Abstract
AIMS:
There is no consensus on impact of time to treatment initiation (TTI) on survival in patients with soft tissue sarcoma (STS). The purpose is to: 1) examine if increased TTI is associated with overall survival (OS), and 2) identify what are the factors associated with the delayed TTI in patients with soft tissue sarcoma.
PATIENTS AND METHODS:
We identified 23,786 localised high-grade STS of the extremity and trunk who underwent definitive surgery from 2004–2015 using the National Cancer Database. The Cox proportional hazards model was used to examine the relationship between each factor and OS. We calculated the incidence rate ratio (IRR) using negative binomial regression models to identify factors affecting TTI.
RESULTS:
Patients in whom TTI was prolonged had poorer OS than those with TTI of 0–30 days: 31–60 days, HR, 1.08; P = 0.011, 61–90 days, HR: 1.11; P = 0.044, 91- days, HR: 1.22; P = 0.003 (vs. 0–30 days). The restricted cubic spline demonstrated the HR increased substantially longer TTI than 50 days. Non-academic centers (vs. academic centers; IRR = 0.64–0.86; P < 0.001) had shorter TTI, while insurance status of Medicaid (vs. private insurance; IRR = 1.34), uninsured (vs. private insurance; IRR = 1.17), and transition in care (IRR = 1.62) had longer TTI.
CONCLUSION:
TTI over 30 days after diagnosis was independently associated with poorer survival. Hazard ratio showed linear increase, especially > 50 days after diagnosis. We recommend initiating treatment within 30 days after diagnosis to achieve the highest likelihood of cure for localised high-grade STS in the extremity and trunk, even when case referral to a specialised center is required.
Keywords: soft tissue sarcoma, time to treatment initiation (TTI), overall survival, National Cancer Database
INTRODUCTION
The interval from diagnosis to the time to treatment initiation (TTI) is increasing in the United States (US) in cancer patients1. The cause of increased TTI includes patient factors, socioeconomic factors, or health care system such as more complicated diagnostic and therapeutic techniques or increasing transitions in care to specialised hospitals. For physicians to reassure patients that current wait times to initiate treatment do not impact long-term outcomes to eliminate patient anxiety and distress associated with delays in TTI, there should be evidence that the length of TTI does not affect the outcome. Although studies in breast, head and neck, gynecologic, and lung cancer suggest that increased TTI is associated with poor survival2–7, the evidence is conflicting in most cancer types. Moreover, it is still unclear if delayed TTI worsens the outcomes of patients with soft tissue sarcoma8, 9.
Soft-tissue sarcomas are heterogeneous neoplasms originating from mesenchymal cells, comprising more than 70 histological subtypes. In high grade soft tissue sarcomas, the tumour grows rapidly and has the potential risk of micro-metastases that cannot be detected by imaging in cases with increased TTI. In addition to this biological background, the rarity and heterogeneity of soft tissue sarcomas inevitably hinders accurate diagnosis and sometimes demands long TTI. Therefore, we hypothesize increased TTI is associated with poor survival in soft tissue sarcoma. However, there have been no reports analysing the impact of TTI on survival in patients with localised high-grade soft tissue sarcoma of the extremity and trunk treated with definitive surgery.
We focused on localised high-grade soft tissue sarcoma of the extremity and trunk treated with definitive surgery and aimed to: 1) examine if the increased TTI is associated with the overall survival (OS), and 2) identify what are the factors influencing the delayed TTI by analysing data from the National Cancer Database (NCDB).
PATIENTS, MATERIALS, AND METHODS
NCDB
The NCDB is a national hospital-based cancer registry jointly sponsored by the American College of Surgeons and the American Cancer Society. Although submissions of patient records to the NCDB were voluntary and open to all cancer facilities in the US, data collection was mandated as a requirement of the Commission on Cancer approved programs since 1997. Currently, NCDB data represent more than 70% of newly diagnosed cancer cases in the US. This study is a STROBE-compliant retrospective study. Due to the anonymous nature of the data, informed consent was waived, and institutional review board approval was not required for this study.
Data extraction
Patients eligible for study inclusion had high-grade, non-metastatic adult soft tissue sarcoma of the extremity or trunk treated with definitive surgery. First, cases of bone and soft tissue sarcoma diagnosed from 2004–2015 were extracted from the NCDB using the International Classification of Diseases-Oncology, Third Edition (ICD-O-3) topography codes (40.x, C41.x, C47.x, C48.x, and C49.x) (n = 136,144). As in the previous reports analysing TTI using NCDB1, 5, 6, 8, 9, TTI was defined as the time from histologic diagnosis of malignancy to initiation of first definitive treatment. Initiation of first definitive treatment is surgery in most of the cases. However, if patients receive preoperative chemotherapy or radiotherapy, initiation of first definitive treatment was defined as the first day of chemotherapy or radiotherapy. Patients with TTI >365 days were excluded because of concerns surrounding miscoding. Inclusion and other exclusion criteria are summarised in Figure 1. We excluded ineligible patients, and the final cohort was composed of 23,786 patients.
Figure 1.
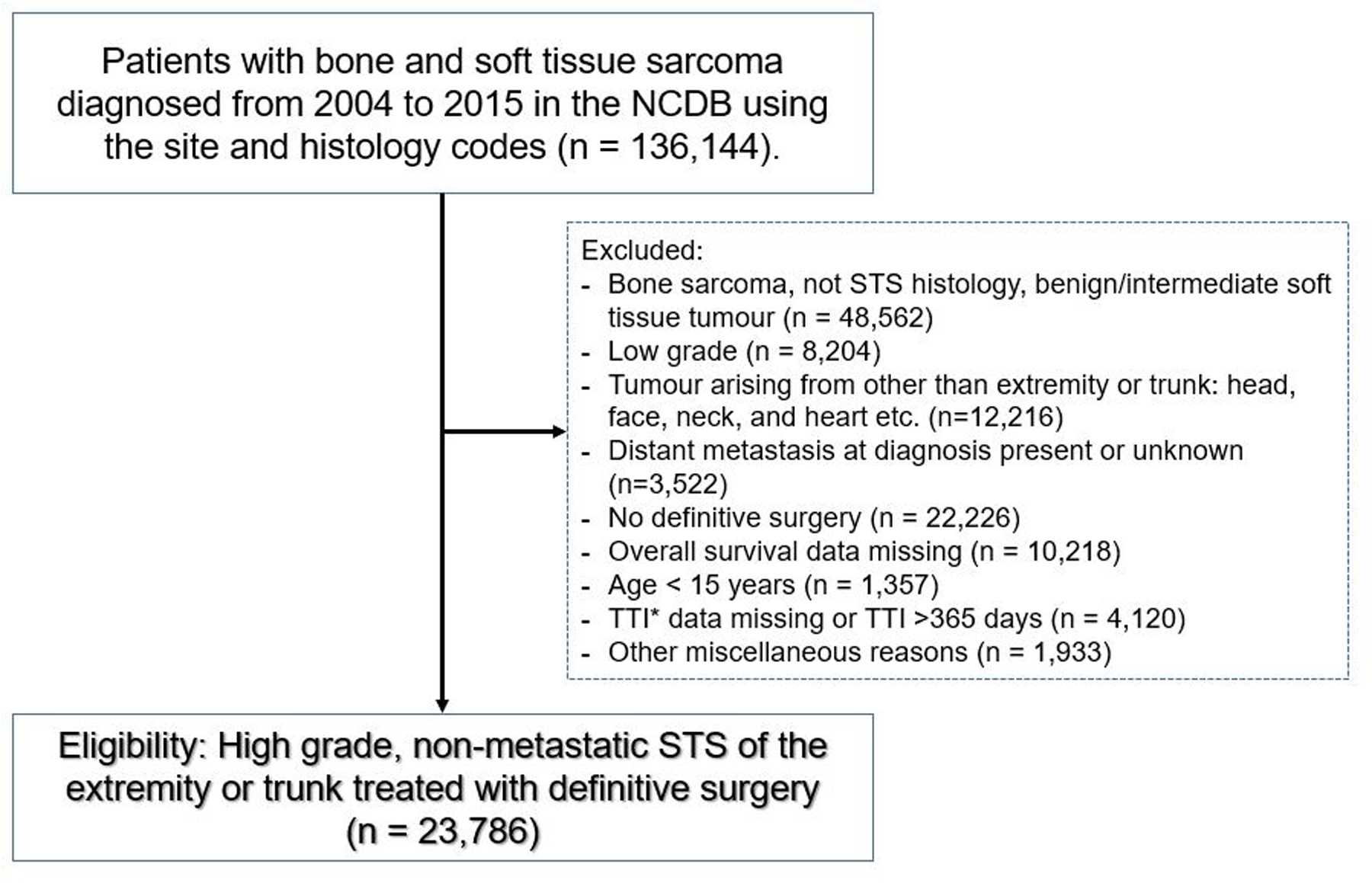
Flow diagram of the study. NCDB = National Cancer Database; STS = soft tissue sarcoma; TTI = time to treatment initiation.
For each patient, we extracted the following data: sex, age (<65 and ≥ 65 years of age), race, insurance status (private insurance, Medicaid, Medicare, other government, uninsured), zip-code level income, zip-code level education, Charlson/Deyo comorbidity scores, tumour location, histological grade, American Joint Committee on Cancer (AJCC) stage, and facility type. Race was categorised into White, Black, Asian, and others, including Pacific Islanders and Native Americans. Type of treatment facility was categorised into community, comprehensive community, academic/research program (includes National Cancer Institute-designated Comprehensive Cancer Centers), or others. Charlson/Deyo comorbidity scores were categorised into 0 (no comorbid conditions reported), 1, or 2 (> 1 comorbid condition reported). Income level was categorised as quartiles based on equally proportioned income ranges among all US zip codes. Education level was defined as the estimated number of adults in a patient’s zip code who did not graduate from high school and is categorised as equally proportioned quartiles among all US zip codes. Histologic grade was categorised into 2 (moderately differentiated), 3 (poorly differentiated), and 4 (undifferentiated). As different AJCC stage editions (sixth and seventh) were used in our dataset, we converted stage from the sixth to seventh as appropriate. These were categorised into stage 2 or 3. The transition in care was defined as a change in facility type from diagnosis to definitive treatment. We categorised TTI into four prespecified groups: 0–30 days, 31–60 days, 61–90 days, and 90– days.
Endpoints
The primary endpoint was OS, which was defined as the period from the date of diagnosis and the date on which the patient was last contacted or died. The secondary endpoints were 30-day and 90-day mortality, which was defined as death 30 or fewer and 90 or fewer days after surgery.
Statistical Analyses
We performed univariate comparisons of proportions using the chi-square test and comparisons of averages using analyses of variance. The OS was estimated using the Kaplan-Meier method, and comparisons were assessed using the log-rank test. Univariate and multivariate analyses were conducted using the Cox proportional hazards model. The TTI was entered into the Cox regression model by using a restricted cubic spline to allow for a nonlinear relationship between TTI and hazard ratio. The model was optimised by testing splines constrained by 3–7 knots, and final model selection was based on the Akaike Information Criteria (AIC) 5, 10. Logistic regression analyses were performed to examine the relationships of each factor with the 30-day and 90-day mortality.
As one of our goals was to determine what factors influenced the TTI, we calculated the incidence rate ratio (IRR) by multivariate analysis using negative binomial regression models, containing all covariates to control for confounders. IRR is defined that for every 1-point increase in the independent variable while holding all other variables in the model constant, the TTI rate (in days) would increase by a factor of that value.
Differences were considered to be statistically significant at P < 0.05. All statistical analyses were conducted using IBM SPSS version 23.0 (IBM SPSS, Armonk, NY, USA) and R version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Demographics
We identified 23,786 eligible patients from 2004–2015. Details of histologic diagnosis and background characteristics of patients according to 30-day interval TTI are shown in Table 1 and Appendix 1. Median and mean TTI was 14.0 days (interquartile range [IQR], 0–32 days) and 20.9 days (standard deviation [SD], 27.5 days), respectively. Median TTI had increased significantly over the year (P < 0.001) (Figure 2). TTI varied significantly according to socioeconomic status, including race, insurance status, income, and education, as well as patient and tumour factors including age, Charlson Deyo score, tumour location/grade, and AJCC stage. Of these, facility type, insurance status, and AJCC stage had the largest difference when compared to each group by median TTI.
Table 1.
Details of histologic diagnosis of all patients (n =23,786).
| Histologic diagnosis | No. (%) |
|---|---|
| UPSa/MFHb | 6,979 (29.3) |
| Leiomyosarcoma | 3,581 (15.1) |
| Liposarcoma | 3,437 (14.4) |
| Myxofibrosarcoma | 1,837 (7.7) |
| Synovial sarcoma | 1,256 (5.3) |
| Spindle cell sarcoma | 1,110 (4.7) |
| Malignant peripheral nerve sheath tumour | 966 (4.1) |
| Hemangiosarcoma (Angiosarcoma) | 504 (2.1) |
| Fibrosarcoma | 421 (1.8) |
| Rhabdomyosarcoma | 393 (1.7) |
| Epithelioid sarcoma | 265 (1.1) |
| Extraskeletal osteosarcoma | 249 (1.0) |
| Others | 2,788 (11.7) |
- UPS = undifferentiated pleomorphic sarcoma
- MFH= malignant fibrous histiocytoma.
Figure 2.
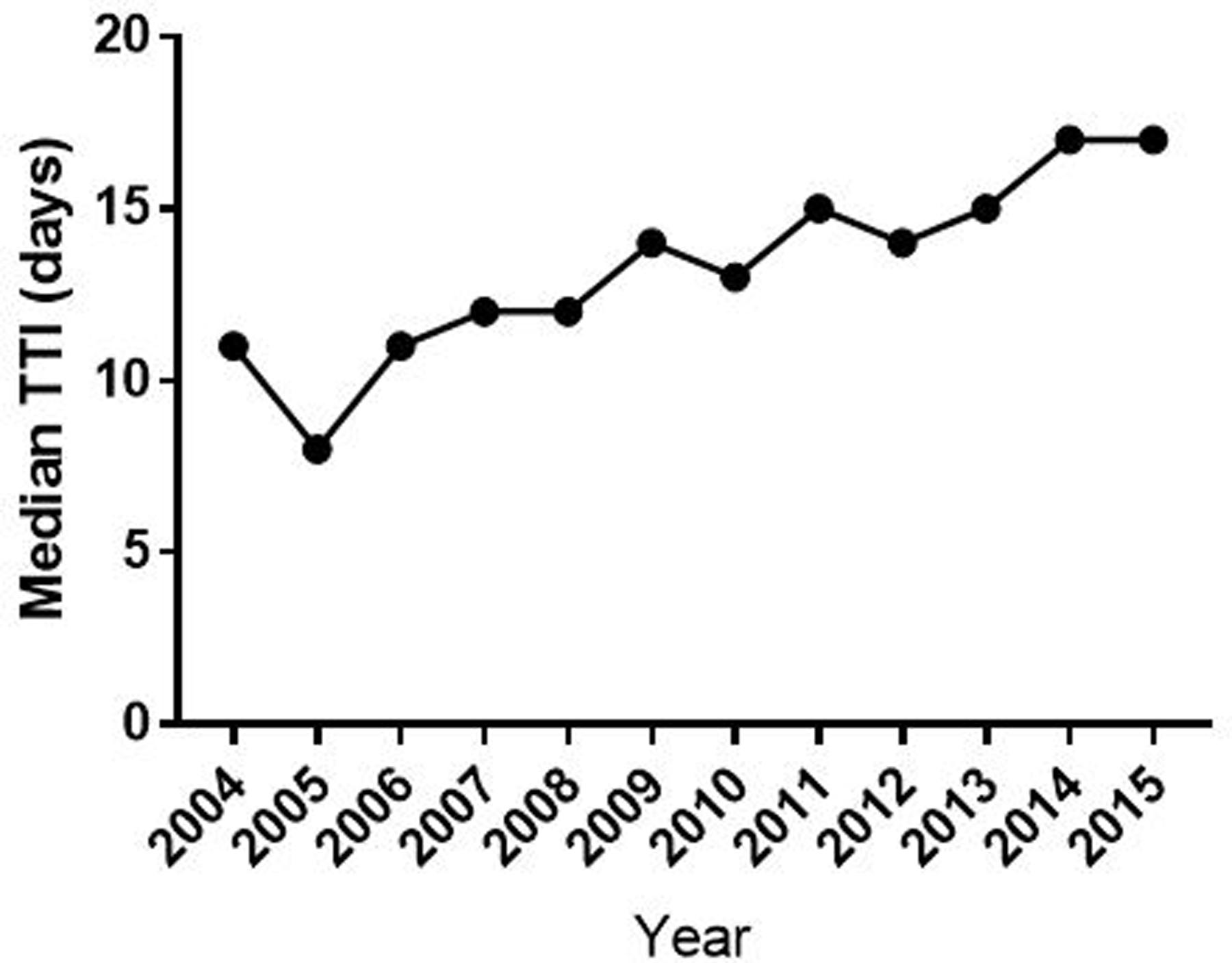
Median TTI by year of diagnosis. TTI = time to treatment initiation.
Impact of TTI on OS and short-term mortality
Figure 3 (left panel) shows the Kaplan–Meier plots for OS stratified by TTI. The five-year OS were 57.7%, 53.9%, 50.6%, and 49.9% in TTI of 0–30 days, 31–60 days, 61–90 days, and 91– days, respectively (log-rank test, P < 0.001). The follow-up rates at 5- and 10-years were 67% and 48%, respectively.
Figure 3.
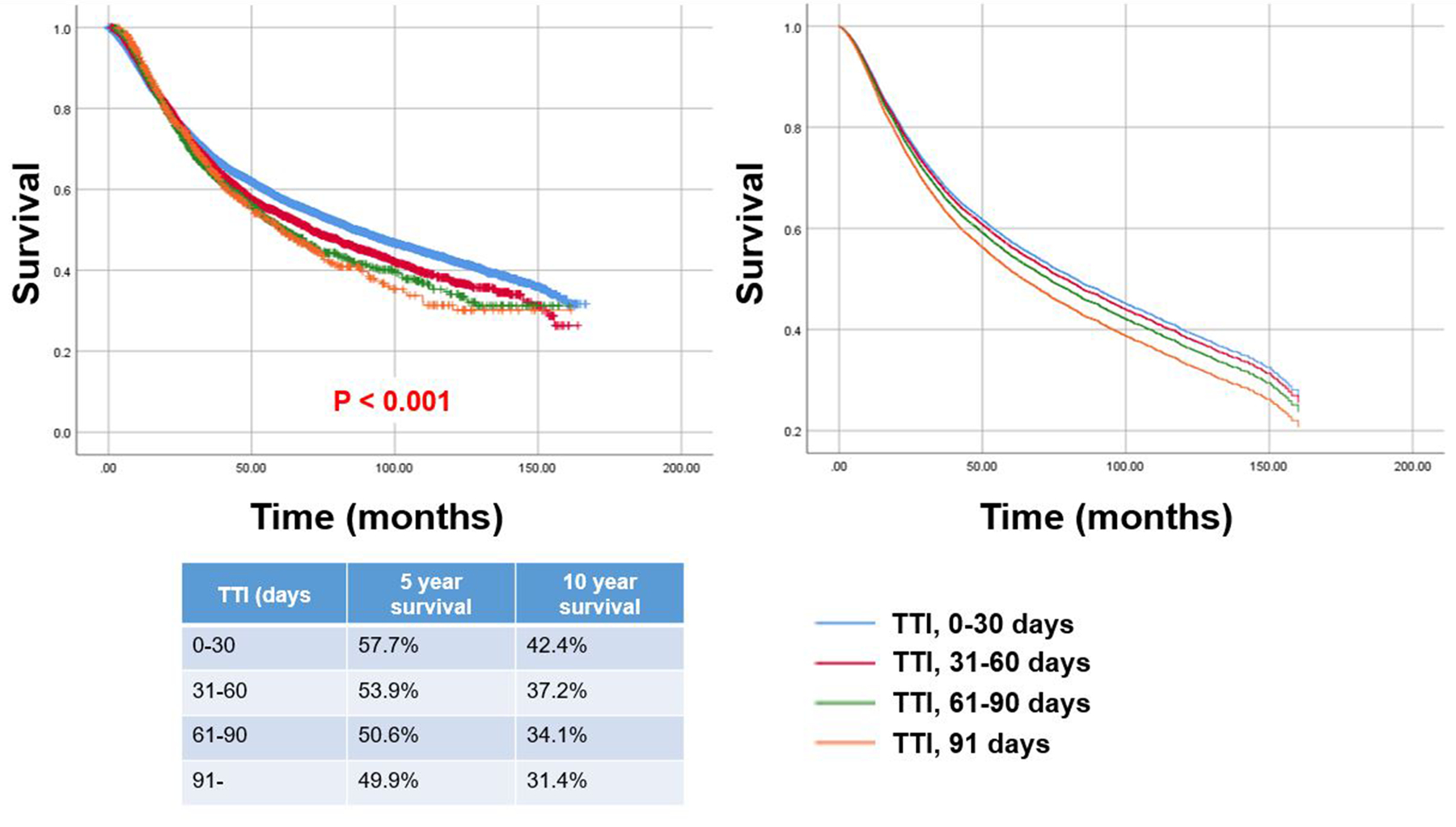
Kaplan-Meier plots of overall survival (OS) for all patients stratified by prespecified TTI groups: 0–30 days, 31–60 days, 61–90 days, and 90– days (left panel) (univariate comparison, log-rank test, P < 0.001). Survival curves according to prespecified time to treatment initiation groups after adjusting for other confounders using Cox regression model (right panel). OS = overall survival; TTI = time to treatment initiation.
Table 2 shows the unadjusted and adjusted hazard ratios obtained from the Cox proportional hazards models for OS. Prolonged TTI was significantly associated with poorer OS compared to TTI of 0–30 days (31–60 days, hazard ratio [HR], 1.08; 95% confidence interval [CI], 1.02–1.14; P = 0.011, 61–90 days, HR: 1.11; 95% CI, 1.00–1.22; P = 0.044, 91– days, HR: 1.22; 95% CI, 1.07–1.39; P = 0.003). Among patient factors, sex (P < 0.001), age (P <0.001), and Charlson/Deyo score (P < 0.001) were significantly associated with OS. Among socioeconomic factors, insurance status, income level, and education level were significantly associated with OS. Tumour or surgical factors, including tumour location (P < 0.001), histologic grade (P < 0.001), AJCC stage (P < 0.001), and surgical margin (P <0.001) were significant predictors for OS. Survival curves according to TTI after adjusting for other confounders using Cox regression model (Figure 3 [right panel]) demonstrated there were clinically meaningful differences in OS.
Table 2.
Univariate and Multivariate Cox Proportional Hazard Model for OS a.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | |
| Total Number of patients | ||||
| Sex | ||||
| Male | Reference | Reference | ||
| Female | 0.92 (0.88–0.95) | < 0.001 | 0.92 (0.88–0.96) | < 0.001 |
| Age | ||||
| ≤64 | Reference | Reference | ||
| ≥65 | 2.00 (1.94–2.06) | < 0.001 | 1.53 (1.43–1.65) | < 0.001 |
| Race | ||||
| White | Reference | Reference | ||
| Black | 1.05 (0.98–1.12) | 0.156 | 0.98 (0.91–1.07) | 0.692 |
| Asian | 0.85 (0.74–0.97) | 0.015 | 0.89 (0.77–1.04) | 0.135 |
| Others | 0.97 (0.80–1.16) | 0.712 | 0.88 (0.70–1.09) | 0.245 |
| Facility type | ||||
| Academic | Reference | Reference | ||
| Comprehensive community | 1.10 (1.05–1.15) | < 0.001 | 1.03 (0.97–1.08) | 0.355 |
| Community | 1.11 (1.01–1.22) | 0.039 | 1.03 (0.92–1.14) | 0.644 |
| Others | 1.02 (0.95–1.08) | 0.612 | 1.01 (0.94–1.08) | 0.773 |
| Insurance status | ||||
| Private insurance | Reference | |||
| Medicaid | 1.50 (1.37–1.64) | < 0.001 | 1.50 (1.34–1.68) | < 0.001 |
| Medicare | 2.06 (1.98–2.15) | < 0.001 | 1.37 (1.27–1.47) | < 0.001 |
| Other government | 1.76 (1.50–2.06) | < 0.001 | 1.57 (1.30–1.89) | < 0.001 |
| Uninsured | 1.39 (1.24–1.56) | < 0.001 | 1.45 (1.25–1.67) | < 0.001 |
| Zip-code level income, $ | ||||
| <38,000 | Reference | |||
| 38,000–47,999 | 0.90 (0.84–0.96) | 0.001 | 0.91 (0.84–0.98) | 0.017 |
| 48,000–62,999 | 0.88 (0.82–0.93) | < 0.001 | 0.91 (0.84–0.99) | 0.023 |
| >63,000 | 0.76 (0.72–0.81) | < 0.001 | 0.89 (0.81–0.97) | 0.006 |
| Zip-code education level | ||||
| ≥21% | Reference | Reference | ||
| 13–20.9% | 0.99 (0.93–1.05) | 0.751 | 1.01 (0.93–1.08) | 0.897 |
| 7–12.9% | 0.90 (0.85–0.96) | 0.001 | 0.94 (0.87–1.02) | 0.135 |
| <7% | 0.81 (0.76–0.86) | < 0.001 | 0.87 (0.80–0.95) | 0.002 |
| Charlson Deyo score | ||||
| 0 | Reference | Reference | ||
| 1 | 1.47 (1.39–1.54) | < 0.001 | 1.22 (1.16–1.29) | < 0.001 |
| 2 | 2.05 (1.89–2.22) | < 0.001 | 1.59 (1.46–1.73) | < 0.001 |
| Tumour location | ||||
| Upper extremity | Reference | Reference | ||
| Lower extremity | 1.03 (0.97–1.09) | 0.340 | 0.99 (0.93–1.06) | 0.763 |
| Trunk | 1.42 (1.34–1.51) | < 0.001 | 1.59 (1.46–1.73) | < 0.001 |
| Histologic grade | ||||
| 2 | Reference | Reference | ||
| 3 | 1.92 (1.78–2.07) | < 0.001 | 1.41 (1.30–1.54) | < 0.001 |
| 4 | 1.89 (1.75–2.04) | < 0.001 | 1.38 (1.26–1.51) | < 0.001 |
| AJCCb stage | ||||
| 2 | Reference | Reference | ||
| 3 | 1.87 (1.80–1.95) | < 0.001 | 1.70 (1.62–1.79) | < 0.001 |
| Surgical margin | ||||
| Negative | Reference | Reference | ||
| Positive | 1.63 (1.55–1.71) | < 0.001 | 1.44 (1.37–1.52) | < 0.001 |
| TTI c | ||||
| 0–30 | Reference | Reference | ||
| 31–60 | 1.10 (1.05–1.16) | < 0.001 | 1.08 (1.02–1.14) | 0.011 |
| 61–90 | 1.18 (1.08–1.29) | < 0.001 | 1.11 (1.00–1.22) | 0.044 |
| 91– | 1.21 (1.07–1.36) | 0.002 | 1.22 (1.07–1.39) | 0.003 |
- OS = overall survival
-AAJCC = American Joint Committee on Cancer
- TTI = time to treatment initiation.
HR of OS adjusted for all covariates according to TTI as a continuous variable by using restricted cubic splines with 5 knots was shown in Figure 4. We found that the HR of death concerning a TTI of zero is the lowest and there is a tendency of increasing HR with TTI being >50 days. Considering substantially increased HR with a TTI > 50 days, we should initiate treatment within 50 days of diagnosis.
Figure 4.
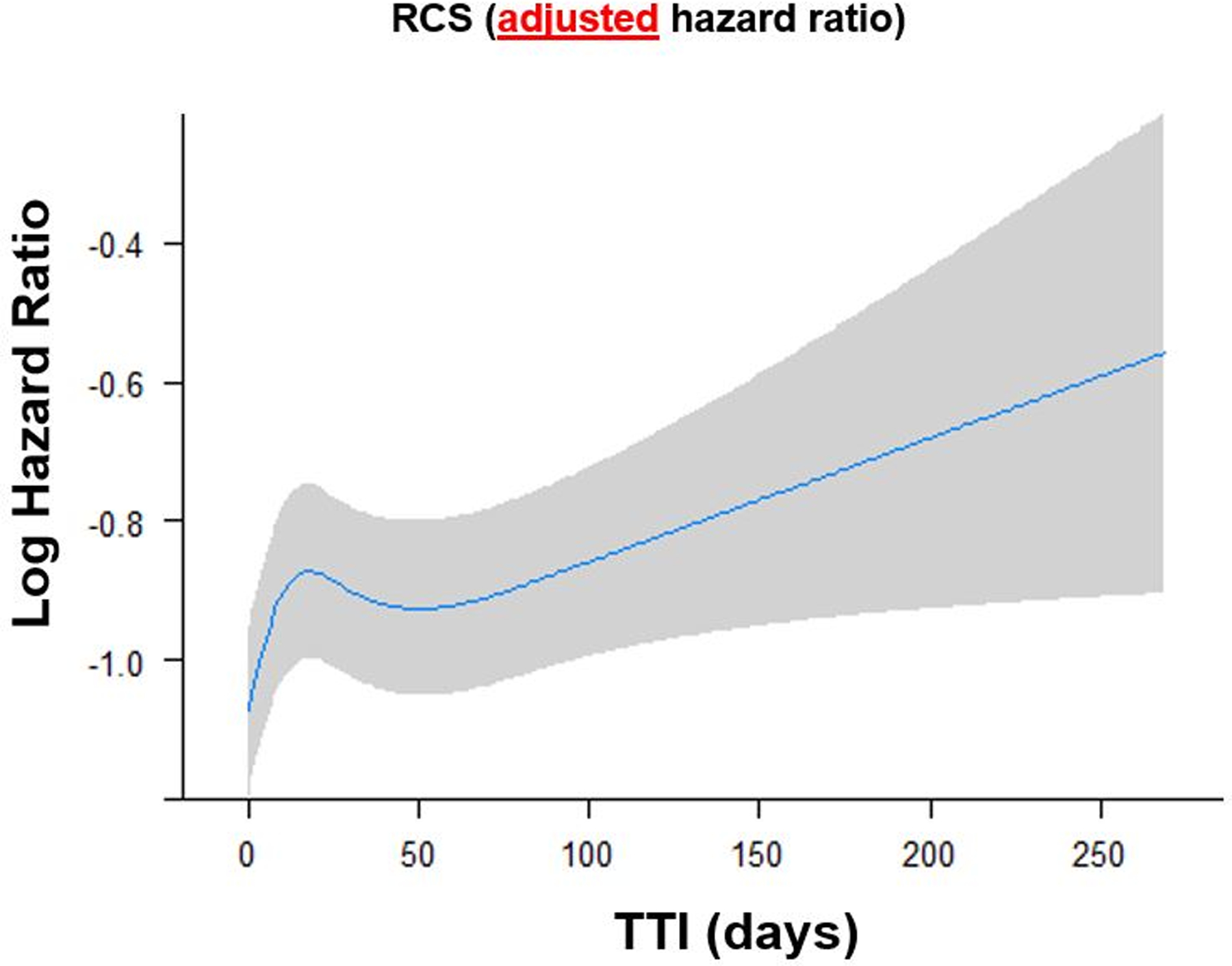
Adjusted HR of overall survival (OS) according to time to treatment initiation (TTI) as a continuous variable by using restricted cubic splines with five knots. The restricted cubic spline allows for a nonlinear relationship of TTI with the log HR of OS after adjusting for other confounders, estimated from the Cox regression model. With a TTI > 50 days, the HR increased substantially. OS = overall survival; TTI = time to treatment initiation.
Data on 30- and 90-day mortality was available in 98.7% (23,465/23,786) and 97.8% (23,260/23,786) of the study population, and these cases were included in the logistic regression analysis to investigate the relationship between short-term mortality and each factor including TTI. Overall short-term mortality was 0.7% and 2.5% at 30-days and 90-days postoperatively, respectively. Multivariate logistic regression analyses did not show significant associations between 30- and 90-day mortality and TTI although some important factors affecting short-term mortality were identified including age, Charlson/Deyo score, tumour location, AJCC stage, and surgical margin (Appendix 2).
Factors affecting TTI
IRRs by multivariate analysis using negative binomial regression models were computed to identify factors affecting TTI (Table 3). Because of high statistical power, all the variables except age were significantly associated with TTI. Of these, facility type, insurance status, AJCC stage, and transition in care had the greatest impact on TTI. Regarding facility type, academic hospital had significantly increased TTI with IRR of other facility types ranging 0.64–0.86. Increased TTI was found in patients with Medicaid (IRR = 1.34; P < 0.001) and those who were uninsured (IRR = 1.17; P < 0.001). Patients with AJCC stage 3 tumour had increased TTI compared to those with stage 2 (IRR = 1.29; P < 0.001). Patients experiencing a transition in care had the greatest increase in TTI (IRR = 1.62; P < 0.001).
Table 3.
Multivariate analysis for incidence rate ratio on TTIa.
| Multivariate analysis | ||
|---|---|---|
| IRRb on TTI (95% CI) | P value | |
| Total No. of patients | ||
| Sex | ||
| Male | Reference | |
| Female | 1.04 (1.03–1.05) | < 0.001 |
| Age | ||
| ≤64 | Reference | |
| ≥65 | 1.00 (0.99–1.01) | 0.554 |
| Race | ||
| White | Reference | |
| Black | 1.09 (1.08–1.10) | < 0.001 |
| Asian | 1.03 (1.01–1.05) | 0.001 |
| Others | 1.12 (1.09–1.15) | < 0.001 |
| Facility type | ||
| Academic | Reference | |
| Comprehensive community | 0.76 (0.75–0.77) | < 0.001 |
| Community | 0.64 (0.63–0.65) | < 0.001 |
| Others | 0.86 (0.85–0.87) | < 0.001 |
| Insurance status | ||
| Private insurance | Reference | |
| Medicaid | 1.34 (1.33–1.36) | < 0.001 |
| Medicare | 1.12 (1.11–1.13) | < 0.001 |
| Other government | 1.18 (1.15–1.21) | < 0.001 |
| Uninsured | 1.17 (1.15–1.19) | < 0.001 |
| Zip-code level income, $ | ||
| <38,000 | Reference | |
| 38,000–47,999 | 1.05 (1.04–1.06) | < 0.001 |
| 48,000–62,999 | 1.07 (1.06–1.08) | < 0.001 |
| >63,000 | 1.06 (1.05–1.08) | < 0.001 |
| Zip-code education level | ||
| ≥21% | Reference | |
| 13–20.9% | 0.95 (0.94–0.96) | < 0.001 |
| 7–12.9% | 0.94 (0.93–0.95) | < 0.001 |
| <7% | 0.90 (0.88–0.91) | < 0.001 |
| Charlson Deyo score | ||
| 0 | Reference | |
| 1 | 1.06 (1.05–1.06) | < 0.001 |
| 2- | 1.15 (1.13–1.16) | < 0.001 |
| Tumour location | ||
| Upper extremity | Reference | |
| Lower extremity | 1.15 (1.14–1.16) | < 0.001 |
| Trunk | 1.03 (1.02–1.04) | 0.020 |
| Histologic grade | ||
| 2 | Reference | |
| 3 | 0.85 (0.84–0.86) | < 0.001 |
| 4 | 0.88 (0.87–0.89) | < 0.001 |
| AJCCc stage | ||
| 2 | Reference | |
| 3 | 1.29 (1.28–1.30) | < 0.001 |
| Transition in care | ||
| No | Reference | |
| Yes | 1.62 (1.61–1.63) | < 0.001 |
- TTI = time to treatment initiation;
- IRR = incident rate ratio;
- AJCC = American Joint Committee on Cancer.
Incidence Rate Ratio means for every 1‐point increase in the independent variable, the rate of TTI (in days) would change by a factor of that value, while holding all other variables in the model constant.
DISCUSSION
The relationship between TTI and outcomes is of current interest, as TTI has increased in the past decade as was shown in our analysis (Figure 2). This could be due to reasons to pursuit better care6, including advances in pretreatment evaluations such as FDG-PET11, 12, and second opinion or transition in care to specialised centers1, 6, 8. Although TTI is shown as a factor to be considered when treating patients in various cancers including breast2, 3, lung4, 7, and head and neck cancers5, 6, information on TTI and its impact on survival in patients with soft tissue sarcoma is limited8, 9.
Curtis et al.8 analysed TTI and influential factors for TTI using 41,592 patients with AJCC stage 1–4 soft tissue sarcoma of the head and neck, extremity, and trunk treated by any treatment modality including palliative surgery and non-surgical treatment. They reported median TTI was 22 days, and transition in care had the greatest impact on increased TTI8. Featherall et al.9 analysed the impact of TTI on OS using 8,648 patients with high-grade soft tissue sarcoma of the head and neck, extremity, and trunk treated by any treatment modality including palliative surgery and non-surgical treatment (13%). Based on their analyses9, there was little to no clinically relevant association of longer TTI with poor survival in contrast to what was observed in other cancer types2–7. However, there have been no reports analysing the impact of TTI on OS in patients with high-grade soft tissue sarcoma of the extremity and trunk treated by definitive surgery. Therefore, we have had no information on what TTI is acceptable to cure this disease. In the present study, median TTI in overall patients was 14 days, which is much shorter than those in previous reports (22 days)8. This is likely because only patients treated with curative intent by definitive surgery were included. The factor that had the greatest impact on increased TTI was a transition in care, which was concordant with the previous study8. We also found facility type, insurance status, and AJCC stage had major impact on TTI. Increased TTI in the academic centers can be explained by the fact that soft tissue sarcoma is rare cancer and usually referred to predominately academic centers.
In contrast to the report by Featherall et al.9, we demonstrated that TTI was associated with OS, and survival difference was evident (Figure 3 and Figure 4). These conflicting conclusions may result from the difference in eligibility. We only included patients treated with curative intent by definitive surgery and excluded patients with soft tissue sarcoma arising in the head and neck where treatment strategy and outcomes are different due to anatomic constraints, leading to difficulty in completely excising tumours with high rates of local recurrence.
Insurance status had a great impact both on prolonged TTI and OS. Given insurance status is considered to directly influence access to health services, its effects to the TTI and OS seems reasonable. Improved insurance reforms, such as Medicaid expansion to nonelderly adults with low incomes in selected states conducted by the Affordable Care Act might improve TTI, as well as OS through better access to health care. As expected, the report analysing the effect of Medicaid expansion on cancer care demonstrated it was associated with a decreased rate of uninsured patients and an increased rate of early-stage cancer diagnosis 13. In the Sarcoma Policy Checklist in Europe, which provides policymakers with priority areas to improve care for sarcoma patients, one of the recommendations is sarcoma patients should have more rapid access to effective treatments14. However, there has not been concrete and quantitative evidence to support this recommendation. We believe our findings reinforce the need of creating the medical care system for STS patients to access to appropriate care in an efficient way.
Notably, the concept and policy of structured referral to specialty centers for high impact rare diseases is standard in the UK and Europe, whereas it is not so widely accepted in the US. Although perverse financial motives and conflicts of interest underly the largely private US system, our findings have the potential to alter the perspective of US physicians and health care systems, and it serves as an affirmation of the UK and European approach. Indeed, the establishment of the National Institute for Health and Care Excellence (NICE) criteria in 2006 for referral of soft tissue masses within two weeks has contributed to the reduction in size of soft tissue sarcomas treated in the UK and improved results15. We hope that similar improvements can be achieved in the US, and critical review of the NCDB data will promote this.
Our study has several limitations. First, database study usually has incomplete or inaccurate data, that can bias the results. There may be an underestimation or overestimation of comorbidities or outcomes due to incomplete reporting. Second, we cannot determine if patient death is cancer-specific or not, as the NCDB does not include the cause of death. Therefore, the relationship between each factor, including TTI and disease-specific survival for soft tissue sarcoma patients, is unknown. Also, the database relies on abstractors to determine whether surgery was performed for curative intent; therefore, it is not known if R2 resected tumours were for curative or palliative intent. Third, the participation rate of small hospitals in the NCDB was relatively low. Hospitals participating in the NCDB represents the American College of Surgeons Commission on Cancer accredited hospitals and does not reflect all inpatient hospitals in the US; this may have resulted in a sample selection bias. Fourth, we may not be able to determine deaths after transfer to another hospital, which may have resulted in an underestimation of outcomes. Fifth, we were not able to identify several important clinical parameters that may have affected the OS, including the severity of preoperative comorbidities, response to chemotherapy, and details of surgery. Sixth, we could not detect the patients’ contribution to treatment delay, or the influence of symptoms-to-treatment interval on OS. Increased TTI is composed of two factors: 1) time from a patient’s first awareness of symptoms to diagnosis (predominantly patient-related), and 2) time from a patient’s first histologic diagnosis to treatment (predominantly physician- or hospital-related). We only focused on physician- or hospital-related TTI because patient-related TTI is not recorded in the NCDB. Additional investigation considering patient-related TTI is warranted to draw a more nuanced statement regarding the effect of TTI on survival in soft tissue sarcoma. Finally, it is possible that undefined comorbid factors could contribute to the delay and affect patient survival. The association of soft tissue doubling time with survival has been reported to be within the relevant span reported in this study (median 26 days; mean 57 days)16. The limited number of factors included in the database preclude testing most potential hypotheses. In addition to reducing patient survival, treatment delays may have other effects such as increased malpractice claims and payments17.
The implications and application of these findings may differ in other medical systems based on insurance system, prevailing patient referral patterns, and the established regionalization of care. For example, significant comorbidity and referral to specialised centers are universal factors affecting longer TTI in many countries and in the US. The prevailing policy guidelines and medical culture influence results as seen in the reduced size and improved outcome of UK soft tissue sarcoma patients since the 2006 NICE criteria mandate for prompt referral to specialized centers. Our finding that insurance was one of the greatest factors affecting delayed treatment initiation cannot be applied to countries that have different insurance systems. New modes of medical care, such as Telemedicine, may help reduce the delays and mitigate these effects regardless of the medical system.
In conclusion, we demonstrated that TTI had a significant impact on OS, but not on short-term mortality, in patients with localised high-grade soft tissue sarcoma in the extremity and trunk treated with definitive surgery. Although differences in patient background characteristics among the groups may have affected outcomes, we identified an independent association between TTI and OS after adjusting for these confounders. We recommend arranging to initiate treatment within 30 days after diagnosis to aim for the cure of localised high-grade soft tissue sarcoma in the extremity and trunk, even though referral to a specialised center is required.
Supplementary Material
TAKE HOME MESSAGE.
Increased time to treatment initiation is associated with poorer overall survival in patients with soft tissue sarcoma.
We recommend initiating treatment within 30 days after diagnosis to achieve the highest likelihood of cure for localised high-grade soft tissue sarcoma in the extremity and trunk, even when case referral to a specialised center is required.
FUNDING
This study was funded in part through the NIH/NCI Cancer Center Support Grant, P30 CA008748.
Footnotes
CONFLICT OF INTEREST STATEMENT
None declared.
ETHICAL COMMITTEE APPROVAL STATEMENT
Due to the anonymous nature of the data, informed consent was waived and institutional review board approval was not required for this study.
REFERENCES
- 1.Khorana AA, Tullio K, Elson P, Pennell NA, Grobmyer SR, Kalady MF, et al. Time to initial cancer treatment in the United States and association with survival over time: An observational study. PLoS One 2019;14(3):e0213209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bleicher RJ, Ruth K, Sigurdson ER, Beck JR, Ross E, Wong YN, et al. Time to Surgery and Breast Cancer Survival in the United States. JAMA Oncol 2016;2(3):330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bleicher RJ, Ruth K, Sigurdson ER, Ross E, Wong YN, Patel SA, et al. Preoperative delays in the US Medicare population with breast cancer. J Clin Oncol 2012;30(36):4485–4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gomez DR, Liao KP, Swisher SG, Blumenschein GR, Erasmus JJ Jr., Buchholz TA, et al. Time to treatment as a quality metric in lung cancer: Staging studies, time to treatment, and patient survival. Radiother Oncol 2015;115(2):257–263. [DOI] [PubMed] [Google Scholar]
- 5.Murphy CT, Galloway TJ, Handorf EA, Egleston BL, Wang LS, Mehra R, et al. Survival Impact of Increasing Time to Treatment Initiation for Patients With Head and Neck Cancer in the United States. J Clin Oncol 2016;34(2):169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murphy CT, Galloway TJ, Handorf EA, Wang L, Mehra R, Flieder DB, et al. Increasing time to treatment initiation for head and neck cancer: an analysis of the National Cancer Database. Cancer 2015;121(8):1204–1213. [DOI] [PubMed] [Google Scholar]
- 7.Samson P, Patel A, Garrett T, Crabtree T, Kreisel D, Krupnick AS, et al. Effects of Delayed Surgical Resection on Short-Term and Long-Term Outcomes in Clinical Stage I Non-Small Cell Lung Cancer. Ann Thorac Surg 2015;99(6):1906–1912; discussion 1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curtis GL, Lawrenz JM, George J, Styron JF, Scott J, Shah C, et al. Adult soft tissue sarcoma and time to treatment initiation: An analysis of the National Cancer Database. J Surg Oncol 2018;117(8):1776–1785. [DOI] [PubMed] [Google Scholar]
- 9.Featherall J, Curtis GL, Lawrenz JM, Jin Y, George J, Scott J, et al. Time to treatment initiation and survival in adult localized, high-grade soft tissue sarcoma. J Surg Oncol 2019;120(7):1241–1251. [DOI] [PubMed] [Google Scholar]
- 10.Gauthier J, Wu QV, Gooley TA. Cubic splines to model relationships between continuous variables and outcomes: a guide for clinicians. Bone Marrow Transplant 2020;55(4):675–680. [DOI] [PubMed] [Google Scholar]
- 11.Hillner BE, Tosteson AN, Song Y, Tosteson TD, Onega T, Goodman DC, et al. Growth in the use of PET for six cancer types after coverage by medicare: additive or replacement? J Am Coll Radiol 2012;9(1):33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lonneux M, Hamoir M, Reychler H, Maingon P, Duvillard C, Calais G, et al. Positron emission tomography with [18F]fluorodeoxyglucose improves staging and patient management in patients with head and neck squamous cell carcinoma: a multicenter prospective study. J Clin Oncol 2010;28(7):1190–1195. [DOI] [PubMed] [Google Scholar]
- 13.Takvorian SU, Oganisian A, Mamtani R, Mitra N, Shulman LN, Bekelman JE, et al. Association of Medicaid Expansion Under the Affordable Care Act With Insurance Status, Cancer Stage, and Timely Treatment Among Patients With Breast, Colon, and Lung Cancer. JAMA Netw Open 2020;3(2):e1921653. [DOI] [PubMed] [Google Scholar]
- 14.Kasper B, Lecointe-Artzner E, Wait S, Boldon S, Wilson R, Gronchi A, et al. Working to improve the management of sarcoma patients across Europe: a policy checklist. BMC Cancer 2018;18(1):424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith GM, Johnson GD, Grimer RJ, Wilson S. Trends in presentation of bone and soft tissue sarcomas over 25 years: little evidence of earlier diagnosis. Ann R Coll Surg Engl 2011;93(7):542–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakamura T, Matsumine A, Matsubara T, Asanuma K, Uchida A, Sudo A. Clinical impact of the tumor volume doubling time on sarcoma patients with lung metastases. Clin Exp Metastasis 2011;28(8):819–825. [DOI] [PubMed] [Google Scholar]
- 17.Hwang R, Park HY, Sheppard W, Bernthal NM. Delayed Diagnosis Is the Primary Cause of Sarcoma Litigation: Analysis of Malpractice Claims in the United States. Clin Orthop Relat Res 2020;478(10):2239–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


