Introduction:
Children are less likely to acquire SARS-CoV-2 infections than adults and when infected, usually have milder disease. True infection and complication rates are, however, difficult to ascertain. In Iceland, a strict test, trace and isolate policy was maintained from the start of the pandemic and offers more accurate information of the number of truly infected children in a nationwide study.
Material and methods:
All children with positive PCR for SARS-CoV-2 infections from February 28, 2020 to August 31, 2021 were followed up through telephone consultations for at least 14 days and their symptoms were registered. Symptom severity and duration were categorized based on age groups and the source of infection was registered.
Results:
A total of 1749 children were infected with SARS-CoV-2 in 3 waves of infections. All waves had similar disease severity whereas the incidence was 5-fold higher in the third wave (3.5 vs. 0.73/1000 children/month). No children had severe symptoms, 81 (4.6%) had moderate symptoms, 1287 (73.9%) had mild and 374 (21.5%) were asymptomatic. Symptoms from upper (n = 839, 48%) and lower respiratory tract (n = 744, 43%) were most common. Median duration of symptoms was 5 days and adolescents had a higher risk of prolonged duration [OR:1.84 (1.39–2.43)]. Nineteen (1.1%) children needed medical attention, but no child was hospitalized. The source of infection was a household member in 65% of cases.
Discussion:
During the first 3 waves of the pandemic, SARS-CoV-2 infections in Icelandic children were mild and none were hospitalized. The most common symptoms were respiratory symptoms followed by fever, headache and tiredness. This study helps shed light on true complication rates of children with confirmed SARS-CoV-2 infection.
Keywords: COVID-19, SARS-CoV-2, children, symptom severity, source of infection
From a pediatric perspective, clinical severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections are less likely to occur when compared with adults1 and most infections have been mild.2,3 With changes in dominating virus variants and increasing vaccination coverage, children may be more susceptible to infection.4 The first reports from China showed that the overall infection rate in children was very low compared with adults (8 vs. 164/100.000) although the total number of truly infected children is hard to measure when mass testing is not performed.5 In a meta-analysis of 1810 pediatric COVID-19 cases, 85% had mild disease. In reports and meta-analyses, the severe disease has been reported in 5% and mortality rates between 0 and 0.3%.6,7 Young age (<2 years) and nonwhite ethnicity were associated with an increased risk of hospital admission. A study reported that from 9478 pediatric hospital admissions due to COVID-19 in the US, 85 died and a 4-fold risk of death was observed if the patients had severe congenital heart disease.8 Pediatric intensive care units in countries with high rates of COVID-19 infection have been highly occupied by children with complications due to COVID-19, including multisystem inflammatory syndrome in children (MIS-C).8,9
The reason for an overall milder disease in children has not been fully explained. Suggestions of the role of the number and/or function of the ACE receptor in the respiratory mucosa have been made, as they are often present in lower numbers in young children.5,10 Also, recent infection with other coronaviruses may provide some cross-reactive T-cell immunity.9 In addition, lower levels of pro-inflammatory cytokines such as IL-6 may decrease the risk of acute respiratory distress syndrome, a well-known complication in adults.9
Iceland had the good fortune of having developed very stringent control of infected patients through isolation and quarantine of exposed individuals as well as mass testing of the population—a factor likely to reduce community spread.11 A total of 1749 children had tested positive for SARS-CoV-19 until August 31st and all of them were monitored closely by hospital staff through regular contacts, telephone calls (TC) and physical assessment if needed during their time in isolation. When a TC raised a concern of clinical symptoms, the children were assessed at the emergency department (ED) of the Children’s Hospital Iceland.
Many previous studies are limited by bias due to varying selection criteria when describing COVID-19 incidence and clinical presentations. The present study however describes the whole pediatric COVID-19 cohort in Iceland and is less subject to bias due to the composition of the well-defined Icelandic population and health care infrastructure and may help filling gaps of valid data on SARS-CoV-2 infections in children using well-defined criteria. The study describes the incidence, source of infection, clinical symptom severity and duration, complications and outcomes but was performed before the emergence of the omicron variant of the SARS-CoV-2 virus.
PATIENTS, MATERIALS AND METHODS
Time Period
The study describes the period from February 28, 2020 until 31st of August 2021.
Database
Through electronic databases, all children who tested positive for SARS-CoV-2 were included in a follow-up clinic. Their parents received a TC at the time of diagnosis and a first TC questionnaire was completed. Demographic information and previous medical history were collected as well as a source of infection, if known. While still symptomatic, a TC was carried out every 1–2 days, and a standard list of questions on clinical symptoms was answered. Once asymptomatic, a TC every 3–4 days was performed with the same list of questions until the child was free from isolation. According to the decision from the health authorities, isolation was required for all infected children for at least 14 days from confirmation of the infection and could only be lifted if the child had been asymptomatic for 7 days.
When analyzing the data, the age groups were defined as younger than 6 months, 6 months–3 years, 4–7 years, 8–13 years and 14–17 years old. Age was counted in months up to 12 months and in whole years in all children older than 1 year. When calculating age-standardized incidence, children were categorized into three groups, 0–3, 4–13 and 14–17-year-olds. The population data were obtained from Statistics Iceland.12
When analyzing the duration of symptoms, the data were analyzed both as continuous variables as well as categorized as <2 days, 3–9 days and 10 days or longer.
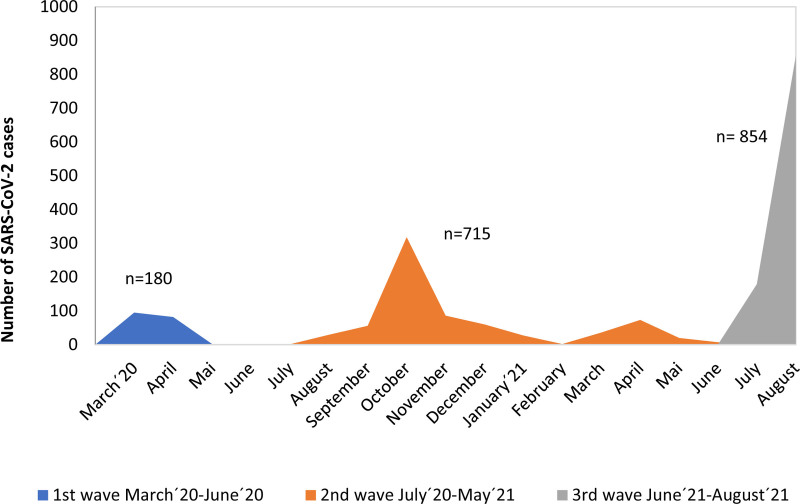
Three waves of the pandemic were defined as March–June 2020 (1st wave), July 2020–May 2021 (2nd wave) and June–August 2021 (3rd wave) as shown in Fig. 1.
FIGURE 1.
Number of SARS-CoV-2 infections in Icelandic children from March 2020–August 2021 Three waves of COVID-19 disease in children in Iceland from March 2020–August 2021. The third wave was dominated by the delta variant of SARS-CoV-2. The median age of children infected with SARS-CoV-2 was 12 years in the first wave and 10 years in both subsequent waves. The mean age-standardized incidence per 1000 children/month was 0.73 during the first wave, 0.73 in the second wave and 3.5/1000 children/month during the third wave.
Case Definition and Sampling
Cases were defined as individuals younger than 18 years of age and qPCR positive for SARS-CoV-2. A cycle threshold level of <35 was defined as a positive result. Samples with a cycle threshold level of 35–40 were categorized as inconclusive by the national reference microbiology laboratory and repeat testing was recommended on the following day. Symptomatic infection was defined as any of the symptoms listed in Table 1. Days with symptoms were counted as the total number of days with symptoms (including the first and last day of symptoms).
TABLE 1.
List of registered symptoms from SARS-CoV-2 infection
| Categories of symptoms from SARS-CoV-2 infection |
|---|
| Upper respiratory (sore throat, runny nose) |
| Lower respiratory (cough, shortness of breath) |
| Abdominal (Diarrhea, vomiting, abdominal pain) |
| Fever |
| Headache |
| Muscle and/or body ache |
| Tiredness/Malaise |
| Loss of smell and taste |
| Loss of appetite |
| Other* |
Light sensitivity, ear pain, dizziness, chills, irritability.
All laboratory-confirmed cases were diagnosed at the National reference laboratory at the Landspitali University Hospital, Department of Clinical Microbiology or the laboratory of Decode Genetics using conventional qPCR methods for SARS-CoV-2 RNA in naso- and/or oropharyngeal swabs.13 Cases were identified through targeted testing of: (1) suspected cases due to symptomatic children in quarantine. (2) Symptomatic children attending health care facilities. (3) Open invitation for screening regardless of symptoms.
Most samples were collected at a defined COVID sampling center in Reykjavik. Other samples were collected from the ED of the Children’s Hospital Reykjavik and smaller health care centers outside the most populated areas (Reykjavik and surroundings).
WHO criteria for the classification of disease severity were applied where the criteria for moderate symptoms were fever, cough and dyspnea for adolescents and cough and dyspnea for younger children.14
The source of infection was classified as household, school, leisure (after school and out-of-home activities) or unknown.
Ethics
The study was approved by the National Bioethics Committee of Iceland (ref: 21-065-S1) and The Institutional Research Committee at Landspitali University Hospital The sample collection was performed on behalf of Icelandic health authorities in agreement with the Act no. 19/1997 on Health Security and Communicable Diseases. Data analysis was performed using study numbers with no personally identifiable information.
Statistical Analysis
The data were expressed as median (with range) or number (percentage). Mann-Whitney U test was used for the comparison of numerical variables and the χ2 test for categorical variables. A P-value of <0.05 was considered statistically significant. A multivariable regression model was used to test for the effects of age and sex on disease severity. The models were adjusted for confounders as shown in the models. A likelihood ratio was calculated and expressed as odds ratios (OR) with 95% confidence intervals. The software Stata (StataCorp, College Station, Texas) version 13.1 was used for statistical analysis.
RESULTS
A total of 1749 children were diagnosed with SARS-CoV-2 infection during the study period. Overall, the age-standardized incidence was 21.5/1000 children. The overall annual incidence was 10.9/1000, 21.5/1000 and 31.8/1000 children for children younger than 4 years old, 4–13 and 14–17-year-olds, respectively. Nineteen (1.1%) patients needed clinical assessment at the Children’s Hospital Emergency Department. No patient required specific treatment (antiviral treatment, corticosteroids or monoclonal antibodies) and there were no hospital admissions. Three were treated with a course of oral antibiotics for a presumed bacterial infection. No child was diagnosed with MIS-C.
Demographics
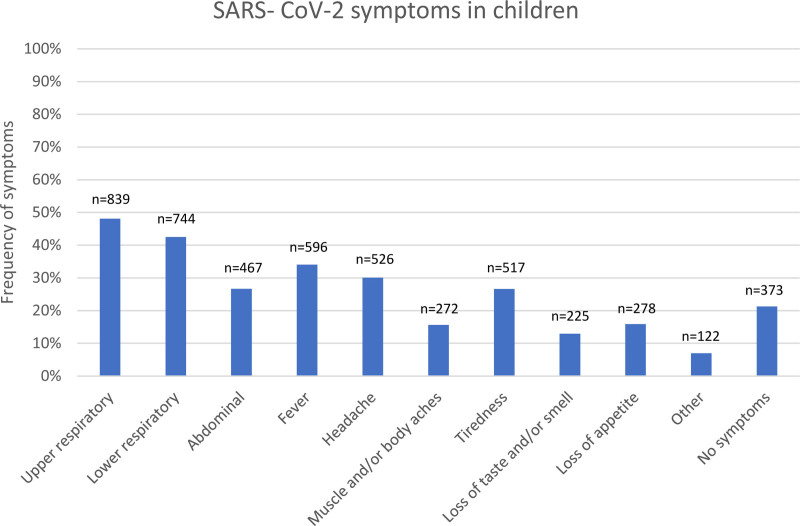
Of the 1749 cases, 919 (52.5%) were male. Children younger than 18 years were 16% of all confirmed COVID-19 cases in Iceland at the completion of data collection (August 2021). The median age of the children was 10 years (range: 1 week–17 years), 433 (24.8%) were older than 13 years of age and 19 children were younger than 6 months. A total of 96 children had underlying conditions (5.5%) which were asthma (only counted in children older than 2 years of age), congenital heart disease or heart failure, type 1 diabetes mellitus, cerebral palsy, immunodeficiency, or trisomy 21. Data on symptoms was missing for 7 children. Asymptomatic children were 373 (21.5%). Of symptomatic children, upper and lower respiratory symptoms were most common and were observed in 48.1% and 42.5%, respectively of all patients. Fever (34%) and headache (30%) were also commonly reported. Abdominal pain, vomiting or diarrhea were reported in 27% as shown in Fig. 2.
FIGURE 2.
Registered symptoms of SARS-CoV-2 infections in 1742 Icelandic children until the end of August 2021. Upper respiratory symptoms were: sore throat and runny nose. Lower respiratory symptoms: cough, shortness of breath. Abdominal symptoms: diarrhea, vomiting, abdominal pain. Other: light sensitivity, ear pain, dizziness, chills, irritability.
Symptom Severity and Duration
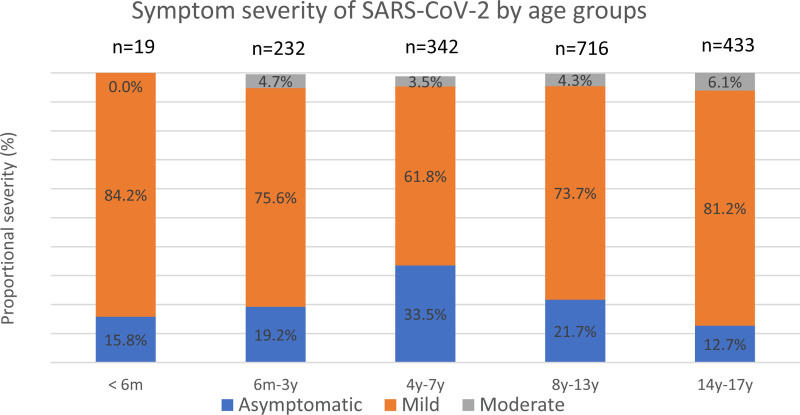
Asymptomatic infection was common in 4–7- and 8–13-year-olds where 31% and 24.4% respectively had no symptoms of infection (Fig. 3). Mild symptoms were reported in 1287 (73.9%) children whereas 81 (4.6%) had moderate symptoms. No child had severe symptoms. Of the 81 children with moderate symptoms, 7 (8.6%) had underlying illness. Underlying medical conditions were not associated with risk of moderate symptoms where 7/96 (7.3%) children with a medical condition had moderate symptoms compared with 74/1646 (4.5%) previously healthy children (P = 0.45). Infants <6 months of age were few (n = 19) and were asymptomatic or had mild disease. The risk of moderate symptoms was similar in all age groups as shown in Table 2.
FIGURE 3.
Symptom severity of SARS-CoV-2 infection in Icelandic children. Classification of severity of SARS-CoV-2 infection in 1742 Icelandic children. No child had severe symptoms.
Table 2.
Odds ratio of higher symptom severity in children with SARS-CoV-2 infection
| N = 381 | N = 1271 | N = 97 | OR of moderate symptoms (95% CI) | |
|---|---|---|---|---|
| Age category | Asymptomatic – no (%) | Mild – no (%) | Moderate – no (%) | |
| <6 months | 3 (0.8) | 16 (1.3) | 0 (0.0) | N/A |
| 6 months–3 years | 45 (12.0) | 176 (13.7) | 11 (13.6) | 1.05 (0.56–1.97) |
| 4–7 years | 116 (31.0) | 214 (16.6) | 12 (14.8) | 0.91 (0.52–1.59) |
| 8–13 years | 156 (41.7) | 529 (41.1) | 31 (38.3) | 0.82 (0.52–1.28) |
| 14–17 years | 54 (14.4) | 352 (27.4) | 27 (33.3) | 1.37 (0.85–2.20) |
| Sex – no (%) | ||||
| Female | 173 (46.3) | 613 (47.6) | 42 (51.9) | 1.12 (0.73–1.72) |
| Male | 201 (53.7) | 674 (52.4) | 39 (48.2) | 0.90 (0.58–1.38) |
No children were severely or critically ill. Data was missing for 7 children.
OR: Odds ratios calculated by binary logistic regression modelling where the reference value was moderate disease. Models were adjusted by source of infection.
CI indicates confidence interval.
Overall, the median duration of illness was 5 days (range:1–33). In total, 210 (12.1%) had symptoms for fewer than 2 days, 864 (49.6%) for 2–9 days and 277 (15.9%) for 10 days or more. An association between age and duration of symptoms was found where adolescents were significantly more likely to have symptoms for ≥10 days of longer duration (OR: 1.84, P < 0.001). The opposite was found for children 4–7 years of age who were significantly less likely to have symptoms ≥10 days as shown in Table 3.
Table 3.
Symptoms from SARS-SCoV-2 infection in children lasting 10 days or longer
| Age | OR of symptoms lasting ≥10 days | 95% CI |
|---|---|---|
| <6m | 1.82 | 0.65–5.13 |
| 6m–3y | 1.03 | 0.79–1.32 |
| 4y–7y | 0.46 | 0.31–0.68 |
| 8y–13y | 0.91 | 0.70–1.18 |
| 14y–17y | 1.84 | 1.39–2.43 |
Odds ratio (OR) and Confidence intervals (CI) of the risk of symptoms lasting 10 days or more based on age category: OR: Odds ratios calculated by binary logistic regression modelling where the reference value was age group. Models were adjusted by sex, severity, and source of infection.
M indicates month; y, years.
Source of Infection
The source of infection was known in 91% of the cases and 1142 were infected in the household (65.3%). In 219 (12.5%) cases, the source of infection was at school or day-care and 231 (13.2%) in leisure activities. For 157 children (9.0%), the source of infection was unknown. For children infected at school or leisure, no information was available whether the children were infected from staff or other pupils. Most of the 157 children with no known source of infection were registered towards the end of the study period where infections were becoming more widespread.
There was no correlation found between symptom severity and source of infection when using logistic regression modelling (data not shown).
Disease Severity and Different Waves of the Pandemic
Three waves of SARS-CoV-2 infections were defined as shown in Fig. 1. In the third wave of infections, the delta variant was dominating. During the 3rd wave, 854 children were diagnosed with SARS-CoV-2 infection despite a much shorter time period than the 2nd wave, which had 715 infections. No significant differences were observed between the waves in terms of the age of infected children, disease severity or symptom duration. The range of observed symptoms between the 3 waves can be seen in Table and Figure, Supplemental Digital Content 1; http://links.lww.com/INF/E765 and 2; http://links.lww.com/INF/E765.
DISCUSSION
In this nationwide study of all children infected in Iceland during the first 18 months of the COVID-19 pandemic (until August 31st, 2021), we found that overall, the symptoms were relatively mild, of short duration and with few complications. No child was admitted to hospital and only 19 needed medical assessment at the Children’s Hospital. Three children were treated with oral antibiotics for presumed bacterial infections. A trend towards more severe symptoms in adolescents is in concordance with other reports.15,16 According to the WHO definition of disease severity, none of the 1749 cases had severe or critical illness.
The source of infection was known for a large part of our cohort (91%) and was from other household members in two-third of cases. Source was school, day-care or in leisure activities in a quarter of the cases, but it is unknown if the infections was contracted from teachers/staff or other children. This strongly indicates that spread is common from adults to children and that spread from other children was probably less common. This is in line with other reports where children are considered less likely to spread the infection.17 Around 20% of the children were asymptomatic throughout the course of infection, but symptomatic children mostly had conventional symptoms of upper respiratory viral infections.
Although this sample size of children is not very large, it has the strong advantage of representing all children with confirmed SARS-CoV-2 infection and most of truly infected children in Iceland during the study period. The source of infection in almost all infected adults and children was traceable due to the strict test, trace, and isolate policy of the Icelandic health authorities at the time. During the study period, most children as well as most adults, who were diagnosed with infection, were already in quarantine and unlikely that many pediatric infections will have been undetected. This cohort also reflects an unselected group of all children with confirmed infections rather than only hospitalized children as in most other studies and therefore helps shed light on the true rates of complications and serious disease in children. There are without doubt some additional asymptomatic cases that were not identified, but according to a serologic survey conducted on randomly selected 30,000 Icelanders in the first half of 2020, only 0.6% of participants had seroconverted but a minority of that cohort were children.11 It also supports suggestions that the disease course was mild in children during the study period and almost all could be managed at home without need for direct medical care. Reflecting the population composition in Iceland, most children in our cohort were however of white Caucasian ethnicity which might bias the results toward a milder disease course.18,19 The fact that in our cohort of more than 1700 pediatric patients, no child needed hospital admission suggests that hospital admission rates in this age groups are no higher than 0.1%–0.3% as reported in a Norwegian study.20 The delta variant, dominating the third wave, did not seem to cause more serious illness in younger children although the ability to better transmit between individuals is probably actual as seen by the rapid rise of cases in a short space of time.21 This trend towards a much higher infectivity rate and accumulation of large groups of children being infected in a short space of time with the emergence of the omicron variant of SARS-CoV-2 has since materialized in the first weeks of 2022.
Adolescents 16–17 years old in Iceland were offered COVID-19 vaccination in April-May 2021, with excellent uptake and 2 dose vaccination rates surpassed 90%. This may have contributed to fewer infections and milder disease course in this age group toward the end of our study period. No children 12–15 years of age were fully vaccinated during the study period although since then around 85% of children >12 years are currently vaccinated.
A study reporting a cohort of children from the region Aragon in Spain shows similar results to ours although hospitalizations were more common (0.5%) and 10% of admitted patients were admitted to intensive care units.22 These differences may reflect that a larger group of unidentified but infected children were not included in the Spanish cohort which affects the rates of hospitalizations. Also, different ethnicity of the cohort could be of importance.
A US study reported that overall mortality in children admitted to hospital with COVID-19 was around 1% and up to 4% in children with severe congenital heart disease.8 Using these numbers of mortality on our cohort, where the admission rates of all infected children are likely to be close to 0.1%, leads to an estimated overall mortality in the pediatric cohort of around 1/100.000 infected. A contributing factor is that no child was diagnosed with MIS-C.23,24
The role of children in transmission has been debated, and although most experts agree that school and preschool closures should be avoided, if possible, many children have suffered from disrupted school activities aimed at halting the spread of the virus. The benefits of such closures remain unproven. Vaccination programs for children 12–17-year-olds have been very successful with low rates of adverse reaction and effectiveness rates of 92%.25 In highly vaccinated communities, such as Iceland, children younger than 12 years are currently the largest group of unvaccinated individuals. This might lead to a different role of children in the spread of disease and younger children may maintain circulation of the virus by serving as a reservoir.26 The decision, whether to vaccinate younger children, is however complex and warrants consideration of several factors as summarized by Zimmerman et al.27
One of the limitations of our study is that the description and registration of symptoms are done through telephone consultations between medical personnel and parents rather than direct clinical contact and this may cause some inaccuracy. In addition, daily TC from health care professionals were made to gather information and give advice. This may well have led to early intervention or advice, decreasing the risk of further deterioration. This may have reduced the risk of development of more severe symptoms and almost certainly reduced the number of children that needed medical care at the hospital. Also, information on the source of infection was missing in 157 cases which may influence interpretation of the importance of the source on population transmission dynamics. The registration of underlying medical illness was only based on the discussion with parents and not confirmed by access to the child’s medical records. In our study, correlation of the viral load (cycle threshold level) and symptom severity was not studied. It is possible that some of the asymptomatic children had false positive tests which would bias the results toward a larger asymptomatic group. These numbers are however likely to be very small due to high specificity of the assay used by the national reference laboratory.28
To conclude, this is a nationwide study on the symptoms in children with SARS-CoV-2 infection and describes the symptoms of all children tested positive through rigorous tracing and testing. This study helps shed light on the true frequency of complications in pediatric SARS-CoV-2 infections and supports the observation that COVID-19 disease in children generally causes nonsevere symptoms and despite around half the cases were during a delta variant of SARS-CoV-2, no hospital admissions were needed although transmission was clearly more potent than in previous variants.
ACKNOWLEDGMENTS
We thank the personnel at COVID-19 ambulatory ward at the Children’s Hospital Iceland and Landspitali University Hospital for data registration and collection.
Supplementary Material
Footnotes
The authors have no funding or conflicts of interest to disclose.
V.T. and A.H. designed the study. K.B. gathered the data and performed the statistical analysis. All authors contributed to interpretation of the study results and revised and approved the manuscript for intellectual content. The corresponding author (V.T.) confirms that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. All authors are employees of the Landspitali University Hospital. V.T., T.L. and A.H. also hold positions at the medical faculty of the University of Iceland.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.pidj.com).
Contributor Information
Thorvardur Love, Email: thorvard@landspitali.is.
Asgeir Haraldsson, Email: asgeir@landspitali.is.
REFERENCES
- 1.Viner RM, Mytton OT, Bonell C, et al. Susceptibility to SARS-CoV-2 infection among children and adolescents compared with adults: a systematic review and meta-analysis. JAMA Pediatr. 2021;175:143–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109:1088–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liguoro I, Pilotto C, Bonanni M, et al. SARS-COV-2 infection in children and newborns: a systematic review. Eur J Pediatr. 2020;179:1029–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Head JR, Andrejko KL, Remais JV. Model-based assessment of SARS-CoV-2 Delta variant transmission dynamics within partially vaccinated K-12 school populations. medRxiv. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145:e20200702. [DOI] [PubMed] [Google Scholar]
- 6.Badal S, Thapa Bajgain K, Badal S, et al. Prevalence, clinical characteristics, and outcomes of pediatric COVID-19: a systematic review and meta-analysis. J Clin Virol. 2021;135:104715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cui X, Zhao Z, Zhang T, et al. A systematic review and meta-analysis of children with coronavirus disease 2019 (COVID-19). J Med Virol. 2021;93:1057–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strah DD, Kowalek KA, Weinberger K, et al. Worse hospital outcomes for children and adults with COVID-19 and congenital heart disease. Pediatr Cardiol. 2022;43:541–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steinman JB, Lum FM, Ho PP, et al. Reduced development of COVID-19 in children reveals molecular checkpoints gating pathogenesis illuminating potential therapeutics. Proc Natl Acad Sci U S A. 2020;117:24620–24626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bunyavanich S, Do A, Vicencio A. Nasal gene expression of angiotensin-converting Enzyme 2 in children and adults. JAMA. 2020;323:2427–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gudbjartsson DF, Norddahl GL, Melsted P, et al. Humoral immune response to SARS-CoV-2 in Iceland. N Engl J Med. 2020;383:1724–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Statistics Iceland. Population by sex, individual age and marital status 1901-2021. 2021. Accessed February 7, 2021.
- 13.Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25:2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization. Clinical Management of COVID-19: Interim Guidance. 2020. [Google Scholar]
- 15.Escosa-García L, Aguilera-Alonso D, Calvo C, et al. Ten key points about COVID-19 in children: the shadows on the wall. Pediatr Pulmonol. 2020;55:2576–2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams N, Radia T, Harman K, et al. COVID-19 Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review of critically unwell children and the association with underlying comorbidities. Eur J Pediatr. 2021;180:689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim J, Choe YJ, Lee J, et al. Role of children in household transmission of COVID-19. Arch Dis Child. 2021;106:709–711. [DOI] [PubMed] [Google Scholar]
- 18.Saatci D, Ranger TA, Garriga C, et al. Association between race and COVID-19 outcomes among 2.6 million children in England. JAMA Pediatr. 2021;175:928–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Antoon JW, Grijalva CG, Thurm C, et al. Factors associated with COVID-19 disease severity in US children and adolescents. J Hosp Med. 2021;16:603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vold L. UK virus variant associated with higher risk of hospital admission. 2021. Available at: www.fhi.no. Accessed April 18, 2021.
- 21.Allen H, Vusirikala A, Flannagan J, et al.; COVID-19 Genomics UK (COG-UK Consortium). Household transmission of COVID-19 cases associated with SARS-CoV-2 delta variant (B.1.617.2): national case-control study. Lancet Reg Health Eur. 2022;12:100252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.García-Vera C, Castejón-Ramírez S, Laín Miranda E, et al. COVID-19 in children: clinical and epidemiological spectrum in the community. Eur J Pediatr. 2022;181:1235–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang L, Tang K, Levin M, et al. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect Dis. 2020;20:e276–e288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheung EW, Zachariah P, Gorelik M, et al. Multisystem inflammatory syndrome related to COVID-19 in previously healthy children and adolescents in New York City. JAMA. 2020;324:294–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lutrick K, Rivers P, Yoo YM, et al. Interim estimate of vaccine effectiveness of BNT162b2 (Pfizer-BioNTech) vaccine in preventing SARS-CoV-2 infection among adolescents Aged 12-17 years - Arizona, July-December 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1761–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haraldsson Á, Love TJ, Thors VS. No one will be safe until our children are safe: parent’s attitude towards COVID-19 childhood immunization. Pediatr Infect Dis J. 2021;40:e388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zimmermann P, Pittet LF, Finn A, et al. Should children be vaccinated against COVID-19? Arch Dis Child. 2022;107:e1. [DOI] [PubMed] [Google Scholar]
- 28.Kostoulas P, Eusebi P, Hartnack S. Diagnostic accuracy estimates for COVID-19 real-time polymerase chain reaction and lateral flow immunoassay tests with bayesian latent-class models. Am J Epidemiol. 2021;190:1689–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.