Abstract
The ResD-ResE signal transduction system is required for aerobic and anaerobic respiration in Bacillus subtilis. The histidine sensor kinase ResE, by functioning as a kinase and a phosphatase for the cognate response regulator ResD, controls the level of phosphorylated ResD. A high level of phosphorylated ResD is postulated to cause a dramatic increase in transcription of ResDE-controlled genes under anaerobic conditions. A mutant ResE, which retains autophosphorylation and ResD phosphorylation activities but is defective in ResD dephosphorylation, allowed partially derepressed aerobic expression of the ResDE-controlled genes. The result indicates that phosphatase activity of ResE is regulated by oxygen availability and anaerobic induction of the ResDE regulon is partly due to a reduction of the ResE phosphatase activity during anaerobiosis. That elimination of phosphatase activity does not result in complete aerobic derepression suggests that the ResE kinase activity is also subject to control in response to oxygen limitation.
In two-component signal transduction systems (for reviews, see references 9 and 29) histidine kinases modulate the activity of response regulators via phosphorylation. Response regulators have autophosphatase activity, and half-lives for various phosphorylated response regulators range from seconds to hours (36). The decay of phosphorylated response regulators is often stimulated by the cognate sensor kinases that possess phosphatase activity (for a review, see reference 30). Since the level of phosphorylation of response regulators is determined by the sum of kinase, phosphotransferase, and phosphatase activities, how each of these activities is regulated is a key issue for understanding the mechanism of signal transduction.
Bacillus subtilis, a gram-positive soil bacterium, can alternate its respiratory systems depending on the growth conditions. When nitrate is present in the absence of oxygen, cells undergo nitrate respiration using nitrate reductase as terminal oxidase (for a review, see references 18 and 23). Anaerobic nitrate respiration, as well as aerobic respiration using cytochrome oxidases, is dependent on the ResDE signal transduction system (24, 31). The sensor kinase ResE and the response regulator ResD are required for transcription of resABCDE (resABC encodes proteins similar to those involved in cytochrome c biogenesis) (31), ctaA (required for cytochrome caa3 oxidase biosynthesis) (31), ctaB (cytochrome caa3 oxidase assembly factor) (13), qcrABC (encoding subunits of menaquinol:cytochrome c oxidoreductase) (31), fnr (encoding anaerobic transcriptional regulator) (24), nasDEF (nitrite reductase) (17), hmp (flavohemoglobin) (12), lctE (lactate dehydrogenase) (4), and sbo-alb (subtilosin biosynthesis) (20). All ResDE-controlled genes so far tested are highly induced by oxygen limitation (21). Recent studies showed that purified ResD directly interacts with promoter regions of some of these genes (22, 37).
We have previously shown that pgk-1, a mutation in pgk (phosphoglycerate kinase gene), suppresses resE but not resD mutations with respect to anaerobic growth in the presence of nitrate and to ResDE-dependent gene expression (21). The pgk-1 mutant displays very low but measurable phosphoglycerate kinase activity compared to the wild-type strain. Accumulation of a glycolytic intermediate, probably 1,3-diphosphoglycerate, was suggested to be responsible for the observed suppressor effect of pgk-1. However, it remains to be examined whether 1,3-diphosphoglycerate can donate phosphate directly to ResD or if a non-cognate kinase is involved in the ResE-independent ResD phosphorylation. During the study we found that aerobic expression of the ResDE-controlled genes was dramatically derepressed in the resE pgk-1 double mutant; however, the expression in the resE+ pgk-1 strain was similar to that of the wild type, showing much lower expression under aerobic than anaerobic conditions. One possible explanation for these results is that ResE has both kinase and phosphatase activities under aerobic conditions but lacks phosphatase activity under anaerobic conditions. In this view, when ResD is phosphorylated by a pathway independent of ResE, and ResD phosphate (ResD∼P) is not dephosphorylated by ResE phosphatase, as in case of the resE pgk-1 mutant, a higher level of ResD∼P would be attained, leading to robust activation of ResD-controlled genes. We also proposed that higher expression of ResDE-controlled genes in the wild-type strain under anaerobic conditions is likely the result of higher ResD∼P due to reduced phosphatase activity of ResE (21). This hypothesis was tested in this study by creating a mutant ResE that possesses kinase activity but lacks phosphatase activity.
MATERIALS AND METHODS
B. subtilis strains and plasmids.
B. subtilis strains and plasmids used in this study are listed in Table 1.
TABLE 1.
B. subtilis strains and plasmids
| Strain or plasmid | Relevant feature(s) | Source or reference |
|---|---|---|
| Strains | ||
| JH642 | trpC2 pheA1 | J. A. Hoch |
| ZB307A | SPβc2Δ2::Tn917::pSK1 | 39 |
| LAB2000 | trpC2 pheA1 SPβc2Δ2::Tn917::pML26 | 12 |
| LAB2234 | trpC2 pheA1 ΔresE::spc | 16 |
| LAB2252 | trpC2 pheA1 SPβc2Δ2::Tn917::pMMN288 | 24 |
| LAB2537 | trpC2 pheA1 amyE::pES17 (resA-lacZ) | 21 |
| LAB2854 | trpC2 pheA1 SPβc2Δ2::Tn917::pMMN392 | 17 |
| LAB3162 | trpC2 pheA1 SPβc2Δ2::Tn917::sbo-lacZ | 38 |
| ORB3303 | resE::neo | This study |
| ORB3304 | trpC2 pheA1 resE::neo | This study |
| ORB3331 | trpC2 pheA1 amyE::pES17 resE::neo | This study |
| ORB3343 | trpC2 pheA1 resE378 amyE::pES17 | This study |
| ORB3362 | trpC2 resE378 | This study |
| ORB3370 | trpC2 resE378 SPβc2Δ2::Tn917::pML26 | This study |
| ORB3371 | trpC2 resE378 SPβc2Δ2::Tn917::pMMN392 | This study |
| ORB3372 | trpC2 resE378 SPβc2Δ2::Tn917::pMMN288 | This study |
| ORB3373 | trpC2 resE378 SPβc2Δ2::Tn917::sbo-lacZ | This study |
| Plasmids | ||
| pPROEX-1 | ColE1 origin with His6, Ampr | GIBCO-BRL |
| pTYB4 | ColE1 origin with intein tag, Ampr | New England Biolabs |
| pUC18 | ColE1 origin, Ampr | 35 |
| pUC19 | ColE1 origin, Ampr | 35 |
| pMMN424 | pTYB4 with resE, Ampr | 22 |
| pMMN425 | pTYB4 with resE378, Ampr | This study |
| pMMN444 | pUC19 with resE, Ampr | This study |
| pMMN446 | pPROEX-1 with resE, Ampr | This study |
| pYZ16 | pUC18 with resE, Ampr | This study |
| pYZ24 | pYZ16 with neo insertion in resE, Ampr Neor | This study |
Construction of plasmids carrying truncated wild-type resE or resE378 gene.
For production of wild-type and mutant ResE proteins, the IMPACT system (New England BioLabs) was used which utilizes the inducible self-cleaving intein tag (3). Plasmid pMMN424, which carries a truncated resE gene in pTYB4, was constructed previously (22). This plasmid was used to produce and purify a soluble form of the wild-type ResE protein lacking the N-terminal 195 amino acids (total amino acids are 589) including two transmembrane regions and the periplasmic region. The truncated protein was shown to have active kinase activity (22, 37). The last amino acid, Arg, is also replaced by Gly and Pro in wild-type and mutant ResE constructs.
Plasmid pMMN425, which was used to purify a truncated form of the mutant ResE (T378R) protein, was constructed as follows. The upstream fragment of resE was amplified by PCR using JH642 chromosomal DNA and two oligonucleotides, oMN98-47 (5′-CATTCTTTTTATCAACCATGGTCACGTACCCT-3′) and oMN98-49 (5′GTCATGAGCTGAGACGACCGATCTCCAT-3′). The downstream fragment of resE was amplified using two oligonucleotides, oMN98-48 (5′-CAGACTCGATTTTACCCGGGTTTTGTCGGAATAT-3′) and oMN98-50 (5′-ATGGAGATCGGTCGTCTCAGCTCATGAC-3′). oMN98-49 and oMN98-50 are complementary and designed to generate the resE378 mutation. The PCR products were denatured at 94°C for 1 min and were successively incubated at 65°C for 2 min and 37°C for 1 min. The annealed mixture was treated with T4 DNA polymerase in the presence of four deoxynucleoside triphosphates at 37°C for 30 min. The aliquot of the reaction mixture was used as a template for PCR using oMN98-47 and oMN98-48 to generate the truncated resE378 gene. The PCR product was digested with NcoI and SmaI and cloned into pTYB4, which was cleaved with the same enzymes to generate pMMN425. The inserted DNA was sequenced to verify the desired mutation, as well as the absence of any extra mutation.
The truncated form of wild-type ResE was also produced as a protein fused to six-histidine residues (His6). This His6-ResE protein was used to purify ResD∼P from His6-ResE by affinity chromatography. Plasmid pMMN424 was digested with SmaI and NcoI (blunt ended with T4 DNA polymerase), and the released resE fragment was cloned into pUC19 digested with SmaI. The resultant plasmid pMMN444 was digested with BamHI and KpnI to release resE, which was subcloned into pPROEX-1 (GIBCO-BRL) that had been digested with the same enzymes to generate pMMN446.
Purification of ResD and ResE proteins.
ResD, wild-type, and mutant ResE proteins were overproduced in Escherichia coli ER2566 (New England Biolabs) as described previously (22, 37). His6-ResE was overproduced in E. coli BL21 carrying pMMN446 and purified using Ni-nitrilotriacetic acid (NTA) resin column chromatography as recommended by the manufacturer. The His6-ResE protein has 44 extra amino acid residues including 6 histidines at the N terminus and 18 extra amino acids at the C terminus of ResE.
Autophosphorylation of ResE.
The truncated wild-type and mutant ResE proteins (60 pmoles) were incubated in 60 μl of TEDG buffer (50 mM Tris-HCl, pH 8.0; 0.5 mM EDTA; 2 mM dithiothreitol [DTT]; 10% glycerol) containing 50 mM KCl, 5 mM MgCl2, and 10 μM [γ-32P]ATP (1 Ci/mmol). After incubation for the indicated periods at room temperature, 10 μl of the reaction mixture was removed and added to 3 μl of a sodium dodecyl sulfate (SDS) sample buffer (250 mM Tris-HCl, pH 6.5; 8% SDS; 8% 2-mercaptoethanol; 40% glycerol; 0.05% bromophenol blue). The proteins were separated by SDS–12% polyacrylamide gel electrophoresis and analyzed using a PhosphorImager (Molecular Dynamics).
Phosphorylation of ResD by ResE.
The wild-type and mutant ResE proteins (960 pmol) were autophosphorylated with [γ-32P]ATP for 30 min at room temperature as described above. The reaction mixture was applied to a Sephadex G-75 column equilibrated with the same buffer. The fractions containing the radioactive ResE, which were free of ATP, were collected. An aliquot of the fractions was incubated with ResD (300 pmol) in 92 μl of TEDG phosphorylation buffer, and ATP was added to 200 μM after 5 min.
Dephosphorylation of ResD∼P.
The His6-ResE protein (0.8 to 1.5 nmol) was autophosphorylated with [γ-32P]ATP as described above, except that DTT in the buffer was replaced by 5 mM 2-mercaptoethanol because DTT reduces the Ni ions of the Ni-NTA resin used for immobilization of His6-ResE. After 30 min at room temperature, Ni-NTA agarose was mixed into the reaction mixture and incubated by gently shaking for 15 min. The Ni-NTA resin was collected by centrifugation and washed with the same buffer to remove unbound His6-ResE and unincorporated ATP until the radioactive signal in the wash buffer became constant. An equal amount of ResD was added to the resin and the mixture was incubated for 10 min at room temperature. The reaction mixture was centrifuged, and the supernatant containing ResD∼P was collected, which was then applied to a Sephadex G-25 column. For the examination of autophosphatase activity, ResD∼P was incubated in the buffer with or without ATP (500 μM) at room temperature. The purified ResD∼P was also incubated with wild-type and mutant ResE (300 to 500 pmol) in the presence or absence of 500 μM ATP or ADP for 10 min.
Construction of B. subtilis strains carrying the resE378 mutation.
The mutant resE allele was introduced into B. subtilis as follows. Two fragments carrying the 5′-part and 3′-part of resE were amplified by using JH642 chromosomal DNA and oligonucleotides oMN98-47 and oMN99-57 (5′-CTGAAGCATGGGGATCCGTGTTCTCAG-3′), as well as oMN98-48 and oMN99-56 (5′-CTGAGAACACGGATCCCCATGCTTCAG-3′). Two complementary oligonucleotides, oMN99-56 and oMN99-57, were designed to create a BamHI site in the resE gene. The PCR products, after annealing and being treated with T4 DNA polymerase as described above, were used as template for the second PCR reaction using oMN98-47 and oMN98-48. The PCR product digested with SmaI and NcoI (the end was filled in) was cloned into pUC18 digested with SmaI and HincII to generate pYZ16. A neomycin-resistant (Neor) cassette isolated from pDZ792 (8) digested with BamHI and BglII was inserted into the BamHI site of pYZ16 to generate pYZ24. B. subtilis strains JH642 (trpC2 pheA1) and ZB307A (trpC2+ pheA1+) were transformed with pYZ24 that was linearized by ScaI cleavage, and Neor transformants were selected as ORB3304 and ORB3303, respectively. The transformants were generated by a double-crossover recombination, as was confirmed by PCR analysis. LAB2537 carrying resA-lacZ was transformed with ORB3304 chromosomal DNA and a chloramphenicol-resistant (Cmr) Neor transformant was chosen as ORB3331. ORB3304 was transformed with ORB3331 chromosomal DNA and pMMN425 with selection for Cmr. A Neos Cmr transformant was chosen as ORB3343. Because ORB3331, like ORB3304, has the Neor cassette in resE, the neomycin sensitivity of ORB3343 is indicative of the replacement of resE::neo by the mutant allele of resE in pMMN425. This was further confirmed by sequencing the PCR product obtained by using ORB3343 chromosomal DNA as a template and oMN98-47 and oMN98-48 as primers. The mutant resE strain without the lacZ fusion was constructed by transforming ORB3343 with ORB3303 chromosomal DNA. After selection with trp+, a Cms Neos transformant was chosen as ORB3362. ORB3362 was used for transduction with phage lysates carrying hmp-lacZ (12), nasD-lacZ (17), fnr-lacZ (24), and sbo-lacZ (38) to construct strains ORB3370, ORB3371, ORB3372, and ORB3373, respectively.
Measurement of β-galactosidase activity.
B. subtilis cells were grown in liquid 2xYT medium (19) with 1% glucose and 0.2% KNO3 or in DS medium (19) with 1% glucose and 0.2% KNO3 (starting optical density at 600 nm was 0.02). Cells were cultured aerobically or anaerobically as previously described (24), and samples were taken every 1 h to measure β-galactosidase activity as described earlier (15). The maximal activity, which was attained at late exponential growth, was listed in Table 2.
TABLE 2.
Aerobic and anaerobic expression of ResDE-controlled genes
| Fusion | β-Galactosidase activity (Miller unit)a in:
|
|||
|---|---|---|---|---|
| DSM
|
2xYT
|
|||
| Aerobic | Anaerobic | Aerobic | Anaerobic | |
| hmp-lacZ | ||||
| Wild type | 1.7 | 6,480 | 3.5 | 6,550 |
| Mutant | 18 | 8,170 | 47 | 5,390 |
| nasD-lacZ | ||||
| Wild type | 4.9 | 540 | 2.5 | 546 |
| Mutant | 45 | 539 | 16 | 491 |
| fnr-lacZ | ||||
| Wild type | 5.5 | 78 | 6.6 | 199 |
| Mutant | 47 | 133 | 42 | 224 |
| resA-lacZ | ||||
| Wild type | 22 | 377 | 16 | 430 |
| Mutant | 227 | 593 | 199 | 605 |
| sbo-lacZ | ||||
| Wild type | 19 | 2,720 | 3.8 | 2,450 |
| Mutant | 440 | 5,370 | 66 | 3,140 |
Maximal activities during growth are shown. Standard deviations are less than 20% for each value.
Western blot analysis.
Cells were grown as above until late exponential growth for anaerobic cultures or T2 (2 h after the onset of the stationary phase) in the case of aerobic cultures. After disruption by French press, cell debris was removed by centrifugation (17,000 × g) for 15 min. The protein concentration in each sample was determined by using the Bio-Rad assay kit. A total of 20 μg of each protein sample was loaded onto SDS–12% polyacrylamide gels. The proteins were detected by Western blot using a chromogenic alkaline phosphatase substrate, anti-ResE antibody (raised against purified ResE by Josman, LLC, Napa, Calif.), and secondary goat anti-rabbit alkaline phosphatase conjugate.
RESULTS AND DISCUSSION
Construction of a mutant ResE (T378R).
The question of how the ResDE signal transduction pathway specifically activates either aerobic or anaerobic respiration was addressed in this study. Because ResD and ResE are needed both for aerobic and anaerobic respiration and yet these genes are highly induced under anaerobic conditions in a ResDE-dependent manner, several possibilities could be envisioned. One possibility is the presence of an unknown regulator that would only be active under anaerobic conditions. This putative regulator may be controlled positively by the ResDE system or it may be a coactivator of ResD when oxygen is limited. Recent studies indicated that ResD binds to the promoter regions of ctaA, resA, hmp, nasD, and fnr, suggesting that ResD activates transcription of these genes by interacting with their regulatory regions (22, 37), arguing against the existence of a coactivator or a ResD-controlled transcription activator. These results, together with studies on a resE suppressor mutant as described above (21), support the conclusion that the level of phosphorylation of ResD is the major factor for determining the induction of these genes.
One possibility for why ResD is phosphorylated to higher levels during anaerobic growth than during aerobic growth is reduced phosphatase activity of ResE under anaerobic conditions. This hypothesis was tested in this study by constructing kinase+ phosphatase− ResE and examining the expression of ResDE-controlled genes in the strain producing the mutant ResE. If the hypothesis is correct, one could expect derepressed aerobic ResDE-dependent gene expression in the strain carrying such a mutant ResE. ResE belongs to the EnvZ subfamily of sensor kinases (6), and kinase+ phosphatase− EnvZ mutants have been isolated (listed in reference 10). One such mutant (envZ11) has an amino acid change (Thr247 to Arg) in the vicinity of the conserved His243 residue (the site for autophosphorylation) (2). Interestingly, an E. coli sensor kinase CpxA with a change of the equivalent residue Thr to Pro displays gain-of-function phenotype. The CpxA101 mutant also lacks phosphatase activity for CpxR∼P (25). Because the corresponding residue (Thr378) is conserved in ResE, we changed the Thr residue to Arg by site-directed mutagenesis using PCR as described in Materials and Methods. To investigate the biochemical activities of wild-type and mutant ResE, the soluble truncated ResE proteins were purified.
Autophosphorylation activities of wild-type and mutant ResE.
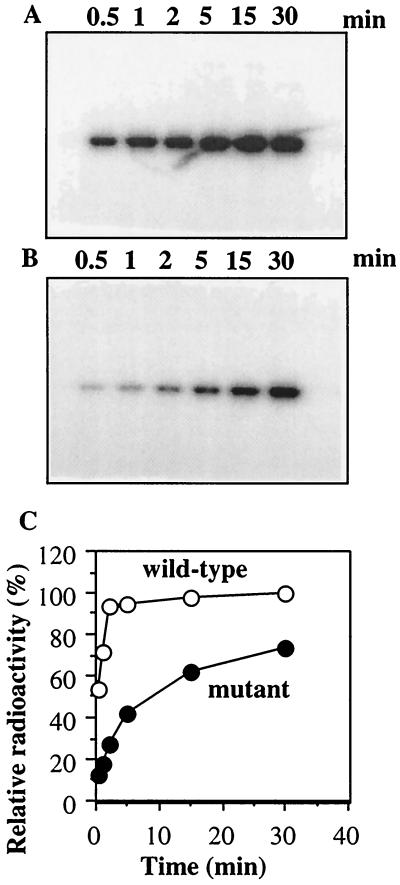
ResE, as with other sensor kinases, can undergo autophosphorylation in the presence of ATP (22, 37). Purified wild-type and mutant (T378R) ResE proteins were examined for autophosphorylation activity. The time course of incorporation of the phosphoryl group from [γ-32P]ATP into ResE is shown in Fig. 1. Both proteins have autophosphorylation activity, although the wild-type ResE was phosphorylated at a higher rate than the mutant. EnvZ11 (2) and CpxA101 (25), carrying the corresponding mutation, exhibited increased or diminished autophosphorylation activity, respectively. This result indicates that the T378R mutation moderately affects the autophosphorylation activity of ResE.
FIG. 1.
Time course of autophosphorylation of ResE. Wild-type (A) and mutant (B) ResE proteins (60 pmol) were incubated at room temperature in 60 μl of TEDG buffer (50 mM Tris-HCl, pH 8.0; 0.5 mM EDTA; 2 mM DTT; 10% glycerol) containing 50 mM KCl, 5 mM MgCl2 and 10 μM [γ-32P]ATP (1 Ci/mmol). At the indicated times, 10-μl samples were taken and analyzed by SDS–12% polyacrylamide gel electrophoresis and autoradiography. (C) Densitometry scanning of the gels.
Phosphorylation and dephosphorylation of ResD by ResE proteins.
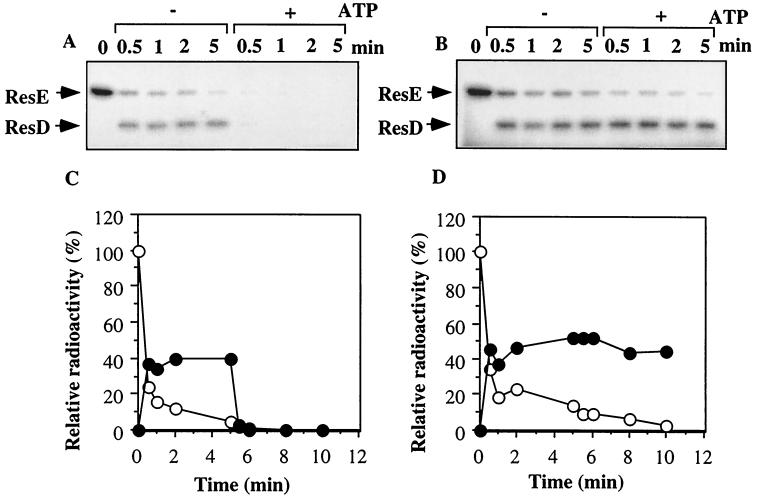
To examine transphosphorylation that is independent of the autophosphorylation reaction, wild-type and mutant ResE∼P proteins free of ATP were purified by gel filtration as described in Materials and Methods. The incubation of ResE∼P with ResD resulted in phosphorylation of ResD (Fig. 2). Phosphorylation of ResD either by wild-type or mutant ResE occurred quickly and reached a maximum level within 0.5 min. EnvZ catalyzes the dephosphorylation of OmpR∼P in the presence of ATP, ADP, or nonhydrolyzable analogs of ATP (1, 11). The addition of ATP to the reaction mixture also stimulated the dephosphorylation of ResD∼P in the reaction containing wild-type ResE, as shown by the absence of radiolabeled ResD and ResE after incubation for 5 min at room temperature (Fig. 2A and C). In contrast, the mutant ResE did not stimulate ResD∼P dephosphorylation by the addition of ATP (Fig. 2B and D). This result indicates that ResE functions as a phosphatase for ResD∼P, which is defective in the case of the T378R mutant.
FIG. 2.
Autophosphorylation and dephosphorylation of ResD. 32P-phosphorylated ResE (A) and ResE (T378R) (B) (960 pmol) were purified and then incubated with purified ResD (300 pmol) in 92 μl of TEDG phosphorylation buffer. After incubation for 0.5, 1, 2, and 5 min at room temperature, 10 μl of the reaction was transferred to the SDS buffer. At 5 min, ATP was added to 200 μM, and the reaction was continued for 0.5, 1, 2, and 5 min. (C and D) Densitometry scanning of the gels in panels A and B, respectively. Symbols: ○, ResE∼P; ●, ResD∼P.
It has been shown that some two-component regulatory proteins dephosphorylate response regulators in two different ways: through the phosphatase activity of sensor kinases, which releases Pi; and through reverse transphosphorylation, which involves transfer of the phosphoryl group from response regulators to sensor kinases. Reverse transphosphorylation has been reported in several two-component regulatory systems, including NRII-NRI (33), CheA-CheY (28), a kinase− phosphatase+ EnvZ mutant (5), ArcB-ArcA (7), and PhoR-PhoP in B. subtilis (27). In an attempt to determine by which process ResD is dephosphorylated, ResD∼P was purified from ResE∼P by using the His6-ResE construct and Ni-chelate chromatography (Materials and Methods).
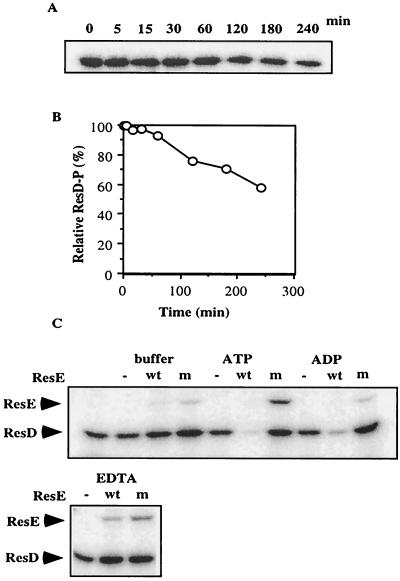
Response regulators have autophosphatase activities, and a key residue in determining the magnitude of the activity is amino acid position 56 (in Spo0F), which is adjacent to the site of phosphorylation of Asp54 (36). Response regulators containing an amino acid residue with a long side chain at the position equivalent to 56 in Spo0F displayed a low autodephosphorylation rate, and those carrying a residue with carboxyamide or carboxylate side chain at that position had high dephosphorylation rates (36). The corresponding amino acid in ResD is Met, the same residue present in PhoB, OmpR, and VanR, which are known to exhibit inefficient autophosphatase activity (36). Consistent with this observation, our result showed that autophosphatase activity of ResD is relatively weak, and the half-life of ResD∼P was calculated to be ca. 4 h (Fig. 3A and B). Addition of ATP did not show any significant effect on autophosphatase activity (data not shown). This long half-life of ResD∼P may explain why the phosphatase activity of ResE is regulated by oxygen tension. When oxygen concentration is increased, the cells need to rapidly decrease the level of ResD∼P by activating ResE phosphatase. A similar possibility was suggested in the case of the FixLJ system, where FixJ∼P has a relatively long half-life (4 h) (14).
FIG. 3.
(A and B) Time course of ResD∼P dephosphorylation. (A) Purified ResD∼P was incubated in TEDG buffer containing 50 mM KCl and 5 mM MgCl2. Samples were transferred at the indicated times to the SDS buffer and subjected to electrophoresis followed by autoradiography. Panel B shows densitometry scanning results of the gel shown in panel A. (C) Dephosphorylation of ResD∼P by ResE. Purified ResD∼P was incubated at room temperature for 10 min in the same buffer in the absence (−) or in the presence of ResE (wt) or ResE (T378R) (m) proteins. ATP and ADP were added to 500 μM, and EDTA was added to 10 mM when indicated.
In the presence of ATP or ADP, wild-type ResE stimulates the dephosphorylation of ResD (Fig. 3C). In contrast, in the presence of the mutant ResE and ATP, reverse transphosphorylation was observed. Reverse transphosphorylation was also detected in wild-type ResE if EDTA was present. This is in sharp contrast to the phosphatase activity of the sensor kinases, which requires Mg2+ and was inhibited by the presence of EDTA. The reverse transphosphorylation from PhoP∼P to PhoR does not require Mg2+ (27) as in the case of the reaction from ResD∼P to ResE.
Effect of the resE378 mutation on transcription of ResDE-controlled genes.
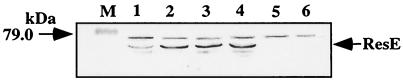
The results using purified ResE proteins indicate that, unlike the wild-type ResE, the mutant ResE lacks phosphatase activity, which could result in higher levels of ResD∼P. Therefore, we examined whether the resE378 mutation affects ResDE-controlled gene expression in vivo. The resE gene was replaced by the mutant allele as described in Materials and Methods. The concentration of wild-type and mutant ResE proteins in aerobic and anaerobic cultures was examined by Western analysis using anti-ResE antibody (Fig. 4). Higher levels of ResE proteins were detected in the wild-type ResE strain grown in 2xYT medium under anaerobic conditions (360%) than under aerobic conditions (100%). In contrast, the level of mutant ResE was similar both in aerobic (450%) and anaerobic cultures (450%) and was as high as the level of the wild-type ResE protein under anaerobic conditions. The levels of ResE protein in the cells grown in DS medium were as follows: aerobic wild-type cultures, 100%; anaerobic wild-type cultures, 410%; aerobic mutant cultures, 200%; and anaerobic mutant cultures, 260% (data not shown). This indicates that the mutant ResE is indeed produced in vivo. The higher mutant ResE concentration compared to that of the wild type during aerobic growth probably reflects the autoregulation of the resE gene because it is transcribed primarily from the resA operon promoter which is dependent on ResDE (31).
FIG. 4.
Western analysis of ResE. B. subtilis cells were grown aerobically or anaerobically in 2xYT with 1% glucose and 0.2% KNO3. A total of 20 μg of each protein sample was separated by SDS–12% polyacrylamide gel electrophoresis. After electrophoresis, the proteins were electrotransferred to a nitrocellulose filter and probed with anti-ResE antibody. Lanes: M, marker (79.0 kDa); 1, JH642 (wild type) grown aerobically; 2, JH642 grown anaerobically; 3, ORB3362 (resE378) grown aerobically; 4, ORB3362 grown anaerobically; 5, LAB2234 (ΔresE) grown aerobically; 6, LAB2234 grown anaerobically. A cross-reacting band is detected both in resE+ and resE strains.
Various lacZ fusions of ResDE-controlled promoters were introduced in the wild-type and mutant strains. Expression of the fusions in both the strains grown aerobically and anaerobically in 2xYT medium or in DS medium was examined (Table 2). Aerobic expression of all genes was partly derepressed in the mutant cells grown in 2xYT or DS medium. Aerobic nasD or fnr expression was six- to ninefold higher in the mutant than in the wild-type ResE strain. In the case of hmp and resA expression, aerobic expression was derepressed by 10- to 13-fold in the mutant strain. The mutation has a more drastic effect on aerobic sbo expression, which resulted in a 17- to 23-fold increase. In contrast, the anaerobic expression of these genes was either not affected at all or only slightly increased (up to twofold) by the mutation.
This result indicates that the resE mutant defective in phosphatase activity leads to the partial derepression of the ResDE-controlled genes under aerobic growth conditions. However, aerobic expression in the mutant strain is still lower than anaerobic expression, unlike the situation in the resE pgk-1 mutant, which showed complete derepression (21). One possible explanation of the difference is that ResD is phosphorylated independently of ResE kinase in the resE pgk-1 mutant, while ResE phosphatase activity is absent, resulting in higher ResD∼P levels than those in the resE378 mutant strain, which has reduced autokinase activity as shown in Fig. 1. An alternative, but not exclusive possibility, is that the kinase activity of ResE, like the phosphatase activity, is also regulated by oxygen limitation. In aerobic cultures of the resE378 strain which lacks phosphatase activity, the level of ResD∼P is high enough to support a 6- to 20-fold induction compared to cultures of wild-type cells; however, the phosphorylation level could still be lower compared to anaerobic cultures, the cells of which not only lack the phosphatase activity but might also have elevated kinase activity. Autophosphorylation activity of the sensor kinase FixL of Rhizobium meliloti is stimulated by low oxygen tension, and the phosphatase activity of FixL∼P (but not that of FixL) is depressed under anaerobic conditions (14). The ResDE regulon could be reciprocally regulated via kinase and phosphatase activity of ResE according to changes in the oxygen level, such as the expression of nitrogen fixation genes in R. meliloti.
ResE is a membrane-associated protein with a type P linker region (periplasmic signal transducing), which is defined by the presence of two amphipathic α-helices (AS1 and AS2) (34). The mechanism of signal transduction in this class of sensors was proposed to involve a conformational change of the periplasmic region brought about by binding to a signal ligand (34). The change in conformation is relayed through the cytoplasmic membrane and causes realignment of the two helices within the linker region, which, in turn, alters the function of the C-terminal cytoplasmic domain. It remains to be determined if the periplasmic region of ResE functions as the signal-sensing domain and what the signal for ResE is that affects kinase and/or phosphatase activity. Interestingly, a PAS domain, which is known to be an important signaling module for sensing changes in light, redox potential, and oxygen (32), was identified in a region adjacent to AS2 (SMART:http://smart.embl-heidelberg.de/ [26]). The involvement of the PAS domain in the redox sensing of ResE remains to be examined. Future studies are also needed to determine whether the kinase and the phosphatase activities are affected by the same signal or whether each activity is modulated by distinct signals.
ACKNOWLEDGMENTS
We are grateful to Peter Zuber for valuable discussions and critical reading of the manuscript. We also thank F. Marion Hulett and Linda Kenney for helpful advice.
This work was supported by NSF grant MCB9996014.
REFERENCES
- 1.Aiba H, Mizuno T, Mizushima S. Transfer of phosphoryl group between two regulatory proteins involved in osmoregulatory expression of the ompF and ompC genes in Escherichia coli. J Biol Chem. 1989;264:8563–8567. [PubMed] [Google Scholar]
- 2.Aiba H, Nakasai F, Mizushima S, Mizuno T. Evidence for the physiological importance of the phosphotransfer between the two regulatory components, EnvZ and OmpR, in osmoregulation in Escherichia coli. J Biol Chem. 1989;264:14090–14094. [PubMed] [Google Scholar]
- 3.Chong S, Mersha F B, Comb D G, Scott M E, Landry D, Vence L M, Perler F B, Benner J, Kucera R B, Hirvonen C A, Pelletier J J, Paulus H, Xu M Q. Single-column purification of free recombinant proteins using a self-cleavable affinity tag derived from a protein splicing element. Gene. 1997;192:271–281. doi: 10.1016/s0378-1119(97)00105-4. [DOI] [PubMed] [Google Scholar]
- 4.Cruz Ramos H, Hoffmann T, Marino M, Nedjari H, Presecan-Siedel E, Dressen O, Glaser P, Jahn D. Fermentative metabolism of Bacillus subtilis: physiology and regulation of gene expression. J Bacteriol. 2000;182:3072–3080. doi: 10.1128/jb.182.11.3072-3080.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dutta R, Inouye M. Reverse phosphotransfer from OmpR to EnvZ in a kinase−/phosphatase+ mutant of EnvZ(EnvZ.N347D), a bifunctional signal transducer of Escherichia coli. J Biol Chem. 1996;271:1424–1429. doi: 10.1074/jbc.271.3.1424. [DOI] [PubMed] [Google Scholar]
- 6.Fabret C, Feher V A, Hoch J A. Two-component signal transduction in Bacillus subtilis: how one organism sees its world. J Bacteriol. 1999;181:1975–1983. doi: 10.1128/jb.181.7.1975-1983.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Georgellis D, Kwon O, De Wulf P, Lin E C C. Signal decay through a reverse phosphorelay in the Arc two-component signal transduction system. J Biol Chem. 1998;273:32864–32869. doi: 10.1074/jbc.273.49.32864. [DOI] [PubMed] [Google Scholar]
- 8.Guérout-Fleury A, Shazand K, Frandsen N, Stragier P. Antibiotic-resistance cassettes for Bacillus subtilis. Gene. 1995;167:335–336. doi: 10.1016/0378-1119(95)00652-4. [DOI] [PubMed] [Google Scholar]
- 9.Hoch J A, Silhavy T J. Two-component signal transduction. Washington, D.C.: ASM Press; 1995. [Google Scholar]
- 10.Hsing W, Russo F D, Bernd K K, Silhavy T J. Mutations that alter the kinase and phosphatase activities of the two-component sensor EnvZ. J Bacteriol. 1998;180:4538–4546. doi: 10.1128/jb.180.17.4538-4546.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Igo M M, Ninfa A J, Stock J B, Silhavy T J. Phosphorylation and dephosphorylation of a bacterial transcriptional activator by a transmembrane receptor. Genes Dev. 1989;3:1725–1734. doi: 10.1101/gad.3.11.1725. [DOI] [PubMed] [Google Scholar]
- 12.LaCelle M, Kumano M, Kurita K, Yamane K, Zuber P, Nakano M M. Oxygen-controlled regulation of flavohemoglobin gene in Bacillus subtilis. J Bacteriol. 1996;178:3803–3808. doi: 10.1128/jb.178.13.3803-3808.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu X, Taber H W. Catabolite regulation of the Bacillus subtilis ctaBCDEF gene cluster. J Bacteriol. 1998;180:6154–6163. doi: 10.1128/jb.180.23.6154-6163.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lois A F, Weinstein M, Ditta G S, Helinski D R. Autophosphorylation and phosphatase activities of the oxygen-sensing protein FixL of Rhizobium meliloti are coordinately regulated by oxygen. J Biol Chem. 1993;268:4370–4375. [PubMed] [Google Scholar]
- 15.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 16.Nakano M M, Dailly Y P, Zuber P, Clark D P. Characterization of anaerobic fermentative growth in Bacillus subtilis: identification of fermentation end products and genes required for growth. J Bacteriol. 1997;179:6749–6755. doi: 10.1128/jb.179.21.6749-6755.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakano M M, Hoffmann T, Zhu Y, Jahn D. Nitrogen and oxygen regulation of Bacillus subtilis nasDEF encoding NADH-dependent nitrite reductase by TnrA and ResDE. J Bacteriol. 1998;180:5344–5350. doi: 10.1128/jb.180.20.5344-5350.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakano M M, Hulett F M. Adaptation of Bacillus subtilis to oxygen limitation. FEMS Microbiol Lett. 1997;157:1–7. doi: 10.1111/j.1574-6968.1997.tb12744.x. [DOI] [PubMed] [Google Scholar]
- 19.Nakano M M, Marahiel M A, Zuber P. Identification of a genetic locus required for biosynthesis of the lipopeptide antibiotic surfactin in Bacillus subtilis. J Bacteriol. 1988;170:5662–5668. doi: 10.1128/jb.170.12.5662-5668.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakano M M, Zheng G, Zuber P. Dual control of sbo-alb operon expression by the Spo0 and ResDE systems of signal transduction under anaerobic conditions in Bacillus subtilis. J Bacteriol. 2000;182:3274–3277. doi: 10.1128/jb.182.11.3274-3277.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakano M M, Zhu Y, Haga K, Yoshikawa H, Sonenshein A L, Zuber P. A mutation in the 3-phosphoglycerate kinase gene allows anaerobic growth of Bacillus subtilis in the absence of ResE kinase. J Bacteriol. 1999;181:7087–7097. doi: 10.1128/jb.181.22.7087-7097.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakano M M, Zhu Y, LaCelle M, Zhang X, Hulett F M. Interaction of ResD with regulatory regions of anaerobically induced genes in Bacillus subtilis. Mol Microbiol. 2000;37:1198–1207. doi: 10.1046/j.1365-2958.2000.02075.x. [DOI] [PubMed] [Google Scholar]
- 23.Nakano M M, Zuber P. Anaerobic growth of a “strict aerobe” (Bacillus subtilis) Annu Rev Microbiol. 1998;52:165–190. doi: 10.1146/annurev.micro.52.1.165. [DOI] [PubMed] [Google Scholar]
- 24.Nakano M M, Zuber P, Glaser P, Danchin A, Hulett F M. Two-component regulatory proteins ResD-ResE are required for transcriptional activation of fnr upon oxygen limitation in Bacillus subtilis. J Bacteriol. 1996;178:3796–3802. doi: 10.1128/jb.178.13.3796-3802.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raivio T L, Silhavy T J. Transduction of envelope stress in Escherichia coli by the Cpx two-component system. J Bacteriol. 1997;179:7724–7733. doi: 10.1128/jb.179.24.7724-7733.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schultz J, Milpetz F, Bork P, Ponting C P. SMART, a simple modular architecture research tool: identification of signalling domains. Proc Natl Acad Sci USA. 1998;95:5857–5864. doi: 10.1073/pnas.95.11.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi L, Liu W, Hulett F M. Decay of activated Bacillus subtilis Pho response regulator, PhoP-P, involved the PhoR-P intermediate. Biochemistry. 1999;38:10119–10125. doi: 10.1021/bi990658t. [DOI] [PubMed] [Google Scholar]
- 28.Stewart R C. Kinetic characterization of phosphotransfer between CheA and CheY in the bacterial chemotaxis signal transduction pathway. Biochemistry. 1997;36:2030–2040. doi: 10.1021/bi962261k. [DOI] [PubMed] [Google Scholar]
- 29.Stock A M, Robinson V L, Goudreau P N. Two-component signal transduction. Annu Rev Biochem. 2000;69:183–215. doi: 10.1146/annurev.biochem.69.1.183. [DOI] [PubMed] [Google Scholar]
- 30.Stock J B, Surette M G, Levit M, Park P. Two-component signal transduction systems: structure-function relationships and mechanisms of catalysis. In: Hoch J A, Silhavy T J, editors. Two-component signal transduction. Washington, D.C.: ASM Press; 1995. pp. 25–51. [Google Scholar]
- 31.Sun G, Sharkova E, Chesnut R, Birkey S, Duggan M F, Sorokin A, Pujic P, Ehrlich S D, Hulett F M. Regulators of aerobic and anaerobic respiration in Bacillus subtilis. J Bacteriol. 1996;178:1374–1385. doi: 10.1128/jb.178.5.1374-1385.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor B L, Zhulin I B. PAS domains: internal sensors of oxygen, redox potential, and light. Microbiol Mol Biol Rev. 1999;63:479–506. doi: 10.1128/mmbr.63.2.479-506.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weiss V, Magasanik B. Phosphorylation of nitrogen regulator I (NRI) of Escherichia coli. Proc Natl Acad Sci USA. 1988;85:8919–8923. doi: 10.1073/pnas.85.23.8919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams S B, Stewart V. Functional similarities among two-component sensors and methyl-accepting chemotaxis proteins suggest a role for linker region amphipathic helices in transmembrane signal transduction. Mol Microbiol. 1999;33:1093–1102. doi: 10.1046/j.1365-2958.1999.01562.x. [DOI] [PubMed] [Google Scholar]
- 35.Yannish-Perron C, Vieira J, Messing J. Improved M12 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 36.Zapf J, Madhusudan M, Grimshaw C E, Hoch J A, Varughese K I, Whiteley J M. A source of response regulator autophosphatase activity: the critical role of a residue adjacent to the Spo0F autophosphorylation active site. Biochemistry. 1998;37:7725–7732. doi: 10.1021/bi9729615. [DOI] [PubMed] [Google Scholar]
- 37.Zhang X, Hulett F M. ResD signal transduction regulator of aerobic respiration in Bacillus subtilis; cta promoter regulation. Mol Microbiol. 2000;37:1208–1219. doi: 10.1046/j.1365-2958.2000.02076.x. [DOI] [PubMed] [Google Scholar]
- 38.Zheng G, Yan L Z, Vederas J C, Zuber P. Genes of the sbo-alb locus of Bacillus subtilis are required for production of the antilisterial bacteriocin subtilosin. J Bacteriol. 1999;181:7346–7355. doi: 10.1128/jb.181.23.7346-7355.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zuber P, Losick R. Role of AbrB in Spo0A- and Spo0B-dependent utilization of a sporulation promoter in Bacillus subtilis. J Bacteriol. 1987;169:2223–2230. doi: 10.1128/jb.169.5.2223-2230.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]