternity and Child Health Hospital, School of Medicine, Shanghai Jiaotong University |
| Notes |
FSH: follicle‐stimulating hormone; HSG: hysterosalpingogram; HyCoSy: hysterosalpingo‐contrast sonography; ICSI: Intracytoplasmic sperm injection; IVF: in vitro fertilisation; OSCM: oil‐soluble contrast media; RCT: randomised controlled trial; WSCM: water‐soluble contrast media.
Differences between protocol and review
In this 2020 update, we updated the outcome measures according to the current Cochrane standard; we used a composite outcome (live birth or ongoing pregnancy) as the primary effective outcome, and intravasation as the primary safety outcome. We used woman per randomised, instead of per pregnancy, as the unit of analysis for all outcomes. We also added clinical pregnancy as a secondary outcome. We removed the sensitivity analysis based on diagnostic and therapeutic aims as it was already included as one subgroup analysis. We checked the data extraction of previously included studies and corrected data errors when applicable.
In the 2015 update we added one new comparison (water‐soluble contrast media (WSCM) versus no treatment).
Contributions of authors
RW, AW, KC, BWJM and LM were involved in screening and/or data extraction in this update. RW and LM performed the analyses. All review authors interpreted the data. RW, AW, KC and LM drafted the update in 2020. CF, NJ and BWJM critically revised the draft. All review authors approved the final version.
Sources of support
Internal sources
None, Other
External sources
None, Other
Declarations of interest
NJ is involved in fertility and endometriosis research with the University of Auckland and the University of Adelaide that is funded by Guerbet, the manufacturer of Lipiodol; he has also been in receipt of research funding from Abb‐Vie and Myovant Sciences;he has private appointments with private medical practice groups called Auckland Gynaecology Group and Repromed Auckland (with both of whom he is a shareholder); he has undertaken paid consultancies for Guerbet, as well as Myovant Sciences, Vifor Pharma and Roche Diagnostics; he was an investigator on two included trials (Johnson 2004 and Johnson 2019).
BWJM is supported by an NHMRC Investigator grant (GNT1176437) and reports consultancy for ObsEva, Merck KGaA, iGenomix and Guerbet ending July 2020; BWJM receives research support from Guerbet through his institute; BWJM was an investigator on one included trial (Dreyer 2017).
AW was an investigator on one included trial (Nugent 2002).
NJ, BWM and AW took no part in selection and data extraction of the studies of which they are authors.
RW, KC, CF and LM have no conflicts of interest to declare.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Al‐Fadhli 2006 {published data only}
- Al-Fadhli R, Sylvestre C, Buckett W, Tulandi T. A randomized study of laparoscopic chromopertubation with lipiodol versus saline in infertile woman. Fertility and Sterility 2006;85(2):505-7. [DOI] [PubMed] [Google Scholar]
Alper 1986 {published data only}
- Alper MM, Garner PR, Spence JE, Quarrington AM. Pregnancy rates after hysterosalpingography with oil- and water-soluble contrast media. Obstetrics and Gynecology 1986;68(1):6-9. [PubMed] [Google Scholar]
De Boer 1988 {published data only}
- De Boer AD, Vemer HM, Willemsen WN, Saunders FB. Oil or aqueous contrast media for hysterosalpingography: a prospective, randomized, clinical study. European Journal of Obstetrics Gynecology and Reproductive Biology 1998;28:65-8. [DOI] [PubMed] [Google Scholar]
Dreyer 2017 {published data only}
- Dreyer K, Berghuis MA, Mijatovic V, Goddijn M, Verhoeve HR, Hoek A, et al. Therapeutic effect of oil-based-versus water-based contrast for hysterosalpingography (HSG) during the fertility work-up (H2Oil study), NTR3270, a nationwide randomised controlled trial. Human Reproduction 2015;30(Suppl1):i120. [Google Scholar]
- Dreyer K, Rijswijk J, Mijatovic V, Goddijn M, Verhoeve HR, Rooij IAJ, et al. Oil-based or water-based contrast for hysterosalpingography in infertile women. New England Journal of Medicine 2017;376(21):2043-52. [DOI] [PubMed] [Google Scholar]
Johnson 2004 {published data only}
- Johnson N, Farquhar C, Suckling J, Yu Y, Sadler L, Hadden W. The FLUSH Trial - a randomised trial of lipiodol flushing for unexplained subfertility by hysterosalpingography. In: Australian and New Zealand Journal of Obstetrics and Gynaecology (refereed abstracts of original oral presentations at the Royal Australian and New Zealand College of Obstetricians and Gynaecologists 5th Scientific Meeting, Auckland). Vol. 43. 2003:406.
- Johnson NP, Farquhar CM, Hadden WE, Suckling J, Yu Y, Sadler L. The FLUSH Trial - Flushing with lipiodol for unexplained (and endometriosis-related) subfertility by hysterosalpingography: a randomised trial. Human Reproduction 2004;19:2043-51. [DOI] [PubMed] [Google Scholar]
- Johnson NP, Kwok R, Stewart AW, Saththianathan M, Hadden WE, Chamley LW. Lipiodol fertility enhancement: two-year follow-up of a randomized trial suggests a transient benefit in endometriosis, but a sustained benefit in unexplained infertility. Human Reproduction 2007;22(11):2857-62. [DOI] [PubMed] [Google Scholar]
Johnson 2019 {published data only}
- Johnson NP, Baidya S, Jessup SO, Print CG, Muthukaruppan A, Chamley LW, et al. Randomised trial of Lipiodol Uterine Bathing Effect (LUBE) in women with endometriosis-related infertility. Fertility & Reproduction 2019;1(1):57-64. [DOI: 10.1142/S2661318219500063] [Google Scholar]
- Johnson NP. Lipiodol fertility enhancement in unexplained and endometriosis-related infertility. Thesis (MD) - University of Auckland 2007.
Letterie 1990 {published data only}
- Letterie GS, Rose GS. Pregnancy rates after the use of oil-based and water-based contrast media to evaluate tubal patency. Southern Medical Journal 1990;83:1402-3. [DOI] [PubMed] [Google Scholar]
Lindborg 2009 {published data only}
- Lindborg L, Thorburn J, Bergh C, Strandell A. Influence of HyCoSy on spontaneous pregnancy: a randomized controlled trial. Human Reproduction 2009;24:1075-9. [DOI] [PubMed] [Google Scholar]
Lindequist 1994 {published data only}
- Lindequist S, Rasmussen F, Larsen C. Use of iotrolan versus ethiodized poppy-seed oil in hysterosalpingography. Radiology 1994;191:513-7. [DOI] [PubMed] [Google Scholar]
Nugent 2002 {published data only}
- Nugent D, Watson AJ, Killick SR, Balen AH, Rutherford AJ. A randomized controlled trial of tubal flushing with lipiodol for unexplained infertility. Fertility and Sterility 2002;77:173-5. [DOI] [PubMed] [Google Scholar]
Ogata 1993 {published data only}
- Ogata R, Nakamura G, Uchiumi Y, Yokoyamay M, Watanabe Y, Nozaki M, et al. Therapeutic efficacy of hysterosalpingography (HSG) in infertility, a prospective, randomized, clinical study. Japanese Journal of Fertility and Sterility 1993;38:91-4. [Google Scholar]
Rasmussen 1991 {published data only}
- Lindequist S, Justesen P, Larsen C, Rasmussen F. Diagnostic quality and complications of hysterosalpingography: oil- versus water-soluble contrast media - a randomized prospective study. Radiology 1991;179:69-74. [DOI] [PubMed] [Google Scholar]
- Rasmussen F, Lindequist S, Larsen C, Justesen P. Therapeutic effect of hysterosalpingography: oil- versus water-soluble contrast media - a randomized prospective study. Radiology 1991;179:75-9. [DOI] [PubMed] [Google Scholar]
Spring 2000 {published data only}
- Spring DB, Barkan HE, Pruyn SC. Potential therapeutic effects of contrast materials in hysterosalpingography: a prospective randomized clinical trial. Radiology 2000;214:53-7. [DOI] [PubMed] [Google Scholar]
Steiner 2003 {published data only}
- Steiner AZ, Meyer WR, Clark RL, Hartmann KE. Oil-soluble contrast during hysterosalpingography in women with proven tubal patency. Obstetrics and Gynecology 2003;101:109-13. [DOI] [PubMed] [Google Scholar]
Yang 1989 {published data only}
- Yang KN, Yeh NG, Pan SB. The therapeutic effects of oil-soluble hysterosalpingography contrast medium following water-soluble hysterosalpingography contrast medium. Chinese Medical Journal 1989;44:293-7. [PubMed] [Google Scholar]
References to studies excluded from this review
Acton 1988 {published data only}
- Acton C, Davitt J, Ryan E. Hysterosalpingography in infertility - an experience of 3631 examinations. Australian and New Zealand Journal of Obstetrics and Gynaecology 1988;28:127-33. [DOI] [PubMed] [Google Scholar]
Barwin 1971 {published data only}
- Barwin N. Hysterosalpingography in infertility. Ulster Medical Journal 1971;41:61-5. [PMC free article] [PubMed] [Google Scholar]
Court 2014 {published data only}
- Court KA, Dare AJ, Weston-Webb M, Hadden WE, Sim RG, Johnson NP. Establishment of lipoidal as a fertility treatment - prospective study of the complete innovative treatment data set. Australian and New Zealand Journal of Obstetrics and Gynaecology 2014;54:13-9. [DOI] [PubMed] [Google Scholar]
DeCherney 1980 {published data only}
- DeCherney AH, Kort H, Barney JB, DeVore GR. Increased pregnancy rate with oil-soluble hysterosalpingography dye. Fertility and Sterility 1980;33:407-10. [PubMed] [Google Scholar]
Gillespie 1965 {published data only}
- Gillespie H. The therapeutic aspect of hysterosalpingography. British Journal of Radiology 1965;38:301-2. [DOI] [PubMed] [Google Scholar]
Mackey 1971 {published data only}
- Mackey RA, Glass RH, Olson LE, Vaidya R. Pregnancy following hysterosalpingography with oil and water soluble dye. Fertility and Sterility 1971;22:504-7. [DOI] [PubMed] [Google Scholar]
Perquin 2006 {published data only}
- Perquin DA, Dorr PJ, Craen AJ, Helmerhorst FM. Routine use of hysterosalpingography prior to laparoscopy in the fertility workup: a multicentre randomized controlled trial. Human Reproduction 2006;21(5):1227-31. [DOI] [PubMed] [Google Scholar]
Pham 2017 {published data only}
- Pham C, Rijswijk J, Dreyer K, Verhoeve H, Karnon J, Mol BW. The cost-effectiveness of tubal flushing: a randomized trial of oil versus water. Fertility and Sterility 2017;108(Suppl 1):e16. [Google Scholar]
Reilly 2019 {published data only}
- Reilly SJ, Glanville EJ, Dhorepatil B, Prentice LR, Mol BW, Johnson NP. The IVF-LUBE trial - a randomized trial to assess Lipiodol® uterine bathing effect in women with endometriosis or repeat implantation failure undergoing IVF. Reproductive Biomedicine Online 2019;38(3):380-6. [DOI] [PubMed] [Google Scholar]
Schwabe 1983 {published data only}
- Schwabe MG, Shapiro SS, Haning RV. Hysterosalpingography with oil contrast medium enhances fertility in patients with infertility of unknown etiology. Fertility and Sterility 1983;40:604-6. [DOI] [PubMed] [Google Scholar]
van Rijswijk 2018a {published data only}
- Rijswijk J, Welie N, Dreyer K, Hooff MH, Bruin JP, Verhoeve H, et al. The FOAM study: is Hysterosalpingo foam sonography (HyFoSy) a cost-effective alternative for hysterosalpingography (HSG) in assessing tubal patency in subfertile women? Study protocol for a randomized controlled trial. BMC Women's Health 2018;18(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Rijswijk 2018b {published data only}
- Rijswijk J, Pham CT, Dreyer K, Verhoeve HR, Hoek A, Bruin JP, et al. Oil-based or water-based contrast for hysterosalpingography in infertile women: a cost-effectiveness analysis of a randomized controlled trial. Fertility and Sterility 2018;110(4):754-60. [DOI] [PubMed] [Google Scholar]
Wolf 1989 {published data only}
- Wolf G, Fitzpal P. Hysterosalpingography with a new contrast medium. Fortschritte auf dem Gebiete der Rontgenstrahlem und der Nuklearmedizin - Erganzungsband 1989;128:190-4. [PubMed] [Google Scholar]
Yaegashi 1987 {published data only}
- Yaegashi N, Kuramoto M, Nakayama C, Nakano M. Pregnancy rates after hysterosalpingography - comparing water soluble contrast medium with oily contrast medium. Acta Obstetrica et Gynaecologica Japonica 1987;39:1812-4. [PubMed] [Google Scholar]
References to ongoing studies
Cai 2019 {published data only}
- ChiCTR1900025866. Ongoing study. 1 January 2020. Contact author for more information.
Hassan 2015 {published data only}
- NCT02433418. Ongoing study. May 2015. Contact author for more information.
Legro 2018 {published data only}
- SHOW Trial (NCT03604549). Ongoing study. 11 January 2019. Contact author for more information.
Mijatovic 2018 {published data only}
- H2Oil-timing study (EUCTR2018-004153-24-NL). Ongoing study. 30 July 2019. Contact author for more information.
Rosielle 2019 {published data only}
- H2Olie2 (NL7925). Ongoing study. August, 20, 2019. Contact author for more information.
Zhang 2020 {published data only}
- ChiCTR2000031612. Ongoing study. 5 April 2020. Contact author for more information.
Additional references
ASRM 2015
- Practice Committee of the American Society for Reproductive Medicine. Diagnostic evaluation of the infertile female: a committee opinion. Fertility and Sterility 2015;103(6):e44-50. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bateman 1987
- Bateman BG, Nunley WC, Kitchin JD, Kaizer DL. Utility of the 24-hour delay hysterosalpingogram. Fertility and Sterility 1987;47:613-7. [DOI] [PubMed] [Google Scholar]
Capitanio 1991
- Capitanio GL, Ferraiolo A, Croce S, Gazzo R, Anserini P, De Cecco L. Transcervical selective salpingography: a diagnostic and therapeutic approach to cases of proximal tubal injection failure. Fertility and Sterility 1991;55:1045-50. [DOI] [PubMed] [Google Scholar]
Chalazonitis 2009
- Chalazonitis A, Tzovara I, Laspas F, Porfyridis P, Ptohis N, Tsimitselis G. Hysterosalpingography: technique and applications. Current Problems in Diagnostic Radiology 2009;38(5):199-205. [PMID: ] [DOI] [PubMed] [Google Scholar]
Coenders‐Tros 2016
- Coenders-Tros R, Kessel MA, Vernooij MM, Oosterhuis GJ, Kuchenbecker WK, Mol BW, et al. Performance of outpatient transvaginal hydrolaparoscopy. Human Reproduction (Oxford, England) 2016;31(10):2285-91. [PMID: ] [DOI] [PubMed] [Google Scholar]
Datta 2016
- Datta J, Palmer MJ, Tanton C, Gibson LJ, Jones KG, Macdowall W, et al. Prevalence of infertility and help seeking among 15 000 women and men. Human Reproduction 2016;31(9):2108-18. [DOI] [PMC free article] [PubMed]
Dreyer 2019
- Dreyer K, Eekelen R, Tjon-Kon-Fat RI, Steeg JW, Steures P, Eijkemans M, et al. The therapeutic effect of hysterosalpingography in couples with unexplained subfertility: a post-hoc analysis of a prospective multi-centre cohort study. Reproductive Biomedicine Online 2019;38(2):233-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Dusak 2013
- Dusak A, Soydinc HE, Onder H, Ekinci F, Görük NY, Hamidi C, et al. Venous intravasation as a complication and potential pitfall during hysterosalpingography: re-emerging study with a novel classification. Journal of Clinical Imaging Science 2013;3:67. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Evers 2002
- Evers JL. Female subfertility. Lancet (London, England) 2002;360(9327):151-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Fang 2018
- Fang F, Bai Y, Zhang Y, Faramand A. Oil-based versus water-based contrast for hysterosalpingography in infertile women: a systematic review and meta-analysis of randomized controlled trials. Fertility and Sterility 2018;110(1):153-60.e3. [PMID: ] [DOI] [PubMed] [Google Scholar]
Farquhar 2019
- Farquhar CM, Bhattacharya S, Repping S, Mastenbroek S, Kamath MS, Marjoribanks J, et al. Female subfertility. Nature Reviews. Disease Primers 2019;5(1):7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Fukui 1999
- Fukui A, Fujii S, Yamaguchi E, Kimura H, Sato S, Saito Y. NK cell subpopulations and cytotoxicity for infertile patients undergoing in vitro fertilisation. American Journal of Reproductive Immunology 1999;41:413-22. [DOI] [PubMed] [Google Scholar]
Grant 1971
- Grant A. Infertility surgery of the oviduct. Fertility and Sterility 1971;22:495-503. [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al, Cochrane Bias Methods Group, Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Inhorn 2015
- Inhorn MC, Patrizio P. Infertility around the globe: new thinking on gender, reproductive technologies and global movements in the 21st century. Human Reproduction Update 2015;21(4):411-26. [DOI] [PubMed] [Google Scholar]
Izumi 2017
- Izumi G, Koga K, Takamura M, Bo W, Nagai M, Miyashita M, et al. Oil-Soluble Contrast Medium (OSCM) for hysterosalpingography modulates dendritic cell and regulatory T cell profiles in the peritoneal cavity: a possible mechanism by Which OSCM enhances fertility. Journal of immunology (Baltimore, Md. : 1950) 2017;198(11):4277-84. [PMID: ] [DOI] [PubMed] [Google Scholar]
Johnson 1992
- Johnson J, Montoya I, Olive D. Ethiodol oil contrast medium inhibits macrophage phagocytosis and adherence by altering membrane electronegativity and microviscosity. Fertility and Sterility 1992;58:511-7. [DOI] [PubMed] [Google Scholar]
Kerin 1991
- Kerin J, Surrey E, Williams D, Daykhovsky L, Grundfest W. Falloposcopic observations of endotubal isthmic plugs as a cause of reversible obstruction and their histologic characterisation. Journal of Laparoendoscopic Surgery 1991;1:103-10. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Lim 2011
- Lim CP, Hasafa Z, Bhattacharya S, Maheshwari A. Should a hysterosalpingogram be a first-line investigation to diagnose female tubal subfertility in the modern subfertility workup? Human Reproduction (Oxford, England) 2011;26(5):967-71. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lindequist 1991
- Lindequist S, Justesen P, Larsen C, Rasmussen F. Diagnostic quality and complications of hysterosalpingography: oil- versus water-soluble contrast media - a randomized prospective study. Radiology 1991;179:69-74. [DOI] [PubMed] [Google Scholar]
Lyons 2006
- Lyons RA, Saridogan E, Djahanbakhch O. The reproductive significance of human Fallopian tube cilia. Human Reproduction Update 2006;12(4):363-72. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mikulska 1994
- Mikulska D, Kurzawa R, Rozewicka L. Morphology of in vitro sperm phagocytosis by rat peritoneal macrophages under influence of oily contrast medium (lipiodol). Acta Europaea Fertilitatis 1994;25:203-6. [PubMed] [Google Scholar]
NICE 2013
- National Institute for Health and Care Excellence. Fertility problems: assessment and treatment. NICE guideline (CG156) 2013. [PubMed]
Novy 1988
- Novy MJ, Thurmond AS, Patton P, Uchida BT, Rosch J. Diagnosis of cornual obstruction by transcervical fallopian tube cannulation. Fertility and Sterility 1988;50:434-40. [PubMed] [Google Scholar]
Roest 2020
- Roest I, Welie N, Mijatovic V, Dreyer K, Bongers M, Koks C, et al. Complications after hysterosalpingography with oil- or water-based contrast: results of a nationwide survey. Human Reproduction Open 2020;2020(1):hoz045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Satoh 2015
- Satoh M, Aso K, Katagiri Y. Thyroid dysfunction in neonates born to mothers who have undergone hysterosalpingography involving an oil-soluble iodinated contrast medium. Hormone Research in Paediatrics 2015;84(6):370-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Saunders 2011
- Saunders RD, Shwayder JM, Nakajima ST. Current methods of tubal patency assessment. Fertility and Sterility 2011;95(7):2171-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sawatari 1993
- Sawatari Y, Hori T, Hoshiai H. Oily contrast medium as a therapeutic agent for infertility because of mild endometriosis. Fertility and Sterility 1993;59:907-11. [DOI] [PubMed] [Google Scholar]
So 2017
- So S, Yamaguchi W, Tajima H, Nakayama T, Tamura N, Kanayama N, et al. The effect of oil and water-soluble contrast medium in hysterosalpingography on thyroid function. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology 2017;33(9):682-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Soules 1982
- Soules MR, Spadoni LR. Oil versus aqueous media for hysterosalpingography: a continuing debate based on many opinions and few facts. Fertility and Sterility 1982;28:1-11. [DOI] [PubMed] [Google Scholar]
Sulak 1987
- Sulak PJ, Letterie GS, Coddington CC, Hayslip CC, Woodward JE, Klein TA. Histology of proximal tubal occlusion. Fertility and Sterility 1987;48:437-40. [PubMed] [Google Scholar]
Thurmond 1990
- Thurmond A, Rosch J. Nonsurgical fallopian tube recanalisation for treatment of infertility. Radiology 1990;174:371-4. [DOI] [PubMed] [Google Scholar]
van Welie 2019
- Welie N, Dreyer K, Rijswijk J, Verhoeve HR, Goddijn M, Nap AW, et al. Treatment effect of oil-based contrast is related to experienced pain at HSG: a post-hoc analysis of the randomised H2Oil study. Human Reproduction (Oxford, England) 2019;34(12):2391-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2019
- Wang R, Welie N, Rijswijk J, Johnson NP, Norman RJ, Dreyer K, et al. Effectiveness on fertility outcome of tubal flushing with different contrast media: systematic review and network meta‐analysis. Ultrasound in Obstetrics & Gynecology 2019;54(2):172-81. [DOI] [PubMed] [Google Scholar]
Watson 1994
- Watson A, Vandekerckhove P, Lilford R, Vail A, Brosens I, Hughes E. A meta-analysis of the therapeutic role of oil soluble contrast media at hysterosalpingography: a surprising result? Fertility and Sterility 1994;61:470-7. [DOI] [PubMed] [Google Scholar]
Yun 2004
- Yun AJ, Lee PY. Enhanced fertility after diagnostic hysterosalpingography using oil-based contrast agents may be attributable to immunomodulation. AJR. American Journal of Roentgenology Diagnostic Imaging and Related Sciences 2004;183(6):1725-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Zhou 2018
- Zhou Z, Zheng D, Wu H, Li R, Xu S, Kang Y, et al. Epidemiology of infertility in China: a population-based study. BJOG: an International Journal of Obstetrics & Gynaecology 2018;125(4):432-41. [DOI: 10.1111/1471-0528.14966] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Johnson 2002
- Johnson N, Venderkerckhove P, Watson A, Lilford R, Harada T, Hughes E. Tubal flushing for subfertility. Cochrane Database of Systematic Reviews 2002, Issue 3. Art. No: CD003718. [CENTRAL: CD003718] [DOI: 10.1002/14651858.CD003718] [DOI] [PubMed] [Google Scholar]
Johnson 2005a
- Johnson N, Vanderkerckhove P, Watson A, Lilford R, Harada T, Hughes E. Tubal flushing for subfertility. Cochrane Database of Systematic Reviews 2005, Issue 2. Art. No: CD003718. [CENTRAL: CD003718] [DOI: 10.1002/14651858.CD003718.pub2] [DOI] [PubMed] [Google Scholar]
Johnson 2007
- Johnson N, Vanderkerchove P, Lilford R, Harada T, Hughes E, Luttjeboer F, etal. Tubal flushing for subfertility. Cochrane Database of Systematic Reviews 2007, Issue 3. Art. No: CD003718. [DOI: 10.1002/14651858.CD003718.pub3] [DOI] [PubMed] [Google Scholar]
Mohiyiddeen 2015
- Mohiyiddeen L, Hardiman A, Fitzgerald C, Hughes E, Mol BW, Johnson N, et al. Tubal flushing for subfertility. Cochrane Database of Systematic Reviews 2015, Issue 5. Art. No: CD003718. [DOI: 10.1002/14651858.CD003718.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vandekerckhove 1996
- Vandekerckhove P, Lilford R, Harada T, Hughes E, Watson A. Oil-soluble versus water-soluble media for assessing tubal patency with hysterosalpingography or laparoscopy in subfertile women. Cochrane Database of Systematic Reviews 1996, Issue 4. Art. No: CD000092. [DOI: 10.1002/14651858.CD000092] [DOI] [PubMed] [Google Scholar]


