Abstract
To understand the risks and outcomes of COVID-19 in the sickle cell disease (SCD) population, our team established a rapid reporting registry to collect data on the course of COVID-19 illness in individuals with SCD. The registry includes cases reported voluntarily by providers. All data are collected through an online case report form available at covidsicklecell.org. The registry helped to recognize patients with SCD as a population at risk of severe COVID-19 illness and to identify comorbidities that put them at higher risk. In this report, we present data on 1045 reported COVID-19 cases based during a two-year long data collection period. Data include 590 (56.5%) children and 455 (43.5%) adults; 51.2% of total population were female. Most individuals (63.1%) had HbSS genotype. Majority of individuals experienced mild symptoms (62.2% of children, 55.6% of adults). We also present a perspective on setting up the registry and experiences through its growth.
Keywords: Sickle cell disease, COVID-19
On March 11th, 2020, the World Health Organization declared COVID-19 a pandemic [1]. In this time of uncertainty, with many questions and concerns, the priority of the clinical and research community interested in improving care and outcomes of individuals with sickle cell disease (SCD) was to keep this population safe and informed. Individuals with SCD are an underserved patient population at high risk of morbidity and mortality due to infectious diseases [2], and there was a need for a unified source of information for both the patients and the providers who care for them. No current existing registries collected information on COVID-19 at the time the pandemic started. Thus, there was a need to create a provider-reported registry to better understand the impact of COVID-19 for multiple chronic diseases, including SCD. We therefore created the SECURE-SCD (www.covidsicklecell.org) registry to collect data on COVID-19 cases among individuals with SCD. This registry was modeled after a similar registry named SECURE-IBD (covidibd.org), a registry that gathered information on COVID-19 in patients with inflammatory bowel disease [3]. Our team of providers, researchers, research coordinators and a program manager directly engaged the Clinical and Translational Science Institute (CTSI) of Southeast Wisconsin and the Institutional Review Board (IRB) at the Medical College of Wisconsin to establish the SECURE-SCD registry. With the help and support of this multidisciplinary team, our SECURE-SCD registry went live at covidsicklecell.org on March 20th, only 9 days after the pandemic proclamation by the World Health Organization.
A link to the registry with case report form (https://covidsicklecell.org) was distributed to healthcare providers and institutions caring for individuals with SCD via email invites, social media platforms, patient advocacy networks and was made available on the Centers for Disease Control and Prevention and American Society of Hematology websites. We also presented information about our registry on various national and international platforms. Providers and institutions were encouraged to report COVID-19 cases in their patients with SCD through an online case report form available on the website. The online case report form included data on the reporter, patient demographics, medical history, existing comorbidities depending on patient's age. Pediatric comorbidities included anxiety, depression, and/or behavioral problems, attention deficit/hyperactivity disorder, developmental delay or learning disability, headaches, seizure disorder, sleep disturbances, stroke, asthma, current cigarette smoking, current use of other tobacco products other than cigarettes, celiac disease, diabetes, kidney disease, obesity, thyroid disease; whereas adult comorbidities included cardiovascular disease including coronary artery disease, heart failure, arrhythmia, etc., asthma, chronic obstructive pulmonary disease, other chronic lung disease, current cigarette smoking, current use of other tobacco products other than cigarettes, hypertension, cancer, chronic liver disease. Information on COVID-19-related symptoms, healthcare utilization, treatments, outcomes and vaccinations (once available) were also collected. The full case report form can be found at https://covidsicklecell.org/wp-content/uploads/2020/04/SecureCOVID19SCD_CovidSickleCe-1.pdf. Data are deidentified, in accordance with Health Insurance Portability and Accountability Act (HIPAA) Safe Harbor De-Identification standards. The Medical College of Wisconsin Human Research Protection Program has determined that storage and analysis of de-identified data does not constitute human subjects research as defined under federal regulations [45 CFR 46.102] and does not require Institutional Review Board (IRB) approval. Study data were collected and managed using REDCap electronic data capture tools hosted at the Medical College of Wisconsin [4,5].
In order to make the data publicly available and easily accessible to everyone, we publish aggregate data at covidsicklecell.org weekly and transitioned to monthly in May 2021. The aggregate data include patient demographics, geographic distribution, medical history, COVID-19 course of infection and deaths due to COVID-19. Aggregated statistics are also split by age groups in years (0–9, 10–18, 19–30, 31–45, 46–64, 65+).
In May 2020, the importance of the registry was recognized by the Centers for Disease Control and Prevention (CDC) who quickly became aware of our registry, and we collaborated with them to publish a report in the journal Emerging Infectious Diseases [6]. This manuscript included data on 178 reported patients with case fatality rate at that time of 7.3%. Soon after this publication, SCD was recognized by the CDC as a high-risk condition for severe COVID-19 outcomes [7] and as a prioritized group for vaccination.
In July 2021 we published a more in-depth analysis of the data from the Registry where we identified SCD patient characteristics that were risk factors for serious COVID-19 illness and hospitalizations [8]. Serious COVID-19 illness included pneumonia with or without clinical symptoms, early respiratory symptoms or gastrointestinal symptoms followed by dyspnea and hypoxia (o2 saturations <92%), acute respiratory distress syndrome, respiratory failure, encephalopathy, shock, coagulopathy, multiorgan impairment (lung, heart, kidney, brain) that may be life threatening [9]. In this analysis that included 750 reported cases we found that children with prior acute care visits for pain, SCD related heart/lung comorbidities or those with SCD related renal comorbidities are at higher risk of serious COVID-19 illness. Children with a history of frequent pain and heart and lung comorbidities are also more likely to require hospitalization due to COVID-19. We also found that adults with a history of frequent pain are at higher risk of both hospitalization and developing serious COVID-19 illness. Genotype and use of hydroxyurea did not impact outcomes. Interestingly, a large electronic health record (EHR) database study showed that after adjusting for differences in comorbidities, hospitalization for COVID-19 illness continued to be significantly higher among individuals with SCD compared to those who do not have SCD. However, it is known that individuals with SCD are at high risk of developing these comorbidities and future work is necessary to understand their risk factors to improve all outcomes for the SCD population [10].
In this report, we describe data gathered from the SECURE-SCD during the first two years of COVID-19 pandemic, from March 20th, 2020 to March 22nd, 2022 and present our perspective of developing and managing this Registry.
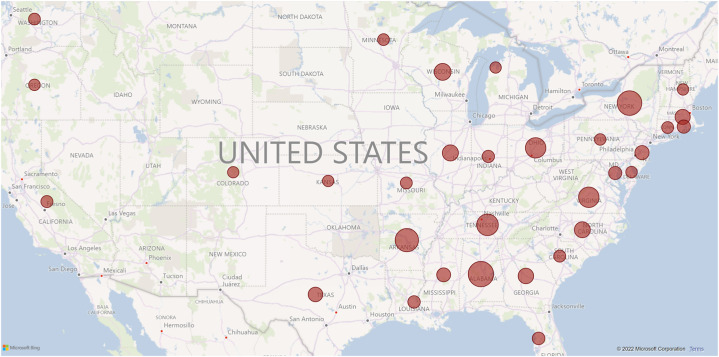
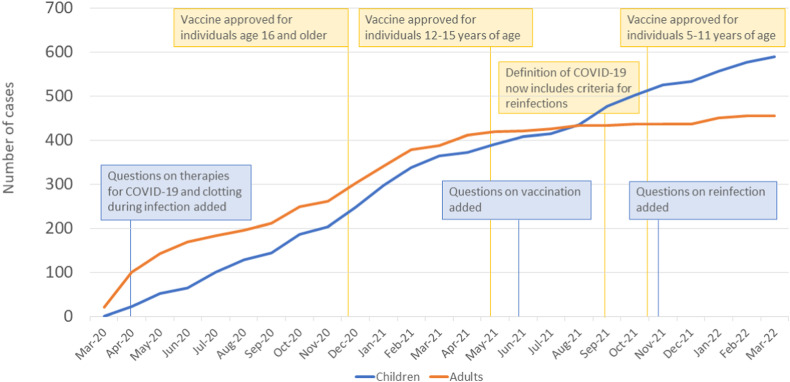
On March 22nd, 2022, when data for this paper were analyzed, there were 1045 cases reported to the Registry. While the majority of the cases are from the United States (913, 87.4%), 17 other countries contributed to the Registry with their reports: Belgium, Brazil, Canada, Dominican Republic, France, French Guiana, Greece, Guadeloupe, Italy, Lebanon, Nigeria, Oman, Saint Martin (French part), Serbia, South Africa, Sweden, Switzerland. Fig. 1 shows the density of reported cases in the United States per individual states. More than 90% of the reported cases came from academic centers. The majority of the reported cases (88.9%) were identified as Black and 536 (51.2%) were female. Out of these, 590 were children (56.5%) and 455 were adults (43.5%). Fig. 2 shows cumulative cases from establishing the Registry in March 2020 to March 2022 for both children and adults. We observe that the number of adult cases increased until early 2021 when they appear to reach a plateau. From March 2021 to March 2022 we observed less than one hundred reported cases in adults. In children, we observe a slower rise in the number of cases in the beginning, but the absence of the plateau in late 2021 and 2022 that was seen in adults. In August 2021, the number of reported pediatric cases surpassed the number of adult cases.
Fig. 1.
Geographic location of cases reported in the SECURE-SCD from March 2020 to March 2022.
Fig. 2.
Cumulative cases reported in SECURE-SCD from March 2020 to March 2022.Blue and orange lines represent number of cumulative cases reported to the SECURE-SCD from March 2020 to March 2022 in children and adults by month. Yellow boxes show timeline of the eligibility of the different age groups for the vaccine since approval in December 2020. Blue boxes represent the timeline of the questions added to the SECURE-SCD report form.
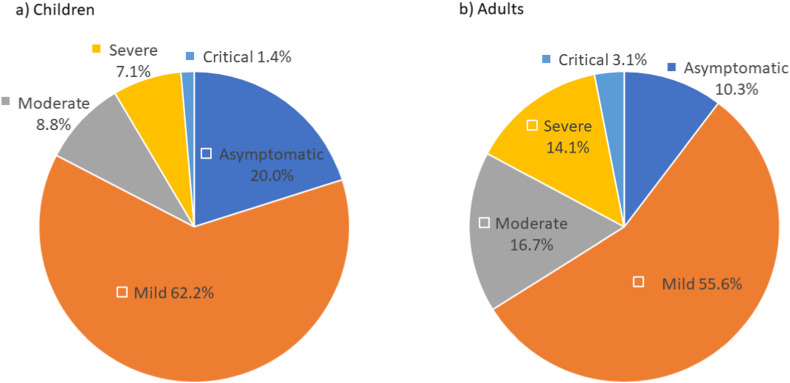
Reported patients have the following genotype distribution: HbSS genotype in 659 (63.1%), HbSβ0 in 44 (4.2%), HbSC in 234 (22.4%) and HbSβ+ in 77 (7.4%) of patients. Data show 576 patients (55.1%) reported using hydroxyurea and 112 (10.6%) had a history of being on chronic transfusions. The most common comorbidity was asthma in both children and adults (25.2% and 15.0%). Of the SCD-related complications reported in the medical history, pain crisis was the most common, reported in 45.6% of children and 69.5% of adults, followed by acute chest syndrome in 29.0% of children and 28.1% of adults. Pain was also the most common SCD-related symptom during COVID-19 illness, reported in 33.6% of children and 67.9% of adults, followed by acute chest syndrome/pneumonia in 15.6% of children and 27.3% of adults. Data are available for 934 patients since introducing the question on thromboembolism. Venous clots occurred in 5 patients (0.05%), pulmonary embolus in 11 patients (1.2%) and arterial clot in 2 patients (0.2%). Fig. 3 shows the severity of the symptoms during COVID-19 infection in children and adults.
Fig. 3.
Severity of the COVID-19 symptoms in children (a) and adults (b). Symptoms were categorized as following: asymptomatic-no clinical signs or symptoms during the positive COVID-19 period; mild-symptoms of acute upper respiratory tract infection, including fever, fatigue, myalgia, cough, sore throat, runny nose, and sneezing or gastrointestinal symptoms or digestive symptoms such as nausea, vomiting, abdominal pain, and diarrhea; moderate-pneumonia with or without clinical symptoms, no hypoxia; severe-early respiratory symptoms or gastrointestinal symptoms followed by dyspnea and hypoxia (O2 saturations <92%); critical-acute respiratory distress syndrome, respiratory failure, encephalopathy, shock, coagulopathy, multiorgan impairment (lung, heart, kidney, brain) that may be life threatening.
Hospitalization occurred in 226 (38.3%) pediatric patients, whereas 262 (57.6%) adults with SCD and COVID-19 required hospitalization. Prior evidence shows that individuals with SCD are at a much higher risk of hospitalization as compared to the general population [10]. However, the observation of adults with SCD having more severe disease and hospitalization compared to children with SCD is similar to that of general population.
Average length of stay in the hospital for children was 5.4 days. Of the hospitalized patients, 95 (42.0%) received transfusion and of those that were transfused, 75 patients (78.9%) received a simple transfusion, and 20 patients (21.1%) received an exchange transfusion. Of those hospitalized, 33 patients (14.6%) were admitted to the intensive care unit and 8 patients (3.5%) required ventilatory support. Death occurred in one child (0.2%).
Average length of hospital stay for adults was 8.7 days. Of the hospitalized patients, 137 (52.3%) received transfusion and of those that were transfused, 99 patients (72.2%) received a simple transfusion, and 36 patients (26.3%) received an exchange transfusion. Of those hospitalized, 44 patients (16.8%) were admitted to the intensive care unit and 14 patients (5.3%) required ventilatory support. Death occurred in 18 adults (4.0%).
Since the beginning of the pandemic, global efforts to identify potential therapeutics for COVID-19 have resulted in the approval of many known and new drugs for use as treatment for COVID-19. As treatments gained FDA approval, we added questions about some of these therapies to the registry. Captured therapies include hydroxychloroquine, azithromycin, convalescent plasma, corticosteroids, tocilizumab, remdesivir, lopinavir/ritonavir, heparin and COVID-19 antibody therapy. We introduced the question on therapies for COVID-19 management in April 2020 and 961 cases have that information reported. The most commonly used COVID-19 therapies during COVID-19 infection are shown in Table 1 . Therapies received by less than 10 cases are not reported in the tables (tocilizumab, lopinavir/ritonavir, and COVID-19 antibody therapy).
Table 1.
Most common therapies (used in more than 10 cases) in hospitalized and not hospitalized pediatric cases (a) and adult cases (b) with SCD and COVID-19 illness.
| a) Pediatric Cases | |||||
|---|---|---|---|---|---|
| Therapy | Hospitalized (N = 218) N (%) | Not hospitalized (N = 356) N (%) | Total (N = 575) N (%) | ||
| Azithromycin | 73 (33.5) | 7 (2.0) | 80 (13.9) | ||
| Corticosteroids | 46 (21.1) | 2 (0.6) | 48 (8.3) | ||
| Heparin | 66 (30.3) | 3 (0.8) | 69 (12.0) | ||
| Remdesivir |
38 (17.4) |
1 (0.3) |
39 (6.8) |
||
| b) Adult Cases | |||||
| Therapy | Hospitalized (N = 215) N (%) | Not hospitalized (N = 166) N (%) | Total (N = 386) N (%) | ||
| Azithromycin | 75 (34.9) | 30 (18.1) | 105 (27.2) | ||
| Corticosteroids | 32 (14.9) | 5 (3.0) | 37 (9.6) | ||
| Heparin | 103 (47.9) | 3 (1.8) | 106 (27.5) | ||
| Remdesivir | 34 (15.8) | 2 (1.2) | 36 (9.3) | ||
| Hydroxychloroquine | 21 (9.8) | 2 (1.2) | 23 (6.0) | ||
| Convalescent plasma | 11 (5.1) | 2 (1.2) | 13 (3.4) | ||
As the pandemic has evolved, numerous public health tools have been recommended to control the spread of COVID-19 and reduce the associated morbidity and mortality. Vaccinations has been one the tools that has shown to be highly effective to prevent severe COVID-19 illness [11,12]. In order to continue to inform our stakeholders, we introduced questions on vaccination status in June 2021. Since then, 225 cases (191 children (84.9%) and 34 adults (15.1%)) were reported to the Registry. Of these, 36 (16%) received at least one dose of the vaccine. There were 25 children (69.4%) and 11 adults (30.6%) among vaccinated patients. The majority of the vaccinated patients (77.8%) experienced only mild COVID-19 symptoms; 11.1% were asymptomatic, 5.6% experienced moderate and 5.6% experienced severe symptoms. None of the vaccinated patients experienced critical symptoms.
In September 2021, CDC updated the national surveillance case definition of COVID-19 to include criteria for capturing reinfections [13]. As a result, we also added a question on reinfection to the registry in November 2021. Since then, 104 cases were reported to the Registry and 4 patients (3.8%) already had previous COVID-19 infection, but they have not been reported in the Registry previously. Future work is needed to understand the impact of vaccinations and treatments on severity of COVID-19 illness and reinfection in individuals with SCD, as well as the trajectory and consequences of long COVID-19 illness in these individuals.
We believe that experiences from developing and managing the SECURE-SCD Registry have provided valuable lessons and insight for potential future infectious diseases epidemics or pandemics. Near-real time reporting of de-identified data via online case report forms is a quick and simple way for gathering information. Public availability of aggregated data raises awareness, encourages providers to report cases and informs the providers and patients. National and international collaboration is imperative, especially for SCD, as well as active dissemination of the data. We shared and presented up-to-date findings at many invited national and international conferences, workshops, and webinars in order to inform the wider SCD and scientific community as the SECURE-SCD Registry was accepted as a robust data source. Most importantly, this collaborative effort helped to inform policy makers thereby protecting vulnerable populations such as individuals with SCD as their care became a priority.
There are some limitations to the SECURE-SCD Registry that should be taken into consideration when interpreting the data. First, these are self-reported cases so only a limited number of cases are reported. However, with COVID-19 being a novel virus and no existing national level SCD registry, the SECURE-SCD registry provided data for a large number of patients in the crucial time period of the pandemic and continues to do so. Also, because cases are reported voluntarily and only by medical providers, it is possible that only the more severe cases might be reported more commonly, while asymptomatic cases could be underreported. However, we do observe a variety in severity of the reported cases. It is also possible that some cases were reported later after they resolved since the Registry has not been created for the purpose of the temporal-trend analysis. Low reporting rates in late 2021 and 2022 might be due to provider reporting fatigue and this should be taken into consideration when interpreting data from later phases of the pandemic. For future efforts, the addition of documenting a specific timeframe (month/year) of the illness for each case would help in understanding the disease dynamic within both individuals and the population and how it changes over time. With more resources, it would be beneficial to increase collaboration with institutions’ bioinformatics teams to include additional details such as laboratory test results that can be extracted directly from electronic health record data, while maintaining the de-identified nature of reporting. Also, creating a centralized national database connected to electronic health records would facilitate the collection of more granular data in addition to overcome concerns about reporting bias. With growing registry and surveillance efforts (i.e., American Society of Hematology Clinical Trials Network, Centers for Disease Control and Prevention, National Alliance for Sickle Cell Centers) – SCD data should be more rapidly available.
In conclusion, SECURE-SCD has been a valuable tool for learning about the impact that COVID-19 has on individuals with SCD. It has also had a pivotal role in informing the sickle cell community and general population in near-real time.
The SECURE-SCD Registry would not be possible without the contribution of reporters worldwide. The authors acknowledge the institutions and individuals for taking the time to contribute their case reports to the Registry. A full list of contributing reporters and institutions is available at covidsicklecell.org.
Funding
This work was supported by Doris Duke Charitable Foundation grant 2020079 and National Institutes of Health National Center for Advancing Translational Sciences award number UL1TR001436.
Practice points
-
•
Individuals with sickle cell disease (SCD) are at higher risk of serious COVID-19 illness and hospitalization compared to general population
-
•
Some sickle cell-related conditions, such as history of frequent pain events, put these individuals at a higher risk of serious COVID-19 illness and hospitalization
-
•
The most frequent sickle cell-related presenting symptoms of COVID-19 illness in individuals with SCD were pain and acute chest syndrome
-
•
SECURE-SCD Registry, a collaborative source which includes de-identified information on the reported COVID-19 cases among individuals with SCD, provides data to understand the impact of COVID19 on the vulnerable population of SCD
Research agenda
-
•
Further research is necessary to understand the effectiveness of recommended treatments for individuals with sickle cell disease (SCD) and severe COVID-19 illness.
-
•
Trajectory of long COVID-19 illness in individuals with SCD is not known.
-
•
Future work should seek to understand the impact of vaccines on COVID-19 severity and reinfections in individuals with SCD
Declaration of competing interest
The authors declare no competing financial interests.
References
- 1.WHO Director General's opening remarks at the media briefing on COVID19. https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020
- 2.Reeves S.L., Jary H.K., Gondhi J.P., Kleyn M., Dombkowski K.J. Health outcomes and services in children with sickle cell trait, sickle cell anemia, and normal hemoglobin. Blood Adv. 2019;3(10):1574–1580. doi: 10.1182/bloodadvances.2018028043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Surveillance epidemiology of coronavirus (COVID-19) under research exclusion SECURE-IBD. https://covidibd.org
- 4.Harris P.A., Taylor R., Minor B.L., Elliott V., Fernandez M., O'Neal L., et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inf. 2019;95 doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inf. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panepinto J.A., Brandow A., Mucalo L., Yusuf F., Singh A., Taylor B., et al. Coronavirus disease among persons with sickle cell disease, United States, March 20-may 21, 2020. Emerg Infect Dis. 2020;26(10):2473–2476. doi: 10.3201/eid2610.202792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention People with certain medical conditions. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html
- 8.Mucalo L., Brandow A.M., Dasgupta M., Mason S.F., Simpson P.M., Singh A., et al. Comorbidities are risk factors for hospitalization and serious COVID-19 illness in children and adults with sickle cell disease. Blood Adv. 2021;5(13):2717–2724. doi: 10.1182/bloodadvances.2021004288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong Y., Mo X., Hu Y., Qi X., Jiang F., Jiang Z., et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145(6) doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 10.Singh A., Brandow A.M., Panepinto J.A. COVID-19 in individuals with sickle cell disease/trait compared with other Black individuals. Blood Advances. 2021;5(7):1915–1921. doi: 10.1182/bloodadvances.2020003741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention Rates of laboratory-confirmed COVID-19 hospitalizations by vaccination status. https://covid.cdc.gov/covid-data-tracker/#covidnet-hospitalizations-vaccination
- 12.Centers for Disease Control and Prevention Rates of COVID-19 cases and deaths by vaccination status. https://covid.cdc.gov/covid-data-tracker/#rates-by-vaccine-status
- 13.Centers for Disease Control and Prevention Coronavirus disease 2019 (COVID-19) 2021 case definition. https://ndc.services.cdc.gov/case-definitions/coronavirus-disease-2019-2021/