Background:
Studies using animal experiments have shown secondary neuronal degeneration in the thalamus after cerebral infarction. Neuroimaging studies in humans have revealed changes in imaging parameters in the thalamus, remote to the infarction. However, few studies have directly demonstrated neuronal changes in the thalamus in vivo. The purpose of this study was to determine whether secondary thalamic neuronal damage may manifest as a decrease in central benzodiazepine receptors in patients with cerebral infarction and internal carotid artery or middle cerebral artery disease.
Methods:
We retrospectively analyzed the data of 140 patients with unilateral cerebral infarction ipsilateral to internal carotid artery or middle cerebral artery disease. All patients had quantitative measurements of 11C-flumazenil binding potential (FMZ-BP), cerebral blood flow, and cerebral metabolic rate of oxygen using positron emission tomography in the chronic stage. Region of interest analysis was performed using NeuroFlexer—an automated region of interest analysis software using NEUROSTAT.
Results:
In the thalamus ipsilateral to the infarcts, the values of FMZ-BP, cerebral blood flow, and cerebral metabolic rate of oxygen were significantly lower than those in the contralateral thalamus. Significant correlations were found between the ipsilateral-to-contralateral ratio of FMZ-BP and the ipsilateral-to-contralateral ratio of cerebral blood flow or cerebral metabolic rate of oxygen in the thalamus. Patients with corona radiata infarcts and striatocapsular infarcts had significantly decreased ipsilateral-to-contralateral FMZ-BP ratio in the thalamus compared with those without. The ipsilateral-to-contralateral ratio of FMZ-BP in the thalamus was significantly correlated with the ipsilateral-to-contralateral cerebral metabolic rate of oxygen ratio in the frontal cortex and showed a significant negative correlation with the number of perseverative errors on the Wisconsin Card Sorting Test.
Conclusions:
Secondary thalamic neuronal damage may manifest as a decrease in central benzodiazepine receptors in patients with cerebral infarction and internal carotid artery or middle cerebral artery disease, which may be associated with frontal lobe dysfunction.
Keywords: cerebral infarction; cerebrovascular disorders; positron emission tomography; receptors, GABA-A; thalamus
Focal cerebral infarction may cause delayed and selective neuronal death in nonischemic, remote brain areas that are connected with the primary lesion site.1,2 Studies using animal experiments have shown that in the aftermath of cerebral infarction, depending on its localization and extent, secondary neuronal damage in the thalamus may occur mainly due to retrograde degeneration, that is neuron destruction following axonal injury that spreads backward along the axon.3,4 In humans, after cerebral infarction in the anterior circulation, a decrease in cerebral blood flow (CBF) or oxygen or glucose metabolism in the ipsilateral thalamus has been demonstrated using positron emission tomography (PET), single-photon emission computed tomography, or computed tomography perfusion,5–8 which may reveal hypofunction in the thalamus due to early functional disconnection or late neurodegeneration. Magnetic resonance imaging (MRI) studies in patients with cerebral infarction sparing the thalamus have revealed changes in signal or diffusion in the thalamus ipsilateral to the infarction, suggesting microstructural tissue alterations with secondary neuronal degeneration.7,9–12 Furthermore, severe neurodegeneration due to large infarcts could cause thalamic atrophy in the long term, visible on computed tomography.13 Thus, several neuroimaging studies have detected secondary thalamic degeneration in humans by revealing changes in various imaging parameters. However, few studies have directly demonstrated neuronal changes in the thalamus in vivo in patients with a history of cerebral infarction. It is unclear whether a decrease in CBF or metabolism in the ipsilateral thalamus reflects functional changes only or involves neuronal degeneration.
The clinical impact of secondary thalamic degeneration due to cerebral infarction is not well understood.10,14,15 Recent MRI studies have shown that in the chronic phase, changes in diffusion or iron accumulation with secondary thalamic degeneration were associated with some cognitive alterations, specifically frontal lobe dysfunction, but the changes in iron accumulation with frontal lobe dysfunction were independent of infarct volume, which suggest that thalamic degeneration may reflect the degree of disruption in the thalamofrontal neuronal network independent of infarct volume.10,15 Quantitative evaluation of neuronal alterations with neuronal degeneration in the thalamus could improve detection of its clinical impact, specifically impairment in the test for frontal lobe function like the Wisconsin Card Sorting Test (WCST).
As most neurons express central benzodiazepine receptors (BZRs), specific imaging of these receptors has enabled the in vivo visualization of neuronal alterations induced by ischemia.16,17 Selective neuronal damage demonstrated as a decrease in BZRs can be quantitatively evaluated in humans using PET and 11C-flumazenil (11C-FMZ), which has been validated against immunohistochemistry in rodent models of cerebral infarction.18
This retrospective study aimed to determine whether secondary thalamic neuronal damage may manifest as a decrease in BZRs in patients with a history of cerebral infarction and internal carotid artery (ICA) or middle cerebral artery (MCA) disease and if so, whether it may be associated with decrease in CBF or metabolism in the thalamus, as well as frontal lobe hypometabolism or frontal lobe dysfunction assessed by the WCST.
Methods
Detailed Methods are available in the Supplemental Material. This article follows the STROBE reporting guideline (Strengthening the Reporting of Observational Studies in Epidemiology; https:www.goodreports.org). Additional data can be made available via the corresponding author to qualified researchers upon reasonable request.
Subjects
In this retrospective study, we used the data of an observational study on 261 patients, which investigated cerebral hemodynamics, metabolism, and BZR. These patients had atherosclerotic occlusion or stenosis of the ICA or MCA, who underwent PET with both 15O-gas and 11C-FMZ for the first time, between January 2003 and December 2013 (Figure S1). They were referred to our PET unit for hemodynamic evaluation as part of a clinical assessment to determine the need for vascular reconstructive surgery. Between August 2004 and December 2005, 81 consecutive patients underwent WCST, for neuropsychological assessments. To obtain a normal control database for the BZR imaging from the observational study, we enrolled 10 healthy control subjects (aged 57±7 years, including 7 men), who had no history of medical or psychiatric disorders and no history of benzodiazepine use.
The inclusion criteria for the present study were (1) unilateral occlusion or stenosis of the extracranial ICA (>60% diameter reduction according to the North American Symptomatic Carotid Endarterectomy Trial criteria19) or intracranial ICA or MCA (>50% diameter reduction according to the WASID criteria [Warfarin-Aspirin Symptomatic Intracranial Disease]20), as documented by conventional or magnetic resonance angiography; (2) unilateral, symptomatic or asymptomatic, cerebral infarction ipsilateral to the ICA or MCA diseases detectable on routine T1-weighted, T2-weighted, or fluid-attenuated inversion recovery MRI; and (3) for symptomatic patients, history of transient ischemic attack (TIA) or minor completed stroke in the ICA or MCA distributions. The exclusion criteria were (1) infarction in the thalamus, cerebral hemisphere contralateral to the arterial lesion or brain stem, detectable on routine MRI; (2) history of TIA or stroke in regions other than the relevant ICA or MCA territory; (3) contralateral ICA or MCA stenosis (>50%); (4) unilateral arterial disease with extensive white matter lesions in both hemispheres, likely caused by bilateral small vessel disease; (5) history of cerebrovascular reconstructive surgery; and (6) history of benzodiazepine use. Finally, 140 patients with unilateral cerebral infarction ipsilateral to atherosclerotic occlusion or stenosis of the ICA or MCA were included in the analysis (Table 1).
Table 1.
Characteristics of the Patients
The protocol for the observational study was approved by the Shiga General Hospital Institutional Review Board of the Human Study Committee (approval number 99). All participants provided written informed consent. All experiments were performed in accordance with the Declaration of Helsinki and the guidelines of Good Clinical Practice.
PET Measurements
PET scans were performed for each subject using an advance whole body scanner (General Electric Medical Systems, Milwaukee, WI).21 First, a series of 15O-gas studies was performed.21 C15O2 and 15O2 were continuously delivered via a mask throughout the 5-minute scan. Cerebral blood volume was measured by bolus inhalation of C15O and scanning for 3 minutes. The 15O-gas study was followed by a study of 11C-FMZ.22 After intravenous injection of 11C-FMZ, a 50-minute dynamic PET scan was initiated simultaneously with tracer administration.
The steady-state method was used to calculate CBF, cerebral metabolic rate of oxygen (CMRO2), and oxygen extraction fraction.23,24 Dynamic data and Logan graphical analysis with reference tissue was used to calculate the binding potential (nondisplaceable) of 11C-FMZ, using the time-activity curves obtained from the pons.22,25
Magnetic Resonance Imaging
MRIs were performed using a Signa unit (General Electric, Milwaukee, WI) operating at a field strength of 1.5T. The imaging protocol consisted of T2-weighted spin echo, T1-weighted spin echo, and fluid-attenuated inversion recovery imaging series. The slice thickness was 5 mm, and the intersection gap was 2.5 mm.
Cerebral infarctions were identified as high-intensity lesions on the fluid-attenuated inversion recovery images. They were classified as pial territory infarcts (infarcts in the frontal, parietal, temporal, or occipital corticosubcortical regions) and deep perforator territory infarcts (infarcts in the corona radiata, combined capsular and basal ganglia infarcts, or solitary basal ganglia infarcts).
The area (mm2) of the infarct on the slice was measured by contouring the high-intensity lesions using the Image J software (https://imagej.nih.gov). Volume of the lesion was calculated by the area multiplied by slice thickness (5 mm). The total volume of the infarcts (cm3) was calculated by summing the volumes for all lesions in the hemisphere with arterial disease.
On the slice with the largest area of thalamus, the area (mm2) was measured by contouring the thalamus on the fluid-attenuated inversion recovery images using the Image J software. When contouring the lateral limit of the thalamus, we referred the T1- and T2-weighted images.
One investigator who was blinded to the clinical status and other imaging data of the patients reviewed all scans.
Wisconsin Card Sorting Test
The WCST is one of the frequently used neuropsychological tests and is sensitive to frontal lobe dysfunction.26 In this study, we used the Keio-Fukuoka-Shimane version of the WCST that can be performed on a personal computer.27,28 The calculated indices for this study included categories achieved, total errors, and perseverative errors, as defined by Nelson.
Data Analysis
For the thalamus, the region of interest (ROI) analysis for 11C-FMZ binding potential (FMZ-BP), CBF, and CMRO2 parametric data was performed using NeuroFlexer29—an automated ROI analysis software using NEUROSTAT.30
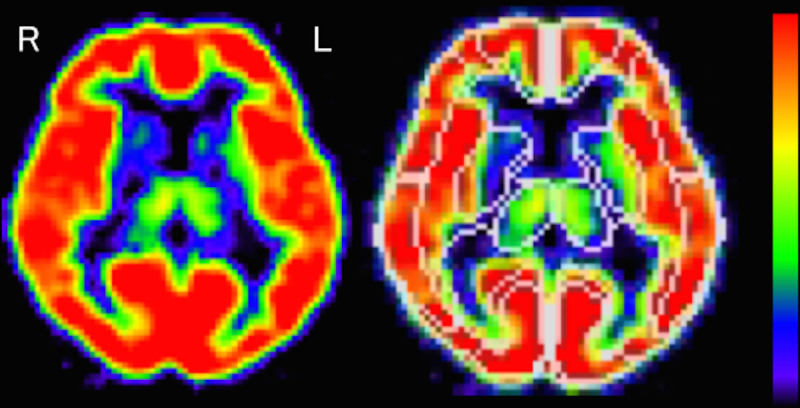
Individual PET images were anatomically standardized to obtain information on voxel transformation using NEUROSTAT.30 This information and inverse transformation were used to modify the VOI template to individual PET images. The inverse transformed VOI templates were extracted from each slice to establish the ROIs. The established ROIs were then set on the PET images (Figure 1).
Figure 1.
Regions of interest in the thalamus on flumazenil positron emission tomography images in a normal volunteer. L indicates left; and R, right.
For the cerebral cortex, a 3-dimensional stereotactic surface projection technique was used to analyze the FMZ-BP, CBF, and CMRO2 parametric data.30 Absolute values in the cerebral cortex of the frontal, parietal, temporal, and occipital lobes were calculated using the stereotactic extraction estimation method.31
We calculated the ipsilateral-to-contralateral ratio of the absolute values of FMZ-BP in the thalamus. The mean±SD values of the left-to-right or the right-to-left ratio of FMZ-BP for the thalamus in the 10 normal controls (7 men and 3 women), aged 57±7 years (mean±SD), were 1.006±0.052 and 0.996±0.053, respectively. Decrease in the ipsilateral-to-contralateral FMZ-BP ratio beyond the lower 95% limit (the mean minus 9t0.05SD) defined in normal subjects (<0.888 for left disease and 0.877 for right) was considered to be a decreased FMZ-BP ratio in the thalamus.
Statistical Analysis
Statistical analysis was performed using the StatView software (SAS Institute, Inc, Cary, NC). PET variable values were compared between groups using the Student t test. Relationships between variables were analyzed using simple or multiple regression analyses.
Hemispheric differences in PET parameters in the thalamus, frontal, parietal, temporal, and occipital cortices were measured using the Student t test. Statistical significance was accepted at P<0.0033 (0.05/15) using a Bonferroni correction. The correlations between ipsilateral-to-contralateral ratio of FMZ-BP and that of CBF, of CMRO2 or that of frontal, parietal, temporal, and occipital CMRO2 were analyzed by a simple regression analysis; significance was established at P<0.025 (0.05/2) and P<0.0125 (0.05/4), respectively. We compared the values of ipsilateral-to-contralateral FMZ-BP ratio between patients with and without 7 locations of cerebral infarction using the Student t test; statistical significance was accepted at P<0.007 (0.05/7). Multiple linear regression analysis (forward stepwise selection) was used to assess the independent predictive value of the location of cerebral infarction with respect to the values of ipsilateral-to-contralateral FMZ-BP ratio. Statistical significance was set at P<0.05. The correlations between ipsilateral-to-contralateral ratio of FMZ-BP and 4 indices of WCST were analyzed by a simple regression analysis; significance was established at P<0.0125 (0.05/4).
Results
Patient Sample
The patients included 106 men and 34 women aged 47 to 85 years (mean±SD, 65±8 years; Table 1). Of the 140 enrolled patients with infarctions, 21 were asymptomatic, 27 had a history of TIA, and 92 had a history of completed stroke. A total of 134 patients showed functional independence in daily life (modified Rankin Scale score, <3), with scores of 3 and 4 in 3 patients each.
Of the 140 patients, 63 underwent the WCST (Table 1). This group included 51 men and 12 women aged 65±9 years. Seven were asymptomatic, whereas 11 and 45 had a history of TIA and completed stroke, respectively. Characteristics of the 63 patients were not significantly different from the total patient sample. However, in the 63 patients, when compared with the total patient sample, the volume of infarcts tended to be smaller and the incidence of ICA diseases tended to be larger. No patient showed functional dependence in their daily lives (modified Rankin Scale score, ≥3). The time interval between the stroke event and PET evaluation tended to be shorter than in the total patient sample. The values obtained in each category, total errors, perseverative errors, and proportion (%) of perseverative errors in total errors were 2.1±1.7 (range, 0–6), 25±8 (range, 10–48), 10±8 (range, 0–47), and 34±20 (range, 0–97.9), respectively.
FMZ-BP in the Thalamus
In the thalamus ipsilateral to the infarcts, the values of FMZ-BP, CBF, and CMRO2 were significantly lower than those in the contralateral thalamus (Table 2; Figure 2).
Table 2.
Positron Emission Tomography Variables for Thalamus and Cerebral Cortex
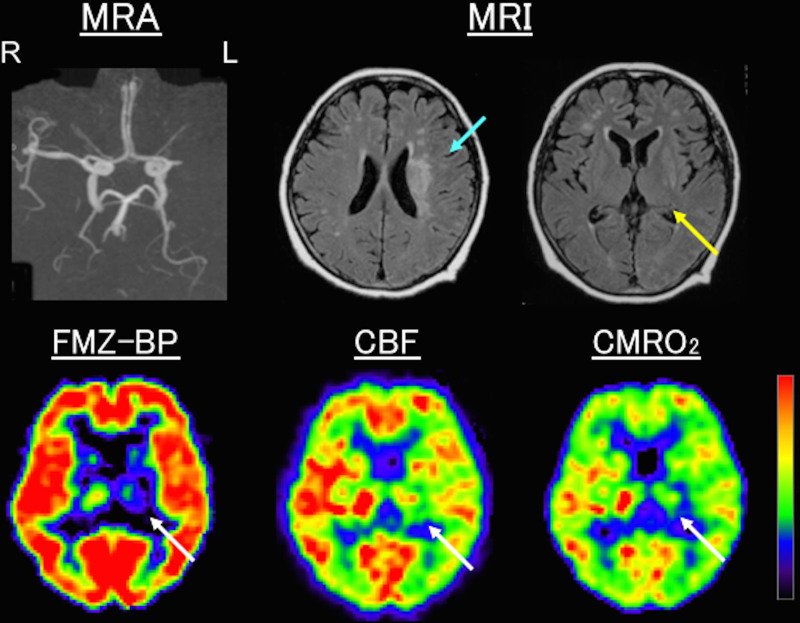
Figure 2.
Examples of positron emission tomography (PET) images in a patient with left (L) middle cerebral artery occlusion (MRA). Magnetic resonance imaging (MRI), 0.5 mo after stroke, showed corona radiata infarction (blue arrow) without ischemic lesions or apparent atrophy in the thalamus (yellow arrow). PET, 0.8 mo after stroke, showed a decrease in flumazenil binding potential (FMZ-BP), cerebral blood flow (CBF), and cerebral metabolic rate of oxygen (CMRO2) in the thalamus (white arrows) ipsilateral to infarction. The ipsilateral-to-contralateral ratios for FMZ-BP, CBF, and CMRO2 were 0.56, 0.76, and 0.61, respectively. R indicates right.
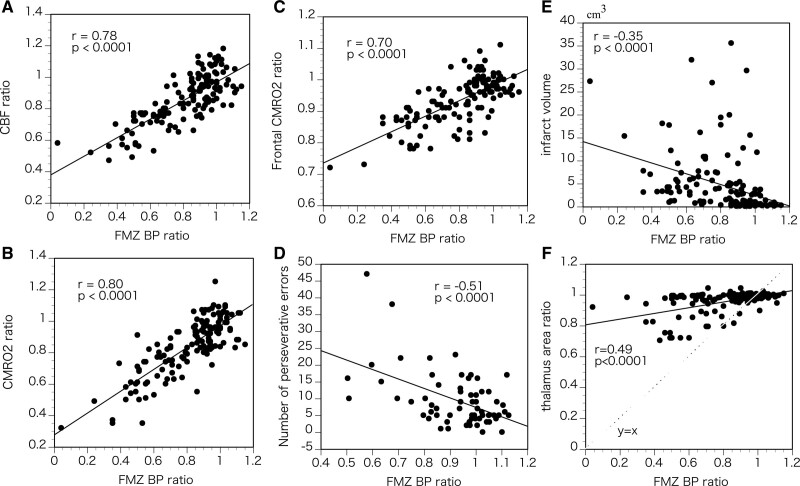
The ipsilateral-to-contralateral ratio of FMZ-BP varied among patients and was below the normal lower 95% limit in 71 patients (51%; 4 asymptomatic patients, 4 with TIAs, and 63 with strokes) or in 9 of the 13 (69%) patients with stroke within 1 (0.6–1.0) month of onset. Significant correlations were found between the ipsilateral-to-contralateral ratios for FMZ-BP, CBF, and CMRO2 in the thalamus (Figure 3A and 3B).
Figure 3.
The relationships between thalamic neuronal damage and other variables. Scatter plots of the ipsilateral-to-contralateral ratios for flumazenil binding potential (FMZ-BP) and those for cerebral blood flow (CBF; A) or cerebral metabolic rate of oxygen (CMRO2; B) in the thalamus, the ipsilateral-to-contralateral ratios for FMZ-BP in the thalamus and those for CMRO2 in the frontal cortex (C), the ipsilateral-to-contralateral ratios for FMZ-BP in the thalamus and the numbers of perseverative errors of Wisconsin Card Sorting Test (D), the ipsilateral-to-contralateral ratios for FMZ-BP in the thalamus and the volume of infarcts (E), and the ipsilateral-to-contralateral ratios for FMZ-BP and those for the area in the thalamus (F).
The ipsilateral-to-contralateral thalamic FMZ-BP ratio showed a significant negative correlation with infarct volume (r=−0.35, P<0.0001) or with the ipsilateral-to-contralateral thalamic area ratio (r=0.49, P<0.0001; Figure 3E and 3F). Age was not significantly correlated with ipsilateral-to-contralateral thalamic FMZ-BP ratios (r=0.09, P=0.25). In patients with stroke, the interval between stroke and PET examinations was not significantly correlated with ipsilateral-to-contralateral thalamic FMZ-BP ratios (r=0.05, P=0.65). This correlation remained insignificant (P=0.11) after controlling for the effect of infarct volume and the ipsilateral-to-contralateral thalamic area ratio using multiple regression analysis.
Location of Infarcts and FMZ-BP Ratio in the Thalamus
Locations of the cerebral infarcts are shown in Table 3. Eight patients had an infarction that spanned across the occipital and parietal or temporal lobes. These infarctions were distal MCA territory infarctions or posterior watershed infarctions involving the lateral occipital lobe. Three patients had ICA diseases, and 5 patients had MCA diseases.
Table 3.
Location of Infarcts and Ipsilateral/Contralateral FMZ-BP Ratio in the Thalamus
Considering that many patients had ≥2 categories of cerebral infarcts, we analyzed the relationship between the presence of cerebral infarcts and ipsilateral-to-contralateral ratios of FMZ-BP in the thalamus (Table 3). Patients with corona radiata infarcts and combined internal capsule with basal ganglia infarcts (striatocapsular infarcts) had significantly decreased FMZ-BP ratio compared with those without. However, the FMZ-BP ratio was not significantly different between patients with and without any other type of cerebral infarcts. When the presence of 7 locations of cerebral infarcts was entered into multiple linear regression analysis (forward stepwise selection), a model including the presence of corona radiata infarctions and striatocapsular infarctions with a correlation coefficient of 0.64 for the FMZ-BP ratio (P<0.0001) was created. In this model, the presence of striatocapsular infarction and corona radiata infarction accounted for 31.6% and 9.4%, respectively, of the variance in the FMZ-BP ratio. The other variables did not significantly contribute to the magnitude of the correlation. After controlling for the effects of age, infarct volume, the ipsilateral-to-contralateral thalamic area ratio, and the presence of the other 5 types of cerebral infarcts using multiple regression analysis, the presence of a corona radiata infarction or striatocapsular infarction was a significant independent predictor for decreased FMZ-BP ratio.
To determine whether a decrease in BZRs was proportional to a decrease in CBF or CMRO2 in the thalamus ipsilateral to the cerebral infarcts, we evaluated the ipsilateral-to-contralateral CBF ratio/ipsilateral-to-contralateral FMZ-BP ratio or ipsilateral-to-contralateral CMRO2 ratio/ipsilateral-to-contralateral FMZ-BP ratio in the thalamus (Table 3). Then, we analyzed the relationship between the location of cerebral infarcts and CBF ratio/FMZ-BP ratio or CMRO2 ratio/FMZ-BP ratio in the thalamus (Table 3). The presence of striatocapsular infarcts was associated with increased CBF ratio/FMZ-BP ratio or CMRO2 ratio/FMZ-BP ratio in the thalamus. After controlling for the effects of age, infarct volume, the ipsilateral-to-contralateral thalamic area ratio, and the presence of the other 6 locations of cerebral infarcts using multiple regression analysis, the presence of striatocapsular infarction was also a significant independent predictor for increased CBF ratio/FMZ-BP ratio or CMRO2 ratio/FMZ-BP ratio.
Cortical Metabolism and FMZ-BP Ratio in the Thalamus
In the frontal, parietal, and temporal cortices ipsilateral to the infarcts, the CBF and CMRO2 values were significantly lower than those in the contralateral cortex (Table 2). The ipsilateral-to-contralateral ratio of FMZ-BP in the thalamus was significantly correlated with the ipsilateral-to-contralateral ratios of CMRO2 in the frontal (r=0.70), parietal (r=0.62), and temporal (r=0.48) cortices (all, P<0.001). A multiple regression model with a forward stepwise selection procedure was used to assess the independent predictive value of the ipsilateral-to-contralateral ratio of CMRO2 in the frontal, parietal, temporal, and occipital cortices with respect to the ipsilateral-to-contralateral ratio of FMZ-BP in the thalamus. It produced a model including the CMRO2 ratio in the frontal or parietal cortex with a correlation coefficient of 0.74 for the FMZ-BP ratio (P<0.0001). In this model, the CMRO2 ratio in the frontal and parietal cortices accounted for 49.3% and 6.7%, respectively, of the variance of the FMZ-BP ratio in the thalamus. The CMRO2 ratio in the frontal or parietal cortex was a significant independent predictor of decreased FMZ-BP ratio, after controlling for the effects of infarct volume and the ipsilateral-to-contralateral thalamic area ratio using multiple regression analysis, The results did not change when 59 patients with cortical infarctions were excluded. A multiple regression model with a forward stepwise selection procedure produced a model including the CMRO2 ratio in the frontal or parietal cortex with a correlation coefficient of 0.76 for the FMZ-BP ratio (P<0.0001). In this model, the CMRO2 ratio in the frontal and parietal cortices accounted for 58.0% and 6.9%, respectively, of variance in the FMZ-BP ratio of the thalamus. The CMRO2 ratio in the frontal or parietal cortex was a significant independent predictor of decreased FMZ-BP ratio.
When the presence of 7 locations of cerebral infarcts was entered into multiple linear regression analysis (forward stepwise selection), a model including the presence of corona radiata infarctions, striatocapsular infarctions, and frontal lobe infarctions with a correlation coefficient of 0.56 for the CMRO2 ratio in the frontal cortex (P<0.0001) was created. In this model, the presence of striatocapsular infarction, corona radiata infarction, and frontal lobe infarction accounted for 23.3%, 5.2%, and 3.0%, respectively, of the variance in the CMRO2 ratio in the frontal cortex. The other locations, including basal ganglia only, did not significantly contribute to the magnitude of the correlation.
WCST Performance and FMZ-BP Ratio in the Thalamus
In 63 patients who underwent WCST, the ipsilateral-to-contralateral thalamic FMZ-BP ratio was significantly and negatively correlated with the number of perseverative errors (r=−0.51, P<0.001; Figure 3D), the proportion of the number of perseverative errors to the number of total errors (r=−0.53, P<0.001), or the number of total errors (r=−0.31, P=0.012). These correlations were significant after controlling for the effect of age, lesion side (left or right), infarct volume, and the ipsilateral-to-contralateral thalamic area ratio (P<0.001, P<0.001, and P<0.05, respectively), using multiple regression analysis. The ipsilateral-to-contralateral thalamic FMZ-BP ratio was not significantly correlated with the number of categories achieved.
The correlations became stronger when 24 patients with cortical infarctions were excluded. The ipsilateral-to-contralateral thalamic FMZ-BP ratio was significantly and negatively correlated with the number of perseverative errors (r=−0.65, P<0.001), the proportion of the number of perseverative errors to the number of total errors (r=−0.69, P<0.001), or the number of total errors (r=−0.43, P<0.01).
Discussion
This study showed neuronal alterations in the context of secondary thalamic degeneration in patients with cerebral infarction and ICA or MCA disease in the chronic stage. Secondary thalamic neuronal damage manifested as a decrease in BZRs, which was accompanied by a decrease in CBF and oxygen metabolism. Infarctions involving the corona radiata or the striatocapsular region contributed to a decrease in the number of thalamic BZRs. Thalamic neuronal damage is associated with frontal lobe hypometabolism and frontal lobe dysfunction assessed by the WCST. Secondary neuronal damage in the thalamus may reflect the degree of disruption of the thalamofrontal neuronal network in patients with ICA or MCA disease.
Secondary thalamic neuronal degeneration may cause a decrease in CBF and metabolism in the thalamus. Decrease in thalamic perfusion and metabolism remote to cerebral infarction have been demonstrated.5–8 This study showed that in the thalamus ipsilateral to cerebral infarcts, a decrease in the number of BZRs was associated with decrease in CBF and CMRO2. Remote decrease in CBF and CMRO2 in the thalamus was accompanied by neuronal alterations in the chronic stage and, thus, may not be caused by a simple decrease in neural input from cerebral cortical or subcortical regions. In contrast, remote depression of CBF and metabolism in the cerebellum contralateral to cerebral infarction (crossed cerebellar diaschisis32) is not accompanied by a decrease in BZRs, suggesting a simple decrease in neural input.33 Decrease in FMZ-BP may be specific to neuronal/synapse loss, while CBF or CMRO2 can be reduced as a result of either neuronal degeneration or diaschisis.1,17
The decreased number of BZRs in the thalamus remote to cerebral infarction may reflect the severity and extent of disconnection between the thalamus and cerebral cortical regions, which may contribute to cortical dysfunction through disruption of the thalamocortical network in patients with cerebral infarction. The thalamus and cortex are reciprocally connected. Corticothalamic and thalamocortical fibers become detached from the corona radiata and the internal capsule and enter the thalamus at its rostral and caudal poles and along its dorsal surface (the thalamic peduncles).34 Thus, infarcts in the internal capsule or the corona radiata can disrupt the connection between the thalamus and the cortex. In the present study, among the 7 locations of infarct, infarcts involving the internal capsule (with basal ganglia) or the corona radiata were associated with decrease in the ipsilateral-to-contralateral ratio of FMZ-BP in the thalamus. This finding implies that even small infarcts can destroy the large number of fibers connecting the thalamus and the cerebral cortex in these regions, as suggested by a previous study.9 The size of infarcts may be another important determinant of the extent of disconnection and was associated with decreases in the ipsilateral-to-contralateral FMZ-BP ratio in the thalamus.
When thalamic degeneration occurs through disruption of the thalamocortical neuronal network due to cerebral infarction, the degree of thalamic degeneration may be correlated with cortical hypometabolism because disruption of the thalamocortical neuronal network may also cause hypometabolism due to decrease in excitatory inputs from the thalamus to the cerebral cortex (diaschisis).1 In the present study, the ipsilateral-to-contralateral ratio of FMZ-BP in the thalamus was correlated with the ipsilateral-to-contralateral ratio of CMRO2 in the frontal or parietal lobes. The results did not change when only patients without cortical infarctions were analyzed, supporting the hypothesis. The correlation with frontal CMRO2 was better than that with parietal CMRO2. Anterior circulation stroke due to ICA or MCA disease may disrupt the connections between the thalamus and the frontal regions through the anterior and superior thalamic peduncle, which may result in frontal cortical hypometabolism.
Secondary thalamic degeneration with frontal cortical hypometabolism may be associated with impairment of frontal lobe function, specifically executive dysfunction. In patients who underwent WCST, the decrease in BZRs was associated with an increase in perseverative errors that are sensitive to frontal lobe dysfunction.26 This association was independent of infarct volume. Several MRI studies demonstrated the association of secondary thalamic degeneration with frontal lobe dysfunction in patients with cerebral infarction at variable locations.10,15 Using R2* mapping, iron accumulation within the entire thalamus was associated with impairment of verbal fluency/set sifting using the Isaac set test at 1-year follow-up.10 The association was independent of initial diffusion-weighted imaging volume.10,15 Using diffusion tensor imaging, decreased fractional anisotropy values in the entire thalamus were associated with lower verbal fluency performance at 3 months poststroke.15 Our study included patients with cerebral infarction and ICA or MCA disease, in whom the connection between the thalamus and the frontal cortex may be susceptible to ischemia, leading to frontal lobe hypometabolism and dysfunction. Stronger correlation between the decrease in BZRs with the number of perseverative errors than with the number of the total errors supports this notion because the number of the total errors increase with impairments in the frontal lobe functions, as well as those of lobes elsewhere.26 Furthermore, the correlation in the decrease in BZRs with the number of perseverative errors became stronger when the effect of focal cortical lesions on WCST performance was excluded by investigating only patients without cortical infarcts. This suggests that disconnection between the thalamus and the frontal regions, not focal cortical lesions, may mainly contribute to the development of frontal lobe dysfunction.
In the thalamus ipsilateral to the cerebral infarcts, a decrease in BZRs was proportional to decreases in thalamic CBF or CMRO2. Thus, in the chronic stage studied here, decreases in CBF and CMRO2 may reflect a decrease in BZRs. However, striatocapsular infarctions were associated with a disproportionate decrease in BZRs in relation to decrease in CBF and CMRO2. An experimental study investigated differences in secondary degeneration of the ventroposterior thalamic nucleus and the reticular thalamic nucleus using 2 stroke models in rats.35 In the photothrombotic model, pure cortical infarcts, without damages in the globus pallidus, only lead to secondary damage in the ventroposterior thalamic nucleus, possibly by retrograde degeneration. MCA occlusion, which leads to infarction of the cortex and globus pallidus, resulted in secondary damage in the ventroposterior thalamic nucleus and reticular thalamic nucleus. The authors suggest that additional reticular thalamic nucleus damage might be caused by the loss of protective inhibitory input from the globus pallidus to the reticular thalamic nucleus. We speculate that the loss of inhibitory input from the globus pallidus might cause relative excitation in the thalamus despite severe neuronal damage from the 2 mechanisms in striatocapsular infarctions, which may lead to a disproportionate decrease in BZRs, relative to decreases in CBF and CMRO2. This finding might be considered when secondary thalamic degeneration is evaluated using perfusion or metabolic imaging.
Severe secondary neurodegeneration due to large infarcts could cause thalamic atrophy in the long term.13 Decrease in the ipsilateral-to-contralateral thalamic FMZ-BP ratio may reflect neuronal damage in patients without atrophy, whereas in patients with atrophy, it may reflect partial volume effects of thalamic atrophy due to thalamic degeneration (ie, neuronal loss), as well as decrease in tissue BZR expression in the residual thalamus. In all patients, the ipsilateral-to-contralateral thalamic FMZ-BP ratio was correlated with the ipsilateral-to-contralateral thalamic area ratio. However, there were some patients showing decrease in ipsilateral-to-contralateral thalamic FMZ-BP ratio despite no thalamic atrophy early after stroke onset (Figure 3F), which may cause lack of correlation between time since onset and thalamic FMZ ratio, despite the wide delay in intervals across the sample. In such patients, long-term follow-up may reveal gradual matching of early decrease in the ipsilateral-to-contralateral thalamic FMZ-BP ratio with delayed decrease in the ipsilateral-to-contralateral thalamic area ratio.
ICA or MCA diseases may cause selective neuronal damage in the cerebral cortex demonstrated as decreased BZR in normal-appearing cerebral cortex due to chronic hemodynamic ischemia.16 However, in patients excluded from this study with unilateral ICA or MCA diseases but without infarction, mild decreases in BZR in the normal-appearing cerebral cortex was seen, but none had the ipsilateral-to-contralateral ratio of FMZ-BP below the normal lower 95% limit (0.998±0.055).
Because secondary degeneration may occur several days or weeks after stroke onset, it may represent a novel target for neuroprotection and stroke management to improve functional outcome.2 Therefore, the evaluation of neuronal alteration in the thalamus with decreased CBF and metabolism may be important to predict if hypoperfusion or hypometabolism is reversible or irreversible. Hypoperfusion and hypometabolism without neuronal damage may indicate the stage of reversible functional depression. This study suggests that secondary thalamic neuronal damage may manifest as a decrease in the number of BZRs 2 weeks after stroke onset. Thus, therapeutic strategies to prevent secondary degeneration may be needed within 2 weeks after the event of stroke.
Limitations
This study has several limitations. First, this study was a retrospective analysis of an observational study. Therefore, we cannot exclude the possibility that unmeasured confounding variables may explain some of our findings. Second, the selected nature of our patients limits the present study. It is of note that patients in the present study had atherothrombotic cerebral infarcts due to ICA or MCA disease with small sizes of cortical infarcts. Most infarcts were located in the anterior circulation. Furthermore, only a few patients who were studied during the 17 months underwent WCST, although their patient characteristics were similar with the total sample. Third, the nature of the study, cross sectional, may prevent a causal interpretation of the associations in our study. However, it may be reasonable to consider the ipsilateral-to-contralateral thalamic FMZ-BP ratio as a reflection of the degree of disruption of the thalamocortical neuronal network. Fourth, our patients were older than normal controls. Because the thalamus is a small oval structure, the ROI method used here might have caused relatively large variations in the left-to-right or the right-to-left ratio of FMZ-BP for the thalamus in normal controls. The variations in older individuals may be larger than in younger individuals, which might affect the incidence of thalamic neuronal degeneration in our patients. Lastly, MRI imaging was done for clinical practice, and the method for measurements of the area of infarcts or the thalamus was subjective. More objective methods using high-resolution MRI might have improved the level of the correlation of MRI measures with PET variables.
Conclusions
In conclusion, secondary thalamic neuronal damage may manifest as a decrease in BZRs in patients with cerebral infarction and ICA or MCA disease, which may be associated with frontal lobe hypometabolism and dysfunction. Secondary neuronal damage in the thalamus may reflect the degree of disruption of the thalamofrontal neuronal network in patients with ICA or MCA disease.
Article Information
Acknowledgments
The authors thank the staff of the PET Division, the Department of Neurology and Neurosurgery, Shiga General Hospital, for their assistance.
Sources of Funding
This study was funded by the Japan Society for the Promotion of Science KAKENHI (grant number 22613001).
Disclosures
None.
Supplemental Material
Supplemental Methods
Figure S1
STROBE Statement
Supplementary Material
Nonstandard Abbreviations and Acronyms
- 11C-FMZ
- 11C-flumazenil
- BZR
- central benzodiazepine receptor
- CBF
- cerebral blood flow
- CMRO2
- cerebral metabolic rate of oxygen
- FMZ-BP
- 11C-flumazenil binding potential
- ICA
- internal carotid artery
- MCA
- middle cerebral artery
- PET
- positron emission tomography
- ROI
- region of interest
- TIA
- transient ischemic attack
- WCST
- Wisconsin Card Sorting Test
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.122.038846.
For Sources of Funding and Disclosures, see page 3162.
Contributor Information
Shinya Kagawa, Email: kagawa@res.med.shiga-pref.jp.
Kuninori Kusano, Email: kusano@res.med.shiga-pref.jp.
Miki Ito, Email: m.ito@res.med.shiga-pref.jp.
Chio Okuyama, Email: okuyama@res.med.shiga-pref.jp.
References
- 1.Feeney DM, Baron JC. Diaschisis. Stroke. 1986;17:817–830. doi: 10.1161/01.str.17.5.817 [DOI] [PubMed] [Google Scholar]
- 2.Zhang J, Zhang Y, Xing S, Liang Z, Zeng J. Secondary neurodegeneration in remote regions after focal cerebral infarction: a new target for stroke management? Stroke. 2012;43:1700–1705. doi: 10.1161/STROKEAHA.111.632448 [DOI] [PubMed] [Google Scholar]
- 3.Fujie W, Kirino T, Tomukai N, Iwasawa T, Tamura A. Progressive shrinkage of the thalamus following middle cerebral artery occlusion in rats. Stroke. 1990;21:1485–1488. doi: 10.1161/01.str.21.10.1485 [DOI] [PubMed] [Google Scholar]
- 4.Iizuka H, Sakatani K, Young W. Neural damage in the rat thalamus after cortical infarcts. Stroke. 1990;21:790–794. doi: 10.1161/01.str.21.5.790 [DOI] [PubMed] [Google Scholar]
- 5.De Reuck J, Decoo D, Lemahieu I, Strijckmans K, Goethals P, Van Maele G. Ipsilateral thalamic diaschisis after middle cerebral artery infarction. J Neurol Sci. 1995;134:130–135. doi: 10.1016/0022-510x(95)00229-2 [DOI] [PubMed] [Google Scholar]
- 6.Nagasawa H, Kogure K, Fujiwara T, Itoh M, Ido T. Metabolic disturbances in exo-focal brain areas after cortical stroke studied by positron emission tomography. J Neurol Sci. 1994;123:147–153. doi: 10.1016/0022-510x(94)90217-8 [DOI] [PubMed] [Google Scholar]
- 7.Ogawa T, Yoshida Y, Okudera T, Noguchi K, Kado H, Uemura K. Secondary thalamic degeneration after cerebral infarction in the middle cerebral artery distribution: evaluation with MR imaging. Radiology. 1997;204:255–262. doi: 10.1148/radiology.204.1.9205256 [DOI] [PubMed] [Google Scholar]
- 8.Reidler P, Thierfelder KM, Fabritius MP, Sommer WH, Meinel FG, Dorn F, Wollenweber FA, Duering M, Kunz WG. Thalamic diaschisis in acute ischemic stroke: occurrence, perfusion characteristics, and impact on outcome. Stroke. 2018;49:931–937. doi: 10.1161/STROKEAHA.118.020698 [DOI] [PubMed] [Google Scholar]
- 9.Hervé D, Molko N, Pappata S, Buffon F, LeBihan D, Bousser MG, Chabriat H. Longitudinal thalamic diffusion changes after middle cerebral artery infarcts. J Neurol Neurosurg Psychiatry. 2005;76:200–205. doi: 10.1136/jnnp.2004.041012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuchcinski G, Munsch F, Lopes R, Bigourdan A, Su J, Sagnier S, Renou P, Pruvo JP, Rutt BK, Dousset V, et al. Thalamic alterations remote to infarct appear as focal iron accumulation and impact clinical outcome. Brain. 2017;140:1932–1946. doi: 10.1093/brain/awx114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li C, Ling X, Liu S, Xu A, Zhang Y, Xing S, Pei Z, Zeng J. Early detection of secondary damage in ipsilateral thalamus after acute infarction at unilateral corona radiata by diffusion tensor imaging and magnetic resonance spectroscopy. BMC Neurol. 2011;11:49. doi: 10.1186/1471-2377-11-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xia C, Zhou J, Lu C, Wang Y, Tang T, Cai Y, Ju S. Characterizing diaschisis-related thalamic perfusion and diffusion after middle cerebral artery infarction. Stroke. 2021;52:2319–2327. doi: 10.1161/STROKEAHA.120.032464 [DOI] [PubMed] [Google Scholar]
- 13.Tamura A, Tahira Y, Nagashima H, Kirino T, Gotoh O, Hojo S, Sano K. Thalamic atrophy following cerebral infarction in the territory of the middle cerebral artery. Stroke. 1991;22:615–618. doi: 10.1161/01.str.22.5.615 [DOI] [PubMed] [Google Scholar]
- 14.Binkofski F, Seitz RJ, Arnold S, Classen J, Benecke R, Freund HJ. Thalamic metbolism and corticospinal tract integrity determine motor recovery in stroke. Ann Neurol. 1996;39:460–470. doi: 10.1002/ana.410390408 [DOI] [PubMed] [Google Scholar]
- 15.Fernández-Andújar M, Doornink F, Dacosta-Aguayo R, Soriano-Raya JJ, Miralbell J, Bargalló N, López-Cancio E, Pérez de la Ossa N, Gomis M, Millán M, et al. Remote thalamic microstructural abnormalities related to cognitive function in ischemic stroke patients. Neuropsychology. 2014;28:984–996. doi: 10.1037/neu0000087 [DOI] [PubMed] [Google Scholar]
- 16.Baron JC, Yamauchi H, Fujioka M, Endres M. Selective neuronal loss in ischemic stroke and cerebrovascular disease. J Cereb Blood Flow Metab. 2014;34:2–18. doi: 10.1038/jcbfm.2013.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sette G, Baron JC, Young AR, Miyazawa H, Tillet I, Barré L, Travère JM, Derlon JM, MacKenzie ET. In vivo mapping of brain benzodiazepine receptor changes by positron emission tomography after focal ischemia in the anesthetized baboon. Stroke. 1993;24:2046–2057; discussion 2057. doi: 10.1161/01.str.24.12.2046 [DOI] [PubMed] [Google Scholar]
- 18.Ejaz S, Williamson DJ, Ahmed T, Sitnikov S, Hong YT, Sawiak SJ, Fryer TD, Aigbirhio FI, Baron JC. Characterizing infarction and selective neuronal loss following temporary focal cerebral ischemia in the rat: a multi-modality imaging study. Neurobiol Dis. 2013;51:120–132. doi: 10.1016/j.nbd.2012.11.002 [DOI] [PubMed] [Google Scholar]
- 19.North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325:445–453. doi: 10.1056/NEJM199108153250701 [DOI] [PubMed] [Google Scholar]
- 20.Samuels OB, Joseph GJ, Lynn MJ, Smith HA, Chimowitz MI. A standardized method for measuring intracranial arterial stenosis. AJNR Am J Neuroradiol. 2000;21:643–646. [PMC free article] [PubMed] [Google Scholar]
- 21.Okazawa H, Yamauchi H, Sugimoto K, Takahashi M, Toyoda H, Kishibe Y, Shio H. Quantitative comparison of the bolus and steady-state methods for measurement of cerebral perfusion and oxygen metabolism: positron emission tomography study using 15O-gas and water. J Cereb Blood Flow Metab. 2001;21:793–803. doi: 10.1097/00004647-200107000-00004 [DOI] [PubMed] [Google Scholar]
- 22.Okazawa H, Yamauchi H, Sugimoto K, Magata Y, Kudo T, Yonekura Y. Effects of metabolite correction for arterial input function on quantitative receptor images with 11C-flumazenil in clinical positron emission tomography studies. J Comput Assist Tomogr. 2004;28:428–435. doi: 10.1097/00004728-200405000-00021 [DOI] [PubMed] [Google Scholar]
- 23.Frackowiak RS, Lenzi GL, Jones T, Heather JD. Quantitative measurement of regional cerebral blood flow and oxygen metabolism in man using 15O and positron emission tomography: theory, procedure, and normal values. J Comput Assist Tomogr. 1980;4:727–736. doi: 10.1097/00004728-198012000-00001 [DOI] [PubMed] [Google Scholar]
- 24.Lammertsma AA, Jones T. Correction for the presence of intravascular oxygen-15 in the steady-state technique for measuring regional oxygen extraction ratio in the brain: 1. Description of the method. J Cereb Blood Flow Metab. 1983;3:416–424. doi: 10.1038/jcbfm.1983.67 [DOI] [PubMed] [Google Scholar]
- 25.Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16:834–840. doi: 10.1097/00004647-199609000-00008 [DOI] [PubMed] [Google Scholar]
- 26.Nelson E. A modified card sorting test sensitive to frontal lobe defects. Cortex. 1976;12:313–324. doi: 10.1016/s0010-9452(76)80035-4 [DOI] [PubMed] [Google Scholar]
- 27.Kashima H, Kashima M, Handa T. Neuropsychological studies on frontal lobe function of the patients with chronic schizophrenia. Results of application of the new modified Wisconsin card sorting test. Japan J Clin Psychiat. 1985;14:1479–1489. [Google Scholar]
- 28.Kobayashi S. Neuropsychological test using personal computer. Japan J Neuropsychol. 2002;18:188–193. [Google Scholar]
- 29.Ogura T, Hida K, Masuzuka T, Saito H, Minoshima S, Nishikawa K. An automated ROI setting method using NEUROSTAT on cerebral blood flow SPECT images. Ann Nucl Med. 2009;23:33–41. doi: 10.1007/s12149-008-0203-7 [DOI] [PubMed] [Google Scholar]
- 30.Minoshima S, Frey KA, Koeppe RA, Foster NL, Kuhl DE. A diagnostic approach in Alzheimer’s disease using three-dimensional stereotactic surface projections of fluorine-18-FDG PET. J Nucl Med. 1995;36:1238–1248. [PubMed] [Google Scholar]
- 31.Mizumura S, Kumita S, Cho K, Ishihara M, Nakajo H, Toba M, Kumazaki T. Development of quantitative analysis method for stereotactic brain image: assessment of reduced accumulation in extent and severity using anatomical segmentation. Ann Nucl Med. 2003;17:289–295. doi: 10.1007/BF02988523 [DOI] [PubMed] [Google Scholar]
- 32.Baron JC, Bousser MG, Comar D, Castaigne P. “Crossed cerebellar diaschisis” in human supratentorial brain infarction. Trans Am Neurol Assoc. 1981;105:459–461. [PubMed] [Google Scholar]
- 33.Dong Y, Fukuyama H, Nabatame H, Yamauchi H, Shibasaki H, Yonekura Y. Assessment of benzodiazepine receptors using iodine-123-labeled iomazenil single-photon emission computed tomography in patients with ischemic cerebrovascular disease. A comparison with PET study. Stroke. 1997;28:1776–1782. doi: 10.1161/01.str.28.9.1776 [DOI] [PubMed] [Google Scholar]
- 34.Nieuwenhuys R, Voogd J, van Huijzen C. Thalamocortical and Corticothalamic Connections. 2nd ed. Springer-Verlag; 1981. [Google Scholar]
- 35.Dihné M, Grommes C, Lutzenburg M, Witte OW, Block F. Different mechanisms of secondary neuronal damage in thalamic nuclei after focal cerebral ischemia in rats. Stroke. 2002;33:3006–3011. doi: 10.1161/01.str.0000039406.64644.cb [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.