Background:
Osteosarcoma (OS) is the primary malignant bone tumor that most commonly affects children, adolescents, and young adults. MicroRNA-34a (miR-34a) is involved in tumor metastasis and may be a prognostic marker for patients with cancer. The aim of the present study was to explore the role of miR-34a in patients with OS. The underlying associations between miR-34a expressions and metastasis, recurrence as well as and prognosis were comprehensively analyzed in OS patients.
Methods:
Reverse transcriptase quantitative PCR (RT-qPCR) was used to investigate serum level of miR-34a between clinical OS patients (n = 162) and age-matched healthy controls (n = 162). Expression of miR-34a in OS tissues and adjacent tissues was analyzed using RT-qPCR. RT-qPCR was used to compare the serum level of miR-34a in patients with OS before and after chemotherapy. Multivariate Cox-regression analysis was used to analyze the association between serum level of miR-34a and chemotherapy resistance, overall survival, as well as recurrence and prognosis of OS patients. Five-year recurrence and survival were estimated using Kaplan–Meier curves.
Results:
Serum level of miR-34a was downregulated in OS patients (n = 86) compared to age-matched healthy controls (n = 86). Expression of miR-34a was downregulated in OS tissue compared to adjacent tissues in clinical patients. The expression of serum miR-34a before and after chemotherapy was positively correlated with the expression of miR-34a in the corresponding tissues. Expression of miR-34a was higher in the group where chemotherapy was effective than that patient where chemotherapy was ineffective. Expression of miR-34a was negatively associated with chemotherapy resistance of OS patients. High serum levels of miR-34a were associated with longer overall survival in OS patients and lower metastasis. Multivariate Cox-regression analysis identified miR-34a serum level with potential prognostic significance.
Conclusion:
The expression level of serum miR-34a in patients with OS is closely related to the chemotherapy resistance, metastasis, recurrence, and survival of osteosarcoma, which can be used as one of the potential biomarkers and prognosis for the treatment of OS patients. Therefore, miR-34a may be a potential molecular for prediction of the efficacy of chemotherapy and prognosis in OS patients.
Keywords: chemotherapy resistance, metastasis, miR-34a, osteosarcoma, recurrence, survival
1. Introduction
Osteosarcoma (OS) is a primary tumor that occurs in the bone or in its associated tissues.[1,2] The potential mechanisms of OS development and progression remain to be elucidated, due to the high complexity of the occurrence and metastasis.[3,4] Clinically, OS is derived from the metastasis of other human cancer, such as breast cancer, gastric cancer, and prostate cancer.[5] OS is a malignant mesenchymal neoplasm that accounts for ~35% primary bone tumors in human.[6,7] Although the introduction of adjuvant chemotherapy and new targeting drugs in the treatment of OS increases survival rates dramatically, side effects, and poor responses of patients with OS contribute to poor 5-year survival (56.31%) and prognosis.[8] Thus, it is necessary to explore novel and effective strategies to improve the survival of patients with OS.
MicroRNA-34a (miR-34a) is involved in tumor cell proliferation, cell cycle, and invasion, and may be a prognostic marker for patients with cancer.[9] MiR-34a can inhibit tumor invasion and metastasis in OS, and its mechanism may be partly related to downregulating the expression of C-IAP2 and Bcl-2 protein directly or indirectly.[10] Overexpression of miR-34a could inhibit the tumor growth and metastasis of OS probably through down regulating c-Met oncolytic gene.[11] MiR-34a contained in bone marrow mesenchymal stem cell-derived extracellular vesicles reduces rheumatoid arthritis inflammation by inhibiting the cyclin I/ATM/ATR/p53 signaling pathway.[12] In addition, Furthermore, downregulated miR-34a expression is a prognostic marker for poor OS in mice.[13] Furthermore, serum miR-34a is a potential diagnostic and prognostic marker for OS patients.[14] Moreover, findings support the development of mechanism-based combination therapy to combat OS and bioengineered miR-34a prodrug represents a new natural miRNA agent.[15]
The present study aimed to investigate the serum level of miR-34a and expression of miR-34a in OS tissues in clinical patients. This study also identified the associations between miR-34 and chemotherapy resistance, metastasis, recurrence, survival, as well as prognosis in OS patients.
2. Methods
2.1. Patients
A total of 176 patients who were diagnosed with OS were included in affiliated Hongqi Hospital of Mudanjiang Medical University between May 2014 and July 2016. Eighty-six age-macheted healthy controls were recruited in affiliated Hongqi Hospital of Mudanjiang Medical University. A total of 14 OS patients with metastasis were excluded. The OS patients included 90 men and 72 women with an average age of 58.6 ± 6.5 years (range, 46–68 years). Exclusion criteria were as follows: age < 18 years; patients with cancer history; the estimated survival time less than 24 months; patients with infectious diseases; patients with distant metastasis; patients had received chemotherapy; and patients with serious other diseases. The protocol was reviewed and approved by the ethical committee of Mudanjiang Medical University. All patients gave their written informed consent.
2.2. Patient samples
A total of 162 biopsy samples (OS and adjacent tissues) from OS patients were collected in this study. All tissue samples immediately stored at −80°C. Total RNA was extracted with Trizol reagent (Invitrogen, Carlsbad, CA) following the manufacturer’s instructions and expression levels of miR-34a were measured using RT-qPCR.
2.3. Reverse transcription-quantitative PCR (RT-qPCR)
The expression levels of miR-34a were measured using TaqMan™ MicroRNA Reverse Transcription kit (Thermo Fisher Scientific, Waltham, USA). Briefly, total RNA was extracted using iPrep™ PureLink™ Total RNA kit (Thermo Fisher Scientific, Inc.) and reverse transcribed to cDNA with Bulge-Loop™ RT primers (Guangzhou RiboBio Co., Ltd.) at 42°C for 2 hours. qPCR was performed using SYBR Premix Ex Taq II (Takara Biotechnology Co., Ltd, Dalian, China), following the manufacturer’s instructions, and the ABI Vii7 Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.). Primers are listed as follow: miR-34a, forward 5′-GCCCTGGCAGTGTCTTAG-3′, reverse 5′-CAGTGCGTGTCGTGGAGT-3′; U6, forward 5′-GCTTCGGCAGCACATATACTAAAAT-3′, reverse 5′-CGCTTCACGAATTTGCGTGTCAT-3′. Relative gene expression was calculated using the 2−ΔΔCq method. Small nuclear U6 purchased from Invitrogen (Thermo Fisher Scientific, Inc.) was used as an internal control.
2.4. Statistical analysis
All data are expressed as the mean ± standard deviation or n (%). Differences in baseline characteristics were calculated using Student t test and the Fisher exact tests. χ2 test was used to test the study variables. Comparison of mRNA levels between healthy controls and OS patients was performed by Wilcoxon rank sum test. Multivariate Cox-regression analysis was used to evaluate the association between level of miR-34a and chemotherapy resistance, metastasis, recurrence, survival, as well as prognosis. Kaplan–Meier method was used to estimate the overall survival and recurrence of OS patients. The difference in OS probability was determined by log-rank test. All data were analyzed using the SPSS 19.0 software (SPSS, Chicago, IL). P < .05 was considered to indicate a statistically significant difference.
3. Results
3.1. Characteristic of OS patients
This study comprised of 162 patients with OS. Fourteen OS patients were excluded according to the exclusion criteria. Male patients were more than female patients (90:72). The demographic and clinicopathological data are shown in Table 1. All patients featured OS with I–II tumor stage and received the same curative resection treatment and chemotherapy. The flow diagram of the patients was shown in Figure 1.
Table 1.
Characteristics of patients with OS.
| Characteristic | Osteosarcoma |
|---|---|
| Age (yr) | 58.6 ± 6.5 |
| Gender, n (%) | |
| Male | 90 (55.6) |
| Female | 72 (44.4) |
| Tumor location, n (%) | |
| Femur | 95 (58.6) |
| Tibia | 67 (41.4) |
| Tumor size (cm), n (%) | |
| <5 | 106 (65.4) |
| ≥5 | 56 (34.6) |
| Clinical stage, n (%) | |
| 0–I | 78 (48.1) |
| IIA | 45 (27.8) |
| IIB | 39 (24.1) |
OS = osteosarcoma.
Figure 1.
The flow diagram of the OS patients in this study. OS = osteosarcoma.
3.2. Expression levels of miR-34a in serum and tissue in OS patients
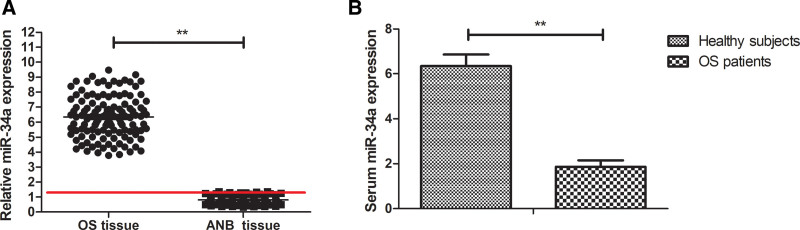
miR-34a profile of 162 diagnostic samples from OS patients was initially assessed the by RT-qPCR. As shown in Figure 2A, the mean expression of miR-34a in OS tissue was significantly lower than that in adjacent normal bone tissues (ANBT, Fig. 2A, 0.81 ± 0.25 vs 6.37 ± 1.66, P < .01). The serum levels of miR-34a in OS patients were significantly lower than that in healthy control subjects (Fig. 2B, 1.80 ± 0.45 vs 5.52 ± 1.75 P < .01).
Figure 2.
Expression of miR-34a in serum and OS tissues in patients with OS. (A) Serum levels of miR-34a were measured in OS patients (n = 162) and healthy individuals (n = 162) by RT-qPCR analysis. A patient with low miR-34a expression below red line, and a patient with high miR-34a expression above red line. (B) Expressional patterns for the indicated miR-34a were evaluated in the OS tissues (n = 162) and paired adjacent normal bone tissues (n = 162) by RT-qPCR analysis. **P < .01. ANB = adjacent normal bone, miR-34a = microRNA-34a, OS = osteosarcoma, RT-qPCR = reverse transcriptase quantitative PCR.
3.3. Expression levels of miR-34a in OS patients before and after chemotherapy
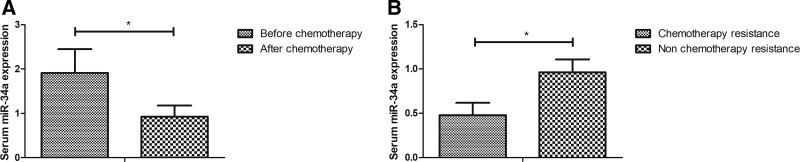
Expression of miR-34a was detected in the serum of OS patients before and after chemotherapy. Results showed that the relative serum miR-34a level was decreased in OS patients after chemotherapy (Fig. 3A, P < .05). Data found that expression of miR-34a was lower in patients with good response to chemotherapy resistance, while miR-34a was higher in OS patients with poor response to chemotherapy (Fig. 3B).
Figure 3.
Expression levels of miR-34a in OS patients before and after chemotherapy. (A) Serum levels of miR-34a were measured in OS patients before and after chemotherapy by RT-qPCR analysis. (B) Serum levels of miR-34a were measured in OS patients with good or poor response to chemotherapy using RT-qPCR analysis. *P < .05. miR-34a = microRNA-34a, OS = osteosarcoma, RT-qPCR = reverse transcriptase quantitative PCR.
3.4. Analysis of the association between miR-34a and recurrence, as well as metastatic status in OS patients
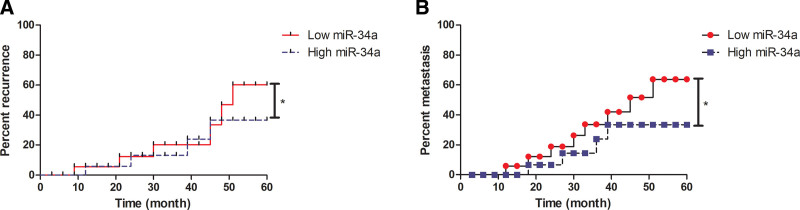
The associations between miR-34a and recurrence, as well as metastatic status were analyzed in OS patients. As shown in Figure 4A, patients with high serum level of miR-34a had lower recurrence of OS than that patients with low serum level of miR-34a (Fig. 4A, P < .05). Besides, patients with higher expression level of miR-34a had lower OS metastasis that that who with lower expression level of miR-34a (Fig. 4B, P < .05). Data demonstrated that high miR-34a expression miR-34a expression was negatively correlated with recurrence of OS patients (R = 0.580, P < .01, Table 2). There was a positive correlation between serum level of miR-34a and metastasis in OS patients (R = 0.680, P < .05).
Figure 4.
Analysis of the association between miR-34a and recurrence or metastatic status in OS patients. (A) Association between miR-148a and recurrence was determined by Kaplan–Meier method. (B) Association between miR-148a and metastasis was determined by Kaplan–Meier method. *P < .05. miR-34a = microRNA-34a, OS = osteosarcoma.
Table 2.
Correlation between miR-34a expression and recurrence or metastasis in patients with OS.
| r | P value | |
|---|---|---|
| Recurrence | −0.580 | .0063 |
| Metastasis | −0.680 | .022 |
miR-34a = microRNA-34a, OS = osteosarcoma.
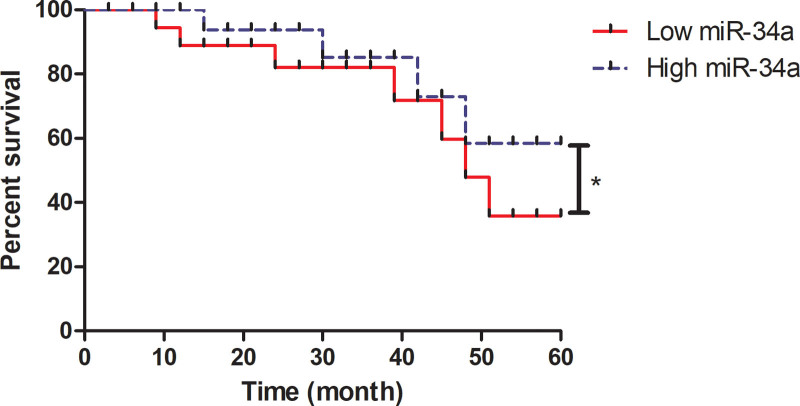
3.5. Analysis of the association between miR-34a and the survival of OS patients
The association between serum level of miR-34a and survival of patients with OS was analyzed in this study. Patients with higher expression level of miR-34a had longer survival that patients who with lower expression level of miR-34a (Fig. 5). During the follow-up period, 45 (27.8%) out of 162 OS patients died of this disease. Distant metastases developed in 64 (39.5%) OS patients. Of these patients, 12 (7.4%) had bone metastases and 52 had lung metastases (2 patients had both bone and lung metastases). Data observed that OS patients with high serum level of miR-34a had lower mortality (18 vs 27) and less metastasis (20 vs 32) than patients who had low level of miR-34a. The median overall survival of patients with OS was 48.5 months (95% confidence interval, 36.8–74.5 months) and 40.2 months (95% CI: 26.8–58.2 months) in the high miR-34a and low miR-34a expression, respectively (Table 3). Besides, serum level of miR-34a was positively correlated with survival of patients with OS (Table 4, R = 0.752, P < .01).
Figure 5.
Analysis of the association between miR-34a and the survival of OS patients. Overall survival curve demonstrated the correlation of miR-34a high expression or low expression with the percent survival in OS patients. miR-34a = microRNA-34a, OS = osteosarcoma.
Table 3.
Comparation of survival and metastasis in OS patients with low or high miR-34a expression during the follow-up period.
| Event | Low miR-34a | High miR-34a | P value |
|---|---|---|---|
| Dead | 27 (16.7%) | 18 (11.1%) | .046 |
| Metastasis | 32 (19.8%) | 20 (12.3%) | .022 |
| Overall survival (mo) | 40.2 (26.8–58.2) | 48.5 (36.8–74.5) | .038 |
miR-34a = microRNA-34a, OS = osteosarcoma.
Table 4.
Correlation between serum level of miR-34a and survival in patients with OS.
| r | P value | |
|---|---|---|
| Survival | 0.752 | .0036 |
miR-34a = microRNA-34a, OS = osteosarcoma.
3.6. Analysis of the association between miR-34a and prognosis of patients with OS
This study analyzed the association between miR-34a and prognosis of patients with OS during the follow-up period. Pearson correlation analysis showed a significant correlation between the expression of miR-34a and prognosis of OS patients (Table 5, R = 0.726, P < .01). Therefore, miR-34a has prognostic significance for patients with OS.
Table 5.
Correlation between serum level of miR-34a and prognosis in patients with OS.
| r | P value | |
|---|---|---|
| Prognosis | 0.726 | .0040 |
miR-34a = microRNA-34a, OS = osteosarcoma.
4. Discussion
The present study analyzed the expression of miR-34a in serum and tissues in patients with OS. In this study, we identified miR-34a whose expressions were significantly downregulated in serum compared to healthy subjects (Fig. 1), suggesting that miR-34a may be involved in the tumorigenesis of OS. In addition, data found that miR-34a expression was downregulated in OS tissues compared to adjacent normal bone tissues, suggesting that miR-34a may be involved in the metastasis of OS (Fig. 1). Furthermore, we identified miR-34a was decreased in serum in OS patients after chemotherapy, suggesting that miR-34a may be predict the efficacy of chemotherapy (Fig. 3). Notably, data in the current study found that miR-34a was negatively associated with metastasis and recurrence, while positively corelated with survival and prognosis of patients with OS (Fig. 5).
MicroRNAs have been identified in OS that possess oncogenic or tumor-suppressing properties and are closely associated with metastasis of OS.[16] Data in a previous study suggest that miR-34a plays a tumor suppressor role in the metastasis of osteosarcoma cells by repressing the expression of CD44.[17] The data from the present study demonstrated that significantly decreasing of serum level of miR-34a was found in the metastatic OS patients compared to OS free patients. MiR-34a suppresses tumor migration and invasion, whereas knockdown of miR-34a expression is associated with metastasis of OS.[18] However, the role of miR-34a as a serum metastatic biomarker has not been previously investigated in OS patients. Consistent with a previous study,[14] we identified that expression of miR-34a was downregulated in OS tissue in clinical patients. In addition, metastatic OS tissues showed lower expression of miR-34a than non-metastatic tissues (Fig. 1). Furthermore, this study quantified the serum levels of miR-34a in OS patients and then indicated that miR-34a was associated with metastatic status in OS patients (Table 4). These data suggest that miR-34a has the potential value of as a serum diagnostic and prognostic marker in osteosarcoma patients.
Chemotherapy resistance is a major cause of OS progression after treatment with chemotherapy.[19] MicroRNAs have been implicated in the development of chemotherapy resistance in OS.[20] However, little is known about the role of miR-34a in chemotherapy resistance of OS. MiR-34a increases cisplatin sensitivity of osteosarcoma cells through up-regulation of c-Myc and Bim signal.[21] In this study, we investigated the serum level of miR-34a in OS patients before and after chemotherapy. Data demonstrated that significantly reduced miR-34a expression was associated with chemotherapeutic resistance in OS patients. Notably, the decreasing expression of miR-34a presented higher chemotherapeutic resistance of OS tissues. MiR-34a suppresses tumor progression and leads to improved prognoses, whereas reduced miR-34a expression is associated with poor overall survival of OS patients.[22] Importantly, this study found that reduced miR-34a expression was observed in serum and tissue, which was associated with poor overall survival and prognosis of OS patients (Fig. 5). Although miR-34a expression could be novel prognosis biomarkers for surgically treated OS,[23] the association between miR-34a and prognosis has not been explored in OS patients. The present study identified the role of miR-34a in the OS patients and outcomes showed that miR-34a was positively associated with survival and prognosis of patients with OS (Table 5). Notably, high serum of miR-34a contributed to longer survival of OS patients, which may be an important prognostic markers of OS patients (Table 3).
Several other limitations of the present study should be addressed. Firstly, this study only analyzed the serum levels and OS tissues expression of miR-34a in a small size OS population. Second, we just analyzed the relationship between serum level of miR-34a and chemotherapy resistance, metastasis, survival, as well as prognosis of OS patients due to the limited data. Third, the patients came from one single hospital, and we did not include all relevant risk factors and all OS patients during observation. Based on the results of this study, we would collect more clinical samples to further investigate the relationship of miR-34a with chemotherapy resistance and explore the regulatory mechanisms of the miR-34a in OS patients.
5. Conclusions
In conclusion, data from the present study systemically investigated the serum level of miR-34a in OS patients and comprehensively analyzed associations between miR-34a expressions with chemotherapy resistance, the metastasis, survival, as well as prognosis of OS patients. Data found that miR-34a were decreased in OS patients with metastatic status and had prognostic significance. This study highlighted the important role of miR-34a in evaluating the therapeutic effects of chemotherapy resistance, metastasis survival prognosis prediction of osteosarcoma.
Author contributions
KXL designed the project, wrote the manuscript, and performed data analysis. HYL, YZ, ZS, and KXL performed experiments. HYL, YZ, and KXL are responsible for confirming the authenticity of the raw data. All authors read and approved the final manuscript.
Conceptualization: Hongyu Lian.
Data curation: Hongyu Lian, Zhang Sun.
Formal analysis: Hongyu Lian, Zhang Sun.
Funding acquisition: Hongyu Lian.
Investigation: Hongyu Lian, Yang Zhou, Zhang Sun.
Methodology: Yang Zhou, Kexin Liu.
Project administration: Kexin Liu.
Resources: Yang Zhou, Zhang Sun, Kexin Liu.
Software: Yang Zhou, Zhang Sun, Kexin Liu.
Supervision: Yang Zhou, Zhang Sun.
Validation: Yang Zhou, Kexin Liu.
Visualization: Kexin Liu.
Writing – original draft: Kexin Liu.
Writing – review & editing: Kexin Liu.
Abbreviations:
- ANBT =
- adjacent normal bone tissues
- miR-34a =
- microRNA-34a
- OS =
- osteosarcoma
- RT-qPCR =
- reverse transcriptase quantitative PCR
This study was funded by the Science and Technology Plan Project of Mudanjiang (grant no. Z2017s0060).
The present study was approved by the Ethical Committee of Mudanjiang Medical University (approval no. 20140216ORX1).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
How to cite this article: Lian H, Zhou Y, Sun Z, Liu K. MicroRNA34a is associated with chemotherapy resistance, metastasis, recurrence, survival, and prognosis in patient with osteosarcoma. Medicine 2022;101:38(e30722).
Contributor Information
Hongyu Lian, Email: hongyu_doctor@126.com.
Yang Zhou, Email: 377042531@qq.com.
Zhang Sun, Email: zhang_sunclinical@163.com.
References
- [1].Yan JP, Xiang RM. Effect assessment of methotrexate in combination with other chemotherapeutic agents for osteosarcoma in children: a protocol for systematic review and meta-analysis. Medicine. 2021;100:e25534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Futani H, Takaki H, Sawai T, et al. Long-term survival following radiofrequency ablation of lung metastases in an elderly patient with calcaneal osteosarcoma: a case report and review of the literature. Medicine (Baltimore). 2021;100:e26681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Higuchi T, Igarashi K, Yamamoto N, et al. Osteosarcoma patient-derived orthotopic xenograft (PDOX) models used to identify novel and effective therapeutics: a review. Anticancer Res. 2021;41:5865–71. [DOI] [PubMed] [Google Scholar]
- [4].Ye ZM, Luo MB, Zhang C, et al. A comprehensive evaluation of single nucleotide polymorphisms associated with osteosarcoma risk: a protocol for systematic review and network meta-analysis. Medicine (Baltimore). 2020;99:e20486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Peng C, Ren Z, Zhu J, et al. Clinical therapeutic effects of combined methotrexate and other chemotherapeutic agents in treating children and young patients with osteosarcoma: a protocol for systematic review and meta-analysis. Medicine. 2021;100:e25564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zhao X, Wu Q, Gong X, et al. Osteosarcoma: a review of current and future therapeutic approaches. Biomed Eng Online. 2021;20:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tang QX, Wang LC, Wang Y, et al. Efficacy of methotrexate, doxorubicin, and cisplatin for osteosarcoma: study protocol for a systematic review of randomized controlled trial. Medicine (Baltimore). 2019;98:e14442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Qiu YQ, Chen YL. Primary meningeal osteoblastic osteosarcoma containing fibroblast osteosarcoma: clinicopathological analysis and literature review. Osteoporosis Int. 2021;32:1007–12. [DOI] [PubMed] [Google Scholar]
- [9].Bahman Soufiani K, Pourfathollah AA, Nikougoftar Zarif M, et al. Tumor microenvironment changing through application of MicroRNA-34a related mesenchymal stem cells conditioned medium: modulation of breast cancer cells toward non-aggressive behavior. Iran J Allergy Asthma Immunol. 2021;20:221–32. [PubMed] [Google Scholar]
- [10].Wen J, Zhao YK, Liu Y, et al. MicroRNA-34a inhibits tumor invasion and metastasis in osteosarcoma partly by effecting C-IAP2 and Bcl-2. Tumour Biol. 2017;39:1010428317705761. [DOI] [PubMed] [Google Scholar]
- [11].Yan K, Gao J, Yang T, et al. MicroRNA-34a inhibits the proliferation and metastasis of osteosarcoma cells both in vitro and in vivo. PloS One. 2012;7:e33778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wu H, Zhou X, Wang X, et al. miR-34a in extracellular vesicles from bone marrow mesenchymal stem cells reduces rheumatoid arthritis inflammation via the cyclin I/ATM/ATR/p53 axis. J Cell Mol Med. 2021;25:1896–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wang W, Hu S, Chang J, et al. Down-regulated microRNA-34a expression as a prognostic marker for poor osteosarcoma in mice: a systematic review and meta-analysis. J Cancer. 2018;9:4179–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wang T, Wu J, Liu X, et al. Serum miR-34a is a potential diagnostic and prognostic marker for osteosarcoma. Int J Clin Exp Path. 2017;10:9683–89. [PMC free article] [PubMed] [Google Scholar]
- [15].Zhao Y, Tu MJ, Yu YF, et al. Combination therapy with bioengineered miR-34a prodrug and doxorubicin synergistically suppresses osteosarcoma growth. Bioche Pharmacol. 2015;98:602–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ebrahimi N, Aslani S, Babaie F, et al. MicroRNAs implications in the onset, diagnosis, and prognosis of osteosarcoma. Curr Mol Med. 2021;21:573–88. [DOI] [PubMed] [Google Scholar]
- [17].Zhao H, Ma B, Wang Y, et al. miR-34a inhibits the metastasis of osteosarcoma cells by repressing the expression of CD44. Oncol Rep. 2013;29:1027–36. [DOI] [PubMed] [Google Scholar]
- [18].Zou Y, Huang Y, Yang J, et al. miR-34a is downregulated in human osteosarcoma stem-like cells and promotes invasion, tumorigenic ability and self-renewal capacity. Mol Med Rep. 2017;15:1631–37. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [19].Benjamin RS. Adjuvant and neoadjuvant chemotherapy for osteosarcoma: a historical perspective. Adv Experimental Med Biol. 2020;1257:1–10. [DOI] [PubMed] [Google Scholar]
- [20].Tang Z, Lu Y, Chen Y, et al. Research progress of MicroRNA in chemotherapy resistance of osteosarcoma. Technol Cancer Res Treat. 2021;20:15330338211034262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Li QC, Xu H, Wang X, et al. miR-34a increases cisplatin sensitivity of osteosarcoma cells in vitro through up-regulation of c-Myc and Bim signal. Cancer Biomarkers. 2017;21:135–44. [DOI] [PubMed] [Google Scholar]
- [22].Wang Y, Jia LS, Yuan W, et al. Low miR-34a and miR-192 are associated with unfavorable prognosis in patients suffering from osteosarcoma. Am J Transl Res. 2015;7:111–9. [PMC free article] [PubMed] [Google Scholar]
- [23].Chen X, Peng D, Shen Y, et al. The potential combinational effect of miR-34a with celecoxib in osteosarcoma. Anti-Cancer Drugs. 2017;28:888–97. [DOI] [PubMed] [Google Scholar]