PURPOSE:
The nearly 90,000 adolescents and young adults (AYAs) diagnosed with cancer in the United States yearly have tended to occupy a no-man's land between medical and pediatric oncology, often reporting that existing models of care are misaligned with their needs and preferences. Although guidelines for optimal AYA cancer care are increasingly available, the implementation of such standards has been varied. This may be in part due to a lack of guidance for implementing specialized AYA care. In this study, we leveraged an implementation science framework to identify barriers and generate practical guidance to inform the implementation of specialized AYA cancer care.
METHODS:
We conducted semistructured qualitative interviews, guided by the Consolidated Framework for Implementation Research, with AYA care stakeholders (N = 32 from 14 cancer programs). Our multidisciplinary research team analyzed interview transcriptions using a template analysis approach and gleaned from interviews practical guidance for implementing specialized AYA care.
RESULTS:
Participants reported barriers to implementing specialized AYA care across all five Consolidated Framework for Implementation Research domains: (1) intervention characteristics (eg, costs), (2) inner setting (eg, difficulties in collaborating between pediatric and medical oncology), (3) outer setting (eg, patient-level barriers to participating in AYA services), (4) individual characteristics (eg, attitudes about AYA oncology), and (5) process (eg, lack of metrics for program evaluation). They also shared practical guidance for addressing these barriers.
CONCLUSION:
Emerging guidance on the core elements of AYA cancer care must be matched with guidance to support the implementation of specialized AYA care. This study contributes to the body of evidence available to inform future implementation efforts.
BACKGROUND
The nearly 90,000 adolescents and young adults (AYAs) diagnosed with cancer in the United States yearly1 have tended to occupy a no-man's land between medical and pediatric oncology,2 often reporting that existing models of care are misaligned with their needs and preferences.3-5 In response to demands for improvements in care for this population from patients, providers, and researchers,6-9 models of specialized (ie, AYA-specific) care have emerged within cancer centers across the country to better meet AYAs' unique needs.10 To provide specialized AYA care, some cancer programs have formally established AYA programs, which include staff or services dedicated to AYAs. Although the scope, composition, and functions of these programs vary greatly, common components include provider expertise, coordination between pediatric and medical oncology, age-appropriate supportive services, efforts to increase clinical trial participation, and patient/family advocacy. The provision of specialized AYA care has been associated with improved outcomes for AYAs including reduced unmet needs,11 increased clinical trial enrollment,12 and the provision of guideline-concordant care.13
Although guidelines for optimal AYA cancer care are increasingly available,8,14,15 the implementation of such standards has been varied. In preliminary studies, half of cancer programs reported having an established AYA program; even among these programs, the availability of key elements of specialized AYA care varied. Such variation may be in large part due to a lack of guidance for implementing specialized AYA care in the context of complex cancer programs, which face multilevel barriers to change.
Although some guidance for implementing specialized AYA care exists in the literature,10,16-18 much of it is constrained to single case studies and, to our knowledge, none of it has been developed through an implementation science lens.19 In this study, we leveraged an implementation science framework20 to identify key barriers and generate practical guidance to inform the implementation of specialized AYA cancer care.
METHODS
We conducted semistructured qualitative interviews with AYA care stakeholders to identify the provider- and organizational-level barriers to implementing specialized AYA care and generate guidance to facilitate implementation. This study was approved by the Wake Forest Institutional Review Board (IRB00074316) before the completion of all data collection activities, and informed consent was obtained from each participant. The Data Supplement (online only) includes the Standards for Reporting Qualitative Research checklist adhered to for these data collection activities.
Sample
In preliminary studies, we surveyed AYA advocates directly involved with AYA care in cancer programs across the country (N = 90). For this study, using survey data, we purposefully sampled cancer programs to maximize variation in (1) having a formalized AYA program (self-reported, yes/no), (2) type of institution (eg, academic medical center v community hospital), (3) geographic location, and (4) AYA patient volume. Within each cancer program, we sought to interview individuals with robust involvement in AYA care (eg, oncology providers, nurses, social workers, patient navigators, etc). The goal was to triangulate across multiple perspectives at each institution, capturing a comprehensive picture of AYA care across diverse health systems.
Recruitment
We recruited interview participants through Teen Cancer America (TCA), an advocacy and consulting organization that works with cancer programs around the country to facilitate the implementation of specialized AYA cancer care. Our TCA partners (H.G. and K.N.) reached out to the AYA advocate who responded to the preliminary survey at each identified cancer program via e-mail using Dillman's21 approach for maximizing response rates. Those who expressed interest in participating were connected to E.H. to schedule interviews and to identify additional relevant interview participants from their institution who were directly involved with AYA care. We recruited until thematic saturation was reached, that is, when subsequent interviews did not generate new information on implementation barriers.
Instrument
We developed a semistructured interview guide (Data Supplement) using constructs from the Consolidated Framework for Implementation Research (CFIR), which identifies implementation determinants across five domains: inner setting (eg, the structural, political, and cultural context within a cancer program), outer setting (eg, a cancer program's broader social, political, and economic context), individual characteristics (eg, characteristics of those involved with AYA care or its implementation), intervention characteristics (eg, features of specialized AYA care), and process (eg, activities related to implementing specialized AYA care).20 Interview guide questions, adapted from those available in the Consolidated Framework for Implementation Research online resources,22 asked interviewees to reflect on the implementation of specialized AYA care at their institution including the barriers that they have faced and the strategies that they have deployed.
Procedure
E.H. conducted 45-minute semistructured interviews via Zoom. We recorded and transcribed interviews for analysis.
Analysis
We analyzed interview transcriptions using template analysis23 based on a priori themes (Data Supplement; ie, CFIR constructs) but allowing for additional themes to emerge. To calibrate our coding schema, E.H. and S.A. independently coded excerpts from one interview transcript. After they met to resolve any discrepancies, E.H. coded the remaining transcripts. This commonly used approach to qualitative analysis24 ensured codebook quality.25 Findings were then summarized by CFIR domain and distilled into guidance for implementing specialized AYA care through collaborative discussion with the study team including experts in implementation science (E.H. and S.A.), AYA research (E.H., L.L., and J.M.S.), and AYA clinical care and program development (L.L., H.G., K.N., B.R., and B.K.).
RESULTS
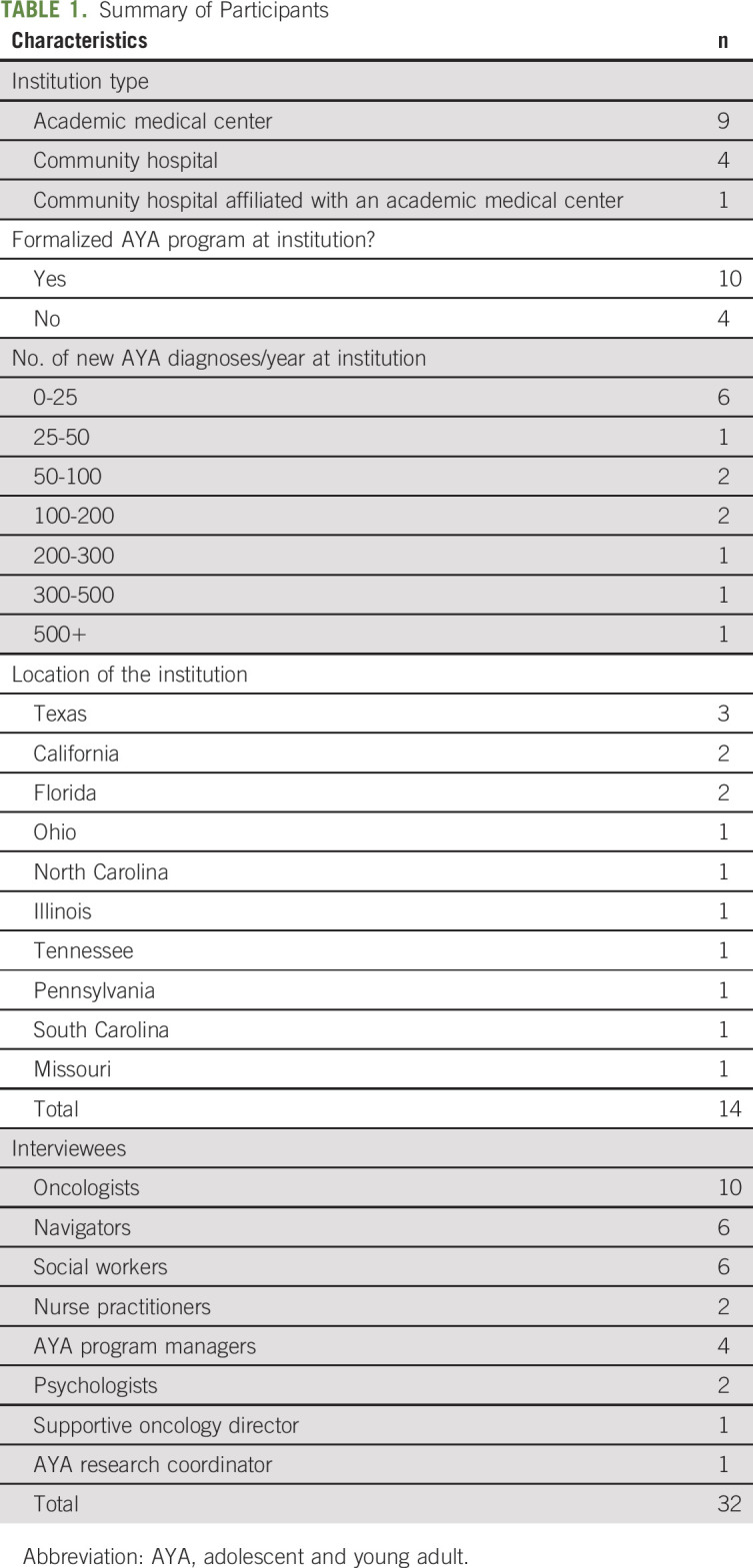
Participants included 32 AYA care stakeholders from 14 cancer programs (1-5 stakeholders per program; Table 1).
TABLE 1.
Summary of Participants
Participants reported barriers to implementing specialized AYA care across all CFIR domains and shared practical guidance for addressing these barriers (Fig 1 and Data Supplement).
FIG 1.
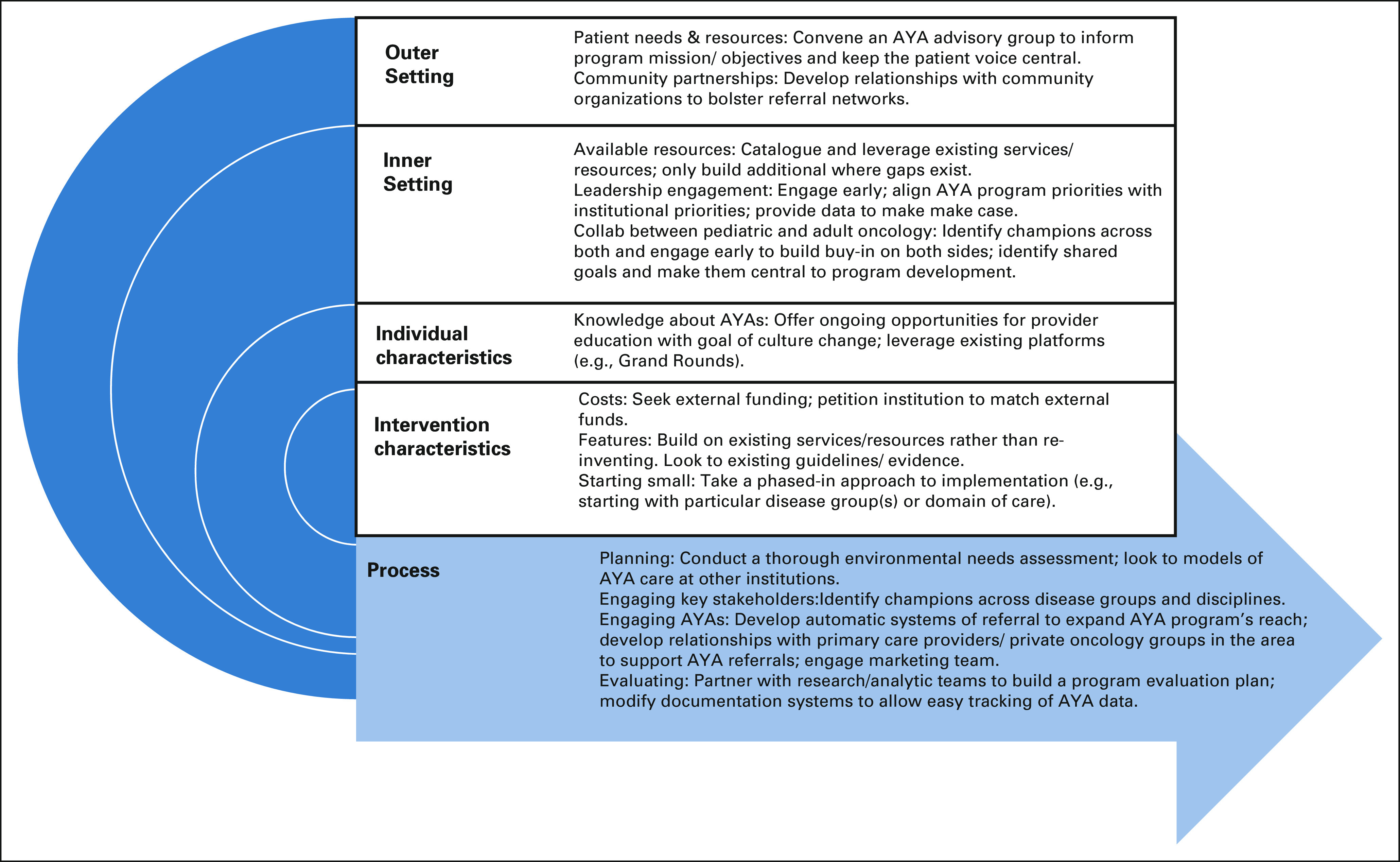
Strategies for implementing specialized AYA care. AYA, adolescents and young adult.
Intervention Characteristics
The Data Supplement contains a brief description of the characteristics of AYA care at each institution interviewed.
Features of specialized AYA care.
Programs interviewed varied tremendously in terms of the structure and functions of AYA care at their institution. AYA services included psychosocial support, educational/vocational support, fertility preservation, support groups or patient events, and efforts to increase clinical trial enrollment. Programs made decisions about where to focus their activities based on patient engagement, clinical practice guidelines (eg, National Comprehensive Cancer Network guidelines), frameworks for AYA care, or other existing evidence. Although most programs focused initial efforts on medical or psychosocial care, more established AYA programs articulated additional goals related to AYA research and education.
Decisions about a program's structure, functions, and model of care often hinged on the services and resources already available at a given institution. Interviewees noted that demonstrating the value-add of specialized AYA care can be challenging; they faced pushback from other providers who felt that the AYA team was stepping on [their] toes. They emphasized the importance of adding value rather than competing with existing provider teams. This was the impetus behind several programs opting for a consultation-based model.
Staffing.
For programs that had AYA-specific staff, their multidisciplinary AYA team was often built over time. One program started with a program director funded through foundational funds and has since expanded to include a multidisciplinary AYA team as their institution recognized the value-add of the AYA program. Here again, interviewees emphasized the importance of capitalizing on existing staff at an institution to avoid reinventing services. Another commonly expressed theme was the importance of having administrative staff to spearhead AYA program development.
Costs.
All programs noted cost as a key barrier. Although some programs benefitted from robust institutional support, most sought external funding from philanthropic donors, foundations, or other groups. Two programs noted that obtaining external funding helped them to galvanize leadership willingness to provide institutional support. One program mentioned that adult providers are judged more closely on their patient billable hours than pediatric providers, resulting in more pushback for nonbillable activities in an adult setting.
Starting small.
Interviewees often discussed the importance of phasing in implementation; they focused initial efforts on specific standards of care or subpopulations before rolling out their program more broadly. Several programs launched their AYA program with a focus on one specific gap in AYA care around which they felt that they could rally broad support (eg, supportive oncology, clinical trial enrollment, and fertility preservation). Three programs started with specific disease groups. For example, one program started with the disease groups for which there was the most overlap between pediatric and adult oncology (eg, leukemia, lymphoma, and sarcoma). This approach allowed them to solidify the collaboration between pediatric and medical oncology. Two programs spoke to the importance of an AYA's program adaptability: they established a program mission and goals upfront but knew that their objectives would have to be flexible and responsive to the dynamic needs of their patients and their institution.
Inner Setting
Leadership engagement.
Leadership buy-in was among the most frequently discussed barriers to implementing specialized AYA care. Programs described changes in leadership as both catalysts for and disruptors of AYA program growth. To garner leadership support, interviewees noted the importance of aligning AYA program goals with institutional priorities and showing administrators the hard numbers to illuminate a few key problems or challenges in AYA care at their cancer center; by approaching leadership with proposed solutions to these challenges, they were able to garner administrative support.
Available resources.
All programs interviewed voiced resource constraints, particularly staffing availability, as a primary barrier to launching or expanding AYA services at their institution. Interviewees often reported that pediatric oncology had more staffing and support resources than adult oncology, making it easier for existing resources to be leveraged for their AYA population.
Provider education.
Interviewees described a variety of methods that they used to educate providers on AYA care and AYA program activities including Grand Rounds, division and team meetings, guest lectures, nursing forums, etc. Three programs emphasized that efforts to educate providers must occur on an ongoing basis to account for provider turnover. Through ongoing education, interviewees hoped to build a culture that was supportive of AYA oncology as its own subspecialty and thus receptive to the implementation of specialized AYA care.
Structural characteristics.
Many interviewees emphasized the importance of the geography of an institution. For example, when pediatric and adult hospitals were colocated, this facilitated greater collaboration. Other programs faced challenges in caring for AYAs across disparate locations. One program addressed these challenges by specifying set days in which their AYA navigator would be at various locations and scheduling patient visits accordingly. Two programs mentioned that telehealth helped to alleviate the challenge of serving patients across disparate locations. Some programs sought to build AYA-specific spaces (eg, lounges and infusion rooms) to address these challenges. However, institutional space limitations hindered some of these efforts.
Collaboration between pediatric and medical oncology.
Interviewees discussed the collaboration between pediatric and medical oncology at length. Programs varied in the extent to which providers and resources from both sides were engaged, with five programs confined completely to one or the other with little collaboration between the two. Programs with more of a shared model emphasized the importance of identifying champions in both pediatric and medical oncology, articulating shared goals, and establishing clear channels of communication. Three programs housed their AYA program in a supportive care department, where there was infrastructure and precedent for offering services across pediatric and adult oncology.
Outer Setting
Patient needs and resources.
In general, interviewees displayed a robust knowledge of the needs and constraints of AYAs. They reported patient-level barriers to participating in AYA services including awareness, insurance, and bandwidth in the face of many competing demands. Two programs discussed their difficulties in engaging minority patients, pointing to the need for a health equity focus in implementing specialized AYA care.
Community partnerships.
Programs partnered with a range of community organizations to launch and grow their AYA program. Interviewees recommended that social workers and other AYA providers develop a robust knowledge of resources available in the community and foster relationships with community organizations to strengthen referral networks.
External pressures.
For some programs, the implementation of specialized AYA care was influenced by existing guidelines or external incentives. Two programs mentioned that the National Comprehensive Cancer Network guidelines for AYA care offered a template for identifying gaps in existing services and resources and building services to address those gaps.
Interviewees disagreed about whether having a formalized AYA program offers or would offer them a competitive advantage over other hospitals in the area. Four programs felt that having an AYA program was a feather in their cap, potentially promoting referrals. Others disagreed, noting that AYAs select their location of care on the basis of geographical convenience, availability of disease-group specialists, and where their primary care provider refers them.
Individual Characteristics
Interviewees discussed the extent to which other providers in their institution were bought into the notion of AYA oncology as its own subspecialty. Although most interviewees reported that providers in their institution were receptive to AYA-specific care, some reported pushback. Interviewees mentioned inertia as a barrier, noting hesitance from other providers to disrupt systems that are already in place.
Implementation Process
Planning.
Interviewees tended to agree that the first critical step to implementing specialized AYA care is conducting a thorough environmental needs assessment, which includes identifying (1) characteristics of the AYA population served by the institution (eg, patient volume, demographics, and clinical characteristics); (2) services and resources available through the institution or community partnerships; (3) attitudes and knowledge on AYA care among leadership, providers, and staff; and (4) organizational capacity or readiness to implement specialized AYA care. In addition to informing the objectives, structure, and functions of the AYA program, this provided an opportunity to collect data to build a case for the value-add of the program. By understanding the needs and resources at their institution, they were able to identify service gaps to focus on. Often, this involved conducting informal interviews with key stakeholders. Many programs convened an advisory board composed of key stakeholders to guide implementation planning or solicited input directly from AYAs about their needs and preferences for care delivery. Two programs mentioned connecting with existing AYA programs to inform their model of care.
Engaging providers and staff.
A critical aspect of conducting an environmental needs assessment was engaging providers and staff from across an institution. This allowed programs to gather information about existing attitudes and processes and build buy-in across departments. The majority of programs relied on referrals from disease group providers to reach AYA patients; they noted the importance of identifying champions across disease groups and disciplines to promote referrals. One program emphasized the importance of engaging nursing staff, including mid-level managers, to promote referrals. Interviewees also discussed the importance of engaging providers in the community to influence referral patterns.
Engaging AYAs.
Interviewees expressed that getting disease group providers to add a step to their workflow was challenging, even when those providers bought into the value-add of AYA-specific care. They noted that, to the extent that referrals can be automated, this will expand the reach of AYA services.
In some cases, programs targeted outreach efforts directly to AYAs. They deployed a range of marketing strategies including developing AYA program brochures/pamphlets across disease group clinics, developing a social media presence, and sending out periodic newsletters.
Program evaluation.
Interview participants used a range of metrics to track the performance of their AYA services and to inform program growth and changes (eg, patient volume and demographics, relative value units, patient satisfaction, etc). Two programs partnered with researchers or other groups within their institution to articulate a plan for program evaluation. Most programs, although, were in the early phases of thinking through program evaluation, pointing to a lack of consensus or guidance on key metrics to track.
DISCUSSION
The provision of specialized AYA care has been associated with improved outcomes for AYAs including reduced unmet needs,11 increased clinical trial enrollment,12 and the provision of guideline-concordant care.13 However, implementing specialized AYA care requires multilevel change, including changes to organizational workflow, interorganizational networks, communication and documentation systems, and culture. In light of these complexities, cancer programs need practical guidance to support decisions about adopting and implementing AYA-specific models of care at their institution. In this study, we synthesized the experiences of 14 cancer programs to provide such guidance.
This study is not without its limitations. As evidenced by their engagement with TCA, programs interviewed had AYA champions; in this sense, they may be ahead of programs without this kind of AYA interest/expertise. Thus, our findings may paint a rosier picture than reality in terms of the availability of specialized AYA care. In many cancer programs around the country, efforts to provide specialized AYA care may be nonexistent or in their infancy. This may be especially true in community-based cancer programs where the majority of AYAs receive care. Indeed, the community hospitals that we interviewed who reported having robust AYA services may be outliers; future research diving deeper into the development of AYA-specific care at these institutions could inform efforts to expand AYA care in other community-based settings. In addition, although interviewees discussed some barriers that AYAs face in participating in AYA services, the focus of this study was on the provider- and organizational-level determinants of implementation; future research exploring AYA-level barriers should include robust engagement of AYAs. AYA care is inherently multidisciplinary, including providers and staff from across many disease groups and disciplines. Although we interviewed a range of AYA stakeholders, there are likely additional perspectives that we did not capture. We aimed to interview cancer programs with and without formalized AYA programs. In the absence of a standardized definition of an AYA program, we relied on self-reported data to make this distinction. Interestingly, during interviews, we discovered that this distinction was not always straightforward. Among the programs that we interviewed, the structure and functions of AYA care varied tremendously. Although guidelines and criteria for optimal AYA cancer care have become increasingly available,8,14,15 more work is needed to define the core components of a comprehensive AYA program. Our interviews suggested that a one-size-fits-all approach to AYA care may not be possible or appropriate; however, interviewees shared elements of their implementation process, which may be generalizable across diverse health systems.
Before implementation planning, cancer programs should conduct a thorough environmental scan of services and resources to identify existing gaps in service availability or capacity. Where gaps exist, additional services may be developed or partnerships with community-based programs may be established to bolster referral networks. Our interview findings highlight the importance of building noncompetitive services for AYAs rather than duplicating existing services or resources. To address concerns about institutional service capacity, many cancer programs took a phased-in approach to implementing specialized AYA care (eg, starting with one disease group and expanding over time). This approach allowed for programs to identify gaps and troubleshoot as they expanded. AYA-specific staffing may also be built over time, but appointing a program manager who is more focused on program development than clinical tasks may be critical to implementation and sustainment.
In addition to leadership buy-in, implementing specialized AYA care requires buy-in from providers beyond just the AYA team, who may not be familiar with AYA-specific services or may view them as redundant with those already provided by the primary oncology team. Educating providers on the added value of AYA-specific care can help build awareness and buy-in. In the early phases of implementation, providers from both pediatric and medical oncology should be engaged to foster a shared ownership of AYA care. Effective collaboration between pediatric and medical oncology may be key to AYA program reach and success.26 Involving AYAs and diverse providers in implementation planning can ensure that AYA program objectives are aligned with stakeholder needs and constraints and help to build a culture that is supportive of AYA oncology as its own subspecialty. Tracking data on AYA experiences and outcomes can inform AYA program growth; although some guidance exists in the literature,27 more consensus and guidance are needed on key structural, process, and outcome measures to track.
In conclusion, emerging guidance on the core elements of AYA cancer care must be matched with guidance to support the implementation of specialized AYA care. This study contributes to the body of evidence available to inform future implementation efforts. Efforts to empirically test the effectiveness of particular strategies in optimizing the implementation of specialized AYA care will only enhance that knowledge base and advance the fledgling field of AYA oncology.
DISCLAIMER
The content of this article is solely the responsibility of the authors and does not necessarily represent the official view of NCATS or NIH.
SUPPORT
E.H.'s effort was supported by Teen Cancer America and by 2T32 CA122061 from the National Cancer Institute. S.A.'s effort was supported by the National Cancer Institute's National Research Service Award sponsored by the Lineberger Comprehensive Cancer Center at the University of North Carolina (T32 CA116339). J.M.S.'s effort was supported by the National Cancer Institute (R01CA242849). S.B.'s effort was supported in part by the Wake Forest Clinical and Translational Science Award UL1TR001420.
J.M.S. and S.B. contributed equally as cosenior authors to this work.
AUTHOR CONTRIBUTIONS
Conception and design: Emily Haines, Lauren Lux, Hilary Gan, Kara Noskoff, Bindu Kumar, Sarah Birken
Administrative support: Kara Noskoff, Bindu Kumar
Provision of study materials or patients: Kara Noskoff
Collection and assembly of data: Emily Haines, Hilary Gan, Kara Noskoff, Bindu Kumar
Data analysis and interpretation: Emily Haines, Sarah Asad, Lauren Lux, Hilary Gan, Bindu Kumar, Betty Roggenkamp, John M. Salsman, Sarah Birken
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Guidance to Support the Implementation of Specialized Adolescent and Young Adult Cancer Care: A Qualitative Analysis of Cancer Programs
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
No potential conflicts of interest were reported.
REFERENCES
- 1.American Cancer Society . American Cancer Society Cancer Prevention and Early Detection Facts and Figures, Tables & Figures 2020. Atlanta, GA: American Cancer Society; 2020. [Google Scholar]
- 2. Pollock BH. Where adolescents and young adults with cancer receive their care: Does it matter? J Clin Oncol. 2007;25:4522–4523. doi: 10.1200/JCO.2007.12.1715. [DOI] [PubMed] [Google Scholar]
- 3. Nass SJ, Beaupin LK, Demark-Wahnefried W, et al. Identifying and addressing the needs of adolescents and young adults with cancer: Summary of an Institute of Medicine workshop. Oncologist. 2015;20:186–195. doi: 10.1634/theoncologist.2014-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Morgan S, Davies S, Palmer S, et al. Sex, drugs, and rock “n” roll: Caring for adolescents and young adults with cancer. J Clin Oncol. 2010;28:4825–4830. doi: 10.1200/JCO.2009.22.5474. [DOI] [PubMed] [Google Scholar]
- 5. Albritton K, Bleyer WA. The management of cancer in the older adolescent. Eur J Cancer. 2003;39:2584–2599. doi: 10.1016/j.ejca.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 6. Wilkinson J. Young people with cancer—How should their care be organized? Eur J Cancer Care. 2003;12:65–70. doi: 10.1046/j.1365-2354.2003.00313.x. [DOI] [PubMed] [Google Scholar]
- 7.Albritton K, Caligiuri M, Anderson B, et al. Closing the Gap: Research and Care Imperatives for Adolescents and Young Adults with Cancer. Bethesda, MD: Department of Health and Human Services, National Institute of Health, National Cancer Institute, and the LiveStrong Young Adult Alliance; 2006. [Google Scholar]
- 8. Ramphal R, Meyer R, Schacter B, et al. Active therapy and models of care for adolescents and young adults with cancer. Cancer. 2011;117:2316–2322. doi: 10.1002/cncr.26048. [DOI] [PubMed] [Google Scholar]
- 9. Johnson RH. AYA in the USA. International perspectives on AYAO, part 5. J Adolesc Young Adult Oncol. 2013;2:167–174. doi: 10.1089/jayao.2012.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reed D, Block RG, Johnson R. Creating an adolescent and young adult cancer program: Lessons learned from pediatric and adult oncology practice bases. J Natl Compr Canc Netw. 2014;12:1409–1415. doi: 10.6004/jnccn.2014.0138. [DOI] [PubMed] [Google Scholar]
- 11. Mitchell L, Tam S, Lewin J, et al. Measuring the impact of an adolescent and young adult program on addressing patient care needs. J Adolesc Young Adult Oncol. 2018;7:612–617. doi: 10.1089/jayao.2018.0015. [DOI] [PubMed] [Google Scholar]
- 12. Shaw PH, Boyiadzis M, Tawbi H, et al. Improved clinical trial enrollment in adolescent and young adult (AYA) oncology patients after the establishment of an AYA oncology program uniting pediatric and medical oncology divisions. Cancer. 2012;118:3614–3617. doi: 10.1002/cncr.26634. [DOI] [PubMed] [Google Scholar]
- 13. Crosswell HE, Quddus F, Khan SS. Adolescent and young adult leukemia and lymphoma care delivery in the community: Metrics and outcomes of a community-based, immersive AYA program. Blood. 2018;132(suppl 1):4733. [Google Scholar]
- 14. Coccia PF, Pappo AS, Beaupin L, et al. Adolescent and young adult oncology, version 2.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16:66–97. doi: 10.6004/jnccn.2018.0001. [DOI] [PubMed] [Google Scholar]
- 15. Kremer LCM, Mulder RL, Oeffinger KC, et al. A worldwide collaboration to harmonize guidelines for the long-term follow-up of childhood and young adult cancer survivors: A report from the international late effects of Childhood Cancer Guideline Harmonization Group. Pediatr Blood Cancer. 2013;60:543–549. doi: 10.1002/pbc.24445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ferrari A, Thomas D, Franklin AR, et al. Starting an adolescent and young adult program: Some success stories and some obstacles to overcome. J Clin Oncol. 2010;28:4850–4857. doi: 10.1200/JCO.2009.23.8097. [DOI] [PubMed] [Google Scholar]
- 17. Linendoll N, Murphy-Banks R, Barthel E, et al. The creation of a comprehensive adolescent and young adult cancer survivorship program: “Lost in transition” no more. J Adolesc Young Adult Oncol. 2021;10:397–403. doi: 10.1089/jayao.2020.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Crosswell HE, Bomar KN, Vickery N, et al. Trials and tribulations for adolescents and young adults with cancer: Measuring the impact of a community-based program. J Natl Compr Cancer Netw. 2017;15:1171–1176. doi: 10.6004/jnccn.2017.0153. [DOI] [PubMed] [Google Scholar]
- 19. Bauer MS, Damschroder L, Hagedorn H, et al. An introduction to implementation science for the non-specialist. BMC Psychol. 2015;3:1–12. doi: 10.1186/s40359-015-0089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Damschroder LJ, Aron DC, Keith RE, et al. Fostering implementation of health services research findings into practice: A consolidated framework for advancing implementation science. Implement Sci. 2009;4:50. doi: 10.1186/1748-5908-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dillman DA. Mail and Telephone Surveys: The Total Design Method. Volume 19. New York, NY: Wiley; 1978. [Google Scholar]
- 22.Interview Guide Tool Consolidated Framework for Implementation Research www.cfirguide.org.
- 23.King N. Cassell C and Symon G (eds): Essential Guide to Qualitative Methods in Organizational Research. Volume 2. London, UK, SAGE Publications Ltd: 2004. Using templates in the thematic analysis of text; pp. 256–270. [Google Scholar]
- 24. Raskind IG, Shelton RC, Comeau DL, et al. A review of qualitative data analysis practices in health education and health behavior research. Health Educ Behav. 2018;46:32–39. doi: 10.1177/1090198118795019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Elo S, Kääriäinen M, Kanste O, et al. Qualitative content analysis: A focus on trustworthiness. SAGE Open. 2014;4:2158244014522633. [Google Scholar]
- 26. Osborn M, Johnson R, Thompson K, et al. Models of care for adolescent and young adult cancer programs. Pediatr Blood Cancer. 2019;66:e27991. doi: 10.1002/pbc.27991. [DOI] [PubMed] [Google Scholar]
- 27. Greenberg M, Klassen A, Gafni A, et al. Outcomes and metrics. Cancer. 2011;117:2342–2350. doi: 10.1002/cncr.26040. [DOI] [PubMed] [Google Scholar]


