Abstract
The objective of this study was to evaluate whether adaptive NKG2C+CD57+ natural killer (adapNK) cell frequencies are associated with pre-clinical coronary atherosclerosis in participants of the Canadian HIV and Aging Cohort Study. This cross-sectional study included 194 Canadian HIV and Aging Cohort Study participants aged ≥ 40 years of which 128 were cytomegalovirus (CMV)+ people living with HIV (PLWH), 8 were CMV−PLWH, 37 were CMV mono-infected individuals, and 21 were neither human immunodeficiency virus nor CMV infected. Participants were evaluated for the frequency of their adapNK cells and total plaque volume (TPV). TPV was assessed using cardiac computed tomography. Participants were classified as free of, or having, coronary atherosclerosis if their TPV was “0” and ">0,” respectively. The frequency of adapNK cells was categorized as low, intermediate or high if they constituted <4.6%, between ≥4.6% and 20% and >20%, respectively, of the total frequency of CD3−CD56dim NK cells. The association between adapNK cell frequency and TPV was assessed using an adjusted Poisson regression analysis. A greater proportion of CMV+PLWH with TPV = 0 had high adapNK cell frequencies than those with TPV > 0 (61.90% vs 39.53%, P = .03) with a similar non-significant trend for CMV mono-infected participants (46.15% vs 34.78%). The frequency of adapNK cells was negatively correlated with TPV. A high frequency of adapNK cells was associated with a relative risk of 0.75 (95% confidence intervals 0.58, 0.97, P = .03) for presence of coronary atherosclerosis. This observation suggests that adapNK cells play a protective role in the development of coronary atherosclerotic plaques.
Keywords: adaptive natural killer cells, aging, atherosclerosis, cytomegalovirus, HIV, natural killer cells
1. Introduction
Antiretroviral therapy (ART) has transformed human immunodeficiency virus (HIV) infection into a treatable, chronic disease. ART stops progression to the acquired immunodeficiency syndrome in most people living with HIV (PLWH), diminishes morbidity, lengthens survival and prevents HIV transmission.[1] However, PLWH have higher levels of immune activation than uninfected individuals, leading to the development of non-AIDS comorbidities including cancer, kidney, liver and cardiovascular disease (CVD).[2] Long term ART treated PLWH have a 1.5 to 2 times higher risk of developing various manifestations of CVD.[3–5] Aside from classic risk factors for CVD, co-infection with herpesviruses may have a substantial effect on the progression of atherosclerosis and cardiovascular risk. Infection with cytomegalovirus (CMV) is thought to be involved in the development of atherosclerosis based on clinical, epidemiological and experimental studies and has been proposed to contribute to the progression of atherosclerotic plaque to heart disease and stroke.[6]
CMV infection drives the persistent expansion of a peripheral blood natural killer (NK) cell subset that expresses the cell surface activating NK receptor NKG2C.[7,8] NKG2C, which forms a heterodimer with CD94 are members of the C-type lectin receptor family.[9] The ligand for NKG2C/CD94, like its inhibitory counterpart, NKG2A/CD94 is the non-classical major histocompatibility complex 1b antigen HLA-E bound to epitopes derived from the leader sequence of several HLA antigens.[10,11] Peptides originating from the UL40 gene product of human CMV complexed with HLA-E together with pro-inflammatory signals control the expansion and differentiation of NKG2C + NK cells.[12] NKG2C + NK cells are called adaptive NK (adapNK) cells because they have properties ascribed to adaptive immune cells such as the ability to expand following acute CMV infection in patients receiving transplants from CMV infected donors,[13–16] ability to expand upon CMV reactivation,[14,16,17] persistence and epigenetic regulation of enhanced effector functions that are similar to those seen in memory CD8+ T cells.[18] Most NKG2C + NK cells also express CD57, which is a marker of mature NK cells.[7,19–22]
Since CMV infection drives the expansion of NKG2C+CD57+ adapNK cells, we questioned whether the frequency of these cells was associated with subclinical CVD. We found that CMV+PLWH and CMV-mono-infected persons without subclinical coronary atherosclerosis had higher frequencies of NKG2C+CD57+ adapNK cells than those with coronary atherosclerosis.
2. Material and Methods
2.1. Ethics statement
This research study was approved by the Research Ethics Boards of the Centre Hospitalier de l’Université de Montréal and the McGill University Health Centre (Project Identification Code 2019-4605). It was conducted according to the principles expressed in the Declaration of Helsinki. Written informed consent was obtained from each study subject for the collection of specimens, subsequent analyses and publication of results obtained from these analyses.
2.2. Study subjects
The current study is a cross sectional analysis, nested within the Canadian HIV and Aging Cohort Study (CHACS), which has been described previously.[23] Briefly, CHACS inclusion criteria were to be ≥40 years of age, or to have lived with HIV for at least 15 years. Consecutive participants from CHACS who were free of clinically overt CVD at baseline and presented a Framingham risk score ranging between 5% and 20% were invited to participate in the cardiovascular imaging sub-study.[24] Of the 219 participants in the imaging sub-study, the 194 participants with complete data and available blood samples were included in our analyses. Of these, 128 were CMV+PLWH, 8 were CMV−PLWH, 37 were CMV mono-infected and 21 individuals were negative for both HIV and CMV infection. Participants were recruited from HIV and sexually transmitted disease clinics in Montreal, QC, Canada. Most were men who have sex with men. The PLWH had been on ART for a median of 15 years and had viral loads <50 copies/mL of plasma. Data on all traditional cardiovascular risk factor were collected prospectively as part of the CHACS study visits.
2.3. Definition of subclinical coronary atherosclerosis
Subclinical coronary atherosclerosis was defined by the presence of atherosclerotic plaque in the coronary arteries, measured using a 256-slice cardiac computed tomography scanner (Brilliance iCT; Philips Healthcare, Cleveland, OH) with injection of contrast media. Details of the imaging procedure are published elsewhere.[24] Briefly, every atherosclerotic plaque in the coronary arteries were identified. Their volume (in mm3) was measured using advanced software and summed to obtain the total plaque volume (TPV). Therefore, TPV represent the total burden of coronary atherosclerosis for every participant. For this analysis, TPV was dichotomized as 0 (absence of subclinical coronary atherosclerosis) or greater than 0 (presence of coronary atherosclerosis).[24]
All imaging studies were performed at the Centre Hospitalier de l’Université de Montréal, Montreal, QC, Canada, and interpreted by a board-certified cardiothoracic radiologist (CC-L). All radiology personnel performing image interpretation and postprocessing were blinded to HIV and CMV status.
2.4. Laboratory measurements
HIV infection was diagnosed by quantifying HIV-1 p24 antigen/antibody in plasma and confirmed by Western blot as previously reported.[25] Blood samples collected into vacutainers containing ethylenediaminetetraacetic acid anticoagulant were obtained from CHACS participants at each study visit. Blood was processed into plasma, which was stored frozen at −80°C until use. Peripheral blood mononuclear cells (PBMC) were isolated from blood by centrifugation over a ficoll-hypaque gradient at 400×g for 30 minutes. Cells were then washed and frozen in 90% fetal bovine serum; 10% dimethyl sulfoxide. Cryovials containing cells were stored in liquid nitrogen until use. CD4 and CD8 T cells counts were measured using 4-color flow cytometry. All participants enrolled in this study were tested for CMV serostatus using commercially available ELISA kits measuring the presence of anti-CMV specific IgG (Abcam, Waltham, MA).
2.5. Staining PBMC for adapNK cells
Frozen PBMCs were thawed and resuspended in RPMI 1640; 5% fetal bovine serum; 2 mM L-glutamine; 50 international units/mL penicillin; 50 mg/mL streptomycin (R5) (all from Wisent, Inc., Saint-Jean-Baptiste, QC, Canada). LIVE/DEAD fixable dead cell stain (Invitrogen, Saint Laurent, QC, Canada) was added to the PBMCs as per manufacturer’s directions before surface staining cells using a panel that included the following fluorochrome conjugated antibodies to CD3-BV785 (clone OKT3), CD14-BV785 (M5E2), CD19-BV785 (HIB19), CD56-BV605 (HCD56) (all from BioLegend, San Diego, CA), CD57-PE (TB01) (Life Technologies, Burlington, ON, Canada), CD16-APC-Cy7 (3G8) (BD Bioscience, Baltimore, MD), NKG2C-PE-Vio770 (REA205) and NKG2A-APC (REA110) (Miltenyi Biotec, Auburn, CA).
2.6. Flow cytometry
A total of 1.5 × 106 to 1.8 × 106 events were acquired for each sample using an LSR Fortessa instrument (BD Bioscience, San Jose, CA). Results were analyzed using FlowJo™ software v10.3 (BD, Ashland, OR). NK cells were identified as live, singlet, CD3−CD14−CD19−CD56dim lymphocytes. AdapNK cells were distinguished from conventional NK (cNK) cells based on expression of cell surface NKG2C. AdapNK cells were defined as CD3−CD14−CD19−CD56dim NKG2C+CD57+ while cNK cells were defined as CD3−CD14−CD19−CD56dim NKG2C− cells. Florescence minus one staining was used to set gates for each experiment. Single-stained beads (BD™ CompBead; BD Bioscience) were used to set compensation. The mean plus 2 standard deviations frequency of NKG2C+CD57+ NK cells in CMV seronegative individuals was 4.6%. CMV seropositive donors having a frequency of adapNK cells <4.6% were classified as having a “low” level of adapNK cell expansion. Those with frequencies of adapNK cells of between 4.6% and <20% and ≥20% were classified as having “intermediate” and “high” levels of adapNK cell expansion, respectively.
2.7. Statistical analysis
GraphPad Prism 6 (GraphPad Software, La Jolla, CA) was used for data analysis and graphical presentation. The significance of difference in the same variable between two, or more than two groups, were tested using non-parametric Mann–Whitney or Kruskal–Wallis tests with Dunn’s post-tests, respectively. The significance of proportional between-group differences in sex, high blood pressure, statin use, anti-platelet use, diabetes, exercise and body mass index was assessed using chi-square tests. Exercise frequency was categorized into two groups. Participants who exercised 30 minutes every day or 3 times per week were considered physically active while those who exercised 30 minutes weekly or less, were classified as not physically active. Smoking status categories included nonsmokers and smokers. Smokers were categorized according to smoking intensity measured as total number of pack-years smoked (with one pack-year representing one year of smoking one pack a day). The significance of correlations between two variables were assessed using non-parametric Spearman tests. Results were considered significant when P values were <.05 (two-tailed).
We used separate univariable and multivariable models of modified Poisson regression with robust variance to assess the association of the frequency of adapNK cells with the presence/absence of subclinical coronary atherosclerosis. A parsimonious approach was used to build the multivariable models. Potential confounders were identified based on a priori knowledge and included HIV status and CVD risk factors (age, high blood pressure, smoking exposure, low-density lipoprotein cholesterol, statin use, and body mass index). Potential confounders were entered into the multivariable model if they showed a univariable association with TPV with a P value ≤.1 and kept in the final model if they were independently associated with TPV or if they modified the point estimate for other predictors by more than 10%. Effect modification by HIV was assessed by inclusion of an interaction term to the fully adjusted model. Although considered potential confounders, sex and diabetes were not included into the final model due to the small number of participants who had diabetes and who were women, which prevented adjusting for these variables. Adjusted odds ratios and prevalence ratios were reported with 95% confidence intervals (CIs). No adjustments were made for multiple comparison. Statistical analyses were performed using R version 3.4.3 software (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
3.1. Study participant characteristics
One hundred and ninety-four CHACS participants were included in the imaging sub-study and in the investigations presented here. These individuals were classified into four groups based on their HIV and CMV infection status. The number of individuals in each of the 4 groups, their median (interquartile range), age and sex distribution as well as their clinical characteristics are provided in Table 1. Between-group differences in sex and age were significant (P = .002 and P = .03, chi-square and Kruskal–Wallis tests, respectively). Participants were more likely to be male. Compared to CMV mono-infected participants, CMV+PLWH had a history of higher smoking intensity, lower levels of low density lipoproteins and lower D-dimer levels, (P < .05 for all, Dunn’s post-tests), fewer had high blood pressure and were physically active (P = .03 and P < .0001, respectively, chi-square tests). Other variable listed in Table 1 did not differ significantly between groups or between CMV+PLWH and CMV−PLWH for HIV related clinical characteristics. CD4+ T and CD8+ T cell counts and CD4/CD8 ratios did not differ significantly between CMV+PLWH and CMV−PLWH participants (Table 1).
Table 1.
Demographic and clinical parameters of the study population (N = 194).
| Characteristic | CMV+PLWH (n = 128) | CMV+HIV− (n = 37) | CMV−PLWH (n = 8) | CMV−HIV− (n = 21) | P value |
|---|---|---|---|---|---|
| Sex, n (%) | |||||
| Males | 119 (92.9) | 28 (75.7) | 8 (100) | 15 (71.4) | .002 |
| Females | 9 (7.0) | 9 (24.3) | 0 (0) | 6 (28.6) | |
| Age (yr), median (IQR) | 55.0 (50.8–60.3) | 58.9 (53.0–65.7) | 55.6 (51.3–57.4) | 58.6 (52.8–63.9) | .03 |
| Hypertension, n (%) | 39 (30.5) | 11 (29.7) | 2 (25.0) | 3 (15.0) | .04 |
| Diabetes mellitus, n (%) | 8 (6.1) | 0 (0) | 0 (0) | 0 (0) | .13 |
| LDL cholesterol (mmol/L), median (IQR) | 2.7 (2.2–3.4) | 3.2 (2.5–3.9) | 2.1 (1.9–3.4) | 3.3 (2.8–4.1) | .01 |
| HDL cholesterol (mmol/L), median (IQR) | 1.3 (1.0–1.5) | 1.3 (1.1–1.7) | 1.2 (1.0–1.4) | 1.4 (1.1–1.6) | .21 |
| Inflammation markers, n (%) | |||||
| D dimer (ng/mL) median (IQR) | 46 (35.9) 270.0 (173.8–340) |
16 (43.2) 333.5 (290.3–484.0) |
5 (62.5) 310 (110.1–495.0) |
7 (33.3) 192 (174.0–382.0) |
.02 |
| Hs.CRP (mg/L) median (IQR) | 91 (71.1) 5 (5–5) |
15 (40.5) 5 (5–5) |
5 (62.5) 5 (4–17.3) |
16 (76.2) 5 (5–5) |
.49 |
| Lipid-lowering medication use, n (%) | |||||
| Statin | 35 (27.3) | 7 (18.9) | 3 (60) | 2 (11.8) | .11 |
| Anti-platelet, n (%) | 29 (22.7) | 2 (5.4) | 1 (25) | 2 (11.8) | .09 |
| Smoking exposure (pack/yr) | |||||
| Smokers, n (%), median (IQR) | 89 (69.5) 18.0 (6.0–30.8) |
17 (45.9) 10.4 (3.9–21.50) |
4 (50) 27.8 (9.0–40.0) |
14 (66.6) 7.7 (3.4–20.2) |
.03 |
| nonsmokers, n (%), median (IQR) | 36 (28.1) 0 (0–0) |
19 (51.4) 0 (0–0) |
4 (50) 0 (0–0) |
7 (33.3) 0 (0–0) |
|
| Exercise, n (%) | |||||
| Physical activity | 39 (30.4) | 32 (86.5) | 3 (37.5) | 15 (71.4) | <.0001 |
| No physical activity | 62 (48.4) | 4 (10.8) | 5 (62.5) | 5 (23.8) | |
| BMI, (kg/m2), n (%) | 120 (93.8) 24.3 (21.9–27.5) |
35 (94.6) 25.6 (23.7–32.0) |
8 (100) 26.6 (22.8–32.8) |
21 (100) 27.1(24.0–30.2) |
.01 |
| Waist circumference (cm), median (IQR) | 93 (86.0–101) | 93 (89.0–100) | 97.5 (88.0–110) | 96.0 (89.0–104) | .35 |
| CD4 current (cells/mL), median (IQR) | 576.0 (406.5––726.0) | – | 693 (324.0–1087) | – | .44 |
| CD8 current (cells/mL), median (IQR) | 693.5 (554.7–1020) | – | 874 (384–924) | – | .29 |
| CD4/CD8 ratio (cells/mL), median (IQR) | 0.9 (0.55–1.1) | – | 0.8 (0.59–0.95) | – | .69 |
| Total years on ART, median (IQR) | 15.1 (13.4–22) | – | 12 (3.8–20.4) | – | .88 |
| Years HIV-infected, median (IQR) | 18.1 (14.3–28.9) | – | 15.2 (4.8–23.3) | – | .38 |
| Undetectable HIV-1 RNA, n (%) | 125 (98) | – | 7 (87.5) | – | .22 |
Kruskal–Wallis tests were used to assess the significance of differences in continuous variables between groups. Chi-square tests were used assess to assess the significance of differences in discrete variables between groups. P values considered significant are shown in bold.
ART = antiretroviral therapy, BMI = body mass index, HDL = high-density lipoprotein cholesterol, HIV = human immunodeficiency virus, Hs.CRP = high sensitivity C-reactive protein, IQR = interquartile range, LDL = low-density lipoprotein cholesterol.
3.2. Frequency of NKG2C+CD57+adapNK cells in CHACS participants
All participants were tested for the frequency of their NKG2C+CD57+CD56dim adapNK cells.
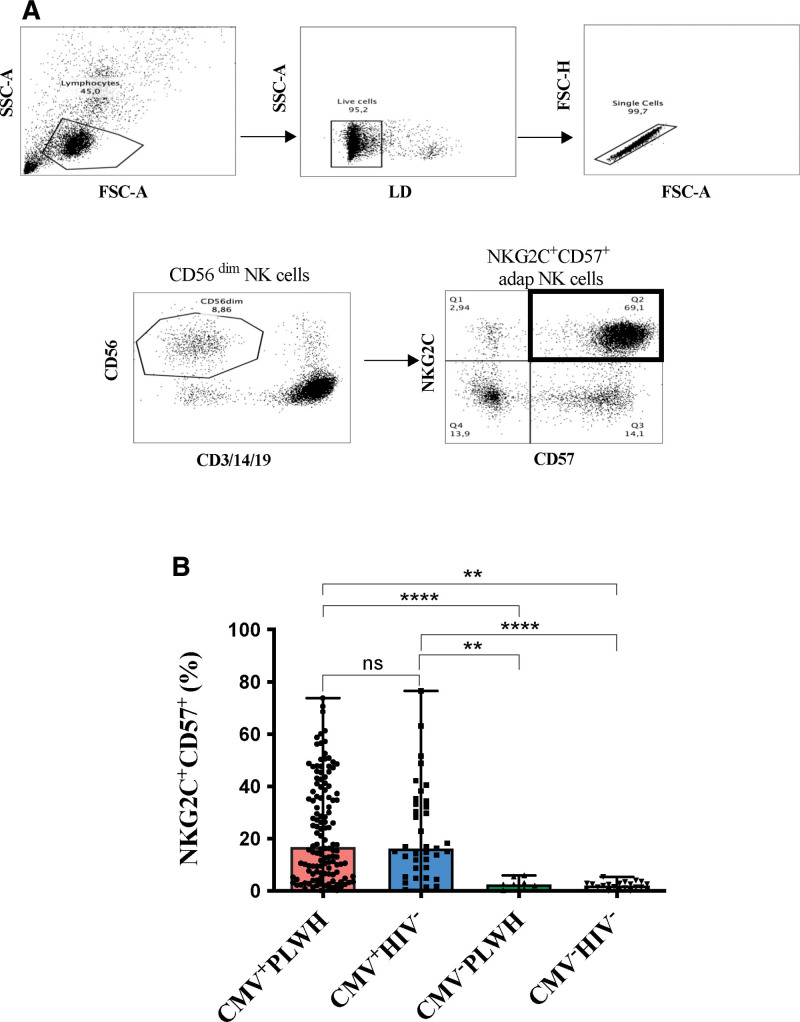
The strategy used to gate on these cells is shown in Figure 1A. The frequency of adapNK cells did not differ significantly between CMV+PLWH (16.8 [6.6–38.5]) and CMV mono-infected (16.3 [8.9–33.9]) participants (Fig. 1B). The frequency of adapNK cells was significantly higher in CMV+PLWH and CMV mono-infected persons than in CMV−PLWH (2.5 [1.0–4.9]) and CMV−HIV− subjects (2.0 [0.9–2.9]) (P < .0001, for all four comparisons, Dunn’s post-tests). The distribution of adapNK cell frequencies in CMV+PLWH and CMV mono-infected individuals categorized as having low, intermediate and high levels of adapNK cells is shown in Supplemental Figure 1, http://links.lww.com/MD/H405.
Figure 1.
Evaluation of the frequency of NKG2C+CD57+ NK cells in HIV+/−CMV+/− participants. (A) Shown is the gating strategy used to detect the frequency of NKG2C+CD57+ expressing NK cells. Peripheral blood mononuclear cells were stained for viability and cell surface CD3, CD14, CD19, CD56, CD57 and NKG2C. CD3−CD14−CD19−CD56dim NK cells were gated on from the live, singlet, lymphocyte gate. From these, we determined the frequency NKG2C+CD57+CD56dim NK cells. (B) The frequency of NKG2C+CD57+ NK cells is shown for cells from CMV+PLWH, CMV mono-infected (CMV+HIV−), CMV−PLWH, and HIV CMV uninfected persons. Each point represents a single individual. Bar graph heights and error bars represent medians and interquartile ranges for the subject groups. A Kruskal–Wallis test with Dunn’s post tests were used to analyze the significance of differences between groups. * = P < .05; ** = P < .01; *** = P < .001; and **** = P < .0001. CMV = cytomegalovirus, FSC-A = forward scatter area, FSC-H = forward scatter height, LD = live/dead, PLWH = people living with HIV, SSC-A = side scatter area.
3.3. The frequency of adapNK cells was higher in CMV+ participants without subclinical coronary atherosclerosis
Supplementary Figure 2, http://links.lww.com/MD/H406 shows the distribution of TPV in 128 CMV+PLWH (A) and 36 CMV mono-infected participants (B) having low, intermediate and high frequencies of adapNK cell. The median TPV tended to decrease (showing a smaller burden of coronary atherosclerosis) as the frequency of adapNK cells increased, with differences being significant for comparisons between participants with low versus high adapNK cell frequencies in CMV+PLWH only.
Table 2 shows the association of presence/absence of coronary atherosclerosis in CMV+ participants (both PLWH and CMV mono-infected) with adapNK cell frequency categories and other variables. A significantly higher frequency of CMV+ persons without coronary atherosclerosis had high frequencies of adapNK cells (P = .01, chi-square test). CMV + persons without, compared to, with coronary atherosclerosis were younger (P = .006, Mann–Whitney test), had a lower exposure to smoking (P = .02, Mann–Whitney test) and lower D-dimer levels (P = .004, Mann–Whitney test). The other variables in Table 2 did not differ significantly between CMV+ participants without and with coronary atherosclerosis.
Table 2.
Characteristic of cytomegalovirus seropositive participants stratified by negative versus positive total plaque volume.
| Characteristic | TPV = 0 (n = 55) | TPV > 0 (n = 109) | P value |
|---|---|---|---|
| AdapNK cells expansion frequency, n (%) | |||
| High | 32 (58.18) | 42 (38.53) | .01 |
| Intermediate | 19 (34.54) | 42 (38.53) | |
| Low | 4 (7.27) | 25 (22.93) | |
| Sex, n (%) | |||
| Males | 52 (94.5) | 97 (88.9) | .24 |
| Females | 3 (5.5) | 12 (11.0) | |
| Age (yr) median, IQR | 54.6 (50.4–58.6) | 57.3 (52.7–62.6) | .006 |
| Hypertension, n (%) | 15 (27.3) | 32 (29.4) | .59 |
| Diabetes mellitus, n (%) | 1 (1.8) | 11 (10.0) | .06 |
| HDL cholesterol (mmol/L) median (IQR) |
46 (83.6) 1.3 (1.02–1.49) |
105 (96.3) 1.27 (1.07–1.47) |
.57 |
| LDL cholesterol (mmol/L) median (IQR) |
44 (80) 2.9 (2.5–3.6) |
104 (95.4) 2.2 (2.2–3.5) |
.56 |
| Markers of inflammation, n (%) | |||
| D-dimer (ng/mL) median (IQR) |
12 (21.8) 200.5 (170–282.8) |
47 (41.9) 303 (269–410) |
.004 |
| Hs-CRP (mg/L) median (IQR) |
30 (54.5) 5 (5–5) |
77 (68.8) 5 (5–5) |
.09 |
| Lipid-lowering medication use, n (%) | |||
| Statin | 11 (20) | 35 (32.1) | .09 |
| Anti-platelet | 5 (9.1) | 24 (22.0) | .09 |
| Smoking exposure (packs/yr), n (%) | |||
| Smokers median (IQR) |
32 (58.1) 6.0 (3.5–25.5) |
73 (66.9) 20.0 (7.7–33.0) |
.002 |
| Nonsmokers median (IQR) |
20 (36.3) 0 (0–0) |
28 (25.7) 0 (0–0) |
|
| Physically active, n (%) | |||
| Yes | 29 (52.7) | 41 (37.6) | .06 |
| No | 25 (45.5) | 67 (61.4) | |
| BMI (kg/m2), n (%) | 51 (92.9) 26.2 (23.1–27.9) |
107 (98.1) 24.2 (22.1–29.2) |
.27 |
| Waist circumference (cm), n (%) median (IQR) |
51 (92.7) 93.0 (89.0–100.0) |
107 (98.2) 92.0 (86.0–101.0) |
.53 |
P values considered significant are shown in bold.
AdapNK cells = adaptive NK cells, BMI = body mass index., HDL = high-density lipoprotein cholesterol, Hs-CRP = high sensitivity C-reactive protein, IQR = interquartile range, LDL = low-density lipoprotein cholesterol, TPV = 0 = negative for subclinical atherosclerosis, TPV > 0 = positive for subclinical atherosclerosis.
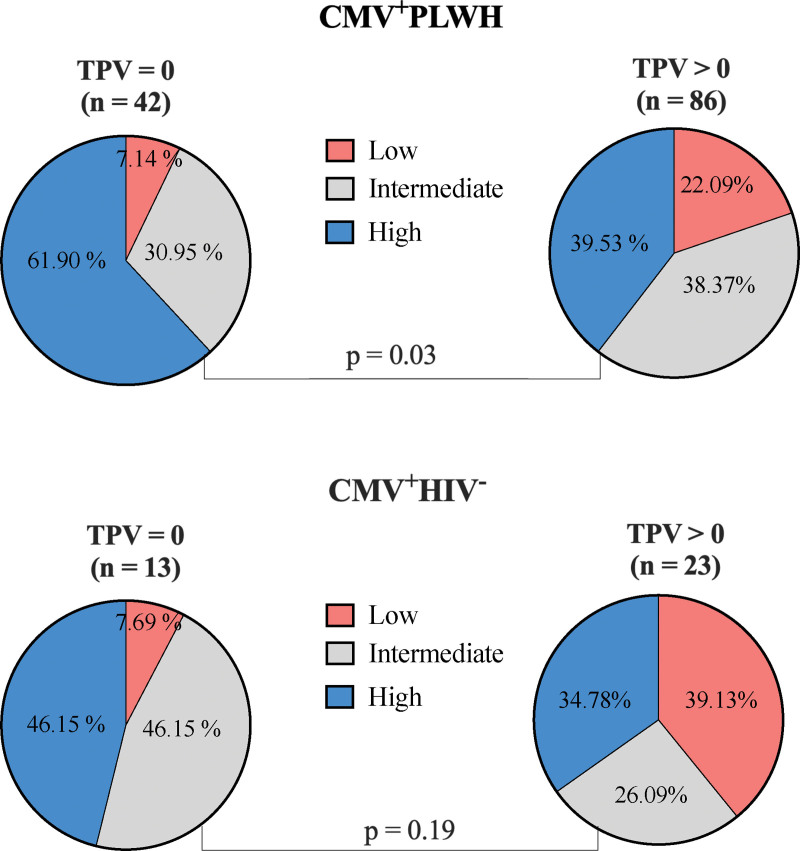
A significantly greater proportion of CMV+PLWH without, compared to with, coronary atherosclerosis (TPV = 0 vs TPV > 0) had a high frequency of adapNK cells (P = .03, chi-square test) (Fig. 2 upper pie chart graphs). In CMV mono-infected participants, there was a non-significant trend towards a higher frequency of adapNK cells in persons without compared to with coronary atherosclerosis (P = .19, chi-square test) (Fig. 2 lower pie chart graphs).
Figure 2.
The proportion of NKG2C+CD57+ adapNK cell frequency categories in TPV negative and positive CMV infected people living with HIV (CMV+PLWH) and CMV mono-infected (CMV+HIV−) individuals. The proportion of NKG2C+CD57+ adapNK cell frequency categories (low, intermediate, and high) was compared in participants with negative (left-hand pie charts) versus positive (right-hand pie charts) TPV in CMV+PLWH (n = 128) (upper pie charts) and CMV mono-infected (CMV+HIV−) individuals (n = 36) (lower pie charts). Chi-square tests were used to test the significance of proportional between-group differences in adapNK cells frequency categories between participants with negative (TPV = 0) versus positive (TPV > 0) for subclinical atherosclerosis. adapNK = adaptive NK, CMV = cytomegalovirus, HIV = human immunodeficiency virus, PLWH = people living with HIV, TPV = total plaque volume.
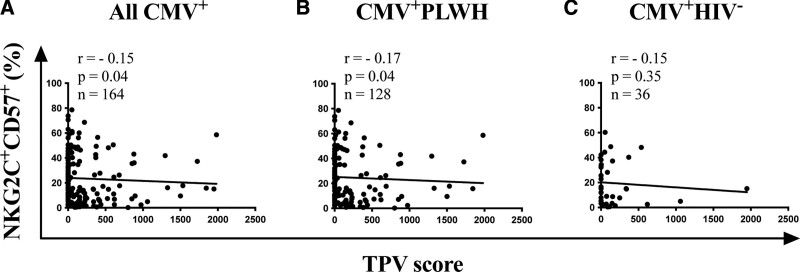
For all CMV+ individuals, there was a weak, though significant negative correlation between the frequency of adapNK cells and TPV (Fig. 3A). Correlations between these 2 parameters were significant for CMV+PLWH (Fig. 3B) but only trended towards significance for CMV mono-infected participants (Fig. 3C). Together, these results show that increasing frequencies of adapNK cells were associated with less subclinical coronary atherosclerosis in all CMV+ persons, a finding that was also apparent when CMV+PLWH and CMV mono-infected participants were considered separately.
Figure 3.
Correlation between NKG2C+CD57+ adapNK cell frequency and TPV in CMV+ PLWH and CMV mono-infected (CMV+HIV−) individuals. (A) The y-axes show the frequency of adapNK cells and the x-axes the TPV in (A) CMV+ (n = 164), (B) CMV+PLWH (n = 128), and (C) CMV mono-infected (n = 36) individuals. The number of subjects tested, the correlation coefficients (r) and the P values for each correlation are shown in the inset at the top left corner of each graph. The statistical significance of the correlations was tested using non-parametric Spearman correlation tests. adapNK = adaptive NK, CMV = cytomegalovirus, HIV = human immunodeficiency virus, PLWH = people living with HIV, TPV = total plaque volume.
3.4. CMV+PLWH and CMV mono-infected participants with high frequencies of adapNK cells have a reduced risk of coronary atherosclerosis
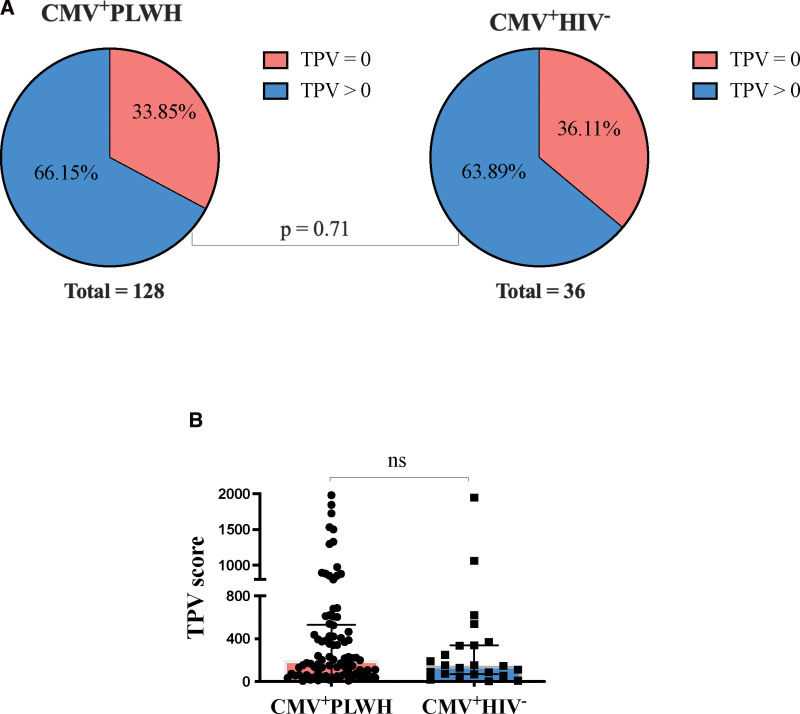
The proportion participants with coronary atherosclerosis did not differ significantly between CMV+PLWH and CMV mono-infected participants (P = .71, chi-square test) (Fig. 4A). There were also no between-group differences in the distribution of TPV when analyzed as a continuous variable (P = .37, Mann–Whitney test) (Fig. 4B). Finally, among CMV+ participants, we did not observe effect modification by HIV status of the association between adapNK cells frequency and presence of coronary atherosclerosis. The P-value of the interaction term between HIV status and adapNK cell frequency was .28.
Figure 4.
Comparison of CMV+PLWH and CMV mono-infected participants for the proportion with negative versus positive TPV and the distribution of these scores. (A) The proportion CMV+PLWH (left-hand panels) and CMV mono-infected individuals (right-hand panels) with negative (TPV = 0) versus positive (TPV > 0) TPV scores. A Chi-square tests was used to determine the statistical significance of proportional between-group differences in positive and negative TPV. (B) Shown on the y-axis are the TPV scores for CMV+PLWH and CMV mono-infected individuals with positive TPV scores. A Mann–Whitney test was used to assess the statistical significance of differences in the distribution of TPV scores in CMV+PLWH versus CMV mono-infected individuals. CMV = cytomegalovirus, PLWH = people living with HIV, TPV = total plaque volume.
This prompted us to combine results for 128 CMV+PLWH and 36 CMV mono-infected participants for the purpose of carrying out an independent multivariable Poisson regression after adjusting for traditional cardiovascular risk factors (Table 3). In this adjusted analysis, a high frequency of adapNK cells was associated with a relative risk (RR) of 0.75 (95% CI, 0.58–0.97, P = .03) for presence of coronary atherosclerosis, independently of other factors. Each 10-year increase in age was associated with an increased RR for coronary atherosclerosis of 1.23 (1.06–1.44, P = .006). Smoking intensity was also associated with a RR of increased coronary plaque of 1.09 (1.05–1.13, P < .001) for each additional pack-year of exposure. On the other hand, there was no evidence of an association between HIV and coronary atherosclerosis (RR 1.08 [0.81–1.42], P = .58). There was no evidence of interaction by HIV status in these analyses.
Table 3.
Univariable and multivariable analysis of association of AdapNK cell frequency with positive total plaque volume score in CMV seropositive participants.
| Characteristic | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| RR (95% CI) | P value | RR (95% CI) | P value | |
| AdapNK cells frequency | ||||
| High (>20%) | 0.74 (0.56–0.95) | .019 | 0.75 (0.58–0.97) | .030 |
| Intermediate (4.6–20%) | 0.90 (0.72–1.14) | .410 | 0.92 (0.72–1.17) | .509 |
| Low (<4.6%) | 1.0 (ref) | 1.0 (ref) | ||
| HIV status | ||||
| Positive | 1.07 (0.81–1.40) | .620 | 1.08 (0.81–1.42) | .582 |
| Negative | 1.0 (ref) | 1.0 (ref) | ||
| Age (per 10 years increase) | 1.26 (1.09–1.45) | .001 | 1.23 (1.06–1.44) | .006 |
| High blood pressure | ||||
| Present | 1.07 (0.85–1.33) | .56 | – | – |
| Absent | 1.0 (ref) | – | – | |
| Smoking exposure (per each increase in 10 pack-years) | 1.09 (1.05–1.13) | <.001 | 1.09 (1.05–1.13) | <.001 |
| LDL-cholesterol (1 mmol/l) | 1.00 (0.91–1.11) | .888 | – | – |
| Statin use | ||||
| Yes | 1.21 (0.98–1.48) | .07 | – | – |
| No | 1.0 (ref) | – | – | |
| BMI (1 kg/m2) | 0.98 (0.96–1.01) | .36 | – | – |
RR per 10-year increase in age.
RR per 10 pack per year increase in smoking.
P values considered significant are shown in bold.
95% CI = 95% confidence intervals, AdapNK cells = adaptive NK cells, BMI, body mass index, LDL-cholesterol = low density lipoprotein cholesterol, RR = relative risk.
4. Discussion
Although ART is effective at controlling HIV replication, aging in PLWH, is associated with greater non-AIDS morbidities, such as CVDs than in age-matched uninfected persons.[26] Increasing morbidity is associated with immune dysfunction, which persists despite treatment that suppresses HIV viral loads below the limit of detection.[27] Persistent co-infections are common in PLWH and likely contribute to this HIV disease related immune dysfunction.[28] Indeed, 94% of PLWH in the CHACS were CMV co-infected. Both HIV and CMV infections are independently associated with inflammation, morbidities related to inflammation and CVD risk, particularly in the elderly.[29] A hallmark of CMV infection is the expansion of a population of adapNK cells.[16,17] In this report, we investigated whether the frequency of these adapNK cells was associated with pre-clinical atherosclerosis measured by TPV in CMV+PLWH and CMV mono-infected individuals with frequencies of adapNK cells that varied widely from 0.19% to 78.6% of CD3−CD14−CD19−CD56dim NK cells. The median interquartile range frequency of adapNK cells was similar in CMV+PLWH and CMV mono-infected individuals who were ≥40 years of age, suggesting that CMV seropositivity, rather than HIV, drives adapNK cell frequency. In this study, CMV+ participants with no subclinical atherosclerosis had higher levels of adapNK cells. This association remained after adjusting for traditional cardiovascular risk factors; CMV+ subjects with high frequencies of adapNK cells had an RR (95% CI) of 0.75 (0.58–0.97, P = .03) for presence of coronary atherosclerosis, indicating a significantly reduced risk.
Atherosclerosis is an inflammatory process in which immune cells and their mediators are important determinants of the disease process.[30–34] HIV infection, even in successfully treated individuals, is characterized by higher immune activation levels than in uninfected persons.[35] This likely predisposes PLWH to the development of atherosclerosis. Using coronary computed tomography angiography, PLWH had a higher prevalence of subclinical atherosclerosis, particularly of non-calcified plaque, which is more vulnerable to rupture, than HIV seronegative persons with similar risk factors.[24] This was the case even for PLWH with low Framingham scores and no evidence of CVD.[36]
CMV infection reconfigures the immune system by driving the expansion of CMV-specific CD8+ T cells that are pathogenic and independently related to higher levels of carotid intima-media thickness in PLWH.[37] Up to 30% of all CD8+ T cells in CMV+ persons can be CMV-specific.[38] When activated, these T cells can contribute to CVD pathogenesis by recognizing CMV epitopes present in plaque where CMV antigens and nucleic acids have been detected.[39–41] AdapNK cells can control CMV infection.[14,18,42] These cells may also regulate CMV-specific CD8+ T cells, which express higher levels of HLA-E, the ligand for NKG2C, than do CD8+ T cells of other specificities.[43] CMV viremia activates CMV-specific CD8+ T cells, further increasing their expression of HLA-E/CMV peptides, which, in turn, activates adapNK cells expressing NKG2C, the receptor for HLA-E/CMV peptide complexes. Once activated, adapNK cells have the potential to kill CMV-specific CD8+ T cells to limit the CD8 T cell inflation observed in CMV+ persons.[43]
NKG2C and NKG2A are inhibitory and activating NK receptors, respectively that are covalently associated with CD94.[44] They share sequence homology and ligands such as HLA-E complexed with epitopes derived from the leader sequence of HLA-A, B, C and G and epitopes from the UL40 CMV protein.[10,12] The interaction of these ligands with NKG2A is more avid than that with NKG2C and inhibitory signals tend to predominate those of activating signals.[11,45] Thus, if NKG2C+CD57+ NK cells co-expressed NKG2A, inhibition of NKG2C+ NK cell functions could ensue. Others have reported that in CD56dim NK cells, expression of NKG2C generally excludes expression of NKG2A.[8,46] NK cell staining of samples from the CHACS studied here showed that among the NKG2C+CD57+CD56dim NK cells <0.3% co-expressed NKG2A. This finding makes it unlikely that NKG2A expression on NKG2C+CD57+ NK cells, dampens the function of these cells. The titer of IgG antibodies to CMV was measured in a subset of 83 study participants. While there was a significant positive correlation between anti-CMV IgG titers and the frequency of NKG2C+CD57+CD56dim NK cells, these antibody titers did not correlate with TPV.
Upon expansion, NKG2C+CD57+ NK cells acquire epigenetic changes that that distinguish them from cNK cells and regulate their effector functions.[47,48] AdapNK cells mediate higher levels of antibody dependent cellular cytotoxicity (ADCC) activity and higher levels of IFN-γ and TNF-α secretion upon activation than cNK cells.[18,48] CMV infection also induces ADCC competent CMV-specific antibodies, which can opsonize CMV antigens present in plaque. Higher frequencies of CD16 expressing adapNK cells would have an advantage over low frequencies of these cells to bind opsonized anti-CMV antibodies and activate adapNK cells to eliminate CMV infected cells within plaques by ADCC. These cells can also limit CD8+ T cell inflation and damage caused by these cytolytic cells. This may explain why CMV+PLWH with the highest levels of adapNK cells are more likely to have no subclinical atherosclerosis.
In addition to their role in anti-tumor and anti-viral responses, NK cells play a crucial role in repairing damaged tissues and maintaining tissue homeostasis.[49] Following myocardial infarction, NK cell expansion from c-kit+ bone marrow derived cells protected the heart by reducing cardiomyocyte apoptosis, deposition of collagen and subsequent fibrosis.[50] In an experimental model of acute myocarditis, activated NK cells accumulated in the heart and released perforin, granzyme B and IFN-γ resulting in decreased fibrosis by inhibiting eosinophil activation and inducing eosinophil apoptosis.[51] While this information is not specific to pre-clinical atherosclerotic plaques, it illustrates the potential of NK cells to repair damaged tissues and limit fibrosis.
Others reported that CMV driven expansion of NKG2C + NK cells was related to carotid atherosclerotic plaque (CAP) instability.[52] They found higher frequencies of NKG2C+ NK cells in persons with high-risk CAP than in those with non-high-risk CAP. This finding was interpreted as evidence that expansion of NKG2C+ cells in subjects with CAP was associated with an increased risk of plaque destabilization.[52] There is no clear explanation for the discrepancy between our results and those of Martinez et al in terms of the role of NKG2C+ NK cells in CVDs. The protective versus pathogenic role of adapNK cells may differ at different stages of the atherosclerotic process. In our investigations, pre-clinical atherosclerosis was assessed in persons with no clinical manifestations of CVD while in Martinez et al, plaque in carotid arteries was symptomatic and severe enough to warrant surgical removal of the CAP. Another possibility that may explain these discrepant results is that Martinez et al enumerated the frequency of NKG2C+ NK cells while we investigated the frequency of NKG2C+CD57+ NK cells. The CD57 marker improves the detection of NK cells with adaptive properties.[16] Although the biology of the atheroscolerotic process is similar in coronary and carotid arteries there exist differences in plaque morphology and characteristics between the two sites. For example, the coronary atherosclerotic plaques are charecterized by a thiner fibrous cap, more intra-plaque hemorrhage and calcified nodules compared to plaque in carotid arteries.[53] Further investigation is needed to explain the discrepancy between our results and those of Martinez et al.
This study had some limitations. The study population size, particularly for CMV mono-infected subjects was small, which may have precluded achieving statistical significance for some of the analyses in which only CMV mono-infected individuals were included. No adjustments were made for multiple comparisons. Therefore, results must be regarded with caution and duplication of these results is desirable. Sex and diabetes were considered as potential confounders due to previous epidemiological knowledge and were investigated as such in the model building strategy, and the text has been modified to reflect this adequately. However, most of the sample were men, and diabetes cases were very rare. Sex and diabetes were not associated with the outcome in univariable analysis, and as such, they could not be confounders of the associations, so they were not included into the final adjusted models. Indeed, due to small cell issues, it would not have been possible to include them into the final models, as it would make estimations unstable. Investigating whether NK cells or adapNK cells or whether CMV antigens were present in atherosclerotic plaques was not feasible in this population with no CVD symptoms being investigated for subclinical CVD. Additionally, the cross-sectional nature of the study limits causal interpretations.
In summary, high frequencies of adapNK cells were associated with a reduced prevalence of coronary atherosclerotic plaque in CMV seropositive individuals, both in the PLWH and in CMV mono-infected groups. Further investigations should focus on determining the directional causality of this link in the setting of pre-clinical atherosclerosis and in other stages in the development of atherosclerosis and other manifestations of CVD.
Acknowledgments
We thank the investigators of the Canadian HIV and Aging Cohort for recruiting and clinically following study participants. We thank Louise Gilbert and Marc Messier-Peet for CHACS coordination and database management and Sylla Mohamed for technical support. We thank the persons enrolled in the CHACS without whose participation this work would not have been possible.
Author contributions
Conceptualization: Khlood Alsulami, Carl Chartrand-Lefebvre, Madeleine Durand, Nicole F. Bernard.
Data curation: Khlood Alsulami, Daniel Tremblay-Sher, Carl Chartrand-Lefebvre, Madeleine Durand.
Formal analysis: Khlood Alsulami, Manel Sadouni, Carl Chartrand-Lefebvre, Nicole F. Bernard.
Funding acquisition: Carl Chartrand-Lefebvre, Cécile Tremblay, Madeleine Durand.
Investigation: Khlood Alsulami, Nicole F. Bernard.
Methodology: Khlood Alsulami, Manel Sadouni, Franck P. Dupuy, Carl Chartrand-Lefebvre.
Project administration: Daniel Tremblay-Sher, Cécile Tremblay, Madeleine Durand, Nicole F. Bernard.
Resources: Baril Jean-Guy, Benoit Trottier, Cécile Tremblay.
Supervision: Manel Sadouni, Daniel Tremblay-Sher, Franck P. Dupuy, Carl Chartrand-Lefebvre, Cécile Tremblay, Madeleine Durand, Nicole F. Bernard.
Validation: Khlood Alsulami, Manel Sadouni, Carl Chartrand-Lefebvre.
Visualization: Khlood Alsulami, Franck P. Dupuy.
Writing – original draft: Khlood Alsulami, Manel Sadouni, Madeleine Durand, Nicole F. Bernard.
Writing – review & editing: Khlood Alsulami, Manel Sadouni, Daniel Tremblay-Sher, Baril Jean-Guy, Benoit Trottier, Franck P. Dupuy, Carl Chartrand-Lefebvre, Cécile Tremblay, Madeleine Durand, Nicole F. Bernard.
Supplementary Material
Abbreviations:
- adapNK =
- adaptive NK
- ADCC =
- antibody dependent cellular cytotoxicity
- ART =
- antiretroviral therapy
- CAP =
- carotid atherosclerotic plaque
- CHACS =
- Canadian HIV and Aging Cohort Study
- CI =
- confidence interval
- CMV =
- cytomegalovirus
- cNK =
- conventional NK
- CVD =
- cardiovascular disease
- HIV =
- human immunodeficiency virus
- PBMC =
- peripheral blood mononuclear cells
- PLWH =
- people living with HIV
- RR =
- relative risk
- TPV =
- total plaque volume
This study was funded by the Canadian Institute of Health Research (CIHR) Team Grants TCO-125276 and HAL-157985, the CIHR Canadian HIV Trials Network CTN 272, the Fonds de Recherche du Québec-Santé (FRQ-S) Network grants, the Département de Radiologie, Radio-Oncologie et Médecine Nucléaire. MD is supported by a Clinician-Researcher Salary award from the FRQ-S.
This research study was approved by the Research Ethics Boards of the Centre Hospitalier de l’Université de Montréal and the McGill University Health Centre (Project Identification Code 2019-4605). It was conducted according to the principles expressed in the Declaration of Helsinki. Written informed consent was obtained from each study subject for the collection of specimens, subsequent analyses and publication of results obtained from these analyses.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
Supplemental Digital Content is available for this article.
How to cite this article: Alsulami K, Sadouni M, Tremblay-Sher D, Baril J-G, Trottier B, Dupuy FP, Chartrand-Lefebvre C, Tremblay C, Durand M, Bernard NF. High frequencies of adaptive NK cells are associated with absence of coronary plaque in cytomegalovirus infected people living with HIV. Medicine 2022;101:38(e30794).
Contributor Information
Khlood Alsulami, Email: khlood.alsulami@mail.mcgill.ca.
Manel Sadouni, Email: sadounim89@gmail.com.
Daniel Tremblay-Sher, Email: daniel.tremblay.sher@gmail.com.
Jean-Guy Baril, Email: jgbaril@videotron.ca.
Benoit Trottier, Email: bentrotte@gmail.com.
Franck P. Dupuy, Email: dura007@hotmail.fr.
Carl Chartrand-Lefebvre, Email: carl.chartrand-lefebvre@umontreal.ca.
Cécile Tremblay, Email: c.tremblay@umontreal.ca.
Madeleine Durand, Email: madeleine.durand@gmail.com.
References
- [1].May MT, Gompels M, Delpech V, et al. Impact on life expectancy of HIV-1 positive individuals of CD4+ cell count and viral load response to antiretroviral therapy. AIDS. 2014;28:1193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hsue PY, Deeks SG, Hunt PW. Immunologic basis of cardiovascular disease in HIV-infected adults. J Infect Dis. 2012;205(suppl 3):S375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Manga P, McCutcheon K, Tsabedze N, et al. HIV and nonischemic heart disease. J Am Coll Cardiol. 2017;69:83–91. [DOI] [PubMed] [Google Scholar]
- [4].Roy Cardinal MH, Durand M, Chartrand-Lefebvre C, et al. Increased carotid artery wall stiffness and plaque prevalence in HIV infected patients measured with ultrasound elastography. Eur Radiol. 2020;30:3178–87. [DOI] [PubMed] [Google Scholar]
- [5].Currier J, Taylor A, Boyd F, et al. Coronary heart disease in HIV-infected individuals. J Acquir Immune Defic Syndr. 2003;33:506–12. [DOI] [PubMed] [Google Scholar]
- [6].Melnick JL, Adam E, Debakey ME. Cytomegalovirus and atherosclerosis. Bioessay. 1995;17:10. [DOI] [PubMed] [Google Scholar]
- [7].Guma M, Cabrera C, Erkizia I, et al. Human cytomegalovirus infection is associated with increased proportions of NK cells that express the CD94/NKG2C receptor in aviremic HIV-1-positive patients. J Infect Dis. 2006;194:38–41. [DOI] [PubMed] [Google Scholar]
- [8].Gumá MA, Vilches C, Gómez-Lozano N, et al. Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood. 2004;104:3664–71. [DOI] [PubMed] [Google Scholar]
- [9].Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–74. [DOI] [PubMed] [Google Scholar]
- [10].Braud VM, Allan DS, O’Callaghan CA, et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature. 1998;391:795–9. [DOI] [PubMed] [Google Scholar]
- [11].Borrego F, Ulbrecht M, Weiss EH, et al. Recognition of human histocompatibility leukocyte antigen (HLA)-E complexed with HLA class I signal sequence–derived peptides by CD94/NKG2 confers protection from natural killer cell–mediated lysis. J. Exp. Med. 1998;187:813–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hammer Q, Ruckert T, Borst EM, et al. Peptide-specific recognition of human cytomegalovirus strains controls adaptive natural killer cells. Nat Immunol. 2018;19:453–63. [DOI] [PubMed] [Google Scholar]
- [13].Mariella Della Chiesa MF, Marina P, Franco L, et al. Phenotypic and functional heterogeneity of human NK cells developing after umbilical cord blood transplantation: a role for human cytomegalovirus? Blood. 2001;119:399–410. [DOI] [PubMed] [Google Scholar]
- [14].Foley B, Cooley S, Verneris MR, et al. Human cytomegalovirus (CMV)-induced memory-like NKG2C(+) NK cells are transplantable and expand in vivo in response to recipient CMV antigen. J Immunol. 2012;189:5082–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Foley B, Cooley S, Verneris MR, et al. Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. Blood. 2012;119:2665–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lopez-Verges S, Milush JM, Schwartz BS, et al. Expansion of a unique CD57(+)NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc Natl Acad Sci USA. 2011;108:14725–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lopez-Verges S, Milush JM, Pandey S, et al. CD57 defines a functionally distinct population of mature NK cells in the human CD56dimCD16+ NK-cell subset. Blood. 2010;116:3865–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Luetke-Eversloh M, Hammer Q, Durek P, et al. Human cytomegalovirus drives epigenetic imprinting of the IFNG locus in NKG2Chi natural killer cells. PLoS Pathog. 2014;10:e1004441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lanier LL, Corliss B, Wu J, et al. Association of DAP12 with activating CD94/NKG2C NK cell receptors. Immunity. 1998;8:693–701. [DOI] [PubMed] [Google Scholar]
- [20].Kared H, Martelli S, Tan SW, et al. Adaptive NKG2C(+)CD57(+) natural killer cell and Tim-3 expression during viral infections. Front Immunol. 2018;9:686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Peppa D, Pedroza-Pacheco I, Pellegrino P, et al. Adaptive reconfiguration of natural killer cells in HIV-1 infection. Front Immunol. 2018;9:474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wu Z, Sinzger C, Frascaroli G, et al. Human cytomegalovirus-induced NKG2C(hi) CD57(hi) natural killer cells are effectors dependent on humoral antiviral immunity. J Virol. 2013;87:7717–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Durand M, Chartrand-Lefebvre C, Baril JG, et al. The Canadian HIV and aging cohort study – determinants of increased risk of cardio-vascular diseases in HIV-infected individuals: rationale and study protocol. BMC Infect Dis. 2017;17:611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Boldeanu I, Sadouni M, Mansour S, et al. Prevalence and characterization of subclinical coronary atherosclerotic plaque with CT among individuals with HIV: results from the Canadian HIV and Aging Cohort Study. Radiology. 2021;299:571–80. [DOI] [PubMed] [Google Scholar]
- [25].Mehraj V, Cox J, Lebouche B, et al. Socio-economic status and time trends associated with early ART initiation following primary HIV infection in Montreal, Canada: 1996 to 2015. J Int AIDS Soc. 2018;21:e25034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hunt PW. HIV and inflammation: mechanisms and consequences. Curr HIV/AIDS Rep. 2012;139:147. [DOI] [PubMed] [Google Scholar]
- [27].Deeks SG, Verdin E, McCune JM. Immunosenescence and HIV. Curr Opin Immunol. 2012;24:501–6. [DOI] [PubMed] [Google Scholar]
- [28].Appay V, Fastenackels S, Katlama C, et al. Old age and anti-cytomegalovirus immunity are associated with altered T-cell reconstitution in HIV-1-infected patients. AIDS. 2011;25:1813–22. [DOI] [PubMed] [Google Scholar]
- [29].Freeman ML, Lederman MM, Gianella S. Partners in crime: the role of CMV in immune dysregulation and clinical outcome during HIV infection. Curr HIV/AIDS Rep. 2016;13:10–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lo J, Plutzky J. The biology of atherosclerosis: general paradigms and distinct pathogenic mechanisms among HIV-infected patients. J Infect Dis. 2012;205(suppl 3):S368–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–95. [DOI] [PubMed] [Google Scholar]
- [32].Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–25. [DOI] [PubMed] [Google Scholar]
- [33].Libby P, Ridker PM, Hansson GK; Leducq Transatlantic Network on Atherothrombosis. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54:2129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–74. [DOI] [PubMed] [Google Scholar]
- [35].Hunt PW. HIV and inflammation: mechanisms and consequences. Curr HIV/AIDS Rep. 2012;9:139–47. [DOI] [PubMed] [Google Scholar]
- [36].Lo J, Abbara S, Shturman L, et al. Increased prevalence of subclinical coronary atherosclerosis detected by coronary computed tomography angiography in HIV-infected men. AIDS. 2010;24:243–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hsue PY, Hunt PW, Sinclair E, et al. Increased carotid intima-media thickness in HIV patients is associated with increased cytomegalovirus-specific T-cell responses. AIDS. 2006;20:2275–83. [DOI] [PubMed] [Google Scholar]
- [38].van den Berg SPH, Pardieck IN, Lanfermeijer J, et al. The hallmarks of CMV-specific CD8 T-cell differentiation. Med Microbiol Immunol. 2019;208:365–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Blum A, Peleg A, Weinberg M. Anti-cytomegalovirus (CMV) IgG antibody titer in patients with risk factors to atherosclerosis. Clin Exp Med. 2003;3:157–60. [DOI] [PubMed] [Google Scholar]
- [40].Melnick JL, Hu C, Burek J, et al. Cytomegalovirus DNA in arterial walls of patients with atherosclerosis. J Med Virol. 1994;170:174. [DOI] [PubMed] [Google Scholar]
- [41].Heybar H, Alavi SM, Farashahi Nejad M, et al. Cytomegalovirus infection and atherosclerosis in candidate of coronary artery bypass graft. Jundishapur J Microbiol. 2015;8:e15476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Vietzen H, Pollak K, Honsig C, et al. NKG2C deletion is a risk factor for human cytomegalovirus viremia and disease after lung transplantation. J Infect Dis. 2018;217:802–6. [DOI] [PubMed] [Google Scholar]
- [43].Ralf Grutza WM, Tina S, Eugen B, et al. NKG2Cpos NK cells regulate the expansion of cytomegalovirus-specific CD8 T cells. J Immunol. 2020;204:2910–17. [DOI] [PubMed] [Google Scholar]
- [44].Lazetic S, Chang C, Houchins JP, et al. Human natural killer cell receptors involved in MHC class I recognition are disulfide-linked heterodimers of CD94 and NKG2 subunits. J Immunol. 1996;157:4741–5. [PubMed] [Google Scholar]
- [45].Aldrich CJ, DeCloux A, Woods AS, et al. Identification of a Tap-dependent leader peptide recognized by alloreactive T cells specific for a class Ib antigen. Cell. 1994;79:649–58. [DOI] [PubMed] [Google Scholar]
- [46].Beziat V, Descours B, Parizot C, et al. NK cell terminal differentiation: correlated stepwise decrease of NKG2A and acquisition of KIRs. PLoS One. 2010;5:e11966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Lee J, Zhang T, Hwang I, et al. Epigenetic modification and antibody-dependent expansion of memory-like NK cells in human cytomegalovirus-infected individuals. Immunity. 2015;42:431–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Schlums H, Cichocki F, Tesi B, et al. Cytomegalovirus infection drives adaptive epigenetic diversification of NK cells with altered signaling and effector function. Immunity. 2015;42:443–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Tosello-Trampont A, Surette FA, et al. Immunoregulatory role of NK cells in tissue inflammation and regeneration. Front Immunol. 2017;8:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Davani S, Deschaseaux F, Chalmers D, et al. Can stem cells mend a broken heart? Cardiovasc Res. 2005;65:305–16. [DOI] [PubMed] [Google Scholar]
- [51].Baci D, Bosi A, Parisi L, et al. Innate immunity effector cells as inflammatory drivers of cardiac fibrosis. Int J Mol Sci. 2020;21:7165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Martinez-Rodriguez JE, Munne-Collado J, Rasal R, et al. Expansion of the NKG2C+ natural killer-cell subset is associated with high-risk carotid atherosclerotic plaques in seropositive patients for human cytomegalovirus. Arterioscler Thromb Vasc Biol. 2013;33:2653–9. [DOI] [PubMed] [Google Scholar]
- [53].Jashari F, Ibrahimi P, Nicoll R, et al. Coronary and carotid atherosclerosis: similarities and differences. Atherosclerosis. 2013;227:193–200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.