Abstract
This study aimed to analyze the changes in brain networks functional connectivity of pilots exposed to simulated hypoxia using resting-state functional magnetic resonance imaging (fMRI). A total of 35 healthy male pilots exposed to 14.5% oxygen concentration (corresponding to an altitude of 3000 m) underwent resting-state fMRI scans. The independent component analysis (ICA) approach was used to analyze changes in the resting-state brain networks functional connectivity of pilots after hypoxic exposure, and 9 common components in brain functional networks were identified. In the functional connections that showed significant group differences, linear regression was used to examine the association between functional connectivity and clinical characteristics. The brain networks functional connectivity after hypoxia exposure decreased significantly, including the left frontoparietal network and visual network 1-area, left frontoparietal network and visual network 2-area, right frontoparietal network and visual network 2-area, dorsal attention network and ventral attention network, dorsal attention network and auditory network, and ventral attention network and visual network 1-area. We found no correlation between the altered functional connectivity and arterial oxygen saturation level. Our findings provide insights into the mechanisms underlying hypoxia-induced cognitive impairment in pilots.
Keywords: brain networks, functional connectivity, hypoxia exposure, pilots, resting-state fMRI
1. Introduction
Flight work is a high-level cognitive activity that requires a continuous and adequate oxygen supply. If a pilot develops hypoxia, he or she may be unable to adequately monitor all the flight systems in real time, which may result in a crash. Malle et al investigated the effects of acute hypobaric hypoxia on working memory (WM) in pilots using the Paced Auditory Serial Addition Test and found that WM was strongly impaired in the hypoxic group.[1] Using a cognitive test battery, Bouak et al found that the occurrence of hypoxic symptoms and decrements in target identification latency and accuracy may negatively affect flight performance in pilots.[2] These findings indicate that hypoxic exposure impairs cognition and memory in pilots. Physiological changes occur in the brain with hypoxia exposure,[3,4] and the main mechanisms underlying hypoxia-induced changes in cognitive function include oxidative stress and neuronal injury.[5]
The human brain is a highly interconnected and integrated network and comprises anatomically separated but functionally linked brain regions. The brain network functional connectivity model at rest reflects the significant coupling of spontaneous fluctuations in ongoing activities and provides a neuroanatomical basis for understanding human behavior and cognitive function.[6]Recently, resting-state functional magnetic resonance imaging (fMRI), which is based on the blood oxygen level dependent signal’s ability to indirectly reflect the activity of neurons in the brain,[7] has been used to explore the effect of hypoxia on cognitive function.[8–10] To investigate the cognitive and brain functional alterations associated with exposure to high-altitude hypoxia, Chen et al used resting-state fMRI to quantify brain functional connectivity alterations and found that differences in functional connectivity before and after hypoxia exposure in multiple brain regions were significantly correlated with cognitive changes.[10] Chen et al investigated the functional connectivity pattern of the default mode network (DMN) in pilots and suggested a potential relationship between flight experience and functional properties of the DMN.[6] More specifically, daily flying practice may activate the pilot’s DMN repeatedly and, ultimately, strengthen its activation level during the resting state.
While hypoxia symptoms experienced by pilots are too minor to affect flight performance and safety at altitudes below 14,000 feet (4267 m), they can occur at lower altitudes. For example, Tristan reported that military aviators recognized hypoxic symptoms at an altitude below 10,000 feet (3048 m).[11] However, few studies have evaluated the resting-state brain networks of pilots after mild hypoxic exposure. Therefore, in this study, by simulating the hypoxic conditions found at an altitude of 3000 m, we evaluated changes in the brain network functional connectivity of pilots after acute mild hypoxia using resting-state fMRI. The present findings may provide insights into the psychophysiological mechanisms behind mild hypoxia-induced cognitive impairment in pilots.
2. Materials and Methods
2.1. Participants
Between 2013 and 2015, 40 male pilots were admitted to the Air Force General Hospital in Beijing. The inclusion criteria were as follows: no known neurological, cardiovascular, or mental health issues, no tobacco or recreational drug use, no history of taking medications that might affect cognitive function, no chronic or genetic diseases, being able to cooperate to complete the study, and provided written informed consent. Based on these criteria, data from 5 participants were excluded because of a history of neurological or psychiatric disorders (n = 3), claustrophobia or inability to complete the examination (n = 1), and lack of written consent (n = 1). Therefore, data from 35 healthy right-handed pilots, aged 24 to 40 years (mean age: 30.6 ± 4.8 years) who had 310–3800 hour of flying experience (mean total flight hour: 1328.1 ± 940.1 hour) were included in the current study. All pilots were lowlanders, that is, born and living in the lowlands, and had at least a 14th grade education.
All procedures were conducted in accordance with the tenets of the Declaration of Helsinki and were approved by the Institutional Review Board of the First Affiliated Hospital of Zhengzhou University (2018-KY-03). Written informed consent was provided by all participants before enrollment in the study.
2.2. Equipment
A 3.0T magnetic resonance imaging (MRI) scanner (Discovery MR750; GE Healthcare, Chicago, IL) was used for imaging, and a CMS-50 finger-pulse oximeter (Contec Medical Systems Co., Ltd., Qinhuangdao City, China) was used to measure pulse rates and blood oxygen saturation. Additionally, an oxygen pillow (Beijing Jinxinxing Medical Device Factory, Beijing, China) was used to control oxygen concentrations to induce hypoxia in participants.
2.3. Hypoxia
The low-oxygen gas mixture comprised nitrogen and oxygen, with the oxygen concentration maintained at 14.5%. The low-oxygen device was composed of high-pressure cylinders, a pressure relief valve, medical oxygen pillow, and a breathing mask. During the experiment, the gas composition inhaled by each participant was equivalent to that experienced at an altitude of 3000 m.[12] Pulse oximetry was performed to monitor the pulse and oxygen saturation in the blood.
2.4. Procedures
Before the magnetic resonance imaging scan, participants were asked to wear a facemask adjusted for appropriate tightness connected with an oxygen pillow with normal air concentration. The pulse rate and peripheral oxygen saturation (SpO2) of each participant were measured using a finger-pulse oximeter.[13] We measured it twice and recorded the average. The resting-state fMRI scans of pre-low-oxygen mixed gas inhalation were first performed with participants wearing rubber earplugs and the tight-fitting facemask connected with an oxygen pillow with normal air concentration. During the scan, participants were asked to close their eyes and stay calm and motionless. All scans were performed by the same radiologist. For the resting-state fMRI scan, gradient-echo sequences were used with the following parameters: repetition time/echo time = 2000/35 ms, flip angle = 90°, layer thickness = 4.0 mm (no gap), layer number = 38, field of view = 24 cm × 24 cm, matrix = 64 × 64, and scanning time = 6 minutes and 50 seconds. Subsequently, we replaced the oxygen pillow with low-oxygen mixed gas and opened the valve to have the participants inhale the gas with an oxygen concentration of 14.5% at the beginning of the T1WI volumetric scanning, and the resting-state fMRI scans of post-low-oxygen mixed gas inhalation were performed successively. For the T1WI volumetric scan, 3D fast scattered gradient-echo brain volume imaging was used to collect the whole brain structure image, including the scalp image, with the following parameters: repetition time/echo time = 8.2/3.2 ms, flip angle = 12°, bandwidth = 31.25 kHz, layer thickness = 1.2 mm (no gap), 100 layers in total, field of view = 24 cm × 24 cm, matrix = 256 × 256, and scanning time = 2 minutes and 59 seconds. At the end of the experiment, pulse rates and oxygen saturation were measured as soon as possible. Likewise, we measured each parameter twice and recorded the mean value.
2.5. Data preprocessing
The scanned images were run in Gretna (www.nitrc.org/projects/gretna) software, within matrix laboratory (r2013b; MathWorks, Natick, MA). To eliminate the influence of machine instability and participant maladjustment, the data of the first 10 time points were excluded from the analysis. Preprocessing included data format conversion, time correction, motion correction, space standardization (standardization of fMRI data to the Montreal Neurological Institute template using the T1 registration method [voxel size, 3 mm3]) and smoothing with a 4-mm Gaussian kernel. The images of participants with head movement translation < 3 mm and rotation movement <3° were included in the analysis. Finally, 66 images were included in the independent component analysis (ICA).
2.6. Functional connectivity analysis
ICA, a data-driven resting-state fMRI analysis method, can effectively remove noise interference without prior hypothesis and traditional model driving; it is especially suitable for static functional connectivity network analysis.[14] The component analysis software, Group ICA of fMRI Toolbox (GIFT) (v1.3i; http://icatb.sourceforge.net), which is based on blind source separation technology, was used to analyze the fMRI data after independent preprocessing. Principal component analysis was used to reduce the data dimension. Within GIFT, the number of independent components was limited to 40 using the software for investigating the reliability of ICA estimates by clustering and visualization,[15] with 100 iterations. The independent components (including spatial maps and time series) of each participant were reconstructed, Fisher z-transformed and, finally, the time courses and spatial maps were normalized into z-scores. The display graphical user interface module of GIFT was used to show all the components of all participants, select the network components with the greatest correlation with the template provided by the GIFT software, and extract the same components of each participant to form a subordinate data package. The comparison of functional network connectivity between the study groups was conducted by multivariate analysis of covariance using the statistical package in GIFT.
2.7. Data and software availability
The raw data will be made available by the authors, without undue reservation, to any qualified researcher.
2.8. Statistical analyses
The average time series of each participant’s 9 functional networks were extracted, and the correlation coefficient between any 2 pairs of the time series was calculated by Pearson correlation analysis. The Pearson correlation coefficient was modified by Fisher z-transform to satisfy the normal distribution. Statistical parametric mapping 12 (Wellcome Centre for Human Neuroimaging, London, UK) was used to analyze the correlation coefficient of the functional network connectivity in the 2 groups by paired sample t tests. For the functional connections that showed significant group differences, we analyzed the associations between functional connectivity and clinical information (SpO2) using linear retrospective analysis. A P value of <.05 was considered statistically significant.
3. Results
3.1. Pulse rate and blood oxygen saturation
Changes in pulse rates and SpO2 of participants between the pre-low-oxygen mixed gas inhalation and post-low-oxygen mixed gas inhalation timepoints are shown in Table 1. Both the pulse rates and SpO2 levels were significantly reduced after hypoxic exposure, indicating that participants were in a state of acute, mild hypoxia (SpO2 = 83.6% ± 12.1%).[5]
Table 1.
Pulse rates and SpO2 levels before and after hypoxic exposure (mean ± SD, n = 35).
| Time points | Pulse rate (beats/min) | SpO2 (%) |
|---|---|---|
| Pre-OI | 71.40 ± 10.85 | 96.27 ± 1.29 |
| Post-OI | 63.97 ± 10.57 | 92.37 ± 3.85 |
| t values | 4.574 | 5.704 |
| P values | .000 | .000 |
Post-OI = post-low-oxygen mixed gas inhalation, Pre-OI = pre-low-oxygen mixed gas inhalation, SpO2 = arterial oxygen saturation.
3.2. Functional connectivity analysis
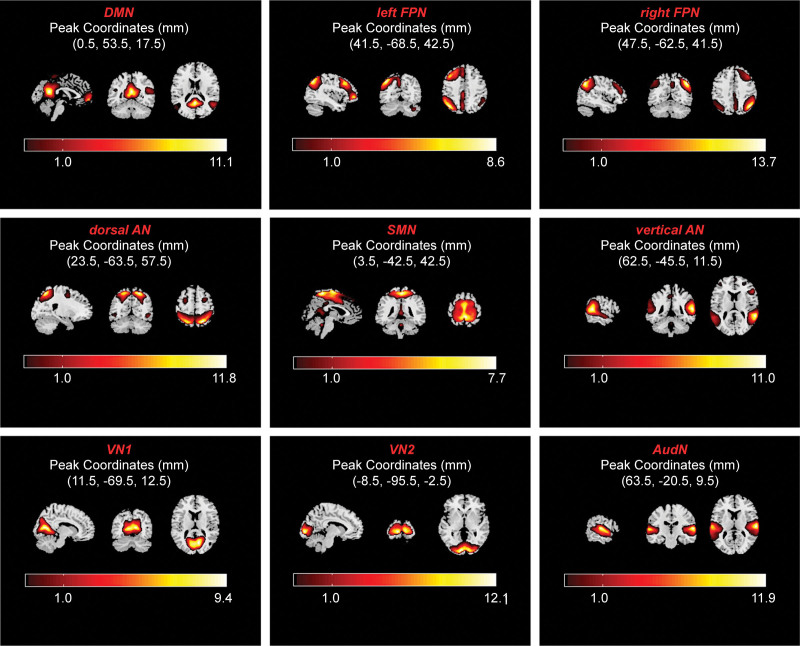
After ICA, a total of 28 resting brain networks were obtained. The components of the white matter, cerebrospinal fluid, and noise were removed. Finally, as shown in Figure 1, according to the degree of association with the template provided by the GIFT software, we selected 9 resting brain networks, including the DMN, left frontal-parietal network (left FPN), right frontal-parietal network (right FPN), sensorimotor network, dorsal attention network (dorsal AN), ventral attention network (ventral AN), visual network 1-area (VN1), visual network 2-area (VN2), and auditory network (AudN).
Figure 1.
Extracted components of the brain networks of interest. Abbreviations: AudN = auditory network, DMN = default mode network, dorsal AN = dorsal attention network, left FPN = left frontal-parietal network, right FPN = right frontal-parietal network, SMN = sensorimotor network, ventral AN = ventral attention network, VN1 = visual network 1-area, VN2 = visual network 2-area.
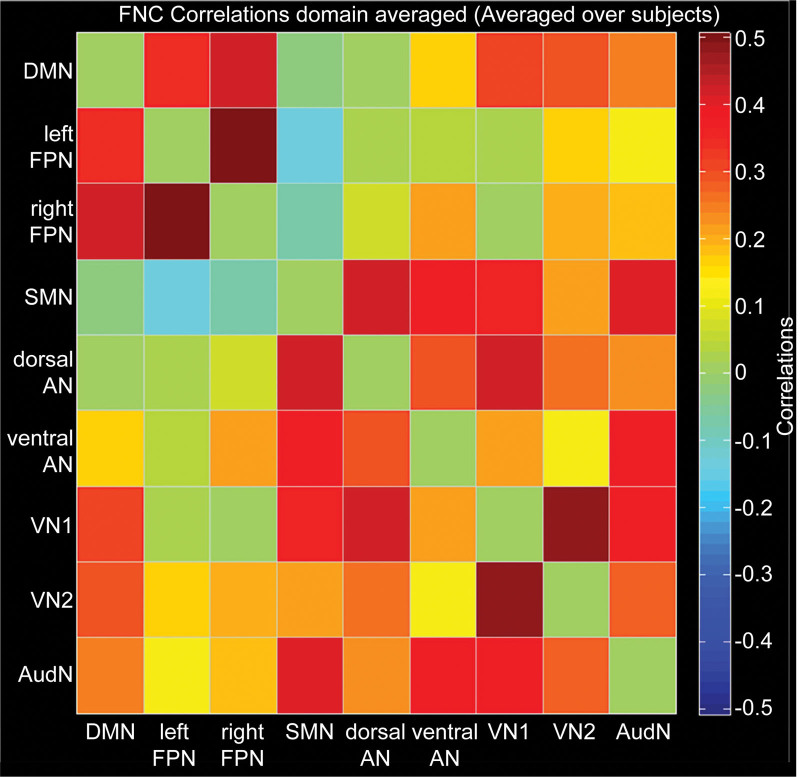
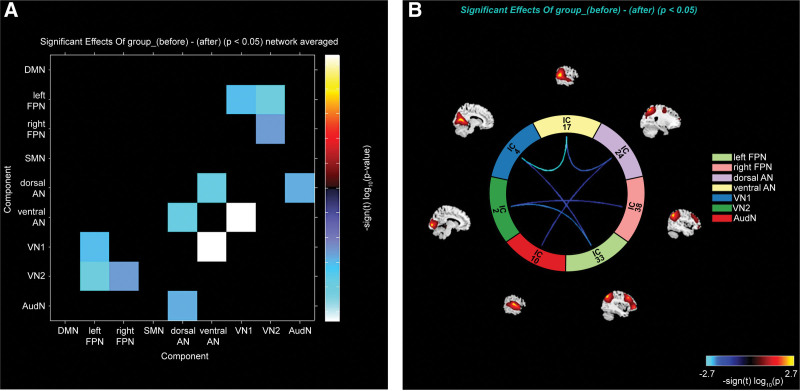
The correlation coefficients of network components are shown in Table 2 and Figure 2. By comparing the resting brain function networks of pilots before and after hypoxic exposures, we found that the connections of the following brain function networks after hypoxic exposure were significantly weakened: left FPN and VN1, left FPN and VN2, right FPN and VN2, dorsal AN and ventral AN, dorsal AN and AudN, ventral AN and VN1 (Fig. 3). There was no enhancement of the connection between other brain networks. In addition, we found no correlation between the changes in functional connectivity and SpO2.
Table 2.
Correlation coefficients of network components and template.
| Network names | Components (correlation) |
|---|---|
| DMN | 23 (0.54) |
| Left FPN | 33 (0.39) |
| Right FPN | 38 (0.5) |
| SMN | 18 (0.55) |
| Dorsal AN | 24 (0.61) |
| Ventral AN | 17 (0.51) |
| VN1 | 4 (0.70) |
| VN2 | 2 (0.6) |
| AudN | 10 (0.7) |
AudN = audit network, DMN = default mode network, dorsal AN = dorsal attention network, left FPN = left frontal-parietal network, right FPN = right frontal-parietal network, SMN = sensorimotor network, ventral AN = ventral attention network, VN1 = visual network 1-area, VN2 = visual network 2-area.
Figure 2.
Average correlation coefficients of network components. The matrix color represents the average correlation coefficients. Abbreviations: AudN = auditory network, DMN = default mode network, dorsal AN = dorsal attention network, left FPN = left frontal-parietal network, right FPN = right frontal-parietal network, SMN = sensorimotor network, ventral AN = ventral attention network, VN1 = visual network 1-area, VN2 = visual network 2-area.
Figure 3.
Decreased functional network connectivity after hypoxia exposure. Results are shown in the forms of matrix graph (A) and ring graph (B). The matrix color and connecting line color of the ring graph are related to changes in connectivity intensity. Values in the scale stand for t values of paired sample t test (P < .05). Abbreviations: AudN = auditory network, DMN = default mode network, dorsal AN = dorsal attention network, left FPN = left frontal-parietal network, right FPN = right frontal-parietal network, SMN = sensorimotor network, ventral AN = ventral attention network, VN1 = visual network 1-area, VN2 = visual network 2-area.
4. Discussion
The current study is a rare example of an investigation into alterations of the resting-state brain network functional connectivity in pilots after acute hypoxic exposure using resting-state fMRI. Our data showed that the pulse rate and blood oxygen saturation levels of participants decreased after hypoxic exposure, which is consistent with the findings of a previous study.[16] Oxygen saturation was maintained at approximately 92%; therefore, these participants were in a state of acute mild hypoxia.[2] SpO2 level was previously reported to be positively correlated with executive performance; that is, decreased SpO2 may lead to executive dysfunction in healthy adults after hypoxic exposure.[17–19] However, we found no correlation between the weakened functional connectivity and decreased SpO2 level. A possible reason is that the etiology of cognitive impairment caused by acute hypoxic exposure is complex. In addition to hypoxia itself, cognitive impairment may also be related to systemic stress response, and individual compensatory mechanisms differ greatly. Besides, as seen previously,[9,10,20] we found decreased resting-state brain network functional connectivity in pilots after acute hypoxic exposure, including the FPN and VN, dorsal AN and ventral AN, dorsal AN and AudN, and ventral AN and VN.
As the first execution network,[21] the FPN integrates motor, sensory and cognitive systems, completes the functional connection between information processing and execution, and participates in the brain’s adjustment and response to the surrounding environment after obtaining sensory information.[22] Notably, as the 2 main components of the FPN, the dorsolateral prefrontal cortex and precuneus are both relatively more sensitive to hypoxia.[6,7,16] Moreover, the FPN, which comprises the frontal eye fields and the lateral intraparietal area, has a pivotal role in complex cognitive control of higher-order brain functions, including oculomotor control, motor preparation, visual attention, and decision-making.[23] In this study, we found that the inter-network connectivities between the FPN and VN were reduced. We speculate that it may be related to the weakening of the synergy between the FPN and VN in the process of performing executive function and visual attention. These changes in functional connectivity between the FPN and VN could explain why abnormal cognitive function occurs in pilots after hypoxic exposure, such as impaired WM and abnormal visual and spatial information processing.[1]
It is well established that the AN chiefly consists of the dorsal and ventral AN. The former is responsible for endogenous goal-directed and exogenous orienting of attention, the latter is involved in reorienting attention to salient stimulation, which together constitute the neural basis of attention. Generally, the 2 anatomically separate subnetworks tend to cooperate with each other by specialized functional connectivity patterns for higher-order cognitive processes.[24] Decreased connectivity between the dorsal AN and ventral AN may reflect disruption of the couplings between them during attentional processing. This alteration may result in attention deficit related to spatial (re-) orientating, which makes it impossible to quickly detect and direct attention towards major changes in the peripheral environment.[25] As 1 of the core regions of dorsal AN, the superior parietal gyrus is critical for sensory perception and interpretation, especially sound processing,[10] and hearing plays an important role in spatial perception and orientation.[26–28] The reduced functional connectivity between the dorsal AN and AudN is considered to be related to the top-down or endogenous attention downregulation of auditory spatial receptive fields in the auditory association cortex. Additionally, we also observed a decrease in connectivity between the ventral AN and VN. It was noted that the decreased functional connectivity between the 2 might suggest the dysfunction of visuospatial attention system.[29] The ventral AN has been shown to be involved in visual attention span; that is, the stimulus-evoked attention significantly activates the ventral AN to reorient the visuospatial attention.[30] A possible explanation for our finding is the decreased perception of endogenous and exogenous stimuli after hypoxia exposure. Overall, decreased functional connectivity may reflect impaired cognitive function of pilots after hypoxia exposure, and such findings seem to provide valuable insights into understanding the psychophysiological mechanisms of flying.
There are some limitations to our study. First, this was a preliminary study evaluating the resting-state functional connectivity in pilots after simulated hypoxic exposure, which differ from the actual flight environment in which complicated environmental variations and flight-task load occurs. Second, we only investigated the impact of hypoxia in male pilots, and it is not clear if there is a similar phenomenon in female pilots. The number of female pilots in China is small; thus, it is difficult to recruit enough female pilots to participate. Third, our study regrettably did not conduct neuropsychological tests to assess the cognitive function of pilots. A previous study using neural ergonomics to study the effect of hypoxia on pilots’ cognitive function found that hypoxia led to cognitive dysfunction at an altitude of approximately 3000 m.[2] Therefore, we felt that the neuropsychological assessment results will be biased. Fourth, our study only simulated 1 flight altitude, the number of subjects was relatively small, and flight experience varied greatly; nevertheless, our resources and the timing of the experiment did not allow us to have additional participants. Hence, further studies with flight-related task fMRI, a large sample size, grouping according to different simulated flight altitudes and flight experience are needed to better understand the changes in brain functional connectivity in pilots during flight.
In conclusion, our findings confirmed the feasibility of simulating hypoxic environmental conditions with MRI equipment and evaluating the alterations in functional connections in pilots after hypoxic exposure using resting-state fMRI; besides, the current study demonstrates decreased inter-network functional connectivity among the FPN, AN, VN, and AudN in pilots after hypoxic exposure, which may help to explain and to provide new insights into the mechanism underlying the impairment of brain function of pilots under hypoxic exposure. This preliminary study sheds light on how hypoxic conditions impact pilots’ cognitive function and provides a new method and experimental reference.
Acknowledgments
We would like to thank GE Healthcare China in Beijing for their support of this work and Yu Tian, Mingyang Ding, and Ling Fang for assistance in data collection. We would like to thank Editage (www.editage.cn) for English language editing.
Author contributions
Conceptualization: Wanshi Zhang.
Data curation: Jie Liu, Shujian Li, Mingxi Liu.
Formal analysis: Jie Liu.
Funding acquisition: Jingliang Cheng.
Investigation: Jie Liu, Wanshi Zhang.
Methodology: Jie Liu, Mingxi Liu, Xianrong Xu, Wanshi Zhang.
Resources: Xianrong Xu.
Software: Yong Zhang, Jingliang Cheng.
Supervision: Yong Zhang, Jingliang Cheng, Wanshi Zhang.
Visualization: Shujian Li, Wanshi Zhang.
Writing – original draft: Jie Liu.
Writing – review & editing: Yong Zhang, Jingliang Cheng, Wanshi Zhang.
Abbreviations:
- AN =
- attention network
- AudN =
- auditory network
- DMN =
- default mode network
- fMRI =
- functional magnetic resonance imaging
- FPN =
- frontal parietal network
- GIFT =
- group ICA of fMRI toolbox
- ICA =
- independent component analysis
- SpO2 =
- peripheral oxygen saturation
- VN =
- visual network
- WM =
- working memory
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
The authors have no funding and conflicts of interest to disclose.
How to cite this article: Liu J, Li S, Liu M, Xu X, Zhang Y, Cheng J, Zhang W. Impaired brain networks functional connectivity after acute mild hypoxia. Medicine 2022;101:38(e30485).
Contributor Information
Jie Liu, Email: 2278880946@qq.com.
Shujian Li, Email: tongnuo@yeah.net.
Mingxi Liu, Email: 2278880946@qq.com.
Xianrong Xu, Email: 349722823@qq.com.
Yong Zhang, Email: 554396376@qq.com.
Wanshi Zhang, Email: 554396376@qq.com.
References
- [1].Malle C, Quinette P, Laisney M, et al. Working memory impairment in pilots exposed to acute hypobaric hypoxia. Aviat Space Environ Med. 2013;84:773–9. [DOI] [PubMed] [Google Scholar]
- [2].Bouak F, Vartanian O, Hofer K, et al. Acute mild hypoxic hypoxia effects on cognitive and simulated aircraft pilot performance. Aerosp Med Hum Perform. 2018;89:526–35. [DOI] [PubMed] [Google Scholar]
- [3].Ma H, Zhang D, Li X, et al. Long-term exposure to high altitude attenuates verbal and spatial working memory: evidence from an event-related potential study. Brain Behav. 2019;9:e01256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Qaid E, Zakaria R, Sulaiman SF, et al. Insight into potential mechanisms of hypobaric hypoxia-induced learning and memory deficit—lessons from rat studies. Hum Exp Toxicol. 2017;36:1315–25. [DOI] [PubMed] [Google Scholar]
- [5].Lefferts WK, DeBlois JP, White CN, et al. Changes in cognitive function and latent processes of decision-making during incremental ascent to high altitude. Physiol Behav. 2019;201:139–45. [DOI] [PubMed] [Google Scholar]
- [6].Chen X, Xu K, Yang Y, et al. Altered default mode network dynamics in civil aviation pilots. Front Neurosci. 2020;13:1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Raichle ME, Macleod AM, Snyder AZ, et al. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Jiang Y, Luo C, Li X, et al. White-matter functional networks changes in patients with schizophrenia. Neuroimage. 2019;190:172–81. [DOI] [PubMed] [Google Scholar]
- [9].Nisha SN, Fathinul Fikri ASF, Aida AR, et al. The objective assessment of the effects on cognition functioning among military personnel exposed to hypobaric-hypoxia: a pilot fMRI study. Med J Malaysia. 2020;75:62–7. [PubMed] [Google Scholar]
- [10].Chen X, Zhang Q, Wang J, et al. Cognitive and neuroimaging changes in healthy immigrants upon relocation to a high altitude: a panel study. Hum Brain Mapp. 2017;38:3865–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Tristan LR. Hypoxia occurrence in a military aviator below 3048 m. Aerosp Med Hum Perform. 2017;88:61–4. [DOI] [PubMed] [Google Scholar]
- [12].Kasai N, Kojima C, Goto K. Metabolic and performance responses to sprint exercise under hypoxia among female athletes. Sports Med Int Open. 2018;2:E71–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Villien M, Bouzat P, Rupp T, et al. Changes in cerebral blood flow and vasoreactivity to CO2 measured by arterial spin labeling after 6 days at 4350m. Neuroimage. 2013;72:272–9. [DOI] [PubMed] [Google Scholar]
- [14].Seewoo BJ, Joos AC, Feindel KW. An analytical workflow for seed-based correlation and independent component analysis in interventional resting-state fMRI studies. Neurosci Res. 2021;165:26–37. [DOI] [PubMed] [Google Scholar]
- [15].Calhoun VD, Adali T, Pearlson GD, et al. A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp. 2001;14:140–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ochi G, Kanazawa Y, Hyodo K, et al. Hypoxia-induced lowered executive function depends on arterial oxygen desaturation. J Physiol Sci. 2018;68:847–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].McMorris T, Hale BJ, Barwood M, et al. Effect of acute hypoxia on cognition: a systematic review and meta-regression analysis. Neurosci Biobehav Rev. 2017;74:225–32. [DOI] [PubMed] [Google Scholar]
- [18].Taylor L, Watkins SL, Marshall H, et al. The impact of different environmental conditions on cognitive function: a focused review. Front Physiol. 2015;6:372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Turner CE, Barker-Collo SL, Connell CJW, et al. Acute hypoxic gas breathing severely impairs cognition and task learning in humans. Physiol Behav. 2015;142:104–10. [DOI] [PubMed] [Google Scholar]
- [20].Chen J, Li JQ, Han QQ, et al. Long-term acclimatization to high-altitude hypoxia modifies interhemispheric functional and structural connectivity in the adult brain. Brain Behav. 2016;6:e00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Liu YF, Kim J, Wilson C, et al. Computer code comprehension shares neural resources with formal logical inference in the fronto-parietal network. eLife. 2020;9:e59340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Assem M, Blank IA, Mineroff Z, et al. Activity in the fronto-parietal multiple-demand network is robustly associated with individual differences in working memory and fluid intelligence. Cortex. 2020;131:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].D’Souza JF, Price NSC, Hagan MA. Marmosets: a promising model for probing the neural mechanisms underlying complex visual networks such as the frontal–parietal network. Brain Struct Funct. 2021;226:3007–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Suo X, Ding H, Li X, et al. Anatomical and functional coupling between the dorsal and ventral attention networks. Neuroimage. 2021;232:117868. [DOI] [PubMed] [Google Scholar]
- [25].Liu S, Zhao B, Shi C, et al. Ocular dominance and functional asymmetry in visual attention networks. Invest Ophthalmol Vis Sci. 2021;62:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Adams WJ. The development of audio-visual integration for temporal judgements. PLOS Comput Biol. 2016;12:e1004865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kaposvári P, Csete G, Bognár A, et al. Audio-visual integration through the parallel visual pathways. Brain Res. 2015;1624:71–7. [DOI] [PubMed] [Google Scholar]
- [28].Oh SY, Boegle R, Ertl M, et al. Multisensory vestibular, vestibular-auditory, and auditory network effects revealed by parametric sound pressure stimulation. Neuroimage. 2018;176:354–63. [DOI] [PubMed] [Google Scholar]
- [29].Hou YB, Wei QQ, Ou RW, et al. Different resting-state network disruptions in newly diagnosed drug-naïve Parkinson’s disease patients with mild cognitive impairment. BMC Neurol. 2021;21:327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zhao J, Wang JK, Huang C, et al. Involvement of the dorsal and ventral attention networks in visual attention span. Hum Brain Mapp. 2022;43:1941–54. [DOI] [PMC free article] [PubMed] [Google Scholar]