Abstract
Objective
This systematic review and meta-analysis aimed to compare the diagnostic performance of transient elastography (TE) and two-dimensional shear wave elastography (2D-SWE) for staging liver fibrosis in patients with chronic viral hepatitis (CVH).
Methods
Pubmed, Embase, Web of Science, and Cochrane Library were searched (-01/08/2021) for studies comparing TE with 2D-SWE in patients with CVH. Other etiologies of chronic liver disease (CLD) and articles not published in SCI journals were excluded. The bivariate random-effects model was used to pool the performance of the TE and 2D-SWE.
Results
Eight articles with a total of 1301 CVH patients were included. The prevalence of significant fibrosis (fibrosis stage ≥ 2), advanced fibrosis (fibrosis stage ≥ 3), and cirrhosis was 50.8%, 44.8%, and 34.7%, respectively. 2D-SWE expressed higher overall accuracy than TE in detecting significant fibrosis (0.93 vs. 0.85, P = 0.04). No significant difference among the overall diagnostic accuracy of TE and 2D-SWE in staging advanced fibrosis and cirrhosis was found.
Conclusion
TE and 2D-SWE express good to excellent diagnostic accuracies to stage fibrosis in CVH patients. 2D-SWE compares favorably with TE especially for predicting significant fibrosis.
1. Introduction
As the leading cause of liver fibrosis and subsequent hepatocellular carcinoma, chronic viral hepatitis (CVH) infection affects approximately 325 million people worldwide and thus contributing significantly to the global health burden [1]. Liver fibrosis is an important pathological basis of liver cirrhosis in patients with chronic viral hepatitis. Accurate assessment of liver fibrosis degree is of great clinical importance in deciding optimal antiviral treatment time, monitoring dynamic changes of chronic viral hepatitis, and identifying candidates for surveillance for hepatocellular carcinoma [2, 3]. Early-stage liver fibrosis is potentially reversible, but precise diagnoses are often difficult to achieve [4]. Liver biopsy is the gold standard, but it is an invasive examination with potential risks, poor repeatability, and certain limitations, limiting its use in routine clinical practice. A rapid, noninvasive, and straightforward method to identify early-stage liver fibrosis has become increasingly popular.
Transient elastography (TE), two-dimensional shear wave elastography (2D-SWE), and pathological biopsy show great mutual agreement. As the most widely used device based on TE, FibroScan is the World Health Organization recommended diagnosis tool for grading liver fibrosis [5, 6]. 2D-SWE is another well-validated elastography technique using conventional ultrasound diagnostic system, which can quantitatively evaluate the elastic modulus of tissue in a certain selected area [7]. 2D-SWE also displays excellent diagnostic utility for liver fibrosis in chronic hepatitis B (CHB) or chronic hepatitis C (CHC) patients [8, 9].
The severity of liver fibrosis has been known to be a crucial risk factor for hepatocellular carcinoma development. Most patients sequentially develop hepatitis, fibrosis, cirrhosis, and then hepatocellular carcinoma. Given that hepatocellular carcinoma is most often associated with CVH infection (including hepatitis B and C) and toxic exposure [10], staging the degree of fibrosis in CVH is vital for stratification of the risk for hepatocellular carcinoma development, contributing to the management of these patients. Deffieux et al. [11] reported that TE expressed similar diagnostic performance to 2D-SWE in CVH patients. However, a meta-analysis based on the individual data implied that 2D-SWE outperformed TE in CVH patients [12]. Whether 2D-SWE is superior to TE for fibrosis, CVH remains under debate. To determine a better imaging technique for the fibrosis of CVH, we conduct a meta-analysis, including only articles with head-to-head comparison between TE and 2D-SWE.
2. Materials and Methods
We performed this systematic review following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guideline [13]. Our review protocol was registered at PROSPERO with number CRD42021272672. This study is a meta-analysis of previous research data and ethics statement is not applicable.
2.1. Articles Search Strategy
The key words “hepatitis B,” “hepatitis C,” “chronic viral hepatitis,” “liver fibrosis,” “FibroScan,” “transient elastography,” “shear wave elastography,” “Supersonic shear imaging,” “SSI,” or “ShearWaveTM elastography” were used to search in Pubmed, Embase, the Web of Science, and the Cochrane Library (-01/10/2021).
2.2. Eligibility Criteria
The inclusion criteria are as follows: (1) the accuracies of 2D-SWE and TE for liver fibrosis in patients with hepatitis B virus (HBV) or hepatitis C virus (HCV) were investigated. The paired data of the studies with head-to-head comparison of TE and 2D-SWE was sufficient to construct 2 × 2 table of test performance; (2) the specific liver fibrosis stage was confirmed by biopsy and the (3) the original articles could be retrieved from SCI journals. The exclusion criteria included the following: (1) studies did not assess the accuracies of TE and 2D-SWE; (2) conference abstracts, review, meta-analysis, case report, and other special types of work were not considered; (3) diagnosis of alcoholic liver disease (ALD), nonalcoholic fatty liver disease (NAFLD), autoimmune liver disease, or hepatic carcinoma.
2.3. Identification of Liver Fibrosis
Regarding the liver histological assessment, the Metavir, Scheuer, and Ishak scoring system were both included in this research. If the fibrosis stage was assessed with the Metavir or Scheuer system, fibrosis was scored as follows: F0, no fibrosis; F1, mild/moderate fibrosis; F ≥ 2, significant fibrosis; F ≥ 3, advanced fibrosis; and F4, cirrhosis. According to the Ishak scoring system, fibrosis was scored as follows: F 0-1, no/mild fibrosis; F ≥ 2, moderate fibrosis; F ≥ 3, significant fibrosis; F ≥ 4, advanced fibrosis; and F ≥ 5, cirrhosis.
2.4. Data Collection and Quality Evaluation
At the initial screening stage, two experienced researchers make preliminary selections following the eligibility criteria. Each reviewed the author then extracted the data individually. We then assessed the quality of the included studies with Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool via Review Manager 5.3 (The Cochrane Collaboration). If discrepancies exist, a third research would independently perform proofreading. Discrepancies were further discussed to achieve a high level of agreement if necessary.
2.5. Statistical Analysis
Based on the constructed 2 × 2 table with the number of true positives (tp), false positives (fp), false negatives (fn), and true negatives (tn), the summary positive likelihood ratio (LR) and negative LR were acquired following corresponding formulas. For diagnostic accuracy meta-analysis, we adopted a random-effects model (Der Simonian and Laird method). The pooled sensitivity, specificity, summary diagnostic odds ratios (DORs), and the nonthreshold heterogeneity of all the included studies were calculated by Stata (version 16.0) using Midas commands [14]. I − squared value > 50% and P value < 0.05 were considered suggestive of statistical heterogeneity [15]. We used Metadisc version 1.4 to explore the potential heterogeneity correlated with threshold effect, based on the Spearman correlation coefficient analysis. P < 0.05 which indicated significant threshold effects. Publication bias was assessed with Deeks' funnel plots in Stata 16.0. Bivariate random-effects model and hierarchical summary receiver operating characteristic (SROC) analysis were processed using Stata version 16.0. To compare the sensitivity and specificity between different approaches, the Z test was performed. The DeLong test was performed to compare ROC curves between TE and 2D-SWE [16]. Statistical significance was assigned as P < 0.05.
3. Results
3.1. Study Characteristics
Figure 1 depicts the study selection process. A total of 6963 potential publications were collected following the search scheme. After deleting duplicates, a total of 4458 publications left. After excluding patent, case report, review, and so forth, 256 articles with full text were downloaded for further screening. By reading the full text, 8 studies [11, 17, 18, 20–24] were ultimately included. Tables 1 and 2 depict both basic and technical characteristics and the technical characteristics of the included studies. Table 3 depicts the data of diagnostic performance of the studies. The prevalence of significant fibrosis, advanced fibrosis, and cirrhosis was 50.8%, 44.8%, and 34.7%, respectively. A total of 1301 subjects (mean age, 41.5 years; 70.2% male) were included. In addition to 1 (12.5%) retrospective studies, the remaining 7 articles were prospective trials. The details of the QUADAS-2 score are presented in Figure 2. Three (37.5%) publications scored 14 points. Two (25%) studies scored 13 points and 3 (37.5%) studies scored 12 points, respectively.
Figure 1.
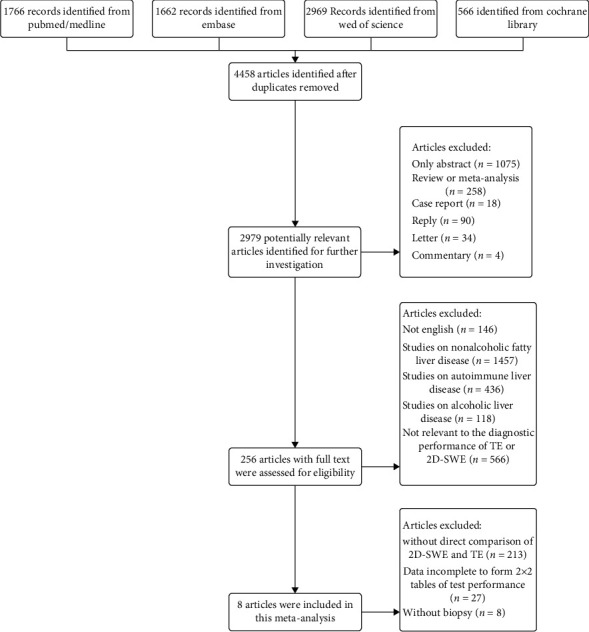
Flow diagram of the study selection. 150 × 160 mm (300 × 300 DPI).
Table 1.
basic characteristics of the included studies.
| Id | Region | Design | Center | Study time | Subject | Mean age | Male (%) | BMI (kg/m2) | ALT (U/L) | Etiology | Scoring system | Length of biopsy samples | QUADAS scores |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leung [17] | China | Prospective | Single center | 2011-2012 | 226 | 48.8 | 65 | 24.2 (21.6-27.3)† | 69 (37.5-105)† | HBV | Metavir | ≥15 mm | 12 |
| Yao [22] | China | Prospective | Single center | 2013-2015 | 54 | 36.7 | 76 | 23.9 (21.9-25)† | 50.4 (28.8-129.2)† | HBV | Ishak | ≥20 mm | 14 |
| Zeng [18] | China | Prospective | Single center | 2013-2015 | 257 | 36.7 | 77.4 | 21.7 (19.7-23.9)† | 42 (28.3-67.8)† | HBV | Metavir | ≥15 mm | 13 |
| Ferraioli [21] | Italy | Retrospective | Single center | 2010-2012 | 121 | 44.8 | 71.9 | 25.4 (17.1-39)§ | 75 (40-126)† | HCV | Metavir | ≥10 mm | 14 |
| Deffieux [11] | France | Prospective | Single center | 2011-2012 | 70 | 46.8 | 79 | 24.1 (16.7-35.9)§ | 81.3 (15-260)§ | HBV (n = 24)HCV (n = 46) | Metavir | ≥9 mm | 14 |
| Xia 2019 | China | Prospective | Single center | 2017 | 158 | 38.55 | 60.1 | NA | NA | HBV | Scheuer | ≥15 mm | 12 |
| Paul [23] | India | Prospective | Single center | 2012-2014 | 240 | 32.6 | 73.3 | 22.3 ± 4.1 | 71.7 ± 89.0 | HBV (n = 172) HCV (n = 68) | Metavir | ≥10 mm | 12 |
| Osman [24] | Egypt | Prospective | Single center | 2019 | 180 | 51.07 | 65 | NA | NA | HBV (n = 32) HCV (n = 148) | Metavir | NA | 13 |
∗Mean ± SD. †Median (interquartile range). §Median (range). BMI: body mass index; NA: not available; QUADAS: quality assessment of studies of diagnostic accuracy studies; TE: transient elastography.
Table 2.
Technical characteristics of the included studies.
| Id | TE | 2D-SWE | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of reader | Experience of operator | Probe | Technical failure rate | No. of reader | Experience of operator | Probe | Diameter of ROI | Technical failure rate | |
| Leung [17] | 1 | More than 5 years | NA | 10.4% (47/454) | 1 | NA | SC6-1 | 3-4 cm | 1.1% (5/454) |
| Yao [22] | 1 | At least 100 TE procedures | M | 0 | 1 | NA | SC6-1 | 2 cm | 0 |
| Zeng [18] | 2 | At least 100 TE procedures | M | 3.1% (8/257) | 2 | More than 6 months | SC6-1 | 2 cm | 0.8% (2/257) |
| Ferraioli [21] | 1 | At least 50 TE procedures | NA | 2.5% (3/121) | 2 | NA | SC6-1 | 2 cm | 1.7% (2/121) |
| Deffieux [11] | 1 | More than 7 years | NA | 5.3% (6/114) | 2 | NA | SC6-1 | 3 cm | 1.7% (2/120) |
| Xia 2019 [19] | NA | NA | M or L | NA | 2 | NA | L5-1 | NA | NA |
| Paul [23] | NA | NA | M or XL | 2.1% (5/240) | NA | NA | SC6-1 | 1-1.5 cm | 1.3% (3/240) |
| Osman [24] | 1 | NA | NA | 14.0% (30/210) | 1 | NA | NA | NA | 5.7% (12/210) |
2D-SWE: two-dimensional shear wave elastography; NA: not available; ROI: region of interest; QUADAS: quality assessment of studies of diagnostic accuracy studies; TE: transient elastography.
Table 3.
Data of diagnostic performance of the studies included in the meta-analysis.
| Study id | Staging fibrosis stage F ≥ 2 | Staging fibrosis stage F ≥ 3 | Staging fibrosis stage F = 4 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sensitivity | Specificity | AUROC | Sensitivity | Specificity | AUROC | Sensitivity | Specificity | AUROC | ||||||||||
| TE | 2D-SWE | TE | 2D-SWE | TE | 2D-SWE | TE | 2D-SWE | TE | 2D-SWE | TE | 2D-SWE | TE | 2D-SWE | TE | 2D-SWE | TE | 2D-SWE | |
| Leung [17] | 0.78 | 0.85 | 0.81 | 0.92 | 0.78 | 0.88 | 0.81 | 0.9 | 0.92 | 0.9 | 0.83 | 0.93 | 0.92 | 0.97 | 0.92 | 0.92 | 0.92 | 0.98 |
| Yao [22] | 0.59 | 0.78 | 0.67 | 0.78 | 0.71 | 0.79 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Zeng [18] | 0.79 | 0.89 | 0.81 | 0.76 | 0.85 | 0.88 | 0.86 | 0.90 | 0.78 | 0.77 | 0.88 | 0.92 | 0.94 | 0.94 | 0.83 | 0.87 | 0.91 | 0.93 |
| Ferraioli [21] | 0.70 | 0.90 | 0.90 | 0.88 | 0.84 | 0.92 | 0.89 | 0.97 | 0.89 | 0.95 | 0.96 | 0.98 | 0.92 | 0.88 | 0.97 | 0.97 | 0.96 | 0.98 |
| Deffieux [11] | 0.84 | 0.87 | 0.82 | 0.76 | 0.89 | 0.85 | 0.81 | 0.86 | 0.83 | 0.74 | 0.83 | 0.82 | 0.78 | 1.00 | 0.86 | 0.79 | 0.87 | 0.90 |
| Xia 2019 [19] | 0.80 | 0.76 | 0.56 | 0.91 | 0.75 | 0.94 | 0.94 | 1.00 | 0.86 | 0.91 | 0.87 | 0.97 | 0.86 | 0.89 | 0.86 | 0.96 | 0.91 | 0.97 |
| Paul [23] | 0.75 | 0.67 | 0.78 | 0.70 | 0.84 | 0.76 | 0.82 | 0.81 | 0.83 | 0.78 | 0.90 | 0.90 | 0.83 | 0.83 | 0.97 | 0.91 | 0.97 | 0.93 |
| Osman [24] | 0.91 | 0.89 | 0.89 | 0.85 | 0.95 | 0.93 | 0.93 | 0.90 | 0.97 | 0.95 | 0.90 | 0.98 | 0.96 | 1.00 | 0.97 | 0.91 | 0.97 | 0.93 |
2D-SWE: two-dimensional shear wave elastography; AUROC: area under summary receiver operating characteristic; NA: not available; TE: transient elastography.
Figure 2.
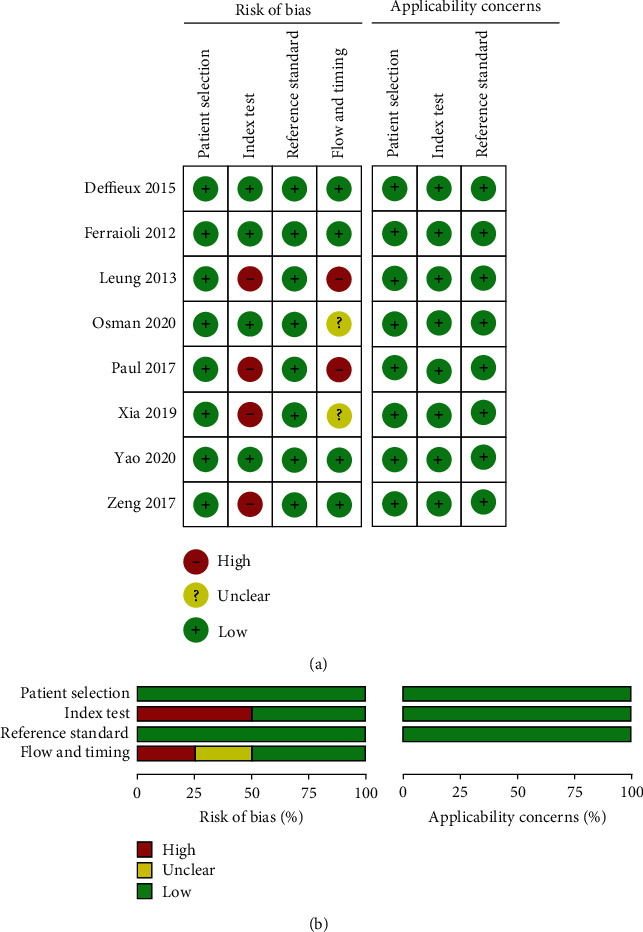
Summary of methodological quality of 8 studies according to Quality Assessment of Diagnostic Studies-2 (QUADAS-2) tool. (a) Overall and (b) study-level of bias. 150 × 160 mm (300 × 300 DPI).
3.2. Diagnosing Significant Fibrosis (F0-1 vs. F2-4)
Eight studies (1301 patients) provided detailed results of head-to-head comparison among TE and 2D-SWE for grading significant fibrosis. Table 4 summarizes the overall diagnostic performance of TE and 2D-SWE for grading significant fibrosis. The pooled sensitivity and specificity of TE were 0.78 (95% CI, 0.72-0.84) and 0.79 (0.71-0.86), respectively. Figure 3(a) shows that the summary AUROC of TE was 0.85 (95% CI, 0.82-0.88). The pooled sensitivity and specificity of 2D-SWE were 0.84 (95% CI, 0.78-0.88) and 0.84 (95% CI, 0.77-0.88). Figure 3(b) shows that the AUROC was 0.90 (95% CI, 0.88-0.93). Compared with TE, 2D-SWE displayed greater sensitivity (0.84 vs. 0.78, P < 0.01) and specificity (0.84 vs. 0.79, P < 0.01). According to the Delong test, 2D-SWE displays higher accuracy than TE (Z = 2.51, P = 0.01).
Table 4.
Meta-analysis of studies with head-to-head comparison of TE and 2D-SWE in staging fibrosis.
| Methods | No. of studies (no. of patients) | Cutoff values range | Sensitivity (95% CI) | Specificity (95% CI) | PLR (95% CI) | NLR (95% CI) | AUROC(95% CI) | DOR (95% CI) |
|---|---|---|---|---|---|---|---|---|
| Staging fibrosis stage F ≥ 2 | ||||||||
| TE | 8 (1301) | 6.1-11.8 | 0.78 (0.72-0.84) | 0.79 (0.71-0.86) | 3.71 (2.43-5.67) | 0.28 (0.21-0.38) | 0.85 (0.82-0.88) | 14.12 (7.88-25.31) |
| 2D-SWE | 8 (1301) | 6-9.58 | 0.84 (0.78-0.88) | 0.84 (0.77-0.88) | 4.87 (3.22-7.36) | 0.2 (0.13-0.32) | 0.90 (0.88-0.93) | 25.19 (11.47-55.32) |
| Staging fibrosis stage F ≥ 3 | ||||||||
| TE | 6 (1089) | 8-8.6 | 0.85 (0.81-0.89) | 0.88 (0.81-0.93) | 7.05 (4.04-12.32) | 0.17 (0.12-0.24) | 0.91 (0.88-0.93) | 44.23 (20.5-95.43) |
| 2D-SWE | 6 (1089) | 7-9.1 | 0.88 (0.82-0.92) | 0.85 (0.77-0.91) | 6.19 (371-10.30) | 0.14 (0.08-0.23) | 0.93 (0.90-0.95) | 50.24 (21.09-119.69) |
| Staging fibrosis stage F = 4 | ||||||||
| TE | 6 (1089) | 11.2-14.6 | 0.90 (0.83-0.94) | 0.91 (0.84-0.95) | 10.7 (5.46-20.99) | 0.11 (0.05-0.24) | 0.95 (0.93-0.97) | 110.24 (40.75-298.24) |
| 2D-SWE | 6 (1089) | 9.7-11.3 | 0.95 (0.85-0.99) | 0.91 (0.86-0.95) | 9.34 (5.91-14.76) | 0.07 (0.02-0.27) | 0.97 (0.95-0.98) | 155.43 (63-383.43) |
2D-SWE: two-dimensional shear wave elastography; AUROC: area under summary receiver operating characteristic; CI: confidence interval; DOR: diagnostic odds ratio; NLR: negative likelihood ratio; PLR: positive likelihood ratio; TE: transient elastography.
Figure 3.
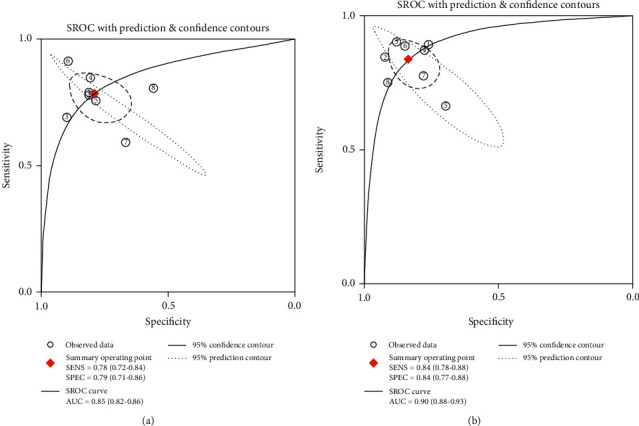
The SROC curves of TE and 2D-SWE showing performance in staging fibrosis stage F ≥ 2. (a) SROC plot of TE for fibrosis stage F ≥ 2. (b) SROC plot of 2D-SWE for fibrosis stage F ≥ 2. 2D-SWE: two-dimensional shear wave elastography; SROC: summary receiver operating characteristic; TE: transient elastography. 150 × 75 mm (300 × 300 DPI).
3.3. Diagnosing Advanced Fibrosis (F0-2 vs. F3-4)
Six studies (1089 patients) provided detailed results of head-to-head comparison among TE and 2D-SWE for determining advanced fibrosis. Table 4 summarizes the overall diagnostic performance of TE and 2D-SWE for grading advanced fibrosis. The pooled sensitivity and specificity of TE were 0.85 (95% CI, 0.81-0.89) and 0.88 (95% CI, 0.81-0.93), respectively. Figure 4(a) shows that the summary AUROC of TE was 0.91 (95% CI, 0.88-0.93). The pooled sensitivity and specificity of 2D-SWE were 0.88 (95% CI, 0.82-0.92) and 0.85 (95% CI, 0.77-0.91). Figure 4(b) shows that the AUROC was 0.93 (95% CI, 0.90-0.95). The sensitivity of 2D-SWE and TE was similar (0.85 vs. 0.88, P = 0.4). 2D-SWE displays similar specificity with TE (0.88 vs. 0.85, P = 0.5). According to the Delong test, the overall diagnostic accuracies of TE and 2D-SWE were comparable (Z = 1.1, P = 0.27).
Figure 4.
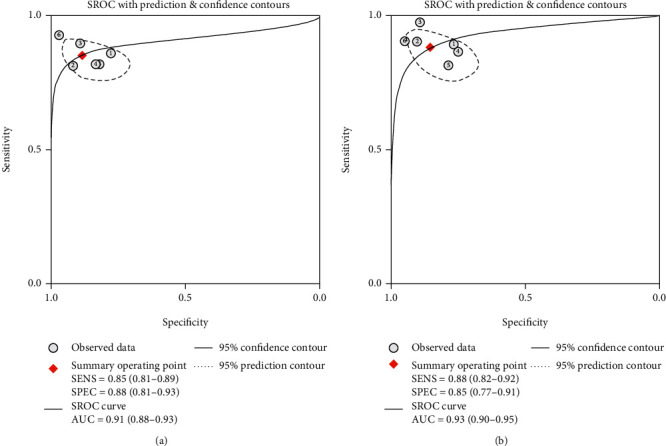
The SROC curves of TE and 2D-SWE showing performance in staging fibrosis stage F ≥ 3. (a) SROC plot of TE for fibrosis stage F ≥ 3. (b) SROC plot of 2D-SWE for fibrosis stage F ≥ 3. 2D-SWE: two-dimensional shear wave elastography; SROC: summary receiver operating characteristic; TE: transient elastography. 150 × 75 mm (300 × 300 DPI).
3.4. Diagnosing Cirrhosis (F0-3 vs. F4)
Six studies (1089 patients) provided detailed results of head-to-head comparison among TE and 2D-SWE in detecting cirrhosis. Table 4 summarizes the overall diagnostic performance of TE and 2D-SWE for grading cirrhosis. The pooled sensitivity and specificity of TE were 0.90 (95% CI, 0.83-0.94) and 0.91 (95% CI, 0.84-0.95). Figure 5(a) shows that the summary AUROC of TE was 0.95 (95% CI, 0.93-0.97). The pooled sensitivity and specificity of 2D-SWE were 0.95 (95% CI, 0.85-0.99) and 0.91 (95% CI, 0.86-0.95). Figure 5(b) shows that the AUROC was 0.97 (95% CI, 0.95-0.98).
Figure 5.
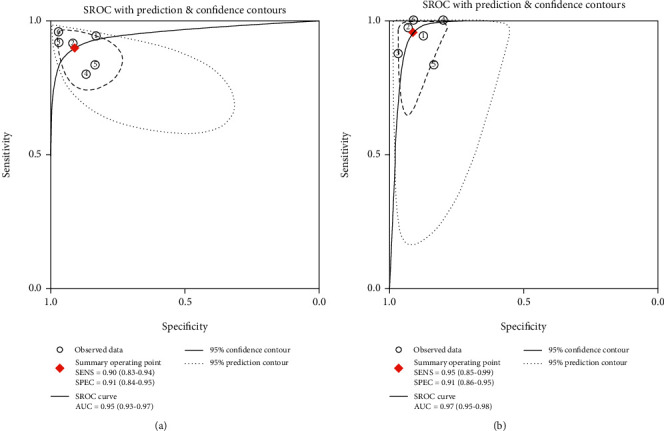
The SROC curves of TE and 2D-SWE showing performance in staging cirrhosis. (a) SROC plot of TE for fibrosis stage F = 4. (b) SROC plot of 2D-SWE for fibrosis stage F = 4. 2D-SWE: two-dimensional shear wave elastography; SROC: summary receiver operating characteristic; TE: transient elastography. 150 × 75 mm (300 × 300 DPI).
The sensitivity of 2D-SWE and TE was similar (0.90 vs. 0.95, P = 0.19). 2D-SWE displays similar specificity with TE (0.91 vs. 0.91, P = 0.87). According to the Delong test, 2D-SWE expressed similar diagnostic accuracy with TE (Z = 1.57, P = 0.12).
3.5. Heterogeneity and Publication Bias
Table 5 summarized the heterogeneity between the included studies. No obvious heterogeneity was observed. The result of Deek's test showed no potential publication bias for TE and 2D-SWE for staging fibrosis.
Table 5.
Heterogeneity of all the included studies.
| Staging fibrosis | Threshold heterogeneity | Nonthreshold heterogeneity | ||
|---|---|---|---|---|
| r s | P value | I 2 | P value | |
| TE | ||||
| Staging fibrosis stage ≥ 2 | -0.12 | 0.78 | 0.54 | 0.06 |
| Staging fibrosis stage ≥ 3 | -0.26 | 0.62 | 0.51 | 0.07 |
| Staging cirrhosis | -0.37 | 0.47 | 0 | 0.25 |
| 2D-SWE | ||||
| Staging fibrosis stage ≥ 2 | -0.1 | 0.82 | 0.56 | 0.05 |
| Staging fibrosis stage ≥ 3 | -0.66 | 0.16 | 0 | 0.24 |
| Staging cirrhosis | -0.14 | 0.79 | 0.47 | 0.08 |
2D-SWE: two-dimensional shear wave elastography; TE: transient elastography.
4. Discussion
It is well known that the progression of liver fibrosis can result in increased mortality, mainly due to esophageal-gastric varices bleeding, hepatic encephalopathy, and hepatocellular carcinoma. Chronic infections due to HBV and HCV are responsible for most cases of hepatocellular carcinoma worldwide. Despite promising advances in treatment of hepatocellular carcinoma, ultimately prevention can reduce the burden of viral hepatitis-related hepatocellular carcinoma. Effective monitoring and surveillance for hepatocellular carcinoma must be offered to patients who already have advanced fibrosis or cirrhosis, so that hepatocellular carcinoma is detected at earlier stages, allowing for curative treatments and longer survival. Though liver biopsy remains the gold standard for grading fibrosis, it is not routinely performed due to the invasiveness, sampling error, interobserver variations, and complications of this procedure. Noninvasive tests for evaluation of liver fibrosis mainly include serum biomarkers and measurement of liver stiffness based on elastography. Though serum biomarkers are good reproducibility and high applicability, most of them are nonspecific of the liver and unable to discriminate between intermediate stages of fibrosis [25]. Unlike MR elastography, elastography based on ultrasound machine is less costly, less time-consuming, and widely available, significantly lowering the barriers of clinical application [26]. Growing evidence indicate that TE and 2D-SWE express excellent diagnostic performance for liver fibrosis in CVH.11-12 Whether 2D-SWE outperforms TE for fibrosis, CVH remains controversial. Therefore, we conduct this meta-analysis to determine a better technique for patients with CVH.
Our meta-analysis concludes that TE and 2D-SWE show acceptable diagnostic accuracies to stage fibrosis in people with CVH. 2D-SWE outperforms TE in predicting significant fibrosis (AUROCs = 0.90 vs. 0.85, P = 0.01). Compared with TE, significantly improved sensitivity (0.84 vs. 0.78, P < 0.01) and specificity (0.84 vs. 0.79, P < 0.01) was seen for detecting significant fibrosis using 2D-SWE. According to hepatitis B management guidances proposed by the American Association for the Study of Liver Diseases (AASLD), we need to initiate antivirus treatment with elevated HBV DNA levels and histologic evidence of significant fibrosis [27]. Therefore, the advantage of 2D-SWE is evident in the management of HBV-infection, which was similar to an individual patient data based meta-analysis [12]. Our result may also help guide the treatment for patients with chronic hepatitis C. According to the guidelines from the American Association of Liver Diseases, HCV patients without cirrhosis can receive pibrentasvir (120 mg)/glecaprevir (300 mg) for 8 weeks. For cirrhotic patients, they are suitable for velpatasvir (100 mg)/sofosbuvir (400 mg) for 12 weeks [28]. Since 2D-SWE expressed similar diagnostic accuracy with TE in predicting cirrhosis, TE and 2D-SWE were both the ideal candidate for patients with HCV. Nevertheless, this finding seems not generalisable to other etiologies. As indicated by another latest meta-analysis, neither TE nor 2D-SWE met the minimum acceptable performance for detecting significant fibrosis in NAFLD. The respective summary AUROCs of 2D-SWE and TE are 75% and 83% [29]. The difference between CVH and NAFLD may be attributed to the higher prevalence of obesity and hepatic steatosis in NAFLD patients. The diagnostic performance of TE would be substantially affected when patients have obesity, ascites, or steatosis [30]. Steatosis is also one of the confounding factors of 2D-SWE. A latest study based on 1306 patients with liver biopsy found that 2D-SWE might be affected by moderate to severe liver steatosis in diagnosing significant fibrosis [31]. No head-to-head comparison between TE and 2D-SWE was performed in patients with ALD for liver fibrosis. Based on the current evidence, only TE is recommend as a noninvasive tool in patients with chronic harmful alcohol use. Liver stiffness measurement by TE < 8 kPa is recommended to rule-out advanced fibrosis in clinical practice [32]. More prospective and multicenter studies on the diagnostic performance of 2D-SWE are needed to provide robust evidence in patients with ALD.
For discriminating advanced fibrosis and cirrhosis, 2D-SWE displays similar overall diagnostic accuracy with TE (AUROCs = 0.93 vs. 0.91, P = 0.27; AUROCs = 0.97 vs. 0.95, P = 0.12). The overall diagnostic performance of TE for staging advanced fibrosis and cirrhosis in CVH is similar to a previous meta-analysis based on 4386 CHB patients, with summary AUROCs of 0.91 and 0.93, respectively [33]. A recent meta-analysis based on 5126 CHB patients shows that the summary AUROC values of 2D-SWE for advanced fibrosis and cirrhosis were 0.93 and 0.94 [34]. The summary AUROCs of TE or 2D-SWE in our results are higher than theirs. Since we only include studies with head-to-head comparison of TE and 2D-SWE, numerous articles were excluded. As a result, the outcome of our meta-analysis is likely to overestimate accuracy, given the small sample sizes examined (n = 1089). TE and 2D-SWE also displays similar diagnostic accuracy for staging advanced fibrosis and cirrhosis in patients with biopsy-proven NAFLD [35, 36].
Except for the overall diagnostic accuracy, we also investigated the rates of reliable liver stiffness measurements of the two examinations in patients with CVH. The technical failure rate of 2D-SWE was below or equal to that of TE (Table 2), indicating that 2D-SWE is a preferable technique providing more reliable measurement. Moreover, compared with TE, 2D-SWE allows an easier access to the certain selected area [37], facilitating the monitoring of the variation of blood flow [38, 39].
With further study, 2D-SWE demonstrates enormous potential for prognosis prediction in patients with CVH. Wu et al. [40] proposed that liver stiffness measured with 2D-SWE is predictive of liver-related events in patients with HBV. 2D-SWE has recently exhibits great potential for redoing the burden of viral hepatitis-related hepatocellular carcinoma. A multivariable model based on age, platelets, and the liver stiffness measured by 2D-SWE can accurately predicts hepatocellular carcinoma in CHB during five-year follow-up, with an AUROC of 0.89 [41]. Another study from Korea implies that liver stiffness value measured by 2D-SWE was a significant predictive factor for overall survival after radiofrequency ablation for hepatocellular carcinoma [42]. Liver stiffness measured by 2D-SWE could also stratify the risk of symptomatic post-hepatectomy liver failure in Child-Turcotte-Pugh grade A patients, regardless of the extent of hepatectomy [43]. This demonstrates that 2D-SWE is a better choice to stage fibrosis.
The strengths of this meta-analysis were summarized as follows: studies comparing the diagnostic performance within the same patient provide a more valid way of comparing different tests. Hence, our meta-analysis is persuasive as we only included studies with head-to-head comparisons between TE and 2D-SWE. Moreover, no substantial heterogeneity was observed. The corresponding results are more convincing and reliable. However, limitations still exist. First, we did not evaluate the potential confounding factors such as obesity, tissue inflammation, and the quantification of viral activity, which may affect the diagnostic accuracies [44]. Although the diagnostic performance of 2D-SWE may not be affected by BMI and liver function indexes [45], TE tends to be affected by inflammation and cholestasis [46] and thus affecting our judgments. Additionally, because of the limited studies in patients with HCV and no substantial heterogeneity observed in the meta-analysis, we did not separately investigate the performance of elastography among patients with HBV or HCV.
5. Conclusion
Collective, TE and 2D-SWE display good to excellent accuracies in staging fibrosis in patients with HBV or HCV. 2D-SWE compares favorably with TE especially for predicting significant fibrosis.
Acknowledgments
The present study was supported by Guangdong Basic and Applied Basic Research Foundation (project No. 2021A1515110799), Jiangxi Province Health Commission Science and Technology Funding Program (project No. 20205015) and Shenzhen Nanshan District Science and Technology Plan Funding Program (project No. NS2021077).
Abbreviations
- AASLD:
American Association for the Study of Liver Diseases
- AUROC:
Area under the receiver operating characteristic curve
- CHB:
Chronic hepatitis B
- CHC:
Chronic hepatitis C
- CVH:
Chronic viral hepatitis
- DOR:
Diagnostic odds ratio
- HBV:
Hepatitis B virus
- HCV:
Hepatitis C virus
- LR:
Likelihood ratio
- NAFLD:
Nonalcoholic fatty liver disease
- NPV:
Negative predictive value
- PPV:
Positive predictive value
- QUADAS:
Quality assessment of diagnostic accuracy studies
- ROC:
Receiver operating characteristic
- ROI:
Region of interest
- 2D-SWE:
Two-dimensional shear wave elastography
- TE:
Transient elastography.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent
Consent is not applicable because it is a meta-analysis of previous studies.
Conflicts of Interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Authors' Contributions
All authors contributed to data contribution, data interpretation, and critical review of the manuscript for important content. Qing-tian Luo, Qing Zhu, and Xiao-dan Zong performed the literature search and data extraction and performed the study quality assessment. Ming-kai Li and Hong-sheng Yu conducted the study design and data analysis. Qing-tian Luo drafted the manuscript. Chang-Yu Jiang and Xiang Liao were responsible for critical revision of the paper. Xiang Liao conducted the study conception and study supervision. Qing-tian Luo and Qing Zhu contributed equally to this work.
References
- 1.Li J., Li Z., Tu J., et al. In vitro and in vivo investigations of a-C/a-C:Ti nanomultilayer coated Ti6Al4V alloy as artificial femoral head. Materials science & engineering: C . 2019;99:816–826. doi: 10.1016/j.msec.2019.02.022. [DOI] [PubMed] [Google Scholar]
- 2.Nahon P., Vo Quang E., Ganne-Carrié N. Stratification of hepatocellular carcinoma risk following HCV eradication or HBV control. Journal of Clinical Medicine . 2021;10(2):p. 353. doi: 10.3390/jcm10020353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salarian M., Turaga R., Xue S., et al. Early detection and staging of chronic liver diseases with a protein MRI contrast agent. Nature Communications . 2019;10(1) doi: 10.1038/s41467-019-11984-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Su C., Yang Y., Lin H. Impact of etiological treatment on prognosis. Hepatology International . 2018;12(S1):56–67. doi: 10.1007/s12072-017-9807-0. [DOI] [PubMed] [Google Scholar]
- 5.Johnson K., Laoveeravat P., Yee E., Perisetti A., Thandassery R., Tharian B. Endoscopic ultrasound guided liver biopsy: recent evidence. World Journal of Gastrointestinal Endoscopy . 2020;12(3):83–97. doi: 10.4253/wjge.v12.i3.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aberra H., Desalegn H., Berhe N., et al. The WHO guidelines for chronic hepatitis B fail to detect half of the patients in need of treatment in Ethiopia. Journal of Hepatology . 2019;70(6):1065–1071. doi: 10.1016/j.jhep.2019.01.037. [DOI] [PubMed] [Google Scholar]
- 7.Grgurevic I., Puljiz Z., Brnic D., et al. Liver and spleen stiffness and their ratio assessed by real-time two dimensional-shear wave elastography in patients with liver fibrosis and cirrhosis due to chronic viral hepatitis. European Radiology . 2015;25(11):3214–3221. doi: 10.1007/s00330-015-3728-x. [DOI] [PubMed] [Google Scholar]
- 8.Chen S., Jiang T. Preoperative noninvasive assessment for liver fibrosis in hepatocellular carcinoma patients with chronic hepatitis B: comparison of two-dimensional shear-wave elastography with serum liver fibrosis models. European Journal of Radiology . 2020;133, article 109386 doi: 10.1016/j.ejrad.2020.109386. [DOI] [PubMed] [Google Scholar]
- 9.Villani R., Cavallone F., Romano A., Bellanti F., Serviddio G. Two-dimensional shear wave elastography versus transient elastography: a non-invasive comparison for the assessment of liver fibrosis in patients with chronic hepatitis C. Diagnostics . 2020;10(5) doi: 10.3390/diagnostics10050313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tseng H., Xiong W., Badeti S., et al. Efficacy of anti-CD147 chimeric antigen receptors targeting hepatocellular carcinoma. Nature Communications . 2020;11(1):p. 4810. doi: 10.1038/s41467-020-18444-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deffieux T., Gennisson J., Bousquet L., et al. Investigating liver stiffness and viscosity for fibrosis, steatosis and activity staging using shear wave elastography. Journal of Hepatology . 2015;62(2):317–324. doi: 10.1016/j.jhep.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 12.Herrmann E., de Lédinghen V., Cassinotto C., et al. Assessment of biopsy-proven liver fibrosis by two-dimensional shear wave elastography: an individual patient data-based meta-analysis. Hepatology . 2018;67(1):260–272. doi: 10.1002/hep.29179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moher D., Liberati A., Tetzlaff J., Altman D., for the PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ . 2009;339 doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang W., Peng F., Meng S., Xu J., Yang Y. Diagnostic value of serum soluble triggering expressed receptor on myeloid cells 1 (sTREM-1) in suspected sepsis: a meta-analysis. BMC Immunology . 2020;21(1):p. 2. doi: 10.1186/s12865-020-0332-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Groenendijk I., den Boeft L., van Loon L., de Groot L. High versus low dietary protein intake and bone health in older adults: a systematic review and meta-analysis. Computational and Structural Biotechnology Journal . 2019;17:1101–1112. doi: 10.1016/j.csbj.2019.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeLong E., DeLong D., Clarke-Pearson D. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics . 1988;44(3):837–845. doi: 10.2307/2531595. [DOI] [PubMed] [Google Scholar]
- 17.Leung V., Shen J., Wong V., et al. Quantitative elastography of liver fibrosis and spleen stiffness in chronic hepatitis B carriers: comparison of shear-wave elastography and transient elastography with liver biopsy correlation. Radiology . 2013;269(3):910–918. doi: 10.1148/radiol.13130128. [DOI] [PubMed] [Google Scholar]
- 18.Zeng J., Zheng J., Huang Z., et al. Comparison of 2-D shear wave elastography and transient elastography for assessing liver fibrosis in chronic hepatitis B. Ultrasound in Medicine & Biology . 2017;43(8):1563–1570. doi: 10.1016/j.ultrasmedbio.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 19.Xia S., Ren X., Ni Z., Zhan W. A Noninvasive Method-Shear-Wave Elastography Compared With Transient Elastography in Evaluation of Liver Fibrosis in Patients With Chronic Hepatitis B. Ultrasound quarterly . 2019;35(2):147–152. doi: 10.1097/RUQ.0000000000000399. [DOI] [PubMed] [Google Scholar]
- 20.Bavu E., Gennisson J., Couade M., et al. Noninvasive in vivo liver fibrosis evaluation using supersonic shear imaging: a clinical study on 113 hepatitis C virus patients. Ultrasound in Medicine & Biology . 2011;37(9):1361–1373. doi: 10.1016/j.ultrasmedbio.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 21.Ferraioli G., Tinelli C., Dal Bello B., et al. Accuracy of real-time shear wave elastography for assessing liver fibrosis in chronic hepatitis C: a pilot study. Hepatology . 2012;56(6):2125–2133. doi: 10.1002/hep.25936. [DOI] [PubMed] [Google Scholar]
- 22.Yao T., Pan J., Qian J., Cheng H., Wang Y., Wang G. Shear wave elastography may be sensitive and more precise than transient elastography in predicting significant fibrosis. World Journal of Clinical Cases . 2020;8(17):3730–3742. doi: 10.12998/wjcc.v8.i17.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paul S., Das P., Mahanta M., et al. Assessment of liver fibrosis in chronic hepatitis: comparison of shear wave elastography and transient elastography. Abdominal Radiology . 2017;42(12):2864–2873. doi: 10.1007/s00261-017-1213-5. [DOI] [PubMed] [Google Scholar]
- 24.Osman A., El Shimy A., Abd El Aziz M. 2D shear wave elastography (SWE) performance versus vibration-controlled transient elastography (VCTE/fibroscan) in the assessment of liver stiffness in chronic hepatitis. Insights Into Imaging . 2020;11(1):p. 38. doi: 10.1186/s13244-020-0839-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.European Association for Study of Liver. EASL-ALEH clinical practice guidelines: non-invasive tests for evaluation of liver disease severity and prognosis. Journal of Hepatology . 2015;63(1):237–264. doi: 10.1016/j.jhep.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y., Fowler K., Ozturk A., et al. Liver fibrosis imaging: a clinical review of ultrasound and magnetic resonance elastography. Journal of Magnetic Resonance Imaging : JMRI . 2020;51(1):25–42. doi: 10.1002/jmri.26716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Terrault N., Lok A., McMahon B., et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology . 2018;67(4):1560–1599. doi: 10.1002/hep.29800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.AASLD-IDSA HCV Guidance Panel. Hepatitis C guidance 2018 update: AASLD-IDSA recommendations for testing, managing, and treating hepatitis C virus infection. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America . 2018;67(10):1477–1492. doi: 10.1093/cid/ciy585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Selvaraj E., Mózes F., Jayaswal A., et al. Diagnostic accuracy of elastography and magnetic resonance imaging in patients with NAFLD: a systematic review and meta-analysis. Journal of Hepatology . 2021;75(4):770–785. doi: 10.1016/j.jhep.2021.04.044. [DOI] [PubMed] [Google Scholar]
- 30.Boursier J., de Ledinghen V., Sturm N., et al. Precise evaluation of liver histology by computerized morphometry shows that steatosis influences liver stiffness measured by transient elastography in chronic hepatitis C. Journal of Gastroenterology . 2014;49(3):527–537. doi: 10.1007/s00535-013-0819-9. [DOI] [PubMed] [Google Scholar]
- 31.Huang Z., Zhou J., Lu X., et al. How does liver steatosis affect diagnostic performance of 2D-SWE.SSI: assessment from aspects of steatosis degree and pathological types. European Radiology . 2021;31(5):3207–3215. doi: 10.1007/s00330-020-07288-5. [DOI] [PubMed] [Google Scholar]
- 32.Panel C. P. G., Berzigotti A., Tsochatzis E., et al. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis - 2021 update. Journal of Hepatology . 2021;75(3):659–689. doi: 10.1016/j.jhep.2021.05.025. [DOI] [PubMed] [Google Scholar]
- 33.Li Y., Huang Y., Wang Z., et al. Systematic review with meta-analysis: the diagnostic accuracy of transient elastography for the staging of liver fibrosis in patients with chronic hepatitis B. Alimentary Pharmacology & Therapeutics . 2016;43(4):458–469. doi: 10.1111/apt.13488. [DOI] [PubMed] [Google Scholar]
- 34.Dong B., Lyu G., Chen Y., et al. Comparison of two-dimensional shear wave elastography, magnetic resonance elastography, and three serum markers for diagnosing fibrosis in patients with chronic hepatitis B: a meta-analysis. Expert Review of Gastroenterology & Hepatology . 2021;15(9):1077–1089. doi: 10.1080/17474124.2021.1880894. [DOI] [PubMed] [Google Scholar]
- 35.Furlan A., Tublin M., Yu L., Chopra K., Lippello A., Behari J. Comparison of 2D shear wave elastography, transient elastography, and MR elastography for the diagnosis of fibrosis in patients with nonalcoholic fatty liver disease. AJR. American Journal of Roentgenology . 2020;214(1):W20–W26. doi: 10.2214/AJR.19.21267. [DOI] [PubMed] [Google Scholar]
- 36.Sharpton S., Tamaki N., Bettencourt R., et al. Diagnostic accuracy of two-dimensional shear wave elastography and transient elastography in nonalcoholic fatty liver disease. Therapeutic Advances in Gastroenterology . 2021;14 doi: 10.1177/17562848211050436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barr R., Ferraioli G., Palmeri M., et al. Elastography assessment of liver fibrosis: Society of radiologists in ultrasound consensus conference statement. Radiology . 2015;276(3):845–861. doi: 10.1148/radiol.2015150619. [DOI] [PubMed] [Google Scholar]
- 38.Dietrich C., Bamber J., Berzigotti A., et al. EFSUMB guidelines and recommendations on the clinical use of liver ultrasound elastography, update 2017 (Long version) Ultraschall in der Medizin (Stuttgart, Germany : 1980) . 2017;38(4):e16–e47. doi: 10.1055/s-0043-103952. [DOI] [PubMed] [Google Scholar]
- 39.Lok A., Perrillo R., Lalama C., et al. Low incidence of adverse outcomes in adults with chronic hepatitis B virus infection in the era of antiviral therapy. Hepatology . 2021;73(6):2124–2140. doi: 10.1002/hep.31554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu M., Wu L., Jin J., et al. Liver stiffness measured with two-dimensional shear-wave elastography is predictive of liver-related events in patients with chronic liver disease due to hepatis B viral infection. Radiology . 2020;295(2):353–360. doi: 10.1148/radiol.2020191481. [DOI] [PubMed] [Google Scholar]
- 41.Zhang T., Zhang G., Deng X., et al. APS (age, platelets, 2D shear-wave elastography) score predicts hepatocellular carcinoma in chronic hepatitis B. Radiology . 2021;301(2):350–359. doi: 10.1148/radiol.2021204700. [DOI] [PubMed] [Google Scholar]
- 42.Lee D., Lee J., Yoon J., et al. Liver stiffness measured by two-dimensional shear-wave elastography: prognostic value after radiofrequency ablation for hepatocellular carcinoma. Liver Cancer . 2018;7(1):65–75. doi: 10.1159/000484445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Long H., Zhong X., Su L., et al. Liver stiffness measured by two-dimensional shear wave elastography for predicting symptomatic post-hepatectomy liver failure in patients with hepatocellular carcinoma. Annals of Surgical Oncology . 2022;29(1):327–336. doi: 10.1245/s10434-021-10563-4. [DOI] [PubMed] [Google Scholar]
- 44.Castera L. Hepatitis B: are non-invasive markers of liver fibrosis reliable? Liver International : Official Journal of the International Association for the Study of the Liver . 2014;34:91–96. doi: 10.1111/liv.12393. [DOI] [PubMed] [Google Scholar]
- 45.Petzold G., Bremer S., Knoop R., et al. Noninvasive assessment of liver fibrosis in a real-world cohort of patients with known or suspected chronic liver disease using 2D-shear wave elastography. European Journal of Gastroenterology & Hepatology . 2020;32(12):1559–1565. doi: 10.1097/MEG.0000000000001675. [DOI] [PubMed] [Google Scholar]
- 46.Oeda S., Tanaka K., Oshima A., Matsumoto Y., Sueoka E., Takahashi H. Diagnostic accuracy of FibroScan and factors affecting measurements. Diagnostics . 2020;10(11):p. 940. doi: 10.3390/diagnostics10110940. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


