Background:
As novel hypoglycemic drugs, the effects of sodium-dependent glucose transporter 2 inhibitors (SGLT-2I) on inflammatory factors such as C-reactive protein (CRP) remain unclear.
Methods:
We conducted a meta-analysis of studies on SGLT-2I in the treatment of type 2 diabetes (T2DM) to observe the changes of CRP in patients with T2DM. We searched 4 electronic databases (CNKI, PubMed, EMBASE, and Cochrane Library) for articles published up to December 31, 2021. Studies were analyzed using a random-effects model to obtain standard deviation mean differences (SMDs) and 95% confidence intervals (CIs). Sensitivity and subgroup analyses were performed. Publication bias was evaluated using funnel plots and Egger test.
Results:
We included data from 927 patients in 13 confirmatory trials that showed a significant decrease in CRP among patients with T2DM treated with SGLT-2I. The decrease was more significant with than without SGLT-2I. In subgroup analysis according to nationality, medication, and comorbidities, CRP reduction was associated with nationality, SGLT-2I type, and the presence of comorbidities. Sensitivity analysis showed that our results were reliable and found no evidence of substantial publication bias.
Conclusions:
SGLT-2I could reduce CRP levels in patients with T2DM.
Registration:
International Prospective Register for Systematic Reviews (PROSPERO) number CRD42021268079.
Keywords: C-reactive protein, meta-analysis, sodium-dependent glucose transporter 2 inhibitor, type 2 diabetes
1. Introduction
The prevalence of type 2 diabetes (T2DM) has reached epidemic levels. It is estimated that >400 million people worldwide are affected by the disease, and the incidence is expected to continue to rise.[1] T2DM and its complications not only increase the mortality and disability burdens worldwide, affecting the life span of patients, these also impose a heavy medical burden on families and society and adversely affect economic development.[2]
A large number of cross-sectional and experimental data suggest that C-reactive protein (CRP), a sensitive biomarker of subclinical systemic inflammation, is associated with hyperglycemia, insulin resistance, and overt T2DM.[3] CRP can induce other proinflammatory factors and local or systemic inflammation, thereby changing central insulin sensitivity, inducing insulin resistance, and destroying insulin metabolic pathways, thus promoting the occurrence of T2DM.[4] Studies have reported that CRP may be used as an independent biomarker to predict diabetes risk.[5] As a new type of hypoglycemic agent, sodium-dependent glucose transporter (SGLT)-2 inhibitors have positive effects in reducing blood sugar, weight, blood pressure, uric acid, urine protein, and improving dyslipidemia.[6] Therefore, since SGLT-2 inhibitors were approved in 2013, these agents have attracted the attention of multidisciplinary experts such as those in endocrinology, cardiology, and nephrology.[6] A large number of animal experiments and cell experiments have found that SGLT-2 inhibitors can reduce postprandial hyperglycemia, lower plasma insulin and blood uric acid levels, increase β-hydroxybutyrate levels, activate adenosine monophosphate-activated protein kinase, and reduce oxidative stress as well as inhibit the advanced glycation end product (AGE)–receptor for AGE axis and other mechanisms to reduce inflammation.[7]
Relevant human clinical data regarding SGLT-2 inhibitors are still relatively scarce, and some clinical findings are somewhat contradictory. Therefore, we reviewed the data published in recent years and conducted a meta-analysis to determine whether SGLT-2 inhibitors can improve T2DM by lowering CRP levels, providing more evidence for the clinical treatment of T2DM and improvement of complications.
2. Methods
2.1. Search strategy and selection criteria
This systematic review of previous systematic reviews with meta-analysis is registered in the International Prospective Register of Systematic Reviews (PROSPERO) trial registry (CRD42021268079). All analyses were based on previous published studies, thus no ethical approval and patient consent are required.
We used the following terms and keywords: diabetes, T2DM, sodium-dependent glucose transporter 2 inhibitors, and C-reactive protein to search 4 electronic databases (CNKI, PubMed, EMBASE, and the Cochrane Library) up to December 31, 2021. Two independent reviewers performed data extraction on the aggregated research data. The literature search process was repeated by other researchers to reduce errors. The literature search process was repeated by other researchers to reduce errors. Additionally, reference lists from the included studies were also manually scanned for additional articles.
We divided patients with T2DM into an SGLT-2 inhibitor group and a placebo group. By observing changes in the CRP levels, we could infer the influence of SGLT-2 inhibitors on the inflammation index.
2.1.1. Inclusion and exclusion criteria
The inclusion criteria were as follows: the study population was clearly diagnosed with T2DM; the intervention group was treated with SGLT-2 inhibitors; the study results included data on changes in CRP levels before and after treatment in patients with T2DM; the included studies provided accurate medication information and the number of participants; there was no direct association among studies; and study design: cohort or case-control study.
The exclusion criteria were as follows: studies were excluded if they used data from a previously published study; the literature data were incomplete, or the original extracted data were insufficient to conduct statistical analysis; case reports and observational studies with no comparisons before and after treatment; no relevant data for CRP before and after SGLT-2 inhibitor treatment; and animal studies.
2.1.1. Data extraction
Different researchers independently reviewed the literature, extracted the required data, and cross-checked the results. Disputes were resolved through mutual consultation and third-party evaluation. We conducted Kappa test on the screening results of the 2 researchers who independently reviewed the literature, and Kappa value = 0.867 was obtained, proving that the results had strong consistency. In addition, other researchers conducted repeated analysis to exclude subjective factors. Data including first author, publication year, research type, research area, sample size, average CRP level, and standard difference were extracted from the articles eventually included in the meta-analysis, and a data extraction table was created.
2.2. Statistical analysis
We sorted the extracted data, established a database, and checked the data carefully. We used Stata/SE15.0 software for statistical analysis (StataCorp LLC, College Station, TX). The selected studies were quantitatively analyzed using standard mean differences (SMDs) and 95% confidence intervals (CIs). The heterogeneity between studies was tested using I2. When I2 ≤50%, the heterogeneity was considered not statistically significant and was analyzed using a fixed-effects model. When I2 >50%, the heterogeneity was considered statistically significant and was analyzed using a random-effects model. Sensitivity analysis was conducted on the data, and the included data were removed one by one and reanalyzed to compare the effect values before and after the elimination to ensure stability of the meta-analysis results. We explored the causes of heterogeneity in subgroup analysis of the factors that may lead to heterogeneity. Evaluation of publication bias was conducted using funnel plots and the Egger test. We set P < .05 to indicate statistical significance, indicating that publication bias could not be excluded.
3. Results
3.1. Data collection
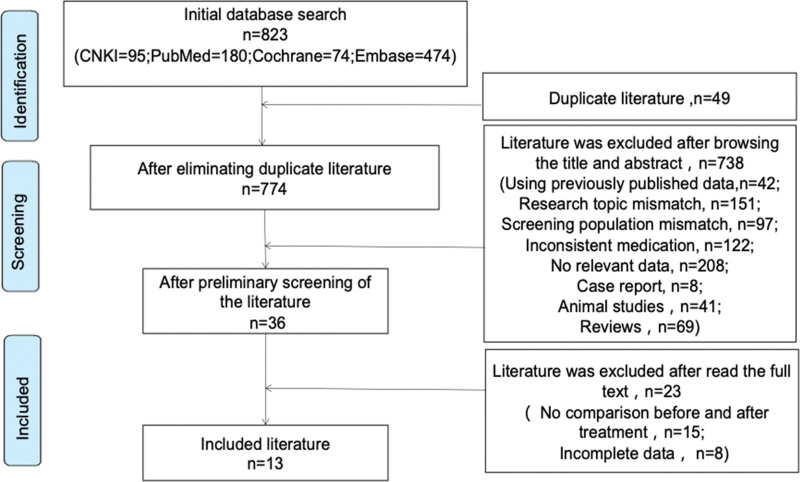
We initially searched 823 related studies using our search terms and keywords; 13 studies finally met the inclusion and exclusion criteria (Fig. 1). Figure 1 outlines the search and selection process. Of the 13 included studies[8–20] (Table 1), 8 were case-control experimental studies and 5 were prospective experimental studies evaluating the changes in CRP before and after treatment with SGLT-2 inhibitors among patients with T2DM (Table 2); these studies were published from 2016 to 2021 and included data for a total of 927 patients. The quality of studies was evaluated with Newcastle-Ottawa Quality Assessment Scale, as shown in Table 3. Among the total patients, 519 were of Chinese nationality and 408 were of non-Chinese nationality. Among the total, 519 patients received SGLT-2 inhibitor treatment (SGLT-2 inhibitors used included dapagliflozin, empagliflozin, luseogliflozin, and canagliflozin) and 408 patients received placebo treatment. Eleven studies calculated the change in CRP levels after 3 months of SGLT-2 inhibitor treatment and 5 studies calculated the change in CRP after 6 months of SGLT-2 inhibitor treatment; 3 studies measured changes in CRP levels after 12 months of SGLT-2 inhibitor treatment.
Figure 1.
Literature screening process and results.
Table 1.
Basic characteristics of the included studies.
| Number | Author | Year | Country | Research type | Medication |
|---|---|---|---|---|---|
| 1 | Zhenfei Ou | 2021 | China | Case-control | Dapagliflozin |
| 2 | Nur Aisyah Zainordin | 2019 | Malaysia | Case-control | Dapagliflozin |
| 3 | Xiaoqing Mo | 2019 | China | Case-control | Dapagliflozin |
| 4 | Sachiko Hattori | 2018 | Japan | Case-control | Engligliflozin |
| 5 | Xiaoying Xia | 2020 | China | Case-control | Dapagliflozin |
| 6 | Daxiang Huang | 2020 | China | Case-control | Dapagliflozin |
| 7 | Wenjun Zhang | 2021 | China | Case-control | Engligliflozin |
| 8 | Nedogoda S.V | 2021 | Russia | Case-control | Engligliflozin |
| 9 | Aki Okamoto | 2016 | Japan | Prospective cohort | Dapagliflozin |
| 10 | Ryotaro Bouchi | 2017 | Japan | Prospective cohort | Luseogliflozin |
| 11 | Hiroshi Tobita | 2017 | Japan | Prospective cohort | Dapagliflozin |
| 12 | Akira Sezai | 2019 | Japan | Prospective cohort | Canagliflozin |
| 13 | Takeshi Osono | 2018 | Japan | Prospective cohort | Canagliflozin |
Table 2.
Results of C-reactive protein before and after the treatment.
| Number | C-reactive protein | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| before treatment | After 3 months of treatment | After 6 months of treatment | After 12 months of treatment | |||||||||||||||||||||
| Test group | Control group | Test group | Control group | Test group | Control group | Test group | Control group | |||||||||||||||||
| n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | |
| 1 | 89 | 3.4057 | 1.1303 | 94 | 3.4648 | 0.9788 | 89 | 2.0409 | 0.7536 | 94 | 2.8352 | 0.6776 | 89 | 1.6704 | 0.6029 | 94 | 2.3352 | 0.6776 | ||||||
| 2 | 36 | 1.93 | 0.6072 | 36 | 2.7 | 0.3146 | 36 | 6.03 | 1.0745 | 36 | 2.96 | 0.5132 | ||||||||||||
| 3 | 30 | 29.58 | 5.37 | 30 | 29.16 | 5.52 | 30 | 10.39 | 3.54 | 30 | 19.82 | 4.46 | ||||||||||||
| 4 | 51 | 1.33 | 1 | 51 | 1.46 | 1.4 | 51 | 1.13 | 0.73 | 51 | 1.43 | 1.56 | 51 | 0.92 | 0.68 | 51 | 1.33 | 1 | 51 | 0.59 | 0.42 | 51 | 1.71 | 1.64 |
| 5 | 40 | 26.26 | 0.56 | 40 | 26.15 | 0.62 | 40 | 24.21 | 0.34 | 38 | 25.64 | 0.48 | ||||||||||||
| 6 | 57 | 8.79 | 0.22 | 57 | 8.62 | 0.29 | 57 | 5.17 | 0.34 | 57 | 8.79 | 0.22 | ||||||||||||
| 7 | 42 | 3.04 | 0.96 | 42 | 2.93 | 0.86 | 42 | 1.8 | 0.6 | 42 | 2.21 | 0.75 | ||||||||||||
| 8 | 61 | 3.1 | 1.2 | 60 | 3.2 | 0.9 | 61 | 1.5 | 0.8 | 61 | 3.1 | 1.2 | ||||||||||||
| 9 | 27 | 2.41 | 2.814 | 27 | 1.607 | 1.96 | ||||||||||||||||||
| 10 | 20 | -0.01 | 0.45 | 20 | -0.1 | 0.33 | ||||||||||||||||||
| 11 | 11 | 3.0413 | 3.5626 | 11 | 2.3825 | 3.5626 | 11 | 1.678 | 2.2902 | |||||||||||||||
| 12 | 35 | 3.9 | 0.7 | 35 | 2 | 0.5 | 35 | 2.1 | 0.4 | 35 | 2.1 | 0.4 | ||||||||||||
| 13 | 20 | 0.9 | 1.3 | 20 | 1.1 | 1.6 | ||||||||||||||||||
Table 3.
Study quality evaluation with Newcastle-Ottawa quality assessment scale.
| Case-control studies | |||||
|---|---|---|---|---|---|
| Reference | Selection | Comparability | Exposure | ||
| Zhenfei Ou 2021 | ★★ | ★★ | ★★★ | ||
| Nur Aisyah Zainordin 2019 | ★ | ★★ | ★★★ | ||
| Xiaoqing Mo 2019 | ★★ | ★★ | ★★★ | ||
| Sachiko Hattori 2018 | ★★ | ★★★ | |||
| Xiaoying Xia 2020 | ★★ | ★★ | ★★★ | ||
| Daxiang Huang 2020 | ★★ | ★★ | ★★★ | ||
| Wenjun Zhang 2021 | ★★ | ★★ | ★★★ | ||
| Nedogoda S.V 2020 | ★ | ★★★ | |||
| Cohort studies | |||||
| Reference | Selection | Comparability | Outcome | ||
| Aki Okamoto 2016 | ★★ | ★★ | ★★★ | ||
| Ryotaro Bouchi 2017 | ★ | ★★ | ★★★ | ||
| Hiroshi Tobita 2017 | ★ | ★ | ★★★ | ||
| Akira Sezai 2019 | ★★ | ★ | ★★★ | ||
| Takeshi Osonoi 2018 | ★★ | ★ | ★★★ | ||
3.2. Meta-analysis of CRP changes in patients with t2dm
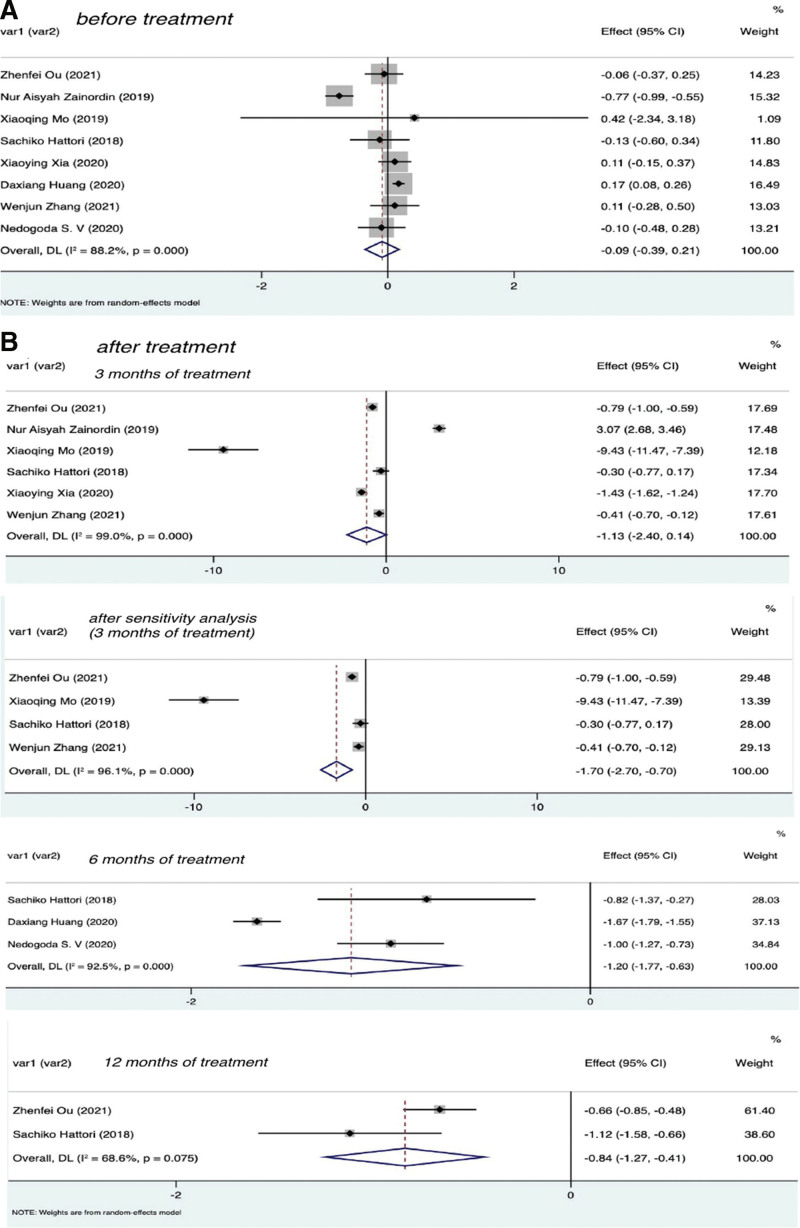
To ensure the validity of our results, before subsequent meta-analysis, the CRP values of the experimental group and control group before treatment were tested for consistency at baseline in 8 selected studies. The results are shown in Figure 2A. As can be seen from the figure, the effect of CRP before treatment was not statistically significant in the experimental and control groups, that is, there was no difference in CRP levels between the 2 groups before treatment; therefore, meta-analysis could be performed.
Figure 2.
Forest plot of CRP in experimental group and control group before and after SGLT-2I treatment. CRP = C-reactive protein, SGLT-2I = sodium-dependent glucose transporter 2 inhibitors.
3.2.1. Effect of SGLT-2 inhibitors on CRP in patients with t2dm
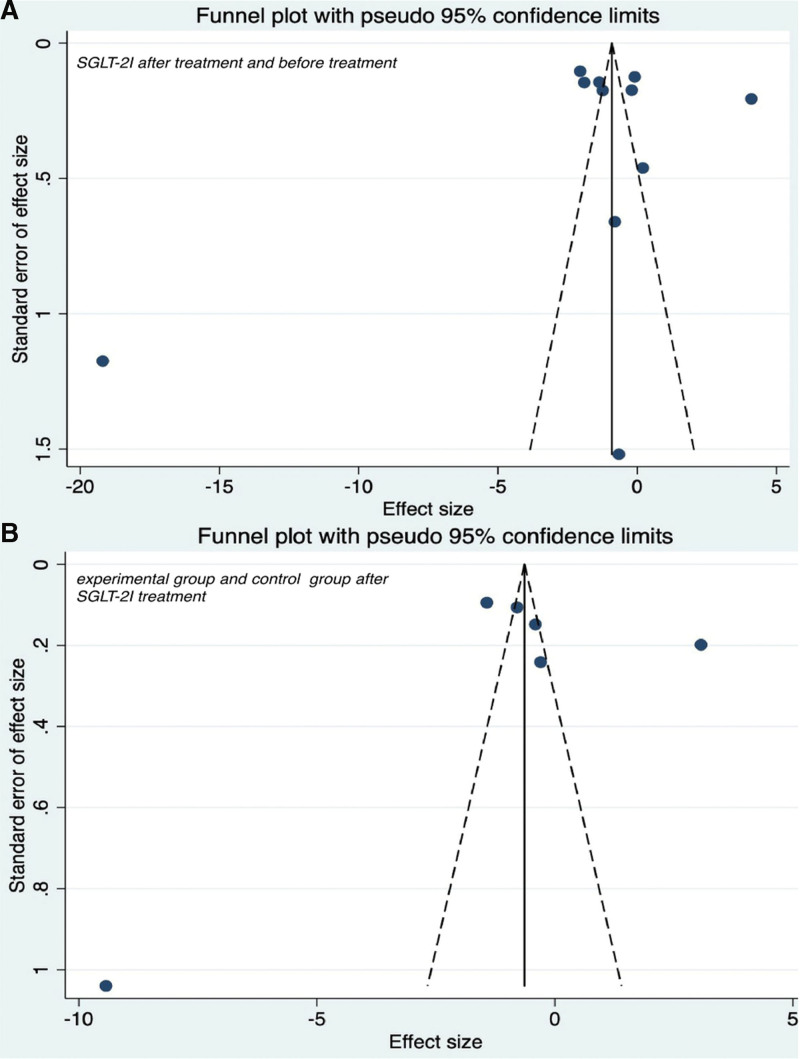
To evaluate the effect of SGLT-2 inhibitors on inflammation in the T2DM group, the data of patients treated for 3, 6, and 12 months were extracted, as shown in Figure 3B. The results were as follows after 3 months of treatment I2 = 99.1% with P < .01, Z = −2.896 with P = .004, SMD = −1.74, 95% CI: −2.92 to −0.56; after 6 months of treatment I2 = 99.2% with P < .01, Z = −2.409 with P = .016, SMD = −1.80, 95% CI: −3.26 to −0.33; after 12 months of treatment I2 = 93.9% with P < .01, Z = −4.330 with P < .01, SMD = −1.43, 95% CI: −2.07 to −0.78. These findings indicated that patients with T2DM who were treated with SGLT-2 inhibitors showed significant differences in CRP levels after 3, 6, and 12 months compared with before treatment. Therefore, the use of SGLT-2 inhibitors was associated with a decrease in CRP. The Egger test (P = .965) showed no significant publication bias. See Figure 4A for funnel diagram.
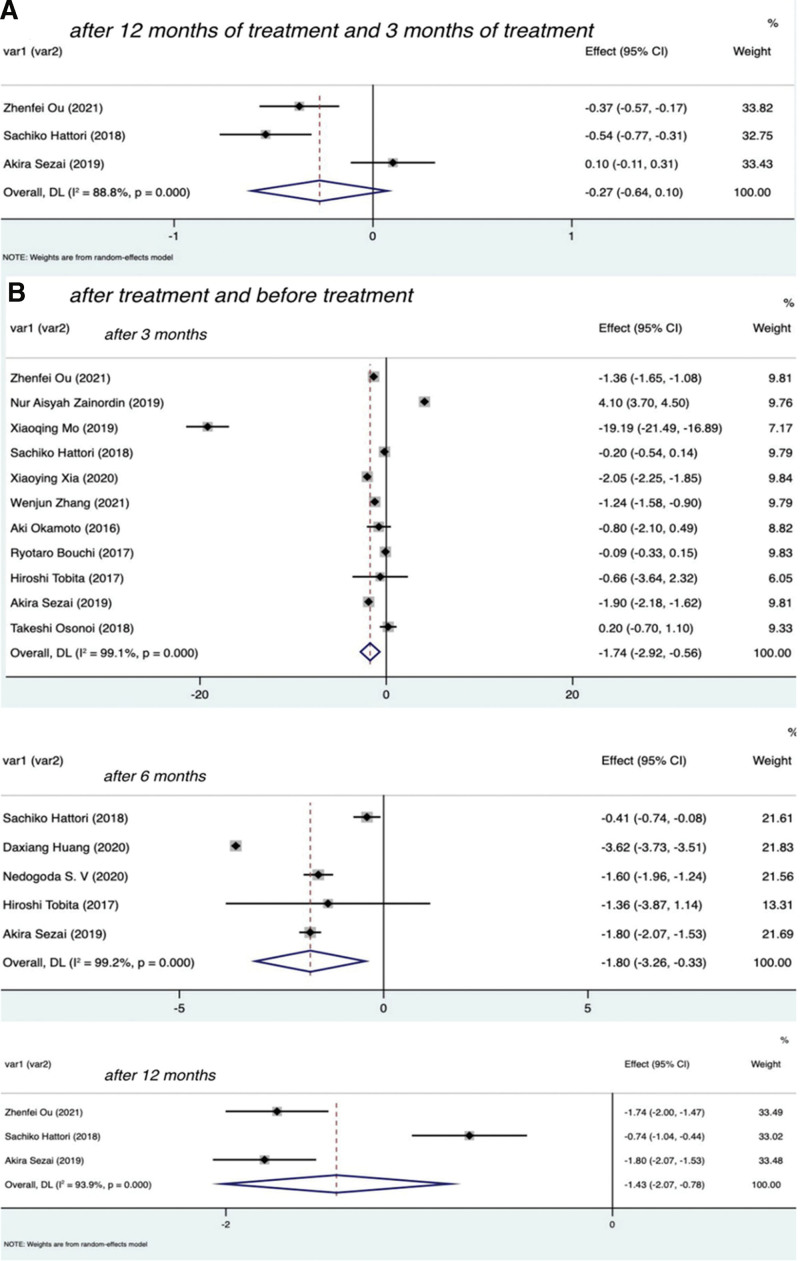
Figure 3.
Forest plot of CRP changes at different time of SGLT-2I treatment. CRP = C-reactive protein, SGLT-2I = sodium-dependent glucose transporter 2 inhibitors.
Figure 4.
Funnel plot of CRP changes after SGLT-2I treatment. CRP = C-reactive protein, SGLT-2I = sodium-dependent glucose transporter 2 inhibitors.
3.2.2. Effect of SGLT-2 inhibitors on CRP in patients with t2dm compared with placebo
To compare the effects of SGLT-2 inhibitors and placebo on inflammation in patients with T2DM, we extracted patient data of the 2 groups at 3, 6, and 12 months after treatment, as shown in Figure 2B. The results were as follows: after 3 months of treatment I2 = 99.0% with P < .01, Z = −1.746 with P = .081, SMD = −1.13, 95% CI: −2.40 to 0.14; after 6 months of treatment I2 = 92.5% with P < .01, Z = −4.120 with P < .01, SMD = −1.20, 95% CI: −1.77 to −0.63; after 12 months of treatment I2 = 68.6% with P = .075, Z = −3.793 with P < .01, SMD = −0.84, 95% CI: −1.27 to −0.41. There was no statistically significant difference in CRP levels between the SGLT-2 inhibitors group and the placebo group at 3 months. In sensitivity analysis, the results showed that SGLT-2i were statistically significant compared with placebo at 3 months (I2 = 96.1% with P < .01; Z = −3.32 with P < .01, SMD = −1.70, 95% CI: −2.70 to −0.70; Fig. 2B). The Egger test (P = .804) showed no significant publication bias. See Figure 4B for funnel diagram. There was a significant difference between the 2 groups at 6 and 12 months. Therefore, the use of SGLT-2I was associated with a significant decrease in CRP compared with no SGLT-2 inhibitors.
3.2.3. Relationship between duration of t2dm treatment with SGLT-2 inhibitors and changes in CRP
To compare the effect of SGLT-2 inhibitors on inflammation in patients with T2DM at different time, the data of patients at 12 and 3 months after treatment were extracted, as shown in Figure 3A. The results were I2 = 88.8% with P < .01, Z = −1.424 with P = .154, SMD = −0.27, 95% CI: −0.64 to −0.10. These findings showed that there was no significant difference in CRP levels among patients with T2DM after 12 and 3 months of SGLT-2 inhibitors treatment.
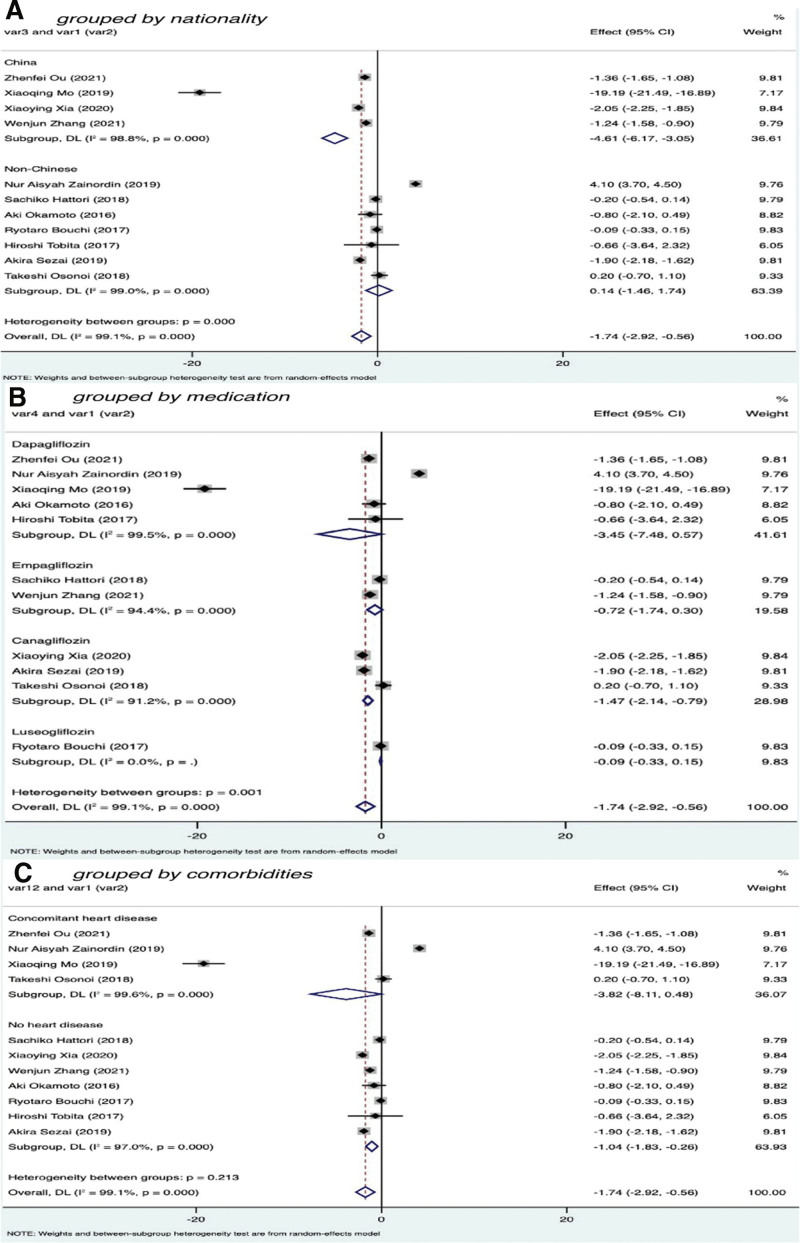
3.2.4. Subgroup analysis
To further improve the reliability of the research and analyze the sources of heterogeneity, we conducted subgroup analysis according to nationality, medication status, and comorbidities in patients with T2DM treated with SGLT-2 inhibitors for 3 months.
The nationality of patients with T2DM was divided into 2 subgroups: Chinese and non-Chinese. Among the total, 4 studies included 201 Chinese participants (I2 = 98.8% with P < .01, Z = −5.787 with P < .01, SMD = −4.61, 95% CI: −6.17 to −3.05), and the difference was statistically significant. Seven studies included 200 non-Chinese participants (I2 = 99.0% with P < .01, Z = 0.172 with P = .864, SMD = 0.14, 95% CI: −1.46 to 1.74); the difference is not statistically significant. These findings showed that the effect of SGLT-2I on reducing CRP levels was more obvious in Chinese than non-Chinese patients (Fig. 5A).
Figure 5.
Subgroup analysis of C-reactive protein after 3 months of treatment:
We divided the different drugs used in the experimental groups into 4 subgroups: dapagliflozin, empagliflozin, canagliflozin, and luseogliflozin. Five studies included 193 patients with T2DM using dapagliflozin (I2 = 99.5% with P < .01, Z = −1.683 with P = .092, SMD = −3.45, 95% CI: −7.48 to 0.57), and the difference was not statistically significant. Two studies included 93 patients with T2DM who were taking empagliflozin (I2 = 94.4% with P < .01, Z = −1.384 with P = .166, SMD = −0.72, 95% CI: −1.74 to 0.30), and the difference was not significant. Three studies included 95 patients with T2DM using canagliflozin (I2 = 91.2% with P < .01, Z = −4.235 with P < .01, SMD = −1.47, 95% CI: −2.14 to −0.79), and the difference was statistically significant. One study involved 20 patients with T2DM using luseogliflozin (I2 < 50% with P < .01, Z = −0.721 with P = .471, SMD = −0.09, 95% CI: −0.33 to 0.15); the difference was not significant. These results indicated that CRP values decreased more significantly after treatment of patients with T2DM using canagliflozin (Fig. 5B).
We divided the included studies into 2 subgroups according to whether the included patients with T2DM had heart disease. Four studies included 175 patients with T2DM who had heart disease; the heterogeneity testing results were I2 = 99.6% with P < .01, Z = −1.741 with P = .082, SMD = −3.82, 95% CI: −8.11 to 0.48; the difference was not significant. Seven studies included 226 patients with T2DM who did not have heart disease; the results of heterogeneity tests were I2 = 97% with P < .01, Z = −2.606 with P = .009, SMD = −1.04, 95% CI: −1.83 to −0.26, and the difference was significant. These results suggest that SGLT-2I have a more significant effect on reducing CRP in T2DM patients who do not have comorbidities (Fig. 5C).
4. Discussion
The results of this meta-analysis showed that the use of SGLT-2 inhibitors to treat T2DM can significantly reduce CRP levels and the reduction in CRP is more significant with treatment than without. However, studies have found that there is no significant difference between the effect of SGLT-2 inhibitors in reducing CRP and the length of treatment. A subgroup analysis of patients’ nationality, medication status, and comorbidities showed that the reduction effect of SGLT-2 inhibitor on CRP was different in different nationalities. Additionally, different types of drugs have different effects. Compared with dapagliflozin, empagliflozin, and luseogliflozin, the effect of canagliflozin in reducing CRP was statistically significant. Moreover, patients with T2DM who did not have heart disease had a significantly greater reduction in CRP levels than those with heart disease. Therefore, the effect of SGLT-2 inhibitors may be affected by multiple factors such as nationality, comorbidities, and type of medication.
The plasma CRP level is a sensitive indicator of systemic inflammatory responses and provides a sensitive and quantitative indicator to evaluate global inflammatory activity.[21] Some studies have found that CRP may be an important additional factor in the pathogenesis of insulin resistance, glucose intolerance, and T2DM.[22] CRP amplifies the inflammatory response by stimulating tissue macrophages to produce TNF-α and IL-1.[21] CRP can reduce the expression and activity of endothelial nitric oxide synthase by inducing the production of inflammatory cytokines in monocytes and promoting the expression of monocyte chemokines and tissue factors, increasing the expression of cell adhesion molecules, chemokines, and endothelin-1 in endothelial cells, enhancing monocyte–endothelial cell adhesion in a proinflammatory role.[23] Some studies have found that insulin has a selective effect on liver protein synthesis. When insulin sensitivity is reduced, the body can increase CRP expression by counteracting the physiological effect of insulin on liver protein synthesis in the acute phase.[24]
The inflammatory process is involved in the pathogenesis of T2DM and the development of related complications such as cardiovascular, kidney, and ophthalmological complications.[25] Insulin resistance plays an important role in the etiology and pathogenesis of T2DM, and studies have found that low levels of tissue-specific inflammation induced by various proinflammatory and/or oxidative stress mediators are closely related to the occurrence of insulin resistance, especially proinflammatory factors.[4] Insulin itself has powerful acute anti-inflammatory effects, including inhibition of cytokine-mediated acute phase protein gene expression and reduction of nuclear factor κB (NF-κB), reactive oxygen species (ROS), plasma intercellular adhesion molecule-1, monocyte chemotaxis protein-1, and plasminogen activator inhibitor-1 (PAI-1); thus, insulin resistance increases levels of inflammatory markers by attenuating these effects.[22] Additionally, insulin resistance is associated with adipose tissue activation, increased release of inflammatory cytokines such as tumor necrosis factor (TNF)-α and interleukin (IL)-6, decreased production of anti-inflammatory cytokine IL-10 by macrophages and lymphocytes, PAI-1, CRP, and monocyte activation.[26] Proinflammatory cytokines and acute phase reactants are involved in various metabolic pathways associated with insulin resistance, including insulin regulation, ROS, lipoprotein lipase action, and adipocyte function.[27] Proinflammatory cytokines may promote insulin resistance in autocrine and paracrine pathways by interfering with insulin signaling in peripheral tissues via activation of the c-Jun N-terminal kinase and NF-κB pathways or induction of β-cell dysfunction and subsequent insulin deficiency by interfering with insulin signaling in peripheral tissues.[28] Insulin resistance has also been found to be accompanied by accumulation of muscle triglycerides and impaired mitochondrial activity.[29] These can all contribute to the progression of diabetes.
Numerous observational studies have shown that CRP levels are increased in patients with T2DM, and CRP is a risk factor for cardiovascular and renal diseases in both patients with diabetes and the general population.[30,31] These studies confirm that CRP contributes to the risk assessment of T2DM and its associated vascular complications. A recent prospective study reconfirmed CRP as a determinant of T2DM risk, particularly after adjusting for obesity, clinical risk factors, and fasting insulin levels, and found CRP to be a strong independent predictor.[32] Therefore, the reduction of CRP level is helpful to the occurrence of T2DM and its related vascular complications, and has important guiding significance for the assessment and prevention of the risk of T2DM and its vascular complications in clinical practice. As one of the clinically common inflammation index, CRP reflects the inflammation and insulin resistance, compared to several other biomarkers of its measuring of low cost, good standardization, there are quite a longer plasma half-life, fairly low subjects within the variation coefficient, which may provide a more stable for subclinical inflammation of indications, to provide a possible auxiliary method for the early detection of risk in patients with diabetes.[33]
The focus of blood glucose intervention using traditional hypoglycemic drugs has been to restore β-cell activity, insulin sensitivity, or tissue glucose uptake so as to restore blood sugar levels to normal.[34] As a new type of hypoglycemic drugs, SGLT-2 inhibitors effectively inhibit the activity of SGLT-2 in the renal proximal tubules by competitively binding with glucose transporters, reduce the reabsorption of glucose by renal tubular epithelial cells, promote the excretion of glucose in urine, improve β cell function and insulin sensitivity, and play a role in lowering blood glucose.[35–37]
A large number of studies have confirmed that oxidative stress and inflammation are closely related and interdependent. The active substance hydrogen peroxide can induce inflammation by activating transcription factor NF-B, and oxidative stress has an important role in the activation of NOD-like receptor protein 3 inflammasome.[38] Recent studies have found that SGLT-2 inhibitors are effective antioxidants that can protect tissues from oxidative damage, not only indirectly through their hypoglycemic effect but also directly by reducing the generation of free radicals or enhancing the antioxidant capacity of cells. SGLT-2 inhibitors stimulate the activity of SIRT1, a major sensor of glucose consumption, and upregulate peroxisome proliferator-activated receptor-γ coactivater-1 α, a downstream target of SIRT1 and a major regulator of mitochondrial biogenesis.[39] SGLT-2 inhibits oxidative stress by modifying the activity of preoxidases (such as NOX, endothelial nitric oxide synthase, and xanthine oxidase), prevents mitochondrial dysfunction by improving the redox state in the brain, reduces the generation of AGEs by lowering blood glucose, and thus reduces the production of free radicals.[40] It has been reported that SGLT-2 can also reduce oxidative stress and inflammation by inhibiting RAS activation.[41] Some animal studies have found that SGLT-2 can reduce oxidative stress by inhibiting ROS production and NADPH (reduced form of nicotinamide-adenine dinucleotide phosphate) oxidase 4 expression and can also reduce inflammation by inhibiting preinflammatory macrophage infiltration.[42]
Meta-analysis is a secondary literature analysis of previous research evidence, which has unavoidable limitations and biases. Previous studies have confirmed that age, smoking, blood lipid level, cardiovascular disease, and body mass index are significantly positively related to CRP.[43] In this study, a subgroup analysis of nationality, medication and comorbidities of the included patients showed that CRP value after SGLT-2 inhibitor treatment was associated with the patient’s nationality, the presence or absence of cardiovascular disease, and the type of drug use. However, other characteristics that may influence CRP levels such as diet status, smoking status, drinking status, treatment status, BMI, lipid level, age, and sex were not analyzed because they were not clearly available in the original literature. Moreover, we included case-control studies, which are inevitably affected by selection bias. A limitation of this analysis is the small number of patients included. Additionally, the number of patients using different drugs varied among studies, and there may be deviations. Other limitations include the different observation times for selected patients and fewer observations for long-term patients.
In summary, the results of this meta-analysis showed that SGLT-2 inhibitors are related to the reduction of CRP and have a certain degree of reliability. Although some limitations affect the accuracy of our results, it can still be considered that the role of SGLT-2 inhibitors in reducing inflammation provides a new target for the treatment of T2DM as well as the prevention and treatment of its complications and other chronic diseases. Further studies are needed to confirm the current results.
5. Conclusion
With the deepening of studies on SGLT-2 inhibitors, although animal experiments have found that SGLT-2 inhibitors can reduce inflammatory response, relevant human clinical data are few and controversial. Therefore, we conducted a meta-analysis of the data published in recent years, and concluded that the use of SGLT-2 inhibitors to treat patients with T2DM can reduce CRP levels, and compared with placebo, CRP levels are reduced more. The CRP reduction effect may not be related to the length of time. The effects of SGLT-2 inhibitors may be affected by many factors, such as nationality, comorbidities, and drug types. These findings expand the understanding of the mechanism of SGLT-2 inhibitors, provide further guidance for the treatment of diabetes mellitus and its related complications, and contribute to the further exploration of the treatment course, drug type, and drug population of SGLT-2 inhibitors.
Acknowledgments
We thank Liwen Bianji (Edanz) (www.liwenbianji.cn) for editing the language of a draft of this manuscript.
Abbreviations:
- AGE =
- advanced glycation end product
- CIs =
- 95% confidence intervals
- CRP =
- C-reactive protein
- IL =
- interleukin
- NF-κB =
- nuclear factor κB
- PAI-1 =
- plasminogen activator inhibitor-1
- PROSPERO =
- International prospective register for systematic reviews
- ROS =
- reactive oxygen species
- SGLT-2I =
- sodium-dependent glucose transporter 2 inhibitors
- SMDs =
- standard deviation mean differences
- T2DM =
- type 2 diabetes
- TNF =
- tumor necrosis factor
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
H.M. and L.Y. contributed equally to this article.
How to cite this article: Sun W, Xing Y, Kong D, Zhang Z, Ma H, Yang L. Meta-analysis of the effect of sodium-dependent glucose transporter 2 inhibitors on C-reactive protein in type 2 diabetes. Medicine 2022;101:38(e30553).
The authors have no funding and conflicts of interest to disclose.
Contributor Information
Wenwen Sun, Email: sunwttd@163.com.
Yuling Xing, Email: xingyl95@163.com.
Dexian Kong, Email: 2033918803@qq.com.
Zhimin Zhang, Email: 1215707816@qq.com.
Linlin Yang, Email: ll.yang@foxmail.com.
References
- [1].Javeed N, Matveyenko AV. Circadian etiology of type 2 diabetes mellitus. Physiology. 2018;33:138–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14:88–98. [DOI] [PubMed] [Google Scholar]
- [3].Pradhan AD. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–34. [DOI] [PubMed] [Google Scholar]
- [4].Rehman K, Akash MSH. Mechanisms of inflammatory responses and development of insulin resistance: how are they interlinked? J Biomed Sci. 2016;23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jafaripour S, Sedighi S, Jokar MH, et al. Inflammation, diet, and type 2 diabetes: a mini-review. J Immunoass Immunochem. 2020;41:768–77. [DOI] [PubMed] [Google Scholar]
- [6].Scheen AJ. Sodium–glucose cotransporter type 2 inhibitors for the treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2020;16:556–77. [DOI] [PubMed] [Google Scholar]
- [7].Bonnet F, Scheen AJ. Effects of SGLT2 inhibitors on systemic and tissue low-grade inflammation: The potential contribution to diabetes complications and cardiovascular disease. Diabetes Metab. 2018;44:457–64. [DOI] [PubMed] [Google Scholar]
- [8].Huang D, Li S, Wu F, et al. The efficacy of cagliazin in the treatment of overweight or obese elderly patients with type 2 diabetes and its effect on inflammatory indicators. Guide China Med. 2020;28:251–5. [Google Scholar]
- [9].Ou Z, Yu T, Guo X, et al. Clinical efficacy evaluation of daglizin in elderly female patients with heart failure complicated with type 2 diabetes mellitus with preserved ejection fraction. Chin J Geriatr Heart Brain Vessel Dis. 2021;23:387–90. [Google Scholar]
- [10].Zainordin NA, Hatta S, Mohamed Shah FZ, et al. Effects of dapagliflozin on endothelial dysfunction in type 2 diabetes with established ischemic heart disease (EDIFIED). J Endocr Soc. 2020;4:bvz017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mo X, Huang C, Zhao Y, et al. Effects of dagaglizin on serum hs-CRP, adiponectin and insulin levels in patients with type 2 diabetes mellitus complicated with coronary heart disease. China Med Phar. 2019;9:193–6. [Google Scholar]
- [12].Hattori S. Anti-inflammatory effects of empagliflozin in patients with type 2 diabetes and insulin resistance. Diabetol Metab Syndr. 2018;10:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Xia X, Li S, Sun S, et al. The efficacy of cagliazin in the treatment of overweight or obese elderly patients with type 2 diabetes and its effect on inflammatory indicators. Guide China Med. 2020;18:57–9. [Google Scholar]
- [14].Zhang W, Sun W, Hu S, et al. Clinical efficacy of engliazin, liraglutide and metformin in the treatment of new-onset type 2 diabetes mellitus complicated with non-alcoholic fatty liver disease and its effect on serum inflammatory factors, D-dimer and liver function. J Clin Exp Med. 2021;20:834–8. [Google Scholar]
- [15].Okamoto A, Yokokawa H, Sanada H, et al. Changes in levels of biomarkers associated with adipocyte function and insulin and glucagon kinetics during treatment with dapagliflozin among obese type 2 diabetes mellitus patients. Drugs R D. 2016;16:255–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Nedogoda SV, Barykina IN, Salasyuk AS, et al. The effect of various classes of glucose-lowering medications on the blood vessel elasticity in patients with type 2 diabetes. Russ J Cardiol. 2020;25:65–71. [Google Scholar]
- [17].Bouchi R, Terashima M, Sasahara Y, et al. Luseogliflozin reduces epicardial fat accumulation in patients with type 2 diabetes: a pilot study. Cardiovasc Diabetol. 2017;16:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Tobita H, Sato S, Miyake T, Ishihara S, et al. Effects of dapagliflozin on body composition and liver tests in patients with nonalcoholic steatohepatitis associated with type 2 diabetes mellitus: a prospective, open-label, uncontrolled study. Curr Ther Res Clin Exp. 2017;87:13–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sezai A, Sekino H, Unosawa S, et al. Canagliflozin for Japanese patients with chronic heart failure and type II diabetes. Cardiovasc Diabetol. 2019;18:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Osonoi T, Gouda M, Kubo M, et al. Effect of canagliflozin on urinary albumin excretion in Japanese patients with type 2 diabetes mellitus and microalbuminuria: a pilot study. Diabetes Technol Ther. 2018;20:681–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Nesto R. C-reactive protein, its role in inflammation, Type 2 diabetes and cardiovascular disease, and the effects of insulin-sensitizing treatment with thiazolidinediones. Diabet Med. 2004;21:810–7. [DOI] [PubMed] [Google Scholar]
- [22].Greenfield JR, Campbell LV. Relationship between inflammation, insulin resistance and type 2 diabetes: “cause or effect”? Curr Diabetes Rev. 2006;2:195–211. [DOI] [PubMed] [Google Scholar]
- [23].Devaraj S, Xu DY, Jialal I. C-reactive protein increases plasminogen activator inhibitor-1 expression and activity in human aortic endothelial cells: implications for the metabolic syndrome and atherothrombosis. Circulation. 2003;107:398–404. [DOI] [PubMed] [Google Scholar]
- [24].Festa A, D’Agostino R, Jr, Howard G, et al. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS). Circulation. 2000;102:42–7. [DOI] [PubMed] [Google Scholar]
- [25].Donath MY. Multiple benefits of targeting inflammation in the treatment of type 2 diabetes. Diabetologia. 2016;59:679–82. [DOI] [PubMed] [Google Scholar]
- [26].Cruz NG, Sousa LP, Sousa MO, et al. The linkage between inflammation and type 2 diabetes mellitus. Diabetes Res Clin Pract. 2013;99:85–92. [DOI] [PubMed] [Google Scholar]
- [27].Navarro JF, Mora C. Role of inflammation in diabetic complications. Nephrol Dial Transplant. 2005;20:2601–4. [DOI] [PubMed] [Google Scholar]
- [28].Esser N, Legrand-Poels S, Piette J, et al. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. 2014;105:141–50. [DOI] [PubMed] [Google Scholar]
- [29].Razak F, Anand SS. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GIN Engl J Med 2004; 350: 664-71. Vasc Med. 2004;9:223–4. [DOI] [PubMed] [Google Scholar]
- [30].Arima H, Kubo M, Yonemoto K, et al. High-sensitivity C-reactive protein and coronary heart disease in a general population of Japanese: the Hisayama study. Arterioscler Thromb Vasc Biol. 2008;28:1385–91. [DOI] [PubMed] [Google Scholar]
- [31].van der Velde M, Bello AK, Brantsma AH, et al. Do albuminuria and hs-CRP add to the International Diabetes Federation definition of the metabolic syndrome in predicting outcome? Nephrol Dial Transplant. 2012;27:2275–83. [DOI] [PubMed] [Google Scholar]
- [32].Pradhan AD, Manson JE, Rifai N, et al. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–34. [DOI] [PubMed] [Google Scholar]
- [33].Ridker PM, Wilson PW, Grundy SM. Should C-reactive protein be added to metabolic syndrome and to assessment of global cardiovascular risk? Circulation. 2004;109:2818–25. [DOI] [PubMed] [Google Scholar]
- [34].Heerspink HJ, Perkins BA, Fitchett DH, et al. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation. 2016;134:752–72. [DOI] [PubMed] [Google Scholar]
- [35].Ni L, Yuan C, Chen G, et al. SGLT2i: beyond the glucose-lowering effect. Cardiovasc Diabetol. 2020;19:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Merovci A, Solis-Herrera C, Daniele G, et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest. 2014;124:509–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ferrannini E, Muscelli E, Frascerra S, et al. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest. 2014;124:499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Peake JM, Suzuki K, Coombes JS. The influence of antioxidant supplementation on markers of inflammation and the relationship to oxidative stress after exercise. J Nutr Biochem. 2007;18:357–71. [DOI] [PubMed] [Google Scholar]
- [39].Packer M. Autophagy-dependent and -independent modulation of oxidative and organellar stress in the diabetic heart by glucose-lowering drugs. Cardiovasc Diabetol. 2020;19:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Yaribeygi H, Atkin SL, Butler AE, et al. Sodium-glucose cotransporter inhibitors and oxidative stress: an update. J Cell Physiol. 2019;234:3231–7. [DOI] [PubMed] [Google Scholar]
- [41].Shin SJ, Chung S, Kim SJ, et al. Effect of sodium-glucose co- transporter 2 inhibitor, dapagliflozin, on renal renin-angiotensin system in an animal model of type 2 diabetes. PLoS One. 2016;11:e0165703e0165703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Terami N, Ogawa D, Tachibana H, et al. Long-term treatment with the sodium glucose cotransporter 2 inhibitor, dapagliflozin, ameliorates glucose homeostasis and diabetic nephropathy in db/db mice. PLoS One. 2014;9:e100777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Koenig W, Sund M, Fröhlich M, et al. C-Reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men: results from the MONICA (Monitoring trends and determinants in cardiovascular disease) Augsburg cohort study, 1984 to 1992. Circulation. 1999;99:237–42. [DOI] [PubMed] [Google Scholar]