Abstract
Objective:
Low-dose and very low-dose intravitreal bevacizumab (IVB) have been reported successful in short-term treatment of type 1 retinopathy of prematurity (ROP), down to an initial dose of 0.004 mg. We now report 12-month outcomes for these infants.
Design:
Masked, multicenter, dose de-escalation study
Participants:
120 prematurely-born infants with type 1 ROP
Methods:
A cohort of 120 infants with type 1 ROP in at least one eye from two sequential dose de-escalation studies of low-dose (0.25, 0.125, 0.063, and 0.031 mg) or very low-dose (0.016, 0.008, 0.004, and 0.002 mg) IVB to the study eye; the fellow eye (if also type 1) received one dose level higher. After primary success or failure at 4 weeks, clinical management was at investigator discretion, including all additional treatment.
Main Outcome Measures:
Reactivation of severe ROP by 6 months corrected age, additional treatments, retinal and other ocular structural outcomes, and refractive error at 12 months corrected age.
Results:
Sixty-two (55%) of 113 study eyes and 55 of 98 (56%) fellow eyes received additional treatment. Of the study eyes, 31 (27%) received additional ROP treatment (6 for initial treatment failure, 4 for reactivation ≤4 weeks, 21 (19%) for later reactivation) and 31 (27%) had prophylactic laser for persistent avascular retina. There was no trend toward a higher risk of additional ROP treatment related to initial IBV doses. However, time to reactivation among study eyes was shorter in eyes that received very low-dose bevacizumab (mean 76.4 days) compared with low dose (mean 85.7 days). At 12 months, poor retinal outcomes and anterior segment abnormalities were both uncommon (3% and 5%, respectively), optic atrophy was noted in 10%, the median refraction was mildly myopic (-0.31D), and strabismus was present in 29% of infants.
Conclusions:
Retinal structural outcomes were very good after low- and very low-dose IVB as initial treatment for type 1 ROP, although many eyes received additional treatment. The rate of reactivation of severe ROP was not associated with dose; however, a post-hoc data driven analysis suggested that reactivation was sooner with very low doses.
Precis:
Low and very-low dose intravitreal bevacizumab down to 0.004 mg for retinopathy of prematurity produces good retinal outcomes and minimal myopia at 12 months, without more reactivation; however, lower doses may lead to earlier reactivation.
Introduction
Intravitreal injection of anti-vascular endothelial growth factor (VEGF) agents for the treatment of retinopathy of prematurity (ROP) has become widespread, and it compares favorably with laser photocoagulation therapy.1–7 Ocular benefits of anti-VEGF treatment vs laser as primary treatment may include continued outward vascularization of retinal vessels, with the unproven potential for improved macular and peripheral visual function, as well as less myopic refractive error.2,3,6–9 Bevacizumab is the most commonly used anti-VEGF drug worldwide, likely due to its availability, low cost, and long prior experience in use for adult retinal diseases. However, it decreases serum VEGF levels for weeks after intravitreal injection, producing unknown long-term effects on developing organ systems and neurologic outcomes for treated infants.2,10–12 Furthermore, anti-VEGF treated eyes require monitoring for extensive periods after treatment because early and later ROP reactivation may occur.2,13–15 Prophylactic laser for persisting peripheral avascular retina in the absence of reactivated ROP is also commonly performed, although with limited evidence-based data.14
We designed and conducted two sequential masked, multicenter, dose de-escalation phase 1 studies to determine the lowest dose of intravitreal bevacizumab (IVB) effective for type 1 ROP.16,17 Doses from 0.25mg down to as low as 0.004 mg (but not 0.002 mg) appeared effective at inducing short-term ROP regression (4 weeks post-intravitreal injection).17 Previously, we reported reactivations,18 additional treatments, and 12-month outcomes for 61 infants treated with de-escalating IVB doses (in mg) of 0.25, 0.125, 0.063, or 0.031.14,19
Herein we report 12-month outcomes for all infants treated in both dose de-escalation studies, with IVB doses as low as 0.002 mg, which is less than 1% of doses commonly used in clinical practice. We included all doses in our analyses, including those previously reported14,16,17,19 to maximize our power to find associations of outcomes with initial IVB dose level, if they existed.
Methods
The study was conducted at 10 institution-based clinical sites and approved by the respective institutional review boards (IRBs). Written informed consent was given by a parent or guardian of each study infant. The study is listed on www.clinicaltrials.gov, under identifier NCT02390531, accessed 6/2/2021. The complete study protocol is available on the PEDIG website (www.pedig.net, accessed 6/2/2021). One hundred twenty infants were treated for ROP with an IVB injection in the study eye in a phase 1 masked study conducted by the Pediatric Eye Disease Investigator Group; 8 different dose levels (in mg) were used for study eyes (0.25, 0.125, 0.063, 0.031, 0.016, 0.008 0.004, 0.002). Among fellow eyes, 109 also had IVB injections, receiving a dose that was one level higher than the study eye (the dose in the fellow eye was 0.625 mg if the study-eye dose was 0.25 mg). Details of drug dilution and injection, and 4-week outcomes, were reported previously,16 as were reactivations and additional treatments at 6 months14 and 12-month clinical and ocular outcomes among the highest four study-eye doses.19
Infants were examined 1-day post-injection (and 4 days if needed), and at 1, 2, 3, and 4 weeks post-injection in the study eye. Beginning 4 weeks after the initial bevacizumab injection, follow-up exams and additional treatment were at investigator discretion. After 6 months corrected age, medical records were reviewed to collect data on ROP reactivations, additional treatments, timing and indications for treatment, and retinal structural outcomes. A study-mandated exam was completed at 12 months (± 2 weeks) corrected age, subsequently referred to as the ‘12-month exam.’
The first additional treatment for each study eye, if it occurred, and for each fellow eye, if it was injected initially and if additional treatment occurred, was recorded, as was the total number of additional treatments per eye. Additional treatment in this manuscript refers to any ROP treatment in the eye following the initial IVB dose, including IVB or laser. Additionally, the reason for additional treatment was recorded as: treatment failure, early reactivation, late reactivation, or persistent avascular retina. “Treatment failure” was defined as no improvement by 3–5 days after the initial injection. “Early reactivation” was defined as initial improvement, but reactivation of type 1 ROP or severe neovascularization requiring additional treatment within 4 weeks. “Late reactivation” was defined as reactivation of plus disease or neovascularization that prompted investigators to give additional treatment after 4 weeks. Treatment for “persistent avascular retina” refers to prophylactic laser to the peripheral avascular retina in the absence of severe recurrent ROP (reactivation).
Statistical Methods
Log-binomial regression was used to calculate the relative risk of additional treatment for treatment failure or early or late reactivation of type 1 ROP comparing 1) initial study-eye dose received at baseline and 2) category of type 1 ROP at enrollment in study eyes. Additionally, a post-hoc analysis was done using a proportional hazards model to calculate the cumulative incidence of any reactivation in study eyes for initial dose groups, accounting for the competing risks of death, initial treatment failure, or receiving laser treatment due to persistent avascular retina. A post-hoc data-driven analysis compared restricted mean time to reactivation among study eyes between the very-low dose group (0.002, 0.004, 0.008, and 0.016 mg) and the low dose group (0.031, 0.063, 0.125, and 0.25 mg) using a time-to-event analysis. The follow-up time used in the analysis was 91 days, the maximum time to reactivation (eAppendix I, (available at https://www.aaojournal.org).
The total dose of IVB included the initial injection in the eye and any repeat injections prior to the 12-month exam. Exploratory analyses were performed to evaluate whether there was any relationship between IVB total dose and each 12-month outcome (eAppendix I, (available at https://www.aaojournal.org). For continuous 12-month outcomes, a linear mixed model adjusting for correlation between eyes (eye-level outcomes), or a linear regression model (infant-level outcomes) was used to evaluate the relationship between total dose and the outcome. For categorical 12-month outcomes, a logistic regression model or Fisher’s exact test was used to evaluate the relationship between total dose and each binary outcome, as appropriate (eAppendix I, (available at https://www.aaojournal.org).
A post-hoc analysis evaluated the relationship between eye-level spherical equivalent (SE) cycloplegic refractive error at 12 months and laser treatment (yes/no) (eAppendix I, (available at https://www.aaojournal.org). Another post-hoc analysis evaluated the relationship between eye-level SE cycloplegic refractive error at 12 months and post-menstrual age (PMA) at the time of the first laser treatment in the eye, if applicable (eAppendix I, (available at https://www.aaojournal.org).
To control for type I error, outcomes with p-values ≤ .01 were considered suggestive of associations which may warrant further exploration. Analyses were conducted using SAS version 9.4 (SAS Inc., Cary, NC). No imputations were performed for missing data.
Results
First Additional Treatment after Initial Bevacizumab Injections
Sixty-two of 113 study eyes (55%, 95% CI: 45% to 64%) and 55 of 98 fellow eyes (56%, 95% CI: 46% to 66%) received additional ROP treatment (Table 1). Causes for additional treatment among the 62 study eyes were treatment failure in 6 eyes (5%, 95% CI: 2% to 11%), early or late reactivation in 25 eyes (22%, 95% CI: 15% to 31%), and persistent avascular retina in 31 eyes (27%, 95% CI: 19% to 37%) (Table 1). Causes for additional treatment among the 55 fellow eyes were similar (Table 1). Among all 45 eyes with early or late reactivation, 18 received bevacizumab (10 study and 8 fellow eyes) at a mean (standard deviation [SD]) of 6.3 (2.4) weeks after the initial injection, while 27 eyes received laser (15 study and 11 fellow eyes) at a mean (SD) of 8.3 (2.6) weeks. Persistent avascular retina was treated with laser in 60 eyes (31 study and 29 fellow eyes) at a mean (SD) of 21.5 (13.7) weeks after the initial IVB injection and a mean (SD) of 58.5 (13.6) weeks post-menstrual age (median 54.7, range 39.4 to 85.1).
Table 1.
| 0.625 mg | 0.250 mg | 0.125 mg | 0.063 mg | 0.031 mg | 0.016 mg | 0.008 mg | 0.004 mg | 0.002 mg | All Eyes | |
|---|---|---|---|---|---|---|---|---|---|---|
| Study Eyes c | ||||||||||
| Enrolled & Treated | 0 | 11 | 14 | 24 | 9 | 13 | 9 | 10 | 23 | 113 |
| No additional treatment d | - | 6 (55%) | 8 (57%) | 13 (54%) | 6 (67%) | 1 (8%) | 4 (44%) | 5 (50%) | 8 (35%) | 51 (45%) |
| Additional Treatment Indication d | - | 5 (45%) | 6 (43%) | 11 (46%) | 3 (33%) | 12 (92%) | 5 (56%) | 5 (50%) | 15 (65%) | 62 (55%) |
| Severe ROP | - | 2 (18%) | 4 (29%) | 8 (33%) | 0 (0%) | 4 (31%) | 3 (33%) | 4 (40%) | 6 (26%) | 31 (27%) |
| Initial treatment failuree | - | 0 (0%) | 0 (0%) | 3 (13%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (10%) | 2 (9%) | 6 (5%) |
| Early reactivatione | - | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 4 (17%) | 4 (4%) |
| Late reactivatione | - | 2 (18%) | 4 (29%) | 5 (21%) | 0 (0%) | 4 (31%) | 3 (33%) | 3 (30%) | 0 (0%) | 21 (19%) |
| Persistent avascular retina | - | 3 (27%) | 2 (14%) | 3 (13%) | 3 (33%) | 8 (62%) | 2 (22%) | 1 (10%) | 9 (39%) | 31 (27%) |
| Fellow Eyes c | ||||||||||
| Enrolled & Treated | 10 | 14 | 21 | 6 | 10 | 8 | 11 | 18 | 0 | 98 |
| No additional treatment d | 5 (50%) | 8 (57%) | 11 (52%) | 4 (67%) | 1 (10%) | 3 (38%) | 5 (45%) | 6 (33%) | - | 43 (44%) |
| Additional Treatment Indication d | 5 (50%) | 6 (43%) | 10 (48%) | 2 (33%) | 9 (90%) | 5 (63%) | 6 (55%) | 12 (67%) | - | 55 (56%) |
| Severe ROP | 2 (20%) | 3 (21%) | 8 (38%) | 0 (0%) | 2 (20%) | 3 (38%) | 4 (36%) | 4 (22%) | - | 26 (27%) |
| Initial treatment failure e | 0 (0%) | 0 (0%) | 3 (14%) | 0 (0%) | 0 (0%) | 1 (13%) | 2 (18%) | 1 (6%) | - | 7 (7%) |
| Early reactivation e | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (6%) | - | 1 (1%) |
| Late reactivation e | 2 (20%) | 3 (21%) | 5 (24%) | 0 (0%) | 2 (20%) | 2 (25%) | 2 (18%) | 2 (11%) | - | 18 (18%) |
| Persistent avascular retina | 3 (30%) | 3 (21%) | 2 (10%) | 2 (33%) | 7 (70%) | 2 (25%) | 2 (18%) | 8 (44%) | - | 29 (30%) |
Re-treatment (additional treatment following initial intravitreal bevacizumab) included laser retinal photocoagulation or intravitreal bevacizumab at investigator discretion.
Table summarizes first re-treatment, if applicable. Gray text indicates doses that were previously reported (14).
Seven enrolled study eyes and 11 enrolled fellow eyes missing from the table due to lack of 4-week follow-up, including death or deviation. Of these eyes, 5 study eyes and 4 fellow eyes were missing due to death. Eleven additional enrolled fellow eyes missing due to lack of need for initial injection.
Any percentages shown are percent of the total N of eyes in each dose group enrolled and treated (at enrollment).
Treatment failure = never improved. Early reactivation = improved but recurred by 4 weeks. Late reactivation = improved but recurred after 4 weeks.
Fifty-two study eyes had zone I ROP at enrollment, and 15 of these (29%) had treatment failure (2) or early/late reactivation (13) (eTable 2, available at https://www.aaojournal.org). Of these 15 eyes, 8 (53%) had progression of retinal vascularization into zone II by the time of additional treatment, which was done at a median of 6.4 weeks (range 5.6 – 9.0) after the initial IVB injection.
The percentage of study eyes with additional treatment for initial treatment failure or early or late reactivation are shown in eTable 3 (available at https://www.aaojournal.org) by initial dose and by category of type 1 ROP at enrollment. The relative risks for additional treatment at each initial dose and for each category of type 1 ROP did not show any clear trend (eTable 3, available at https://www.aaojournal.org). A similar analysis calculating the cumulative incidence of additional treatment for reactivation in study eyes yielded similar results (data not shown). Among all study eyes, there appeared to be a trend toward the very low doses having earlier reactivation (Figure 1). Among study eyes with reactivation within 91 days of the initial injection, the mean time to reactivation in the very low dose group was 76.4 days (standard error=3.4) compared with 85.7 days (standard error=1.6) in the low dose group (Figure 2). No reactivations were observed after 91 days; however, as many infants were censored between 91 days and the 12-month exam, the possibility of reactivation after 91 days cannot be definitively ruled out.
Figure 1: Dose vs Days Until Additional Treatment for Treatment Failure or Reactivation.
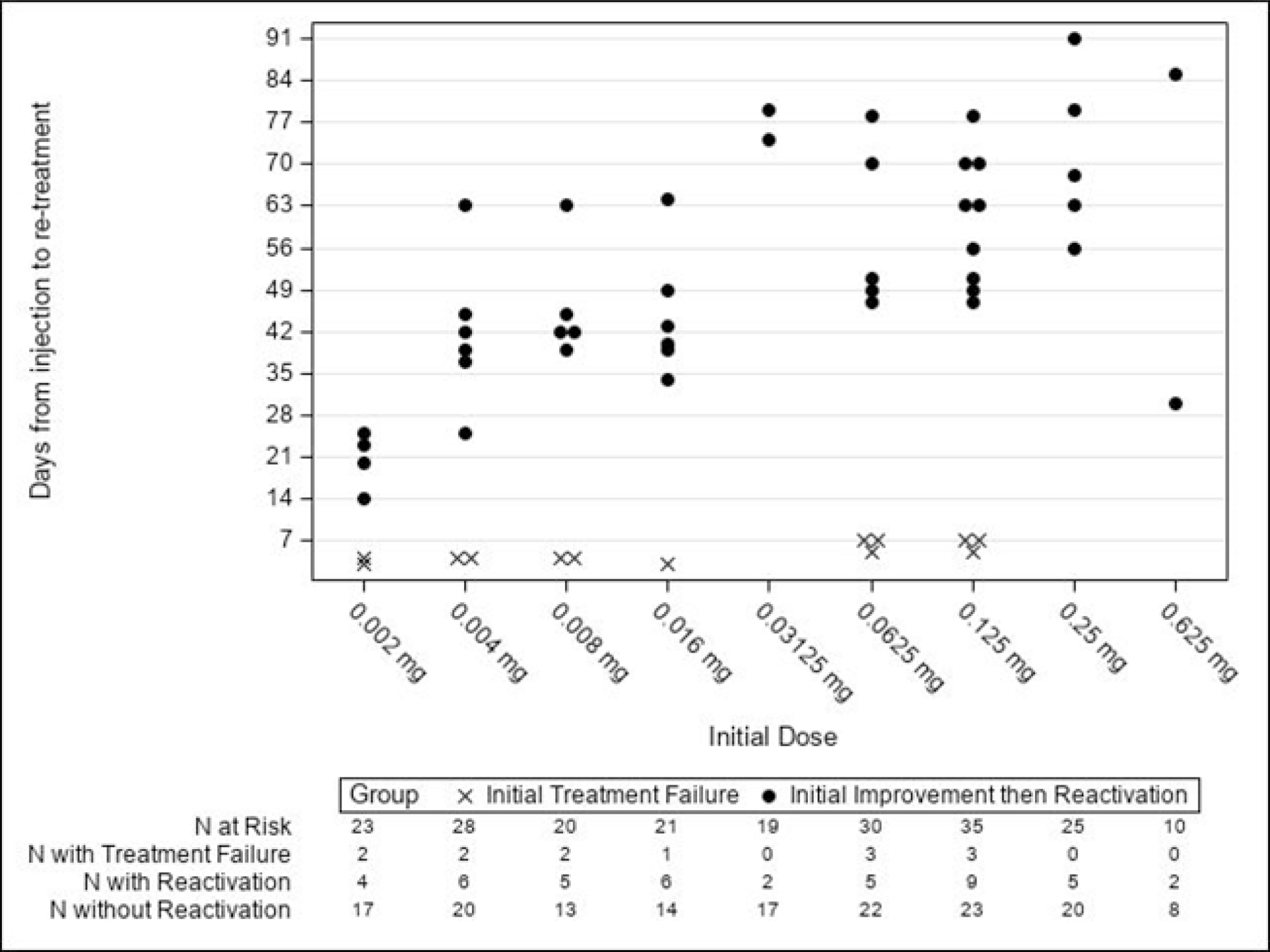
Figure includes both study and fellow eyes requiring additional treatment, and shows the first additional treatment for each eye. Initial treatment failure = never improved. Initial improvement then reactivation = improved but reactivated by or after 4 weeks; this includes both early and late reactivation.
Figure 2: Probability of No Reactivation Over Time Following Initial Injection.
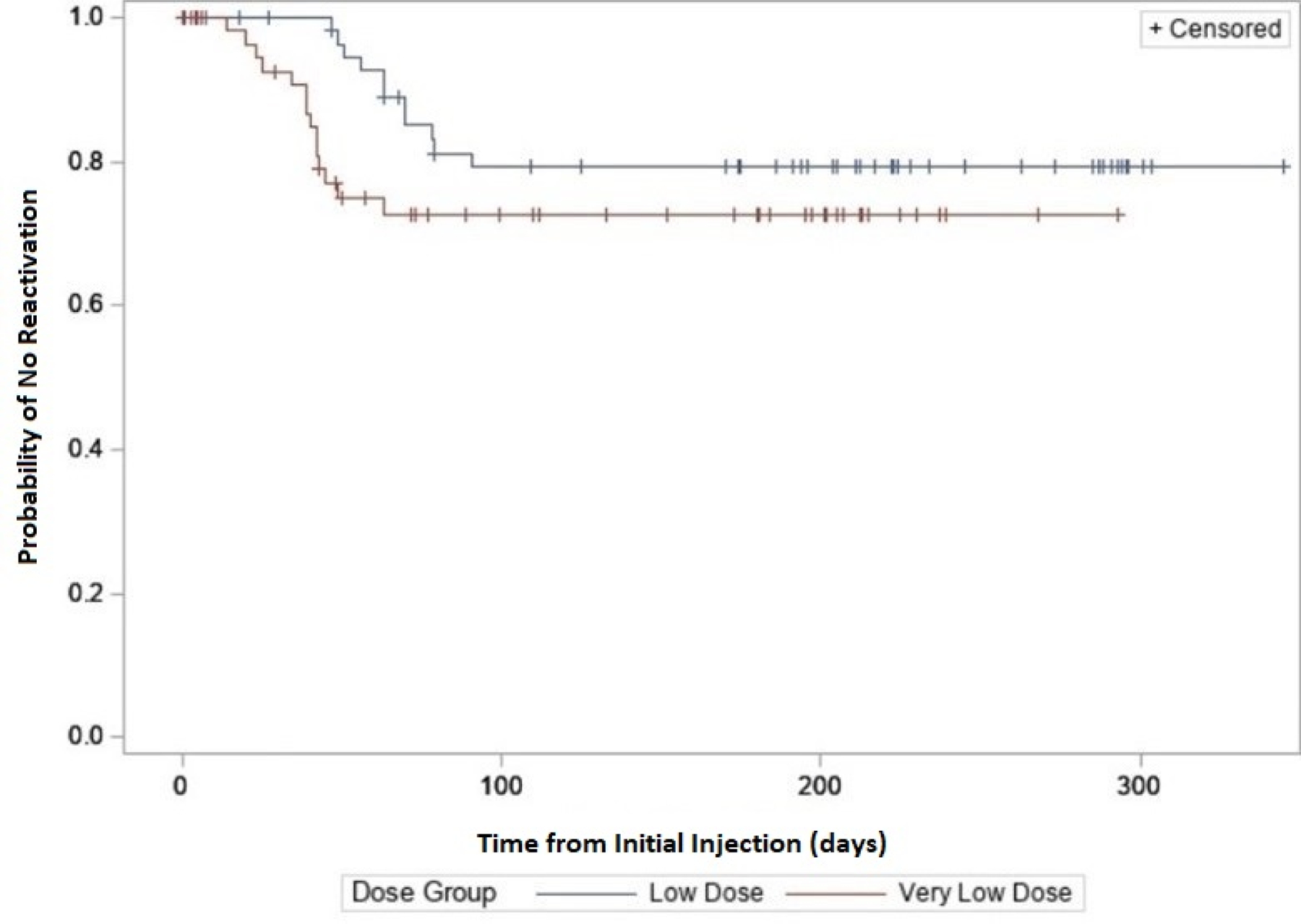
The restricted mean time to reactivation among study eyes with reactivation within 91 days was compared between the very-low dose (0.002, 0.004, 0.008, and 0.016 mg) and low dose (0.031, 0.063, 0.125, and 0.250 mg) groups using a time-to-event analysis, where reactivation was considered the event. Data of eyes treated for persistent avascular retina or treatment failure were censored at the time of treatment. Average time to reactivation was 76.4 days in the very-low dose group and 85.7 days in the low dose group based on this analysis.
Structural Outcomes
Structural outcomes of the eyes receiving the 4 higher doses (low-dose group) were previously reported.14 Of 102 treated eyes of 57 infants surviving to at least 6 months and having been initially treated with the 4 lowest IVB doses, 98 had regression of ROP with no retinal detachment and normal macular structure by indirect ophthalmoscopy, 3 eyes (2 infants) developed stage 4A, and 1 eye (1 infant) developed stage 5 retinal detachment. One infant initially treated with 0.016 mg IVB in the study eye and 0.031 mg in the fellow eye was later treated with laser for late reactivation of severe ROP, developed stage 4A detachments in both eyes, and had macular ectopia without retinal detachment at 12 months. A second infant initially treated with 0.016 mg IVB in the study eye was later treated with laser for late reactivation of severe ROP, after which the eye developed a stage 4A detachment treated with lens-sparing vitrectomy and had straightening of temporal vessels at 12 months. The fellow eye did not develop type 1 ROP, did not receive any treatment, and had a normal macular outcome. A third infant was initially treated with 0.002 mg in the study eye; after initial improvement, there was reactivation of severe ROP 3 weeks after initial IVB, prompting additional treatment with 0.25 mg IVB. Despite initial regression, severe ROP reactivated (for the second time) and was treated with laser, again with good initial response but ultimately stage 4A retinal detachment. Lens sparing vitrectomy was initially successful, but ultimately stage 5 retinal detachment developed, and the parents declined further treatment. The fellow eye was initially treated with 0.004 mg IVB, was additionally treated with 0.25 mg IVB and laser for reactivation of severe ROP, and had regression with normal macular structure.
12-month Examination
Among the 120 infants in the study, 98 (82%) completed the 12-month examination. Of these 98, 41% were female with a mean gestational age of 24.9 weeks (SD=1.7) and a mean birthweight of 698 grams (SD=302). Characteristics of infants completing vs. those not completing the 12-month examination were similar (eTable 4, available at https://www.aaojournal.org).
Cycloplegic Refractive Error at 12 Months
Cycloplegic refractive error was measured by retinoscopy in 180 eyes (study and fellow); 14 other eyes did not have retinoscopy measured and 2 other eyes were aphakic and excluded from analyses. The mean (SD) and median (quartiles) SE refractive error were -1.31D (3.81D) and -0.31D (-2.50D, +1.13D), respectively (Table 5). Seventy-one eyes had refractive error ≤ - 1.00D (mean ± SD: -4.86D ± 3.56D, median -3.75D), 60 had >-1.00D and <+1.00D (mean ± SD: +0.06D ± 0.54D, median 0.00D), and 49 had ≥ +1.00D (mean ± SD: +2.17D ± 1.36D, median +1.75D). Fifteen infants (15%) had anisometropia >1.50D SE (eTable 6, available at https://www.aaojournal.org). No relationship was identified between total IVB dose in the eye and cycloplegic refractive error at 12 months (p=0.09), or between total IVB dose in the infant and anisometropia at 12 months (p=0.47) (Table 5 and eTable 6, respectively. available at https://www.aaojournal.org).
Table 5.
Cycloplegic Refraction Findings at the 12-Month Outcome Examination
| Overall | Total Dose of Bevacizumab Injected Into Eye Prior to 12 Months | P-Value | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| >0.25mg | >0.125 to 0.25mg | >0.063 to 0.125mg | >0.031 to 0.063mg | >0.016 to 0.031mg | >0.008 to 0.016mg | >0.004 to 0.008mg | >0.002 to 0.004mg | >0 to 0.002mg | 0mg | ||||||||||||||
| N=196 Eyesa |
N=33 Eyesa |
N=26 Eyesa |
N=24 Eyesa |
N=18 Eyesa |
N=16 Eyesa |
N=17 Eyesa |
N=16 Eyesa |
N=21 Eyesa |
N=16 Eyesa |
N=9 Eyesa |
|||||||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | ||
| Cycloplegic Refraction (Spherical Equivalent) b | .09c | ||||||||||||||||||||||
| >+5.00D | 2 | 1% | 2 | 6% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | |
| >=+1.00D to <=+5.00D | 47 | 24% | 4 | 12% | 9 | 35% | 9 | 39% | 4 | 22% | 4 | 25% | 4 | 24% | 4 | 25% | 5 | 24% | 2 | 13% | 2 | 22 % | |
| >−1.00D to <+1.00D | 60 | 31% | 8 | 24% | 8 | 31% | 8 | 35% | 8 | 44% | 5 | 31% | 5 | 29% | 3 | 19% | 7 | 33% | 5 | 31% | 3 | 33 % | |
| −5.00D to <= −1.00D | 48 | 24% | 11 | 33% | 3 | 12% | 3 | 13% | 4 | 22% | 4 | 25% | 5 | 29% | 7 | 44% | 5 | 24% | 4 | 25% | 2 | 22 % | |
| <−5.00D | 23 | 12% | 7 | 21% | 4 | 15% | 3 | 13% | 2 | 11% | 2 | 13% | 2 | 12% | 2 | 13% | 1 | 5% | 0 | 0% | 0 | 0% | |
| Not Doneb | 16 | 8% | 1 | 3% | 2 | 8% | 1 | 4% | 0 | 0% | 1 | 6% | 1 | 6% | 0 | 0% | 3 | 14% | 5 | 31% | 2 | 22 % | |
| Mean (SD) | −1.31D (3.81D) | −2.48D (5.30D) | −0.73D (3.53D) | −0.50D (3.24D) | −1.31D (3.59D) | −1.58D (4.75D) | −1.46D (3.79D) | −2.04D (3.36D) | −0.74D (2.62D) | −0.86D (2.43D) | −0.09D (1.67D) | ||||||||||||
| Median Quartiles | −0.31D −2.50D, +1.13D |
−1.31D −4.88D, +0.13D |
0.00D −2.38D, +1.69D |
+0.75D −1.38D, +1.5D |
+0.19D −2.50D, +0.75D |
−0.13D −2.38D, +1.25D |
−0.25D −3.38D, +1.31D |
−1.88D −3.94D, +0.81D |
−0.25D −1.25D, +1.25D |
0.00D −2.50D, +0.50D |
0.13D −2.00D, +1.63D |
||||||||||||
| Range | −16.25D to +7.50D | −13.75D to +7.50D | −10.50D to +4.50D | −8.88D to +3.00D | −10.00D to +2.25D | −16.25D to +3.50D | −10.00D to +3.50D | −8.75D to +2.00D | −7.50D to +2.50D | −5.00D to +2.50D | −2.63D to +1.75D | ||||||||||||
CI = Confidence Interval
Percentages are not adjusted for any correlation between eyes.
Number of eyes initially examined at the 12-month exam. Gray text indicates doses that were previously reported (19).
Cycloplegic refraction analysis excludes 2 aphakic eyes, included in the tabulation under the “not done” category.
P-value from linear mixed model accounting for any correlation between eyes for an association between the continuous outcome and total dose as a categorical factor.
Laser treatment after initial IVB was performed in 104 eyes (study and fellow eyes) prior to the 12-month exam, for reactivation of severe ROP (n=31 eyes) or persistent avascular retina (n=73 eyes), at a mean of 45.0 weeks PMA (SD=3.6) and 60.0 weeks PMA (SD=17.1), respectively. The mean (SD) and median (quartiles) SE cycloplegic refractive error of the 102 phakic eyes receiving laser treatment were -1.80D (3.92D) and -0.81D (-3.63D, +0.75D), respectively, compared with -0.76D (3.63D) and +0.25D (-1.50D, +1.50D) in the 92 eyes not receiving laser treatment (adjusted mean difference of -0.34D, 99% CI: -2.36 to 1.67D, p=0.63). There was no relationship identified between PMA at the time of laser treatment and refractive error at 12 months (p=0.38), or between PMA at the time of laser and high myopia at 12 months (p=0.09) (Figure 3). However, we noted that of the 31 eyes receiving laser after 60 weeks PMA, no eye had myopia higher than -5.0 diopters. Spherical equivalent refractive error at 12 months stratified by zone at the time of laser (for any indication) can be found in eFigure 4 (available at https://www.aaojournal.org).
Figure 3: Spherical Equivalent (SE) at 12 Months vs Post-menstrual Age (PMA) by Primary Indication for Laser.
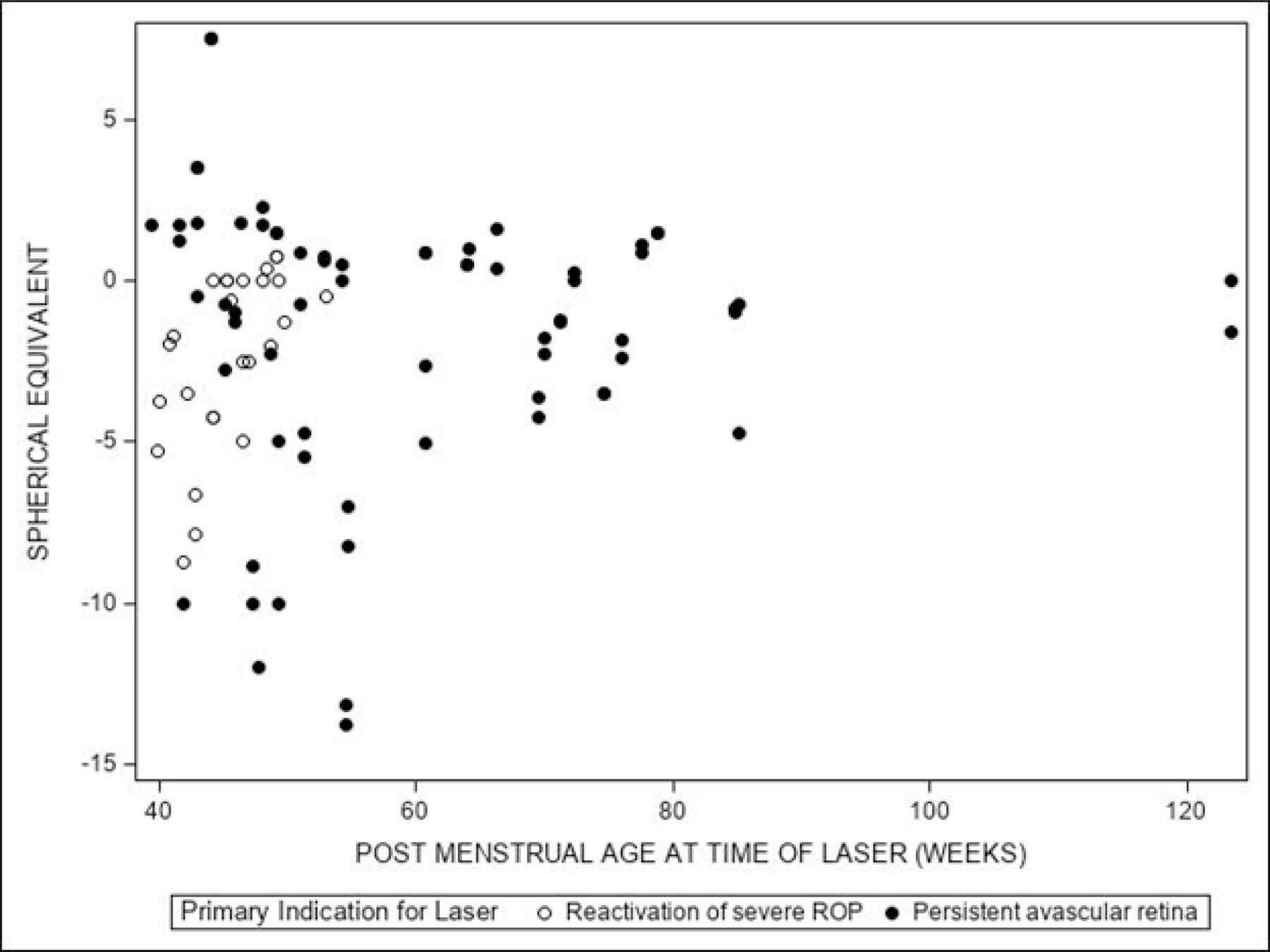
Figure shows first laser treatment for each eye (study and fellow), excluding two phakic eyes. The relationship between eye-level SE cycloplegic refractive error at 12 months and post-menstrual age (PMA) at the time of the first laser treatment in the eye (if applicable) was evaluated using a linear mixed model adjusted for the correlation between eyes as well as the zone (i.e., specific location in the eye) at the time of laser treatment. P-value for the association between refractive error and PMA was p=0.38. Additionally, a logistic regression model adjusted for the correlation between eyes and the zone at the time of laser treatment, was used to test for the association between high myopia (refractive error ≤ -5.0D) and PMA at the time of the first laser treatment in the eye, if applicable. P-value for the association between high myopia (Refractive error ≤ -5.0D) and PMA was p=0.09.
Ocular Examination at 12 Months
Abnormalities of the cornea, lens, or other anterior segment locations were reported in <1%, 3%, and 2% of eyes, respectively (eTable 7, available at https://www.aaojournal.org). Optic nerve atrophy was present in 19 (10%) eyes. Among the 19 eyes with optic atrophy, 13 (68%) had periventricular leukomalacia and/or hydrocephalus with shunt.
Constant or intermittent strabismus at distance or near fixation was reported in 28 infants (29%), manifest nystagmus in 12 (12%), and amblyopia in 7 (7%) (eTable 6, available at https://www.aaojournal.org). Visual fixation was assessed in 91 infants; of those, 85 (93%) had central fixation with both eyes; 1 (1%) had central fixation with one eye only, and 5 (5%) did not have central fixation with either eye. There were no relationships identified between any 12-month exam findings with total dose of IVB (Table 5 and eTables 6-7, available at https://www.aaojournal.org).
Adverse Events
Eight infants died from preexisting medical conditions associated with prematurity; six of these were previously reported.14 These infants did not complete the 12-month exam. Causes of death were acute respiratory failure (2), necrotizing enterocolitis (2), chronic lung disease (2), liver failure (1), and cardiac arrest (1).
Discussion
Twelve-month follow-up of 98 infants with type 1 ROP (82% of initial cohort) enrolled into two sequential dose de-escalation studies demonstrated good initial response to IVB doses as low as 0.004 mg. By the 12-month examination, 179 eyes (91%) had a normal macular outcome, 3 eyes had macular ectopia, and 3 eyes had stage 5 retinal detachment. These structural outcomes are similar to those reported in the literature with higher initial IVB doses2 as well as low-dose initial IVB from 0.25 mg down to 0.031 mg.19
Additional treatment for reactivation of ROP (rather than laser for persistent avascular retina in the absence of reactivation) was occurred for 21% of eyes across all doses. Reported additional treatment rates for reactivation of ROP following higher doses of IVB vary, from 0% with 0.25 mg in a small prospective study (n=25) by Khoadabande et al,20 to 4% in the Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity (BEAT-ROP) study (using 0.625 mg and up to only 54 weeks PMA),1 and to 15% reported in a large retrospective series (n=130) by Comez et al. (using 0.625 mg) with >1 year follow-up.21 In our study, early or late reactivation occurred in 25/113 (22%) of study eyes. Reactivation did not seem to be associated with lower initial IBV dose, because similar rates were present at the highest IVB doses (e.g., fellow eyes treated at 0.625 mg). Our data are consistent with the true reactivation rate being as low as 15% or as high as 31% (based upon the 95% CI). Possible explanations for our apparently higher rate of retreatment for reactivation compared with some other studies include: (1) our multi-center study design with different investigators, in a real-world setting without strict guidelines limiting additional treatments, (2) the fact that when ROP reactivation occurred, most cases involved both eyes of the infant, and/or (3) chance, due to our limited sample size.
Study eyes were treated for reactivation of severe ROP at a mean of 7.1 weeks after initial IVB injection with additional IVB or laser, while laser for persistent avascular retina occurred at a mean of 21.5 weeks after initial injection. While there is variation in practice, and this study did not dictate treatment after initial IVB, infants with persistent avascular retina in the absence of severe ROP reactivation are commonly treated with prophylactic laser, often prior to discharge from initial hospitalization. Presumably, this is done to avoid requirements for prolonged outpatient follow-up examinations with risks of late reactivation or loss to follow-up2,13 and because other investigators have reported that earlier prophylactic laser decreases the required number of outpatient ROP examinations after IVB for type 1 ROP.22 Additionally, performing accurate assessments of the peripheral retina in older premature infants in the clinic becomes progressively challenging.
While the reactivation rate for severe ROP did not seem to increase as the initial IVB progressed to a lower dose, a scatter plot of days to reactivation versus initial dose suggested that there might be a trend toward earlier reactivation with lower doses (Figure 1). A time-to-event analysis also suggested that the restricted mean time to reactivation may be shorter with very low doses than with low doses (Figure 2). Therefore, there may be a downside to very low-dose IVB, since several eyes treated with 0.031 mg or lower initial IVB had ROP reactivations by 6–8 weeks post-injection. From a practical clinician’s perspective, on the other hand, shorter time to reactivation may obviate prolonged post-bevacizumab follow-up of larger babies, many of whom will be returning from an outpatient setting. Because additional treatment for ROP reactivation was chosen by the clinician and could include either laser or repeat IVB, laser at an earlier (rather than later) PMA may treat more of the peripheral retina, potentially having a negative effect on peripheral visual field and possibly producing more myopia. Such a potential disadvantage may be offset by lower doses, which may be more physiologic and result in better retinal development.2,23 Additionally, if IVB has adverse systemic effects, a lower dose has theoretical benefit for the premature infant’s overall development. Further studies are needed to confirm these findings. Our analysis of restricted mean time to reactivation had some caveats, namely that a) it was both a post-hoc and a data-driven analysis and b) it did not allow us to factor in competing events (such as treatment for persistent avascular retina). In addition, our study was designed to stop when we reached a dose with a larger proportion of initial treatment failures or early severe reactivations. Because of this, we were more likely to see early reactivation at the lowest dose.
The overall 12-month cycloplegic refraction was mildly myopic (median -0.31 D) with no relationship found between total dose of IVB and either refractive error (per eye) or anisometropia (total dose per infant) at 12 months. Several studies (including BEAT-ROP) report less severe myopia after anti-VEGF vs. laser, 8,9 and our current results are consistent with these findings. Additionally, secondary analyses did not reveal more myopia in eyes receiving laser for reactivation of severe ROP or for persistent avascular retina vs. those eyes never receiving laser, or eyes of infants receiving laser after IVB at earlier (vs. later) PMA. Although it was interesting to note that no eyes receiving laser after 60 weeks PMA developed high myopia by 12 months, we may be underpowered to find small differences, and it was a post-hoc exploratory outcome. The data were further confounded by the fact that eyes receiving laser prior to 60 weeks PMA included those treated for ROP reactivation as well as persistent avascular retina, while those treated after 60 weeks PMA included only those with persistent avascular retina. Further studies are needed to confirm that eyes receiving late laser (after 60 weeks PMA) do not develop high myopia.
Ocular abnormalities were uncommon, with clinically-diagnosed optic nerve atrophy highest among them at 10% of eyes; periventricular leukomalacia and hydrocephalus were common in these infants. This rate of optic atrophy is similar to our findings in the low-dose cohort previously reported19 and in the ETROP study.24 Because optic atrophy likely reflects CNS abnormalities in these infants,2,19,25 attributing poor neurodevelopmental outcomes to IVB is problematic in non-randomized series of IVB vs. laser, where sicker infants often receive the former treatment.
No relationship was identified between any eye-level or infant-level ocular exam or clinical test findings and the total dose of IVB received prior to the 12-month examination. Unanswered questions regarding the optimal dose of initial IVB for type 1 ROP include the relationship between initial IVB dose and the potential for more normal foveal and peripheral vascular development, and ultimately the relationship to final visual acuity and neurodevelopmental outcomes.
This study must be viewed in light of some limitations. First, this dose-finding study was designed to determine the short-term (4-week) response to different initial doses of IVB, without requiring any particular treatment regimen after that time. Therefore, there were a variety of management strategies for early- and late-reactivation and persistent peripheral avascular retina in the absence of severe reactivation. Second, the limited numbers of infants and eyes in each cohort reduce our power to find a difference among initial or total IVB doses in terms of secondary outcomes. Third, we lacked photographic documentation of initial category of Type 1 ROP as well as quantitative final extent of vascularization. As this was a real-world study and did not dictate clinical care after four weeks of study-related activities, and because we did not include anesthetized retinal examination at a later age as a required study activity, we have no way to verify that eyes not receiving laser reached (or did not reach) full vascular maturity. Hence, we do not actually know how many eyes were left with persistent avascular retina. Additionally, we acknowledge that we cannot rule out that in the determination of the lowest effective IVB dose for type 1 ROP,16 the higher-dosed fellow eyes may have improved the outcome of the lower-dosed study eyes through systemic crossover of the IVB. Finally, as noted above, we were unable to definitively link initial IVB dose with time to ROP reactivation when it did occur.
Conclusions
Ocular outcomes at 12 months are encouraging for initial doses of IVB from 0.25 mg down to as low as 0.004 mg as initial treatment for type 1 ROP. Reactivation rates and 12-month outcomes across all tested IVB doses (down to 0.004 mg) were similar. However, our data suggest a trend toward earlier reactivation with very low doses. While implications for wider clinical use must be considered, further studies are needed to confirm this trend. Although lower doses of IVB seem quite effective at producing regression of type 1 ROP, at the present time, they require dilution by inpatient pharmacies using sterile procedures to allow a delivery volume of at least 0.010 ml (and ideally perhaps 0.020). Additional studies of low-dose IVB and other anti-VEGF agents for type 1 ROP are warranted, with particular attention to timing and characteristics of reactivation, extent of vascularization, need for additional treatment, and longer-term outcomes such as visual acuity, macular structure by imaging, and neuro-development.
Supplementary Material
eFigure 4: Spherical Equivalent (SE) Refractive Error at 12 Months by Zone at Time of Laser. Distribution of SE refractive error (in diopters) shown by zone at the time of laser, for those eyes which received laser for any reason. Mean (standard deviation) of SE refractive error was -4.25 (0) diopters for 2 eyes in Zone I, -1.62 (3.80) diopters for 65 eyes in Zone II, and -1.57 (4.61) diopters for 18 eyes in Zone III.
Study Acknowledgements
Participating members of the Pediatric Eye Disease Investigator Group. Number of patients in parenthesis. Includes all investigators (I) and all coordinators (C) active in the study at any time.
Durham, NC - Duke University Eye Center (34)
Sharon F. Freedman (I); Sasapin G. Prakalapakorn (I); David K. Wallace (I); Sarah K. Jones (C); Navajyoti R. Barman (C); Robert J. House (C); David A. Nasrazadani (C)
Virginia Beach, VA - Virginia Pediatric Eye Center (14)
Eric Crouch (I); Earl R. Crouch, Jr. (I), Gaylord G. Ventura (C)
Cincinnati, OH - Cincinnati Children`s Hospital (12)
Michael B. Yang (I); Eniolami O. Dosunmu (I); Michael E. Gray (I); William W. Motley (I); Katherine Castleberry (C); Patricia Cobb (C); Patricia Hirsch (C); Melissa Reed (C); Monica A. Sandoval (C); Neil Vallabh (C)
Columbus, OH - Pediatric Ophthalmology Associates, Inc. (12)
David L. Rogers (I); Don. L. Bremer (I), Richard P. Golden (I); Catherine O. Jordan (I); Mary Lou McGregor (I); Rachel E. Reem (I), Amanda N. Schreckengost (C); Sara A. Maletic (C); Rachel T. Miller (C)
Houston, TX - Texas Children’s Hospital - Dpt. Of Ophthalmology (12)
Amit R. Bhatt (I); David K. Coats (I); Gihan Romany (C); Ann B. Demmy (C); Lingkun X. Kong (C)
Salt Lake City, UT - University of Utah/Moran Eye Center (12)
Mary E. Hartnett (I); David C. Dries (I); Robert O. Hoffman (I); Susan Allman (C); Katie J. Farnsworth (C); Barbara Hart (C); Kelliann Ordonez (C)
Atlanta, GA - The Emory Eye Center (9)
Amy K. Hutchinson (I); George B. Hubbard, III (I); Prethy Rao (I); Joshua E. Robinson (I); Judy L. Brower (C)
Indianapolis, IN - Indiana University Department of Ophthalmology (7)
Kathryn M. Haider (I); Charline S. Boente (I); Heather A. Smith (I); Elizabeth A. Hynes (C); Michele E. Whitaker (C)
Boston, MA - Boston Children`s Hospital (6)
Deborah K. VanderVeen (I); Jason S. Mantagos (I); Carolyn Wu (I); Samantha Goldstein (C); Tamar Winter (C); Grace X. Yoon (C)
Oklahoma City, OK - Dean A. McGee Eye Institute, University of Oklahoma (3)
R. Michael Siatkowski (I); Janine E. Collinge (I); Kelli J. Satnes (C); Michelle H. Blunt (C)
Baltimore, MD – Wilmer Eye Institute (0)
Michael X. Repka (I); Courtney Kraus (I); Jennifer A. Shepard (C)
PEDIG Coordinating Center - Tampa, FL
Raymond T. Kraker, Roy W. Beck, Darrell S. Austin, Nicole M. Boyle, Danielle L. Chandler, Patricia L. Connelly, Courtney L. Conner, Quayleen Donahue, Brooke P. Fimbel, Robert J. Henderson, Amra Hercinovic, James E. Hoepner, Joseph D. Kaplon, Zhuokai Li, B. Michele Melia, Gillaine Ortiz, Julianne L. Robinson, Kathleen M. Stutz, Desirae R. Sutherland, David O. Toro, Victoria C. Woodard, Rui Wu.
PEDIG Executive Committee
Susan A. Cotter (Co-chair), Jonathan M. Holmes (Co-chair), Roy W. Beck, Eileen E. Birch, Angela M. Chen (2017–2018), Stephen P. Christiansen (2018–2020), Eric R. Crouch III (2014–2015), Laura B. Enyedi (2014–2016), S. Ayse Erzurum (2016–2017, 2020–2021), Donald F. Everett, Sharon F. Freedman (2016–2018), William V. Good (2017–2019), Erin C. Jenewein (2021-present), Raymond T. Kraker, Katherine A. Lee (2014–2016), Richard London (2018–2020), Vivian M. Manh (2016–2018, 2020–2022), Ruth E. Manny (2013–2016, 2017–2019), Beth A. Morrell (2021-present), David G. Morrison (2018–2019), David B. Petersen (2020–2022), Stacy L. Pineles, Hantamalala Ralay Ranaivo (2019–2021), Michael X. Repka, Tawna L. Roberts, Scott T. Ruark (2018–2019), Bonita R. Schweinler (2016–2018), Jayne L. Silver (2014–2016), Donny W. Suh (2021-present), Allison I. Summers (2019–2021), Lisa C. Verderber (2015–2017), David K. Wallace, Katherine K. Weise.
National Eye Institute - Bethesda, MD
Donald F. Everett
Data and Safety Monitoring Committee
Marie Diener-West (chair), John D. Baker, Barry Davis, Dale L. Phelps, Stephen W. Poff, Richard A. Saunders, Lawrence Tychsen
Funding/Support:
Supported by National Eye Institute of National Institutes of Health, Department of Health and Human Services EY011751, EY023198, and EY018810. The funding organization had no role in the design or conduct of this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meeting Presentation:
This work was presented at the American of Ophthalmology’s Annual Meeting 2021 in New Orleans, LA, and the American Academy of Optometry’s Annual Meeting 2021 in Boston, MA.
Conflict of Interest:
No conflicting relationships exist for any authors.
Taxonomy topics (4–12):
pediatric ophthalmology, retinopathy of prematurity
Address for Reprints:
An address for reprints will not be provided.
References
- 1.Mintz-Hittner HA, Kennedy KA, Chuang AZ, Group B-RC. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med 2011;364(7):603–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.VanderVeen DK, Melia M, Yang MB, Hutchinson AK, Wilson LB, Lambert SR. Anti-Vascular Endothelial Growth Factor Therapy for Primary Treatment of Type 1 Retinopathy of Prematurity: A Report by the American Academy of Ophthalmology. Ophthalmology 2017;124(5):619–633. [DOI] [PubMed] [Google Scholar]
- 3.Mueller B, Salchow DJ, Waffenschmidt E, et al. Treatment of type I ROP with intravitreal bevacizumab or laser photocoagulation according to retinal zone. Br J Ophthalmol 2017;101(3):365–370. [DOI] [PubMed] [Google Scholar]
- 4.Stahl A, Lepore D, Fielder A, et al. Ranibizumab versus laser therapy for the treatment of very low birthweight infants with retinopathy of prematurity (RAINBOW): an open-label randomised controlled trial. Lancet 2019;394(10208):1551–1559. [DOI] [PubMed] [Google Scholar]
- 5.Chen TA, Schachar IH, Moshfeghi DM. Outcomes of Intravitreal Bevacizumab and Diode Laser Photocoagulation for Treatment-Warranted Retinopathy of Prematurity. Ophthalmic Surg Lasers Imaging Retina 2018;49(2):126–131. [DOI] [PubMed] [Google Scholar]
- 6.Hwang CK, Hubbard GB, Hutchinson AK, Lambert SR. Outcomes after Intravitreal Bevacizumab versus Laser Photocoagulation for Retinopathy of Prematurity: A 5-Year Retrospective Analysis. Ophthalmology 2015;122(5):1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lepore D, Quinn GE, Molle F, et al. Intravitreal bevacizumab versus laser treatment in type 1 retinopathy of prematurity: report on fluorescein angiographic findings. Ophthalmology 2014;121(11):2212–2219. [DOI] [PubMed] [Google Scholar]
- 8.Geloneck MM, Chuang AZ, Clark WL, et al. Refractive outcomes following bevacizumab monotherapy compared with conventional laser treatment: a randomized clinical trial. JAMA Ophthalmol 2014;132(11):1327–1333. [DOI] [PubMed] [Google Scholar]
- 9.Harder BC, Schlichtenbrede FC, von Baltz S, Jendritza W, Jendritza B, Jonas JB. Intravitreal bevacizumab for retinopathy of prematurity: refractive error results. Am J Ophthalmol 2013;155(6):1119–1124.e1111. [DOI] [PubMed] [Google Scholar]
- 10.Sato T, Wada K, Arahori H, et al. Serum concentrations of bevacizumab (avastin) and vascular endothelial growth factor in infants with retinopathy of prematurity. Am J Ophthalmol 2012;153(2):327–333 e321. [DOI] [PubMed] [Google Scholar]
- 11.Kong L, Bhatt AR, Demny AB, et al. Pharmacokinetics of bevacizumab and its effects on serum VEGF and IGF-1 in infants with retinopathy of prematurity. Invest Ophthalmol Vis Sci 2015;56(2):956–961. [DOI] [PubMed] [Google Scholar]
- 12.Wu WC, Lien R, Liao PJ, et al. Serum levels of vascular endothelial growth factor and related factors after intravitreous bevacizumab injection for retinopathy of prematurity. JAMA Ophthalmol 2015;133(4):391–397. [DOI] [PubMed] [Google Scholar]
- 13.Fierson WM, Ophthalmology AAOPSo, American Academy Of O, American Association For Pediatric O, Strabismus, American Association Of Certified O. Screening Examination of Premature Infants for Retinopathy of Prematurity. Pediatrics 2018;142(6). [DOI] [PubMed] [Google Scholar]
- 14.Wallace DK, Dean TW, Hartnett ME, et al. A dosing study of bevacizumab for retinopathy of prematurity: late recurrences and additional treatments. Ophthalmology 2018;125(12):1961–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hajrasouliha AR, Garcia-Gonzales JM, Shapiro MJ, Yoon H, Blair MP. Reactivation of Retinopathy of Prematurity Three Years After Treatment With Bevacizumab. Ophthalmic Surg Lasers Imaging Retina 2017;48(3):255–259. [DOI] [PubMed] [Google Scholar]
- 16.Wallace DK, Kraker RT, Freedman SF, et al. Assessment of Lower Doses of Intravitreous Bevacizumab for Retinopathy of Prematurity: A Phase 1 Dosing Study. JAMA Ophthalmol 2017;135(6):654–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wallace DK, Kraker RT, Freedman SF, et al. Short-term Outcomes After Very Low-Dose Intravitreous Bevacizumab for Retinopathy of Prematurity. JAMA Ophthalmol 2020;138(6):698–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiang MF, Quinn GE, Fielder AR, et al. International Classification of Retinopathy of Prematurity, Third Edition. Ophthalmology 2021;128(10):e51–e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crouch ER, Kraker RT, Wallace DK, et al. Secondary 12-Month Ocular Outcomes of a Phase 1 Dosing Study of Bevacizumab for Retinopathy of Prematurity. JAMA Ophthalmol 2020;138(1):14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khodabande A, Niyousha MR, Roohipoor R. A lower dose of intravitreal bevacizumab effectively treats retinopathy of prematurity. J AAPOS 2016;20(6):490–492. [DOI] [PubMed] [Google Scholar]
- 21.Comez A, Karakucuk Y, Ozmen MC, Celemler P, Saygili O. The results of intravitreal bevacizumab monotherapy for treating aggressive posterior retinopathy of prematurity and Type 1 retinopathy of prematurity. Eye (Lond) 2021. [DOI] [PMC free article] [PubMed]
- 22.Hong GJ, Stinnett SS, Freedman SF, Wallace DK, Prakalapakorn SG. Prophylactic laser versus continued surveillance after initial bevacizumab treatment for retinopathy of prematurity. J aapos 2021;25(3):177–180. [DOI] [PubMed] [Google Scholar]
- 23.Hartnett ME. Pathophysiology and mechanisms of severe retinopathy of prematurity. Ophthalmology 2015;122(1):200–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Early Treatment for Retinopathy of Prematurity Cooperative G, Good WV, Hardy RJ, et al. Final visual acuity results in the early treatment for retinopathy of prematurity study. Arch Ophthalmol 2010;128(6):663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaushal M, Razak A, Patel W, Pullattayil AK, Kaushal A. Neurodevelopmental outcomes following bevacizumab treatment for retinopathy of prematurity: a systematic review and meta-analysis. J Perinatol 2020. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 4: Spherical Equivalent (SE) Refractive Error at 12 Months by Zone at Time of Laser. Distribution of SE refractive error (in diopters) shown by zone at the time of laser, for those eyes which received laser for any reason. Mean (standard deviation) of SE refractive error was -4.25 (0) diopters for 2 eyes in Zone I, -1.62 (3.80) diopters for 65 eyes in Zone II, and -1.57 (4.61) diopters for 18 eyes in Zone III.


