Abstract
We examined the impact of COVID-19 pandemic on the emergency department length of stay (EDLOS) and clinical outcomes of patients with severe pneumonia admitted to the intensive care unit (ICU) through the emergency department (ED). This single-center retrospective observational study included adult patients with pneumonia admitted to the ICU through the ED between January and December 2019 (pre-pandemic) and between March 2020 and February 2021 (during-pandemic). We compared and analyzed the EDLOS by dividing it into pre-, mid-, and post-EDLOS and in-hospital mortality of patients with pneumonia admitted to the ICU according to the time of ED visits before and during the COVID-19 pandemic. Risk factors for in-hospital mortality according to the time of ED visits were analyzed using multiple logistic regression analysis. In total, 227 patients (73 patients pre-pandemic and 154 patients during the pandemic) with pneumonia admitted to the ICU through the ED were analyzed. During the COVID-19 pandemic, pre-, mid-, and post-EDLOS increased (P < .05), and the in-hospital mortality rate increased by 10.4%; however, this was not significant (P = .155). Multivariate logistic regression analysis revealed post-EDLOS (ED waiting time after making ICU admission decision) as an independent risk factor for in-hospital mortality of patients with pneumonia admitted to the ICU, pre-pandemic (odds ratio [OR] = 2.282, 95% confidence interval [CI]: 1.367–3.807, P = .002) and during the pandemic (OR = 1.126, 95% CI: 1.002–1.266, P = .047). Mid-EDLOS (ED time to assess, care, and ICU admission decision) was an independent risk factor for in-hospital mortality of patients with pneumonia admitted to the ICU during the COVID-19 pandemic (OR = 1.835, 95% CI: 1.089–3.092, P = .023). During the pandemic of emerging respiratory infectious diseases, to reduce in-hospital mortality of severe pneumonia patients, it is necessary to shorten the ED waiting time for admission by increasing the number of isolation ICU beds. It is also necessary to accelerate the assessment and care process in the ED, and make prompt decisions regarding admission to the ICU.
Keywords: COVID-19, emergency department, length of stay, pneumonia
1. Introduction
The World Health Organization declared a global pandemic of COVID-19 on March 11, 2020.[1] The COVID-19 pandemic has impacted daily life and society at multiple levels,[2] including the emergency department (ED) care system. Many patients with COVID-19 have mild infections, including upper respiratory symptoms with fever. However, approximately 15% cases develop severe disease, and 5% become critically ill.[3] SARS-CoV-2 spreads via droplets and direct contacts. Additionally, the recently identified Omicron variant is highly transmissible. Therefore, early diagnosis, isolation, and supportive care are essential for patients because of the high contagiousness and possibility of rapid deterioration.[3,4] Therefore, when a patient with fever and respiratory symptoms visits the ED, preliminary isolation and screening tests are conducted using separate mobile lines and negative-pressure isolation rooms (IRs). Subsequently, a seriously ill patient hospitalized due to complications, such as pneumonia, or is epidemiologically suspected of COVID-19 infection, undergoes isolation until release after check of a negative COVID-19 polymerase chain reaction (PCR) test.
During the current COVID-19 pandemic, the shortage of ED and inpatient isolation beds for patients with severe pneumonia persisted in the course of ED treatment, where these preemptive isolation measures were essential. In particular, the shortage of isolation beds in the intensive care unit (ICU) remains an ongoing issue for critically ill patients. In February 2020, Daegu, South Korea, the surge in COVID-19 cases resulted in complete depletion of ICU and negative-pressure isolation beds, and 3 patients died at home while waiting for hospital admission.[5] As the surge of COVID-19 infections and bed crisis intensified, the government issued executive orders to hospitals to allocate more beds for critically ill patients with COVID-19. However, on December 15, 2020, in the most heavily affected area (metropolitan Seoul), only 2 ICU beds were available for patients with COVID-19, and 580 patients were waiting at home even after a confirmed COVID-19 diagnosis.[6]
Similarly, in the ED care process for patients with fever/respiratory symptoms or symptoms epidemiologically related to COVID-19 infection, we experienced an increase in waiting patients and waiting time for entry to IRs outside the ED, difficulties in determining the priority of isolation according to the severity of waiting patients, increased concerns regarding the efficient use of insufficient IRs, and increased workload. In addition, there was overcrowding and congestion in the ED owing to an increased ED length of stay (EDLOS) for patients with pneumonia whose hospitalization was due to the lack of inpatient isolation beds.
The EDLOS is a well-established indicator of ED overcrowding.[7,8] An increase in the EDLOS has been reported to cause delayed assessment and care.[9–13] Furthermore, it is associated with poor prognosis of critically ill patients because of an increased length of hospital stay and mortality rate of patients.[14–17] A report demonstrated that the EDLOS increased despite the decreased ED visits during the COVID-19 pandemic,[18] and a multicenter study reported an increase in the EDLOS for patients managed in isolation.[19] In addition, data from Hong Kong public hospitals and clinics databases have revealed increases in the overall mortality rate of non-COVID-19 diseases and length of hospitalization.[20]
Based on these facts and our experience, we hypothesized that prolonged EDLOS could negatively affect outcomes of patients with severe pneumonia who were admitted to the ICU through the ED. Therefore, we aimed to investigate the clinical outcomes and changes in the EDLOS according to changes in ED care patterns of patients before and during the COVID-19 pandemic.
2. Methods
2.1. Study design and population
Our hospital is a university-affiliated training hospital with approximately 700 beds, and approximately 50,000 patients visit the ED annually. The ED consists of a regional level 1 trauma center (6 general care beds, 2 resuscitation room beds, and 2 operating room beds) and a local emergency medical center (24 general care beds, 2 resuscitation room beds, and 3 IR beds). The number of beds remained the same in 2019 and 2020. In 2019, there were no limits imposed on the number of inpatient beds for patients with pneumonia, however, in February 2020, a limit of 58 inpatients beds was imposed (16 single-person negative pressure IRs, 33 pneumonia cohort beds, and 9 IRs in the ICU) in the hospital. The number of doctors working in the non-traumatic local emergency medical center included 5 emergency medicine board physicians, 7 emergency medicine residents, and 2 interns, and there was no change during the study period. The number of nurses in the non-traumatic local emergency medical center was 23 to 31 in 2019 and 29 to 37 in 2020. ED care for patients with pneumonia is the same on weekdays and weekends. The ED staff is in charge of initial evaluation and treatment, and the final admission is decided on via internal medicine consultation.
Since January 20, 2020, after the first reported infection of COVID-19 in Korea, medical staff working in the ED were required to screen and treat patients with potential COVID-19 infection. After 1-month preparation period, a separate COVID-19 screening clinic was installed and operated at the entrance of the ED. From February 10, 2020, during the daytime (9 AM to 6 PM), ED care was provided after screening at the screening clinic by non-ED medical staff. At nighttime (6 PM to 9 AM), ED medical staff directly screened and provided ED care. For this reason, January and February 2020 were excluded from the study period as the time window for determining COVID-19 screening protocols for emergency patients. To minimize seasonal deviation in pneumonia incidence, the study period was set to 1 year before and during the COVID-19 pandemic. This study was approved by the Institutional Review Board of the hospital (approval number: 2021-10-009). Due to the retrospective design of the study, the ethics committee granted an informed consent waiver.
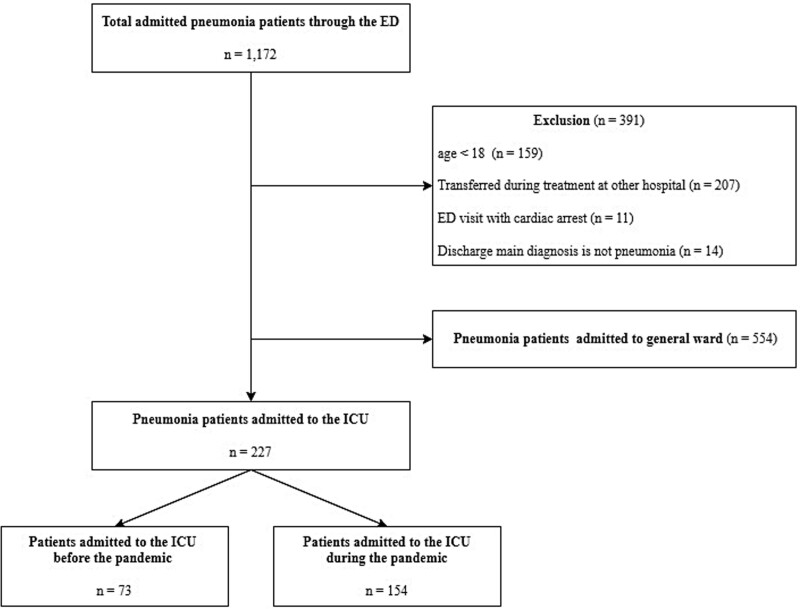
From January to December 2019 (control period) and from March 2020 to February 2021 (COVID-19 pandemic period), the International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10) code was used to first extract inpatients through the ED with the main diagnosis of pneumonia (ICD-10 code: J10.0, J11.0, J12~18, J69). After excluding patients based on the exclusion criteria, the remaining patients who were admitted to the ICU were selected as the final study participants. The exclusion criteria included: Patients aged <18 years, those who were transferred during treatment after being diagnosed with pneumonia at other hospitals, those who visited the ED initially in cardiac arrest, and those who were admitted to the ICU with pneumonia as the main diagnosis according to ICD-10 codes but for whom pneumonia was not the primary diagnosis at discharge (Fig. 1).
Figure 1.
Flow chart of study patients. ED = emergency department, ICU = intensive care unit.
2.2. Data collection and definitions
Data were collected by reviewing medical records. Age, sex, initial vital signs, state of consciousness, past medical history (hypertension, diabetes mellitus, tuberculosis, liver disease, myocardial infarction, congestive heart failure, cerebrovascular disease, chronic obstructive pulmonary disease, asthma, chronic renal disease, active neoplastic disease, and dementia), number of medicines taken, residency before ED visit, means of ED visit, presenting symptoms, initial ED laboratory results (first measured arterial blood gas analysis, white blood cell counts, hemoglobin, hematocrit, platelets, electrolytes, creatinine, total bilirubin, albumin, blood urea nitrogen, blood glucose, C-reactive protein, blood culture, and sputum culture results), initial chest x-ray and computed tomography readings, time of first antibiotic administration, time of ED visit, time of first ED assessment and care, time of ICU admission decision, time of exit from ED, total hospitalization days and days in ICU, in-hospital mortality, ED triage and acuity scale, and procedures at the ED or after admission (central venous catheterization, tracheal intubation, use of inotrope, and hemodialysis). To evaluate and compare the severity and mortality risk of patients at the time of ED visit and after admission to the ICU using the aforementioned variables, the pneumonia severity index, CURB-65 score, acute physiology and chronic health evaluation II, and sequential organ failure assessment scores were calculated and compared.
The EDLOS was defined as the previous stay time (duration from the time of ED visit to the first assessment and care in the ED; pre-EDLOS), intermediate stay time (duration from the time of the first assessment and care to the time of decision to ICU admission; mid-EDLOS), and post-stay time (duration from the time of ICU admission decision to the time of exit from the ED; post-EDLOS). The EDLOS was calculated based on emergency medical records.
2.3. Statistical analysis
Data were analyzed using SPSS version 22.0 (IBM Corp., Armonk, NY). The normality of continuous variables was tested using the Kolmogorov-Smirnov test. Normally distributed continuous variables are presented as means ± standard deviations and were compared using Student’s t test. Non-normally distributed continuous variables are presented as medians and interquartile ranges and were compared using the Mann–Whitney U test. Categorical variables are described as numbers (%) and were compared using the chi-square test or Fisher’s exact test. Logistic regression analysis was performed to identify the in-hospital mortality risk factors of patients with pneumonia admitted to the ICU through the ED throughout the 2-year study period (before and during the COVID-19 pandemic period). Variables with a statistically significant difference (P-value <.1) in univariate analysis were included in the multivariate logistic regression model. Multivariate logistic regression analysis was performed with the forward elimination method using the likelihood ratio. The suitability of the multivariate logistic regression model was determined using the Hosmer and Lemeshow test. Results are expressed as adjusted odds ratios (OR) and their 95% confidence intervals (CIs). For all tests, P values <.05 were considered statistically significant.
3. Results
The total number of patients that visited the ED during the pandemic (March 2020 to February 2021) and control period (January to December 2019) was 35,561 (24,056 non-traumatic ED patients vs 11,505 regional level 1 trauma center patients) and 52,369 (37,702 non-traumatic ED patients vs 14,667 regional level 1 trauma center patients), respectively. Throughout the study period, 1172 patients (551 patients during the control period vs 621 patients during the pandemic period) were admitted through the ED with pneumonia. Among them, we excluded 159 patients aged <18 years, 207 patients who were transferred to the ED from other hospitals due to worsening condition during inpatient treatment, 11 patients who visited the ED with initial cardiac arrest and were diagnosed with pneumonia, and 14 patients who were admitted with an initial primary diagnosis of pneumonia but whose final diagnosis at discharge was not pneumonia. Ultimately, 73 and 154 patients who were admitted to the ICU before and during the pandemic, respectively, were included in the study (Fig. 1).
3.1. Patient characteristics according to ED visit period
Table 1 shows the comparison of baseline characteristics over the 2 periods. There were no differences between patients with severe pneumonia admitted to the ICU through the ED over the 2 periods in median values of age, ED first antibiotics administration time, hospital day, and ICU admission day, rates of sex, comorbidities, polypharmacy, presenting symptoms, radiologic results, and in-hospital procedures. No differences were observed over the 2 periods in severity and mortality prediction scores, rate of ED triage acuity, mean pneumonia severity index, and median values of CURB-65, APACE II, and sequential organ failure assessment score at the ED and ICU. Compared to that during the control period, the in-hospital mortality rate during the pandemic increased by 10.4% (35.1% from 24.7%), but this was not statistically significant (P = .155). Differences were observed in the median values of systolic blood pressure, diastolic blood pressure, mean arterial pressure, and saturation in patients with pneumonia admitted to the ICU through the ED during the pandemic (P < .05). The rates of residency prior to the ED and means of ED visits were different between the pandemic and control periods. The median values of all EDLOSs (total-, pre-, mid-, and post-EDLOS) during the pandemic increased significantly compared to those in the control period (Table 1).
Table 1.
Baseline patient characteristics according to ED visit periods.
| Variable | Before pandemic (Jan. 2019–Dec. 2019) (n = 73) |
During pandemic (Mar. 2020–Feb. 2021) (n = 154) |
P value |
|---|---|---|---|
| Age (yr) | 78 [66–83] | 77 [67–84] | .957 |
| Sex | .980 | ||
| Male | 44 (60.3) | 91 (59.1) | |
| Female | 29 (39.7) | 63 (40.9) | |
| Initial vital signs | |||
| Glasgow coma scale | 15 [13–15] | 15 [14–15] | .950 |
| Systolic blood pressure (mm Hg) | 110 [89–131] | 124 [103–149] | .001* |
| Diastolic blood pressure (mm Hg) | 64 [51–80] | 73 [60–89] | .008* |
| Mean arterial pressure | 81.2 ± 22.4 | 91.4 ± 23.7 | .002* |
| Heart rates (bpm) | 109.5 ± 24.3 | 102.9 ± 26.6 | .075 |
| Respirator rates (bpm) | 22 [20–26] | 22 [20–25] | .074 |
| Body temperature (℃) | 37.1 [36.1–37.9] | 36.9 [36.2–37.6] | .584 |
| Saturation (%) | 90.0 [80.5–95.0] | 92.5 [88.0–97.0] | .011* |
| Comorbidities | |||
| Hypertension | 36 (49.3) | 71 (46.1) | .756 |
| Diabetes mellitus | 23 (31.5) | 55 (35.7) | .636 |
| Tuberculosis | 1 (1.4) | 8 (5.2) | .310 |
| Liver disease | 5 (6.8) | 9 (5.8) | 1.000 |
| Myocardial infarction | 5 (6.8) | 10 (6.5) | 1.000 |
| Congestive heart failure | 14 (19.2) | 33 (21.4) | .829 |
| Cerebrovascular disease | 18 (24.7) | 29 (18.8) | .403 |
| Chronic obstructive pulmonary disease | 5 (6.8) | 10 (6.5) | 1.000 |
| Asthma | 4 (5.5) | 8 (5.2) | 1.000 |
| Chronic renal disease | 9 (12.3) | 17 (11.0) | .951 |
| Active neoplastic disease | 1 (1.4) | 8 (5.2) | .310 |
| Dementia | 4 (5.5) | 10 (6.5) | .999 |
| Polypharmacy (≥5 medications) | 30 (41.1) | 69 (44.8) | .702 |
| Residency prior to ED | <.001* | ||
| Home | 39 (53.4) | 112 (72.7) | |
| Nursing home | 16 (21.9) | 35 (22.7) | |
| Other hospital | 18 (24.7) | 7 (4.5) | |
| Means of ED visit | <.001* | ||
| On foot | 29 (39.7) | 46 (29.9) | |
| EMS (119) ambulances | 20 (27.4) | 89 (57.8) | |
| Other ambulance | 24 (32.9) | 19 (12.3) | |
| Presenting symptoms | |||
| Mental change | 13 (17.8) | 32 (20.8) | .729 |
| General weakness | 19 (26.0) | 26 (16.9) | .151 |
| Poor oral intake | 2 (2.7) | 7 (4.5) | .774 |
| Dyspnea | 41 (56.2) | 75 (48.7) | .364 |
| Chest discomfort | 7 (9.6) | 8 (5.2) | .338 |
| Fever | 14 (19.2) | 30 (19.5) | 1.000 |
| Cough | 12 (16.4) | 24 (15.6) | 1.000 |
| Sputum | 13 (17.8) | 22 (14.3) | .624 |
| Rhinorrhea | 7 (9.6) | 9 (5.8) | .452 |
| Laboratory results | |||
| Arterial pH | 7.4 [7.3–7.4] | 7.4 [7.3–7.5] | .241 |
| PaO2 (mm Hg) | 56.0 [45.0–66.0] | 63.0 [50.0–92.5] | .006* |
| PaCO2 (mm Hg) | 35.0 [30.5–41.5] | 37.0 [30.0–45.0] | .236 |
| HCO3- (mEq/L) | 23.1 [19.6–26.6] | 22.8 [18.2–27.4] | .969 |
| White blood cell (cells/µL) | 10.4 [7.3–14.3] | 12.3 [8.1–16.7] | .080 |
| Hemoglobin (g/dL) | 11.6 [10.3–13.6] | 11.9 [10.3–13.7] | .799 |
| Hematocrit (%) | 35.0 [31.2–41.1] | 37.5 [32.3–43.0] | .107 |
| Platelets (×103cell/µL) | 200 [153–288] | 224 [146–295] | .499 |
| Sodium (mEq/L) | 135 [131–138] | 134 [130–137] | .176 |
| Potassium (mEq/L) | 4.0 [3.6–4.7] | 4.0 [3.6–4.6] | .681 |
| Creatinine (mg/dL) | 1.3 [0.8–2.0] | 1.1 [0.7–1.9] | .191 |
| Bilirubin (mg/dL) | 0.8 [0.5–1.1] | 0.7 [0.4–1.0] | .060 |
| Albumin (g/dL) | 3.6 ± 0.6 | 3.6 ± 0.7 | .639 |
| Blood urea nitrogen (mg/dL) | 21.0 [14.0–37.0] | 22.5 [13.0–36.0] | .738 |
| Blood glucose (mg/dL) | 159 [113–195] | 143 [113–190] | .550 |
| C-reactive protein (mg/L) | 13.5 [6.8–23.9] | 5.8 [1.2–16.3] | <.001* |
| Blood culture | 7 (9.6) | 15 (9.7) | 1.000 |
| Sputum culture | 5 (6.8) | 6 (3.9) | .531 |
| Radiologic results | |||
| Infiltration on chest X-ray | 62 (84.9) | 126 (81.8) | .695 |
| Effusion on chest X-ray | 18 (24.7) | 44 (28.6) | .646 |
| Bilateral involvement on chest CT | 58 (79.5) | 125 (81.2) | .900 |
| Multi-lobar involvement on chest CT | 53 (72.6) | 99 (64.3) | .274 |
| ED first antibiotics administration (min) | 187.0 [146.0–247.5] | 208.0 [165.0–273.0] | .069 |
| EDLOS | |||
| Pre-EDLOS (min) | 0.0 [0.0–0.0] | 0.0 [0.0–14.0] | <.001* |
| Mid-EDLOS (h) | 3.0 [2.3–3.9] | 3.4 [2.8–4.3] | .011* |
| Post-EDLOS (h) | 1.2 [0.9–3.5] | 3.5 [1.7–6.1] | <.001* |
| Total-EDLOS (h) | 4.8 [3.7–7.2] | 8.2 [6.2–10.1] | <.001* |
| Hospital days | 17 [8–27] | 14 [9–21] | .330 |
| ICU admission days | 6 [3–14] | 4 [2–10] | .098 |
| In-hospital mortality | .155 | ||
| Alive | 55 (75.3) | 100 (64.9) | |
| Death | 18 (24.7) | 54 (35.1) | |
| Triage acuity | .397 | ||
| 1 (Resuscitation) | 20 (27.4) | 36 (23.4) | |
| 2 (Emergent) | 22 (30.1) | 56 (36.4) | |
| 3 (Urgent) | 28 (38.4) | 47 (30.5) | |
| 4 (Less urgent) | 3 (4.1) | 13 (8.4) | |
| 5 (No urgent) | 0 (0) | 2 (1.3) | |
| Pneumonia severity index | 123.0 ± 35.8 | 125.7 ± 33.3 | .597 |
| CURB-65 score | 2 [1–3] | 2 [1–3] | .155 |
| APACHE II score at ED | 15 [13–20] | 16 [12–19] | .719 |
| APACHE II score at ICU | 19 [16–22] | 19 [15–22] | .734 |
| SOFA score at ED | 4 [2–5] | 4 [2–5] | .329 |
| SOFA score at ICU | 6 [4–8] | 5 [3–8] | .095 |
| Procedures at or after ED | |||
| Intubation | 35 (47.9) | 65 (42.2) | .503 |
| Central vein catheterization | 36 (49.3) | 61 (39.6) | .216 |
| Inotrope | 47 (64.4) | 89 (57.8) | .423 |
| Hemodialysis | 1 (1.4) | 10 (6.5) | .178 |
APACHE = acute physiology and chronic health evaluation, CT = computed tomography, ED = emergency department, EDLOS = emergency department length of stay, EMS = emergency medical services, ICU = intensive care unit, SOFA = sequential organ failure assessment.
P value <.05.
3.2. Comparison of EDLOS by ED visit period and survival status
Separate comparison of the EDLOSs of patients with pneumonia admitted to the ICU through the ED throughout the study period according to the ED visit period and survival status revealed that during the pandemic period of March 2020 to February 2021, the median values of mid-, post-, and total-EDLOS were longer in the death patient group, and there was no difference in the median value of pre-EDLOS between the survival and death groups. During the 2019 control period, the median values of post- and total-EDLOS were longer in the death group, but there were no differences in the median values of pre- and mid-EDLOS between the 2 groups. The median value of mid-EDLOS was significantly different in the death patient group only during the COVID-19 pandemic compared to the control periods (Table 2).
Table 2.
Comparison of EDLOS by ED visit period and survival status.
| Study population EDLOS by ED visit period | |||
|---|---|---|---|
| Before pandemic (Jan. 2019–Dec. 2019) (n = 73) |
During pandemic (Mar. 2020–Feb. 2021) (n = 154) |
P value | |
| Pre-EDLOS (min) | 0.0 [0.0–0.0] | 0.0 [0.0–14.0] | <.001 |
| Mid-EDLOS (min) | 177.0 [137.0–232.0] | 207.0 [165.0–259.0] | .011 |
| Post-EDLOS (min) | 75.0 [55.0–230.0] | 213.0 [100.0–368.0] | <.001 |
| Total-EDLOS (min) | 286.8 [219.6–434.0] | 493.5 [369.0–604.2] | <.001 |
| Study population EDLOS by survival status | |||
| Alive discharge (n = 155) |
Death (n = 72) |
P value | |
| Pre-EDLOS (min) | 0.0 [0.0–9.5] | 0.0 [0.0–7.0] | .556 |
| Mid-EDLOS (min) | 180.0 [136.5–248.0] | 219.5 [185.0–260.5] | <.001 |
| Post-EDLOS (min) | 100.0 [75.0–183.5] | 334.0 [254.0–490.0] | <.001 |
| Total-EDLOS (min) | 366.0 [285.9–475.8] | 593.4 [495.6–735.9] | <.001 |
| 2019 (before pandemic period) patients EDLOS by survival status | |||
| Alive discharge (n = 55) |
Death (n = 18) |
P value | |
| Pre-EDLOS (min) | 0.0 [0.0–0.0] | 0.0 [0.0–0.0] | .092 |
| Mid-EDLOS (min) | 179.0 [135.0–257.5] | 173.0 [164.0–196.0] | .868 |
| Post-EDLOS (min) | 64.0 [52.0–121.5] | 323.0 [235.0–990.0] | <.001 |
| Total-EDLOS (min) | 267.0 [209.9–365.7] | 553.8 [286.2–1203.0] | <.001 |
| Mar. 2020–Feb. 2021 (during pandemic period) patients EDLOS by survival status | |||
| Alive discharge (n = 100) |
Death (n = 54) |
P value | |
| Pre-EDLOS (min) | 0.0 [0.0–16.5] | 0.0 [0.0–10.0] | .452 |
| Mid-EDLOS (min) | 190.5 [138.5–248.0] | 240.5 [203.0–275.0] | <.001 |
| Post-EDLOS (min) | 119.0 [92.0–220.0] | 342.5 [263.0–450.0] | <.001 |
| Total-EDLOS (min) | 399.6 [335.1–532.2] | 601.8 [512.0–730.8] | <.001 |
ED = emergency department, EDLOS = emergency department length of stay.
3.3. Independent risk factors for in-hospital mortality of patients with severe pneumonia admitted to the ICU through the ED according to the ED visit period
The risk factors of in-hospital mortality in the study population and patients classified according to the ED visit period identified using univariate logistic regression analysis are presented in Table 3.
Table 3.
Univariate logistic regression analysis of in-hospital mortality risk factors in patients with pneumonia admitted to the ICU through the ED.
| Odds ratio | 95% confidence interval | P value | |
|---|---|---|---|
| Study population | |||
| Mid-EDLOS (h) | 1.471 | 1.166–1.855 | .001 |
| Post-EDLOS (h) | 1.316 | 1.183–1.465 | <.001 |
| Active neoplastic disease (yes) | 6.955 | 1.368–35.358 | .019 |
| Blood glucose | 0.995 | 0.991–1.000 | .036 |
| Albumin | 0.292 | 0.177–0.480 | <.001 |
| C-reactive protein | 1.037 | 1.011–1.065 | .006 |
| Infiltration on chest X-ray | 2.416 | 1.011–5.774 | .047 |
| Multi-lobar involvement on chest CT | 2.151 | 1.131–4.093 | .020 |
| Intubation (yes) | 5.796 | 3.124–10.752 | <.001 |
| Central vein catheterization (yes) | 3.301 | 1.845–5.908 | <.001 |
| Inotrope (yes) | 16.701 | 6.380–43.718 | <.001 |
| Pneumonia severity index | 1.011 | 1.003–1.020 | .011 |
| APACHE II score at ED | 1.057 | 1.003–1.113 | .038 |
| APACHE II score at ICU admission | 1.084 | 1.032–1.138 | .001 |
| SOFA score at ICU admission | 1.179 | 1.082–1.284 | <.001 |
| 2019 (before pandemic) patients | |||
| Post-EDLOS (h) | 2.007 | 1.348–2.988 | .001 |
| Liver disease (yes) | 15.429 | 1.596–149.157 | .018 |
| COPD (yes) | 15.429 | 1.596–149.157 | .018 |
| Albumin | 0.347 | 0.138–0.876 | .025 |
| Intubation (yes) | 8.750 | 2.255–33.952 | .002 |
| Central vein catheterization (yes) | 3.617 | 1.131–11.565 | .030 |
| Inotrope (yes) | 14.167 | 1.760–114.017 | .013 |
| SOFA score at ICU admission | 1.169 | 1.012–1.351 | .034 |
| 2020 (during pandemic) patients | |||
| Mid-EDLOS (h) | 1.719 | 1.278–2.312 | <.001 |
| Post-EDLOS (h) | 1.202 | 1.080–1.338 | .001 |
| Active neoplastic disease (yes) | 6.125 | 1.192–31.485 | .030 |
| Albumin | 0.264 | 0.145–0.483 | <.001 |
| C-reactive protein | 1.050 | 1.014–1.088 | .007 |
| Multi-lobar involvement on chest CT | 2.284 | 1.090–4.784 | .029 |
| Intubation (yes) | 5.597 | 2.721–11.513 | <.001 |
| Central vein catheterization (yes) | 3.561 | 1.780–7.126 | <.001 |
| Inotrope (yes) | 19.551 | 6.542–58.433 | <.001 |
| Pneumonia severity index | 1.010 | 1.000–1.021 | .049 |
| APACHE II score at ED | 1.068 | 1.003–1.137 | .041 |
| APACHE II score at ICU admission | 1.095 | 1.030–1.163 | .003 |
| SOFA score at ICU admission | 1.214 | 1.087–1.356 | .001 |
APACHE = acute physiology and chronic health evaluation, CT = computed tomography, ED = emergency department, EDLOS = emergency department length of stay, ICU = intensive care unit, SOFA = sequential organ failure assessment.
3.3.1. Independent risk factors of in-hospital mortality for the entire study population.
Multivariate logistic regression analysis (receiver operating characteristic [ROC] area under the curve [AUC] for the prediction model = 0.891, P < .001) revealed post-EDLOS (hours) (OR: 1.233, 95% CI: 1.106–1.374, P < .001), serum albumin (OR: 0.360, 95% CI: 0.171–0.757, P = .007), endo-tracheal intubation (OR: 4.884, 95% CI: 1.970–12.109, P = .001), and inotropic use (OR: 9.371, 95% CI: 2.352–37.339, P = .002) as risk factors for in-hospital mortality of patients with pneumonia admitted to the ICU through the ED (Table 4).
Table 4.
Multivariate logistic regression analysis of in-hospital mortality risk factors in patients with pneumonia admitted to the ICU through the ED.
| Odds ratio | 95% confidence interval | P value | |
|---|---|---|---|
| Study population | |||
| Post-EDLOS (h) | 1.233 | 1.106–1.374 | <.001 |
| Albumin | 0.360 | 0.171–0.757 | .007 |
| Intubation (yes) | 4.884 | 1.970–12.109 | .001 |
| Inotrope (yes) | 9.371 | 2.352–37.339 | .002 |
| 2019 (before pandemic) patients | |||
| Post-EDLOS (h) | 2.282 | 1.367–3.807 | .002 |
| C-reactive protein | 1.122 | 1.015–1.241 | .024 |
| Albumin | 0.067 | 0.009–0.523 | .010 |
| 2020 (during pandemic) patients | |||
| Mid-EDLOS (h) | 1.835 | 1.089–3.092 | .023 |
| Post-EDLOS (h) | 1.126 | 1.002–1.266 | .047 |
| Albumin | 0.357 | 0.133–0.958 | .041 |
| Intubation (yes) | 8.811 | 2.614–26.692 | <.001 |
| Inotrope (yes) | 11.752 | 2.217–62.298 | .004 |
ED = emergency department, EDLOS = emergency department length of stay, ICU = intensive care unit.
3.3.2. Independent risk factors for in-hospital mortality in patients with severe pneumonia admitted to the ICU through the ED before the COVID-19 pandemic.
Multivariate analysis (ROC AUC for the prediction model = 0.935, P < .001) revealed post-EDLOS (hours) (OR: 2.282, 95% CI: 1.367–3.807, P = .002), serum C-reactive protein (OR: 1.122, 95% CI: 1.015–1.241, P = .024), and serum albumin (OR: 0.067, 95% CI: 0.009–0.523, P = .010) as risk factors for in-hospital mortality before the COVID-19 pandemic (Table 4).
3.3.3. Independent risk factors for in-hospital mortality in patients with severe pneumonia admitted to the ICU through the ED during the COVID-19 pandemic.
Multivariate analysis (ROC AUC for the prediction model = 0.905, P < .001) revealed mid-EDLOS (hours) (OR: 1.835, 95% CI: 1.089–3.092, P = .023), post-EDLOS (hours) (OR: 1.126, 95% CI: 1.002–1.266, P = .047), serum-albumin (OR: 0.357, 95% CI: 0.133–0.958, P = .041), endo-tracheal intubation (OR: 8.811, 95% CI: 2.614–26.692, P < .001), and inotropic use (OR: 11.752, 95% CI: 2.217–62.298, P = .004) as risk factors for in-hospital mortality during the COVID-19 pandemic (Table 4).
4. Discussion
We assessed the relationship between the EDLOS and outcome in severe pneumonia patients who admitted to the ICU through the ED before and during the emerging respiratory infectious disease, COVID-19 pandemic. The pre-, mid-, post-, and total-EDLOS of the group of patients admitted during the pandemic were significantly longer than those of patients admitted before the pandemic. Multivariate logistic regression showed that the post-EDLOS was an independent risk factor for in-hospital mortality for both the before and during COVID-19 pandemic groups. Unlike the before-pandemic severe pneumonia patients, mid-EDLOS was an additional independent risk factor for death in patients with severe pneumonia during the pandemic period.
Before the COVID-19 pandemic, isolation was unnecessary except for certain infectious diseases, such as active pulmonary tuberculosis. Moreover, even if the disease was contagious, it was relatively easy to prevent infections by implementing mask-wearing or adjusting the distance between patients. Therefore, there were few restrictions on visiting the ED and hospitalization for patients who presented with fever and respiratory symptoms that could be pneumonia. In addition, if the condition of patients with pneumonia worsened, there were few restrictions on transfer between hospitals. Our ED patient volume during the COVID-19 pandemic decreased from 52,369 to 35,561, in line with previous studies.[18,21–24] However, during the same period, the number of patients hospitalized to the general ward for pneumonia through the ED increased from 248 to 306, and the number of patients admitted to the ICU increased from 73 to 154. We attributed this change to the avoidance of patients with fever, respiratory symptoms, or dyspnea in small and medium-sized hospitals, where securing IRs is difficult, and to the concentration of these patients in tertiary hospitals. In particular, visits to tertiary hospitals for critically ill patients had been concentrated. During the COVID-19 pandemic, after being diagnosed with pneumonia at other clinics or hospitals, the rate of patients who were transferred to our ED and admitted to the ICU decreased from 24.7% to 4.5%. This was attributed to the shortage of IRs in the ED and lack of inpatient isolation beds. Indeed, our ED may have accommodated fewer pneumonia patients diagnosed in other hospitals compared to those before the COVID-19 pandemic. During the control period, the rates of mean ED visits via on foot (39.7% vs 29.9%) and ambulances (32.9% vs 12.3%) were relatively high. During the control period, 27.4% (n = 20) of patients with severe pneumonia admitted to the ICU through our ED visited via emergency medical services (EMS), whereas during the COVID-19 pandemic, 57.8% (n = 89) of all patients visited our ED via EMS. This highlights an increase in ED inflow through EMS in patients with severe pneumonia requiring ICU care during the COVID-19 pandemic. This result is consistent with previous findings that the increase in EMS calls and corresponding dispatches during the pandemic is an indicator of the increase in COVID-19-related patients requiring ICU care.[25] For other reasons, we proposed that since increased need for preemptive isolation for emergency treatment of patients with fever, respiratory symptoms, or dyspnea, more ED visits occurred through EMS, which may have afforded easier access to real-time information concerning isolation beds using the national emergency medical information system rather than individually. In this regard, previous studies have reported that EMS calls and dispatches increased during the COVID-19 pandemic.[26,27]
In most previous studies on EDLOS, the reasons for the increased EDLOS included a shortage of available ICU beds, followed by an increased number of critically ill patients and delayed decisions for ICU admission in the ED.[15,16,28] In particular, insufficient beds in the ICU are a contributing factor to an increase in post-EDLOS. Comparison of pre- and post-COVID-19 pandemic periods revealed that the median post-EDLOS of patients with severe pneumonia requiring intensive care increased significantly from 75 to 213 min. This result can be attributed to the effect of a more limited number of ICU beds (9 isolated beds), accelerated by the need for isolation after the COVID-19 pandemic, compared to the control period when there were no limits on the ICU beds for patients with pneumonia. In Korea, it was only recently (March 2022) that the number of confirmed cases exceeded PCR testing capacity, and expert rapid antigen tests were finally recognized as a confirmed test for COVID-19. During the pandemic period included in our study (March 2020 to February 2021), the only confirmed test recognized by the Korea Centers for Disease Control and Prevention was the real-time RT-PCR test. If ICU isolation beds were empty, patients could be admitted immediately without assessing the PCR test results. However, if isolation beds were full, the post-EDLOS may have been longer due to the need to wait for PCR results in the ED. As the pandemic continued, a rapid PCR test (XpertⓇ Xpress SARS-CoV-2 assay) was introduced in our ED. However, we conjecture that the rapid PCR test was predominantly used for emergency surgical patients and was not actively used in patients with pneumonia. Thus, it did not contribute substantially to reducing post-EDLOS. Regardless of the timing of ED visits, post-EDLOS was a risk factor for in-hospital mortality in all patients with severe pneumonia and did not exhibit any differences between patients throughout the study period. The delay in ICU admission of patients with severe pneumonia in the ED partly underpinned the increase in in-hospital mortality because the ED has a lower proportion of doctors and nurses per patient compared to the ICU and is not well equipped for intensive care.[16] In addition, ED staff tend to focus more on the initial evaluation and treatment of new emergency patients, which could result in a continuous decrease in optimal care owing to less interest in critically ill patients who have already been decided ICU admission.[28]
In this study, the biggest difference among in-hospital mortality risk factors in patients with severe pneumonia admitted to the ICU through the ED during the COVID-19 pandemic was that mid-EDLOS was identified as a risk factor for in-hospital mortality. In the control period, mid-EDLOS was not significantly different between surviving and deceased patients. However, during the pandemic, there was a significant prolongation for deceased patients (median = 240.5 min) compared to survivors (median = 190.5 min). Mid-EDLOS is affected by examination equipment, ED staff manpower for diagnosis and treatment, and transport personnel manpower for patient movement.[29] Mid-EDLOS delay during the pandemic was inevitable, especially for patients with fever, respiratory symptoms, or dyspnea during the initial assessment and care because of the need for blood sampling for ED basic tests, additional COVID-19 PCR tests, and diagnostic imaging tests (portable X-ray in IRs, chest computed tomography, etc). This was at least partly attributed to the time needed for medical personnel to wear personal protective equipment, the disinfection of inspection equipment and space, and the requirement to secure a mobile line within the hospital to avoid overlap with patients in the ED with general disease who were less likely to be infected with COVID-19.
The pre-EDLOS also increased during the COVID-19 pandemic. However, there was no significant difference in the comparison according to the survival status during each period, and it was not an in-hospital mortality risk factor of patients with severe pneumonia admitted to the ICU through the ED. We think that this is the result of flexible use of the cardiopulmonary resuscitation room (capable of maintaining negative pressure), which is usually reserved for emergency patients, such as those with cardiac arrest, or critically ill patients who need immediate care, such as those with unstable vital signs, severe hypoxia or change of consciousness, even during the pandemic with insufficient isolation beds.
A growth in infectious diseases has occurred over the past few decades, affecting the overall health care system, including the ED environment.[30–32] The fight against COVID-19 has afforded many scientific lessons, such as the need for rapid vaccine development and establishment of effective quarantine policies. However, even after overcoming COVID-19, the threat of emerging infectious diseases still remains. Accordingly, we emphasize the need to continuously change and adapt the ED environment in preparation for new infectious disease outbreaks through research on emergency medical care systems based on the current pandemic. Specifically, we propose that efforts are warranted to prepare for future challenges, such as temporary plans to expand ED isolation beds during the pandemic, and develop protocols for the efficient use of limited isolation beds and utilization of emergency medical personnel and equipment to minimize EDLOS delay.
This study had several limitations. First, it was a single-center study with a relatively small sample size because we only included patients with severe pneumonia admitted to the ICU through our ED who could be traced until discharge and death. Thus, the findings may not be generalizable to other medical institutions with different environments. External validation is needed by performing multicenter studies of large cohorts. Second, there were inherent limitations concerning the selection bias because it was a retrospective study. We reviewed medical records of patients, and inappropriate data entry may have occurred. Third, the final clinical outcomes of patients who refused active treatment and were finally admitted to the general ward despite ED medical staff recommendations and those of patients who chose to terminate treatment and were discharged against medical advice may have been excluded. In addition, we did not include the final clinical outcomes of patients transferred to other hospitals due to the lack of ICU beds while waiting in our ED. Fourth, there is a possibility of bias due to the lack of comparison with factors, such as waiting time at other hospitals before ED visits, EMS transfer time, and distance.
5. Conclusions
Both before and during the COVID-19 pandemic, the post-EDLOS delay (ED waiting time after a decision to be admitted due to the lack of ICU beds) was a common risk factor for in-hospital mortality in patients with severe pneumonia. However, we also identified mid-EDLOS delay (ED time to assess, care, and ICU admission decision) as a risk factor for in-hospital mortality in patients with severe pneumonia during the COVID-19 pandemic. In the midst of pandemics of emerging respiratory infectious diseases, in order to reduce in-hospital mortality of severe pneumonia patients, it is necessary to shorten the ED waiting time for admission by increasing the number of isolation ICU beds. It is also necessary to accelerate the assessment and care process in the ED, and make prompt decisions regarding admission to the ICU.
Author contributions
Conceptualization: Won Young Sung.
Formal analysis: Won Young Sung.
Investigation: Jun Young Ha.
Methodology: Won Young Sung.
Project administration: Jun Young Ha.
Supervision: Won Young Sung.
Writing – original draft: Jun Young Ha, Won Young Sung.
Writing – review & editing: Jun Young Ha, Won Young Sung.
Abbreviations:
- CI =
- confidence interval
- ED =
- emergency department
- EDLOS =
- emergency department length of stay
- EMS =
- emergency medical services
- ICD-10 =
- International Statistical Classification of Diseases and Related Health Problems 10th Revision
- ICU =
- intensive care unit
- IR =
- isolation room
- OR =
- odds ratio
- PCR =
- polymerase chain reaction
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
The authors received no financial support for the research, authorship, and publication of this article.
Supplemental Digital Content is available for this article.
The authors have no conflicts of interest to disclose.
The need to obtain informed consent was waived due to the retrospective design of the study. The consent waiver was approved by the Institutional Review Board of Daejeon Eulji University Hospital (EMC 2021-10-009).
This study was approved by the Institutional Review Board of Daejeon Eulji University Hospital (EMC 2021-10-009).
How to cite this article: Ha JY, Sung WY. Impact of COVID-19 pandemic on emergency department length of stay and clinical outcomes of patients with severe pneumonia: A single-center observational study. Medicine 2022;101:38(e30633).
Contributor Information
Jun Young Ha, Email: 20210687@eulji.ac.kr.
Won Young Sung, Email: sage77@hanmail.net;sage77@eulji.ac.kr.
References
- [1].World Health Organization. Coronavirus disease (COVID-19): situation report-51. Available at: http://apps.who.int/iris/bitstream/handle/10665/331475/nCoVsitrep11Mar2020-eng.pdf?sequence=1&isAllowed=y. [Access date March 14, 2022].
- [2].Haleem A, Javaid M, Vaishya R. Effects of COVID-19 pandemic in daily life. Curr Med Res Pract. 2020;10:78–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Rahman S, Montero MTV, Rowe K, et al. Epidemiology, pathogenesis, clinical presentations, diagnosis and treatment of COVID-19: a review of current evidence. Expert Rev Clin Pharmacol. 2021;14:601–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zhai P, Ding Y, Wu X, et al. The epidemiology, diagnosis and treatment of COVID-19. Int J Antimicrob Agents. 2020;55:105955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kim SW, Lee KS, Kim K, et al. A brief telephone severity scoring system and therapeutic living centers solved acute hospital-bed shortage during the COVID-19 outbreak in Daegu, Korea. J Korean Med Sci. 2020;35:e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kim A. Korea marks single-day record for COVID-19 deaths. The Korea Herald. December 15, 2020. Available at: http://www.koreaherald.com/view.php?ud=20201215000860. [Access date March 14, 2022].
- [7].Di Somma S, Paladino L, Vaughan L, et al. Overcrowding in emergency department: an international issue. Intern Emerg Med. 2015;10:171–5. [DOI] [PubMed] [Google Scholar]
- [8].Tekwani KL, Kerem Y, Mistry CD, et al. Emergency department crowding is associated with reduced satisfaction scores in patients discharged from the emergency department. West J Emerg Med. 2013;14:11–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Liu SW, Chang Y, Weissman JS, et al. An empirical assessment of boarding and quality of care: delays in care among chest pain, pneumonia, and cellulitis patients. Acad Emerg Med. 2011;18:1339–48. [DOI] [PubMed] [Google Scholar]
- [10].Hwang U, Richardson L, Livote E, et al. Emergency department crowding and decreased quality of pain care. Acad Emerg Med. 2008;15:1248–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gaieski DF, Agarwal AK, Mikkelsen ME, et al. The impact of ED crowding on early interventions and mortality in patients with severe sepsis. Am J Emerg Med. 2017;35:953–60. [DOI] [PubMed] [Google Scholar]
- [12].Kulstad EB, Kelley KM. Overcrowding is associated with delays in percutaneous coronary intervention for acute myocardial infarction. Int J Emerg Med. 2009;2:149–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Richardson D, McMahon KL. Emergency department access block occupancy predicts delay to surgery in patients with fractured neck of femur. Emerg Med Australas. 2009;21:304–8. [DOI] [PubMed] [Google Scholar]
- [14].Liew D, Liew D, Kennedy MP. Emergency department length of stay independently predicts excess inpatient length of stay. Med J Aust. 2003;179:524–6. [DOI] [PubMed] [Google Scholar]
- [15].Hung SC, Kung CT, Hung CW, et al. Determining delayed admission to intensive care unit for mechanically ventilated patients in the emergency department. Crit Care. 2014;18:485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zhang Z, Bokhari F, Guo Y, et al. Prolonged length of stay in the emergency department and increased risk of hospital mortality in patients with sepsis requiring ICU admission. Emerg Med J. 2019;36:82–7. [DOI] [PubMed] [Google Scholar]
- [17].Boudi Z, Lauque D, Alsabri M, et al. Association between boarding in the emergency department and in-hospital mortality: a systematic review. PLoS One. 2020;15:e0231253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lucero A, Sokol K, Hyun J, et al. Worsening of emergency department length of stay during the COVID-19 pandemic. J Am Coll Emerg Physicians Open. 2021;2:e12489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].O’Reilly GM, Mitchell RD, Mitra B, et al. Impact of patient isolation on emergency department length of stay: a retrospective cohort study using the Registry for Emergency Care. Emerg Med Australas. 2020;32:1034–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Xiong X, Wai AKC, Wong JYH, et al. Impact of varying wave periods of COVID-19 on in-hospital mortality and length of stay for admission through emergency department: a territory-wide observational cohort study. Inf Other Respir Viruses. 2022;16:193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Boserup B, McKenney M, Elkbuli A. The impact of the COVID-19 pandemic on emergency department visits and patient safety in the United States. Am J Emerg Med. 2020;38:1732–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Çikrikçi Işik G, Çevik Y. Impact of COVID-19 pandemic on visits of an urban emergency department. Am J Emerg Med. 2021;42:78–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lai YW, Hsu CT, Lee YT, et al. Analysis of COVID-19 pandemic impact on the presenting complaints of the emergency department visits. Medicine (Baltim). 2021;100:e28406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kim S, Ro YS, Ko SK, et al. The impact of COVID-19 on the patterns of emergency department visits among pediatric patients. Am J Emerg Med. 2022;54:196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].By the COVID-19 APHP-Universities-INRIA-INSERM Group. Early indicators of intensive care unit bed requirement during the COVID-19 epidemic: a retrospective study in Ile-de-France region, France. PLoS One. 2020;15:e0241406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Saberian P, Conovaloff JL, Vahidi E, et al. How the COVID-19 epidemic affected prehospital emergency medical services in Tehran, Iran. West J Emerg Med. 2020;21:110–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Al Amiry A, Maguire BJ. Emergency Medical Services (EMS) calls during COVID-19: early lessons learned for systems planning (a narrative review). Open Access Emerg Med. 2021;13:407–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Chalfin DB, Trzeciak S, Likourezos A, et al. DELAY-ED study group. Impact of delayed transfer of critically ill patients from the emergency department to the intensive care unit. Crit Care Med. 2007;35:1477–83. [DOI] [PubMed] [Google Scholar]
- [29].Asplin BR, Magid DJ, Rhodes KV, et al. A conceptual model of emergency department crowding. Ann Emerg Med. 2003;42:173–80. [DOI] [PubMed] [Google Scholar]
- [30].Huang CC, Yen DH, Huang HH, et al. Impact of severe acute respiratory syndrome (SARS) outbreaks on the use of emergency department medical resources. J Chin Med Assoc. 2005;68:254–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Costello BE, Simon HK, Massey R, et al. Pandemic H1N1 influenza in the pediatric emergency department: a comparison with previous seasonal influenza outbreaks. Ann Emerg Med. 2010;56:643–8. [DOI] [PubMed] [Google Scholar]
- [32].Paek SH, Kim DK, Lee JH, et al. The impact of middle east respiratory syndrome outbreak on trends in emergency department utilization patterns. J Korean Med Sci. 2017;32:1576–80. [DOI] [PMC free article] [PubMed] [Google Scholar]