Abstract
Low-temperature-adapted archaea are abundant in the environment, yet little is known about the thermal adaptation of their proteins. We have previously compared elongation factor 2 (EF-2) proteins from Antarctic (Methanococcoides burtonii) and thermophilic (Methanosarcina thermophila) methanogens and found that the M. burtonii EF-2 had greater intrinsic activity at low temperatures and lower thermal stability at high temperatures (T. Thomas and R. Cavicchioli, J. Bacteriol. 182:1328–1332, 2000). While the gross thermal properties correlated with growth temperature, the activity and stability profiles of the EF-2 proteins did not precisely match the optimal growth temperature of each organism. This indicated that intracellular components may affect the thermal characteristics of the EF-2 proteins, and in this study we examined the effects of ribosomes and intracellular solutes. At a high growth temperature the thermophile produced high levels of potassium glutamate, which, when assayed in vitro with EF-2, retarded thermal unfolding and increased catalytic efficiency. In contrast, for the Antarctic methanogen adaptation to growth at a low temperature did not involve the accumulation of stabilizing organic solutes but appeared to result from an increased affinity of EF-2 for GTP and high levels of EF-2 in the cell relative to its low growth rate. Furthermore, ribosomes greatly stimulated GTPase activity and moderately stabilized both EF-2 proteins. These findings illustrate the different physiological strategies that have evolved in two phylogenetically related but thermally distinct methanogens to enable EF-2 to function satisfactorily.
Despite the knowledge that low-temperature-adapted (psychrophilic or psychrotolerant) archaea are abundant and are suspected to play key ecological roles in low-temperature environments (9, 10, 25, 26), studies into the molecular and physiological mechanisms of low-temperature adaptation in archaea is a field in its infancy (7). Progress has mainly been hampered by the marginal success in isolating and cultivating archaea from these environments. One of the few free-living psychrotolerant species is Methanococcoides burtonii (minimum growth temperature, −2.5°C; optimal growth temperature [Topt], 23°C), which was isolated from saline, methane-saturated water in Ace Lake, Antarctica, at an in situ temperature of 1 to 2°C (13). Other species include the psychrophile Methanogenium frigidum (12), the psychrotolerant species Halorubrum lacusprofundii (14), and the sponge symbiont Cenarchaeum symbiosum (28). The extent of knowledge in this field (reviewed in reference 7) is limited to studies on the low-temperature regulation of a DEAD-box RNA helicase gene and the role of CspA-like proteins from M. burtonii (22), DNA sequencing of genome sections and biochemical characterization of a DNA polymerase from C. symbiosum (33), and structural and biochemical studies of elongation factor 2 (EF-2) from M. burtonii (37, 38).
EF-2 is a GTPase, which, like its bacterial homologue elongation factor G (EF-G), interacts in its GTP-bound state with ribosomes and catalyzes translocation. During this interaction, GTP is hydrolyzed and the GDP-bound form of the elongation factor releases the ribosome and is available to bind GTP and enter a new translocation cycle. The interaction of the elongation factor with the ribosome and the ability to hydrolyze GTP are part of a cooperative process. This is illustrated by the low levels of GTP hydrolysis by elongation factor proteins in the absence of ribosomes (11, 29) and the greatly increased peptidyl-chain elongation with the addition of EF-G to ribosomes (27). Elongation factor proteins (EF-2 and EF-G) are therefore crucial as accessory proteins to ribosomes and for normal cell function.
During cold shock or growth at low temperature, translation becomes limiting (reviewed in references 16, 32, and 41). Ribosomes are involved in sensing cold shock (40), and modifying the translation machinery to facilitate protein synthesis at low temperature is a key factor in the cold shock responses of Escherichia coli (41) and Bacillus subtilis (16). In psychrophilic Pseudomonas spp. and Bacillus spp., the translation apparatus appears to be adapted to activity at low temperatures (reviewed in reference 32). At the other end of the temperature spectrum, the thermal profile of activity of EF-2 from the thermophilic archaeon Sulfolobus solfataricus matches well with its optimal growth temperature (29).
In a recent study, members of our group characterized the ribosome-independent GTPase activity and stability of the EF-2 proteins from M. burtonii and the closely related, moderate thermophile, Methanosarcina thermophila (38). These studies showed that EF-2 from M. burtonii had a decreased activation energy for GTP hydrolysis and for protein unfolding in comparison to its thermophilic counterpart, thereby possessing biochemical properties that should assist function at low temperatures. However, comparison of these intrinsic properties with the growth temperature range of the parent organism indicated that intracellular factors were likely to be important for EF-2 function in the cell. Specifically, the EF-2 from M. burtonii showed a relatively low initial reaction rate of GTP hydrolysis at its optimal growth temperature, with the highest level of activity occurring at higher temperatures, whereas the EF-2 from M. thermophila was most active at temperatures below its optimal growth temperature. In this study we examined the effects of ribosomes and intracellular solutes on the activities and stabilities of the EF-2 proteins from both methanogens to identify the factors that may contribute to the difference between the observed intrinsic activities and the physiological activities that might be expected. As a result, we identified growth-temperature-dependent properties and intracellular components that may be important for the ability of EF-2 proteins to function effectively at optimal physiological growth temperatures.
MATERIALS AND METHODS
Growth conditions.
M. burtonii (strain DSM 6242) and M. thermophila (strain DSM 1825) were originally isolated from a water sample from Ace Lake, Antarctica (13), and thermophilic digester sludge (42), respectively. The strains were grown anaerobically in a modified methanogen growth medium (MFM) and a gas phase of 80:20 N2-CO2 (13). MFM is equivalent to MGM (13), with the concentrations modified as follows: for sodium chloride, 23.37 g liter−1; for trimethylammonium chloride, 5 g liter−1; for sodium hydrogen actetate, 2.52 g liter−1 and for yeast extract, 2 g liter−1. When cells were grown for intracellular solute extraction, the yeast extract was omitted to provide a completely defined medium. Cells were grown from frozen glycerol stocks in the modified medium and passaged three times before being used as the inocula for cell growth for ribosome preparations and intracellular solute extraction. One-liter culture volumes were grown without shaking in 2-liter Schott bottles sealed with butyl-rubber stoppers. Growth was monitored by measuring the optical density at 600 nm.
Ribosome preparation.
Ribosomes were purified from M. burtonii and M. thermophila by a modification of the method described by Matthaei and Nirenberg (24). One-liter volumes of M. burtonii and M. thermophila grown at 23 and 42°C, respectively, were harvested in mid-logarithmic growth by centrifugation at 10,000 × g for 10 min at 4°C. The supernatant was removed, and the pellet was immediately resuspended in 3 ml of buffer (10 mM Tris-HCl, pH 7.5; 10 mM magnesium acetate; 60 mM potassium chloride; 6 mM phenylmethylsulfonyl fluoride). Cells were lysed by sonication on ice using a power setting of 4 and a 40% duty cycle with a Branson Sonifier. Cell extracts were centrifuged three times at 30,000 × g for 20 min at 4°C each time, and the final supernatant was centrifuged at 105,000 × g for 4 h at 4°C. The pellet was resuspended in 300 μl of high-salt-content buffer (20 mM Tris-HCl, pH 7.5; 10 mM magnesium acetate; 500 mM ammonium chloride; 0.5 mM dithiothreitol). Using 1.5-ml microcentrifuge tubes, the suspension was layered over 800 μl of high-salt-content buffer supplemented with 0.5 M sucrose and centrifuged at 105,000 × g for 12 h at 4°C. The pellet was resuspended in 300 μl of high-salt-content buffer and covered with a layer of sucrose, and the centrifugation step was repeated. The final pellet, containing the purified ribosomes, was resuspended in 100 μl of storage buffer (20 mM morpholinepropanesulfonic acid [MOPS], pH 7.5; 10 mM ammonium chloride; 10 mM magnesium acetate; 1 mM dithiothreitol; 50% [vol/vol] glyerol) and stored at −20°C. The ribosome quality was checked by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (20), and the ribosome quantity was assessed using spectrophotometry, with assessments based on the assumption that one absorption unit at 260 nm was equal to 25 pmol of ribosomes (29).
Intracellular solute analysis by nuclear magnetic resonance (NMR) spectroscopy.
Intracellular solutes were obtained using an ethanol extraction protocol establishsed for methanogens (21). One-liter culture volumes (mid-logarithmic growth phase) of M. burtonii grown at 8 and 23°C and M. thermophila grown at 30 and 42°C were extracted. Cell extracts were freeze-dried and resuspended in deuterium oxide.
13C and 1H NMR spectra were recorded at room temperature in 5-mm-diameter tubes on a Bruker DPX 300 or DMX 500 spectrometer, using the deuterium signal of the solvent as the lock. The chemical shifts were read from the residual protonated solvent.
One-dimensional 1H NMR experiments were carried out on a Bruker DPX 300 spectrometer with a spectral width of ca. 3,500 Hz, a 45° pulse angle, 32,000 data points, and a repetition delay of 10.0 s. One-dimensional 13C NMR spectra were recorded in the pulsed Fourier transfer mode (64,000 data points for the flame ionization detector [FID]) at 298 K on a Bruker DPX 300 spectrometer operating at 75.47 MHz or a Bruker DMX 500 spectrometer operating at 125.77 MHz. DEPT 135 experiments were carried out on a Bruker DPX 300 spectrometer using the typical DEPT 135 sequence, and the following acquisition parameters: AQ = 1.37 s, D1 = 3 s, SI = 64,000, P1 = 7.5 s, P3 = 9.84 s, PL1 = −3dB, PL2 = −3dB, PL12 = −17.6dB, PCPD = 105 s.
The DQF-COSY spectra were recorded on a Bruker DMX 600 spectrometer (TXI 5-mm probe) using a spectral width of 3,500 Hz and a repetition delay of 2.0 s. For each FID, four scans were accumulated. The two-dimensional data were acquired with 512 increments in the F1 dimension and 2,048 data points in the F2 dimension and were zero filled prior to Fourier transformation. A q-sine-bell window function was applied in both dimensions. 1H-detected gradient HSQC and HMBC experiments were recorded on a Bruker DMX 600 MHz spectrometer using the pulse sequence invieagssi and inv4gplplrnd micro programs of the Bruker software, respectively. The spectral widths used in the experiments were ca. 2,800 Hz and 200 ppm in the F2 (1H) and F1 (13C) dimensions, respectively. The spectra were acquired with 512 increments in the F1 dimension with four scans and 2,048 data points in the F2 dimension. After zero filling the F1 dimension, the data was processed using q-sine-bell weighting functions in both dimensions.
The structures of the solutes were elucidated from a combination of one-dimensional and two-dimensional (DQF-COSY, HSQC, and HMBC) NMR spectroscopy experiments and comparison of 1H and 13C chemical shifts with the chemical shifts described elsewhere (21, 23, 31). Solutes were quantified and correlated to total cell protein as described previously (23).
Stability and activity assays.
EF-2 proteins from M. burtonii and M. thermophila were purified as described previously (38). The ribosome-dependent GTPase activities of the EF-2 proteins were determined in a solution containing 20 mM MOPS (pH 7.5), 10 mM ammonium chloride, 10 mM magnesium actetate, 1 mM dithiothreitol, 1 mM spermine, 3 μM EF-2 protein, 10 μM ribosomes, and 50 μM [α-32P]GTP (0.5 μCi), with the addition of specific salts as indicated below. The amount of GDP formed was determined from radioactive images generated on phosphor screens as described previously (38). For Michaelis-Menten kinetics the [α-32P]GTP concentration used ranged from 15 to 400 μM. GTP hydrolysis was monitored over time, and the initial reaction rate (V) was determined from the linear portion of the graph. Values for V were plotted against the substrate concentration in a double-reciprocal Lineweaver-Burk plot. Michaelis-Menten constants (Km values) and maximum reaction velocities (Vmax) were calculated from the slope and the y-axis intercept, respectively. Duplicate measurements within an experiment showed less than 10% variation, and experimental values varied less than 10% between experiments. The data reported here are averages from all experiments.
The thermal unfolding of the EF-2 proteins was determined by differential scanning calorimetry (DSC) as described previously (38). To examine the effect of salts on thermal unfolding (see Results), EF-2 proteins were dialyzed with a solution containing 20 mM MOPS (pH 7.5), 1 mM β-mercaptoethanol, and a specified concentration of glutamate or aspartate. The final dialysate was kept as a reference for the DSC experiments. The activation energy of unfolding was determined for two different scan rates (1.5 and 0.1 K per min) as described previously (38).
Western blot analysis and enzyme-linked immunosorbent assay (ELISA).
Antibodies against purified M. burtonii EF-2 were raised in rabbits by the Institute of Medical and Veterinary Science (Adelaide, Australia). Cellular proteins were extracted from 100-ml batches of mid- and late-logarithmic-phase cultures of M. burtonii cells grown in MFM at 8 and 23°C. Cells were harvested by centrifugation at 10,000 × g for 10 min at 4°C, resuspended in 20 mM Tris-HCl (pH 7.5), and lysed by three freeze-thaw cycles. Ten micrograms of total protein was separated by SDS-PAGE (20) and transferred by electroblotting onto a polyvinylidene difluoride membrane (Bio-Rad) (39). The membrane was blocked by incubation at room temperature in phosphate-buffered saline (PBS) (8 g of sodium chloride liter−1, 0.2 g of potassium chloride liter−1, 1.15 g of disodium hydrogen phosphate liter−1) containing 5% (wt/vol) skim milk powder (SMP). Anti-EF-2 antibody binding was performed overnight at 4°C in a solution containing a 1:500 dilution of the rabbit antiserum in PBS plus 1% (wt/vol) SMP. The blot was washed extensively with PBS containing 0.05% (vol/vol) Tween 20 (Sigma). The membrane was incubated at room temperature with a 1:5,000 dilution of a secondary antibody, donkey anti-rabbit horseradish peroxidase (Jackson Immuno Research Lab. Inc.) in PBS with 1% (wt/vol) SMP. The membrane was washed as described above, and antibody detection was performed with a Renaissance Western Blot Chemiluminescence kit (NEN) according to the manufacturer's specifications. Bands were visualized by exposing the blot to photographic film.
Quantitative ELISA was performed to accurately determine the amount of EF-2 in cell extracts. Whole cell protein extracts and purified EF-2 were serially diluted in carbonate buffer (1.538 g of disodium carbonate liter−1, 2.93 g of sodium hydrogen carbonate liter−1 [pH 9.6]) and immobilized onto MaxiSorb microtiter plates (NUNC) by incubation overnight at 4°C. The washing steps and primary and secondary antibody binding were performed as described above, except that a donkey anti-rabbit antibody conjugated to alkaline phosphatase (Pierce) was used. Detection was performed with the TMB-peroxidase Kit (Kirkegaard and Perry). After blue color development, the reaction was stopped by the addition of an equal volume of 2 M sulfuric acid and the absorption at 450 nm was measured. A standard curve was generated using purified EF-2. For determining the level of EF-2 in cell extracts, only concentrations of the cell extracts that fell within the linear range of the response curve were used.
RESULTS
EF-2 proteins from M. burtonii and M. thermophila have relatively low intrinsic GTPase activities (38). The activities were temperature dependent and stimulated by the presence of aliphatic alcohols and divalent cations (38), characteristics that have also been observed for EF-G from E. coli (11) and EF-2 from S. solfataricus (30). To extend our understanding of the potential role of physiological components in thermal adaptation, we examined the effects of ribosomes and intracellular solutes.
Effect of temperature on ribosome-dependent GTPase activity.
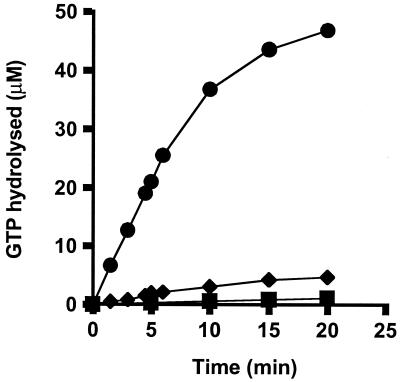
Ribosomes greatly stimulated GTPase activity of the EF-2 from M. burtonii (Fig. 1) and M. thermophila (data not shown). Rates of hydrolysis in the presence of ribosomes were at least 200-fold higher than those in the absence of ribosomes. Ribosome preparations showed a weak intrinsic GTPase activity, as has been reported for S. solfataricus (29). This activity was calculated for all GTPase experiments and used for baseline subtraction.
FIG. 1.
Stimulation of M. burtonii EF-2 activity by ribosomes. GTP hydrolysis was measured at 35°C in the presence of ribosomes (10 μM) and EF-2 (3 μM) from M. burtonii (●), ribosomes alone (10 μM) (⧫), or EF-2 alone (3 μM) (■).
The GTPase activity of the M. burtonii EF-2 was equivalent to that of ribosomes isolated from M. burtonii and M. thermophila (data not shown). Similar results were observed for the ribosomal stimulation of the M. thermophila EF-2. This indicates that interactions between EF-2 and the ribosome are structurally and functionally similar for these organisms.
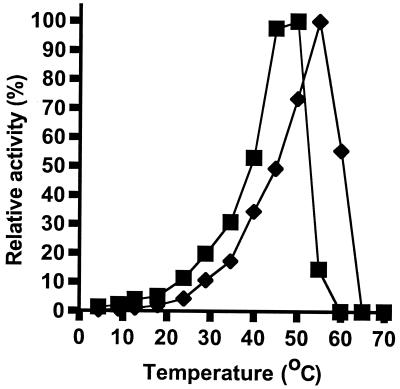
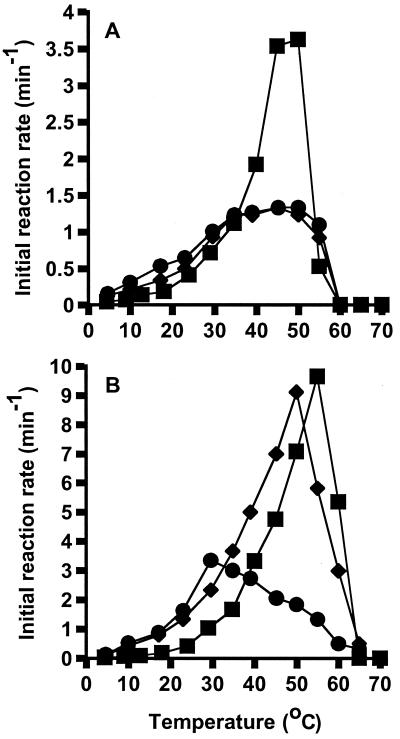
The combined effect of ribosomes and temperature on GTPase activity was assessed by determining initial rates of hydrolysis in the presence of 50 μM GTP over a temperature range from 4 to 70°C (Fig. 2). The overall effect of temperature was a shift of the M. burtonii EF-2 activity profile towards a temperature lower than that to which M. thermophila shifted.
FIG. 2.
Effect of ribosomes and temperature on EF-2 activity. Relative GTPase activity for combinations of EF-2 and ribosomes from M. burtonii (■) and M. thermophila (⧫).
The maximal activities for the EF-2 proteins were 3.5 and 9.6 mol of GTP hydrolyzed mol−1 min−1 for the M. burtonii EF-2 at 50°C and the M. thermophila EF-2 at 55°C, respectively. This indicates that under the conditions tested the M. thermophila EF-2 is a more active protein than the M. burtonii EF-2.
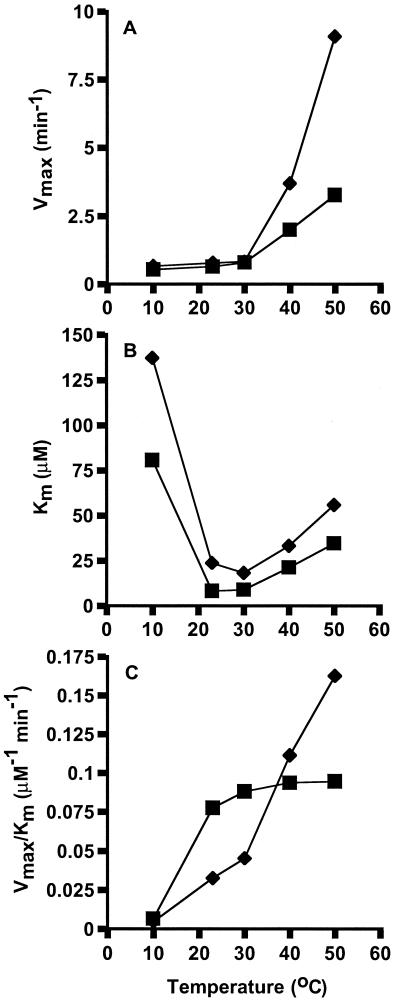
To examine the effect of temperature on the kinetics of ribosome-dependent EF-2 GTPase activity, Michaelis-Menten constants and catalytic efficiencies (Vmax/Km) were determined (Fig. 3). In the temperature range between 10 and 30°C, both EF-2 proteins had low Vmax values (Fig. 3A). Above 30°C, the Vmax values increased rapidly, particularly for the M. thermophila EF-2 (9.1 min−1 at 50°C compared to 3.3 min−1 for M. burtonii EF-2). At all temperatures tested the Km values for the M. burtonii EF-2 were lower than those for the M. thermophila EF-2 (Fig. 3B). Values for Km were lowest for both proteins between 23 and 30°C and increased with temperatures above 30°C. At 10°C, the Km for EF-2 proteins increased to 137 μM for M. thermophila and 81 μM for M. burtonii. The values for Vmax and Km show that EF-2 from the thermophile can achieve a higher rate of GTP hydrolysis at relatively high temperatures, while the affinity for GTP is highest with the EF-2 from the psychrotolerant strain, especially at relatively low temperatures.
FIG. 3.
Temperature dependence on Michaelis-Menten kinetics. The maximum reaction velocity (Vmax) (A), Michaelis-Menten constant (Km) (B), and catalytic efficiency (Vmax/Km) (C) were determined for the ribosome-dependent GTPase activity of M. burtonii EF-2 (■) and M. thermophila EF-2 (⧫).
As a result of the different effects of temperature on Vmax and Km for the two proteins, the catalytic efficiency (Vmax/Km) was higher for the M. burtonii EF-2 at low temperatures, whereas the catalytic efficiency for the M. thermophila EF-2 was minimal at low temperatures and greater at higher temperatures, reaching peak levels at the highest temperature tested (Fig. 3C). In combination, these data indicate that the M. burtonii EF-2 has the capacity to function at a low temperature by increasing affinity for GTP and improving catalytic efficiency.
Characterization of intracellular solutes and their effect on stability and activity of EF-2 proteins.
The intracellular solutes from M. burtonii and M. thermophila were examined to determine if there were compositional differences between the organisms or temperature-induced changes for either organism. These data were then related to EF-2 activity and stability.
MFM, the medium used for M. thermophila, supported growth in a disaggregated form (35), which was confirmed by microscopy. Growth could therefore be monitored by measuring the optical density, and the cells could be harvested at specific growth phases (e.g., mid-logarithmic). Using these culture conditions, the apparent temperature optimum for growth for M. thermophila was found to be 40 to 45°C, with no growth occurring at 50°C, which is consistent with results obtained in previous studies (35).
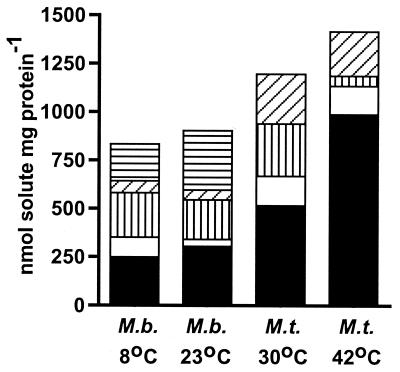
Using NMR spectroscopy analysis, the intracellular solute composition was determined for M. thermophila grown at 42 and 30°C and for M. burtonii grown at 23°C (Topt) and 8°C (Fig. 4). The organic solutes identified were compounds that are known to be synthesized by methanogens, including l-α-glutamate, l-alanine, Nɛ-acetyl-β-lysine and l-aspartate (reviewed in reference 31), and a novel compound, β-alanine betaine. It is unlikely that the β-alanine betaine was accumulated from the medium in a fashion similar to that previously described for glycine betaine (reviewed in reference 15), since the growth medium was a completely defined medium. A detailed description of β-alanine betaine from the two methanogens will appear elsewhere (T. Thomas et al., unpublished data).
FIG. 4.
Intracellular, organic solute composition for M. burtonii (M. b.) and M. thermophila (M.t.) grown in MFM at Topt (23 and 42°C, respectively) and low temperatures (8 and 30°C, respectively). Solid black bars, l-α-glutamate; solid white bars, l-alanine; horizontal striped bars, l-aspartate; vertical striped bars, Nɛ-acetyl-β-lysine; diagonal striped bars, β-alanine betaine.
A striking compositional difference between the organic solutes from M. burtonii and M. thermophila relates to the levels of l-α-glutamate and l-aspartate (Fig. 4). The levels of l-α-glutamate were higher in M. thermophila than they were in M. burtonii, and the concentration in M. thermophila increased with the growth temperature. In contrast, l-aspartate was not detected in M. thermophila but was present at relatively high levels in M. burtonii.
To examine the effects of these two amino acids on EF-2 activity and stability, potassium salts were chosen due to the intracellular preference for potassium, rather than sodium, in methanogens (31). Based on our analysis and values reported for intracellular concentrations of solutes in M. thermophila (36) and taking into account the similar coccoid morphology for M. burtonii (0.8- to 1.8-μm diameter) (13) and M. thermophila (0.8- to 1.6-μm diameter) (36), 500 mM K glutamate and 100 mM K aspartate were calculated to be the average levels present in M. thermophila growing at 42°C and M. burtonii growing at 8 or 23°C, respectively. While other solutes (Nɛ-acetyl-β-lysine and β-alanine betaine) were differentially produced, they were not commercially available and were not further examined in this study.
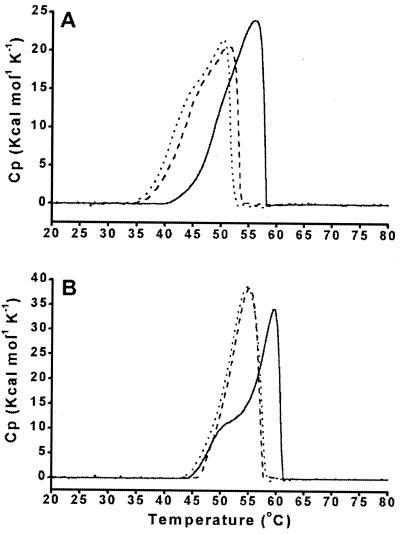
Differential scanning calorimetry was used to assess thermal unfolding of the EF-2 proteins (Fig. 5). Under all conditions tested, protein unfolding was irreversible, indicating that the solutes did not facilitate refolding of the unfolded protein. In the presence of 500 mM K glutamate the unfolding profile for M. thermophila EF-2 shifted to a higher temperature, with the maximum value of heat absorption increasing from 55°C in the absence of K glutamate to 60°C in its presence (Fig. 5B). The activation energy for the unfolding process was calculated to be 351 or 491 kJ mol−1 in the absence or presence, respectively, of K glutamate. In contrast to glutamate, aspartate (100 mM) had little effect on protein stabilization, and the activation energy of unfolding was 374 kJ mol−1. Potassium glutamate also stabilized M. burtonii EF-2 (Fig. 5A). The activation energy for the unfolding process was 364 kJ mol−1, 250 kJ mol−1, and 203 kJ mol−1 for 500 mM K glutamate, 100 mM K aspartate, and EF-2 with nothing added, respectively.
FIG. 5.
Temperature-dependent unfolding of EF-2 proteins. Differential scanning calorimetry thermograms from M. burtonii (A) and M. thermophila (B) in the absence of salts (dotted line) and in the presence of 100 mM K aspartate (dashed line) or 500 mM K glutamate (solid line) are shown. The protein concentration was 2 mg/ml in 20 mM MOPS (pH 7.5)–1 mM β-mercaptoethanol, and the scan rate was 1.5 K min−1. Cp, heat capacity.
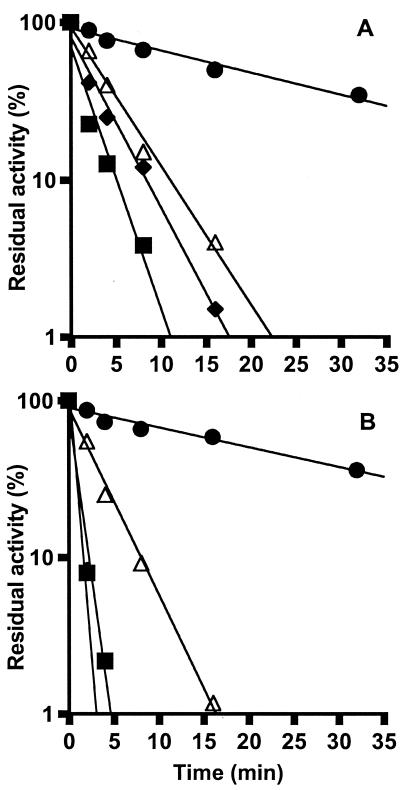
The thermal stabilizing effect of the solutes was further assessed by measuring the GTPase activities remaining for the EF-2 proteins from M. burtonii and M. thermophila after incubation at 55 and 60°C, respectively (Fig. 6). Five hundred millimolar K-glutamate dramatically increased the retention of GTPase activity of both EF-2 proteins; the half-life of activity increased from less than 2 min in the absence of glutamate to greater than 30 min in its presence. At approximate intracellular concentrations of K glutamate in M. burtonii (100 mM), there was a slight stabilizing effect (data not shown), similar to that observed for 100 mM K aspartate (Fig. 6). One hundred millimolar K aspartate had essentially no effect on M. thermophila EF-2 activity. The data indicate that the concentrations of K glutamate that are estimated to be present in M. thermophila growing at 42°C enabled GTPase activity to be substantially retained at high temperatures (60°C), while K aspartate had little effect on stabilizing the activity of the M. thermophila EF-2.
FIG. 6.
Thermal inactivation of the GTPase activities of M. burtonii EF-2 (A) and M. thermophila EF-2 (B) at 55 and 60°C, respectively, in the absence of additional solutes (■) and in the presence of K aspartate (100 mM) (⧫), K glutamate (500 mM) (●), or ribosomes (45 μM) (▵). The EF-2 protein-concentration in 20 mM MOPS (pH 7.5)–1 mM β-mercaptoethanol was 13.6 μM. Residual GTPase activity was determined at 35°C as described in Materials and Methods. Activity assays were performed in the presence of equivalent concentrations of K glutamate and K aspartate. The residual activity was calculated as a percentage of the original activity.
In comparative experiments, GTPase activity was measured in the presence of ribosomes (Fig. 6). Ribosomes appeared to exert a moderate stabilizing effect on both EF-2 proteins as the half-lives of GTPase activity for M. burtonii and M. thermophila were approximately 3 and 4 min, respectively, compared with less than 2 min in the absence of ribosomes.
Combined effects of ribosomes, solutes, and temperature on GTPase activity.
The temperature profiles of initial reaction rates were determined for the EF-2 proteins in the presence of ribosomes and in the absence or presence of K aspartate or K glutamate (Fig. 7). The inclusion of aspartate or glutamate marginally increased the activity of the M. burtonii EF-2 at temperatures below 35°C, while above this temperature the initial reaction rates were reduced. The response of the M. thermophila EF-2 to K aspartate was minimal in comparison to the effect of K glutamate. The GTPase activity profile was shifted towards lower temperatures by the addition of 100 mM K aspartate; however, the maximum initial reaction rates were very similar. In contrast, the effect of K glutamate was to shift the maximum initial reaction rate from 9.6 min−1 at 55°C to 3.3 min−1 at 30°C.
FIG. 7.
Initial reaction rates of ribosome-dependent GTPase activities of M. burtonii EF-2 (A) and M. thermophila EF-2 (B) in the absence of salts (■) and in the presence of 100 mM K aspartate (⧫) or 500 mM K glutamate (●).
It was striking that initial reaction rates were low for the M. thermophila EF-2 in the presence of 500 mM K glutamate, since this concentration is likely to occur in cells growing at 42°C (Fig. 4).
To rationalize the effects of the solutes on the initial reaction rates with the stabilizing effects of the solutes on GTPase activity (Fig. 6), analyses of the Michaelis-Menten kinetics were performed (Table 1). For the M. burtonii EF-2, the addition of solutes led to a decrease in the Vmax and Km values. The net effect of both Vmax and Km decreasing in the presence of K aspartate led to a catalytic efficiency (Vmax/Km) that was essentially the same as that without the solute. The effect of K glutamate, however, was to increase catalytic efficiency from 0.09 min−1 μM−1 to 0.14 min−1 μM−1. While K glutamate clearly affects the catalytic efficiency of the M. burtonii EF-2, it is important to note that this concentration would not be expected inside the cell (Fig. 4). Physiological relevance, however, may be attributed to the effect of 100 mM K aspartate, which led to a decrease in Km from 21.2 μM to 13.1 μM. The improved binding efficiency with K aspartate may compensate for the large increase in Km that occurs below 23°C in the absence of solute (Fig. 3), thereby partially rescuing the loss of catalytic efficiency.
TABLE 1.
Effects of K glutamate and K aspartate on Michaelis-Menten kinetics for ribosome-dependent GTPase activity at 40°C
| Protein | Solute (concn)a | Vmax (min−1) | Km (μM) | Vmax/Km (min−1 μM−1) |
|---|---|---|---|---|
| M. burtonii EF-2 | None | 2.0 | 21.2 | 0.09 |
| K-asp (100) | 1.2 | 13.1 | 0.09 | |
| K-glu (500) | 1.3 | 9.4 | 0.14 | |
| M. thermophila EF-2 | None | 3.7 | 33.2 | 0.11 |
| K-asp (100) | 5.3 | 33.1 | 0.16 | |
| K-glu (500) | 2.3 | 12.1 | 0.19 |
K-asp, K aspartate; K-glu, K glutamate. Concentrations are micromolar amounts.
For the M. thermophila EF-2, K aspartate also affected catalytic efficiency; however, unlike what was observed for the M. burtonii EF-2, the effect was not on Km, but on Vmax (Table 1). While K aspartate was not detected in the cells (Fig. 4), these results highlight the individual responses of each EF-2 protein to specific solutes. In the presence of 500 mM K glutamate, the Vmax and Km values were reduced in comparison to the values obtained in the absence of solute. Importantly, however, the catalytic efficiency at 40°C was higher (0.19 min−1 μM−1 compared to 0.11 min−1 μM−1 in the absence of the solute). This improvement in catalytic efficiency, generated by an increased affinity for GTP, at the expense of maximum reaction velocity, may be an adaptive mechanism for improved function of M. thermophila EF-2 at higher growth temperatures.
Growth-temperature-dependent levels of EF-2 in M. burtonii.
The presence of K glutamate and ribosomes appears to provide adequate stability and activity for M. thermophila EF-2 at its optimal growth temperature (40 to 50°C). However, while the catalytic efficiency of M. burtonii EF-2 at its optimal growth temperature (23°C) is reasonably high (Fig. 3C), it is reduced at a low temperature (8°C). To examine if intracellular levels of EF-2 changed during growth at low temperatures, Western blot and ELISA analyses were performed.
Single bands which comigrated with the purified EF-2 as a marker were detected in all cell extracts in Western blot analysis (data not shown). Using quantitative ELISA, the EF-2 concentration was calculated as 5 or 4 mg of EF-2 g of cell protein−1 for M. burtonii grown at 8°C to the mid-logarithmic- or late-logarithmic-growth phase, respectively, and 6 mg of EF-2 g of cell protein−1 for cultures grown at 23°C to the mid- and late-logarithmic-growth phases. These data show that similar intracellular levels of EF-2 were present, irrespective of growth temperature. In contrast to relatively constant levels of EF-2, the growth rate of M. burtonii decreased from 0.046 h−1 at 23°C to 0.013 h−1 at 8°C. Therefore, relative to the growth rate there is about three times as much EF-2 in the cell at 8°C as there is at 23°C, and this may compensate for the reduced activity of EF-2 at the lower growth temperature.
DISCUSSION
Microorganisms are capable of growth at temperatures from above 110°C to below 0°C (8); however, the range of temperatures that permit the survival of an individual microorganism generally does not rise above 50°C at its upper limit (2). While it is known that an important constraint is the thermal adaptation of proteins, comparatively little is known about the effects of intracellular, physiological modifiers of protein stability and activity, particularly for low-temperature-adapted archaea. This study describes the responses of EF-2 proteins to ribosomes and solutes from two closely related methanogens and highlights the important role that intracellular components may play in thermal adaptation.
M. burtonii and M. thermophila accumulate a variety of intracellular solutes (Fig. 4). Although the proportions of the compounds vary for each organism, the overall composition was determined to be similar, with the only compound distinguishing the genera being l-aspartate, which was unique to M. burtonii. Individual genera of methanogens have been found to contain unique combinations of solutes (36), and the similarity between these two methanogens likely reflects their close phylogenetic relationship (37), despite their being representatives of different genera.
For M. thermophila, potassium glutamate was produced at higher levels in response to higher growth temperatures (Fig. 4), and in the presence of physiological levels of the solute, the activity of the EF-2 protein was stabilized (Fig. 5 and 6). While various compounds may have stabilizing properties, a physiological benefit can only be gained if the solutes do not interfere with the activity of the protein. Our results indicate that despite decreasing the maximum velocity of GTPase hydrolysis, K glutamate exerts a positive effect on the catalytic efficiency of the protein by reducing its Km value at 40°C (Table 1). Potassium glutamate is therefore a compatible solute, as its stabilizing properties do not adversely effect EF-2 activity. Similar properties have also been observed for potassium-2,3-diphosphoglycerate with enzymes from thermophilic and hyperthermophilic archaea (17, 34). Sodium and potassium glutamate have also been reported to effect the stability of tubulin (1), bovine serum albumin and lysozyme (19), proteins involved in cell-free transcription (18), Rho protein activity (43), and gyrase activity (3), indicating that it has an important function in a broad range of biological systems.
Nɛ-acetyl-β-lysine and K glutamate have previously been shown to accumulate in M. thermophila in response to increasing extracellular osmolality of the growth medium (36). Our results indicate that accumulation is also affected by temperature. However, while the highest levels of Nɛ-acetyl-β-lysine were observed during growth at 30°C, the highest levels of K glutamate occurred at 42°C (Fig. 4). The capacity of this species to differentially regulate intracellular solute concentrations in response to changes in temperature and osmotic pressure indicates it has a sensory system that can respond to diverse abiotic changes in the environment.
Ribosomes greatly stimulated (Fig. 1) and moderately stabilized (Fig. 2 and 6) the GTPase activities of the EF-2 proteins. This demonstrates the potential importance of intracellular protein-protein interactions in the thermal adaptation of individual proteins. This may be particularly important for protein complexes, such as EF-2 and the ribosome. In the absence of detailed structural information on the interactions of archaeal EF-2 proteins and ribosomes, it is difficult to know what causes the changes in thermal properties. However, it is possible that during a temperature increase, specific functional domains of EF-2 may undergo structural alterations, as long as the protein is not associated with the ribosome. This may explain why the temperature profile of ribosome-independent catalysis shifted to a lower temperature (38) than the profile of ribosome-dependent activity (Fig. 2). In support of this, the DSC analysis shows that the protein begins to unfold at temperatures (e. g., for M. thermophila EF-2, at 50°C [Fig. 5]) at which the ribosome-dependent activity is still increasing (Fig. 2 and 3C).
The EF-2 protein from M. burtonii is thermally stabilized by K glutamate (Fig. 5 and 6) and ribosomes (Fig. 1), indicating that many aspects of its biochemistry and structure are similar to those of its thermophilic counterpart. While the protein retains these properties even at low temperatures, there are at least three factors that appear to contribute towards improving its activity at such temperatures. Firstly, M. burtonii does not produce sufficient concentrations of K glutamate (Fig. 4) to alter the thermal properties of the protein, most likely because thermal denaturation is not a major factor in a low-temperature environment. Secondly, the affinity for GTP is high, resulting in an increased catalytic efficiency at low temperatures (Fig. 3). This is illustrated by the Km values for GTP-binding for M. burtonii EF-2 at its optimum growth temperature of 23°C (8 μM), which is lower than those for M. thermophila EF-2 at 40°C (33 μM) and 50°C (55 μM), S. solfataricus EF-2 at 87°C (32 μM [29]) and E. coli EF-G at 30°C (41 μM [11]). At temperatures approaching in situ growth conditions for M. burtonii (13), the affinity for GTP-binding increases rapidly, with a concomitant decrease in catalytic efficiency (Fig. 3). This indicates that the rate-limiting step for GTP hydrolysis at low temperatures even for EF-2 from a psychrotolerant species, could be the binding of GTP to the protein. To further compensate for the increased Km it is possible that K aspartate, which is only produced in detectable levels by M. burtonii (Fig. 4), may provide an adaptive mechanism by increasing GTP affinity (Table 1).
The third factor that may compensate for loss of activity at low temperature is the regulation of cellular levels of EF-2. The translation system is essential to cell growth and has been shown to be a critical target in the cold shock response and for adapting to growth at low temperatures (4, 5, 16, 40, 41). In the absence of other adaptive mechanisms that may increase the activity of EF-2, maintaining EF-2 levels steady at low temperatures may ensure that ribosome translocation remains adequate. It is possible that the levels of other components of the translation apparatus are also regulated in a similar temperature-dependent fashion. Consistent with this is the finding that the gene encoding EF-2 is located in a streptomycin-like operon structure encoding the ribosomal protein S7 and elongation factor 1α (T. Thomas et al., unpublished results), implying that ribosomal and ribosome-associated proteins have coordinated synthesis at low temperatures.
Finally, it may also be envisaged that archaea growing under different thermal constraints may synthesize proteins that interact with the ribosome or associated proteins (e.g., EF-2) and alter their stability and activity, thereby optimizing translation. We recently examined the ribosomes from M. burtonii and M. thermophila grown at different temperatures and found a number of differences between ribosomal protein bands on SDS-PAGE, particularly between the two methanogens (T. Thomas and R. Cavicchioli, unpublished results). It has also recently been reported that EF-G and initiation factor 2 function as chaperones, fulfilling a role in protein folding and protection from stress (6). These findings indicate that a variety of cellular mechanisms exist to modify the function of ribosomes and associated proteins and may also be relevant in thermal adaptation.
ACKNOWLEDGMENTS
We wish to thank Hilde Stender for expert assistance with NMR spectroscopy, Martin Wisemann for advice regarding the Western blot analysis and the ELISA, and Paul March, Tassia Kolesnikow, Neil Saunders, Scott Rice, and Suhelen Egan for critical comments on the manuscript.
This work was funded by the Australian Research Council.
REFERENCES
- 1.Arakawa T, Timasheff S N. The mechanism of action of Na glutamate, lysine HCl, and piperazine-N,N′-bis(2-ethanesulfonic acid) in the stabilization of tubulin and microtubule formation. J Biol Chem. 1984;259:4979–4986. [PubMed] [Google Scholar]
- 2.Atlas R M, Bartha R. Microbiol ecology. 4th ed. Menlo Park, Calif: Benjamin/Cummings Publishing Company, Inc.; 1998. [Google Scholar]
- 3.Blanche F, Cameron B, Bernard F X, Maton L, Manse B, Ferrero L, Ratet N, Lecoq C, Goniot A, Bisch D, Crouzet J. Differential behaviors of Staphylococcus aureus and Escherichia coli type II DNA topoisomerase. Antimicrob Agents Chemother. 1996;40:2714–2720. doi: 10.1128/aac.40.12.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bobier S R, Ferroni G D, Innis W E. Protein synthesis by the psychrophiles Bacillus psychrophilus and Bacillus insolitus. Can J Microbiol. 1972;18:1837–1843. doi: 10.1139/m72-287. [DOI] [PubMed] [Google Scholar]
- 5.Broeze R J, Solomon C J, Pope D H. Effects of low temperature on the in vivo and in vitro protein synthesis in Escherichia coli and Pseudomonas fluoresecens. J Bacteriol. 1978;134:861–874. doi: 10.1128/jb.134.3.861-874.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caldas T, Laalami S, Richarme G. Chaperone function of bacterial elongation EF-G and initiation factor IF2. J Biol Chem. 2000;275:855–860. doi: 10.1074/jbc.275.2.855. [DOI] [PubMed] [Google Scholar]
- 7.Cavicchioli R, Thomas T, Curmi P M G. Cold stress response in archaea. Extremophiles. 2000;4:321–331. doi: 10.1007/s007920070001. [DOI] [PubMed] [Google Scholar]
- 8.Cavicchioli R, Thomas T. Extremophiles. In: Lederberg J, editor. Encyclopedia of microbiology. Vol. 2. San Diego, Calif: Academic Press; 2000. pp. 317–337. [Google Scholar]
- 9.DeLong E F. Marine microbiol diversity: the tip of the iceberg. Trends Biotechnol. 1997;15:203–207. doi: 10.1016/S0167-7799(97)01044-5. [DOI] [PubMed] [Google Scholar]
- 10.DeLong E F, Taylor L T, Marsh T L, Preston C M. Visualization and enumeration of marine planktonic archaea and bacteria by using polyribonucleotide probes and fluorescent in situ hybridization. Appl Environ Microbiol. 1999;65:5554–5563. doi: 10.1128/aem.65.12.5554-5563.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.deVendittis E, Masullo M, Bocchini V. The elongation factor G carries a catalytic site for GTP-hydrolysis which is revealed by using 2-propanol in the absence of ribosomes. J Biol Chem. 1986;261:4445–4450. [PubMed] [Google Scholar]
- 12.Franzmann P D, Liu Y, Balkwill D L, Aldrich H C, Conway de Macario E, Boone D R. Methanogenium frigidum sp. nov., a psychrophilic, H2-using methanogen from Ace Lake, Antarctica. Int J Syst Bacteriol. 1997;47:1068–1072. doi: 10.1099/00207713-47-4-1068. [DOI] [PubMed] [Google Scholar]
- 13.Franzmann P D, Springer N, Ludwig W, Conway de Macario E, Rohde M. A methanogenic archaeon from Ace Lake, Antarctica: Methanococcoides burtonii sp. nov. Syst Appl Microbiol. 1992;15:573–581. [Google Scholar]
- 14.Franzmann P D, Stackebrandt E, Sanderson K, Volkmann J K C, Stevenson D E, P L, McMeekin T A, Burton H R. Halobacterium lacusprofundi sp. nov., a halophilic bacterium, isolated from Deep Lake, Antarctica. Syst Appl Microbiol. 1988;11:20–27. [Google Scholar]
- 15.Galinski E A. Compatible solutes of halophilic eubacteria: molecular principles, water-solute interaction, stress protection. Experimentia. 1993;49:487–496. [Google Scholar]
- 16.Graumann P, Wendrich T M, Weber M H W, Schroder K, Marahiel M A. A family of cold shock proteins in Bacillus subtilis is essential for cellular growth and for efficient protein synthesis at optimal and low temperatures. Mol Microbiol. 1997;25:741–756. doi: 10.1046/j.1365-2958.1997.5121878.x. [DOI] [PubMed] [Google Scholar]
- 17.Hensel R, Koenig H. Thermoadaptation of methanogenic bacteria by intracellular ion concentration. FEMS Microbiol Lett. 1988;49:75–79. [Google Scholar]
- 18.Hethke C, Bergerat A, Hausner W, Forterre P, Thomm M. Cell-free transcription at 95°C: Thermostability and transcriptional components and DNA-topology requirements of Pyrococcus transcription. Genetics. 1999;152:1325–1333. doi: 10.1093/genetics/152.4.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kita Y, Arakawa T, Lin T Y, Timasheff S N. Contribution of the surface free energy perturbation to protein-solvent interactions. Biochemistry. 1994;33:15178–15189. doi: 10.1021/bi00254a029. [DOI] [PubMed] [Google Scholar]
- 20.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 21.Lai M-C, Ciulla R, Roberts M F, Sowers K R, Gunsalus R P. Extraction and detection of compatible intracellular solutes. In: Sowers K R, Schreier H J, editors. Archaea: a laboratory manual (methanogens). Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1995. pp. 349–368. [Google Scholar]
- 22.Lim J, Thomas T, Cavicchioli R. Low temperature regulated DEAD-box RNA helicase from the Antarctic archaeon, Methanococcoides burtonii. J Mol Biol. 2000;297:553–567. doi: 10.1006/jmbi.2000.3585. [DOI] [PubMed] [Google Scholar]
- 23.Martins L O, Huber R, Huber H, Stetter K O, Da Costa M S, Santos H. Organic solutes in hyperthermophilic archaea. Appl Environ Microbiol. 1997;63:896–902. doi: 10.1128/aem.63.3.896-902.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matthaei J H, Nirenberg W M. Characteristics and stabilization of DNase-sensitive protein synthesis in E. coli extracts. Proc Natl Acad Sci USA. 1961;47:1580–1588. doi: 10.1073/pnas.47.10.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murray A E, Wu K Y, Moyer C L, Karl D M, DeLong E F. Evidence for circumpolar distribution of planktonic Archaea in the Southern Ocean. Aquat Microb Ecol. 1999;18:263–273. [Google Scholar]
- 26.Pace N R. A molecular view of microbial diversity and the biosphere. Science. 1997;276:734–740. doi: 10.1126/science.276.5313.734. [DOI] [PubMed] [Google Scholar]
- 27.Petska S. Studies on the formation of transfer ribonucleic acid-ribosome complex. J Biol Chem. 1969;244:1533–1539. [PubMed] [Google Scholar]
- 28.Preston C M, Wu K Y, Molinski T F, DeLong E F. A psychrophilic crenarchaeon inhabits a marine sponge—Cenarchaeum symbiosum gen. nov., sp. nov. Proc Natl Acad Sci USA. 1996;93:6241–6246. doi: 10.1073/pnas.93.13.6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raimo G, Masullo M, Bocchini V. Studies on the polypeptide elongation factor 2 from Sulfolobus solfataricus. J Biol Chem. 1995;270:21082–21085. doi: 10.1074/jbc.270.36.21082. [DOI] [PubMed] [Google Scholar]
- 30.Raimo G, Masullo M, Scarano G, Bocchini V. The site for GTP hydrolysis on the archaeal elongation factor 2 is unmasked by aliphatic alcohols. Biochimie. 1996;78:832–837. doi: 10.1016/s0300-9084(97)84335-0. [DOI] [PubMed] [Google Scholar]
- 31.Robertson D E, Roberts M F. Organic osmolytes in methanogenic archaebacteria. BioFactors. 1991;3:1–9. [PubMed] [Google Scholar]
- 32.Russel N J, Hamamoto T. Psychrophiles. In: Hosikoshi K, Grant W D, editors. Extremophiles: microbial life in extreme environments. New York, N.Y: Wiley-Liss, Inc.; 1998. pp. 25–45. [Google Scholar]
- 33.Schleper C, Swanson R V, Mathur E L, DeLong E F. Characterization of a DNA polymerase from the uncultivated psychrophilic archaeon Cenarchaeum symbiosum. J Bacteriol. 1997;179:7803–7811. doi: 10.1128/jb.179.24.7803-7811.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shima S, Herault D A, Berkessel A, Thauer R K. Activation and thermostabilization effects of cyclic 2,3-diphosphoglycerate on enzymes from the hyperthermophilic Methnaopyrus kandleri. Arch Microbiol. 1998;170:469–472. doi: 10.1007/s002030050669. [DOI] [PubMed] [Google Scholar]
- 35.Sowers K R, Gunsalus R P. Adaptation for growth at various saline concentrations by the archaebacterium Methanosarcina thermophila. J Bacteriol. 1988;170:998–1002. doi: 10.1128/jb.170.2.998-1002.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sowers K R, Gunsalus R P. Halotolerance in Methanosarcina spp.: Role of Nɛ-acetyl-β-lysine, α-glutamate, glycine betaine and K+ as compatible solutes for osmotic adaptation. Appl Environ Microbiol. 1995;61:4382–4388. doi: 10.1128/aem.61.12.4382-4388.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomas T, Cavicchioli R. Archaeal cold-adapted proteisn: structural and evolutionary analysis of the elongation factor 2 proteins from psychrophilic, mesophilic and thermophilic methanogens. FEBS Lett. 1998;439:281–286. doi: 10.1016/s0014-5793(98)01375-1. [DOI] [PubMed] [Google Scholar]
- 38.Thomas T, Cavicchioli R. Effect of temperature on the stability and activity of elongation factor 2 proteins from Antarctic and thermophilic methanogens. J Bacteriol. 2000;182:1328–1332. doi: 10.1128/jb.182.5.1328-1332.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;82:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.VanBogelen R A, Neidhardt F C. Ribosomes as sensors of heat and cold shock in Escherichia coli. Proc Natl Acad Sci USA. 1990;87:5589–5593. doi: 10.1073/pnas.87.15.5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamanaka K. Cold shock response in Escherichia coli. J Mol Microbiol Biotechnol. 1999;1:193–202. [PubMed] [Google Scholar]
- 42.Zinder S H, Sowers K R, Ferry J G. Methanosarcina thermophila sp. nov., a thermophilic, acetotrophic, methane-producing bacterium. Int J Syst Bacteriol. 1985;35:522–523. [Google Scholar]
- 43.Zou L, Richardson J P. Enhancement of transcription termination factor rho activity with potassium glutamate. J Biol Chem. 1991;266:10201–10209. [PubMed] [Google Scholar]