Abstract
Helicobacter pylori, an oxygen-sensitive microaerophile, contains an alkyl hydroperoxide reductase homologue (AhpC, HP1563) that is more closely related to 2-Cys peroxiredoxins of higher organisms than to most other eubacterial AhpC proteins. Allelic replacement mutagenesis revealed ahpC to be essential, suggesting a critical role for AhpC in defending H. pylori against oxygen toxicity. Characterization of the ahpC promoter region divulged two putative regulatory elements and identified the transcription initiation site, which was mapped to 96 and 94 bp upstream of the initiation codon. No homologue of ahpF, which encodes the dedicated AhpC reductase in most eubacteria, was found in the H. pylori genome. Instead, homologues of Escherichia coli thioredoxin (Trx) reductase (TrxR, HP0825) and Trx (Trx1, HP0824) formed a reductase system for H. pylori AhpC. A second Trx homologue (Trx2, HP1458) was identified but was incapable of AhpC reduction, although Trx2 exhibited disulfide reductase activity with other substrates [insulin and 5,5′-dithiobis(2-nitrobenzoic acid)]. AhpC interactions with each substrate, Trx1 and hydroperoxide, were bimolecular and nonsaturable (infinite Vmax and Km values) but rapid enough (at 1 × 105 to 2 × 105 M−1 s−1) to suggest an important role for AhpC in cellular peroxide metabolism. AhpC also exhibited a wide specificity for hydroperoxide substrates, which, taken together with the above results, suggests a minimal binding site for hydroperoxides composed of little more than the cysteinyl (Cys49) active site. H. pylori AhpC was not reduced by Salmonella typhimurium AhpF and was slightly more active with E. coli TrxR and Trx1 than was S. typhimurium AhpC, demonstrating the specialized catalytic properties of this peroxiredoxin.
Infection with Helicobacter pylori, a microaerophilic, gram-negative bacterium, is associated with type B gastritis and peptic ulcer disease and is a risk factor for gastric carcinomas in humans (8, 17, 27). It so prevalently affects the world population that H. pylori has been described as “the most common chronic infection” (http://www.cdc.gov/ncidod/dbmd/diseaseinfo/hpylori_t.html), with some developing countries experiencing nearly 100% infection rates. While H. pylori infection is generally controlled with a cocktail of antibiotics and bismuth (60), the specter of emerging antibiotic resistance necessitates the search for alternative drug strategies and a clearer understanding of bacterial defense systems.
H. pylori colonizes the mucosal layer of the stomach and secretes immunogenic products that recruit macrophages and polymorphonuclear leukocytes to the site of infection (59). Here, the resulting oxidative burst by the phagocytic cells produces reactive oxygen species (ROS), such as superoxide anion (O2⋅−), hydrogen peroxide (H2O2), and the hydroxyl radical (OH⋅−), that damage gastric tissues. Increased ROS levels are present in H. pylori patients (19), and it is thought that long-term exposure to ROS contributes to the development of cancerous gastric cells (16).
To resist oxidative damage from chronic inflammation, H. pylori relies on a variety of protective enzymatic systems, including catalase (the katA gene product) (47) and superoxide dismutase (the sodB gene product) (63), which eliminate H2O2 and O2⋅−, respectively; however, it is unknown how H. pylori coordinates its oxidative stress response because the bacterium lacks the oxidatively activated regulatory genes, soxRS and oxyR, common to other eubacteria (67). We report herein that H. pylori also expresses a thioredoxin (Trx)-dependent alkyl hydroperoxide reductase (AhpC) protein, a member of the peroxiredoxin (Prx) family (62), whose activity can detoxify lipid hydroperoxides analogous to those created in membranes exposed to ROS (32). All Prx enzymes are dimers, decamers, or, in some cases, higher-order aggregates (1, 38, 61) with one essential, N-terminal Cys residue per subunit (Cys46 of Salmonella typhimurium AhpC) (21). Some Prxs contain only this single, conserved Cys (the 1-Cys Prxs), but most include another conserved Cys residue (the 2-Cys Prxs) analogous to Cys165 in S. typhimurium AhpC, which links the two subunits via an intersubunit disulfide bond with the N-terminal Cys in the oxidized protein (21, 53). Reduced S. typhimurium AhpC directly converts hydroperoxides to alcohols with concomitant formation of a sulfenic acid (Cys-SOH) at Cys46; condensation between the two active-site cysteinyl derivatives then regenerates the stable intersubunit disulfide bond of the oxidized protein (20, 21).
Our search of the H. pylori genomic database for an AhpC homologue revealed a gene (ahpC, HP1563) that had previously been reported to encode a species-specific antigen by O'Toole et al. (48). Although not identified as a peroxidase at that time, the H. pylori AhpC sequence containing the two conserved Cys residues was later classified as a 2-Cys Prx (12). To reduce AhpC, most bacteria, including S. typhimurium, express a specialized flavoprotein reductase, AhpF, which is homologous to Escherichia coli Trx reductase (TrxR), except that AhpF contains an additional N-terminal region directly involved in AhpC reduction (54, 55). Alternatively, some eukaryotic Prx systems utilize TrxR and Trx to reduce the peroxidase (sometimes referred to as Trx-dependent Prxs or TPxs), where TrxR catalyzes the reduction of Trx, which, in turn, reduces the Prx disulfide bond. In these cases, electron transfer proceeds along the following path: NADPH → TrxR → Trx → AhpC (TPx) → ROOH.
While eukaryotic examples of Trx-dependent Prx proteins abound (e.g., Entamoeba histolytica AhpC [52], Saccharomyces cerevisiae TPx [10], and multiple human Prx homologues [11]), to our knowledge, only one other bacterial Trx-dependent Prx, besides the H. pylori AhpC described herein, has been experimentally demonstrated [TPx from the cyanobacterium Synechocystis (75)]. A distantly related Prx family member, E. coli bacterioferritin comigratory protein, has also been shown to exhibit low levels of Trx-dependent peroxidase activity (34). Inspection of the H. pylori genome yielded no ahpF, but an E. coli trxA homologue (trx1, HP0824 in the annotation of Tomb et al. [67]) encoding Trx1 was identified along with an E. coli trxB homologue (trxR, HP0825) encoding TrxR. Further examination uncovered HP1164, annotated as a TrxR locus due to its similarity to Plasmodium falciparum trxR (21% identity). HP1164 does not, however, encode a putative catalytic disulfide motif (CXXC or CXXXXC) indicative of such a redox center and was therefore excluded from these studies. Further genomic searches revealed a second Trx locus (trx2, HP1458) encoding Trx2. Therefore, we considered TrxR (HP0825)-Trx1 (HP0824) and TrxR (HP0825)-Trx2 (HP1458) to be good candidates as AhpC-reducing systems.
Herein, we present the first example of a Trx-dependent alkyl hydroperoxide reductase system from a gastric pathogen and describe the cloning, purification, and kinetic characterization of AhpC, Trx1, Trx2, and TrxR from H. pylori. Along with the genetic characterization of H. pylori ahpC, we have also shown that AhpC plays a critical role in the defense against oxygen toxicity that is essential for survival and growth, even in microaerophilic environments.
(Abstracts reporting some of this information have been published earlier [5, 6]).
MATERIALS AND METHODS
Materials.
Sodium dodecyl sulfate (SDS), ultrapure glycine, ultrapure urea, EDTA, dithiothreitol, Tris base, and other buffer reagents were purchased from Research Organics (Cleveland, Ohio). Bacteriological medium components were from Difco Laboratories (Detroit, Mich.). Ethanol was obtained from Warner Graham Company (Cockeysville, Md.). Isopropyl-β-d-thiogalactopyranoside (IPTG) and 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) stocks were from Inalc (Milan, Italy). Vent DNA polymerase, Moloney murine leukemia virus reverse transcriptase, and T4 polynucleotide kinase were purchased from New England Biolabs (Beverly, Mass.). Restriction enzymes, ligase, calf intestinal phosphatase, and restriction buffers were obtained from Promega (Madison, Wis.). Agarose medium EEO (electrophoresis grade) and H2O2 were from Fisher (Fairlawn, N.J.). Acrylamide-Bis (40%) solution was purchased from Bio-Rad (Hercules, Calif.). Ampicillin (AMP) powder, cumene hydroperoxide (CHP), glucose-6-phosphate, glucose-6-phosphate dehydrogenase, linoleic acid, lipoxygenase, and insulin were from Sigma (St. Louis, Mo.). Ethyl hydroperoxide (EtOOH) was from Polysciences, Inc. (Warrington, Pa.), and tert-butyl hydroperoxide (t-BOOH) was from Aldrich (Milwaukee, Wis.). NADPH and NADH were from Roche Molecular Biochemicals (Mannheim, Germany).
RNA isolation and primer extension analysis.
For RNA isolation, 50 ml of H. pylori strain HP 26695 (67) was grown in Brucella broth supplemented with 10% fetal calf serum (Sigma) in a microaerobic environment (7% O2, 5% CO2) at 37°C to an optical density at 590 nm of 1.0 and harvested by centrifugation at 4°C for 10 min. Cells were lysed in 50 mM Tris-HCl (pH 8.0)–1 mM EDTA–50 mM NaCl buffer supplemented with 1.5% SDS for 5 min at 95°C. Following phenol extraction and precipitation, the RNA was dissolved in diethylpyrocarbonate-treated water and the total RNA concentration was determined at 260 nm. For the primer extension studies, an oligonucleotide (5′-GCCAGAAGAAAAGAATCGCACCGT-3′; Gibco-BRL Custom Primers) was 5′- end labeled in the presence of [γ-32P]ATP (5,000 Ci/mmol; Amersham) and 20 U of T4 polynucleotide kinase. Total H. pylori RNA (100 μg) was incubated with the 32P-labeled oligonucleotide and annealed under the following conditions: 80°C for 5 min, 65°C for 5 min, 42°C for 10 min, and 37°C for 20 min. Following annealing, the RNA was precipitated with ethanol, dried, and resuspended in 7 μl of diethylpyrocarbonate-treated water and 13 μl of reverse transcriptase buffer (50 mM Tris-HCl at pH 8.3, 30 mM KCl, 10 mM MgCl2, 1 μg of actinomycin D [Sigma], 1 mM dithiothreitol, 2.5 mM each deoxynucleoside triphosphate). One microliter of Moloney murine leukemia virus reverse transcriptase (50 U/μl) was added to the sample, and reverse transcription was carried out at 42°C for 60 min; 1 μl of 0.5 M EDTA and 1 μl of RNase (10 mg/ml) were added, and the reaction mixture was incubated at 37°C for an additional 30 min. The reaction was stopped by phenol-chloroform extraction, followed by ethanol precipitation. The sample was then dried and resuspended in 10 μl of sequencing loading buffer. In parallel, dideoxy DNA sequencing of the cloned ahpC gene and the upstream region was performed using the same oligonucleotide primer to determine the transcriptional start site. After denaturation at 95°C for 2 min, aliquots were subjected to 6% urea polyacrylamide gel electrophoresis (PAGE) and autoradiographed.
Allelic replacement mutagenesis of ahpC.
Genomic DNA isolated from H. pylori strain 26695 (67) was used to amplify ahpC with forward and reverse oligonucleotide primers (Table 1) under typical PCR conditions to generate a product 930 bp long. Isolated ahpC was digested at flanking EcoRI and SacI sites and then inserted into pBluescript-SK (+) plasmid vector (Stratagene, La Jolla, Calif.) digested with the same restriction enzymes. Vector derivatives of pBluescript were stably maintained in E. coli strains but were not replicated in H. pylori hosts. The ahpC-containing plasmid was then digested with XbaI (located approximately 330 bp from the ahpC translational start site), and a chloramphenicol resistance (camR) cassette originating from Campylobacter coli (70) digested with XbaI was inserted to interrupt the coding sequence of ahpC. Four H. pylori strains were transformed with the ahpC::camR construct: 26695 (67), SS1 (40), HP1 (28), and HP1061 (25). The same strains were also transformed with a construct in which the nitroreductase gene (rdxA) was interrupted with camR (26). H. pylori transformation (electroporation) and subsequent evaluation of any colonies obtained after up to 7 days of incubation in selective medium were carried out as described elsewhere (57).
TABLE 1.
Primers used to amplify and subclone H. pylori structural genes
| Expression vector and gene | Primer
(5′–3′)
|
|
|---|---|---|
| Forward | Reverse | |
| pPROK1 | ||
| ahpC | CGCCCGGGAGGAGGAAGAATAGATGTTAGTTACAAAACTTGCC | CCCTGCAGCTACAGCTTGATGGAATTTTCTTTAAGAT |
| trx1 | CGCCCGGGAGGAGGAAGAATAGATGAGTCACTATATTG | CCCTGCAGTTAGCCTAAAAGTTTGTTCAATTGC |
| trx2 | GCGAATTCAGGAGGAAGAATAGATGTCAGAAATGATTAACGGG | GCAAGCTTCCCCTTACAATAACGCTTTTAGAGC |
| trxR | CGCCCGGGAGGAGGAAGAATAGATGATAGATTGCGCGATTATTGG | CCAAGCTTGATTTAATGGTG |
| pBluescript-SK | ||
| ahpC | TGGAATTCGCCCAATAACGATGAAACAAG | GAGAGCTCCGATTAAAGCTTAATGGA |
Cloning of ahpC, trx1, trx2, and trxR into expression vectors.
The H. pylori genomic database was searched for genes corresponding to the E. coli sequences for ahpC, trxA, trxB, and trxC (www.tigr.org/tdb/mdb/hpdb/hpdb.html). Clones GHPDQ26, GHPAB86, GHPCL04, and GHPAE26, containing trx1, trx2, trxR, and ahpC, respectively, were ordered from The Institute of Genomic Research-ATCC Microbial Genome Special Collection. Plasmid DNA was purified from the E. coli host using the Wizard Miniprep Kit (Promega). The genes of interest were amplified using PCR primers (listed in Table 1) synthesized in the DNA Synthesis Core Laboratory of the Comprehensive Cancer Center of Wake Forest University. PCR mixtures (50 μl) contained 300 μM deoxynucleoside triphosphates, 1 U of vent polymerase, 10 pmol each of the forward and reverse oligonucleotides, 0.5 mM MgCl2, and 0.5 μg of template DNA. Reactions were carried out as follows in a Mini Cycler (MJ Research, Waltham, Mass.): 95°C for 30 s, 40°C for 45 s, and 72°C for 1.5 min (5 cycles); 98°C for 30 s and 72°C for 1 min (35 cycles); and then 72°C for 15 min. The PCR products were purified using the QIAquick PCR Cleanup kit (Qiagen, Studio City, Calif.). The PCR products, 600 bp for ahpC, 300 bp for trx1 and trx2, and 932 bp for trxR, were each separately ligated into the pCR2.1 TA cloning vector (Invitrogen, Carlsbad, Calif.). Plasmids containing each insert were digested with the restriction enzymes corresponding to the engineered restriction sites (Table 1), and then DNA fragments of interest were excised from agarose gels and purified using the Gene Clean II Kit (Bio 101, Inc., Vista, Calif.). These fragments were inserted, using T4 DNA ligase, into pPROK1 expression vectors (Clontech, Palo Alto, Calif.; expression under control of the tac promoter), appropriately digested, isolated from agarose gels, and pretreated with calf intestinal phosphatase.
Bacterial strains and culture procedures.
Ligated DNA was transformed into competent E. coli XL-1 Blue cells (Stratagene), except the AhpC-expressing plasmid, which was transformed into an ahpCF deletion strain of E. coli, TA4315 (65). Single colonies were selected on Luria-Bertani (LB) plates containing AMP at 50 μg/ml, and those containing the recombinant DNA were evaluated for protein expression on SDS-polyacrylamide gels after induction with 0.4 mM IPTG. Isolated plasmid DNA for each construct was sequenced throughout the coding region by automated DNA sequencing at the Comprehensive Cancer Center of Wake Forest University. Bacterial stocks containing each plasmid with the subcloned gene were prepared from a single colony and stored at −80°C in LB broth containing 15% (vol/vol) glycerol. Culture procedures were generally the same as reported earlier (53).
Purification of recombinant AhpC.
Purification of AhpC was performed as described earlier (53), with some modifications. All procedures were carried out in a standard buffer (pH 7.0) consisting of 25 mM potassium phosphate with 1.0 mM EDTA. Briefly, 100 ml of E. coli TA4315 harboring the pPROK1/ahpC plasmid was added to 10 liters of LB medium containing 0.5 g of AMP supplemented with 0.2% glucose in a BioFlo 2000 fermentor (New Brunswick Scientific, Edison, N.J.). IPTG (0.4 mM) was added at A600 = 0.9, and bacteria were harvested by centrifugation 16 h after induction. Pelleted bacteria were disrupted with a Bead Beater (BioSpec Products, Bartlesville, Okla.), cell extracts were treated with streptomycin sulfate to precipitate nucleic acids, and 20 and 60% ammonium sulfate treatments were carried out to precipitate proteins as described previously (53). The protein resuspended in standard buffer containing 20% ammonium sulfate was applied to a Phenyl Sepharose 6 Fast Flow Column (24 by 2.5 cm; Pharmacia LKB Biotechnology Inc.), washed with 20% ammonium sulfate buffer, and eluted with a linear gradient of 20 to 0% ammonium sulfate in standard buffer. Protein fractions were evaluated for purity of overexpressed AhpC by SDS-PAGE, and the purest fractions were pooled. After dialysis against 10 mM potassium phosphate buffer (pH 7.0), the protein was loaded onto a DEAE-cellulose column (24 by 2.5 cm; Whatman DE52) and eluted with a linear gradient of 10 to 100 mM potassium phosphate (1-liter total volume). Again, fractions were analyzed for AhpC by SDS-PAGE and pure fractions were pooled, concentrated, and aliquotted for storage at −20°C.
Purification of recombinant Trx1.
A 10-liter culture of E. coli XL-1 Blue harboring the pPROK1/trx1 plasmid was grown in the fermentor and induced as described above for AhpC expression. The crude cell extract obtained as described above was treated with streptomycin sulfate, and following centrifugation, the supernatant was subjected to heat denaturation at 70°C for 4 min to remove contaminating proteins. Denatured proteins were removed via centrifugation at 23,000 × g for 20 min, and the supernatant was dialyzed overnight in three changes (6 liters each) of 5 mM potassium phosphate buffer (pH 7.0). The dialyzed protein was applied to a DEAE-cellulose column (24 by 2.5 cm; Whatman DE52) pre-equilibrated with 10 mM potassium phosphate (pH 7.0). Trx1 eluted upon application of a linear gradient of 10 to 100 mM potassium phosphate (1-liter total volume) and was identified by SDS-PAGE. Pooled fractions were concentrated to 2 ml, and the concentrate was applied to a BioGel A-0.5 m agarose column (Bio-Rad) equilibrated in the standard buffer. The fractions were collected, assessed for purity using SDS-PAGE, pooled, and aliquotted for storage at −20°C. Further molecular weight and purity analysis required the use of Tris-Tricine gels as described by Ausubel et al. (3).
Purification of recombinant TrxR.
A 10-liter culture of XL-1 transformed with the pPROK1/trxR plasmid was grown, induced, and harvested; the crude extract was prepared essentially as described for AhpC. After the 60% ammonium sulfate treatment, the resuspended and dialyzed protein was loaded onto the DEAE-cellulose column and eluted as described for Trx1. Purified fractions, as assessed by the ratio of A280 to A450, were pooled and loaded onto an Affi-Gel Blue column (24 by 2.5 cm; Bio-Rad). Fractions were eluted with a linear gradient of 25 mM potassium phosphate buffer to 600 mM potassium phosphate and 0.3 M NaCl (pH 7.0; 700-ml total volume). Protein purity was assessed by A280/A450 ratio determination and SDS-PAGE. Pure fractions were pooled, dialyzed in standard buffer, and aliquotted for storage at −20°C.
Purification of recombinant Trx2.
A 10-liter culture of XL-1 transformed with the pPROK1/trx2 plasmid was grown under the conditions described for Trx1. Bacteria containing Trx2 were treated as described for Trx1, except for the following modifications. Instead of heat denaturation, the supernatant from an initial treatment with 50% ammonium sulfate was brought to 80%. The pelleted protein was resuspended in 5 mM potassium phosphate buffer, dialyzed overnight in the same buffer, and applied to the DEAE-cellulose column pre-equilibrated with 5 mM potassium phosphate buffer (pH 7.0). The nonbinding fraction was then applied to a carboxymethyl cellulose column (24 by 2.5 cm; Whatman CM) pre-equilibrated with 5 mM potassium phosphate buffer (pH 7.0); this was followed by elution with a linear gradient of 5 to 60 mM potassium phosphate (700-ml total volume). SDS-PAGE was again used to assess purity, and concentrated protein was aliquotted for storage at −20°C in standard buffer.
Other protein purifications.
Purification of E. coli TrxR and Trx1 was done essentially as described by Poole et al. (54). S. typhimurium AhpF and AhpC were purified as reported previously (53).
Sequence analysis.
AhpC homologues were identified through BLAST (2) searches with the H. pylori AhpC sequence. Pairwise comparisons of the H. pylori AhpC sequence were generated with AhpC sequences from other organisms using the BLAST Two Sequences program at the National Center for Biotechnology Information website (http://www.ncbi.nlm.nih.gov/).
Spectroscopic experiments.
Most spectral assays were carried out using a thermostatted Gilford 220 updated recording spectrophotometer with a Beckman DU monochromator and a Kipp and Zonen (Delft, The Netherlands) chart recorder. Extinction coefficient determination using microbiuret assays for proteins at 280 nm and for bound flavin at 450 nm, thiol content determination using 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB), and disulfide detection using 2-nitro-5-thiosulfobenzoate (NTSB) were all performed as described previously (51, 53). Further quantification of H. pylori proteins relied on the following extinction coefficients experimentally obtained at 280 nm (except where noted): AhpC, 24,800 ± 300 M−1 cm−1; Trx1, 17,200 ± 1,000 M−1 cm−1; Trx2, 23,300 ± 300 M−1 cm−1; TrxR, 11,900 ± 110 M−1 cm−1 (450 nm). To quantitate the concentration of other proteins and reagents by absorbance, the following extinction coefficients were used: E. coli TrxR, 11,300 M−1 cm−1 (454 nm) (73); E. coli Trx1, 13,700 M−1 cm−1 (280 nm) (31); S. typhimurium AhpF, 13,100 M−1 cm−1 (450 nm); AhpC, 24,300 M−1 cm−1 (280 nm) (53); TNB2−, 14,150 M−1 cm−1 (412 nm) (58); NADPH, 6,200 M−1cm−1 (340 nm); NADH, 6,220 M−1 cm−1 (340 nm).
Determination of H2O2 content using ferrithiocyanate.
To monitor the disappearance of hydrogen peroxide in solution, experiments were conducted essentially as described by Thurman et al. (66), except that peroxidase reactions (0.5 ml of AhpC [20 μM], Trx1 [2.0 μM], and TrxR [0.2 μM]) were initiated with the addition of H2O2 (1 mM). Reactions mixtures were incubated at 37°C, and aliquots were removed at various time points up to 30 min. Reactions were terminated by the addition of 0.5 ml of 12.5% trichloroacetic acid, precipitated proteins were removed by centrifugation, and peroxide content was analyzed by ferrithiocyanate complex formation [0.2 ml of 10 mM Fe(NH4)2(SO4)2 and 0.1 ml of 2.5 mM KSCN were added to a 50-μl aliquot of supernatant]. Red ferrithiocyanate complex was measured at 480 nm, and peroxide concentration was determined from a standard curve with H2O2.
AhpC activity assays and reductase system determination.
All AhpC activity assays were conducted on an Applied Photophysics DX.17 MV stopped-flow spectrophotometer at 25°C, except where noted otherwise. Aerobic, steady-state AhpC activity assays were monitored by following the decrease in A340 due to NADPH oxidation. All peroxidase assays were conducted with 50 mM potassium phosphate buffer (pH 7.0)–0.1 M ammonium sulfate–0.5 mM EDTA. Proteins in one syringe were mixed with substrates in the other syringe, except where indicated otherwise. The rate of change in A340 was assessed by linear regression analysis of the first 10% of the linear region of the resulting trace. Preparation of the reaction chamber for anaerobic assays was conducted essentially as described previously (44).
Steady-state kinetic analysis of TrxR with Trx1.
Km and kcat measurements of TrxR for NADPH and Trx1 were conducted essentially as previously described (41), except that they were carried out on the stopped-flow spectrophotometer. Assays contained 0 to 40 μM NADPH (0.2 μM at the lowest concentration), 0 to 100 μM Trx1, and 34 nM TrxR (final concentrations are indicated), and activity was followed by monitoring the change in NADPH fluorescence over time (excitation at 340 nm and emission recorded at 90° using a 400-nm filter). The decrease in fluorescence over time was converted to absorbance units per minute using a standard curve. Primary rate data were fitted to a rectangular hyperbolic function obtained using the Marquardt-Levenberg curve-fitting algorithm in SigmaPlot (Jandel Scientific, San Rafael, Calif.). The rate data obtained after varying each substrate were transformed and displayed in a primary Hanes-Wolf plot and yielded lines that intersected on the y axis, indicating a substituted mechanism for TrxR. A substituted (ping-pong) mechanism was then assumed for all equations, and the initial rate in the absence of products was represented by the following equation (15): v = Vab/(KmBa + KmAb + ab), where a is the concentration of Trx1, b is the concentration of NADPH, V is the maximum velocity, and Km is the Michaelis constant. Slopes and y intercepts obtained from the linear regression lines for each concentration of Trx1 were replotted in a secondary Hanes-Wolf plot to obtain the true Vmax and Km for each substrate. The y intercept of the secondary plot was equivalent to KmB/V, and the slope was equal to 1/V. To solve for KmA, the following relationship was used: V(app)/Km(app) = V/KmA, where the V(app)/Km(app) ratio was obtained from Michaelis-Menten plots of the initial-rate data.
The apparent Km and Vmax of TrxR for Trx1 and Trx2 were also determined on a thermostatted Milton Roy Spectronic 3000 diode array spectrophotometer at 340 nm using an insulin reduction assay as described by Holmgren and Bjornstedt (30). Reactions (500 μl) were conducted at 25°C in 100 mM potassium phosphate buffer (pH 7.5) with EDTA (1.5 mM) containing Trx1 or Trx2 (0 to 50 μM), insulin (80 μM), NADPH (150 μM), and TrxR (7.0 nM; added last). Kinetic parameters were obtained using the curve-fitting function in Sigma Plot as described above. Assays using DTNB (200 μM) instead of insulin were conducted essentially as described above, except that the reaction was monitored at 412 nm to observe the rate of formation of TNB2−.
The oxidase activity of TrxR was measured as described earlier (53). Briefly, H. pylori TrxR (30 to 240 nM), S. typhimurium AhpF (15 to 60 nM), or E. coli TrxR (30 to 120 nM) was added to 500 μl of air-saturated standard buffer containing NADPH (150 μM) and the change in A340 was monitored. The TrxR activity of H. pylori TrxR or Trx1 mixed with E. coli TrxR or Trx1 in heterologous mixtures was determined on the Gilford spectrophotometer using the DTNB reduction assay described above. Reaction mixtures contained 0.5 to 100 μM Trx1 (from either H. pylori or E. coli) and were started with the addition of 0.5 μM TrxR (from either H. pylori or E. coli).
Bisubstrate steady-state kinetic analysis of AhpC.
Aerobic peroxidase assays monitoring the A340 change of NADPH were carried out on a stopped-flow spectrophotometer as described above. In one syringe, peroxide substrates (10 to 40 μM) were incubated in the peroxidase assay buffer. (All concentrations shown are final concentrations achieved upon mixing.) In the other syringe, AhpC (0 to 2 μM), Trx1 (20 μM), and E. coli TrxR (2 μM) were incubated with NADPH (150 μM) for 5 min prior to assay with the peroxide substrate. (Use of E. coli TrxR rather than H. pylori TrxR in this assay did not significantly affect the results but did greatly decrease the problem of AhpC-independent NADPH oxidation.) The kinetic coefficients were obtained as described by Forstrom et al. (24). Data were analyzed using the integrated Dalziel rate equation for a two-substrate enzymatic system in which enzyme-substrate complexes were not experimentally observed: E0(t/[ROH]t) = φ1 ln([ROOH]t/([ROOH]0−[ROH]t)/[ROH]t) + φ2/[Trx1] + φ0, where [ROOH]0 is the initial hydroperoxide concentration, [ROH]t is the product (alkyl alcohol) concentration at time t, and φ1, φ2, and φ0 are the Dalziel coefficients. Data were chosen and analyzed as described previously for tryparedoxin peroxidase from Crithidia fasciculata (46). Linoleic acid hydroperoxide (LOOH) was prepared by enzymatic oxidation with soybean lipoxygenase as described by Maiorino et al. (43).
RESULTS
Amino acid sequence alignments.
In an amino acid sequence comparison of the putative AhpC from H. pylori with the more extensively studied S. typhimurium enzyme, Cys49 in H. pylori AhpC aligns perfectly with the essential, conserved Cys46 from S. typhimurium AhpC (21). Cys49 in H. pylori AhpC is, therefore, most likely the site of interaction with peroxides and the site of sulfenic acid formation. The H. pylori AhpC amino acid sequence was also compared to the deduced amino acid sequences for other 1- and 2-Cys AhpC homologues from a wide range of organisms (Table 2). Of all the known homologues, H. pylori AhpC shares the most sequence identity with a select group of bacterial Prxs including AhpC from Campylobacter jejuni (67%), TPx from Rickettsia prowazekii (54%), and AhpC from Legionella pneumophila (52%). Other than the proteins from these three bacterial sources, however, H. pylori AhpC is more similar to 2-Cys Prx protein sequences from higher organisms than to other bacterial AhpC proteins (Table 2). Of the sequences shown, S. cerevisiae Prx (2-Cys), C. elegans Prx, the human proliferation-associated gene (2-Cys), and Synechocystis sp. strain PCC6803 (a cyanobacterium) TPx have all been shown experimentally to be recycled by a Trx-reducing system (10, 36, 52).
TABLE 2.
Pairwise sequence alignments (percent identities) of H. pylori AhpC with AhpC homologues from other organisms
| Organisma | Eubacteria
|
Archaeon, M. jannaschii (anaerobic) | Eukaryota
(aerobic)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H. pylori (microaerophilic) | Aerobic
|
Anaerobic
|
Yeast
|
C. elegans | Human
|
|||||||
| M. tuberculosis | Synechocystis | C. pasteurianum | S. mutans | S. typhimurium | 1-Cys S. cerevisiae | 2-Cys S. cerevisiae | 1-Cys H. sapiens | 2-Cys H. sapiens | ||||
| H. pylori | 100 | 41 | 46 | 39 | 34 | 32 | 32 | 30 | 46 | 47 | 27 | 45 |
| M. tuberculosis | 100 | 37 | 38 | 32 | 33 | 26 | 32 | 36 | 35 | 32 | 39 | |
| Synechocystis | 100 | 51 | 36 | 35 | 34 | 29 | 47 | 55 | 26 | 57 | ||
| C. pasteurianum | 100 | 32 | 34 | 38 | 32 | 50 | 53 | 30 | 52 | |||
| S. mutans | 100 | 62 | 27 | 23 | 34 | 37 | 22 | 36 | ||||
| S. typhimurium | 100 | 33 | 30 | 31 | 37 | 27 | 37 | |||||
| M. jannaschii | 100 | 45 | 33 | 36 | 41 | 40 | ||||||
| 1-Cys S. cerevisiae | 100 | 29 | 31 | 48 | 34 | |||||||
| 2-Cys S. cerevisiae | 100 | 66 | 28 | 60 | ||||||||
| C. elegans | 100 | 29 | 74 | |||||||||
| 1-Cys H. sapiens | 100 | 31 | ||||||||||
| 2-Cys H. sapiens | 100 | |||||||||||
The accession numbers (National Center for Biotechnology Information database) of the sequences are as follows: AhpC of H. pylori, P21762; open reading frame C of Clostridium pasteurianum, P23161; AhpC of Streptococcus mutans, BAA25695; AhpC of Salmonella typhimurium, P19479; AhpC of Mycobacterium tuberculosis, CAB03768; SLL0755 of Syechocystis sp. strain PCC6803, Q55624; AhpC of Methanococcus jannaschii, AAB98731; Prx1 of Saccharomyces cerevisiae, CAA80784; TSA-I (type Iα TPx) of S. cerevisiae, P34760; AhpC/TSA of Caenorhabditis elegans, AAA79342; Prx (hORF6) of Homo sapiens, BAA03496; proliferation-associated protein TPx2 of H. sapiens, Q06830.
TrxR and Trx1 from H. pylori share a moderate amount of sequence identity (37 and 51%, respectively) with the corresponding E. coli homologues and a high percentage of identity (60 and 68%, respectively) with the homologues from the closely related species C. jejuni. As in H. pylori, the absence of ahpF in C. jejuni makes TrxR and Trx1 reasonable candidates as C. jejuni's AhpC reductase system. H. pylori Trx2 shares the highest sequence identity with a Trx homologue from Archaeoglobus fulgidus (Trx4, 42%) (39) and is more closely related to E. coli Trx2 (41% identity) (45) than to E. coli Trx1 (33% identity) (29). Known redox centers and binding motifs of other Trx and TrxR proteins are completely conserved in the H. pylori proteins. Unlike the vast majority of TrxR-Trx1 systems from other bacteria, the gene encoding TrxR is positioned just downstream from trx1 in the H. pylori genome. The clustering of trx1 and trxR in this order has also been observed in Streptomyces clavuligerus (14); however, in mycobacteria (72) and in C. jejuni (49), the opposite orientation, trxR and then trx1, is observed. In most other species, trxR and trx1 are widely separated on the chromosome. Interestingly, H. pylori trx2 (HP1458) is not found near trx1 (HP0824) or trxR (HP0825).
Characterization of purified H. pylori proteins.
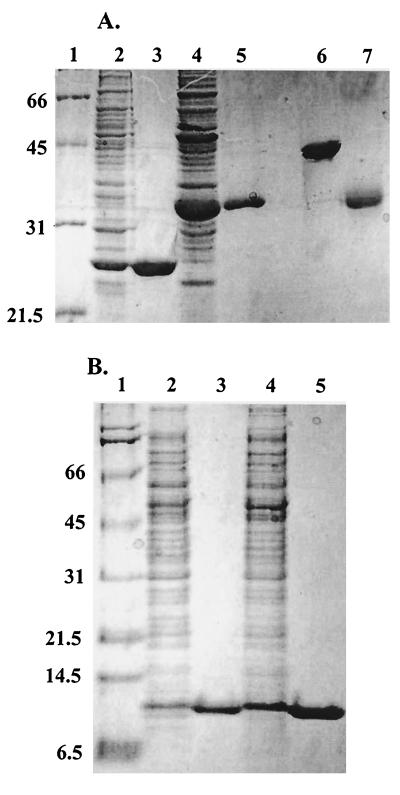
AhpC, Trx1, Trx2, and TrxR expressed from pPROK1 in the respective E. coli strains were purified to homogeneity (Fig. 1). All proteins were obtained in high yields as soluble proteins after induction with IPTG at 37°C. Migration of the H. pylori proteins was compared to that of pure S. typhimurium or E. coli homologues during SDS-PAGE, which allowed the detection of each protein during purification and assessment of purity. Pure, reduced AhpC corresponded to an apparent molecular mass of 26 kDa, which is slightly higher than its expected molecular mass of 22,235 Da. When prepared in nonreducing sample buffer and analyzed by SDS-PAGE, AhpC migrated as a 50-kDa protein, indicating that the purified protein is oxidized and contains one or more intersubunit disulfide bonds (Fig. 1A, lane 6). TrxR migrated as a 33-kDa protein on SDS-PAGE under both reducing and nonreducing conditions (Fig. 1A, lanes 5 and 7; expected mass, 33,538 Da). Previously, an intersubunit disulfide bond between TrxR subunits was suggested by Windle et al. (74); our results, however, rule out such a linkage between subunits. Spectral analyses (data not shown) of TrxR revealed a strong A454, which is typical of enzymes containing a bound flavin cofactor. Trx1 and Trx2 both migrated as monomers in the presence or absence of β-mercaptoethanol and gave masses of approximately 12 kDa, close to their expected masses of 11,854 and 11,744 Da, respectively.
FIG. 1.
SDS-PAGE analysis of purified AhpC, Trx1, Trx2, and TrxR from H. pylori. (A) Crude lysates and recombinant purified AhpC and TrxR were analyzed on the same 12% polyacrylamide gel in reducing sample buffer (except where noted) as follows: lane 1, molecular mass markers (Broad Range Molecular Weight Standards; Bio-Rad); lane 2, crude extracts of E. coli cells transformed with pPROK1/ahpC and induced with 0.4 mM IPTG for at least 3 h at 37°C; lane 3, pure, recombinant AhpC; lane 4, crude extracts of E. coli cells transformed with pPROK1/trxR and induced as described above; lane 5, pure, recombinant TrxR protein; lane 6, pure AhpC in nonreducing sample buffer; lane 7, pure TrxR in nonreducing sample buffer. (B) Crude lysates and recombinant Trx1 and Trx2 were analyzed on a 10% Tris-Tricine gel as follows: lane 1, molecular mass markers; lane 2, crude extracts of E. coli cells transformed with pPROK1/trx1 after induction with IPTG; lane 3, pure, recombinant Trx1; lane 4, crude extracts of E. coli cells transformed with pPROK1/trx2 after induction with IPTG; lane 5, recombinant purified Trx2 after purification. Equivalent protein masses (10 μg) were loaded in all lanes of both gels. Molecular masses (in kilodaltons) are indicated on the left.
Kinetic characterization of Trx1, Trx2, and TrxR.
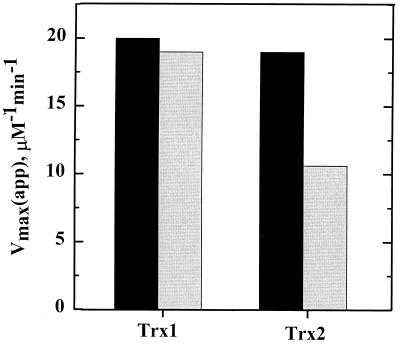
H. pylori TrxR and Trx1 were shown in a series of assays to act as a general disulfide reductase system analogous to their counterparts from E. coli. When assayed with small-molecule (DTNB) or protein (insulin) disulfide-containing substrates, both Trx1 and Trx2 from H. pylori exhibited TrxR-dependent reductase activities that were hyperbolically dependent on the Trx1 and Trx2 concentrations. Insulin reduction in vitro by TrxR-Trx1 gave a Vmax(app) of 19.9 ± 1.4 μM min−1 and a Km(app) for Trx1 of 13.4 ± 2.7 μM (Fig. 2), while the TrxR-Trx2 system gave a Vmax(app) of 10.6 ± 1.5 μM min−1 and a Km(app) for Trx2 of 11.0 ± 1.9 mM (Fig. 2). While Trx2 exhibited about half of the turnover rate of Trx1 in insulin assays, both Trx1 and Trx2 reduced the small, non-protein disulfide substrate DTNB with about the same catalytic parameters as the TrxR-Trx1-insulin system (Fig. 2). In TrxR-Trx1-insulin experiments in which NADPH was replaced with NADH, no decrease in the NADH A340 was observed upon addition of TrxR to the reaction mixture, demonstrating the high specificity of TrxR for NADPH. H. pylori TrxR and Trx1 were also capable of forming an efficient reducing system when mixed with their E. coli counterparts (data not shown). Using the DTNB-linked TrxR assay, reaction mixtures containing one H. pylori reductase component mixed with a corresponding E. coli partner protein gave catalytic efficiencies (kcat/Km for Trx1) which were just a fewfold lower for the heterologous systems compared with the natural systems. The fact that the H. pylori TrxR and Trx1 proteins can interact efficiently with the E. coli proteins suggests that the H. pylori proteins share a great deal of structural similarity with their E. coli counterparts and also illustrates the functional homology H. pylori Trx1 and TrxR share with other TrxR systems.
FIG. 2.
Comparative kinetic parameters of Trx1 and Trx2 from H. pylori. Trx activity assays using either 200 μM DTNB (black bars) or 80 μM insulin (gray bars) as a substrate for 0 to 50 μM Trx1 or Trx2 were conducted in a standard buffer of 100 mM potassium phosphate and 2 mM EDTA, pH 7.4, with 150 μM NADPH. Assays were started with the addition of 7.0 nM TrxR and were monitored at 340 nm when insulin was used as the substrate or at 412 nm when DTNB was used as the substrate to observe the release of TNB2−. Rates were determined from the first 10% of the reaction and then fitted to a hyperbola to determine the Vmax(app) and Km(app) values for each.
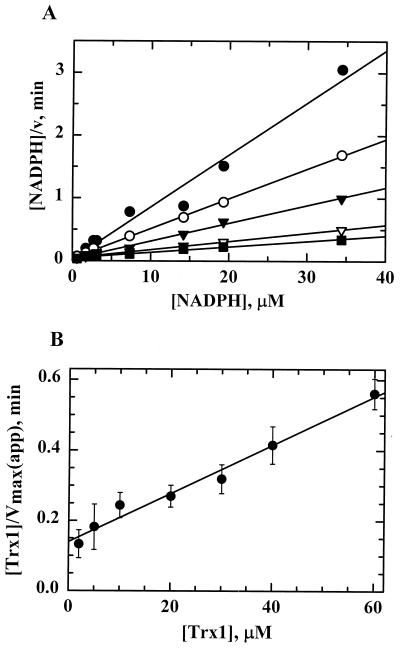
To establish the true kcat and Km values for each substrate of H. pylori TrxR, assays varying NADPH at different fixed concentrations of Trx1 were conducted in the absence of additional electron acceptors and were monitored on the stopped-flow spectrophotometer by the time-dependent decrease in NADPH fluorescence. A representative primary Hanes-Wolf plot of the initial-rate data over a range of NADPH concentrations (Fig. 3A) showed that lines representing five different Trx1 concentrations intersected at the y axis, denoting a substituted (i.e., ping-pong) reaction mechanism for TrxR (15). The replot of slopes with respect to Trx1 concentration (Fig. 3B) yielded kcat and Km values, reported in Table 3, which were only slightly different from those for the corresponding E. coli proteins. Nonetheless, the catalytic efficiencies (kcat/Km) of both systems and both substrates are in a similar range, indicating that TrxR and Trx1 act as an effective general protein disulfide reductase system similar to the homologous E. coli system.
FIG. 3.
Steady-state kinetic analysis of Trx1 and TrxR from H. pylori. Assays were conducted using a stopped-flow spectrophotometer that monitored the decrease in NADPH fluorescence as described in Materials and Methods. NADPH concentrations (0.8 to 34 μM) were varied at fixed concentrations of Trx1 in the presence of 0.1 μM TrxR. (A) Representative primary Hanes-Wolf plot of one set of initial-rate data for 2 μM (closed circles), 5 μM (open circles), 10 μM (closed triangles), 20 μM (open triangles), and 60 μM (closed squares) Trx1. (B) A replot of the averaged data from three separate experiments was used to calculate the steady-state kinetic parameters for H. pylori TrxR as summarized in Table 3.
TABLE 3.
Steady-state kinetic parameters of TrxR from H. pylori and E. coli
AhpC cysteine thiol and disulfide quantification.
In previous work, our laboratory has demonstrated that peroxidase activity in S. typhimurium AhpC is reliant on an essential, conserved cysteine residue (Cys46) while a second active-site cysteine (Cys165′) contributed by a different AhpC subunit stabilizes the oxidized protein through the formation of an intersubunit disulfide bond (21). Thiol quantification of reduced AhpC revealed the presence of two cysteine thiol groups per monomer (2.10 ± 0.17). Denaturation of reduced H. pylori AhpC did not change the thiol titer, indicating a high degree of accessibility of the cysteine thiol groups in the reduced protein. As isolated from E. coli, overexpressed H. pylori AhpC was in its oxidized form and lacked free thiol groups; NTSB assays revealed that oxidized AhpC contained one disulfide bond per monomer (0.90 ± 0.07). Taken together, along with the presence of intersubunit disulfide bonds in nonreducing SDS-PAGE gels of AhpC (see above), these results support a head-to-tail arrangement of monomers to form two active sites per dimer in H. pylori AhpC, as is the case with S. typhimurium AhpC.
Peroxidase activity of AhpC, Trx1 or Trx2, and TrxR.
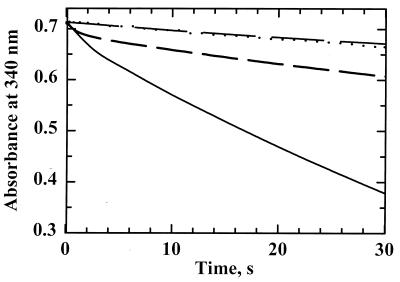
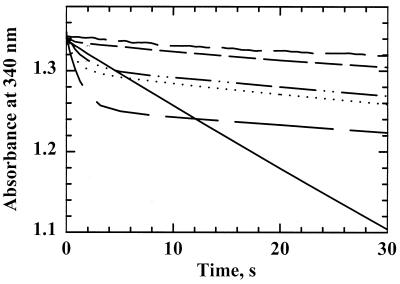
To test the ability of H. pylori AhpC to reduce hydroperoxides with H. pylori Trx1 and TrxR acting as the reducing system, the proteins were mixed with NADPH and cumene hydroperoxide and the change in A340 was monitored. The maximal sustained rate of NADPH oxidation was observed when all three proteins, TrxR, Trx1, and AhpC, were included in the assay mixture (Fig. 4). Alone, TrxR possessed oxidase activity (Fig. 4, long, dashed lines), which was observed as a steady decrease in A340 at 0.70 s−1 in the absence of peroxide; E. coli TrxR exhibited a much slower rate of NADPH oxidation (0.01 s−1) under these conditions. In the presence of peroxide, NADPH oxidation by H. pylori TrxR alone increased to 0.80 s−1, which is indicative of weak peroxidatic activity of TrxR. In the absence of AhpC, a small burst in NADPH oxidation was first observed (between 0.3 and 1 s) at a rate of 5.1 s−1 relative to the TrxR concentration, which tapers off to a rate similar to that of TrxR alone (0.9 s−1) once all of the Trx1 had been reduced (reduction of 5 μM Trx1 accounts for a decrease in A340 of 0.03). With all three H. pylori proteins present, an initial burst of NADPH oxidation (∼6.6 s−1 relative to TrxR), at a rate similar to that of TrxR and Trx1 alone, was observed. The somewhat slower but sustained rate observed between 10 and 20 s (2.5 s−1) was not significantly changed when AhpC concentrations were varied, suggesting that both Trx1 reduction by TrxR and AhpC reduction by Trx1 were partially rate limiting under these conditions.
FIG. 4.
AhpC activity assays with H. pylori AhpC, TrxR, and Trx1. NADPH oxidation was monitored at 340 nm on a stopped-flow spectrophotometer for assay mixtures containing TrxR (0.5 μM), Trx1 (5 μM), and AhpC (20 μM; solid line). Other assays were conducted similarly but in the absence of AhpC (short, dashed line), in the absence of Trx1 (dotted line), or in the absence of both AhpC and Trx1 (long, dashed line). NADPH and CHP (150 μM and 1 mM, respectively, after mixing) were incubated in one syringe with peroxidase assay buffer, and assays were initiated when substrates were mixed with proteins incubated in the second syringe with peroxidase buffer.
Investigation of the H. pylori genome yielded two Trx homologues, Trx1 and Trx2, both of which contain the catalytic WCXXC site required for reductase activity. To determine if Trx2 could act as a reductant for H. pylori AhpC, stopped-flow peroxidase experiments in which Trx2 replaced Trx1 in the assay mixture were conducted (Fig. 5). Initially, NADPH oxidation rates were quite fast for reactions with Trx2 (6.0 and 14.7 s−1 for 5 and 10 μM Trx2, respectively, for data from 0.1 to 1 s), compared to the slower rates occurring at later time points in the same assays (0.8 s−1 from 3 to 10 s). After about 5 s, the rates observed for TrxR alone, for TrxR plus 5 μM Trx1, and for AhpC in the presence of TrxR plus 5 or 10 μM Trx2 were all about the same; only the TrxR-Trx1-AhpC system showed a significantly higher sustained rate of NADPH oxidation (Fig. 5). These data indicate that while Trx2 is a good substrate for TrxR, it fails to act as a reductase for H. pylori AhpC.
FIG. 5.
AhpC activity assay with Trx1 or Trx2 as the reductant. The decrease in NADPH absorbance was monitored on a stopped-flow spectrophotometer when AhpC (20 μM) and TrxR (0.5 μM) were assayed with Trx1 (5.0 μM, solid line) or with Trx2 (5.0 μM, dotted line; 10 μM, long dashes) in peroxidase assay buffer as described in the legend to Fig. 4. Assays of TrxR-Trx1 excluding the AhpC protein (dashed-dotted line) and assays of TrxR alone (small dashes) were also conducted. AhpF (0.5 μM) from S. typhimurium was included in place of TrxR-Trx1 with H. pylori AhpC (medium dashes), and in this case, NADH rather than NADPH was used as the reducing substrate under anaerobic conditions.
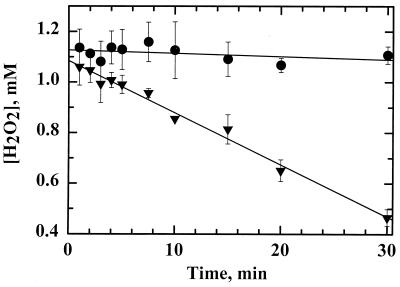
Proof that AhpC reduces peroxides was obtained using an endpoint assay in which the peroxide concentration was monitored over the course of the incubation. In an assay mixture containing TrxR, Trx1, AhpC, NADPH, and H2O2, ferrithiocyanate complex formation with H2O2 decreased over time (5.0 s−1 relative to TrxR), indicating that peroxide was continually consumed in the presence of H. pylori AhpC (Fig. 6). When AhpC was not included in the reaction mixture, no decrease in the peroxide levels was observed. Again, reaction rates were not linear with respect to AhpC concentrations due to partially rate-limiting reduction by TrxR and Trx1 (data not shown). Full kinetic characterization of AhpC with its reducing system TrxR-Trx1 is described in a later section.
FIG. 6.
Peroxide consumption by H. pylori AhpC. The disappearance of peroxide was monitored by ferrithiocyanate complex formation following incubations of a mixture containing 20 μM AhpC (triangles) or 0 μM AhpC (circles) with TrxR (0.2 μM), Trx1 (2.0 μM), and an NADPH-regenerating system consisting of glucose-6-phosphate (10 mM), glucose-6-phosphate dehydrogenase (0.2 U/ml), and NADPH (10 μM) in 500-μl volumes. All reactions were started with the addition of H2O2 (1 mM) as described in Materials and Methods.
Specificity of H. pylori AhpC for TrxR-Trx1 or AhpF-like reductase systems.
In S. typhimurium and most other bacterial systems, the AhpC component is reduced by a specialized flavoprotein related to TrxR and known as AhpF, NADH oxidase, Nox-1, or PrxR (55). In a set of experiments designed to test the specificity of H. pylori AhpC for its own reductase system, S. typhimurium AhpF replaced H. pylori Trx1 and TrxR in the stopped-flow peroxidase assay mixture with H. pylori AhpC. No significant rate of NADH oxidation was observed in an anaerobic S. typhimurium AhpF and H. pylori AhpC system (Fig. 5, medium dashes). Higher concentrations of AhpF still showed no activity with H. pylori AhpC (data not shown), indicating a clear specificity of the cysteine-based peroxidase for reduction by Trx1 rather than by AhpF from a different bacterial source. S. typhimurium AhpC (10 μM) also exhibited considerable specificity for its own reductase, AhpF (0.5 μM), compared with reduction by E. coli TrxR (0.5 μM) plus E. coli Trx1 (5 μM); turnover rates were 42 s−1 with AhpF and 1.7 s−1 with E. coli TrxR-Trx1 (data not shown). Nonetheless, the rate of turnover of S. typhimurium AhpC with the E. coli TrxR-Trx1 system was only about twofold lower than that of H. pylori AhpC with its own TrxR-Trx1 system (3.2 s−1 under the same conditions) while H. pylori AhpC and S. typhimurium AhpF interaction was undetectable. Among the proteins under investigation, H. pylori TrxR and Trx1 and E. coli TrxR and Trx1 were the most interchangeable in peroxidase assays. H. pylori AhpC (2 μM) assayed with the E. coli proteins TrxR (2 μM) and Trx1 (25 μM) exhibited a rate of NADPH oxidation that was about the same as that obtained with H. pylori TrxR-Trx1 under the same conditions (1.9 versus 3.2 s−1).
Steady-state kinetics of AhpC.
To further investigate the peroxidase activity of H. pylori AhpC, reaction conditions were first established under which initial rates were directly proportional to AhpC at a Trx1 concentration (30 μM) that was at least 10-fold higher than the maximal concentration of AhpC (3 μM). Because the very low intrinsic NADPH oxidase activity of E. coli TrxR allowed the observation of low peroxidase rates above background NADPH turnover, E. coli TrxR replaced H. pylori TrxR using concentrations that were high enough (2 to 3 μM) to support rapid H. pylori Trx1 recycling (i.e., additional TrxR did not further increase observed rates of NADPH oxidation). AhpC-dependent rates of NADPH oxidation measured over a range of Trx1 concentrations (15 to 30 μM) suggested a simple bimolecular interaction between reduced Trx1 and oxidized AhpC at a rate of 1.0 × 105 M−1 s−1 (data not shown).
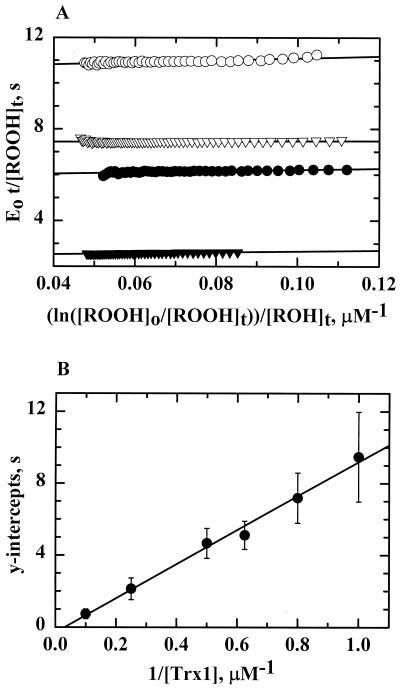
Given the putative nonsaturable interaction between Trx1 and AhpC suggested by the studies described above and the need for information concerning interactions between reduced AhpC and hydroperoxide substrates, kinetic data were generated and analyzed as described previously by Dalziel (18). Because the secondary Dalziel plots (e.g., Fig. 7B) intersect the y axis at the origin (within experimental error), the Km values of AhpC for peroxides and Trx1 and Vmax values are infinite. Parallel lines in the primary plot (Fig. 7A) also indicate a substituted (ping-pong) mechanism for H. pylori AhpC by which AhpC does not form detectable enzyme-substrate complexes with either substrate and displays nonsaturating kinetics, a kinetic pattern that has been observed for other nonheme peroxidases, such as glutathione peroxidase (23) and tryparedoxin peroxidase (46).
FIG. 7.
Kinetic analysis of AhpC from H. pylori by the Dalziel method. Assays of AhpC were conducted on a stopped-flow spectrophotometer using AhpC (4.0 μM), Trx1 (2.0 μM), and E. coli TrxR (1.0 μM) with NADPH (150 μM) and limiting amounts of peroxide (10 to 40 μM). Proteins were incubated with NADPH for 5 min prior to mixing with peroxide. The resulting reaction traces were used to calculate [ROOH] and [ROH] over time. (A) In the Dalziel primary plot, a least-squares linear regression line was obtained for the calculated data points for five different concentrations of Trx1: 4.0 μM (closed triangles), 2.0 μM (closed circles), 1.6 μM (open triangles), and 1.0 μM (open circles). The resulting lines give slopes of φ1 and intercepts of φ2/[Trx1]. (B) The apparent maximum velocities (y intercepts from the primary plot) were replotted in a Dalziel secondary plot against the reciprocal of the [Trx1] at which they were obtained. The slope of the resulting linear regression line is φ2.
With the above observations in mind, the rate data were plotted using the Dalziel equation for two-substrate reactions in which enzyme-substrate complexes are not observed: [E]0/v = φ1/[ROOH] + φ2/[Trx1], where [E] is the concentration of AhpC and φ1 and φ2 are the Dalziel kinetic coefficients. Because enzyme-substrate complexes are not observed experimentally, the interactions of AhpC with its two substrates can be depicted as a sequence of consecutive, bimolecular, nonreversible reactions: AhpCred + ROOH → AhpCox + ROH + H2O and AhpCox + TrxRed → AhpCred + Trx1ox. The apparent limiting rates for these two reactions are characterized by their kinetic rate constants, k1′ and k2′, respectively, which are the reciprocals of the Dalziel coefficients, φ1 and φ2. To obtain the rate constants, the rate data were evaluated using the integrated Dalziel equation (24). After elucidating product and reactant amounts at various times, the data were substituted into the integrated rate equation (given in Materials and Methods) and plotted (Fig. 7A). The slopes of the lines in the primary plot are equal to φ1: y intercepts from Fig. 7A replotted in Fig. 7B versus the reciprocal of the Trx1 concentration produced a line with a slope of φ2. By taking the reciprocal of φ2, the second-order rate constant, k2′, of 1.0 × 105 M−1 s−1 was determined for the interaction between AhpC and Trx1, giving the same value as that obtained using the AhpC-dependent assays described above, in which the peroxide substrate was in excess. Using a similar strategy, the value for k1′ (2.0 × 105 M−1 s−1) was determined for the interaction of AhpC with H2O2 and was of a magnitude similar to that of k2′ for the oxidation of Trx1. Therefore, both steps are partially rate limiting under these conditions.
AhpC was also tested for the ability to reduce peroxides other than H2O2, including EtOOH, t-BOOH, CHP, and linoleic acid hydroperoxide (LOOH). Using one concentration of Trx1 (2.0 μM), the slopes from the primary plot were determined for each peroxide to yield the following apparent k1′ values: H2O2, 2.0 × 105 M−1 s−1; EtOOH, 1.7 × 105 M−1 s−1; t-BOOH, 1.6 × 105 M−1 s−1; CHP, 1.2 × 105 M−1 s−1; LOOH, 1.1 × 105 M−1 s−1. AhpC was capable of reducing the different peroxides, including the more structurally complex compounds t-BOOH and LOOH, with similar apparent rate constants. No change in k2′ reflecting the interaction between Trx1 and AhpC was observed for the different hydroperoxide substrates.
Essentiality of H. pylori ahpC.
Allelic replacement mutagenesis of ahpC was conducted to study the physiological role of AhpC in H. pylori and to determine the effect of AhpC removal on H. pylori's responses to oxidative stress. When ahpC interrupted with the camR cassette was introduced into H. pylori strains HP26695, SS1, HP1061, and HP1, there was no growth of colonies on selective medium after 7 days of incubation, whereas a few thousand colonies are normally obtained after 2 to 3 days for nonessential-gene knockouts carried out in this manner (e.g., with the rdxA knockout control). While single crossover events have been described in H. pylori using this method of transformation (57), no such phenomenon was observed even after several repeated transformation attempts. A similar construct was successfully generated in which the nitroreductase (rdxA) gene was knocked out with the same camR cassette (26) and introduced by transformation and homologous recombination into the same H. pylori strains. Both Camr and Mtzr (metronidazole-resistant) colonies were formed, indicating that rdx is not essential for growth. Therefore, the loss of growth under microaerobic conditions with the loss of ahpC strongly suggests an essential role for AhpC in H. pylori viability.
Genetic characterization of ahpC.
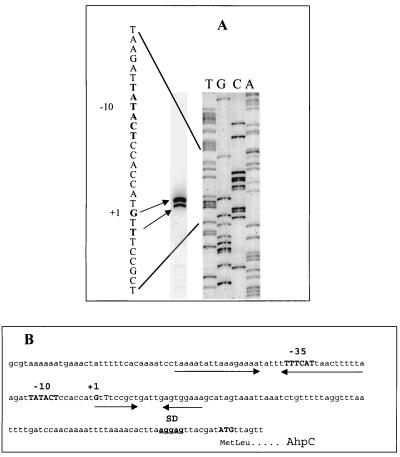
The gene encoding AhpC is located between ceuE (HP1562, encoding an iron III ABC transporter) transcribed in the opposite direction and a gene encoding an outer membrane protein (HP1564) in the same orientation as ahpC and containing endogenous promoter sequences (71). The location of the transcriptional start site of ahpC was determined by primer extension analysis. Two prominent reverse transcript bands migrate parallel to G and T residues, 96 and 94 bp upstream, respectively, from the AUG translation initiation codon (Fig. 8A). Sequences resembling the H. pylori ς70 consensus sequence (25) were located at the expected distance from the transcriptional start site (Fig. 8B, bold). The potential −10 hexamer of the putative ahpC promoter, TATACT, displays a high degree of identity (5 of 6 bp) to the −10 consensus sequence for H. pylori, TAtaaT (25). Other putative regulatory sequence elements were identified in the promoter region centered around −40 and +10 (underlined, Fig. 8B), underscoring the possibility that ahpC transcription is regulated. However, no Fur (ferric uptake regulator) binding site (69) was evident in the H. pylori ahpC promoter region and the H. pylori genome lacks a structural gene for OxyR, the redox-sensitive transcription factor which upregulates AhpC production in response to oxidative stress in other eubacteria (65). Although the deduced H. pylori AhpC amino acid sequence has already been published, the protein was not identified as an alkyl hydroperoxide reductase and the translational start site of ahpC was not correctly positioned by O'Toole et al. (48).
FIG. 8.
Primer extension analysis of ahpC. (A) To map the transcriptional start site of H. pylori AhpC mRNA, a [γ−32P]ATP-labeled oligonucleotide complementary to the 5′ end of ahpC was hybridized to 100 μg of total RNA and extended using reverse transcriptase. DNA sequencing reactions carried out with the same primer (right) were electrophoresed concomitantly with the primer extension products (left) to the left on a 6% urea polyacrylamide sequencing gel. Two potential 5′ ends of the ahpC transcript are 96 (G) and 94 (T) bp upstream from the AUG translation initiation codon. The potential −10 hexamer of the putative ahpC promoter is indicated in bold upstream of the transcriptional start site. (B) Shown is the DNA sequence of the region upstream of ahpC from H. pylori strain 26695. The potential ahpC transcription and translation start signals are shown in bold, as well as the Shine-Dalgarno (SD) ribosome binding site and the putative promoter sites centered at −10 and −35. The inverted repeats are indicated by arrows.
DISCUSSION
Antioxidant systems critical to the defense of H. pylori against ROS generated by the oxidative burst of macrophages and polymorphonuclear leukocytes are central to the ability of this organism to establish chronic infections in gastric tissues and to combat the high degree of inflammatory responses mounted by the host. To date, Fe-dependent superoxide dismutase (63), catalase (47), and the Trx-dependent peroxidase (AhpC) described herein have been characterized as H. pylori antioxidant enzymes. Deletion of the gene encoding catalase (katA) from the H. pylori chromosome did not affect the viability of the organism (71), whereas we have found that ahpC is essential for H. pylori survival under microaerobic conditions. Previous studies with other organisms in which the ahpC locus was deleted or mutated have not demonstrated AhpC essentiality. For example, in studies of the closely related, but more aerotolerant, enteric pathogen C. jejuni, deletion of sodB decreased its capacity to survive in macrophages (50), while deletion of ahpC increased susceptibility to oxidative stress (4); nonetheless, viability under conditions of low oxygen tension was not affected for either mutant. In support of our findings, two other groups have recently reported attempts to knock out H. pylori ahpC and have reached similar conclusions regarding its essentiality (42, 13). H. pylori sensitivity toward oxidative damage is highlighted by its dependence on microaerophilic growth conditions. Its inability to grow in the absence of ahpC is a clear reflection of the delicate redox balance required to support, yet not inactivate, key metabolic enzymes (37).
Given the apparent requirement of TrxR-Trx1 for AhpC activity, the TrxR-Trx1 reductase system is also likely to be indispensable for H. pylori viability, as well, although this hypothesis has yet to be tested. The alternative, that an as-yet-unidentified reductase is also capable of AhpC reduction, is also a possibility. Interestingly, no homologues for the E. coli glutathione reductase system, such as glutaredoxin (grxA), γ-l-glutamyl-l-cysteine synthetase (gshA), or glutathione reductase (gorA), can be found in the H. pylori genome. Therefore, H. pylori does not have the main other reductase system that can serve as a compensatory mechanism in most other organisms (56), highlighting the possible fundamental requirement for a functional TrxR-Trx1 system in H. pylori.
Our demonstration that the 26-kDa protein previously described as an abundant “species-specific antigen” by O'Toole et al. (48) is a cysteine-based peroxidase reactivated by reduced Trx agrees nicely with the sequence-based comparisons of H. pylori AhpC with a wide variety of other Prxs. A subset of these proteins, some of which have been classified as TPxs, are not reducible by bacterial AhpF proteins and rely on reducing equivalents from Trx to support turnover with cellular peroxides (10, 33, 36, 52). We have found that this capacity to accept electrons from reduced Trx is a property common to bacterial AhpC proteins as well, which nonetheless show much greater reactivity toward their specialized flavoprotein reductase, AhpF. Some eukaryotic Prxs, on the other hand, are incapable of turnover with reduced Trx proteins, at least under the conditions tested (22, 35). The specificity of such cysteine-based peroxidases for reduced Trx, as implied by the TPx designation, is therefore of questionable validity as a unique functional description distinguishing this group among the diverse Prx proteins.
In cases in which the reactivation of Prx proteins by their electron donor proteins has been investigated in detail, two different kinetic patterns have been observed. For AhpF-AhpC interactions, reduction of AhpC by AhpF is a saturable phenomenon characterized by a Km for AhpC of around 15 μM and a kcat/Km for the flavoprotein of ∼107 M−1 s−1 (55). For the Prx system from C. fasciculata, interaction of reduced tryparedoxin with its peroxidase is a bimolecular process with infinite kcat and Km values and a second-order rate constant of 1.5 × 106 to 3.5 × 106 M−1 s−1 (46). Here, we have demonstrated that the interaction of Trx1 with H. pylori AhpC is also bimolecular (∼105 M−1 s−1) and that therefore no enzyme-substrate complexes between Trx1 and AhpC can be detected kinetically. Presumably, this kinetic pattern will hold for other Trx-dependent Prx proteins as well when such kinetic profiles are investigated.
Using kinetic studies akin to those previously applied to another Prx, tryparedoxin peroxidase from C. fasciculata, the interaction of H. pylori AhpC with peroxides was also shown to be a bimolecular process (like Trx-Prx interactions) lacking detectable enzyme-substrate complexes. This ping-pong mechanism has also been observed for the distantly related glutathione peroxidases (23, 68). The higher rate of enzyme-peroxide interaction for glutathione peroxidase (∼108 M−1 s−1) has been attributed to the unique reactivity of the selenocysteine at the active site (64), although at 105 to 106 M−1 s−1, the rate of peroxide reduction is still quite high and indicative of an important role for H. pylori AhpC in cellular peroxide metabolism. Using k1′ to characterize the protein-peroxide interaction, our experiments demonstrated essentially no specificity when AhpC was tested with a wide variety of small, bulky, aromatic, or lipid hydroperoxide substrates, as was true of the C. fasciculata peroxidase (46). Differential reactivities toward particular hydroperoxide substrates have been reported for some other Prx enzymes based on less quantitative analyses (9, 33, 34). In addition, peroxynitrite (OONO−) has recently been shown to be a substrate for H. pylori AhpC, with the rate of decomposition occurring at a second-order rate constant of 1.21 × 106 M−1 s−1 (7). These results are all consistent with a minimal binding site on AhpC for hydroperoxides (and peroxynitrite) consisting of little more than the catalytic residue (Cys49) at the active site.
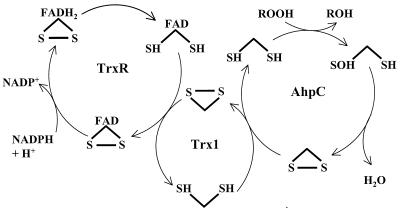
On the basis of results reported here and mechanisms outlined for other 2-Cys AhpC homologues (10, 51), electrons from NADPH proceed along the path outlined in Fig. 9 for the reduction of peroxides to alcohols. This scheme is highly analogous to that for electron transfer through the S. typhimurium AhpC system, except that TrxR and Trxl replace AhpF in the H. pylori system. Nonetheless, one tightly bound flavin and three disulfide redox centers mediate electron transfer from pyridine nucleotide to peroxide in both cases.
FIG. 9.
Pathway for transfer of reducing equivalents from NADPH to hydroperoxide in the alkyl hydroperoxide reductase system from H. pylori. Note that the enzyme species shown do not necessarily represent actual catalytic intermediates. FAD, flavin adenine dinucleotide.
Little information exists on potential redox or iron regulation of H. pylori AhpC expression, although in other studies of TrxR and Trx1 from H. pylori, Windle et al. (74) observed that Trxl expression dramatically increased under conditions of oxidative stress; Trx1 was therefore classified as a stress response element in H. pylori. While the proximal location of trxR and trx1 in the chromosome could provide the bacterium with a mechanism for a coordinated response eliciting expression of both proteins, an increase in TrxR expression did not accompany increased Trx1 expression under oxidative stress conditions (74). Interestingly, reductase activity of the TrxR-Trx1 reductase system was also reportedly present in the media of culture supernatants and could be available to support extracellular peroxide reduction by AhpC if the latter protein is also exported.
In conclusion, this is the first report of a Trx-dependent alkyl hydroperoxide system from a gastric pathogen. To our knowledge, AhpC from H. pylori is only the second known bacterial AhpC to be demonstrated experimentally to require the Trx- reducing system for reduction of the oxidized AhpC active site. An essential role for AhpC in H. pylori has been established with our ahpC mutagenesis experiments, and this is, to our knowledge, the only case in which an ahpC locus has been shown to be required for viability. The presence of this essential Trx-dependent peroxidase in H. pylori suggests important roles for TrxR, Trx1, and AhpC in the removal of alkyl hydroperoxides to protect against oxidative stress and in the preservation of the microaerobic environment required for H. pylori viability.
ACKNOWLEDGMENTS
Funding for this work was provided in part by grants from the National Institutes of Health (NIH R01 GM50389) to L.B.P. and from the Canadian Institutes for Health Research (CIHR MT11318 and RP14292) to P.S.H.
We are particularly grateful to C. M. Reynolds for the purification of E. coli Trx1 and TrxR and to Desnee Wynn, Lois LaPrade, and Louis Bryden for technical assistance. Special thanks to Joseph O'Flaherty for his assistance with the fatty acid hydroperoxide preparation and to James Luba for helpful discussions of the kinetic data.
REFERENCES
- 1.Alphey M S, Bond C S, Tetaud E, Fairlamb A H, Hunter W N. The structure of reduced tryparedoxin peroxidase reveals a decamer and insight into reactivity of 2-Cys peroxiredoxins. J Mol Biol. 2000;300:903–916. doi: 10.1006/jmbi.2000.3881. [DOI] [PubMed] [Google Scholar]
- 2.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 3.Ausubel F M, Brent R B, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Short protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1999. [Google Scholar]
- 4.Baillon M L, van Vliet A H, Ketley J M, Constantinidou C, Penn C W. An iron-regulated alkyl hydroperoxide reductase (AhpC) confers aerotolerance and oxidative stress resistance to the microaerophilic pathogen Campylobacter jejuni. J Bacteriol. 1999;181:4798–4804. doi: 10.1128/jb.181.16.4798-4804.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker L M S, Poole L B. An alkyl hydroperoxide reductase system from the gastric pathogen, Helicobacter pylori: cloning, purification, and kinetic studies. FASEB J. 1999;13:A1447. [Google Scholar]
- 6.Baker L M S, Poole L B. Thioredoxin 1, not Thioredoxin 2, selectively reduces the peroxidase component of Helicobacter pylori'salkyl hydroperoxide reductase system. FASEB J. 2000;14:A1525. [Google Scholar]
- 7.Bryk R, Griffin P, Nathan C. Peroxynitrite reductase activity of bacterial peroxiredoxins. Nature. 2000;407:211–215. doi: 10.1038/35025109. [DOI] [PubMed] [Google Scholar]
- 8.Buck G K, Gourley W K, Lee W K, Subramanyam K, Latimer J M, DiNuzzo A R. Relation of Campylobacter pyloridisto gastritis and peptic ulcer. J Infect Dis. 1986;153:664–669. doi: 10.1093/infdis/153.4.664. [DOI] [PubMed] [Google Scholar]
- 9.Cha M K, Yun C H, Kim I H. Interaction of human thiol-specific antioxidant protein 1 with erythrocyte plasma membrane. Biochemistry. 2000;39:6944–6950. doi: 10.1021/bi000034j. [DOI] [PubMed] [Google Scholar]
- 10.Chae H Z, Chung S J, Rhee S G. Thioredoxin-dependent peroxide reductase from yeast. J Biol Chem. 1994;269:27670–27678. [PubMed] [Google Scholar]
- 11.Chae H Z, Kang S W, Rhee S G. Isoforms of mammalian peroxiredoxin that reduce peroxides in presence of thioredoxin. Methods Enzymol. 1999;300:219–226. doi: 10.1016/s0076-6879(99)00128-7. [DOI] [PubMed] [Google Scholar]
- 12.Chae H Z, Robinson K, Poole L B, Church G, Storz G, Rhee S G. Cloning and sequencing of thiol-specific antioxidant from mammalian brain: alkyl hydroperoxide reductase and thiol-specific antioxidant define a large family of antioxidant enzymes. Proc Natl Acad Sci USA. 1994;91:7017–7021. doi: 10.1073/pnas.91.15.7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chalker A F, Minehart H W, Hughes N J, Koretke K K, Lonetto M A, Brinkman K K, Warren P V, Lupas A, Stanhope M J, Brown J R, Hoffman P S. Systematic identification of selective genes in Helicobacter pyloriby genome prioritization and allelic replacement mutagenesis. J Bacteriol. 2001;183:1259–1268. doi: 10.1128/JB.183.4.1259-1268.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen G, Yanko M, Mislovati M, Argaman A, Schreiber R, Av-Gay Y, Aharonowitz Y. Thioredoxin-thioredoxin reductase system of Streptomyces clavuligerus: sequences, expression, and organization of the genes. J Bacteriol. 1993;175:5159–5167. doi: 10.1128/jb.175.16.5159-5167.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cornish-Bowden A. Fundamentals of enzyme kinetics. London, England: Portland Press, Ltd.; 1999. [Google Scholar]
- 16.Correa P. Helicobacter pyloriand gastric carcinogenesis. Am J Surg Pathol. 1995;19:S37–S43. [PubMed] [Google Scholar]
- 17.Correa P. A human model of gastric carcinogenesis. Cancer Res. 1988;48:3554–3560. [PubMed] [Google Scholar]
- 18.Dalziel K. Initial steady state velocities in the evaluation of enzyme-coenzyme-substrate reaction mechanisms. Acta Chem Scand. 1957;11:1706–1723. [Google Scholar]
- 19.Davies G R, Simmonds N J, Stevens T R, Sheaff M T, Banatvala N, Laurenson I F, Blake D R, Rampton D S. Helicobacter pylori stimulates antral mucosal reactive oxygen metabolite production in vivo. Gut. 1994;35:179–185. doi: 10.1136/gut.35.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ellis H R, Poole L B. Novel application of 7-chloro-4-nitrobenzo-2-oxa-1,3-diazole to identify cysteine sulfenic acid in the AhpC component of alkyl hydroperoxide reductase. Biochemistry. 1997;36:15013–15018. doi: 10.1021/bi972191x. [DOI] [PubMed] [Google Scholar]
- 21.Ellis H R, Poole L B. Roles for the two cysteine residues of AhpC in catalysis of peroxide reduction by alkyl hydroperoxide reductase from Salmonella typhimurium. Biochemistry. 1997;36:13349–13356. doi: 10.1021/bi9713658. [DOI] [PubMed] [Google Scholar]
- 22.Fisher A B, Dodia C, Manevich Y, Chen J W, Feinstein S I. Phospholipid hydroperoxides are substrates for non-selenium glutathione peroxidase. J Biol Chem. 1999;274:21326–21334. doi: 10.1074/jbc.274.30.21326. [DOI] [PubMed] [Google Scholar]
- 23.Flohe L, Loschen G, Gunzler W A, Eichele E. Glutathione peroxidase. V. The kinetic mechanism. Hoppe Seyler's Z Physiol Chem. 1972;353:987–999. doi: 10.1515/bchm2.1972.353.1.987. [DOI] [PubMed] [Google Scholar]
- 24.Forstrom J W, Stults F H, Tappel A L. Rat liver cytosolic glutathione peroxidase: reactivity with linoleic acid hydroperoxide and cumene hydroperoxide. Arch Biochem Biophys. 1979;193:51–55. doi: 10.1016/0003-9861(79)90007-9. [DOI] [PubMed] [Google Scholar]
- 25.Forsyth M H, Cover T L. Mutational analysis of the vacA promoter provides insight into gene transcription in Helicobacter pylori. J Bacteriol. 1999;181:2261–2266. doi: 10.1128/jb.181.7.2261-2266.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goodwin A, Kersulyte D, Sisson G, Veldhuyzen van Zanten S J, Berg D E, Hoffman P S. Metronidazole resistance in Helicobacter pylori is due to null mutations in a gene (rdxA) that encodes an oxygen-insensitive NADPH nitroreductase. Mol Microbiol. 1998;28:383–393. doi: 10.1046/j.1365-2958.1998.00806.x. [DOI] [PubMed] [Google Scholar]
- 27.Graham D Y, Klein P D. Campylobacter pyloridisgastritis: the past, the present, and speculations about the future. Am J Gastroenterol. 1987;82:283–286. [PubMed] [Google Scholar]
- 28.Guruge J L, Falk P G, Lorenz R G, Dans M, Wirth H P, Blaser M J, Berg D E, Gordon J I. Epithelial attachment alters the outcome of Helicobacter pyloriinfection. Proc Natl Acad Sci USA. 1998;95:3925–3930. doi: 10.1073/pnas.95.7.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holmgren A. Thioredoxin. 6. The amino acid sequence of the protein from Escherichia coli B. Eur J Biochem. 1968;6:475–484. doi: 10.1111/j.1432-1033.1968.tb00470.x. [DOI] [PubMed] [Google Scholar]
- 30.Holmgren A, Bjornstedt M. Thioredoxin and thioredoxin reductase. Methods Enzymol. 1995;252:199–306. doi: 10.1016/0076-6879(95)52023-6. [DOI] [PubMed] [Google Scholar]
- 31.Holmgren A, Reichard P. Thioredoxin. 2. Cleavage with cyanogen bromide. Eur J Biochem. 1967;2:187–196. doi: 10.1111/j.1432-1033.1967.tb00125.x. [DOI] [PubMed] [Google Scholar]
- 32.Jacobson F S, Morgan R W, Christman M F, Ames B N. An alkyl hydroperoxide reductase from Salmonella typhimuriuminvolved in the defense of DNA against oxidative damage. Purification and properties. J Biol Chem. 1989;264:1488–1496. [PubMed] [Google Scholar]
- 33.Jeong J S, Kwon S J, Kang S W, Rhee S G, Kim K. Purification and characterization of a second type thioredoxin peroxidase (type II TPx) from Saccharomyces cerevisiae. Biochemistry. 1999;38:776–783. doi: 10.1021/bi9817818. [DOI] [PubMed] [Google Scholar]
- 34.Jeong W, Cha M K, Kim I H. Thioredoxin-dependent hydroperoxide peroxidase activity of bacterioferritin comigratory protein (BCP) as a new member of the thiol-specific antioxidant protein (TSA)/alkyl hydroperoxide peroxidase C (AhpC) family. J Biol Chem. 2000;275:2924–2930. doi: 10.1074/jbc.275.4.2924. [DOI] [PubMed] [Google Scholar]
- 35.Kang S W, Baines I C, Rhee S G. Characterization of a mammalian peroxiredoxin that contains one conserved cysteine. J Biol Chem. 1998;273:6303–6311. doi: 10.1074/jbc.273.11.6303. [DOI] [PubMed] [Google Scholar]
- 36.Kang S W, Chae H Z, Seo M S, Kim K, Baines I C, Rhee S G. Mammalian peroxiredoxin isoforms can reduce hydrogen peroxide generated in response to growth factors and tumor necrosis factor-alpha. J Biol Chem. 1998;273:6297–6302. doi: 10.1074/jbc.273.11.6297. [DOI] [PubMed] [Google Scholar]
- 37.Kelly D J. The physiology and metabolism of the human gastric pathogen Helicobacter pylori. Adv Microb Physiol. 1998;40:137–189. doi: 10.1016/s0065-2911(08)60131-9. [DOI] [PubMed] [Google Scholar]
- 38.Kitano K, Niimura Y, Nishiyama Y, Miki K. Stimulation of peroxidase activity by decamerization related to ionic strength: AhpC protein from Amphibacillus xylanus. J Biochem (Tokyo) 1999;126:313–319. doi: 10.1093/oxfordjournals.jbchem.a022451. [DOI] [PubMed] [Google Scholar]
- 39.Klenk H P, Clayton R A, Tomb J F, White O, Nelson K E, Ketchum K A, Dodson R J, Gwinn M, Hickey E K, Peterson J D, Richardson D L, Kerlavage A R, Graham D E, Kyrpides N C, Fleischmann R D, Quackenbush J, Lee N H, Sutton G G, Gill S, Kirkness E F, Dougherty B A, McKenney K, Adams M D, Loftus B, Venter J C, et al. The complete genome sequence of the hyperthermophilic, sulphate-reducing archaeon Archaeoglobus fulgidus. Nature. 1997;390:364–370. doi: 10.1038/37052. [DOI] [PubMed] [Google Scholar]
- 40.Lee A, O'Rourke J, De Ungria M C, Robertson B, Daskalopoulos G, Dixon M F. A standardized mouse model of Helicobacter pyloriinfection: introducing the Sydney strain. Gastroenterology. 1997;112:1386–1397. doi: 10.1016/s0016-5085(97)70155-0. [DOI] [PubMed] [Google Scholar]
- 41.Lennon B W, Williams C H., Jr Reductive half-reaction of thioredoxin reductase from Escherichia coli. Biochemistry. 1997;36:9464–9477. doi: 10.1021/bi970307j. [DOI] [PubMed] [Google Scholar]
- 42.Lundstrom A M, Bolin I. A 26 kDa protein of Helicobacter pylorishows alkyl hydroperoxide reductase (AhpC) activity and the mono-cistronic transcription of the gene is affected by pH. Microb Pathog. 2000;29:257–266. doi: 10.1006/mpat.2000.0388. [DOI] [PubMed] [Google Scholar]
- 43.Maiorino M, Gregolin C, Ursini F. Phospholipid hydroperoxide glutathione peroxidase. Methods Enzymol. 1990;186:448–457. doi: 10.1016/0076-6879(90)86139-m. [DOI] [PubMed] [Google Scholar]
- 44.Mallett T C, Claiborne A. Oxygen reactivity of an NADH oxidase C42S mutant: evidence for a C(4a)-peroxyflavin intermediate and a rate-limiting conformational change. Biochemistry. 1998;37:8790–8802. doi: 10.1021/bi9803630. [DOI] [PubMed] [Google Scholar]
- 45.Miranda-Vizuete A, Damdimopoulos A E, Gustafsson J, Spyrou G. Cloning, expression, and characterization of a novel Escherichia colithioredoxin. J Biol Chem. 1997;272:30841–30847. doi: 10.1074/jbc.272.49.30841. [DOI] [PubMed] [Google Scholar]
- 46.Nogoceke E, Gommel D U, Kiess M, Kalisz H M, Flohe L. A unique cascade of oxidoreductases catalyses trypanothione-mediated peroxide metabolism in Crithidia fasciculata. Biol Chem. 1997;378:827–836. doi: 10.1515/bchm.1997.378.8.827. [DOI] [PubMed] [Google Scholar]
- 47.Odenbreit S, Wieland B, Haas R. Cloning and genetic characterization of Helicobacter pyloricatalase and construction of a catalase-deficient mutant strain. J Bacteriol. 1996;178:6960–6967. doi: 10.1128/jb.178.23.6960-6967.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O'Toole P W, Logan S M, Kostrzynska M, Wadstrom T, Trust T J. Isolation and biochemical and molecular analyses of a species-specific protein antigen from the gastric pathogen Helicobacter pylori. J Bacteriol. 1991;173:505–513. doi: 10.1128/jb.173.2.505-513.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parkhill J, Wren B W, Mungall K, Ketley J M, Churcher C, Basham D, Chillingworth T, Davies R M, Feltwell T, Holroyd S, Jagels K, Karlyshev A V, Moule S, Pallen M J, Penn C W, Quail M A, Rajandream M A, Rutherford K M, van Vliet A H, Whitehead S, Barrell B G. The genome sequence of the food-borne pathogen Campylobacter jejunireveals hypervariable sequences. Nature. 2000;403:665–668. doi: 10.1038/35001088. [DOI] [PubMed] [Google Scholar]
- 50.Pesci E C, Cottle D L, Pickett C L. Genetic, enzymatic, and pathogenic studies of the iron superoxide dismutase of Campylobacter jejuni. Infect Immun. 1994;62:2687–2694. doi: 10.1128/iai.62.7.2687-2694.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Poole L B. Flavin-dependent alkyl hydroperoxide reductase from Salmonella typhimurium. 2. Cystine disulfides involved in catalysis of peroxide reduction. Biochemistry. 1996;35:65–75. doi: 10.1021/bi951888k. [DOI] [PubMed] [Google Scholar]
- 52.Poole L B, Chae H Z, Flores B M, Reed S L, Rhee S G, Torian B E. Peroxidase activity of a TSA-like antioxidant protein from a pathogenic amoeba. Free Radic Biol Med. 1997;23:955–959. doi: 10.1016/s0891-5849(97)00066-x. [DOI] [PubMed] [Google Scholar]
- 53.Poole L B, Ellis H R. Flavin-dependent alkyl hydroperoxide reductase from Salmonella typhimurium. 1. Purification and enzymatic activities of overexpressed AhpF and AhpC proteins. Biochemistry. 1996;35:56–64. doi: 10.1021/bi951887s. [DOI] [PubMed] [Google Scholar]
- 54.Poole L B, Godzik A, Nayeem A, Schmitt J D. AhpF can be dissected into two functional units: tandem repeats of two thioredoxin-like folds in the N-terminus mediate electron transfer from the thioredoxin reductase-like C-terminus to AhpC. Biochemistry. 2000;39:6602–6615. doi: 10.1021/bi000405w. [DOI] [PubMed] [Google Scholar]
- 55.Poole L B, Reynolds C M, Wood Z A, Karplus P A, Ellis H R, Li Calzi M. AhpF and other NADH:peroxiredoxin oxidoreductases, homologues of low Mrthioredoxin reductase. Eur J Biochem. 2000;267:6126–6133. doi: 10.1046/j.1432-1327.2000.01704.x. [DOI] [PubMed] [Google Scholar]
- 56.Prieto-Alamo M J, Jurado J, Gallardo-Madueno R, Monje-Casas F, Holmgren A, Pueyo C. Transcriptional regulation of glutaredoxin and thioredoxin pathways and related enzymes in response to oxidative stress. J Biol Chem. 2000;275:13398–13405. doi: 10.1074/jbc.275.18.13398. [DOI] [PubMed] [Google Scholar]
- 57.Raudonikiene A, Zakharova N, Su W W, Jeong J Y, Bryden L, Hoffman P S, Berg D E, Severinov K. Helicobacter pyloriwith separate beta- and beta′-subunits of RNA polymerase is viable and can colonize conventional mice. Mol Microbiol. 1999;32:131–138. doi: 10.1046/j.1365-2958.1999.01336.x. [DOI] [PubMed] [Google Scholar]
- 58.Riddles P W, Blakeley R L, Zerner B. Ellman's reagent: 5,5′-dithiobis(2-nitrobenzoic acid)—a reexamination. Anal Biochem. 1979;94:75–81. doi: 10.1016/0003-2697(79)90792-9. [DOI] [PubMed] [Google Scholar]
- 59.Rieder G, Hatz R A, Moran A P, Walz A, Stolte M, Enders G. Role of adherence in interleukin-8 induction in Helicobacter pylori-associated gastritis. Infect Immun. 1997;65:3622–3630. doi: 10.1128/iai.65.9.3622-3630.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Salyers A A, Whitt D D. Bacterial pathogenesis—molecular approach. Washington, D.C.: ASM Press; 1994. pp. 273–281. [Google Scholar]
- 61.Schröder E, Littlechild J A, Lebedev A A, Errington N, Vagin A A, Isupov M N. Crystal structure of decameric 2-Cys peroxiredoxin from human erythrocytes at 1.7 A resolution. Structure. 2000;8:605–615. doi: 10.1016/s0969-2126(00)00147-7. [DOI] [PubMed] [Google Scholar]
- 62.Schröder E, Ponting C P. Evidence that peroxiredoxins are novel members of the thioredoxin fold superfamily. Protein Sci. 1998;7:2465–2468. doi: 10.1002/pro.5560071125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Spiegelhalder C, Gerstenecker B, Kersten A, Schiltz E, Kist M. Purification of Helicobacter pylorisuperoxide dismutase and cloning and sequencing of the gene. Infect Immun. 1993;61:5315–5325. doi: 10.1128/iai.61.12.5315-5325.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stadtman TC. Selenocysteine. Annu Rev Biochem. 1996;65:83–100. doi: 10.1146/annurev.bi.65.070196.000503. [DOI] [PubMed] [Google Scholar]
- 65.Storz G, Jacobson F S, Tartaglia L A, Morgan R W, Silveira L A, Ames B N. An alkyl hydroperoxide reductase induced by oxidative stress in Salmonella typhimurium and Escherichia coli: genetic characterization and cloning of ahp. J Bacteriol. 1989;171:2049–2055. doi: 10.1128/jb.171.4.2049-2055.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thurman R G, Ley H G, Scholz R. Hepatic microsomal ethanol oxidation. Hydrogen peroxide formation and the role of catalase. Eur J Biochem. 1972;25:420–430. doi: 10.1111/j.1432-1033.1972.tb01711.x. [DOI] [PubMed] [Google Scholar]
- 67.Tomb J F, White O, Kerlavage A R, Clayton R A, Sutton G G, Fleischmann R D, Ketchum K A, Klenk H P, Gill S, Dougherty B A, Nelson K, Quackenbush J, Zhou L, Kirkness E F, Peterson S, Loftus B, Richardson D, Dodson R, Khalak H G, Glodek A, McKenney K, Fitzegerald L M, Lee N, Adams M D, Venter J C, et al. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 68.Ursini F, Maiorino M, Gregolin C. The selenoenzyme phospholipid hydroperoxide glutathione peroxidase. Biochim Biophys Acta. 1985;839:62–70. doi: 10.1016/0304-4165(85)90182-5. [DOI] [PubMed] [Google Scholar]
- 69.van Vliet A H, Baillon M L, Penn C W, Ketley J M. Campylobacter jejuni contains two furhomologs: characterization of iron-responsive regulation of peroxide stress defense genes by the PerR repressor. J Bacteriol. 1999;181:6371–6376. doi: 10.1128/jb.181.20.6371-6376.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang Y, Taylor D E. Chloramphenicol resistance in Campylobacter coli: nucleotide sequence, expression, and cloning vector construction. Gene. 1990;94:23–28. doi: 10.1016/0378-1119(90)90463-2. [DOI] [PubMed] [Google Scholar]
- 71.Westblom T U, Phadnis S, Langenberg W, Yoneda K, Madan E, Midkiff B R. Catalase negative mutants of Helicobacter pylori. Eur J Clin Microbiol Infect Dis. 1992;11:522–526. doi: 10.1007/BF01960807. [DOI] [PubMed] [Google Scholar]
- 72.Wieles B, van Soolingen D, Holmgren A, Offringa R, Ottenhoff T, Thole J. Unique gene organization of thioredoxin and thioredoxin reductase in Mycobacterium leprae. Mol Microbiol. 1995;16:921–929. doi: 10.1111/j.1365-2958.1995.tb02318.x. [DOI] [PubMed] [Google Scholar]
- 73.Williams C H, Jr, Zanetti G, Arscott L D, McAllister J K. Lipoamide dehydrogenase, glutathione reductase, thioredoxin reductase, and thioredoxin. J Biol Chem. 1967;242:5226–5231. [PubMed] [Google Scholar]
- 74.Windle H J, Fox A, Ni Eidhin D, Kelleher D. The thioredoxin system of Helicobacter pylori. J Biol Chem. 2000;275:5081–5089. doi: 10.1074/jbc.275.7.5081. [DOI] [PubMed] [Google Scholar]
- 75.Yamamoto H, Miyake C, Dietz K J, Tomizawa K, Murata N, Yokota A. Thioredoxin peroxidase in the cyanobacterium Synechocystissp. PCC 6803. FEBS Lett. 1999;447:269–273. doi: 10.1016/s0014-5793(99)00309-9. [DOI] [PubMed] [Google Scholar]