ABSTRACT
OBJECTIVE
To determine whether changes in skin temperature can affect the integrity of skin.
METHODOLOGY
The authors conducted a systematic literature search as per the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) guidelines. They searched the CINAHL (Cumulative Index to Nursing and Allied Health Literature), Cochrane, MEDLINE Complete, Academic Search Ultimate, and HyDi databases for articles examining the effects of skin temperature on skin integrity published through April 2020. Two independent reviewers scored the methodologic quality of the 13 included studies.
RESULTS
Only 11 studies were included in the qualitative analysis, as the other two articles had a critical risk of bias. There is strong evidence to indicate that an increase in skin temperature leads to changes in skin structure and function. However, ulcer formation was more affected by intrinsic and extrinsic factors, rather than by temperature alone.
CONCLUSION
Further high-quality randomized controlled trials are required to investigate the direct effect of skin temperature on ulceration.
KEYWORDS: integrity, skin, structure, temperature, ulceration, wound care
INTRODUCTION
The loss of skin integrity and development of localized ulcerations can have serious consequences and significantly impact quality of life.1 Research involving animal studies, engineered muscle tissue, and finite element modeling has shown that tissue deformation due to mechanical loading is an important cause of ulceration in the short term.2,3 Typically, cell death starts in the muscle and subcutaneous fat, beneath intact skin, when cell resistance is insufficient to withstand the deformation incurred. Research focused on pressure injury (PI) prevention has found that higher skin temperatures reduce cell resilience and lead to a higher susceptibility to direct deformation injury.3,4
The temperature of the human body adapts when exposed to temperature changes caused by internal or external factors. Vasodilation or vasoconstriction are triggered to maintain a relatively constant core body temperature5,6 to keep the body functioning efficiently and protect the normal integrity of the skin. If such physiologic responses do not occur, skin integrity may be compromised because the skin’s ability to withstand mechanical stress, balance homeostasis,7,8 and maintain its immunologic function becomes impaired.9 In this state, the skin becomes vulnerable to external and internal mechanical factors, possibly resulting in tissue breakdown. Further, elevated skin temperatures have been associated with a reduction of the cohesive strength of the integrity of the stratum corneum.10 Therefore, an unfavorable microclimate consisting of uncontrolled temperature, humidity, and airflow at the skin surface may affect the structure and function of the skin,5,11 potentially increasing its susceptibility to damage when subjected to external mechanical factors.
In recent years, interest in research regarding skin temperature and ulceration has increased, mostly focusing on the early detection of inflammation before tissue breakdown occurs. Using temperature as an indicator of underlying tissue damage, Lavery et al12 found that an increase in temperature by 2.2° C detected by a home monitoring infrared thermometer was indicative of underlying inflammation. Iaizzo13 found that an increase in skin temperature by extrinsic or intrinsic stimuli causes a disruption in skin integrity in a swine model. In another porcine study, Kokate et al4 found that the application of temperatures greater than 35° C caused full-thickness cutaneous and deep tissue injury with necrosis at temperatures greater than 45° C.4 Studies investigating skin responses to changes in temperature that lead to skin tissue breakdown have primarily been limited to animal studies13,14 and experiments on cadaveric skin.10 Although in vitro and animal studies have provided significant insight into skin properties with regard to tissue breakdown, they are limited in the context of human skin integrity. Thus, the evidence is still unclear. Studies investigating human skin resilience in relation to temperature changes have mostly used measures of other parameters, namely, transepidermal water loss (TEWL) and stratum corneum hydration (SCH), which are known to affect skin resilience.
Advances in technology have improved investigations of the subtle changes occurring within human skin, which might not be identified during a visual inspection with the naked eye. Skin temperatures can be measured by either contact (eg, thermistors, thermocouples) or noncontact (eg, infrared thermal cameras) methods. Other changes occurring within the skin layers such as TEWL and SCH have also been investigated objectively using different measurement tools for specific purposes. For instance, capacitive sensors have been used to measure the hydration level of the superficial layers of the skin (ie, SCH), whereas open-chamber devices have been used to measure the amount of water that passively evaporates through skin to the external environment (ie, TEWL).15 These measurements quantify the structural changes occurring to further understand skin function.
Given the increased interest in the relationship between temperature and skin integrity and its potential implications in the prevention and management of ulcerations, a thorough systematic review following a critical evaluation of the literature is warranted. Further, analyses of the clinical value of temperature in identifying decreased tissue resilience and the role of elevated temperature as a key causative factor in skin tissue breakdown merit consideration. Understanding these relationships can support clinicians and caregivers in optimizing the environment and monitoring skin temperature to prevent skin breakdown and ulcer complications.
METHODS
This systematic review was designed and conducted according to guidelines outlined by PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses).16 This review was registered with the Prospective Register of Systematic Reviews (no. CRD42020177560). The protocol for this review can be accessed from https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=177560.
Information Source and Search Strategy
Two independent researchers conducted database searches in January 2021 with the final search conducted in April 2021. The following databases were searched for articles examining the effects of skin temperature on the integrity of the skin: CINAHL (Cumulative Index to Nursing and Allied Health Literature), Cochrane, MEDLINE Complete, Academic Search Ultimate, and HyDi (a University of Malta search engine incorporating several databases including PubMed and EBSCO). The standardized electronic literature search strategy was performed using the following keywords: skin integrity, skin breakdown, pressure injury, skin resilience, thermography, infrared imaging, and skin temperature measurement. No restrictions were placed on the publication year; however, only human studies and articles published in the English language were included. Reference lists of the included and eligible articles were also hand searched to identify any additional studies. Retrieved references were exported to a reference manager to identify and remove any duplicates present. Figure 1 illustrates the full search strategy.
Figure 1.
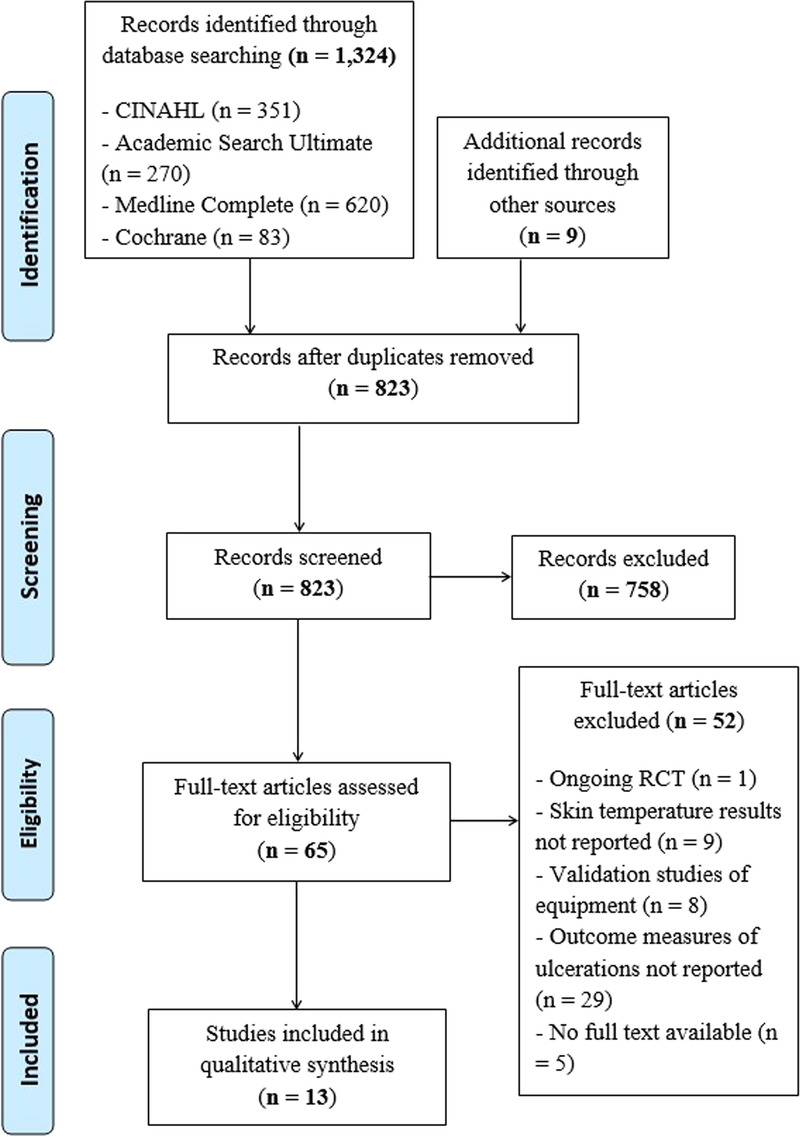
PRISMA FLOW DIAGRAM
Eligibility Criteria
All identified articles were screened for eligibility according to the PICOS framework: population, intervention, comparator, outcome measures, and study type. Studies were included if participants were older than 18 years and had intact skin with no skin breakdown at the start of the study; the interventions included any changes in skin temperature caused by external factors such as prolonged loading, hot and cold baths, and skin contact with cushions or mattresses made of different materials; the comparator (control) was either a group of healthy participants with healthy skin or an intact skin region from participants undergoing intervention; and the outcome measures included TEWL, SCH, skin erythema, skin deformability, skin breakdown, or ulcer formation. Studies investigating ulcerated skin were excluded, unless these studies also included data from participants with intact skin.
The types of studies selected for this review included randomized and nonrandomized controlled trials, cohort studies, cross-sectional studies, and observational studies. Only scientific articles in peer-reviewed journals were included. Non-peer-reviewed articles, such as editorials, letters to the editor, narrative reviews, and tool development or validation studies, were excluded.
Study Selection
Two independent researchers initially screened article titles and abstracts to identify studies that met the inclusion criteria. The full text of the shortlisted articles was then reviewed to further assess eligibility. Any disagreement over the eligibility of particular studies was resolved through discussion between the two researchers. If an agreement was not reached, a third and fourth reviewer intervened.
Data Extraction
Key data were extracted independently by the same two researchers for each of the included studies using structured data extraction sheets. The data extracted included the article title, year of publication, study design, setting, method of assessment, length of study, population characteristics, outcome measures, and results. During the shortlisting process, there was no blinding of study authors, research institution, or place of publication.
Methodologic Quality
Two researchers assessed the methodologic quality of the included articles using the Cochrane ROBINS-I (Risk of Bias in Nonrandomized Studies of Interventions)17 tool or Cochrane Risk of Bias (RoB 2.0),18 a tool depending on the type of study being analyzed.
Randomized trials were assessed using RoB 2.0,18 which involves judgments of the risk of bias across five domains, as well as an overall risk of bias. Trials were classified as having an overall low risk of bias, some concerns of bias, or a high risk of bias (Figure 2).
Figure 2.
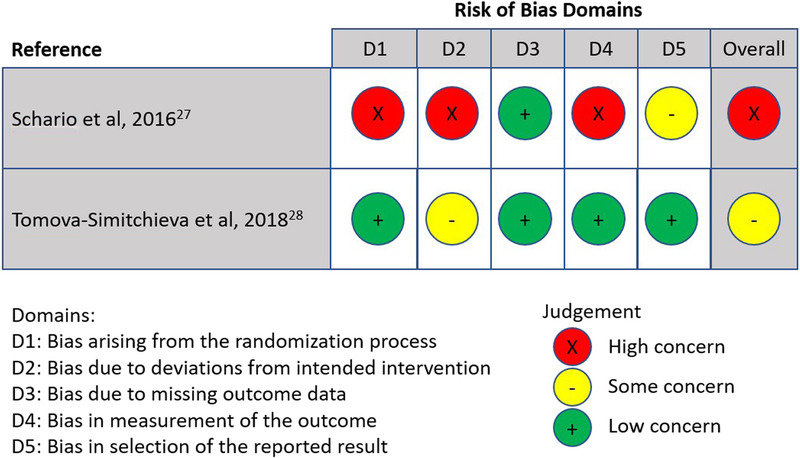
METHODOLOGIC QUALITY OF INCLUDED RANDOMIZED TRIALS
Nonrandomized trials were assessed using ROBINS-I. This tool involves judgments on the risk of bias on seven domains and overall risk of bias.17 Trials were classified overall as having either no information, low risk, moderate risk, serious risk, or critical risk of bias (Figure 3). Any discrepancies between researchers were discussed and resolved via consensus with a third researcher.
Figure 3.
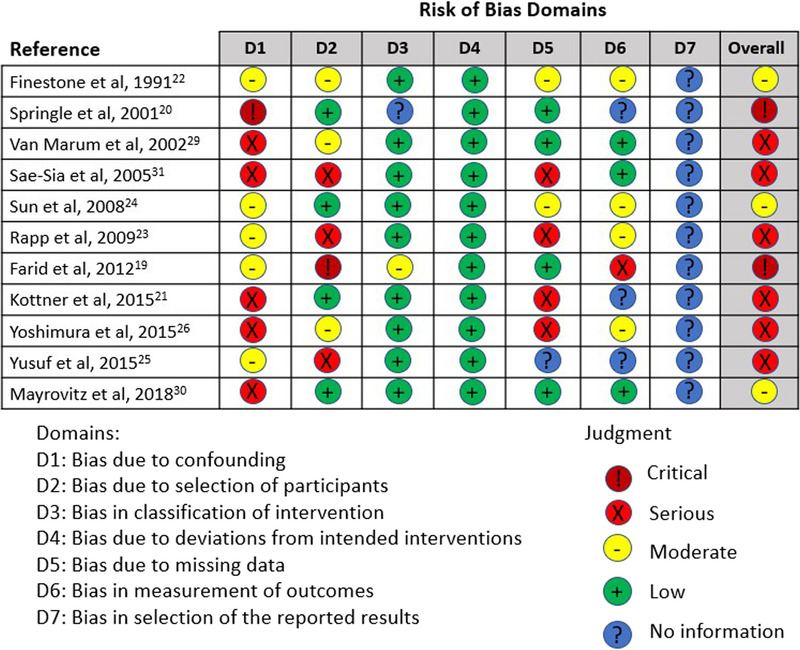
METHODOLOGIC QUALITY OF INCLUDED NONRANDOMIZED STUDIES
RESULTS
Overview of All Included Studies
A total of 1,324 articles were identified through database searches, and an additional nine articles were identified through referencing. After duplicates were removed (n = 510), the remaining 823 articles were screened by title and abstract. Of these, 758 did not meet the inclusion criteria and were excluded. Following full-text assessment of the 65 articles remaining, a further 52 articles were excluded based on the exclusion criteria. Thirteen articles met the full inclusion criteria and were included in the qualitative evaluation of this review (Figure 1).
Study Designs and Characteristics
One of the included articles19 was a retrospective observational study; the remaining 12 articles were all prospective studies.20–31 Of these prospective studies, eight were observational cohort studies,21,22,24–26,29–31 one used a repeated-measures design,20 one used a time-series design,23 and two were randomized controlled trials27,28 with a crossover design. There was a wide range of participant ages and mixed populations among the studies, including healthy participants, participants with comorbidities, individuals with neurological impairment following spinal cord injury, individuals with diabetes, and nursing home residents.
Data Synthesis
Because of the heterogeneity of the study designs, participant populations, and reported outcome measures in the included studies, meta-analysis could not be performed. The extracted data are summarized in Tables 1 and 2, and the results were compared narratively. The data collected were grouped in the tables according to the type of outcome measures analyzed: short-term effects (eg, changes in skin temperature, structure, and function) or long-term effects (eg, end-stage skin damage with ulcer formation with long-term patient follow-up).
Table 1.
SHORT-TERM EFFECTS: THE INFLUENCE OF TEMPERATURE ON SKIN STRUCTURE AND FUNCTION
| Reference | Participants | Method | Outcome Measures | Investigated Area | Control | Results |
|---|---|---|---|---|---|---|
| Kottner et al (2015)21 | - 20 Women - Mean age, 69.9 y - Median age, 70.5 y - Range, 60–80 y |
- Observational clinical study - Skin temperature measured before and after a 90-min immobilization protocol and 150 min in a supine position - Temperature differences induced by different loading times - Temperature measured with a contactless infrared skin thermometer |
- Skin temperature - TEWL - SCH - Erythema |
- Sacral skin - Right lateral heel |
Sternal skin | - Baseline heel skin temperature (29° C) was lower than sternal and sacral area (32° C) and after 90 min, the increase in temperature was statistically significant (+2.2° C in the sacrum and + 1.4° C in the heel). Temperature increased further after 150 min of loading (+2.5° and + 3.1° C, respectively) - As temperature increased, TEWL and erythema also increased. After 20-min recovery, temperature, TEWL, and erythema remained high in the heel but returned to baseline in the sacrum - The greatest increase in temperature corresponded to the greatest increase in TEWL and erythema |
| Tomova-Simitchieva et al (2018)28 | - 15 Women - Median age, 66 y - Range, 60–80 y |
- RCT with crossover design - Skin temperature recorded before and after an immobilization protocol of 2 h in supine position - Measurements were repeated again after 20 min of recovery - Temperature differences induced by using three different support surfaces - Temperature measured with a contactless infrared skin thermometer |
- Skin temperature - TEWL - SCH - Hydration of deeper epidermal and dermal skin layers - Erythema - Skin deformability/stiffness and elasticity |
- Sacral skin - Right lateral heel |
Compared with baseline | - After 2 h, increase in temperature was statistically significant (+3.1° C in the sacrum and + 1.7° C in the heel) - As temperature increased, erythema, TEWL and SCH also increased, but decreased after offloading - Greatest increase in temperature corresponded to greatest increase in TEWL, SCH, moisture, and erythema in the heel area (foam surface) - After loading, the maximum extensibility increased and decreased back to baseline after 20 min - Elasticity decreased as temperature increased |
| Schario et al (2017)27 | - 6 Women - Median age, 65 y - Range, 60–80 y |
- RCT crossover design - Skin temperature measured before and after a 45-min seated immobilization protocol with contactless infrared thermometer - Temperature differences induced by using two different fabrics on support surfaces |
- Skin temperature -TEWL - SCH - Erythema - Skin deformability/stiffness and elasticity |
Gluteal skin | Compared both sides to baseline | - Temperature increase was significant on both sides (+2.5° C and +0.7° C; P = .028) with the greatest changes with cotton fabric - As temperature increased with loading, TEWL and erythema increased significantly when using 3D cotton - Skin elasticity and relative elastic recovery remained stable after loading |
| Finestone et al (1991)22 | - 5 Men, 1 woman - Age range, 18–30 y - Patients with spinal cord injury |
Observational clinical study - Thermistor probe measurements taken after sitting for an average of 2.5 h without pressure relief and repeated after 1 h - Temperature was measured at the site of highest erythema immediately after offloading every 10 min for 60 min |
- Erythema was visually graded on a predetermined 1-10 redness intensity scale - Maximum and minimum diameters of the erythematous area were measured |
Ischial tuberosity | Surrounding skin | - Skin temperature of experimentally induced erythematous areas remained elevated, even 1 h after pressure relief - Erythematous area decreased in size between the first inspection held immediately after pressure relief and the second inspection 1 h later - No linear relationship was found between temperature and erythema |
Abbreviations: RCT, randomized controlled trial; SCH, stratum corneum hydration; TEWL, transepidermal water loss.
Table 2.
LONG TERM EFFECTS: THE INFLUENCE OF TEMPERATURE ON SKIN BREAKDOWN
| Reference | Participants | Method | Outcome measures | Investigated Area | Control | Results |
|---|---|---|---|---|---|---|
| Van Marum et al (2002)29 | - 82 Nursing home residents - Aged ≥60 y |
- Observational longitudinal prospective study - Skin temperature measured before and after application of a local cold stimulus (17° C for 7 min) to observe sympathetic nerve function (thermoregulation) by blood flow response without loading - Temperature sensors were attached directly to the skin |
Development of new PI (in relation to time of blood flow response after cold stimulus) | Major trochanter | N/A | - No significant relationship was found between the difference in the final and initial temperature measurements and the risk of PI development - Participants who developed a PI showed a significantly longer blood flow response time than those who did not develop a PI |
| Rapp et al (2009)23 | - 8 Men, 12 women - Aged 71–101 y - Nursing facility residents |
- Time-series study - Ulcer risk was calculated on enrollment and then weekly for 3 wk - Skin temperature was continuously recorded for 5 d and then weekly for 3 wk - Thermistor probes were used |
Difference in skin temperature regularity (MSE) and PI risk category (using Braden Scale) or development of PI using the NPUAP Scale | Right midaxillary line, fifth Intercostal space | N/A | - The temperature spectral exponent predicted PI development - Skin temperature mean MSE was lowest among those who developed PIs - Skin temperature mean MSE for the high-risk group was higher compared with the low-risk group - The mean time for temperature to return to baseline was longer for the high-risk group |
| Sun et al (2008)24 | - 94 Men, 61 women - 119 with diabetes and 36 without (control) |
- Prospective cohort study - Skin temperature and SSR were assessed at the start of the study and repeated every few months for 4 y During every visit, ulcer formation was recorded - Skin temperature was measured using a medical thermal imaging radiometer system |
Development of foot ulcer using the Seattle Wound Classification System | Plantar aspect of feet in individuals with diabetes (at-risk group with preulcerative lesions, SSR− group and SSR+ group) | Plantar aspect of healthy individuals | - At baseline, the at-risk group had significantly higher mean temperatures compared with other groups - Over time, temperature increased in the SSR− group but decreased in the at-risk group - There was no significant time-related temperature change between the baseline and 4-y study in the SSR+ and control groups - After 4 y, mean plantar temperature was higher in the at-risk and SSR− compared with the SSR+ and healthy group - The plantar ulceration rate was not significantly different in patients with absent SSR and those with SSR possibly due to well-controlled diabetes mellitus |
| Mayrovitz et al (2018)30 | 58 Men (mean age, 70.4 y), and 42 women (mean age, 74 y) admitted to a critical care unit |
- Prospective cohort study - Skin temperature was measured after patients were offloaded for 4 min to assess whether a decrease in temperature after loading is associated with risk of PI development - Temperature was measured with infrared thermal imaging |
- PI recorded with no specific guidelines - Difference in temperature by > −1.5° C compared with control site as an indication of PI risk |
Sacral skin | Remote skin area (at least 10 cm proximal to the sacrum) | - 32 Participants had a lower temperature compared with control site by >1.5° C; 6 participants had a higher temperature by >1.98° C; 63 participants showed no clinically significant difference in temperature between the two sites - There was no significant difference between temperature in the sacral area and remote skin in patients with PIs - Of the 16 participants who developed a PI, 7 were in the sacral region, but only 2 of these had prior sacral-to-control area temperature differences of −1.5° C or less |
| Yoshimura et al (2015)26 | 13 Men, 16 women who underwent elective surgery (mean age, 44.4 y) |
- Prospective observational study - Skin temperature was measured every 30 min from the beginning to the end of surgery and repeated again 30 min after surgery ended - Skin was reassessed 1 wk postoperation for PI formation - Temperature probes attached to the skin were used to measure temperature |
- Development of park-bench position PI or deep tissue injury after surgery - The International NPUAP-EPUAP Pressure Ulcer Classification System was used - Level of perspiration using a perspiration meter - Peak pressure measured by a pressure mapping device |
Thorax area; fourth to eighth rib | N/A | The baseline values in the patients with PIs were significantly lower than those observed in the group without PIs (34.9° ± 0.5° C vs 35.3° ± 0.4° C) - The increase in temperature was also significantly higher in the group who developed PIs compared with those who did not - The increase in skin temperature was found to be significantly and independently associated with PI development - Skin temperature consistently increased over time until the end of surgery |
| Sae-Sia et al (2005)31 | 17 Participants with spinal cord injury or cerebrovascular accident; average age, 61.1 ± 15.6 y; range, 33-89 y |
- Observational cohort study - Skin temperature was measured within 24-96 h of admission and 48-72 h after the initial assessment while lying supine and repeated after turning on lateral side for 15 min - Thermocouple probes were used |
Development of PI using the NPUAP classification system | Sacral skin | N/A | - Skin temperature may increase at least 1.2° C 24-96 h before sacral PI development - Mean skin temperature at initial assessment in those developing a PI was higher (P = .001) compared with those who did not develop PIs - Skin temperature remained elevated (P = .002) 15 min after beginning offloading and even 48–72 h later, regardless of reclining position in those who developed PIs |
| Yusuf et al (2015)25 | 42 Men, 29 women; aged ≥18 y; patients in an acute care setting | Observational prospective cohort study - Skin temperature was measured every 3 d from admission until the 15th day or whenever a PI developed or superficial skin changes occurred - An infrared digital noncontact thermometer was used |
- PI development using the EPUAP staging system - Skin temperature difference between sacrum and control site - Skin moisture - Erythema |
Sacral area | Periumbilical area | - There was only a marginal difference in temperature between the sacrum and the control site (0.9° ± 0.6° C) in the group with superficial skin changes compared with the group with no skin changes (P = .071). - 20 Participants had PIs and superficial skin changes; 51 had no skin changes - No data for skin moisture or erythema were reported |
Abbreviations: EPUAP, European Pressure Ulcer Advisory Panel; MSE, multiscale entropy; NPUAP, National Pressure Ulcer Advisory Panel; PI, pressure injury; SSR, sympathetic skin response.
Short-term Effects: The Influence of Temperature on Skin Structure and Function
Four articles investigated short-term effects, noting changes in skin temperature after 45 to 150 minutes of loading and after 20 minutes of recovery.21,22,27,28 These articles focused on skin functionality, including TEWL, SCH, and erythema. In addition, two articles examined the influence of temperature on the structural components of the skin, namely, stiffness and elasticity,27,28 and one studied the recovery rate of the erythematous area.22
Temperature
In all the areas investigated, skin temperature increased with prolonged loading time. After 45 minutes of loading, Schario et al27 reported an increase in temperature by 1.6° C. With prolonged loading of 15021 and 120 minutes,28 increases in skin temperature of 1.7° to 3.1° C were reported in the sacrum and 1.9° C in the heel, respectively.
Transepidermal water loss
Transepidermal water loss, SCH, and skin erythema are objective assessments of skin functionality, indicating or predicting early-stage skin damage. Whereas TEWL values less than 70 g/m2 per hour are indicative of intact skin and a fully functional barrier, higher TEWL values are generally considered to be an indicator of a disturbed or disrupted skin barrier.21 All the studies included in this systematic review that assessed TEWL as a measure of skin function reported that as temperature increased, TEWL also increased. The greatest increase in TEWL (16.5 g/m2 per hour) was observed in the heel with an increase of 1.7° C. Schario et al27 reported an increase in TEWL in the gluteal region of 8.1 g/m2 per hour with a temperature change of 1.1° C. Lower but significant increases in TEWL were also reported in the sacrum (7 g/m2 per hour).28 Although the included studies differ in loading times, contact materials used, and areas of skin investigated, there is a general agreement that as temperature increased, TEWL also increased.32
Erythema
Studies that investigated erythema as a measure of change in skin resilience found that as temperature increased, erythema also increased.22,27,28 The largest increase in erythema (+225 AU) was reported in the gluteal area following 45 minutes of loading.27 Kottner et al21 reported an increase of 117 AU in the heel area compared with an increase of 59 AU in the sacrum. Three of the four studies that measured erythema reported that the highest increase in temperature corresponded to the highest increase in erythema in the sacral, gluteal, and heel areas, respectively.21,27,28 Finestone et al22 reported only visual inspection of erythema and stated that after offloading, skin temperature decreased by 3° C, and the size and intensity of erythema also decreased. However, no linear relationship was found between decrease in temperature and decrease in observed erythema. After 20 minutes of recovery, temperature and erythema remained high in the heel, decreasing only marginally, but returned to baseline in the sacrum.33 However, Tomova-Simitchieva et al28 found that temperature and erythema decreased significantly in the sacrum and the heel following 20 minutes of recovery.
Stratum corneum hydration
Three studies investigated SCH in relation to skin temperature and reported conflicting results.21,27,28 Tomova-Simitchieva et al28 reported that SCH increased as temperature increased, with the highest increase in SCH corresponding to the highest increase in temperature when using a foam support surface. In addition, as temperature decreased after offloading, SCH also decreased, but the difference was not statistically significant. Schario et al27 also noted that SCH increased as temperature increased, but this change in skin hydration was not statistically significant. In contrast, Kottner et al21 reported that as temperature increased, SCH remained unaffected. The use of different supporting surface materials may have contributed to the observed differences. As a result, the effect of temperature on SCH remains unclear.
Skin elasticity
Although Schario et al27 and Tomova-Simitchieva et al28 both examined structural changes in relation to skin elasticity, the authors reported different results. Schario et al27 found that although there was a significant temperature increase, skin elasticity remained stable after 45 minutes. However, Tomova-Simitchieva et al28 found that elasticity increased after 2 hours in a lying position, whereas the temperature increased by 1.7° and 3.1° C in the heel and sacrum, respectively. As with SCH, these reported differences in skin elasticity might have been due to the different loading time protocol or type of material used. Moreover, the 45 minutes allocated in the study by Schario and colleagues might not have been sufficient to enable a significant change in skin elasticity, whereas the 2-hour period allocated by Tomova-Simitchieva et al28 resulted in these changes. Perhaps a longer period is required to cause skin elasticity changes.
Long-term Effects: The Influence of Temperature on Skin Breakdown
Seven articles reported the long-term effects of temperature on skin breakdown with the endpoint being ulceration.23–26,29–31 Skin integrity was investigated via visual inspection to determine the level of breakdown in the layers of the skin (dermis and epidermis). Four of the studies23,25,26,31 followed the International NPUAP-EPUAP Pressure Ulcer Classification System guideline for staging the level of tissue breakdown present in their cohorts; the Seattle Wound Classification System was used in one study,24 and the other two studies29,30 recorded only the presence or absence of ulceration (Table 2).
Methodologic approaches included the application of local cold stimulus,29 repeated loading in specific body locations,25,31 and prolonged loading during surgery.26 To determine whether skin temperature regulation can be used to predict ulcer risk formation, one study23 used continuous temperature monitoring, whereas the others investigated specific time points. Three of the seven studies investigated healthy participants with no comorbidities.23,25,29 One study investigated patients who underwent elective surgery.26 In the other studies, participants had comorbidities such as diabetes,24 cerebrovascular accident,31 and cardiovascular disease,30 which led to shorter time for ulcer formation.
Van Marum et al29 investigated the relationship between blood flow response time (measured by temperature change after applying a local cold stimulus) and the risk of ulcer development. They reported that the group who developed ulcers showed a significantly longer blood flow response time (4.5 ± 1.2 minutes) and therefore a longer time to cause a change in temperature, compared with the group who did not develop ulcers (3.7 ± 0.7 minutes). This delay in temperature change in participants who developed ulcers was also found by Rapp et al,23 who performed continuous monitoring of temperature data. Skin temperature fluctuations over time were evident, and those who developed ulcers had the lowest multiscale entropy, meaning that patients with a lower level of skin integrity demonstrated a slower change in temperature. This finding can also be linked to recovery time responses in studies that investigated the return of skin temperature to baseline measures.23,29 This recovery time was delayed in older adults23,29 and in patients with neurologic impairment.26
Using a different methodology, Yusuf et al25 collected temperature data every 3 days for 15 days in patients admitted in an acute care setting. The development of ulcers and superficial skin changes were observed with daily visual inspection of the skin. An increase in temperature of 0.9° ± 0.6° C was reported in participants who developed superficial skin changes such as skin tears and friction-associated blisters compared with a control group, but the difference was not statistically significant (P = .071).
Similarly, Mayrovitz et al30 found no significant temperature differences between participants who developed ulcerations and those who did not. This was the only study that compared the site of interest with adjacent skin. In six patients, the ulcerated site had an increase in temperature of more than 1.5° C, whereas in 32 patients, the ulcerated site had a temperature decrease of more than 1.5° C. In the patients who did not ulcerate, the temperature difference from the control site averaged 0.13° ± 0.58° C. These measurements were taken after the area under investigation was offloaded for approximately 4 minutes to acclimatize to room temperature and eliminate loading factors. However, this offloading period might not have been long enough to eliminate the effect of loading on temperature.28
In the study by Sun et al,24 participants with diabetes and a healthy control group were followed up for a period of 4 years and plantar skin temperature was monitored. Participants in this study were grouped according to the sudomotor sympathetic response as a determinant of autonomic disorders. No significant temperature change over time occurred between baseline and the 4-year follow-up period between the healthy control group (at baseline, 26.7° ± 2.0° C; at 4-year follow-up, 26.6° ± 2.2° C) and the group of patients with diabetes who had no further diabetic complications (at baseline, 27.0° ± 2.3° C; at 4-year follow-up, 27.3° ± 2.5° C). However, in the group of patients with diabetes who had absent sudomotor sympathetic response, plantar mean foot temperature increased (at baseline, 27.8° ± 2.1° C; at 4-year follow-up, 29.0° ± 2.4° C; P < .05).
Similarly, Sae-Sia et al31 found that skin temperature increased by 1.2° C in the 24 to 96 hours before ulcers developed in patients with neurologic impairment but healthy skin. Researchers recorded the sacral skin temperature while participants lay supine in bed and then assessed ulcer formation after 2 or more days from the initial examination for a maximum of 2 weeks. Mean skin temperature at the initial assessment (baseline) was higher for patients who later developed an ulcer (37.3° C) compared with those who did not (36° C; P = .001), suggesting that the skin is more susceptible to acute injury with higher temperatures.
Moreover, Yoshimura et al26 noted that when participants underwent a lengthy surgical procedure with prolonged loading of more than 6 hours, the change in skin temperature from baseline to the end of surgery was significantly higher in the patients who developed ulceration than in those who did not (2.7° ± 0.3° C vs 1.9° ± 0.8° C; P < .05). In fact, elevated skin temperature was found to be an independent PI risk factor. However, patients who developed ulcers within a week after the surgery had significantly lower baseline skin temperatures than those without ulceration (34.9° ± 0.5° C vs 35.3° ± 0.4° C; P = .026). The authors explain that this lower baseline caused a high temperature change following loading, which is associated with heat-related cytotoxicity.
DISCUSSION
This systematic review examined the current evidence regarding the effect of skin temperature in the pathway leading to tissue breakdown. Although the heterogeneity of the studies identified and the small participant groups make it difficult to establish defined conclusions, the results indicate that there is a clear correlation between elevated skin temperature and detrimental effects in the skin in both the short and long term.
The findings of this review support continuous monitoring of skin temperature in patients at risk of ulceration because it can contribute to the early detection of impending skin failure and, therefore, help prevent subsequent ulceration. This model may be particularly relevant to in-shoe temperature monitoring as a tool for foot ulcer prevention.
Short-term Effects of Skin Temperature on Skin Structure and Function
Strong evidence indicates that an increase in skin temperature leads to an increased TEWL and, therefore, damage to the epidermal barrier function. This finding was reported in all the reviewed articles.21,27,28 The association between damage to the epidermis and TEWL is well known, and TEWL greater than 70 g/m2 per hour may indicate impaired skin barrier function.21 In stable ambient conditions, TEWL oscillates between 4 and 10 g/m2 per hour, depending on the skin area, and should ideally be as low as possible in healthy skin. However, it may increase up to 30 times when the epidermis is damaged, resulting in increased water loss and poor barrier function. The water content of the stratum corneum is also necessary for proper stratum corneum maturation and skin desquamation. Increased TEWL impairs the enzymatic functions required for normal desquamation, resulting in the visible appearance of dry, flaky skin.34 Humectants and occlusive moisturizers have been reported to significantly decrease TEWL and are useful in the management of areas of PI.35
Moreover, TEWL causes changes within the dermal layer as well, leading to a decrease in structural stiffness. With increased temperature, skin elasticity increases because of the decreased stiffness.28 Several decades ago, Gibson et al36 proposed that the dermis has a natural mesh-like arrangement of collagen fibers at the molecular level that enables repeated rearrangement of individual fibers to resist severe stretch under minimal stresses associated with normal activity and loading. At rest, the fibers appear randomly oriented, but when an increasing load is applied, the fibers become parallel. In this histologic study,36 the authors showed that because of fluid being displaced from the meshwork, the fibers failed to return to their previous random arrangement. These mechanical property changes of the skin contribute to the skin being less responsive to change with temperature and unable to adapt, leading to tissue weakening and eventually breakdown. Although some evidence indicates that an increase in skin temperature leads to the skin decreasing its stiffness,28 other research27 did not support this finding. However, the shorter loading time used27 may have influenced the results and potentially limited the value of directly comparing these results.
Skin alteration in response to a change in skin temperature might lead to serious impairment in the tissue’s mechanical properties, resulting in breakdown due to a reduced ability to sustain potentially damaging loads.37 An increase in skin stiffness over bony prominences might also lead to a higher chance of skin breakdown because the skin is already approaching its maximum ability to stretch (terminal stiffness). Further, for every 1° C increase in skin temperature, blistering time was lowered by approximately 13%, leading to quicker separation of the dermal-epidermal layers, blister formation, and weakened skin integrity.38 In addition, an increase in temperature has been associated with increased oxygen and nutrient requirements, which may be insufficient in areas of pressure.
Long-term Effects of Skin Temperature on End-Stage Skin Damage and Ulcer Formation
This review shows evidence of differences in heat dissipation between the skin of healthy individuals and those with thermoregulatory problems. The temperature of healthy skin decreased to baseline within 20 minutes after off-loading occurred.28 However, skin temperatures did not recover to baseline in the presence of ulcer formation in individuals with comorbidities who were at increased risk of skin breakdown.22,26,30,31 Prolonged recovery was also present in patients who developed skin changes, and ulceration and was found to have a significant relationship with ulcer formation.23,24,31 There is a strong association between impaired thermoregulatory function and increased risk of ulceration. Because the role of skin temperature as a measure of thermoregulatory function is well known, patients with underlying abnormal intrinsic neurologic response may benefit from continuous skin temperature monitoring as a useful predictor of ulceration. Patients with Charcot deformity are also susceptible to an increase in skin temperature via an intrinsic pathway because of localized inflammation12 and may potentially benefit from continuous temperature monitoring as well, although further research is required. New developments in mobile health technology show promise in improving management and optimizing timely intervention for patients who are at risk of ulceration, potentially increasing time in remission and improving quality of life.39
This review did not address confounding factors that may have influenced the study results because most were not reported, including the influences of room and ambient humidity, patient sweat, and air convection.40 Skin microclimate conditions beneath dressings were also not addressed. Evidence indicates that different dressing materials have different properties for heat dissipation. Using infrared thermography to characterize skin microclimate may be effective in the development of treatment strategies.41
Variations in methodologic design did not allow for meta-analysis. The studies identified were longitudinal, nonrandomized studies lasting from a few days to years. Randomized controlled trials using ulceration incidence as a clinical endpoint are time-consuming, costly, and of limited external validity.42 Consequently, observational studies are undertaken to provide evidence and encourage the use of large, routinely collected data sets assembled through data linkage. Inclusion of these articles was deemed suitable because nonrandomized studies can still provide evidence about long-term outcomes and populations that are typical of real-world practice.
Most of the included studies had a small sample size; consequently, the pooled sample sizes are comparably small, which limits the generalizability of the results across the entire population. In addition, although none of the studies included in this review occurred during the COVID-19 pandemic, evidence suggests that the cytokine storm associated with COVID-19 is linked to an increased risk of deep tissue injury,43,44 which needs to be considered in future research. Nevertheless, the information presented here may promote new research theories and increase understanding of the role skin temperature plays in the development of ulceration.
Future research should not only focus on prediction of ulcer development using temperature changes, but also analyze the time of recovery and time taken for temperature changes because this information can provide a better indication of loss of skin integrity, skin breakdown, and ulceration. Further research using robust methodologies with good stratification of patient population is warranted.
CONCLUSION
The aim of this review was to determine whether elevated skin temperature is implicated in the pathway for tissue breakdown. The authors conclude that there is strong evidence indicating that elevated skin temperature immediately effects skin resilience. There is also important evidence showing a relationship between increased risk of ulceration and increased skin temperature in patients with impaired thermoregulation.
Continuous temperature monitoring may be useful for early detection of ulcer development risk. This may be potentially useful in patients with diabetes as a preventive measure for diabetic foot ulceration, because thermoregulatory function is often impaired at early stages in this patient group. It may also help determine risk for foot ulceration and early detection of skin failure, enabling prompt management to avoid deterioration.
Footnotes
The authors have disclosed no financial relationships related to this article.
REFERENCES
- 1.Renner R, Erfurt-Berge C. Depression and quality of life in patients with chronic wounds: ways to measure their influence and their effect on daily life. Chron Wound Care Manage Res 2017;4:143–51. [Google Scholar]
- 2.Gefen A. How much time does it take to get a pressure ulcer? Integrated evidence from human, animal, and in vitro studies. Ostomy Wound 2008;54(10):26–8. [PubMed] [Google Scholar]
- 3.Oomens CW, Bader DL, Loerakker S, Baaijens F. Pressure induced deep tissue injury explained. Ann Biomed Eng 2015;43:297–305. [DOI] [PubMed] [Google Scholar]
- 4.Kokate JY Leland KJ Held AM, et al. Temperature-modulated pressure ulcers: a porcine model. Arch Phys Med Rehabil 1995;76:666–73. [DOI] [PubMed] [Google Scholar]
- 5.Gefen A. How do microclimate factors affect the risk for superficial pressure ulcers: a mathematical modeling study. J Tissue Viability 2011;20(3):81–88. [DOI] [PubMed] [Google Scholar]
- 6.Loerakker S Manders E Strijkers GJ, et al. The effects of deformation, ischemia, and reperfusion on the development of muscle damage during prolonged loading. J Appl Physiol 2011;111:1168–77. [DOI] [PubMed] [Google Scholar]
- 7.Romanovsky AA. Skin temperature: its role in thermoregulation. Acta Phys 2014;210:498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schlader ZJ Sarker S Mündel T, et al. Hemodynamic responses upon the initiation of thermoregulatory behavior in young healthy adults. Temperature 2016;3:271–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moncrieff G, van Onselen J, Young T. The role of emollients in maintaining skin integrity. Wounds UK 2015;11(1):68–74. [Google Scholar]
- 10.Wu KS, van Osdol WW, Dauskardt RH. Mechanical properties of human stratum corneum: effects of temperature, hydration, and chemical treatment. Biomaterials 2006;27:785–95. [DOI] [PubMed] [Google Scholar]
- 11.Edsberg LE, Black JM, Goldberg M, McNichol L, Moore L, Sieggreen M. Revised National Pressure Ulcer Advisory Panel pressure injury staging system: revised pressure injury staging system. J Wound Ostomy Continence Nurs 2016;43(6):585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lavery LA Higgins KR Lanctot DR, et al. Home monitoring of foot skin temperatures to prevent ulceration. Diabetes Care 2004;27:2642–7. [DOI] [PubMed] [Google Scholar]
- 13.Iaizzo PA. Temperature modulation of pressure ulcer formation: using a swine model. Wounds 2004;16:336–43. [Google Scholar]
- 14.Patel S, Knapp CF, Donofrio JC, Salcido R. Temperature effects on surface pressure-induced changes in rat skin perfusion: implications in pressure ulcer development. J Rehabil Res Dev 1999;36:189–201. [PubMed] [Google Scholar]
- 15.Zaidi Z, Lanigan SW. Skin: structure and function. In: Dermatology in Clinical Practice. Springer: London; 2010:1–15. [Google Scholar]
- 16.Moher D Liberati A Tetzlaff J Altman DG, PRISMA Group . Preferred Reporting Items for Systematic Reviews and Meta-analyses: the PRISMA statement. PLoS Med 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sterne JA Hernán MA Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomized studies of interventions. BMJ 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins JP Sterne JA Savovic J, et al. A revised tool for assessing risk of bias in randomized trials. Cochrane Database Syst Rev 2016;10(Suppl 1):29–31. [Google Scholar]
- 19.Farid KJ, Winkelman C, Rizkala A, Jones K. Using temperature of pressure-related intact discolored areas of skin to detect deep tissue injury: an observational, retrospective, correlational study. Ostomy Wound 2012;58(8):20–31. [PubMed] [Google Scholar]
- 20.Sprigle S, Linden M, McKenna D, Davis K, Riordan B. Clinical skin temperature measurement to predict incipient pressure ulcers. Adv Skin Wound Care 2001;14:133–7. [DOI] [PubMed] [Google Scholar]
- 21.Kottner J Dobos G Andruck A, et al. Skin response to sustained loading: a clinical explorative study. J Tissue Viability 2015;24(3):114–122. [DOI] [PubMed] [Google Scholar]
- 22.Finestone HM, Levine SP, Carlson GA, Chizinsky KA, Kett RL. Erythema and skin temperature following continuous sitting in spinal cord injured individuals. J Rehabil Res Dev 1991;28(4):27–32. [DOI] [PubMed] [Google Scholar]
- 23.Rapp MP, Bergstrom N, Padhye NS. Contribution of skin temperature regularity to the risk of developing pressure ulcers in nursing facility residents. Adv Skin Wound Care 2009;22:506–513. [DOI] [PubMed] [Google Scholar]
- 24.Sun PC, Lin HD, Jao SH, Chan RC, Kao MJ, Cheng CK. Thermoregulatory sudomotor dysfunction and diabetic neuropathy develop in parallel in at-risk feet. Diabetic Med 2008;25:413–8. [DOI] [PubMed] [Google Scholar]
- 25.Yusuf S Okuwa M Shigeta Y, et al. Microclimate and development of pressure ulcers and superficial skin changes. Int Wound J 2015;12(1):40–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshimura M Nakagami G Iizaka S, et al. Microclimate is an independent risk factor for the development of intraoperatively acquired pressure ulcers in the park-bench position: a prospective observational study. Wound Repair Regen 2015;23:939–47. [DOI] [PubMed] [Google Scholar]
- 27.Schario M Tomova-Simitchieva T Lichterfeld A, et al. Effects of two different fabrics on skin barrier function under real pressure conditions. J Tissue Viability 2017;26:150–5. [DOI] [PubMed] [Google Scholar]
- 28.Tomova-Simitchieva T, Lichterfeld-Kottner A, Blume-Peytavi U, Kottner J. Comparing the effects of 3 different pressure ulcer prevention support surfaces on the structure and function of heel and sacral skin: an exploratory cross-over trial. Int Wound J 2018;15:429–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Marum RJ, Meijer JH, Ribbe MW. The relationship between pressure ulcers and skin blood flow response after a local cold provocation. Arch Phys Med Rehabil 2002;83(1):40–3. [DOI] [PubMed] [Google Scholar]
- 30.Mayrovitz HN, Spagna PE, Taylor MC. Sacral skin temperature assessed by thermal imaging. J Wound Ostomy Continence Nurs 2018;45(1):17–21. [DOI] [PubMed] [Google Scholar]
- 31.Sae-Sia W, Wipke-Tevis DD, Williams DA. Elevated sacral skin temperature (Ts): a risk factor for pressure ulcer development in hospitalized neurologically impaired Thai patients. Appl Nurs Res 2005;18(1):29–35. [DOI] [PubMed] [Google Scholar]
- 32.Kottner J, Lichterfeld A, Blume-Peytavi U. Transepidermal water loss in young and aged healthy humans: a systematic review and meta-analysis. Arch Dermatol Res 2013;305:315–23. [DOI] [PubMed] [Google Scholar]
- 33.Kottner J, Black J, Call E, Gefen A, Santamaria N. Microclimate: a critical review in the context of pressure ulcer prevention. Clin Biomech 2018;59:62–70. [DOI] [PubMed] [Google Scholar]
- 34.Verdier-Sévrain S, Bonté F. Skin hydration: a review on its molecular mechanisms. J Cosmet Dermatol 2007;6(2):75–82. [DOI] [PubMed] [Google Scholar]
- 35.Sethi A, Kaur T, Malhotra SK, Gambhir ML. Moisturizers: the slippery road. Indian J Dermatol 2016;61:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gibson T, Kenedi RM, Craik JE. The mobile micro-architecture of dermal collagen: a bio-engineering study. Br J Surg 1965;52:764–70. [DOI] [PubMed] [Google Scholar]
- 37.Stark HL. Directional variations in the extensibility of human skin. Br J Plast Surg 1977;30:105–14. [DOI] [PubMed] [Google Scholar]
- 38.Van der Leun JC, Lowe LB, Jr, Beerens E. The influence of skin temperature on dermal-epidermal adherence: evidence compatible with a highly viscous bond. J Invest Dermatol 1974;62(1):42–6. [Google Scholar]
- 39.Najafi B, Reeves ND, Armstrong DG. Leveraging smart technologies to improve the management of diabetic foot ulcers and extend ulcer-free days in remission. Diabetes Metab Res Rev 2020;36:e3239. [DOI] [PubMed] [Google Scholar]
- 40.Gefen A. How medical engineering has changed our understanding of chronic wounds and future prospects. Med Eng Phys 2019;72:13–8. [DOI] [PubMed] [Google Scholar]
- 41.Amrani G, Peko L, Hoffer O, Ovadia-Blechman Z, Gefen A. The microclimate under dressings applied to intact weight-bearing skin: infrared thermography studies. Clin Biomech 2020;75:104994. [DOI] [PubMed] [Google Scholar]
- 42.Beeckman D, Hecke AV. RCTs best for evidence on wound care: fact or fiction? Br J Nurs 2012;21(Sup12):S3. [DOI] [PubMed] [Google Scholar]
- 43.Gefen A, Ousey K. COVID-19: pressure ulcers, pain and the cytokine storm. J Wound Care 2020;29:540–2. [DOI] [PubMed] [Google Scholar]
- 44.Gefen A. Infrared thermography, COVID-19 and pressure ulcer risk. J Wound Care 2020;29:483–4. [DOI] [PubMed] [Google Scholar]


