Abstract
Introduction:
Decision aids (DAs) for breast cancer screening are increasingly being used by physicians, but the association of physician practice DA use and mammography rates remains uncertain. Using national data, we examine the association of practice-level DA use and mammography use among older women.
Methods:
Physician practice responses to the 2017/2018 National Survey of Healthcare Organizations and Systems (NSHOS) (n=1,236) were linked to 2016 and 2017 Medicare fee-for-service (FFS) beneficiary data from eligible beneficiaries (n=439,684) ages 65–74. In 2021, multivariable generalized linear models estimated the association of practice DA use for breast cancer screening and advanced HIT functions with mammography use, controlling for practice and beneficiary characteristics.
Results:
Overall, 60.1% of eligible beneficiaries had a screening mammography and 37.3% of physician practices routinely used DAs for breast cancer screening. In adjusted analyses, advanced HIT functions (odds ratio (OR)=1.19, p=0.04) were associated with mammography use, but practice use of DAs was not (OR=0.95, p=0.21). Beneficiary clinical and socioeconomic characteristics, including race, comorbidities, Medicare and Medicaid eligibility, and median household income were more strongly associated with mammography use than practice-level DA use or advanced HIT functions.
Conclusions:
HIT-enabled automation of mammography reminders and other advanced HIT functions may support mammography, while breast cancer DAs may reduce patients’ propensities to be screened through the alignment of their preferences and screening decision. More resources may be needed for DAs to be routinely implemented to improve solicitation of patient preferences and targeting of mammography services.
Introduction
Breast cancer is the most common cancer among women in the United States, constituting the second leading cause of cancer death among women overall.1 United States Preventive Services Task Force (USPSTF) guidelines recommend eligible women receive biennial mammograms until age 74,2 although evidence is mixed regarding the importance of screening beyond age 70.3–5 While mammography can enable early detection of cancer, false positive results are risks. Over-diagnosis occurs when malignancies are detected that would not have resulted in clinical significance; it is estimated that up to 25% of breast cancer cases may be over-diagnosed.6–8 Decisions about whether to receive a mammography should depend on patients’ risks and preferences and include a structured discussion about the risks and benefits of screening.6,9
Decision aids (DAs) can help clinicians structure conversations about screening decisions, costs and benefits of various choices,10 and patients’ health goals.11 Breast cancer screening DAs can increase patient awareness of risks associated with over-diagnosis.12–15 To date, there is mixed evidence about the association of DAs with mammography use. For example, one systematic review found that DAs have minimal impact on screening decisions,16 while another found that DAs reduce screening intentions.17 Although DA use is low overall,18 practices that integrate them into clinic workflows use DAs more consistently.19 Practices with more advanced health information technology (HIT) may be more likely to use HIT-enabled DAs and patient reminders, which can impact mammography screening rates.
No national evidence exists about whether physician practice adoption of DAs for breast cancer screening or HIT functions are associated with mammography use among older adult women. This analysis fills a critical gap in evidence by analyzing a national sample of physician practices and claims data from eligible, attributed Medicare fee-for-service (FFS) beneficiaries.
Methods
Data
Physician practice responses to the 2017/2018 National Survey of Healthcare Organizations and Systems (NSHOS) were linked to 2017 Medicare FFS beneficiary and claims data using National Provider Identifiers. NSHOS used a stratified-cluster sampling design to select eligible physician practices and yielded a response rate of 47%.20
Analytic Sample
The analytic sample includes female beneficiaries ages 65–74 with Part B eligibility and no HMO enrollment in 2017 or 2016. Practices with <100 attributed beneficiaries (n=493) were excluded to ensure the reliable estimation of practice effects on mammography use.21 See Supplement Table 1 for analytic sample exclusions. The final sample includes 439,684 Medicare FFS beneficiaries attributed to one of 1,236 NSHOS physician practice responses (average beneficiaries per practice= 285, standard deviation (SD)=344).
Measures
The dependent variable is receipt of screening mammography as indicated in Medicare FFS claims data from the cohort of eligible beneficiaries in 2017, with a 2-year look-back period of 2016–2017 to assess mammography use. The independent variables are 1) a dichotomous measure of physician practice use of DAs for breast cancer screening and 2) a composite measure of advanced HIT functions (range: 0–100). Practice-level control variables include practice size, ownership, specialist-to-primary care physician (PCP) ratio, and advanced practice clinician count. Patient-level control variables include patient age, race/ethnicity, Hierarchical Condition Category (HCC) risk scores (i.e., comorbidities), dual eligibility for Medicare and Medicaid, high-poverty zip code, and median household income (see Supplement Table 2 for measure definitions).
Statistical Analysis
Multivariable generalized linear models were estimated to examine the association of practices’ DA use and HIT functions with patient-level receipt of mammography. Complete case analyses were conducted. Model 1 examined the association of practice- and patient-level variables with mammography use. Model 2 extends Model 1 and includes an interaction term between HIT and DA use. Predicted probabilities of mammography use were calculated to illustrate how use varied by DA use and HIT in adjusted analyses. All statistical analyses were conducted using STATA statistical software.22 The study was approved by the Institutional Review Board.
Results
Overall, 60.1% of eligible beneficiaries had a mammogram, 37.3% of practices routinely used DAs for breast cancer screening, and advanced HIT functions averaged 0.60 (SD=0.47). In unadjusted analyses, beneficiaries who did not have a mammogram were more likely to be attributed to practices that routinely used DAs compared to beneficiaries who had a mammogram (37.9% vs. 37.0%, p<0.001) (Table 1).
Table 1.
Patient and Practice Characteristics, by Practice-Level Mammography Use
| Overall | Beneficiaries without a screening mammography in the past 2 years | Beneficiaries with a screening mammography in the past 2 years | Difference | |
|---|---|---|---|---|
| Patient N (% of analytic sample) | 439,684 (100%) | 171,984 (39.1%) |
267,700 (60.9%) |
- |
| Mean | Mean | Mean | p-value | |
| Main Predictors | ||||
| Practice Use of Breast Cancer Screening Decision Aids (%) | 37.3 | 37.9 | 37.0 | *** |
| Practice Advanced Health Information Technology (HIT) Functions (Mean (SD)) | 0.600 (0.470) | 0.590 (0.480) |
0.600 (0.470) |
*** |
| Practice Characteristics | ||||
| Practice Size | ||||
| 3–7 Physicians (%) | 43.2 | 43.4 | 43.1 | - |
| 8–12 Physicians (%) | 19.1 | 18.4 | 19.6 | *** |
| 13–19 Physicians (%) | 8.20 | 8.50 | 8.10 | *** |
| More than 20 Physicians (%) | 29.4 | 29.8 | 29.2 | * |
| Specialty Mix | ||||
| No Specialists (%) | 26.9 | 26.5 | 27.2 | ** |
| Low (%) | 24.3 | 26.0 | 23.2 | *** |
| Moderate (%) | 23.7 | 22.9 | 24.3 | *** |
| High (%) | 25.0 | 24.6 | 25.3 | *** |
| Total Advanced Practice Clinicians (Mean, SD) | 5.40 (18.1) | 5.10 (16.0) |
5.60 (19.4) |
*** |
| Practice Ownership | ||||
| Independent-owned (%) | 39.3 | 40.9 | 38.2 | *** |
| Medical Group-owned (%) | 13.4 | 12.8 | 13.7 | *** |
| System-owned (%) | 47.3 | 46.3 | 48.0 | *** |
| Patient Characteristics | ||||
| Age | 69.2 | 68.5 | 69.7 | *** |
| Race/Ethnicity | ||||
| White (%) | 86.3 | 85.2 | 87.0 | *** |
| Black (%) | 7.10 | 7.70 | 6.60 | *** |
| Asian (%) | 1.80 | 2.20 | 1.60 | *** |
| Latinx (%) | 0.700 | 0.900 | 0.600 | *** |
| Other (%) | 4.00 | 4.00 | 4.10 | - |
| Hierarchical Condition Categories (HCC) Score (Mean (SD)) | 0.710 (0.760) | 0.790 (0.440) |
0.650 (0.00) |
*** |
| Dual Medicare/Medicaid Eligibility (%) | 2.90 | 4.10 | 2.10 | *** |
| Frail Elder (%) | 2.80 | 3.30 | 2.50 | *** |
| Mental Illness (%) | 21.0 | 22.6 | 20.0 | *** |
| High Poverty Zip Code (%) | 14.8 | 16.1 | 13.9 | *** |
| Annual Median Household Income (Mean (SD)) | $61,921 ($42,676) | $60,790 ($42,505) |
$62,267 (42,726) |
*** |
Note:
p<0.05
p<0.01
p<0.001
In adjusted analyses, routine DA use was not significantly associated with patient-level mammography use (OR=0.95, p=0.21) (Table 2, Model 1). Beneficiaries attributed to practices with higher specialist-to-PCP ratios (OR=0.61, p<0.01) were less likely to have a mammogram, while beneficiaries of practices owned by a hospital or health system (OR=1.18, p<0.01) and more advanced HIT functions (OR=1.19, p<0.05) were more likely to have a mammogram.
Table 2.
Association of Practice Adoption of Decision Aids and Advanced Health Information Technology Capabilities with Mammography
| Model 1: Full Model with Patient & Practice Characteristics |
Model 2: Full Model with DA*HIT Interaction |
|
|---|---|---|
| Main Predictors (Practice Variables) | ||
| Practice Use of Breast Cancer Screening Decision Aids (DAs) | 0.95 (0.87, 1.03) | 0.94 (0.85, 1.03) |
| Practice Advanced Health Information Technology (HIT) Functions |
1.19 (1.01, 1.40) * |
1.17 (0.970, 1.40) |
| Practice Use of DAs * Practice HIT Functions | - | 1.05 (0.890, 1.24) |
| Practice Characteristics | ||
| Practice Size | ||
| 3–7 Physicians (reference) | - | - |
| 8–12 Physicians | 1.10 (0.95, 1.29) | 1.10 (0.95, 1.29) |
| 13–19 Physicians | 0.96 (0.83, 1.10) | 0.96 (0.84, 1.10) |
| More than 20 Physicians | 1.06 (0.92, 1.22) | 1.06 (0.92, 1.22) |
| Specialist Ratio | 0.61 (0.43, 0.85) ** | 0.61 (0.43, 0.86) ** |
| Total Advanced Practice Clinicians | 0.95 (0.82, 1.11) | 0.95 (0.82, 1.11) |
| Practice Ownership | ||
| Independent-owned (reference) | - | - |
| Medical group-owned | 1.16 (1.00, 1.34) | 1.16 (0.99, 1.34) |
| System-owned | 1.18 (1.06, 1.31) ** | 1.18 (1.06, 1.31) ** |
| Patient Characteristics | ||
| Age | 0.95 (0.92, 0.99) * | 0.95 (0.92, 0.99) * |
| Race/Ethnicity | ||
| White (reference) | - | - |
| Black | 1.13 (1.04, 1.23) ** | 1.13 (1.04, 1.23) ** |
| Asian | 0.78 (0.71, 0.86) *** | 0.78 (0.71, 0.86) *** |
| Latinx | 0.89 (0.76, 1.05) | 0.89 (0.76, 1.05) |
| Others | 0.99 (0.92, 1.06) | 0.99 (0.92, 1.06) |
| Hierarchical Condition Categories (HCC) Score | 0.81 (0.80, 0.82) *** | 0.81 (0.80, 0.82) *** |
| Dual Medicare/Medicaid | 0.60 (0.56, 0.65) *** | 0.60 (0.56, 0.65) *** |
| High Poverty Zip Code | 0.91 (0.87, 0.95) *** | 0.91 (0.87, 0.95) *** |
| Annual Median Household Income | 1.06 (1.04, 1.08) *** | 1.06 (1.04, 1.08) *** |
| Constant | 2.31 (2.02, 2.63) *** | 2.33 (2.02, 2.69) *** |
| Total Beneficiaries | 439,684 | 439,684 |
| Total Physician Practices | 1,236 | 1,236 |
Note:
p<0.05
p<0.01
p<0.001
Note: Outcome is patient-level screening mammography use
Note: Odds ratios and 95% confidence intervals are reported
Older age (OR=0.95, p<0.05), Asian race (OR=0.78, p<0.001), more comorbidities (OR=0.81, p<0.001), dual Medicare and Medicaid eligibility (OR=0.60, p<0.001), high-poverty zip code of residence (OR=0.91, p<0.001) were associated with lower odds of mammography use (Table 2, Model 1). Black race (OR=1.13, p<0.01) and greater median household income (OR=1.06, p<0.001) were associated with higher odds of mammography use. There was no interaction effect between DA use and HIT (Table 2, Model 2). Predicted probabilities based on Model 2 are presented in the Figure. Results were largely consistent for models that 1) included all practices with attributed beneficiaries, irrespective of volume (Supplement Tables 3–4) and 2) when DA use for any preference-sensitive condition was considered (Supplement Table 5).
Figure. Mammography Use, by Practice Adoption of Breast Cancer Decision Aids and Advanced Health Information Technology (HIT) Capabilities.
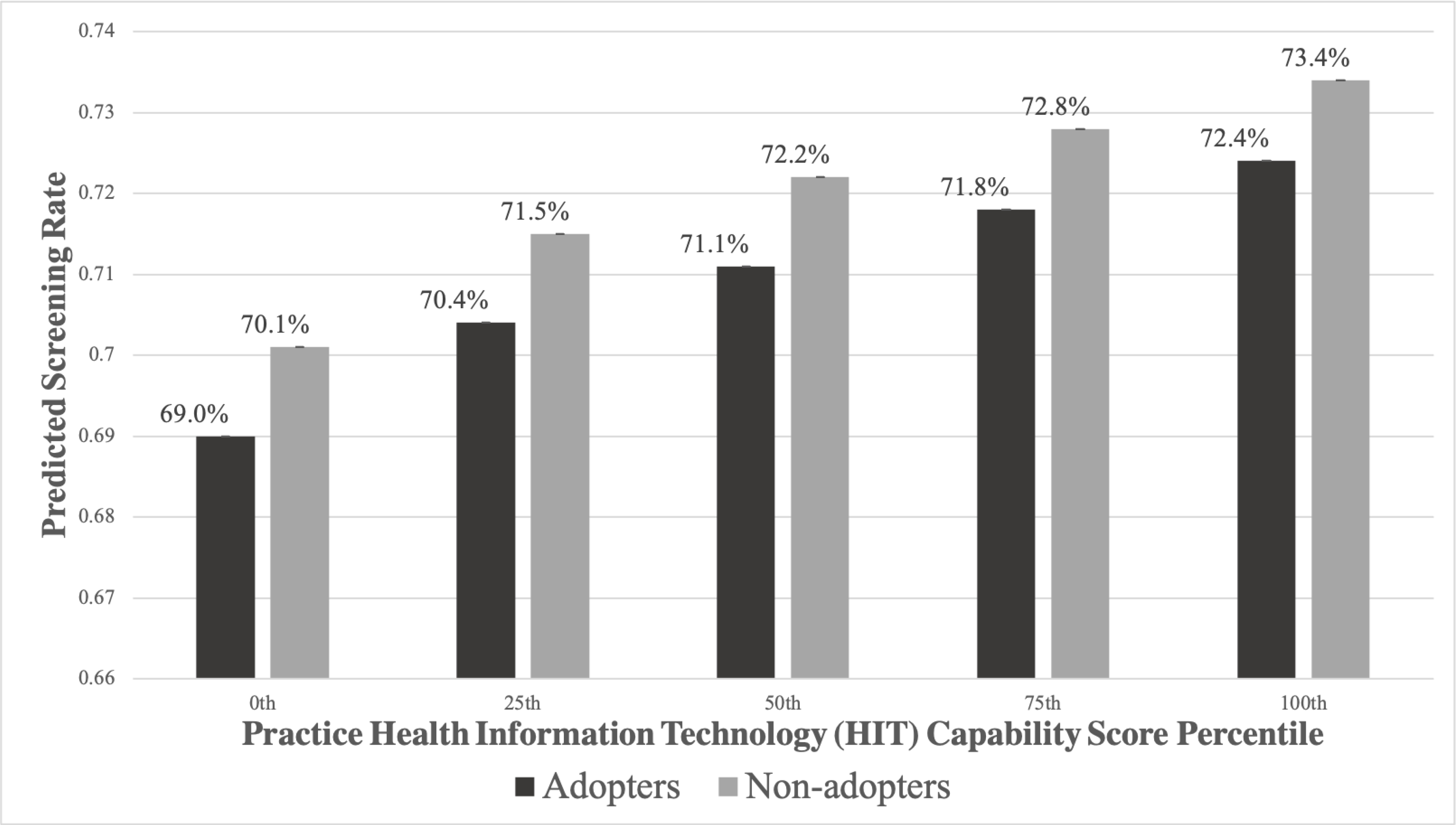
Legend: Adopters = Practice use of decision aids for breast cancer screening (use for “all” or “most” eligible patients), Non-adopters =< Practice does not use decision aids for breast cancer screening (use for “none” or “some” eligible patients).
Note: The advanced HIT scores (range: 0–100) associated with each percentile cutpoint are as follows: 0th percentile cutpoint = 0, 25th percentile cutpoint = 38.1, 50th percentile cutoff = 52.4, 75th percentile cutoff = 66.7, 100th percentile cutoff = 100.
Discussion
DAs are encouraged by payers because of their potential to reduce costs and improve quality.23 We hypothesized that advanced HIT would enable DA use and patient reminders for mammography, which could impact mammography rates. Although practice DA use was associated with patient-level mammography use in unadjusted analyses, the DA effect attenuates once advanced HIT and other practice characteristics are considered. The results suggest that HIT and DAs may have countervailing relationships with mammography use among older adult women. HIT-enabled automation of mammography reminders and other advanced HIT functions may support mammography,18 while breast cancer DAs may reduce patients’ propensities to be screened through the alignment of their preferences and screening decision. This may be why we found relatively small associations between DA use and advanced HIT functions with mammography use in adjusted analyses that consider both variables simultaneously.
Specialty mix of physician practices were associated with lower mammography rates, suggesting that having proportionally more specialist physicians may not specifically incentivize breast cancer screening activity. Alternatively, hospital or health system ownership was associated with greater mammography rates, suggesting that availability of organizational resources may enable greater screening capacity.
The present study has some limitations. First, NSHOS assessed breast cancer screening DA use with a single question; DA design, implementation strategies, and patient populations targeted were not assessed. Second, NSHOS does not include small (<3 primary care physicians) or federally-owned practices, so the results may not generalize to them. Third, the 47% NSHOS survey response rate may bias results; however, respondent and non-respondent practices do not substantially differ (Supplement Table 6). Fourth, racial/ethnic diversity is low because Medicare FFS data were analyzed; inclusion of Medicare Advantage data may improve generalizability. Finally, although mammography was assessed over two years, we could not account for delays/refusals at the patient-level. Future work should include a more robust assessment of patient-level factors associated with DA use and mammography, with an emphasis on understanding how DA use shifts patients differentially toward and away from mammography use to impact overall patterns of use.24
Conclusions
USPSTF guidelines recommend breast cancer screening through age 74, but concerns about overdiagnosis and harm underscore the importance of patient-provider communication regarding risks-benefit trade-offs. 25–27 Despite increasing awareness of breast cancer screening DAs, our results indicate that they likely have not been implemented consistently enough to have an impact on mammography use among older women. More resources may be needed for physician practices to routinely implement DAs and improve elicitation of patients’ preferences and targeting of mammography services.
Supplementary Material
Financial Disclosure:
This study was supported by the Agency for Healthcare Research and Quality’s (AHRQ’s) Comparative Health System Performance Initiative under Grant #1U19HS024075, which studies how health care delivery systems promote evidence-based practices and patient-centered outcomes research in delivering care. Ms. Ross received support from the Agency for Healthcare Research and Quality (T32HS022241). The statements, findings, conclusions, views, and opinions contained and expressed in this article are based in part on data obtained under license from IQVIA information services: OneKey subscription information services 2010–2017, IQVIA incorporated all rights reserved. The statements, findings, conclusions, views, and opinions contained and expressed herein are not necessarily those of IQVIA Incorporated or any of its affiliated or subsidiary entities. AMA is the source for these data; statistics, tables or tabulations were prepared using AMA Masterfile data.
Footnotes
Conflict of Interest Statement: None of the authors have conflicts of interest to disclose.
References
- 1.CDCBreastCancer. Basic Information About Breast Cancer Centers for Disease Control and Prevention. Published September 21, 2021. https://www.cdc.gov/cancer/breast/basic_info/index.htm [Google Scholar]
- 2.Recommendation: Breast Cancer: Screening | United States Preventive Services Taskforce. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/breast-cancer-screening#fullrecommendationstart.
- 3.Nelson HD, Tyne K, Naik A, et al. Screening for breast cancer: an update for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;151(10):727–737, W237–242. doi: 10.7326/0003-4819-151-10-200911170-00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fedewa SA, de Moor JS, Ward EM, et al. Mammography Use and Physician Recommendation After the 2009 U.S. Preventive Services Task Force Breast Cancer Screening Recommendations. Am J Prev Med. 2016;50(5):e123–e131. doi: 10.1016/j.amepre.2015.10.010 [DOI] [PubMed] [Google Scholar]
- 5.McCarthy EP, Burns RB, Freund KM, Ash AS, Shwartz M, Marwill SL, Moskowitz MA. Mammography use, breast cancer stage at diagnosis, and survival among older women. J Am Geriatr Soc. 2000. Oct;48(10):1226–33. [DOI] [PubMed] [Google Scholar]
- 6.Welch HG, Black WC. Overdiagnosis in Cancer. JNCI: J Natl Cancer Inst. 2010;102(9):605–613. doi: 10.1093/jnci/djq099 [DOI] [PubMed] [Google Scholar]
- 7.de Gelder R, Heijnsdijk EAM, van Ravesteyn NT, Fracheboud J, Draisma G, de Koning HJ. Interpreting Overdiagnosis Estimates in Population-based Mammography Screening. Epidemiol Rev. 2011;33(1):111–121. doi: 10.1093/epirev/mxr009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monticciolo DL, Helvie MA, Hendrick RE. Current issues in the overdiagnosis and overtreatment of breast cancer. AJR. 2018. Feb;210(2):285–91 [DOI] [PubMed] [Google Scholar]
- 9.Hersch J, Jansen J, Barratt A, et al. Women’s views on overdiagnosis in breast cancer screening: a qualitative study. BMJ. 2013;346:f158. doi: 10.1136/bmj.f158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen EO, Weaver OO, Tso HH, Gerlach KE, Leung JWT. Breast Cancer Screening via Digital Mammography, Synthetic Mammography, and Tomosynthesis. Am J Prev Med. 2020;58(3):470–472. doi: 10.1016/j.amepre.2019.09.016 [DOI] [PubMed] [Google Scholar]
- 11.O’Connor AM, Rostom A, Fiset V, et al. Decision aids for patients facing health treatment or screening decisions: systematic review. BMJ. 1999;319(7212):731–734. doi: 10.1136/bmj.319.7212.731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martínez-Alonso M, Carles-Lavila M, Pérez-Lacasta MJ, Pons-Rodríguez A, Garcia M, Rué M. Assessment of the effects of decision aids about breast cancer screening: a systematic review and meta-analysis. BMJ Open. 2017;7(10):e016894. doi: 10.1136/bmjopen-2017-016894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu L, Li P, Yang S, et al. Web-based decision aids to support breast cancer screening decisions: systematic review and meta-analysis. J Comp Eff Res. 2020;9(14):985–1002. doi: 10.2217/cer-2020-0052 [DOI] [PubMed] [Google Scholar]
- 14.Hersch J, Barratt A, Jansen J, et al. Use of a decision aid including information on overdetection to support informed choice about breast cancer screening: a randomised controlled trial. Lancet. 2015;385(9978):1642–1652. doi: 10.1016/S0140-6736(15)60123-4 [DOI] [PubMed] [Google Scholar]
- 15.Schonberg MA, Davis RB, Karamourtopoulos MC, et al. A Pre-Test–Post-Test Trial of a Breast Cancer Risk Report for Women in Their 40s. Am J Prev Med. 2020;59(3):343–354. doi: 10.1016/j.amepre.2020.04.014 [DOI] [PubMed] [Google Scholar]
- 16.Esmaeili M, Ayyoubzadeh SM, Javanmard Z, Niakan R Kalhori SA systematic review of decision aids for mammography screening: Focus on outcomes and characteristics. Int J Med Inform. 2021;149:104406. doi: 10.1016/j.ijmedinf.2021.104406 [DOI] [PubMed] [Google Scholar]
- 17.Ivlev I, Hickman EN, McDonagh MS, Eden KB. Use of patient decision aids increased younger women’s reluctance to begin screening mammography: a systematic review and meta-analysis. J Gen Intern Med. 2017;32(7):803–812. doi: 10.1007/s11606-017-4027-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Légaré F, Witteman HO. Shared Decision Making: Examining Key Elements And Barriers To Adoption Into Routine Clinical Practice. Health Aff. 2013;32(2):276–284. doi: 10.1377/hlthaff.2012.1078 [DOI] [PubMed] [Google Scholar]
- 19.Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database of Syst Rev. 2017;(4). doi: 10.1002/14651858.CD001431.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fisher ES, Shortell SM, O’Malley AJ, et al. Financial Integration’s Impact On Care Delivery And Payment Reforms: A Survey Of Hospitals And Physician Practices. Health Aff (Millwood). 2020;39(8):1302–1311. doi: 10.1377/hlthaff.2019.01813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sequist Thomas D., et al. Reliability of medical group and physician performance measurement in the primary care setting. Medical Care (2011): 126–131. [DOI] [PubMed] [Google Scholar]
- 22.StataCorp. Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC.; 2021. [Google Scholar]
- 23.Hansen SF. The Role of Decision Aids in the Affordable Care Act. Stanford Journal of Public Health. Published October 21, 2013. Accessed May 8, 2020. https://www.stanford.edu/group/sjph/cgi-bin/sjphsite/the-role-of-decision-aids-in-the-affordable-care-act/
- 24.Hurley VB, Wang Y, Rodriguez HP, Shortell SM, Kearing S, Savitz LA. Decision aid implementation and patients’ preferences for hip and knee osteoarthritis treatment: insights from the high value healthcare collaborative. Patient Prefer. Adherence. 2020;14:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pashayan N, Morris S, Gilbert FJ, Pharoah PDP. Cost-effectiveness and Benefit-to-Harm Ratio of Risk-Stratified Screening for Breast Cancer: A Life-Table Model. JAMA Oncol. 2018;4(11):1504. doi: 10.1001/jamaoncol.2018.1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Løberg M, Lousdal ML, Bretthauer M, Kalager M. Benefits and harms of mammography screening. Breast Cancer Res. 2015;17(1):63. doi: 10.1186/s13058-015-0525-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barratt AL, Irwig LM, Salkeld GP, Glasziou PP, Houssami N. Benefits, harms and costs of screening mammography in women 70 years and over: a systematic review. Med J Aust. 2002;176(6):266–271. doi: 10.5694/j.1326-5377.2002.tb04405.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


