PURPOSE
Aumolertinib (formerly almonertinib; HS-10296) is a novel third-generation epidermal growth factor receptor tyrosine kinase inhibitor approved in China. This double-blind phase III trial evaluated the efficacy and safety of aumolertinib compared with gefitinib as a first-line treatment for locally advanced or metastatic EGFR-mutated non–small-cell lung cancer (NSCLC; ClinicalTrials.gov identifier: NCT03849768).
METHODS
Patients at 53 sites in China were randomly assigned 1:1 to receive either aumolertinib (110 mg) or gefitinib (250 mg) once daily. The primary end point was progression-free survival (PFS) per investigator assessment.
RESULTS
A total of 429 patients who were naïve to treatment for locally advanced or metastatic NSCLC were enrolled. PFS was significantly longer with aumolertinib compared with gefitinib (hazard ratio, 0.46; 95% CI, 0.36 to 0.60; P < .0001). The median PFS with aumolertinib was 19.3 months (95% CI, 17.8 to 20.8) versus 9.9 months with gefitinib (95% CI, 8.3 to 12.6). Objective response rate and disease control rate were similar in the aumolertinib and gefitinib groups (objective response rate, 73.8% and 72.1%, respectively; disease control rate, 93.0% and 96.7%, respectively). The median duration of response was 18.1 months (95% CI, 15.2 to not applicable) with aumolertinib versus 8.3 months (95% CI, 6.9 to 11.1) with gefitinib. Adverse events of grade ≥ 3 severity (any cause) were observed in 36.4% and 35.8% of patients in the aumolertinib and gefitinib groups, respectively. Rash and diarrhea (any grade) were observed in 23.4% and 16.4% of patients who received aumolertinib compared with 41.4% and 35.8% of those who received gefitinib, respectively.
CONCLUSION
Aumolertinib is a well-tolerated third-generation epidermal growth factor receptor tyrosine kinase inhibitor that could serve as a treatment option for EGFR-mutant NSCLC in the first-line setting.
INTRODUCTION
Epidermal growth factor receptor (EGFR) mutations are one of the most common oncogenic driver mutations in non–small-cell lung cancer (NSCLC). Osimertinib, a third-generation EGFR tyrosine kinase inhibitor (TKI), was initially approved on the basis of the clinical efficacy demonstrated by the AURA program for the treatment of NSCLC patients with an EGFR T790M mutation and was subsequently approved for first-line treatment of patients with advanced NSCLC and EGFR exon 19 deletion or L858R mutations.1-4 In the pivotal FLAURA study, treatment with osimertinib resulted in a 54% reduction in the risk of disease progression or death as compared with treatment with a first-generation EGFR TKI. However, the toxicities of rash and diarrhea are strongly associated with inhibition of wild-type EGFR.3 There is a need for additional third-generation EGFR inhibitors that both offer effective first-line treatment of EGFR-mutant NSCLC and are well-tolerated.
CONTEXT
Key Objective
ANEAS is a randomized, double-blind, phase III trial evaluating the efficacy and safety of aumolertinib compared with gefitinib as first-line treatment for locally advanced or metastatic epidermal growth factor receptor–mutated non–small-cell lung cancer (NSCLC; ClinicalTrials.gov identifier: NCT03849768).
Knowledge Generated
Aumolertinib is a novel third-generation epidermal growth factor receptor tyrosine kinase inhibitor that has been approved in China for patients with EGFR-mutant NSCLC. The results suggest the possible use of aumolertinib as first-line treatment for EGFR-mutant NSCLC, particularly given the encouraging low rates of EGFR wild-type–mediated toxicity.
Relevance
This study conducted exclusively in China has findings broadly applicable to this genomically defined, global patient population as the natural history of and approach to evaluation and treatment of patients with EGFR-mutant NSCLC are similar worldwide, as evidenced by the guidelines of the National Comprehensive Cancer Network, European Society for Medical Oncology, and The Pan-Asian Guidelines Adaptation.
Aumolertinib (proposed international nonproprietary name; formerly almonertinib; HS-10296) is a novel, irreversible, third-generation EGFR TKI developed by Hansoh Pharmaceutical Group Co, Ltd (Shanghai, China). Aumolertinib demonstrated higher selectivity against both EGFR-sensitizing and T790M mutations with less inhibition against wild-type EGFR than osimertinib.5 In March 2020 aumolertinib was approved in China, on the basis of APOLLO (ClinicalTrials.gov identifier: NCT0298110),6,7 for the treatment of patients with advanced NSCLC and an EGFR T790M mutation.
On the basis of the preclinical and promising clinical profile of aumolertinib in the second-line setting, the AENEAS trial, a phase III randomized, double-blind study comparing aumolertinib with gefitinib in the first-line setting for patients with advanced EGFR-mutant NSCLC, was initiated and the results of the primary analysis are reported herein.
METHODS
Study Design and Patients
AENEAS was a multicenter, double-blind, randomized phase III trial conducted at 53 study sites in mainland China (Data Supplement, online only). The trial was conducted in accordance with the protocol, applicable local regulations, and the Declaration of Helsinki and International Conference on Harmonisation Guidelines for Good Clinical Practices principles. The Protocol (online only) was approved by each institution's research ethics board, and all patients provided written informed consent before initiating any study-related procedure.
Patients with histologic or cytologic confirmation of locally advanced or metastatic NSCLC harboring an EGFR mutation (sensitizing mutations, such as exon 19 deletion and L858R mutations, as detected by a central laboratory using the Cobas EGFR Mutation Test [version 2; Roche Molecular Systems Inc, Pleasanton, CA]) were eligible, as detected in tissue (preferred) or blood. No prior systemic therapy was permitted except for treatment in the adjuvant/neoadjuvant setting, and no prior treatment with an EGFR inhibitor was permitted.
At baseline, patients were required to have at least one measurable lesion, defined as ≥ 10 mm. Baseline assessment was performed using RECIST 1.1. Prestudy CNS imaging was mandatory. Patients with asymptomatic, stable CNS metastases that did not require steroids for at least 2 weeks before starting the study drug (ie, aumolertinib or gefitinib) were included.
Study Procedures
An interactive web response system randomly assigned eligible patients 1:1 to receive either 110 mg aumolertinib or 250 mg gefitinib, administered once daily orally, stratified by the type of EGFR mutation (exon 19 deletion or L858R) and CNS metastases status (with or without). Treatment with study drug was continued until disease progression, withdrawal of consent, the development of unacceptable side effects, or the fulfillment of other discontinuation criteria. Treatment with study drug beyond disease progression was permitted if the patients continued to derive clinical benefits as assessed by the treating investigator. Upon disease progression, patients in the gefitinib group who acquired an EGFR T790M mutation were eligible to crossover to aumolertinib treatment.
Outcomes
The primary end point was progression-free survival (PFS), as determined by investigator assessment. Secondary end points included overall survival (OS), objective response rate (ORR), duration of response (DoR), disease control rate (DCR), and depth of response.
Systemic response was assessed by the investigators and by blinded independent central review and was classified according to RECIST 1.1. Computed tomography imaging was performed at baseline and every 6 weeks (±7 days) from the start of aumolertinib until the 15-month time point, after which imaging was performed at 12-week intervals. Prestudy CNS imaging was mandatory.
All adverse events (AEs) were evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03 for up to 28 days beyond the last dose of any protocol treatment.
Statistical Analysis
Approximately 262 events of 410 randomly assigned patients can achieve 90% power to detect a hazard ratio (HR) of 0.67 (median PFS [mPFS] from 10 to 15 months) at a 2-sided alpha level of .05 with an enrollment ratio of 1:1, an accrual time of 8 months, and a longest follow-up time of 23 months.
Primary efficacy analysis was performed on the full analysis set, including all randomly assigned patients who received the study drug at least once. The primary end point of PFS was summarized using the Kaplan-Meier method. A log-rank test that was stratified by EGFR mutation type and CNS metastases status was used to detect the statistical difference of PFS between the two groups.
The stratified Cox proportional hazards model, as adjusted by EGFR mutation type and CNS metastases status, was used to estimate the PFS HR, along with the two-sided 95% CI of the two treatment groups. The proportional hazards assumption was assessed by the use of the model from the study by Lin et al.8 Other time-to-event end points were analyzed in a similar manner. Both ORR and DCR were summarized using the point estimate with the two-sided 95% CI. A logistic regression model, stratified by EGFR mutation type and CNS metastases status, was used for odds ratio and P value calculation to compare ORR and DCR between the two treatment groups. Safety analyses were performed on the safety analysis set, which included all randomly assigned patients who received the study drug at least once. All the statistical analysis was performed using SAS v9.4.
RESULTS
The results herein are presented using the data cutoff date of January 15, 2021.
Study Patients and Study Drug Treatment
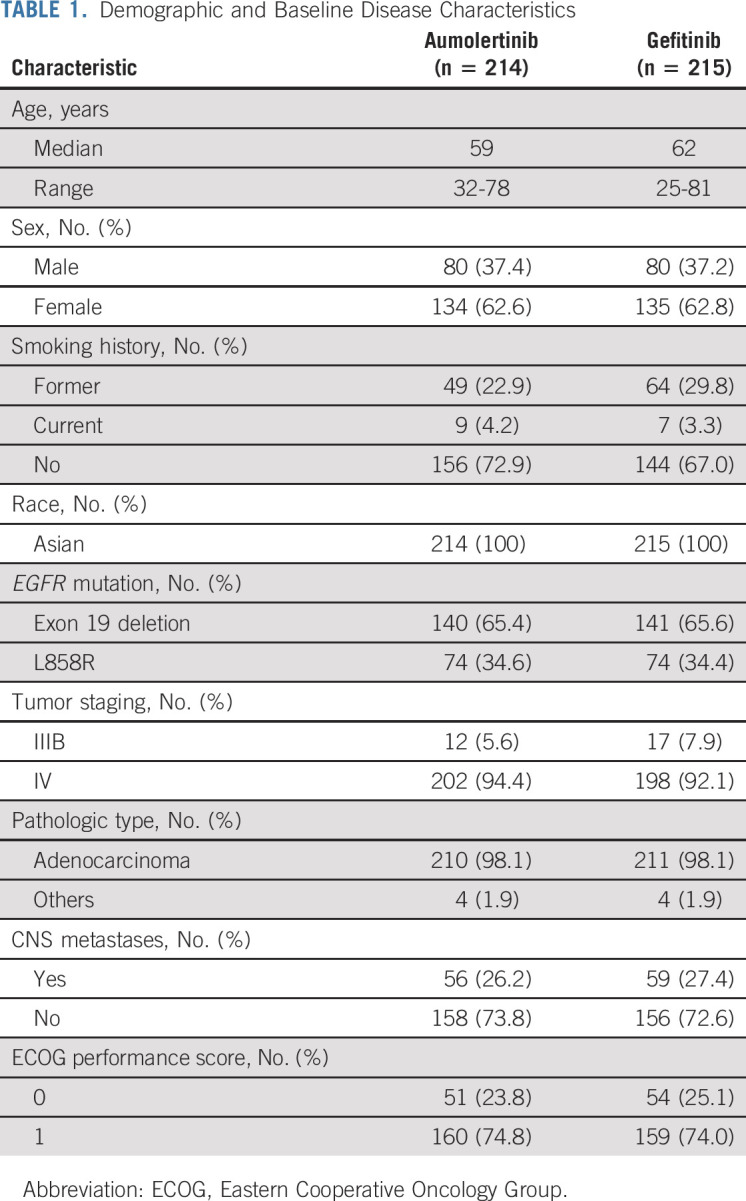
Between November 30, 2018, and September 06, 2019, 838 patients were screened at 53 centers in China. A total of 409 patients failed screening, and 429 patients were enrolled and randomly assigned (Data Supplement). Forty-one patients (19.1%) on the gefitinib arm had crossed over and received at least one dose of aumolertinib as of the data cutoff date, the disposition of which is presented in the Data Supplement. All randomly assigned patients received one or more doses of study drug (ie, aumolertinib or gefitinib). All enrolled patients had baseline measurable disease according to RECIST 1.1 assessment. Demographic and baseline characteristics were well-balanced between the groups (Table 1). In the total population, 115 patients (26.8%) had brain metastases at baseline—26.2% in the aumolertinib group and 27.4% in the gefitinib group (Table 1).
TABLE 1.
Demographic and Baseline Disease Characteristics
At the data cutoff of January 15, 2021, the median duration of drug total exposure was 463.5 (range, 1-715) days for aumolertinib and 254.0 (range, 1-714) days for gefitinib. The medication compliance was 99.3% and 98.5% for patients receiving aumolertinib and gefitinib, respectively. A total of 105 patients in the aumolertinib group (data maturity of 49.1%) and 158 patients in the gefitinib group (data maturity of 73.5%) had experienced an event of RECIST-defined progression or death. A total of 93 patients (43.5%) in the aumolertinib group and 34 patients (15.8%) in the gefitinib group had continued to receive study drug treatment as of the data cutoff date.
Efficacy
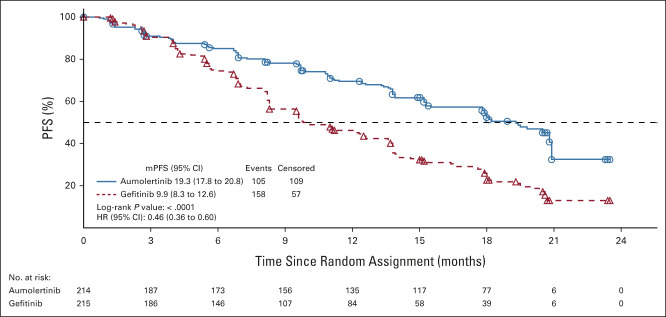
The median follow-up time in the aumolertinib group was 20.5 months (95% CI, 18.0 to 20.6), compared with 20.7 months (95% CI, 19.3 to 20.8) in the gefitinib group, respectively. The median duration of PFS (as defined by either progressive disease or death) was 19.3 months (95% CI, 17.8 to 20.8) in the aumolertinib group, which was significantly longer (P < .0001) than that of the gefitinib group at 9.9 months (95% CI, 8.3 to 12.6; Fig 1). The proportional hazard assumption was not violated per the testing results with a P value of .094. The HR between the two groups was 0.46 (95% CI, 0.36 to 0.60; Fig 1). As such, improvements in both 1-year (69.5% v 46.3%) and 2-year (32.5% v 12.9%) PFS rates were observed for the aumolertinib and gefitinib groups, respectively. Analysis of PFS by blinded independent central review was consistent with these results and is presented in the Data Supplement.
FIG 1.
Kaplan-Meier estimates of PFS. The duration of PFS by investigator assessment is estimated via Kaplan-Meier methods. In tandem, hazard in the aumolertinib arm divided by the hazard in the gefitinib arm provided the hazard ratio. For the patients who discontinued study treatment or received new antitumor therapy treatment before progression or death, the patient was censored at the latest evaluable examination date of imaging before the discontinuation date or the date of starting new antitumor therapies. HR, hazard ratio; mPFS, median PFS; PFS, progression-free survival.
Aumolertinib demonstrated a consistent PFS benefit across all prespecified stratification factors, namely, EGFR mutation type and the presence or absence of known or treated CNS metastases (Fig 2).
FIG 2.
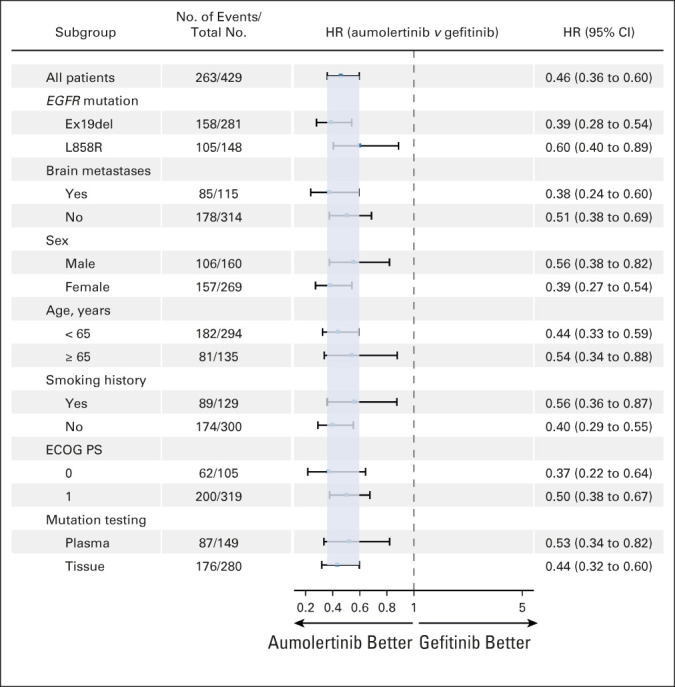
PFS by subgroup analysis. PFS was estimated as shown in Figure 1 for the following prespecified subgroups: EGFR mutation type, brain/CNS metastases status, sex, age, smoking history, baseline ECOG PS, and mutation test methods. ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; PFS, progression-free survival.
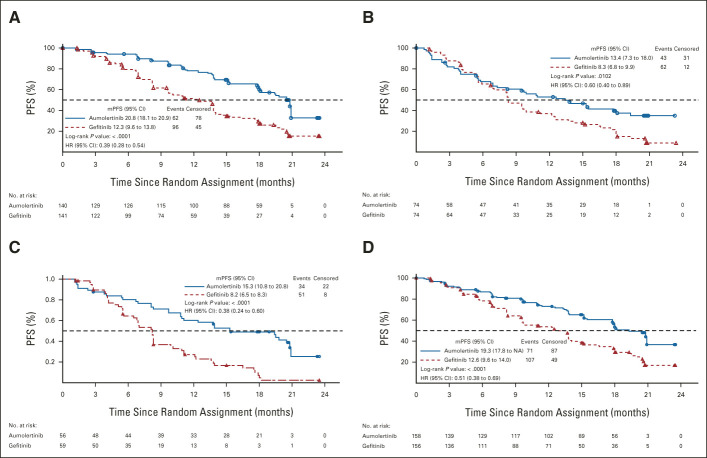
Among all patients with an EGFR exon 19 deletion mutation, the mPFS for the aumolertinib and gefitinib groups was 20.8 months and 12.3 months, respectively (HR, 0.39; P < .0001; Fig 3A). Among all patients with EGFR L858R, the mPFS for the aumolertinib and gefitinib groups was 13.4 months and 8.3 months, respectively (HR, 0.60; P = .0102; Fig 3B). Among all patients with CNS metastases, the mPFS for the aumolertinib and gefitinib groups was 15.3 months and 8.2 months, respectively (HR, 0.38;P < .0001; Fig 3C). By comparison, among patients without CNS metastases, the mPFS for both groups was 19.3 months and 12.6 months, respectively (HR, 0.51; P < .0001; Fig 3D). Analysis of PFS by other subgroups such as sex, age, smoking history, and baseline ECOG PS is shown in Figure 2.
FIG 3.
Kaplan-Meier estimates of PFS by stratification factors. PFS was estimated as shown in Figure 1 for the following prespecified groups: (A) patients with EGFR exon 19 deletion, (B) patients with EGFR L858R, (C) patients with CNS metastases, and (D) patients without CNS metastases. HR, hazard ratio; mPFS, median PFS; NA, not applicable; PFS, progression-free survival.
The summary of secondary efficacy end points is shown in Table 2. Preliminary analysis of OS at the time of the final PFS analysis showed that 123 patients had died (data maturity of 28.7%), including 54 patients (data maturity of 25.2%) in the aumolertinib group and 69 patients (data maturity of 32.1%) in the gefitinib group.
TABLE 2.
Summary of Secondary Efficacy End Points
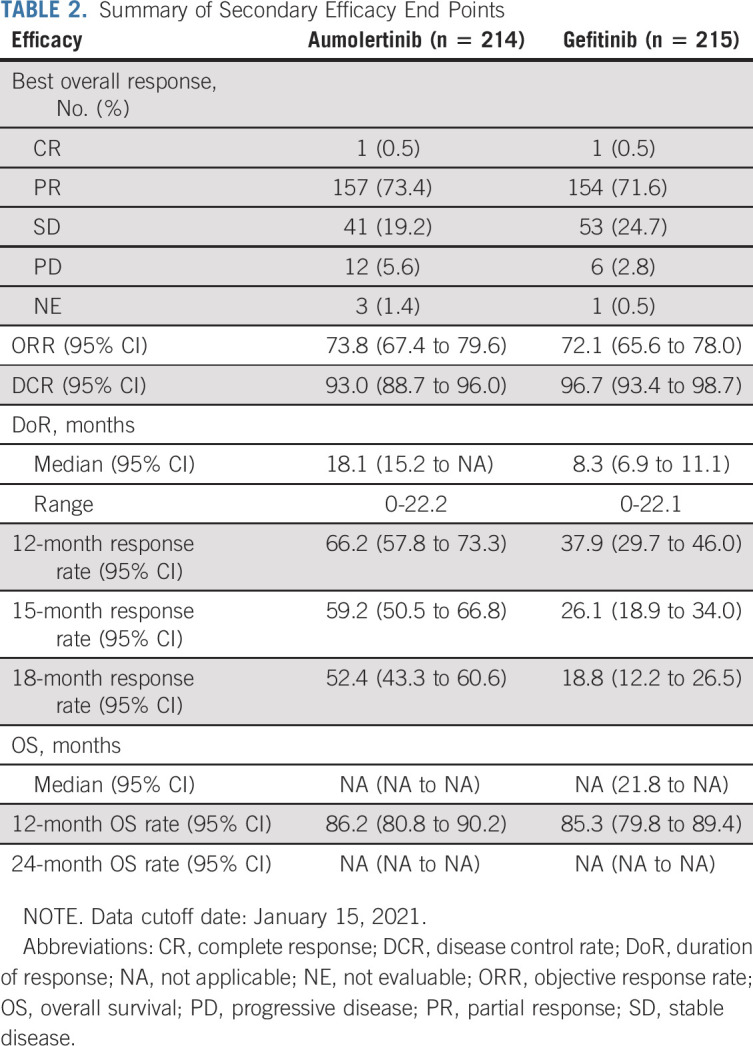
Both ORR and DCR were similar in the aumolertinib and gefitinib groups, respectively, with ORRs of 73.8% (95% CI, 67.4 to 79.6) and 72.1% (95% CI, 65.6 to 78.0), respectively, and DCRs of 93.0% (95% CI, 88.7 to 96.0) and 96.7% (95% CI, 93.4 to 98.7), respectively.
For both ORR and DCR, there was no statistically significant difference between the two groups (P = .6939 and .0884, respectively). The mean percentage of depth of best response of the aumolertinib group was 45% (range, –100 to 50), whereas that of the gefitinib group was 42% (range, –85.5 to 24.4), with no significant difference between the groups (P = .1688). The maximum percentage change in sum of the diameters of target lesions compared with baseline for aumolertinib and gefitinib is shown in the Data Supplement.
The median DoR was longer in the aumolertinib group than the gefitinib group at 18.1 months (95% CI, 15.2 to not applicable) versus 8.3 months (95% CI, 6.9 to 11.1; HR, 0.38; 95% CI, 0.28 to 0.51; P < .0001). Among patients who had responded to study drug treatment, an event of disease progression or death occurred in 72 of 158 patients (45.6%) in the aumolertinib group and 113 of 155 patients (72.9%) in the gefitinib group as of the data cutoff date.
Safety and AEs
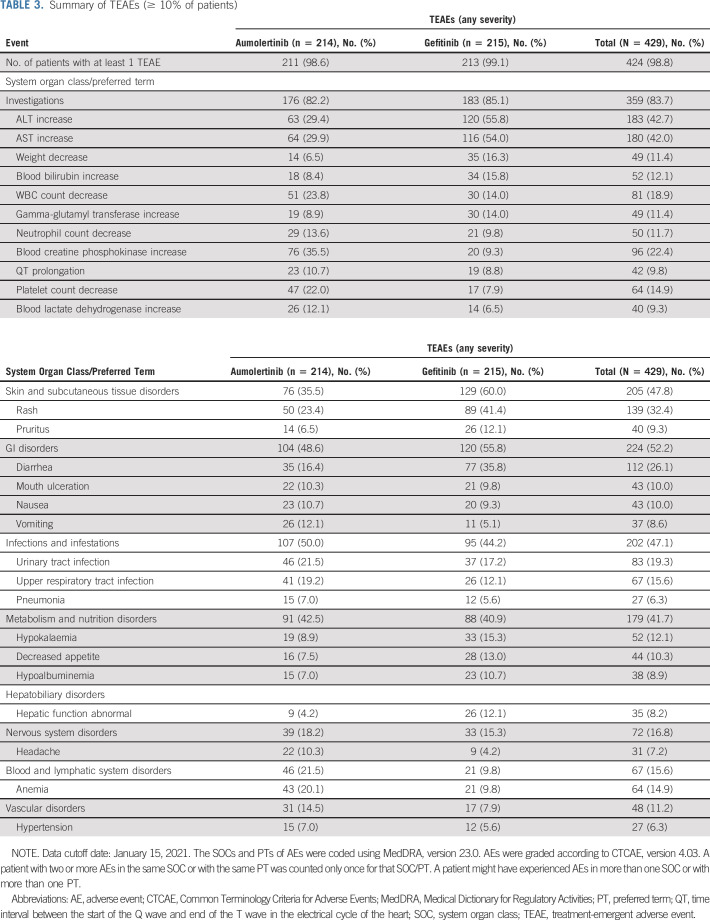
Of the 429 patients, 424 (98.8%) had experienced at least one AE during the study treatment period as of the data cutoff date, January 15, 2021. Of total, 211 patients (98.6%) in the aumolertinib group and 213 patients (99.1%) in the gefitinib group had experienced at least one treatment-emergent adverse event (TEAE; Table 3). TEAEs of grade ≥ 3 were similar in the aumolertinib and gefitinib groups (36.4% v 35.8%), respectively (Data Supplement). The most common TEAEs of grade ≥ 3 were ALT increase (2.8% v 12.1%) and aspartate transaminase increase (1.4% v 9.3%), respectively (Data Supplement). Incidence of serious adverse events was similar in the aumolertinib and gefitinib groups (22.0% v 21.4%), respectively (Data Supplement).
TABLE 3.
Summary of TEAEs (≥ 10% of patients)
The most common AEs in the aumolertinib and gefitinib groups were blood creatine phosphokinase (CPK) increase (35.5% v 9.3%), aspartate transaminase increase (29.9% v 54.0%), ALT increase (29.4% v 55.8%), rash (23.4% v 41.4%), and diarrhea (16.4% v 35.8%; Table 3), respectively. Treatment-related AEs (incidence ≥ 10%) and serious adverse events (any incidence) assessed by the investigator are summarized in the Data Supplement. Two (0.9%) patients in the aumolertinib group experienced interstitial lung disease (which was grade 2) assessed by the investigator as treatment-related, whereas one (0.5%) patient in the gefitinib group had interstitial lung disease.
Aumolertinib was associated with a lower rate of AEs leading to permanent discontinuation (3.7% compared with 5.1% with gefitinib). Dose interruptions were implemented in 36 (16.8%) patients in the aumolertinib group and in 53 (24.7%) patients in the gefitinib group. Nine (4.2%) patients in the aumolertinib group and 10 (4.7%) in the gefitinib group experienced AEs leading to dose reduction.
A total of eight patients—five in the aumolertinib group and three in the gefitinib group—experienced AEs leading to death between the start of initial study drug and up to 28 days after their last dose of study drug treatment. For the five aumolertinib-treated patients, the events leading to death were cardiac arrest (n = 2) and cerebral infarction, cardiogenic pulmonary edema complicated with upper GI hemorrhage, and unexplained (n = 1 each), respectively; the investigator-assessed relationship of the AE to study drug was unrelated in four patients and unable to be determined in one patient. For the three gefitinib-treated patients, the events leading to death were suicide, infectious pneumonia, and unexplained, respectively; the investigator-assessed relationship of the AE to study drug was unrelated in two patients and unable to be determined in one patient.
DISCUSSION
In this article we describe a randomized, phase III trial assessing aumolertinib as an intervention in the context of first-line treatment of patients with advanced EGFR-mutant NSCLC using gefitinib as an active comparator. First-generation EGFR inhibitors (eg, gefitinib) were traditionally considered the standard of care for disseminated and recurrent EGFR-mutated NSCLC, until the approval of osimertinib by the US Food and Drug Administration in April 2018 for first-line use as demonstrated by the FLAURA trial.3,9 Subsequently, in December 2020, osimertinib was also approved for use as an adjuvant therapy for resected NSCLC,10 on the basis of the results of the ADAURA trial. Inherent to this robust progress is the observation that a longer duration of therapy magnifies the importance of tolerability, toxicity, and the cost of component therapy. Given the approval of only one third-generation EGFR inhibitor, there is a standing need for another highly efficacious and well-tolerated agent to diversify the treatment armamentarium.
In AENEAS, aumolertinib met the primary objective of the trial by demonstrating a 9.4-month improvement in mPFS relative to gefitinib. Benefits with aumolertinib were noted across all prespecified stratification factors, including patients stratified by the presence (or absence) of CNS metastases (HR, 0.38). These results were particularly encouraging given that CNS metastases remain a major cause of morbidity and mortality in this population. The superior efficacy observed in patients with exon 19 deletion mutations relative to those with L858R mutations is a consistent feature of the class of third-generation EGFR inhibitors.3 The median DoR was nearly 10 months longer with aumolertinib, but no significant difference in ORR or DCR was observed.
Toxicities believed to be mediated by the inhibition of wild-type EGFR in cutaneous and GI epithelium were observed at lower rates in the aumolertinib group than in the gefitinib group. The rates of both rash and diarrhea were significantly lower in the aumolertinib group than in the gefitinib group (23.4% v 41.4% and 16.4% v 35.8%, respectively). The observation suggesting less EGFR WT inhibition correlates with preclinical data, including the observation that the primary metabolite of aumolertinib retains comparable selectivity for mutant over wild-type EGFR as the parent compound7; it also correlates with the toxicity profile noted in APOLLO.6 Notably, these signals should be interpreted in light of the longer median total exposure time of aumolertinib relative to gefitinib, 464 versus 254 days, respectively.
Blood CPK (skeletal muscle isoform) increase was relatively common with aumolertinib (34% of patients), but this event was predominately mild to moderate in severity. Only 7% of patients had a blood CPK increase of grade ≥ 3 severity. No patient had a CPK increase associated with rhabdomyolysis, and CPK increase did not manifest as a serious adverse event. Thus, CPK increase was a laboratory finding only rarely associated with mild muscle pain. Although CPK increase led to temporary interruption of aumolertinib in 11 patients (5.1%) and to dose reduction in six patients (2.8%), no patients discontinued aumolertinib because of CPK increase.
Importantly, this study was conducted exclusively in China with ethnic Chinese patients. The extrapolation of these study results to patients of other geographic regions is supported by the globally consistent approach to evaluation and treatment of patients with EGFR-mutant NSCLC evident in the high similarity of the National Comprehensive Cancer Network, European Society for Medical Oncology, and The Pan-Asian Guidelines Adaptation.11 Furthermore, multiple studies suggest similar outcomes for this patient population when controlling for widely recognized baseline characteristics such as ECOG PS, EGFR mutation type, and sex. Analyses of the outcomes of patients with CNS metastases and CNS progressive disease will be reported in a separate publication. Similarly, given the low maturity of OS, these data along with crossover analyses will be reported in a subsequent publication.
In conclusion, aumolertinib is a well-tolerated third-generation EGFR TKI that is approved in China for the second-line treatment of advanced EGFR T790M mutation-positive NSCLC patients in 2020 and recently for the first-line treatment of locally advanced or metastatic NSCLC harboring TKI sensitizing mutations. The results presented suggest that the use of aumolertinib may be warranted as the first-line treatment for EGFR-mutant NSCLC, particularly given the encouraging rates of EGFR wild-type-mediated toxicity. Additional studies of aumolertinib are ongoing in the adjuvant setting (ClinicalTrials.gov identifier: NCT04687241) and in the metastatic setting in combination with chemotherapy (ClinicalTrials.gov identifier: NCT04923906).
ACKNOWLEDGMENT
We thank all patients in this study and their families, along with the investigators and staff at the participating centers. We also thank the study teams from Hansoh Pharmaceutical Group Co. Ltd, including Sui-Sui Dong, Jiawei Wei, Xiaoling Qian, Xue Sun, Zhenzhong Su, Qiu Sun, and Ziqiang Chen for their support and the Teddy Clinical Research Laboratory (Shanghai, China) for EGFR mutation testing and Fantastic Bioimaging (Shanghai, China) for ICR assessment. We thank Rakesh Ojha and Catherine Despot for medical writing assistance.
Shun Lu
Consulting or Advisory Role: AstraZeneca, Pfizer, Boehringer Ingelheim, Hutchison MediPharma, Simcere Pharmaceutical Group, Zai Lab, GenomiCare, Yuhan, Prime Oncology, Roche
Speakers' Bureau: AstraZeneca, Roche, Hansoh Pharma, Hengrui Therapeutics
Research Funding: AstraZeneca (Inst), Hutchison MediPharma (Inst), BMS (Inst), Hengrui Therapeutics (Inst), BeiGene (Inst), Roche (Inst)
Xiaorong Dong
Consulting or Advisory Role: Hansoh Pharma
Research Funding: Hansoh Pharma (Inst)
Yuping Sun
Honoraria: Roche, Pfizer, AstraZeneca
Consulting or Advisory Role: AstraZeneca, Roche
Research Funding: Roche, Pfizer
Yinghua Ji
Research Funding: AoSaikang, CSPC Pharma, Hualan Bio (Inst)
Jianhua Shi
Honoraria: Roche, BeiGene
Consulting or Advisory Role: Hansoh Pharma
Research Funding: Hansoh Pharma (Inst)
Junguo Lu
Research Funding: Hansoh (Inst), Akesobio (Inst), Shanghai Henlius Biotech (Inst)
Shaoshui Chen
Consulting or Advisory Role: Hansoh Pharma
Research Funding: Hansoh Pharma (Inst)
Xinmin Yu
Research Funding: BeiGene, Innovent Biologics, BMS, MSD, Hansoh
Zhehai Wang
Consulting or Advisory Role: Hansoh Pharma
Research Funding: Hansoh Pharma (Inst)
Xingxiang Xu
Consulting or Advisory Role: Hansoh Pharma
Research Funding: Hansoh Pharma (Inst)
Jian Fang
Consulting or Advisory Role: Hansoh Pharma
Research Funding: Hansoh Pharma (Inst)
Yanping Hu
Consulting or Advisory Role: Hansoh Pharma
Research Funding: Hansoh Pharma (Inst)
Nong Yang
Consulting or Advisory Role: Hansoh Pharmaceutical Group Co, Ltd
Research Funding: Hansoh Pharmaceutical Group Co, Ltd
Xiangdong Zhou
Research Funding: MSD, AZ, Hansoh, Hengrui, Sanofi, J&J, BMS, BeiGene, Pfizer, GSK, Novartis, Chiesi (Inst)
You Lu
Honoraria: Roche/Genentech, AstraZeneca, Pfizer, Bristol Myers Squibb, Merck Sharp & Dohme, BeiGene
Consulting or Advisory Role: Roche/Genentech, AstraZeneca, Pfizer, Bristol Myers Squibb, Merck Sharp & Dohme, BeiGene
Qiming Wang
Consulting or Advisory Role: Hansoh Pharma
Research Funding: Hansoh Pharma (Inst)
Jianying Zhou
Consulting or Advisory Role: Hansoh Pharma
Research Funding: Hansoh Pharma (Inst)
Yuan Chen
Consulting or Advisory Role: Hansoh Pharma
Research Funding: Hansoh Pharma (Inst)
Haihua Yang
Consulting or Advisory Role: Hansoh Pharma
Research Funding: Hansoh Pharma (Inst)
Changan Sun
Employment: Jiangsu Hansoh Pharmaceutical Group Co, Ltd
Leadership: Jiangsu Hansoh Pharmaceutical Group Co, Ltd
Hongying Wei
Employment: Hansoh Pharma
Chuan Li
Employment: Hansoh Pharma
Siraj M. Ali
Employment: EQRX, EQRX
Leadership: Incysus, Elevation Oncology, Pillar Biosciences, Droplet Biosciences
Stock and Other Ownership Interests: Exelixis, Merus NV, Pfizer, Pfizer
Consulting or Advisory Role: Azitra, Princeptx, Archer
Patents, Royalties, Other Intellectual Property: patents via Foundation Medicine, patents via Seres Health on microbiome stuff in non-neoplastic disease
Vincent A. Miller
Employment: Foundation Medicine, EQRX
Leadership: Revolution Medicines
Stock and Other Ownership Interests: Foundation Medicine, Mirati Therapeutics, Revolution Medicines, EQRX
Patents, Royalties, Other Intellectual Property: Received periodic royalties related to T790M patent awarded to the Memorial Sloan Kettering Cancer Center
Qiong Wu
Employment: Hansoh Pharma
No other potential conflicts of interest were reported.
See accompanying editorial on page 3103
PRIOR PRESENTATION
Presented in part at the 2021 ASCO conference, Chicago, IL, June 4-8, 2021.
SUPPORT
Supported by Hansoh Pharmaceutical Group Co, Ltd.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
Upon request, and subject to certain criteria, conditions, and exceptions, Hansoh will provide access to individual deidentified participant data from Hansoh-sponsored interventional clinical studies conducted for medicines for indications that have been approved. Data requests may be submitted to medical@hspharm.com.
AUTHOR CONTRIBUTIONS
Conception and design: Shun Lu, Hongying Wei, Qiong Wu
Administrative support: Hongying Wei, Changan Sun
Provision of study materials or patients: Xiaorong Dong, Hong Jian, Jianhua Chen, Gongyan Chen, Yuping Sun, Yinghua Ji, Ziping Wang, Jianhua Shi, Junguo Lu, Shaoshui Chen, Dongqing Lv, Guojun Zhang, Chunling Liu, Juan Li, Xinmin Yu, Zhong Lin, Zhuang Yu, Zhehai Wang, Jiuwei Cui, Xingxiang Xu, Jian Fang, Jifeng Feng, Zhi Xu, Rui Ma, Jie Hu, Nong Yang, Xiangdong Zhou, Xiaohong Wu, Zhihong Zhang, You Lu, Yanping Hu, Liyan Jiang, Qiming Wang, Renhua Guo, Jianying Zhou, Baolan Li, Chunhong Hu, Wancheng Tong, Helong Zhang, Lin Ma, Yuan Chen, Zhijun Jie, Yu Yao, Longzhen Zhang, Weng Jie, Weidong Li, Jianping Xiong, Xianwei Ye, Jianchun Duan, Meili Sun, Changan Sun, Hongying Wei, Qiong Wu
Collection and assembly of data: Shun Lu, Xiaorong Dong, Hong Jian, Jianhua Chen, Gongyan Chen, Yuping Sun, Yinghua Ji, Ziping Wang, Jianhua Shi, Junguo Lu, Shaoshui Chen, Dongqing Lv, Guojun Zhang, Chunling Liu, Juan Li, Xinmin Yu, Zhong Lin, Zhuang Yu, Zhehai Wang, Jiuwei Cui, Xingxiang Xu, Jian Fang, Jifeng Feng, Zhi Xu, Rui Ma, Jie Hu, Nong Yang, Xiangdong Zhou, Xiaohong Wu, Chengping Hu, Zhihong Zhang, You Lu, Yanping Hu, Liyan Jiang, Qiming Wang, Renhua Guo, Jianying Zhou, Baolan Li, Chunhong Hu, Wancheng Tong, Helong Zhang, Lin Ma, Yuan Chen, Zhijun Jie, Yu Yao, Longzhen Zhang, Weng Jie, Weidong Li, Jianping Xiong, Xianwei Ye, Jianchun Duan, Haihua Yang, Meili Sun, Changan Sun, Hongying Wei, Qiong Wu
Data analysis and interpretation: Shun Lu, Hongying Wei, Chuan Li, Siraj M. Ali, Vincent A. Miller, Qiong Wu
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
AENEAS: A Randomized Phase III Trial of Aumolertinib Versus Gefitinib as First-Line Therapy for Locally Advanced or Metastatic Non–Small-Cell Lung Cancer With EGFR Exon 19 Deletion or L858R Mutations
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Shun Lu
Consulting or Advisory Role: AstraZeneca, Pfizer, Boehringer Ingelheim, Hutchison MediPharma, Simcere Pharmaceutical Group, Zai Lab, GenomiCare, Yuhan, Prime Oncology, Roche
Speakers' Bureau: AstraZeneca, Roche, Hansoh Pharma, Hengrui Therapeutics
Research Funding: AstraZeneca (Inst), Hutchison MediPharma (Inst), BMS (Inst), Hengrui Therapeutics (Inst), BeiGene (Inst), Roche (Inst)
Xiaorong Dong
Consulting or Advisory Role: Hansoh Pharma
Research Funding: Hansoh Pharma (Inst)
Yuping Sun
Honoraria: Roche, Pfizer, AstraZeneca
Consulting or Advisory Role: AstraZeneca, Roche
Research Funding: Roche, Pfizer
Yinghua Ji
Research Funding: AoSaikang, CSPC Pharma, Hualan Bio (Inst)
Jianhua Shi
Honoraria: Roche, BeiGene
Consulting or Advisory Role: Hansoh Pharma
Research Funding: Hansoh Pharma (Inst)
Junguo Lu
Research Funding: Hansoh (Inst), Akesobio (Inst), Shanghai Henlius Biotech (Inst)
Shaoshui Chen
Consulting or Advisory Role: Hansoh Pharma
Research Funding: Hansoh Pharma (Inst)
Xinmin Yu
Research Funding: BeiGene, Innovent Biologics, BMS, MSD, Hansoh
Zhehai Wang
Consulting or Advisory Role: Hansoh Pharma
Research Funding: Hansoh Pharma (Inst)
Xingxiang Xu
Consulting or Advisory Role: Hansoh Pharma
Research Funding: Hansoh Pharma (Inst)
Jian Fang
Consulting or Advisory Role: Hansoh Pharma
Research Funding: Hansoh Pharma (Inst)
Yanping Hu
Consulting or Advisory Role: Hansoh Pharma
Research Funding: Hansoh Pharma (Inst)
Nong Yang
Consulting or Advisory Role: Hansoh Pharmaceutical Group Co, Ltd
Research Funding: Hansoh Pharmaceutical Group Co, Ltd
Xiangdong Zhou
Research Funding: MSD, AZ, Hansoh, Hengrui, Sanofi, J&J, BMS, BeiGene, Pfizer, GSK, Novartis, Chiesi (Inst)
You Lu
Honoraria: Roche/Genentech, AstraZeneca, Pfizer, Bristol Myers Squibb, Merck Sharp & Dohme, BeiGene
Consulting or Advisory Role: Roche/Genentech, AstraZeneca, Pfizer, Bristol Myers Squibb, Merck Sharp & Dohme, BeiGene
Qiming Wang
Consulting or Advisory Role: Hansoh Pharma
Research Funding: Hansoh Pharma (Inst)
Jianying Zhou
Consulting or Advisory Role: Hansoh Pharma
Research Funding: Hansoh Pharma (Inst)
Yuan Chen
Consulting or Advisory Role: Hansoh Pharma
Research Funding: Hansoh Pharma (Inst)
Haihua Yang
Consulting or Advisory Role: Hansoh Pharma
Research Funding: Hansoh Pharma (Inst)
Changan Sun
Employment: Jiangsu Hansoh Pharmaceutical Group Co, Ltd
Leadership: Jiangsu Hansoh Pharmaceutical Group Co, Ltd
Hongying Wei
Employment: Hansoh Pharma
Chuan Li
Employment: Hansoh Pharma
Siraj M. Ali
Employment: EQRX, EQRX
Leadership: Incysus, Elevation Oncology, Pillar Biosciences, Droplet Biosciences
Stock and Other Ownership Interests: Exelixis, Merus NV, Pfizer, Pfizer
Consulting or Advisory Role: Azitra, Princeptx, Archer
Patents, Royalties, Other Intellectual Property: patents via Foundation Medicine, patents via Seres Health on microbiome stuff in non-neoplastic disease
Vincent A. Miller
Employment: Foundation Medicine, EQRX
Leadership: Revolution Medicines
Stock and Other Ownership Interests: Foundation Medicine, Mirati Therapeutics, Revolution Medicines, EQRX
Patents, Royalties, Other Intellectual Property: Received periodic royalties related to T790M patent awarded to the Memorial Sloan Kettering Cancer Center
Qiong Wu
Employment: Hansoh Pharma
No other potential conflicts of interest were reported.
REFERENCES
- 1.Mok TS, Wu YL, Ahn MJ, et al. : Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med 376:629-640, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goss G, Tsai CM, Shepherd FA, et al. : Osimertinib for pretreated EGFR Thr790Met-positive advanced non-small-cell lung cancer (AURA2): A multicentre, open-label, single-arm, phase 2 study. Lancet Oncol 17:1643-1652, 2016 [DOI] [PubMed] [Google Scholar]
- 3.Soria JC, Ohe Y, Vansteenkiste J, et al. : Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med 378:113-125, 2018 [DOI] [PubMed] [Google Scholar]
- 4.Ramalingam SS, Vansteenkiste J, Planchard D, et al. : Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med 382:41-50, 2020 [DOI] [PubMed] [Google Scholar]
- 5.Bao R, Zhang F, Tong Z, et al. : Discovery of a third-generation EGFR inhibitor, which is highly selective and potent against both resistant and activating EGFR mutations for NSCLC therapy. Cancer Res 76, 2016. (suppl; abstr 3036) [Google Scholar]
- 6.Lu S, Wang Q, Zhang G, et al. : Efficacy of aumolertinib (HS-10296) in patients with advanced EGFR T790M+ NSCLC: Updated Post-National Medical Products Administration Approval results from the APOLLO registrational trial. J Thorac Oncol 17:411-422, 2022 [DOI] [PubMed] [Google Scholar]
- 7.Yang JCH, Camidge DR, Yang CT, et al. : Safety, efficacy, and pharmacokinetics of almonertinib (HS-10296) in pretreated patients with EGFR-mutated advanced NSCLC: A multicenter, open-label, phase 1 trial. J Thorac Oncol 15:1907-1918, 2020 [DOI] [PubMed] [Google Scholar]
- 8.Lin DY, Wei LJ, Ying Z: Checking the Cox model with cumulative sums of martingale-based residuals. Biometrika 80:557-572, 1993 [Google Scholar]
- 9.Nagasaka M, Zhu VW, Lim SM, et al. : Beyond osimertinib: The development of third-generation EGFR tyrosine kinase inhibitors for advanced EGFR+ NSCLC. J Thorac Oncol 16:740-763, 2021 [DOI] [PubMed] [Google Scholar]
- 10.Wu YL, Tsuboi M, He J, et al. : Osimertinib in resected EGFR-mutated non-small-cell lung cancer. N Engl J Med 383:1711-1723, 2020 [DOI] [PubMed] [Google Scholar]
- 11.Wu YL, Planchard D, Lu S, et al. : Pan-Asian adapted clinical practice guidelines for the management of patients with metastatic non-small-cell lung cancer: A CSCO-ESMO initiative endorsed by JSMO, KSMO, MOS, SSO and TOS. Ann Oncol 30:171-210, 2019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Upon request, and subject to certain criteria, conditions, and exceptions, Hansoh will provide access to individual deidentified participant data from Hansoh-sponsored interventional clinical studies conducted for medicines for indications that have been approved. Data requests may be submitted to medical@hspharm.com.