Abstract
The skin microbiome plays a critical role in skin homeostasis and disorders. UV-radiation is the major cause for nonmelanoma skin cancer, but other risk factors including immune suppression, chronic inflammation, and antibiotic usage, suggest the microbiome as an additional, unexplored risk factor and potential disease biomarker. The overarching goal was to study the skin microbiome in squamous cell carcinoma (SCC) and pre-malignant actinic keratosis (AK) compared to healthy skin to identify skin cancer-associated changes in the skin microbiome. We performed a high-resolution analysis of shotgun metagenomes of AK and SCC to healthy skin, revealing microbial community shifts specific to AK and SCC. Most prominently, the relative abundance of pathobiont Staphylococcus aureus was increased at the expense of commensal Cutibacterium acnes in SCC compared to healthy skin, and enrichment of functional pathways in SCC reflected this shift. Notably, C. acnes associated with lesional vs. healthy skin differed at the strain level, suggesting specific functional changes associated with its depletion in SCC. Our study revealed a transitional microbial dysbiosis from healthy skin to AK to SCC, supporting further investigation of the skin microbiome for use as a biomarker and providing hypotheses for studies investigating how these microbes might influence skin cancer progression.
Keywords: Cutaneous Squamous Cell Carcinoma, Actinic Keratosis, Skin Microbiome, Non-Melanoma Skin Cancer
INTRODUCTION
Nonmelanoma skin cancer (NMSC), including basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), is the most common cancer worldwide with a high and increasing health care burden. The prognosis for NMSCs is favorable if detected early, but the metastatic rate can approach 10% (Didona et al., 2018, Leiter et al., 2014). Ultraviolet (UV) radiation via sun exposure is the top risk factor for NMSC causing DNA damage, ROS (reactive oxygen species) and inflammatory cytokines leading to immunosuppression and cancer development (Didona et al., 2018, Nehal and Bichakjian, 2018). Because of its ubiquity and potential severity, it is important to identify new risk factors, early diagnostic biomarkers, and new clinical interventions for NMSC.
Increasing evidence has linked the gut microbiome to several cancers (Boleij and Tjalsma, 2012, Kostic et al., 2012, Voigt et al., 2017, Wang et al., 2017, Zeller et al., 2014), either directly via the production of carcinogens or indirectly through stimulation of (chronic) inflammation or immunosuppression (Cuevas-Ramos et al., 2010, Goodwin et al., 2011, Grivennikov et al., 2010, Rubinstein et al., 2013, Sepich-Poore et al., 2021). The skin microbiome, despite its central role in cutaneous immunity, has remained largely uncharacterized in skin cancer, though its role in disease progression, severity, and risk of skin cancer (Kullander et al., 2009, Madhusudhan et al., 2020, Nakatsuji et al., 2018, Woo et al., 2022, Wood et al., 2018) and skin-related liquid cancers such as cutaneous T-cell lymphoma (Harkins et al., 2020) has become of significant interest.
Because squamous cells are the most superficial epidermal cells and thusly in closest proximity to the skin microbiome, we hypothesized that the skin microbiome might be implicated in SCC progression, severity, and treatment response. Skin microbes interact closely with specialized cells expressing pattern-recognition receptors and may modulate immunity and inflammatory response (i.e., T lymphocyte function) (Miller and Modlin, 2007, Naik et al., 2015, Naik et al., 2012). Moreover, immunosuppression, chronic inflammation, and viral infections can increase susceptibility to skin carcinogenesis (Didona et al., 2018, Matinfar et al., 2018, Neagu et al., 2019). Taken together, these factors suggest that the skin microbiome is a potential disease biomarker. However, a deep characterization of the SCC-associated microbiome that would propel such investigations is lacking.
Here, we characterized the skin microbiome in SCC and its precursor, actinic keratoses (AKs), compared to normal, healthy skin (HS) in SCC patients and control individuals (CTRLs) to identify microbes associated with SCC and its progression from AKs. Using whole genome shotgun (WGS) sequencing, we found a pronounced decrease of a keystone commensal, Cutibacterium (C.) acnes, accompanied by an increase of a pathobiont, Staphylococcus (S.) aureus in AK and SCC, indicating disease-associated changes in the relative abundance of key members of the skin microbiome. This study provides a high-resolution baseline characterization of the microbial association with SCC, laying the groundwork for further studies investigating mechanistic contributions of such associations to SCC etiology.
RESULTS
Study design and analysis strategy
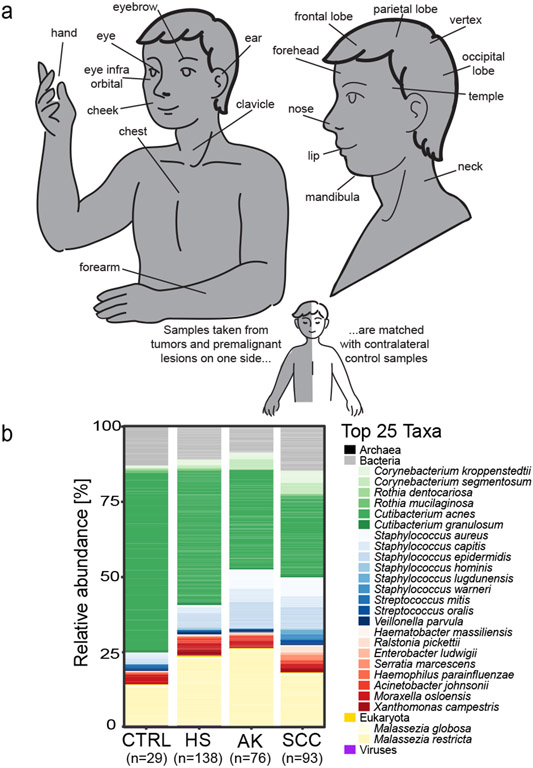
We recruited 81 patients with diagnosed cutaneous squamous cell carcinoma (SCC) and actinic keratosis (AK), and 25 healthy control individuals (CTRLs) at the University Hospital Heidelberg, Germany (Table 1, Table S1). Per patient, 2-6 skin swabs matched by sampling site were obtained from 1-2 healthy skin (HS), 1-2 AK, and 1-2 SCC sites (Figure 1a). Additionally, HS samples from CTRLs were matched by age, gender, and sampling site to a subset of the patients. After quality control, we analyzed 336 samples representing 138 HS, 76 AK, 93 SCC samples and 29 HS CTRL samples (Supplemental Material, Table S2). Clinical classification of AKs was based on the Olsen grade, which distinguishes AKs based on the thickness and degree of hyperkeratosis (Grade I-III). SCCs were classified based on the TNM system for cancer staging. Most AKs were early-stage lesions (Olsen grade I) as were most SCC tumors (Stage I (T1)) without signs of metastasis, with mild surface severity and without ulceration. Sampling sites were mainly sebaceous (e.g., forehead), with few dry (e.g., hands), moist (e.g., lips) and moist/sebaceous (e.g., inner eye corner) sites (Figure 1a, Table S1) – physiologic differences that affect skin microbiome composition (Grice and Segre, 2011). Finally, we employed a within-individual sampling design to account for skin site-specific differences. For most analyses, we included samples matched within-patient, per site without replicates (e.g., 1 HS and 1 SCC sample per patient for HS-SCC comparisons, or gender/site/age matching for CTRL-SCC), hereafter called ‘matched’ datasets. For select analyses, we used the full dataset scaled by sampling site to remove the dependency of pseudoreplicates within patients and different sampling sites, allowing all samples to be included (‘scaled dataset’). We additionally employed mixed effect models to account for pseudoreplicates within patients and sampling site, which were largely concordant. For simplicity, we reported results in which the matched dataset was used and called attention only to when results from the ‘scaled dataset’ or mixed effect models (Table S7) were significantly discordant or of particular note.
Table 1. Overview of minimal metadata of the present study population.
Data are summarized by median with the range in brackets, n.a.: data not available. HS: healthy skin, AK: Actinic keratosis, SCC: Squamous cell carcinoma, Surface severity: SCC surface topography and texture: mild (I), moderate (II), severe (III). More details can be found in Table S1.
| Cohort | Disease status (# samples) |
Gender (M / F) |
Age in years (range) |
BMI (kg / m2) (range) |
Olsen Grade | T-Stage | Surface severity | Ulceration | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | n.a. | T1 | T2 | n.a. | I | II | III | n.a. | yes | no | n.a. | |||||
| Patients (n=81) | HS (n=138) | 55/26 | 77 (49 – 101) | 25.0 (19.0-38.0) | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| AK (n=76) | 62 | 9 | 2 | 3 | - | - | - | - | - | - | - | - | - | - | ||||
| SCC (n=93) | - | - | - | - | 87 | 3 | 3 | 50 | 34 | 6 | 3 | 25 | 64 | 4 | ||||
| Controls (n=25) | HS (n=29) | 19/6 | 77 (64-86) | 25.00 (15.0-35.0) | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
Figure 1: Study design and overall microbial skin community description.
a: Skin swabs from actinic keratoses (AK) or squamous cell carcinoma (SCC) tumors were collected from the skin sites shown, with a contralateral unaffected, healthy (HS) sample collected for each individual, where possible. b: Relative abundance plot of the most abundant bacteria, fungi, and viruses are shown for HS, AK and SCC from skin cancer patients and healthy skin swabs from control individuals (CTRL). Non-bacterial species were grouped by their respective kingdom. Composite data across the cohorts are shown for simplicity; exemplary individual patient breakdown is shown in Figure S2a.
The skin microbiome composition in healthy skin and lesions
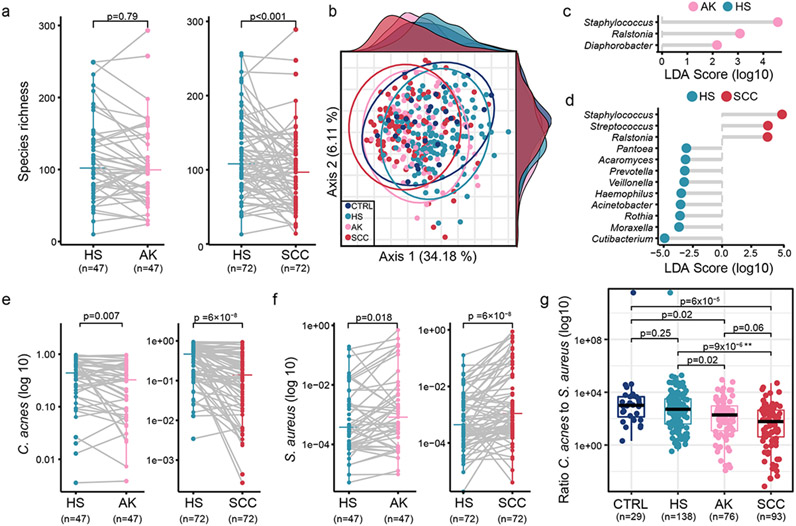
We first studied the overall community composition in CTRLs and skin cancer patients. Microbial diversity is associated with numerous diseases, including inflammatory skin diseases (e.g., Le Chatelier et al., 2013, Nakatsuji and Gallo, 2019, Schwimmer et al., 2019). No significant differences were observed in the Shannon diversity index between CTRLs, HS, AK, and SCC (Figure S1a-b, Table S7). In contrast, species richness (number of unique species) was significantly increased in HS vs. SCC, but not HS vs. AK (Figure 2a; the latter also significantly increasing when scaled (Figure S1c) or modeled for sampling site and patient (Table S7)). This suggested a loss of diversity in the tumor environment driven by low abundance species. Supporting broader microbial changes specific to SCC, principal coordinate analysis (PCoA) of all samples did not show grouping by skin site or site characteristic (Figure S1d), but rather by disease progression (Figure 2b, PERMANOVA, FDR-corrected p<0.05 for all pairwise comparisons, except p>0.05 for HS vs. CTRL, as anticipated). Overall, we concluded that there is a dysbiosis associated with disease progression, driven by factors other than skin site. Therefore, in downstream analyses, samples from different sites were treated as replicates of the corresponding disease type (HS, AK, or SCC).
Figure 2: AK- and SCC-associated biomarkers.
a: Richness comparing matched (gray lines) HS vs. AK, and SCC. b: PCoA clustering of scaled data. Ellipses and density plots are described in Supplementary Materials and Methods, c and d: LDA score plot showing differentially abundant genera in HS vs. AK, and SCC (matched datasets). Scores ≥2 for HS vs. AK and ≥3 for HS vs. SCC (full datasets in Figure S4) were ranked by effect size, e: Abundances of C. acnes, f: S. aureus for matched HS vs. AK and SCC. g: C. acnes:S. aureus ratio in scaled dataset. P-values were calculated by paired (a, e, f) and unpaired (g) Wilcoxon test. For (g), p-values were FDR-corrected. P-value of mixed effects models: *<0.1, **<0.05, ***<0.01 (Table S7).
Detection of skin cancer-associated biomarkers
Overall, the skin microbiome composition was consistent with other Western skin microbiome cohorts (Byrd et al., 2018, Findley et al., 2013, Grice et al., 2009, Grice and Segre, 2011, Oh et al., 2016). Cutibacterium (C.) acnes, C. granulosum, Staphylococcus (S.) species and Corynebacteria species constituted the largest portion of the bacterial community, while the fungal community was dominated by Malassezia globosa and restricta. Viruses, such as human polyomavirus, phages (e.g., Cutibacterium phage) and human papilloma viruses (HPV) comprised a small proportion of the microbiome (Figure 1b; representative data in Figure S2a). Deeper analysis of beta HPV types, the HPV type more commonly linked with SCC (Tampa et al., 2020), revealed an increased viral presence in HS than in AK or SCC and a striking predominance in eyebrows (Supplementary Data, Figure S3). Mites, particularly Demodex folliculorum, had decreased prevalence in SCC but not AK (Supplementary Data, Table S3).
To identify microbes associated with AK or SCC (potential biomarkers of interest), we used linear discriminant analysis (LDA) (Segata et al., 2011), comparing taxa at multiple phylogenetic levels (genus, species) to allow for potential functional redundancy at these levels. The most discriminant genera were Cutibacterium (HS-associated) and Staphylococcus (AK- and SCC-associated, Figure 2c-d, Figure S4a, matched datasets). We also identified potential new skin microbial markers, including AK-associated Ralstonia and Diaphorobacter and SCC-associated Ralstonia and Streptococcus. These are relatively rarer skin microbes but have been reported in healthy and atopic subjects (Fyhrquist et al., 2014) albeit potentially also as kit contaminants (Salter et al., 2014).
At species level, multiple Lactobacillus (L.) species (e.g., L. rhamnosus and L. plantarum) were less abundant in AK and SCC while Ralstonia (R.), particularly R. pickettii, was positively associated with AK and SCC (Figure S4b-c). Interestingly, lactobacilli exhibit anti-inflammatory and probiotic properties, promoting homeostasis in multiple organs including skin and delayed tumor development in a mouse model (Supplementary Data). For HS vs. SCC, C. acnes and S. aureus were the most discriminant species. Interestingly, additional staphylococcal and cutibacteria species were also differentially abundant (Table S4, Figure S4, Supplementary Data), which we believe collectively contributed to AK- and SCC-associated dysbiosis. For example, C. granulosum, one of the three most prevalent cutibacterium species, is hypothetically antitumorigenic (Milas et al., 1974, Paslin et al., 1974) while S. hominis, similar to C. acnes, can harbor anti-S. aureus activity (Supplementary Data) (Nakatsuji et al., 2017).
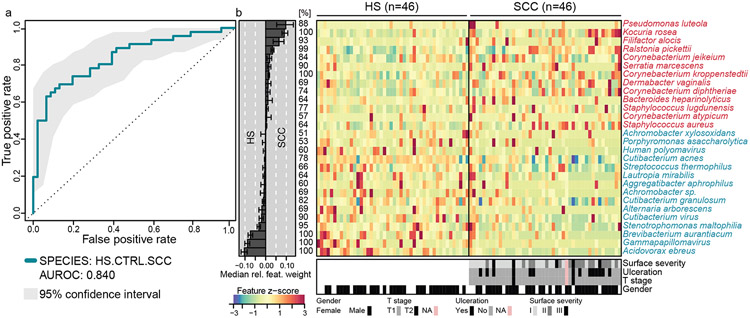
To complement results from LDA, we performed a lasso model on matched samples from patients and CTRLs to identify additional SCC-specific features. Of microbial species that contributed the most weight to the model, 13 were enriched in SCC, including S. aureus, multiple Corynebacteria species, and Serratia (S.) marcescens (Figure 3, Supplementary Data). Interestingly, S. marcescens was previously associated with the skin microbiome of immunodeficient patients (Oh et al., 2013), and multiple of our skin cancer patients are immunosuppressed due to previous organ transplants (Table S1), a strong risk factor for skin cancer.
Figure 3: Metagenomic model for variable selection.
a: Test accuracy of the model based on the species-level dataset “HS.CTRL.SCC” identifying variables discriminating HS vs. SCC is depicted as ROC curve and area under the ROC (AUROC). For details on resampling and cross-validation see Supplementary Materials and Methods and Supplementary Data). b: Relative abundances of the most discriminatory 27 species are displayed as a heatmap as fold change over the median relative abundance observed in the matched samples (z-score). The bar plot shows the mean contribution (bar width: median relative weight) of each species. Percentages indicate the fraction of models containing the species during cross-validation. Bars underneath the heatmap show the distribution of tumor surface severity, ulceration, T stage, and gender across samples.
Next, we tested if C. acnes and S. aureus had an antagonistic relationship through tumor progression. The decline of C. acnes was concomitant with an increase of S. aureus from HS to AK to SCC (Figure 2e-f, Figure S5a-b, scaled dataset in Figure S5c-d, modelled data in Table S7), indicating that early signs of microbial alterations are detectable in AK. We further investigated the magnitude of this antagonism by calculating the ratio of C. acnes:S. aureus, analogous to the Bacteroides:Firmicutes ratio often used in gut microbiome studies to describe microbial dysbiosis. While our data does not measure absolute abundances, the observed changes in relative abundance suggested an antagonistic relationship, supported by Francuzik et al., 2018, possibly through propionic acid, the main fermentation product of C. acnes, which inhibits S. aureus growth in atopic dermatitis (Shu et al., 2013). Indeed, the C. acnes:S. aureus ratio gradually declined with disease progression (scaled dataset, Figure 2g, HS vs. SCC significant and HS vs. AK trending to significance in model (Table S7)), suggesting an imbalance in the interactions of these two species in premalignant vs. cancerous skin.
The ratios of C. acnes or S. hominis to S. aureus both declined with disease progression for most major skin sites (Figure S6), but they were not associated with lesional characteristics such as Olsen grade, T-stage, ulceration, and tumor surface severity. One exception was a noted decrease in the S. hominis:S. aureus ratio in ulcerated tumors (scaled data (Figure S7); not significant for modeling (Table S7)). This is likely because C. acnes and S. hominis declined in relative abundance with tumor advancement (Figure S7i-1), while S. aureus remained consistently high in SCC irrespective of ulceration or degree of tumor surface severity (Figure S7m-n, Table S7). Finally, no microbial features were specifically associated with previous therapies (dialysis or immunosuppression, Supplementary Data). Overall, these data support our analyses showing a gradual change in the skin microbiome from HS to AK, with more striking changes manifesting in SCC.
C. acnes strain population
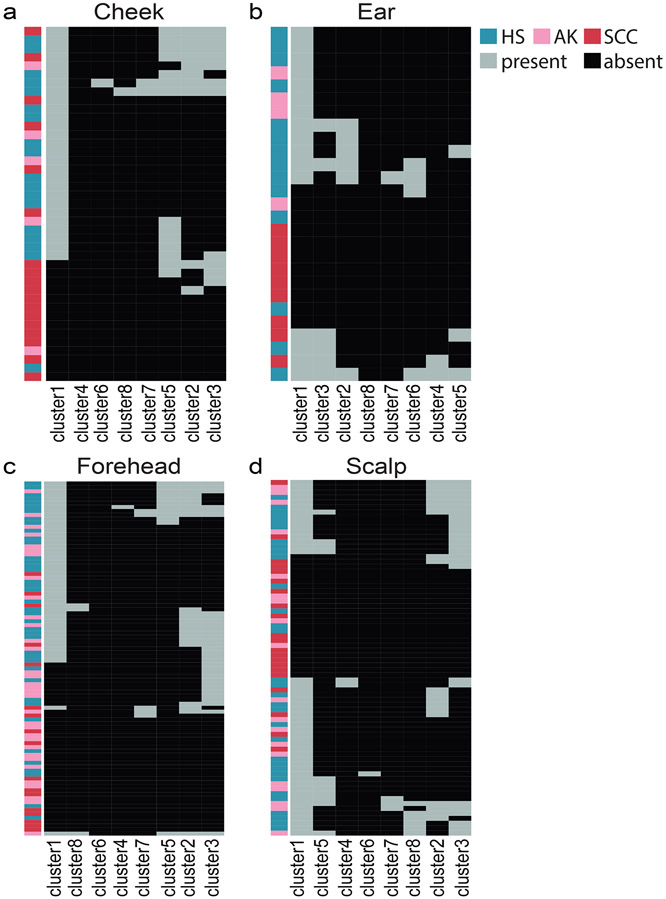
Because different C. acnes strains have different inflammatory characteristics (Jasson et al., 2013), it is plausible that the C. acnes-SCC association is strain-specific, with depletion of beneficial strains in SCC. Here, we defined the C. acnes strain composition across our sample cohort (other species of interest had insufficient read coverage). Notably, hierarchical clustering of all patient samples revealed a consistent depletion of a phylogenetic clade (“cluster 1”, Figure S8) in SCC, across skin sites (Figure 4).
Figure 4: C. acnes strain diversity between HS, AK, and SCC at different sampling sites.
Heatmap of all samples from the a: cheek (n=41), b: ear (n=27), c: forehead (n=90) and d: scalp (n=72). Strains from cluster 1 were less likely to be identified in SCC vs. HS in each skin site, indicating strain preferences specific to the disease. For visualization purposes, we subdivided the dataset by sampling site with a focus on skin sites with the greatest sample size.
Next, we identified genes that were differentially prevalent in at least 2/3 of cluster 1 strains vs. other clusters, as gene content differences could infer functional aspects relevant for the SCC association. We identified five genes, including a putative ribosomal N-acetyltransferase (YdaF, GCN5-related N-acetyltransferase (GNAT) family), a 6-O-methylguanine DNA-methyltransferase (MGMT) family protein (ogt1), an ABC transporter transmembrane region, and two hypothetical proteins. YdaF contributes to regulatory post-translational modifications (Favrot et al., 2016), MGMT family proteins contribute to DNA repair mechanisms, and ABC transporters are crucial for cell viability and virulence.
Genes absent in cluster 1 but present in at least 2/3rds of the remaining clusters included an acyl-CoA carboxylase epsilon subunit, a pyridoxal phosphate biosynthetic protein (pdxA2 gene), a putative nucleotide-binding of sugar-metabolizing enzyme, DeoR C terminal sensor domain (srlR2 gene) and 5 hypothetical proteins. While such gene lists lacked overt functionalities mediating C. acnes colonization or SCC predisposition, we emphasize that investigating strain differences is important for follow-up studies testing the role of C. acnes in SCC carcinogenesis, as much of patient-specific microbial (and thus, phenotypic) diversity is manifested at strain level (Zhou et al., 2020).
Microbial gene functions in the SCC-associated skin microbiome
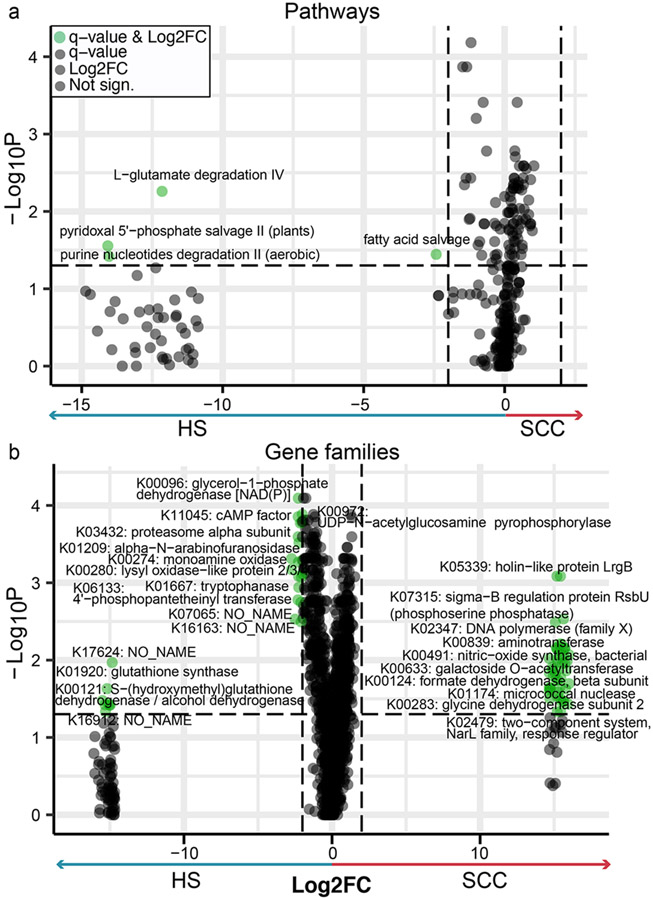
To infer microbial functions associated with growth in a tumor environment, we compared the relative abundances of microbial gene families between HS and SCC (Figure 5a-b; no differentially abundant features in HS vs. AK). 143 gene families were more abundant in SCC and 183 in HS, most of which were enzymes or components of transport processes (Table S5). For example, two HS-associated gene families are involved in glutathione metabolism (K01920: glutathione (GSH) synthase and K00121: S-(hydroxymethyl)glutathione dehydrogenase/alcohol dehydrogenase). GSH is an enzymatic cellular antioxidant (Godic et al., 2014, Telorack et al., 2016), protecting UV-exposed skin from ROS and oxidative stress. However, such UV-induced stressors can overwhelm the skin’s inherent antioxidant and immune responses, leading to DNA damage, immunotoxicity, and ultimately cancer (Godic et al., 2014). Several pathways were depleted in SCC, including fatty acid salvage, nucleotide degradation, and amino acid metabolism (L-glutamate degradation, pyridoxal 5’-phosphate salvage-II). Of note, pyridoxal metabolism was also identified in our C. acnes strain analysis (Supplementary Data). In addition, three holin-/antiholin-like KOs (lrgA, lrgB and cidA) were enriched in SCC. This finding likely reflects the increased representation of S. aureus, which possesses cid and lrg operons, in SCC. These control cell lysis and death, leading to the release of genomic DNA that becomes a structural part of biofilm matrices (Ranjit et al., 2011).
Figure 5: Gene family and pathway enrichment in HS vs. SCC.
Volcano plots show differentially abundant pathways (a) and gene families (b) identified in matched HS and SCC samples through paired Wilcoxon test with FDR-corrected p-values (q-value). For legibility, only a subset of gene families is labelled in the plot. For b, the comprehensive list of all differentially abundant gene families is listed in Table S5. For pathways and gene families, a q-value <0.05 with a log2-fold change (Log2FC) of 2 were deemed significant (green). Data points to the far right or far left indicate zero abundance in HS or SCC, respectively.
Finally, we examined if there were functions that would suggest a transitional progression from HS to AK to SCC. We listed features that either reached the fold change cutoff or significance level (Table S5) and highlighted gene families that overlapped in HS vs. SCC. This included amino acid metabolism-related enzymes and two holin-like proteins, again likely resulting from increased S. aureus. Overall, these examples identify changes in the skin microbiome’s functional potential that likely result from changes in the relative abundance of key skin microbes during tumorigenesis.
DISCUSSION
We present a large-scale WGS study of the AK- and SCC-associated skin microbiome, including a multi-kingdom, strain, and gene content analysis. Our results build on earlier reports (16S rRNA gene sequencing- or PCR-based) and a review that described Staphylococcus and Cutibacterium changes in SCC and AK (Kullander et al., 2009, Squarzanti et al., 2020, Woo et al., 2022, Wood et al., 2018). Taken together with recent studies showing that S. aureus can promote tumor cell growth via modulation of antimicrobial peptide human β-Defensin-2 expression (Madhusudhan et al., 2020), and C. acnes’ antitumorigenic, immunostimulatory and anti-angiogenic properties in different cancer types or cell line studies (Talib and Saleh, 2015, Wang et al., 2018), we hypothesize that the shift in the C. acnes:S. aureus ratio might promote a doubly deleterious effect in the SCC environment.
With the caveat that skin microbiota, with the exception of a specific skin Staphylococcus that produces antitumorigenic agents (Nakatsuji et al., 2018), have not yet been shown to influence the onset or progression of SCC in vivo, we speculate that any potential role in SCC progression might arise from the impact of these two microbes on the skin’s immune milieu. S. aureus possesses numerous virulence factors and pro-inflammatory agents (Thammavongsa et al., 2015, Zheng et al., 2021). Secretomes from clinical S. aureus isolates cultivated from AK and SCC samples were recently shown to activate SCC-associated inflammatory mediators in a human keratinocyte cell line and also elicited skin inflammation in mice (Krueger et al., 2021). C. acnes and S. aureus exhibit antagonistic properties (e.g., in atopic dermatitis (Francuzik et al., 2018, Kong et al., 2012)), potentially mediated via propionic acid, the main fermentation product of C. acnes (Shu et al., 2013). C. acnes could be depleted because premalignant or malignant tissue may be less favorable for C. acnes growth due to a loss of sebaceous properties (drier and scaly tissue), simultaneously generating a niche favorable for S. aureus. Thusly, C. acnes’ protective effects against S. aureus colonization would be reduced, and simultaneously the increased susceptibility of the tissue to S. aureus colonization (Kullander et al., 2009) might promote a pro-inflammatory environment.
This hypothesis, however, is complicated by studies that show both positive and negative associations of inflammation with cancer risk. Environmental influences such as UV radiation generating DNA damage and ROS can lead to skin inflammation and tumorigenesis. Generally, acute inflammation, e.g., due to acute UV exposure (sunburn), elicits an anti-tumorigenic response by Type 1 helper cells. In contrast, chronic inflammation is triggered by ROS (e.g., in response to chronic UV exposure or pathogens) and is controlled by regulatory T cells and Type 2 helper cells that secrete pro-tumorigenic molecules and is further enhanced by the secretion of pro-inflammatory cytokines from keratinocytes and sebocytes (reviewed in Ciazynska et al., 2021, Neagu et al., 2019). Future studies will be needed to separate these risk factors.
In recent years, the gut microbiome has been increasingly exploited for its clinical diagnostic and prognostic potential (Wirbel et al., 2019, Wirbel et al., 2021, Zeller et al., 2014). The skin microbiome has not (yet) been evaluated in this regard. Our study provides a baseline characterization of the skin microbiome composition, showing a striking change from HS to SCC. However, we observed fewer changes from HS to AK, suggesting a more gradual microbial shift insufficient to leverage for diagnostic purposes, possibly since most AKs were of Olsen grade I. The skin microbiome could gain clinical relevance if a microbial signature could distinguish high from low risk AKs and SCCs with potential to metastasize, or if the skin microbiota could identify SCCs that might fail to be responsive to non-surgical interventions, such as immunotherapy. Typically, AKs are removed preemptively by topical (e.g., 5-fluorouracil) or surgical (cryosurgery, dermabrasion) treatment since it is currently impossible to predict when/which AK lesions transition into malignant SCC (up to 16% of AKs (Didona et al., 2018)). For our study, SCCs were histopathologically confirmed while AK diagnosis relied on dermoscopical identification of typical AK features, which is the current clinical procedure, although histopathological analysis of all lesions would have been ideal.
We acknowledge that the presented data are correlative and do not determine whether these observations are a cause or effect of SCC progression. An alternative explanation is that local cutaneous changes and altered metabolism of tumor cells as seen in AK and SCC may sufficiently alter the microbial habitat, leading to the observed microbial and inferred functional changes (discussed in Woo et al., 2022). For example, physiologic alterations such as hair follicle loss and sebaceous gland destruction in SCC could contribute to the microbial changes observed. From histopathologic analysis, terminal hair follicles were generally present and intact, especially in early, superficial SCC lesions, which constituted most of our lesions with T-stage 1. Furthermore, a few studies showed that tissue scarring (Liu et al., 2018) did not result in microbial differences to HS in the same patient, and that hair loss was, rather, associated with increased C. acnes (Ho et al., 2019) vs. the decrease observed in our cohort. Future studies examining the microbiome associated with other skin alterations such as scars, keloids, BCC, or other NMSCs and melanoma will be of interest to see if they are associated with similar skin microbiome dysbiosis.
The gut microbiome is now recognized both for its role in cancer progression, such as Fusobacterium nucleatum and others (Cuevas-Ramos et al., 2010, Goodwin et al., 2011, Grivennikov et al., 2010, Kostic et al., 2013, Rubinstein et al., 2013), and as a powerful modulator of cancer immunotherapeutics (Gopalakrishnan et al., 2018, Routy et al., 2018). Research into a potential role of the skin microbiome in skin cancer progression and treatment response is in its infancy, but given its role in modulating the local immune milieu, it is plausible that it might influence these processes. These first forays into characterizing the skin microbiome of AK and SCC at high resolution provide strain-level hypotheses to inform mechanistic studies into whether microbes such as S. aureus and C. acnes might be involved in SCC development, progression, severity, and possibly treatment response.
MATERIALS AND METHODS
Patient recruitment
We recruited 81 patients with established evidence of non-melanoma skin cancer and 29 healthy individuals at the Department of Dermatology, Venereology, and Allergology, University Medical Center, Ruprecht-Karl University, Heidelberg, Germany. Written informed consent was obtained from all participants and the study protocol was approved by the ethics committee of the Medical Faculty at the University of Heidelberg, Germany and the Human Subjects Institutional Review Board at The Jackson Laboratory, Farmington, USA. Please see Supplementary Materials and Methods for details.
Skin swab collection
Skin swabs from SCC patients were collected from SCC, AK, and matched HS sites as well as HS from healthy individuals (Table S1). Samples were shipped on dry ice to The Jackson Laboratory for Genomic Medicine, Farmington, Connecticut, USA and stored at −80°C until processing. Please see Supplementary Materials and Methods for details.
DNA extraction and sequencing
DNA was extracted using the GenElute Bacterial DNA Isolation kit (Sigma Aldrich, NA2110-1KT, St. Louis, MO, USA) and sequencing libraries were prepared using the Nextera XT DNA Library Prep Kit (Illumina, San Diego, CA, USA) according to the manufacturer’s instructions with minor modifications. Whole genome sequencing (WGS) was predominantly carried out using a 2x150bp (paired end) sequencing protocol for the Illumina HiSeq 2500 (Illumina, San Diego, CA, USA) at the Genome Technologies core facility at the Jackson Laboratory for Genomic Medicine, Farmington, USA. Please see Supplementary Materials and Methods for details.
Dataset overview
In total, we collected 429 swabs from 81 patients and 25 healthy control individuals (Table S1) of which 348 samples were sequenced. Data from 336 skin swabs was of sufficient quality for analysis. 14 negative (negative extraction, PCR and library control) and 10 positive controls (mock community) were sequenced (Supplementary Materials and Methods). This adds up to 372 sequenced samples (Table S1 and S2).
mWGS data processing for taxonomic and functional classification
Metagenomic whole genome shotgun sequencing (mWGS) data was processed with the GATK pipeline Pathseq (v2.0. (Kostic et al., 2011, Walker et al., 2018)) for taxonomic classification and HPViewer for HPV genotyping. The HUMAnN 2.0 pipeline was used for functional classification. Strain identification and growth rate analysis were conducted using SMEG (Emiola et al., 2020) and GRiD (Growth Rate InDex) (Emiola and Oh, 2018). Details are described in the Supplementary Materials and Methods.
Biomarker discovery
To identify SCC and AK-associated biomarkers we used the Linear discriminant analysis of Effect Size (LEfSe) pipeline (Segata et al., 2011) and Siamcat (Wirbel et al., 2019, Wirbel et al., 2021, Zeller et al., 2014). Please see Supplementary Materials and Methods for details.
Statistical analysis
R (version 4 (R Core Team, 2020)) and RStudio (version 1.4.94) were used for statistical analysis and data visualization. Statistical significance for matched samples (matched by sampling site, gender, age) was assessed using the paired Wilcoxon signed-rank test while scaled data was subjected to the unpaired Wilcoxon signed-rank test. For datasets containing unmatched (but not scaled) data we used mixed effect models to account for pseudo-replicates within patients (random effect) and sampling site (fixed effect). Multiple test correction was carried out for comparisons with ≥ 4 groups using the post-hoc false discovery rate (FDR) method. Only groups with ≥ 6 samples were included in statistical analysis. Uncorrected p-values and FDR-adjusted p-values (q-values) <0.05 were deemed significant. Details are described in the Supplementary Materials and Methods.
Supplementary Material
ACKNOWLEDGEMENTS AND FUNDING
We are thankful to the Oh group for inspiring discussions and acknowledge the contribution of the Genome Technologies Service at The Jackson Laboratory for expert assistance with sample sequencing for the work described in this publication. We are grateful to George W. Weinstock, Jennifer C. Chen, Paul Igor Costea, Luis Sordo Vieira and Parithi Balachandran for helpful discussions. This work was funded by the American Cancer Society (RSG-16-255-01-MPC and IRG-82-003-33 NCCC-01), the Leo Foundation, the JAX Cancer Center grant (P30 CA03419), the Scott R. MacKenzie Foundation, and the Mark Foundation for Cancer Research. JO is additionally supported by the NIH (1 R01 AR078634-01, DP2 GM126893-01 and K22 AI119231-01, 1U54NS105539, 1 U19 AI142733, 1 R21 AR075174). We are grateful for the funding of AYV through the Pyewacket PostDoc Fund.
Abbreviations:
- SCC
Squamous Cell Carcinoma
- AK
Actinic Keratosis
- HS
Healthy Skin
- mWGS
Metagenomic Whole Genome Sequencing
- NMSC
Non-Melanoma Skin Cancer
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
The authors state no conflict of interest.
DATA AVAILABILITY
The dataset related to this article can be found on SRA under the BioProject accession number PRJNA736108. Abundance tables used for taxonomic analysis are provided in Table S6.
REFERENCES
- Boleij A, Tjalsma H. Gut bacteria in health and disease: a survey on the interface between intestinal microbiology and colorectal cancer. Biological reviews of the Cambridge Philosophical Society 2012;87(3):701–30. [DOI] [PubMed] [Google Scholar]
- Byrd AL, Belkaid Y, Segre JA. The human skin microbiome. Nature Reviews Microbiology 2018. [DOI] [PubMed] [Google Scholar]
- Ciazynska M, Olejniczak-Staruch I, Sobolewska-Sztychny D, Narbutt J, Skibinska M, Lesiak A. Ultraviolet Radiation and Chronic Inflammation-Molecules and Mechanisms Involved in Skin Carcinogenesis: A Narrative Review. Life (Basel) 2021;11(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas-Ramos G, Petit CR, Marcq I, Boury M, Oswald E, Nougayrede JP. Escherichia coli induces DNA damage in vivo and triggers genomic instability in mammalian cells. Proc Natl Acad Sci U S A 2010;107(25):11537–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didona D, Paolino G, Bottom U, Cantisani C. Non Melanoma Skin Cancer Pathogenesis Overview. Biomedicines 2018;6(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emiola A, Oh J. High throughput in situ metagenomic measurement of bacterial replication at ultra-low sequencing coverage. Nat Commun 2018;9(1):4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emiola A, Zhou W, Oh J. Metagenomic growth rate inferences of strains in situ. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favrot L, Blanchard JS, Vergnolle O. Bacterial GCN5-Related N-Acetyltransferases: From Resistance to Regulation. Biochemistry 2016;55(7):989–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findley K, Oh J, Yang J, Conlan S, Deming C, Meyer JA, et al. Topographic diversity of fungal and bacterial communities in human skin. Nature 2013;498(7454):367–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francuzik W, Franke K, Schumann RR, Heine G, Worm M. Propionibacterium acnes Abundance Correlates Inversely with Staphylococcus aureus: Data from Atopic Dermatitis Skin Microbiome. Acta Derm Venereol 2018;98(5):490–5. [DOI] [PubMed] [Google Scholar]
- Fyhrquist N, Ruokolainen L, Suomalainen A, Lehtimaki S, Veckman V, Vendelin J, et al. Acinetobacter species in the skin microbiota protect against allergic sensitization and inflammation. J Allergy Clin Immunol 2014;134(6):1301–9 e11. [DOI] [PubMed] [Google Scholar]
- Godic A, Poljsak B, Adamic M, Dahmane R. The role of antioxidants in skin cancer prevention and treatment. Oxid Med Cell Longev 2014;2014:860479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin AC, Destefano Shields CE, Wu S, Huso DL, Wu X, Murray-Stewart TR, et al. Polyamine catabolism contributes to enterotoxigenic Bacteroides fragilis-induced colon tumorigenesis. Proc Natl Acad Sci U S A 2011;108(37):15354–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018;359(6371):97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grice EA, Kong HH, Conlan S, Deming CB, Davis J, Young AC, et al. Topographical and temporal diversity of the human skin microbiome. Science 2009;324(5931):1190–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grice EA, Segre JA. The skin microbiome. Nature reviews Microbiology 2011;9(4):244–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010;140(6):883–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkins CP, MacGibeny MA, Thompson K, Bubic B, Huang X, Brown I, et al. Cutaneous T-cell lymphoma skin microbiome is characterized by shifts in certain commensal bacteria but not viruses when compared to healthy controls. The Journal of investigative dermatology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho BS, Ho EXP, Chu CW, Ramasamy S, Bigliardi-Qi M, de Sessions PF, et al. Microbiome in the hair follicle of androgenetic alopecia patients. PLoS One 2019;14(5):e0216330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasson F, Nagy I, Knol AC, Zuliani T, Khammari A, Dreno B. Different strains of Propionibacterium acnes modulate differently the cutaneous innate immunity. Exp Dermatol 2013;22(9):587–92. [DOI] [PubMed] [Google Scholar]
- Kong HH, Oh J, Deming C, Conlan S, Grice EA, Beatson MA, et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res 2012;22(5):850–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostic AD, Chun E, Robertson L, Glickman JN, Gallini CA, Michaud M, et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 2013;14(2):207–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostic AD, Gevers D, Pedamallu CS, Michaud M, Duke F, Earl AM, et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res 2012;22(2):292–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostic AD, Ojesina AI, Pedamallu CS, Jung J, Verhaak RG, Getz G, et al. PathSeq: software to identify or discover microbes by deep sequencing of human tissue. Nat Biotechnol 2011;29(5):393–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger A, Zaugg J, Chisholm S, Linedale R, Lachner N, Teoh SM, et al. Secreted Toxins From Staphylococcus aureus Strains Isolated From Keratinocyte Skin Cancers Mediate Pro-tumorigenic Inflammatory Responses in the Skin. Front Microbiol 2021;12:789042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullander J, Forslund O, Dillner J. Staphylococcus aureus and squamous cell carcinoma of the skin. Cancer Epidemiol Biomarkers Prev 2009;18(2):472–8. [DOI] [PubMed] [Google Scholar]
- Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013;500(7464):541–6. [DOI] [PubMed] [Google Scholar]
- Leiter U, Eigentler T, Garbe C. Epidemiology of skin cancer. Adv Exp Med Biol 2014;810:120–40. [DOI] [PubMed] [Google Scholar]
- Liu SH, Huang YC, Chen LY, Yu SC, Yu HY, Chuang SS. The skin microbiome of wound scars and unaffected skin in patients with moderate to severe burns in the subacute phase. Wound Repair Regen 2018;26(2):182–91. [DOI] [PubMed] [Google Scholar]
- Madhusudhan N, Pausan MR, Halwachs B, Durdevic M, Windisch M, Kehrmann J, et al. Molecular Profiling of Keratinocyte Skin Tumors Links Staphylococcus aureus Overabundance and Increased Human beta-Defensin-2 Expression to Growth Promotion of Squamous Cell Carcinoma. Cancers (Basel) 2020;12(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matinfar M, Shahidi S, Feizi A. Incidence of nonmelanoma skin cancer in renal transplant recipients: A systematic review and meta-analysis. J Res Med Sci 2018;23:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milas L, Hunter N, Basic I, Withers HR. Complete regressions of an established murine fibrosarcoma induced by systemic application of Corynebacterium granulosum. Cancer Res 1974;34(10):2470–5. [PubMed] [Google Scholar]
- Miller LS, Modlin RL. Toll-like receptors in the skin. Semin Immunopathol 2007;29(1):15–26. [DOI] [PubMed] [Google Scholar]
- Naik S, Bouladoux N, Linehan JL, Han SJ, Harrison OJ, Wilhelm C, et al. Commensal-dendritic-cell interaction specifies a unique protective skin immune signature. Nature 2015;520(7545):104–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik S, Bouladoux N, Wilhelm C, Molloy MJ, Salcedo R, Kastenmuller W, et al. Compartmentalized control of skin immunity by resident commensals. Science 2012;337(6098):1115–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsuji T, Chen TH, Butcher AM, Trzoss LL, Nam SJ, Shirakawa KT, et al. A commensal strain of Staphylococcus epidermidis protects against skin neoplasia. Sci Adv 2018;4(2):eaao4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsuji T, Chen TH, Narala S, Chun KA, Two AM, Yun T, et al. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci Transl Med 2017;9(378). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsuji T, Gallo RL. The role of the skin microbiome in atopic dermatitis. Ann Allergy Asthma Immunol 2019;122(3):263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neagu M, Constantin C, Caruntu C, Dumitru C, Surcel M, Zurac S. Inflammation: A key process in skin tumorigenesis. Oncol Lett 2019;17(5):4068–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehal KS, Bichakjian CK. Update on Keratinocyte Carcinomas. The New England journal of medicine 2018;379(4):363–74. [DOI] [PubMed] [Google Scholar]
- Oh J, Byrd AL, Park M, Program NCS, Kong HH, Segre JA. Temporal Stability of the Human Skin Microbiome. Cell 2016;165(4):854–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J, Freeman AF, Program NCS, Park M, Sokolic R, Candotti F, et al. The altered landscape of the human skin microbiome in patients with primary immunodeficiencies. Genome Res 2013;23(12):2103–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paslin D, Dimitrov NV, Heaton C. Regression of a transplantable hamster melanoma by intralesional injections of Corynebacterium granulosum. J Natl Cancer Inst 1974;52(2):571–3. [DOI] [PubMed] [Google Scholar]
- R Core Team. A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2020. [Google Scholar]
- Ranjit DK, Endres JL, Bayles KW. Staphylococcus aureus CidA and LrgA proteins exhibit holin-like properties. J Bacteriol 2011;193(10):2468–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillere R, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018;359(6371):91–7. [DOI] [PubMed] [Google Scholar]
- Rubinstein MR, Wang X, Liu W, Hao Y, Cai G, Han YW. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/beta-catenin signaling via its FadA adhesin. Cell Host Microbe 2013;14(2):195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter SJ, Cox MJ, Turek EM, Calus ST, Cookson WO, Moffatt MF, et al. Reagent and laboratory contamination can critically impact sequence-based microbiome analyses. BMC Biol 2014;12:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwimmer JB, Johnson JS, Angeles JE, Behling C, Belt PH, Borecki I, et al. Microbiome Signatures Associated With Steatohepatitis and Moderate to Severe Fibrosis in Children With Nonalcoholic Fatty Liver Disease. Gastroenterology 2019;157(4):1109–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, et al. Metagenomic biomarker discovery and explanation. Genome Biol 2011;12(6):R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepich-Poore GD, Zitvogel L, Straussman R, Hasty J, Wargo JA, Knight R. The microbiome and human cancer. Science 2021;371(6536). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu M, Wang Y, Yu J, Kuo S, Coda A, Jiang Y, et al. Fermentation of Propionibacterium acnes, a commensal bacterium in the human skin microbiome, as skin probiotics against methicillin-resistant Staphylococcus aureus. PLoS One 2013;8(2):e55380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squarzanti DF, Zavattaro E, Pizzimenti S, Amoruso A, Savoia P, Azzimonti B. Non-Melanoma Skin Cancer: news from microbiota research. Crit Rev Microbiol 2020;46(4):433–49. [DOI] [PubMed] [Google Scholar]
- Talib WH, Saleh S. Propionibacterium acnes Augments Antitumor, Anti-Angiogenesis and Immunomodulatory Effects of Melatonin on Breast Cancer Implanted in Mice. PLoS One 2015;10(4):e0124384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tampa M, Mitran CI, Mitran MI, Nicolae I, Dumitru A, Matei C, et al. The Role of Beta HPV Types and HPV-Associated Inflammatory Processes in Cutaneous Squamous Cell Carcinoma. J Immunol Res 2020;2020:5701639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telorack M, Abplanalp J, Werner S. Low levels of glutathione are sufficient for survival of keratinocytes after UV irradiation and for healing of mouse skin wounds. Arch Dermatol Res 2016;308(6):443–8. [DOI] [PubMed] [Google Scholar]
- Thammavongsa V, Kim HK, Missiakas D, Schneewind O. Staphylococcal manipulation of host immune responses. Nature reviews Microbiology 2015;13(9):529–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt AY, Zeller G, Bork P. [Microbial Biomarkers for Early Cancer Detection]. Dtsch Med Wochenschr 2017;142(4):267–74. [DOI] [PubMed] [Google Scholar]
- Walker MA, Pedamallu CS, Ojesina AI, Bullman S, Sharpe T, Whelan CW, et al. GATK PathSeq: a customizable computational tool for the discovery and identification of microbial sequences in libraries from eukaryotic hosts. Bioinformatics 2018;34(24):4287–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Funchain P, Bebek G, Altemus J, Zhang H, Niazi F, et al. Microbiomic differences in tumor and paired-normal tissue in head and neck squamous cell carcinomas. Genome Med 2017;9(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Choi JE, Wu CC, Di Nardo A. Skin commensal bacteria S. epidermidis promote survival of melanocytes bearing UVB-induced DNA damage, while bacteria P. acnes inhibit survival of melanocytes by increasing apoptosis. Photodermatol Photoimmunol Photomed 2018. [DOI] [PubMed] [Google Scholar]
- Wirbel J, Pyl PT, Kartal E, Zych K, Kashani A, Milanese A, et al. Meta-analysis of fecal metagenomes reveals global microbial signatures that are specific for colorectal cancer. Nature medicine 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirbel J, Zych K, Essex M, Karcher N, Kartal E, Salazar G, et al. Microbiome meta-analysis and cross-disease comparison enabled by the SIAMCAT machine learning toolbox. Genome Biol 2021;22(1):93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo YR, Cho SH, Lee JD, Kim HS. The Human Microbiota and Skin Cancer. Int J Mol Sci 2022;23(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood DLA, Lachner N, Tan JM, Tang S, Angel N, Laino A, et al. A Natural History of Actinic Keratosis and Cutaneous Squamous Cell Carcinoma Microbiomes. MBio 2018;9(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller G, Tap J, Voigt AY, Sunagawa S, Kultima JR, Costea PI, et al. Potential of fecal microbiota for early-stage detection of colorectal cancer. Mol Syst Biol 2014;10:766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Marsman G, Lacey KA, Chapman JR, Goosmann C, Ueberheide BM, et al. The cell envelope of Staphylococcus aureus selectively controls the sorting of virulence factors. Nat Commun 2021;12(1):6193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Spoto M, Hardy R, Guan C, Fleming E, Larson PJ, et al. Host-Specific Evolutionary and Transmission Dynamics Shape the Functional Diversification of Staphylococcus epidermidis in Human Skin. Cell 2020;180(3):454–70 e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset related to this article can be found on SRA under the BioProject accession number PRJNA736108. Abundance tables used for taxonomic analysis are provided in Table S6.