A Perspective on “Noncanonical Role of Telomerase in Regulation of Microvascular Redox Environment with Implications for Coronary Artery disease”
Endothelial dysfunction is the inability of vascular endothelial cells to perform their normal biological functions and has been implicated in the development of cardiovascular diseases such as coronary artery disease and hypertension. Nitric oxide (NO) is the major mediator of endothelial function, which also has antiinflammatory and antioxidant effects, attenuating endothelial as well as smooth muscle cell pathology associated with cardiovascular diseases.1 In addition to NO and prostacyclin, endothelium-derived hyperpolarizing factor (EDHF) is a critical mediator of vasodilation. Under normal circulatory conditions, where NO acts as the main vasodilator, EDHF plays little role in the dilation associated with coronary autoregulatory capacity. An EDHF, H2O2, acts as a compensatory mechanism against loss of NO to maintain a residual vasodilatory response. Accordingly, during postischemic reperfusion injury of the coronary circulation, H2O2 is produced to protect further myocardial damage. Thus, the presence of coronary risk factors or coronary artery disease increases the contribution of H2O2 to flow-mediated dilation (FMD).2 While this dilatory mechanism plays an important role in maintaining myocardial perfusion when NO is reduced, it also exposes the already dysfunctional vessels to inflammatory H2O2.
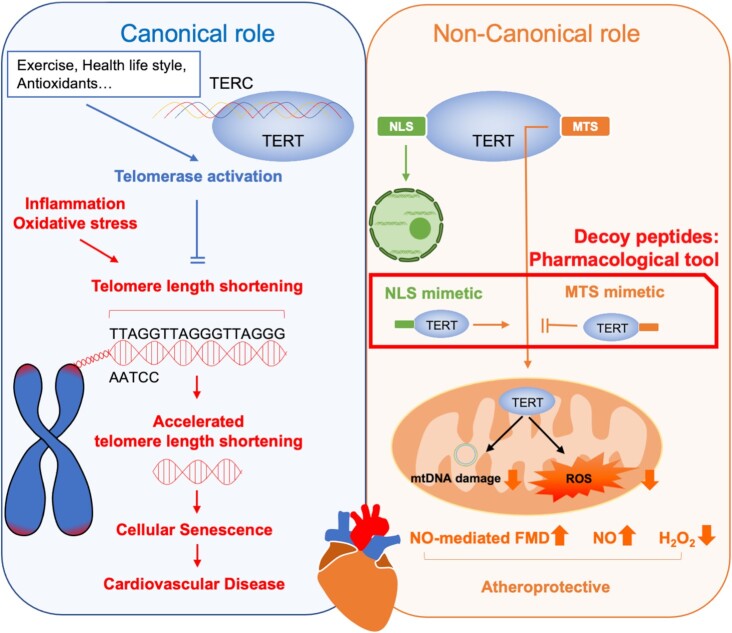
Telomerase is composed of telomerase RNA component (TERC) and telomerase reverse transcriptase (TERT), the catalytic component, and is responsible for the maintenance and elongation of telomere length. Telomere length gradually shortens with each cell division, and when shortened to a critical length, cellular senescence occurs with subsequent transition to apoptosis. In addition to the chronological aging, several environmental stress conditions are known to induce early telomere shortening and cellular senescence (Figure 1, left panel). Cardiovascular risk factors such as smoking, inactivity, obesity, and hypertension have been found to be associated with shorter telomeres.3 In TERT KO mice, telomeres were shortened, and age-related pathologies were observed.4 In addition to this canonical role of TERT in the maintenance of telomere homeostasis, evidence for noncanonical functions for TERT have been demonstrated. Interestingly, in the first-generation TERT-deficient mice, physical exercise did not protect against doxorubicin-induced vascular apoptosis. These mice are deficient in telomerase activity but have normal telomere length indicating that the beneficial cardiovascular effects of physical exercise require the noncanonical TERT function.5,6 Similarly, the noncanonical TERT function appears to preserve endothelial function and protect against vascular stress induced by angiotensin II.7 Importantly, in addition to its conventional nuclear localization, TERT also localized in mitochondria interacting with mitochondrial DNA, and thereby protecting mitochondrial DNA from damage.3 Mitochondrial TERT thus contributes to preserve mitochondrial respiratory capacity and reduces mitochondrial reactive oxygen species. However, it remained unclear whether either nuclear or mitochondrial TERT is involved in the aforementioned endothelial protection. Furthermore, it was difficult to distinguish the overlapping functions of TERT in the nucleus and mitochondria because prior studies were performed in TERT-deficient cells or mice or after expression of TERT mutants targeting specific cellular organelles while maintaining endogenous protein expression.
Figure 1.
Canonical and Noncanonical Effects of Telomerase. Telomerase consists of telomerase RNA component (TERC) and telomerase reverse transcriptase (TERT), the catalytic component, and is responsible for maintenance and elongation of telomere length. Several cardiovascular stress conditions are known to induce early telomere shortening and cellular senescence. In addition to this, important role of TERT in maintaining telomere homeostasis, evidence has accumulated for a nontelomeric function of this enzyme: TERT improves mitochondrial membrane potential and complex I activity of the respiratory chain, reduces mitochondrial reactive oxygen species, interacts with mitochondrial DNA, thereby protecting mitochondrial DNA from damage. The decoy peptides presented in this study represent the first pharmacological means to separate the mitochondrial and nuclear effects of TERT and represent a potential new therapeutic target for mitochondrial TERT. NLS, nuclear target sequence; MTS, mitochondrial target sequence; NO, nitric oxide; mtDNA, mitochondrial DNA; ROS, reactive oxygen species; H2O2, hydrogen peroxide.
In the present study, using vessels from patients with coronary artery disease, Ait-Aissa et al. demonstrated that increasing mitochondrial TERT, but not nuclear TERT, restores the physiological mechanisms of FMD whereas nuclear TERT promotes pathological H2O2-mediated cell proliferation.8 Providing a major advancement in the field, the authors introduced a novel decoy peptide that can manipulate intracellular TERT localization and demonstrated that pathological vasodilation seen in patients with coronary artery disease can be restored to physiological vasodilation. The decoy peptides presented in this study represent the first pharmacological means to separate the mitochondrial and nuclear effects of TERT and represent a potential new therapeutic target for mitochondrial TERT (Figure 1, right panel). These findings are in line with a prior study utilizing mouse models containing TERT only in the nucleus or mitochondria, demonstrating that mitochondrial TERT is important in cardiac protection against ischemia-reperfusion injury.3
Although the transcriptional regulation of human TERT (hTERT) has been extensively studied, the regulation of hTERT by mRNA processing events is less well known. More than 20 hTERT transcription variants have been detected with the two most studied TERT alternative splice variants being spliced at α- and β-sites. Interestingly, overexpression of β-deletion competes with telomerase RNA and inhibits endogenous telomerase activity.9 Overexpressed β-deletion proteins also localize to the nucleus and mitochondria. In the present study, the authors showed that α- and β-deletion TERT levels in cardiac vessels increased and full-length TERT mRNA levels decreased in the patients. They also showed that increased β-deletion TERT regulates microvascular endothelial function toward pathological vasodilation, while silencing of β-deletion TERT regulates physiological vasodilation. However, the relationship between β-deletion TERT and mitochondrial function remains unclear. Further, elucidation of these splicing variants of TERT may provide new therapeutic targets for the treatment of cardiovascular disease.
The signaling pathways initiating flow-mediated endothelial function vary by species and tissues, and are primarily sensed by membrane glycocalyx, mechano-sensitive ion channels, and focal adhesions.10 Secondary effector pathways include protein kinases that activate eNOS and other vasoactive mediators.10 The present study provides evidence that mitochondrial TERT is involved in these pathways and regulates vasodilatory mediator switching. Although mitochondrial reactive oxygen species were implicated in these mechanisms, the precise mechanism remains unclear. Therefore, further studies are needed in future to elucidate the mechanisms by which the intracellular distribution of TERT and its splice variants modulates microvascular endothelial function, including differences in signaling pathways, and the relationship between the antioxidant system and mitochondrial metabolism.
Funding
None declared.
Contributor Information
Keiichi Torimoto, Cardiovascular Research Center, Lewis Katz School of Medicine at Temple University, Philadelphia, PA 19140, USA.
Satoru Eguchi, Cardiovascular Research Center, Lewis Katz School of Medicine at Temple University, Philadelphia, PA 19140, USA.
Conflict of Interest
S.E. holds the position of Editorial Board Member for Function and is blinded from reviewing or making decisions for the manuscript.
References
- 1. Xu S, Ilyas I, Little PJet al. Endothelial dysfunction in atherosclerotic cardiovascular diseases and beyond: from mechanism to pharmacotherapies. Pharmacol Rev. 2021;73(3):924–967. [DOI] [PubMed] [Google Scholar]
- 2. Miura H, Bosnjak JJ, Ning G, Saito T, Miura M, Gutterman DD.. Role for hydrogen peroxide in flow-induced dilation of human coronary arterioles. Circ Res. 2003;92(2):e31–40. [DOI] [PubMed] [Google Scholar]
- 3. Hoffmann J, Richardson G, Haendeler J, Altschmied J, Andres V, Spyridopoulos I.. Telomerase as a therapeutic target in cardiovascular disease. Arterioscler Thromb Vasc Biol. 2021;41(3):1047–1061. [DOI] [PubMed] [Google Scholar]
- 4. Jaskelioff M, Muller FL, Paik JHet al. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature. 2011;469(7328):102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Werner C, Furster T, Widmann Tet al. Physical exercise prevents cellular senescence in circulating leukocytes and in the vessel wall. Circulation. 2009;120(24):2438–2447. [DOI] [PubMed] [Google Scholar]
- 6. Werner C, Hanhoun M, Widmann Tet al. Effects of physical exercise on myocardial telomere-regulating proteins, survival pathways, and apoptosis. J Am Coll Cardiol. 2008;52(6):470–482. [DOI] [PubMed] [Google Scholar]
- 7. Ait-Aissa K, Kadlec AO, Hockenberry J, Gutterman DD, Beyer AM.. Telomerase reverse transcriptase protects against angiotensin II-induced microvascular endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2018;314(5):H1053–H1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ait-Aissa K., Norwood-Toro LE, Terwoord Jet al. Non-canonical role of telomerase in regulation of microvascular redox environment with implications for coronary artery disease. Function. 2022:zqac043. 10.1093/function/zqac043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Listerman I, Sun J, Gazzaniga FS, Lukas JL, Blackburn EH.. The major reverse transcriptase-incompetent splice variant of the human telomerase protein inhibits telomerase activity but protects from apoptosis. Cancer Res. 2013;73(9):2817–2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Davies PF, Zilberberg J, Helmke BP.. Spatial microstimuli in endothelial mechanosignaling. Circ Res. 2003;92(4):359–370. [DOI] [PubMed] [Google Scholar]