Abstract
Objective.
Post-stroke cognitive impairment (PSCI) is associated with etiology, severity, and functional outcome of stroke. The risks of recurrent stroke and death in patients with PSCI and insulin resistance (IR) is unknown. The goal of this study was to determine whether global and domain-specific cognitive impairment after stroke in patients with IR was associated with recurrent stroke and death.
Materials and Methods:
We studied patients with recent stroke or transient ischemic attack (TIA) and IR with a baseline Modified Mini-Mental State Examination (3MS) cognitive exam at median of 79 days after stroke. We considered a baseline score of ≤ 88 on the 3MS to indicate global cognitive impairment, and domain-specific summary scores in the lowest quartile to indicate language, attention, orientation, memory and visuospatial impairments. The primary endpoint was fatal or non-fatal recurrent stroke, and the secondary endpoints were all-cause mortality, and fatal or non-fatal myocardial infarction (MI).
Results.
Among studied n=3,338 patients 13.6% had global cognitive impairment. During the median 4.96 years of follow-up, 7.4% patients experienced recurrent stroke, 3.5% MI, and 7.3% died. In the fully adjusted model, impairment in language (HR 1.35; 95% CI 1.01–1.81) and orientation (HR 1.41; 95% CI: 1.06–1.87) were associated with a higher risk of recurrent stroke, while attention impairment was associated with all-cause mortality (HR 1.34; 95% CI: 1.01–1.78).
Discussion/Conclusion:
In patients with recent stroke/TIA and IR, post-stroke language and orientation impairments independently predicted recurrent stroke, while attention deficit was associated with increased risk of all-cause mortality.
Keywords: Stroke, Insulin Resistance, Cognitive Impairment, Cognitive Testing
Introduction
Post-stroke cognitive impairment (PSCI) occurs in 20–40% of patients after stroke (1, 2). Despite more than 25 years of research, the question regarding risk of recurrent stroke and death in patients with PSCI remains controversial. The majority of published literature reported higher risk that was independent of demographics, education, and cardiovascular risk factors (3–13). However, some studies with relatively large sample sizes showed no association between PSCI and the risk of stroke recurrence and death (14, 15). Interestingly, both neutral studies are population-based as opposed to the cohorts enrolled in randomized clinical trials where higher risks were reported (4, 13, 16).
Insulin resistance (IR) is a known risk factor for stroke (17). The Insulin Resistance Intervention after Stroke (IRIS) trial was a double-blind, placebo controlled, randomized clinical trial, which showed that in patients without diabetes who had IR along with a recent ischemic stroke and without dementia, pioglitazone decreased the risk of stroke, myocardial infarction and type 2 diabetes (18). On the other hand, in subgroup analysis of the IRIS trial, pioglitazone has failed to show efficacy in preventing PSCI (19). However, risks of stroke recurrence and death, stratified according global and domain-specific cognitive performance were not reported. These are clinically relevant questions as in a recently completed study, we have shown that not only global but also domain-specific cognitive impairment may predict future vascular events as in patients with small vessel (lacunar) stroke, memory impairment was an independent predictor of recurrent stroke and death, while global and non-memory impairment were associated with death (16).
Given the lack of data on association of PSCI and the risk of stroke recurrence and mortality in ischemic stroke patients with IR, we aimed to study whether global and domain-specific cognitive impairment after stroke in patients with IR is associated with recurrent stroke and death. We hypothesized that PSCI with global and domain-specific dysfunction will be associated with higher risks of stroke recurrence and death in patients with stroke and IR. Here we report the results of subgroup analysis of the IRIS trial assessing the risk of recurrent stroke, death, and MI according to the cognitive performance at the time of randomization.
Materials/Methods
Study Population
IRIS was a randomized, double-blind, placebo-controlled clinical trial that tested pioglitazone for prevention of stroke and MI in patients with IR but without diabetes who had an ischemic stroke or TIA within 180 days of trial entry (18, 19, 20, 21). Major exclusion criteria include dementia, diabetes, age < 40 years of age and a history of heart failure or bladder cancer. At time of randomization, participants were assigned to either the treatment arm (pioglitazone) or control (placebo) arm. Institutional review boards approved the protocol at each site prior to data collection. Written informed consent was obtained from the participants. The George Washington University Office of Human Research determined that no further review by the Institutional Review Board is required for this subgroup analysis. All data generated or analyzed during this study are included in this article and its supplementary material files. Further enquiries can be directed to the corresponding author.
Assessment of Cognitive Function
The Modified Mini-Mental State Examination (3MS) was administered during the baseline interview at study entry and annually throughout follow-up. The 3MS has a scoring system that ranges from 0 to 100, with higher scores indicating better function (22). A 3MS score of less than 78 has 88% sensitivity, 90% specificity, 29% positive predictive value and 99% negative predictive value for detecting dementia (19). Similar to IRIS, global cognitive impairment was defined by a cutoff score of ≤ 88 on the 3MS, which accurately identifies patients with impaired cognitive function (23). Domain-specific cognitive impairment was defined as scoring below the 25th percentile in the sample for each of five domains including: attention (3MS < 7), language (3MS < 34), memory (3MS < 18), orientation (3MS < 25), and visuospatial (3MS < 10).
Outcome Assessment
The primary outcome of interest was fatal or non-fatal recurrent stroke, and the secondary outcomes of interest were (1) all-cause mortality, and (2) fatal or non-fatal MI. Participants were contacted every 2 weeks during the dose titration period, and then, starting at month 4, every 4 months during follow-up. All outcomes were adjudicated by the members of independent committee in a blinded fashion.
Covariates
Before randomization, demographics including race/ethnicity, age, and gender, were self-reported. The Modified Rankin Scale (mRS), which measures degree of disability post-stroke, the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification of the qualifying stroke, and measurement of Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) were also determined by site investigators. Participants had either one or two results for HOMA-IR; where they had 2 results, we used the average of the two (20). Additionally, trial participants were assessed using the NIH Stroke Scale (NIHSS) at baseline during the randomization. After randomization, at the baseline interview, participants self-reported their education level, smoking status, alcohol use, and patient health history (for prior stroke/TIA, myocardial infarction, hypertension, and elevated cholesterol). Smoking status was defined as 1) current smoker, 2) former smoker, and 3) never smoked. Alcohol usage was dichotomized into those who ever drank and those who never drank. Education was defined as years of formal education and grouped into three categories: 0–8 years, 9–12 years, and > 12 years.
Statistical Methods
Characteristics of the sample were summarized using means and standard deviations or medians and interquartile ranges for numerical variables; frequencies and proportions for categorical variables. To assess differences in characteristics between the cognitively impaired and cognitively normal groups, we used two-sample t-tests or Mann-Whitney U tests for numerical variables and Pearson’s Chi-squared tests for categorical variables.
Participants accumulated follow-up time from their baseline examination until either the outcome of interest or censorship due to death, loss to follow-up, or the final follow-up interview, whichever came first (Fig. 1). We used Cox proportional hazards models to quantify the impact of overall and domain-specific cognitive impairment on risk of recurrent stroke, all-cause mortality, and myocardial infarction. The details of Cox proportional hazards analyses are provided in supplementary Appendix A.
Figure 1.
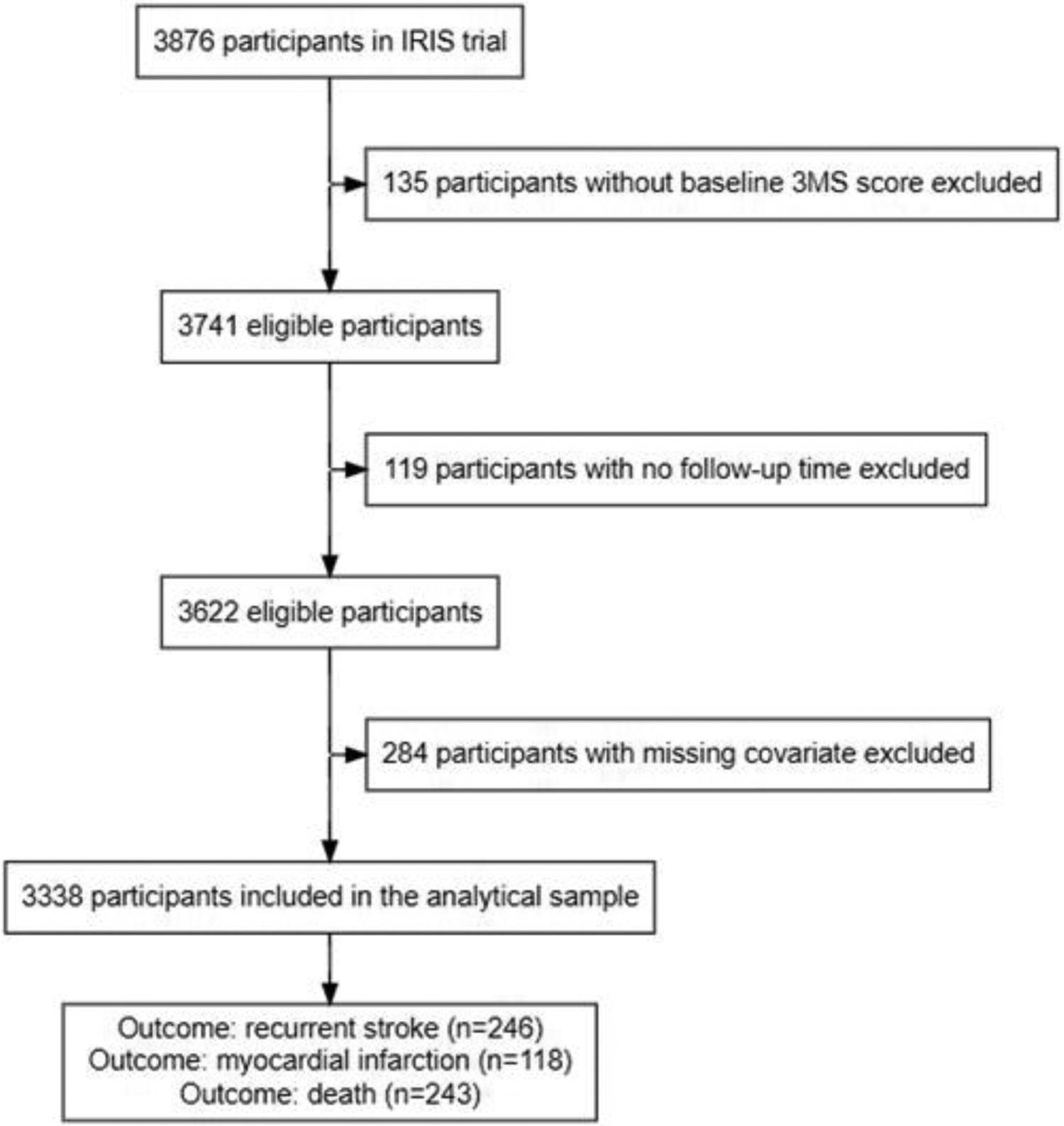
Flowchart showing final analytical sample. Out of the 3,876 total participants in the IRIS trial, 135 were excluded on the basis of missing a baseline 3MS score; 119 were excluded on the basis of having no follow-up time; 284 were excluded on the basis of missing covariate data. The final analytical sample consisted of 3,338 participants, out of which 246 experienced at least one recurrent stroke, 118 experienced at least one MI, and 243 died, during the study period.
We used four sequential models to assess the influence of potential confounders: Model 1 corresponds to a crude model, with overall (or domain-specific) cognitive impairment as the only predictor; Model 2 adjusts for age at study entry, sex, race/ethnicity, years of education, and treatment arm (pioglitazone vs. placebo); Model 3 additionally adjusts for stroke history, hypertension history, hyperlipidemia history, MI history, HOMA-IR index, smoking status, and weekly alcohol use; and Model 4 additionally adjusts for stroke etiology (TOAST criteria), baseline NIHSS, and baseline mRS. We chose variables likely to meet the definition of confounders for inclusion in our models and provide multiple levels of adjustment to illustrate the influence of adjusting for different types of confounders. Missingness on covariates was addressed by using multiple imputation in sensitivity analyses (24). An additional sensitivity analysis adjusting for time between qualifying entry event and baseline interview was performed. Additional details are available in online supplementary Appendix A. Statistical analyses were performed using R (R version 4.1.2 (2021-11-01) “Bird Hippie”, Austria, Vienna, https://www.R-project.org). We reported 95% confidence intervals and considered 2-sided p values of <0.05 to be statistically significant.
Results
We included all n=3,741 IRIS participants who were administered their baseline cognitive exam at the time of their baseline interview (median time after qualifying ischemic stroke or TIA was 79 days). We then excluded 119 participants (3.2%) who had no follow-up time (they did not have any follow-up interviews, nor record of experiencing an event) and 284 participants (7.6%) with missing covariate data for a final sample size of n= 3,338 participants (88.7% stroke, 11.3% TIA) (Fig. 1). Comparison of the eligible sample to the full IRIS cohort is available in online supplementary Appendix B and online supplementary Table B1.
Of the n=3,338 patients in the main analysis, n=246 (7.4%) experienced at least one recurrent stroke, n=118 (3.5%) experienced at least one MI, and n=243 (7.3%) died during the study period. The median follow-up time was 4.96 years with last contact either at an annual interview, or at an event, whichever date came last.
Characteristics of the sample are provided in Table 1. There were n=453 (13.6%) patients with global cognitive impairment (3MS < 89). On average, those with cognitive impairment tended to be older, were more likely to be female, non-white, have no more than 12 years of education, and to report no alcohol usage. They were also more likely to report a history of prior stroke or TIA (prior to the entry event), more likely to have a stroke rather than TIA as their qualifying entry event, and to have higher median and mean scores on the NIHSS and the mRS, respectively.
Table 1.
Baseline Characteristics of Patients.
| Overall N=3338 | Normal N=2885 | Impaired (3MS < 89) n=453 | p | |
|---|---|---|---|---|
| Qualifying Entry Event = TIA, n (%) | 378 (11.3) | 347 (12.0) | 31 (6.8) | 0.0023 |
| Age (mean (SD)) | 62.96 (10.60) | 62.47 (10.38) | 66.07 (11.42) | <0.0011 |
| Race/Ethnicity, n (%) | <0.0013 | |||
| White, non-Hispanic | 2761 (82.7) | 2462 (85.3) | 299 (66.0) | |
| Black, non-Hispanic | 349 (10.5) | 243 (8.4) | 106 (23.4) | |
| Hispanic/Latino | 113 (3.4) | 84 (2.9) | 29 (6.4) | |
| Other, non-Hispanic | 115 (3.4) | 96 (3.3) | 19 (4.2) | |
| Female, n (%) | 1142 (34.2) | 965 (33.4) | 177 (39.1) | 0.023 |
| Treatment = Pioglitazone, n (%) | 1669 (50.0) | 1430 (49.6) | 239 (52.8) | 0.233 |
| Smoking Status, n (%) | 0.553 | |||
| Never | 1143 (34.2) | 998 (34.6) | 145 (32.0) | |
| Current | 538 (16.1) | 461 (16.0) | 77 (17.0) | |
| Former | 1657 (49.6) | 1426 (49.4) | 231 (51.0) | |
| Alcohol Use, n (%) | 1785 (53.5) | 1582 (54.8) | 203 (44.8) | <0.0013 |
| Education, n (%) | <0.0013 | |||
| 0–8 years | 184 (5.5) | 126 (4.4) | 58 (12.8) | |
| 9–12 years | 1541 (46.2) | 1276 (44.2) | 265 (58.5) | |
| 12+ years | 1613 (48.3) | 1483 (51.4) | 130 (28.7) | |
| HOMA-IR (median [IQR]) | 4.65 [3.80, 6.30] | 4.70 [3.80, 6.30] | 4.60 [3.70, 6.00] | 0.092 |
| History of elevated cholesterol, n (%) | 2292 (68.7) | 1990 (69.0) | 302 (66.7) | 0.353 |
| History of hypertension, n (%) | 2387 (71.5) | 2054 (71.2) | 333 (73.5) | 0.343 |
| History of myocardial infarction, n (%) | 286 (8.6) | 245 (8.5) | 41 (9.1) | 0.763 |
| History of hospitalization for prior stroke or TIA, n (%) | <0.0013 | |||
| No prior history of stroke or TIA | 2799 (83.9) | 2457 (85.2) | 342 (75.5) | |
| Prior history of stroke or TIA, and 1 hospitalization | 292 (8.7) | 233 (8.1) | 59 (13.0) | |
| Prior history of stroke or TIA, and >1 hospitalization | 124 (3.7) | 92 (3.2) | 32 (7.1) | |
| Prior history of stroke or TIA, but no hospitalization | 123 (3.7) | 103 (3.6) | 20 (4.4) | |
| TOAST, n (%) | 0.103 | |||
| Atherosclerosis | 869 (26.0) | 733 (25.4) | 136 (30.0) | |
| Lacunar | 1008 (30.2) | 893 (31.0) | 115 (25.4) | |
| Embolic | 249 (7.5) | 218 (7.6) | 31 (6.8) | |
| Other | 83 (2.5) | 72 (2.5) | 11 (2.4) | |
| Uncertain | 1129 (33.8) | 969 (33.6) | 160 (35.3) | |
| NIH Stroke Scale (median [IQR]) | 0.00 [0.00, 1.00] | 0.00 [0.00, 1.00] | 1.00 [0.00, 2.00] | <0.0012 |
| Modified Rankin Scale (mean (SD)) | 0.95 (0.98) | 0.89 (0.95) | 1.33 (1.11) | <0.0011 |
| Attention Domain Score (median [IQR]) | 7.00 [7.00, 7.00] | 7.00 [7.00, 7.00] | 6.00 [4.00, 7.00] | <0.0012 |
| Language Domain Score (median [IQR]) | 36.00 [34.00, 37.00] | 36.00 [35.00, 37.00] | 31.00 [28.00, 33.00] | <0.0012 |
| Memory Domain Score (median [IQR]) | 20.00 [18.00, 21.00] | 21.00 [19.00, 21.00] | 15.00 [11.00, 17.00] | <0.0012 |
| Orientation Domain Score (median [IQR]) | 25.00 [25.00, 25.00] | 25.00 [25.00, 25.00] | 25.00 [23.00, 25.00] | <0.0012 |
| Visuospatial Domain Score (median [IQR]) | 10.00 [10.00, 10.00] | 10.00 [10.00, 10.00] | 10.00 [8.00, 10.00] | <0.0012 |
Student t-test;
Mann-Whitney U test;
Pearson’s chi-square test.
IQR- interquartile range
In our analytical sample of n=3,338, the median time from qualifying entry event to baseline cognitive exam is 79 days overall, 74 days for participants with cognitive impairment, and 80 days for participants withoutAt the baseline cognitive exam, the prevalence of global cognitive impairment was 13.6% (453/3338), and domain-specific cognitive impairment distribution was as follows: n=678 (20.3%) attention (3MS < 7), n=781 (23.4%) language (3MS < 34), n=708 (21.2%) memory (3MS < 18), n=703 (21.1%) orientation (3MS < 25), and n=716 (21.4%) with visuospatial (3MS < 10).
In the crude analysis, global (HR 1.65; 95% CI 1.21–2.25), attention (HR 1.60; 95% CI 1.21, 2.11), language (HR 1.65; 95% CI 1.26–2.15), memory (HR 1.43; 95% CI 1.08–1.89), and orientation (HR 1.60; 95% CI 1.22–2.10) impairments but not visuospatial (HR 1.26; 95% CI 0.94–1.68) were associated with a significantly higher risk of recurrent stroke (Table 2). In the fully adjusted model (Model 4), language (HR 1.35; 95% CI 1.01–1.81) and orientation (HR 1.41; 95% CI 1.06–1.87) impairments remained associated with a higher risk of recurrent stroke.
Table 2.
Crude and Adjusted Hazard Ratios (95% CI) for Recurrent Stroke, Based on the Global Cognitive Impairment and Domain-specific Cognitive Impairment as Predictors.
| Cognitive domain | Model 11 | Model 22 | Model 33 | Model 44 | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | |
| Global impairment (n=453) | 1.65 (1.21, 2.25) | 0.002 | 1.43 (1.03, 1.99) | 0.03 | 1.38 (0.99, 1.92) | 0.06 | 1.24 (0.88, 1.74) | 0.22 |
| Attention impairment (n=678) | 1.60 (1.21, 2.11) | <0.001 | 1.44 (1.08, 1.92) | 0.01 | 1.38 (1.03, 1.84) | 0.03 | 1.32 (0.99, 1.77) | 0.06 |
| Language impairment (n=781) | 1.65 (1.26, 2.15) | <0.001 | 1.54 (1.16, 2.03) | 0.003 | 1.48 (1.11, 1.96) | 0.01 | 1.35 (1.01, 1.81) | 0.04 |
| Memory impairment (n=708) | 1.43 (1.08, 1.89) | 0.01 | 1.24 (0.93, 1.66) | 0.14 | 1.27 (0.95, 1.70) | 0.10 | 1.22 (0.91, 1.63) | 0.19 |
| Orientation impairment (n=703) | 1.60 (1.22, 2.10) | <0.001 | 1.51 (1.15, 2.00) | 0.003 | 1.51 (114, 1.99) | 0.004 | 1.41 (1.06, 1.87) | 0.02 |
| Visuospatial impairment (n=716) | 1.26 (0.94, 1.68) | 0.12 | 1.19 (0.89, 1.60) | 0.25 | 1.16 (0.86, 1.56) | 0.33 | 1.07 (0.79, 1.45) | 0.65 |
Abbreviations: 3MS, Modified Mini-Mental State Examination; Q1, first quartile (<25th percentile); HR, hazard ratio; CI, 95% confidence interval.
Unadjusted model; main predictor only.
Adjusted for age, sex, race/ethnicity, years of education, treatment arm (pioglitazone vs. placebo).
Adjusted for model 2 + stroke history, hypertension history, hyperlipidemia history, myocardial infarction history, HOMA-IR index, smoking status, weekly alcohol use.
Adjusted for model 3 + stroke etiology (TOAST criteria), baseline NIHSS, baseline modified Rankin score.
In the crude analysis, global, attention, memory, and visuospatial impairments were associated with a higher risk of death while language and orientation impairments were not (Table 3). In the fully adjusted model (Model 4), only attention impairment remained associated with a higher risk of death (HR 1.34; 95% CI: 1.01–1.78).
Table 3.
Crude and Adjusted Hazard Ratios (95% CI) for All-Cause Mortality, Based on the Global Cognitive Impairment and Domain-specific Cognitive Impairment as Predictors.
| Cognitive domain | Model 11 | Model 22 | Model 33 | Model 44 | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | |
| Global impairment (n=453) | 1.62 (119, 2.21) | 0.002 | 1.29 (0.93, 1.79) | 0.13 | 1.19 (0.85, 1.66) | 0.31 | 1.05 (0.75, 1.47) | 0.78 |
| Attention impairment (n=678) | 1.77 (1.35, 2.33) | < 0.001 | 1.49 (1.12, 1.97) | 0.006 | 1.40 (1.06, 1.86) | 0.02 | 1.34 (1.01, 1.78) | 0.045 |
| Language impairment (n=781) | 1.29 (0.97, 1.70) | 0.08 | 1.18 (0.88, 1.58) | 0.28 | 1.12 (0.83, 1.50) | 0.46 | 0.99 (0.74, 1.34) | 0.96 |
| Memory impairment (n=708) | 1.82 (139, 2.37) | < 0.001 | 1.38 (1.04, 1.83) | 0.02 | 1.36 (1.03, 1.80) | 0.03 | 1.28 (0.97, 1.70) | 0.09 |
| Orientation impairment (n=703) | 1.30 (0.98, 1.74) | 0.07 | 1.20 (0.90, 1.61) | 0.21 | 1.16 (0.87, 1.55) | 0.31 | 1.07 (0.79, 1.43) | 0.67 |
| Visuospatial impairment (n=716) | 1.41 (1.06, 1.87) | 0.02 | 1.35 (101, 1.80) | 0.04 | 1.32 (0.98, 1.76) | 0.06 | 1.21 (0.90, 1.63) | 0.20 |
Abbreviations: 3MS, Modified Mini-Mental State Examination; Q1, first quartile (<25th percentile); HR, hazard ratio; CI, 95% confidence interval.
Unadjusted model; main predictor only.
Adjusted for age, sex, race/ethnicity, years of education, treatment arm (pioglitazone vs. placebo).
Adjusted for model 2 + stroke history, hypertension history, hyperlipidemia history, myocardial infarction history, HOMA-IR index, smoking status, weekly alcohol use.
Adjusted for model 3 + stroke etiology (TOAST criteria), baseline NIHSS, baseline modified Rankin score.
In the crude analysis, attention-domain impairment was associated with a higher risk of MI; however, this association did not remain significant in the fully adjusted model 4 (Table 4).
Table 4.
Crude and Adjusted Hazard Ratios (95% CI) for Myocardial Infarction, Based on the Global Cognitive Impairment and Domain-specific Cognitive Impairment as Predictors.
| Cognitive domain | Model 11 | Model 22 | Model 33 | Model 44 | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | |
| Global impairment (n=453) | 1.13 (0.68, 1.86) | 0.64 | 0.91 (0.54, 1.54) | 0.72 | 0.90 (0.53, 1.52) | 0.69 | 0.91 (0.53, 1.55) | 0.73 |
| Attention impairment (n=678) | 1.53 (1.02, 2.29) | 0.04 | 1.38 (0.91, 2.09) | 0.13 | 1.32 (0.87, 2.01) | 0.19 | 1.38 (0.91, 2.10) | 0.13 |
| Language impairment (n=781) | 1.10 (0.73, 1.67) | 0.64 | 0.99 (0.64, 1.53) | 0.95 | 0.97 (0.63, 1.50) | 0.90 | 0.99 (0.64, 1.54) | 0.96 |
| Memory impairment (n=708) | 1.04 (0.68, 1.61) | 0.85 | 0.85 (0.54, 1.33) | 0.46 | 0.81 (0.51, 1.27) | 0.35 | 0.81 (0.51, 1.28) | 0.36 |
| Orientation impairment (n=703) | 1.05 (0.68, 1.63) | 0.82 | 0.98 (0.63, 1.52) | 0.92 | 0.97 (0.63, 1.51) | 0.91 | 0.96 (0.62, 1.51) | 0.87 |
| Visuospatial impairment (n=716) | 1.10 (0.72, 1.69) | 0.66 | 1.04 (0.67, 1.61) | 0.87 | 1.00 (0.64, 1.55) | 1.00 | 0.99 (0.64, 1.55) | 0.97 |
Abbreviations: 3MS, Modified Mini-Mental State Examination; Q1, first quartile (<25th percentile); HR, hazard ratio; CI, 95% confidence interval.
Unadjusted model; main predictor only.
Adjusted for age, sex, race/ethnicity, years of education, treatment arm (pioglitazone vs. placebo).
Adjusted for model 2 + stroke history, hypertension history, hyperlipidemia history, myocardial infarction history, HOMA-IR index, smoking status, weekly alcohol use.
Adjusted for model 3 + stroke etiology (TOAST criteria), baseline NIHSS, baseline modified Rankin score.
Findings of sensitivity analysis adjusting for time between qualifying entry event and baseline interview were consistent with the primary analysis (Appendix C, eTables C1, C2, C3). Findings of sensitivity analyses using mulitple imputation to account for missing covariate data were generally consistent with those of primary analyses, both in direction of effect and significance. One notable exception is that the association between attention impairment and mortality was attenuated (HR 1.28; 95% CI 0.97–1.69; p = 0.08) (online supplemental Appendix C and eTable C5).
Discussion
The main findings of this large, well-characterized sample of nondisabled and nondemented patients with stroke and IR were that post-stroke language and orientation impairments predicted stroke recurrence, while impaired attention carried higher risk of all-cause mortality. These associations were independent of demographics, cardiovascular risk factors including history of hypertension, MI, and stroke, pioglitazone treatment, as well as stroke etiology, severity and outcome.
The novelty of this study is the fact that it reported effects of domain-specific cognitive impairment on the risk of recurrent stroke and death in patients with stroke and IR. In this study, impaired language was independently associated with 35% increased risk of stroke recurrence. Although this is similar to a previous report from the IRIS trial where aphaisa on a baseline general neurological exam was associated with increased stroke and MI, the scope of that study was different (21). In that subgroup analysis of IRIS trial, Kernan et al. aimed to study the efficacy of pioglitazone hydrochloride on secondary stroke prevention according to pretreatment risks of recurrent vascular events. The authors found that patients at higher risk had greater absolute benefit from pioglitazone treatment (21). The reported association of aphasia with stroke recurrence was an incidental finding and neither were further analyses conducted nor were explanations for this association discussed (21).
In the current study, we provided a comprehensive analysis on the risks of recurrent stroke and death according to five cognitive domains assessed by the 3MS. In the 3MS battery, language assessment included naming, category fluency of animals, similarities, repetition, reading, writing, and following 3 stage command (22). Therefore, these data cover the whole spectrum of language functions rather than aphasia observed during routine clinical neurological examination reported by Kernan et al (21).
The reported association between language impairment with increased risk of stroke recurrence is intriguing and there are a few possible explanations. Given that reported association was strong for language and orientation in prediction of recurrent stroke, while essentially there was no association for MI, dominant hemispheric localization rather than overlap between cerebrovascular and neurodegeneration pathologies is the most likely explannation. Since both language and orientation impairments are mostly localized in the left hemisphere, increased risk of stroke recurrence in these cognitive impairments might be localization related. We adjusted for stroke etiology (TOAST) which included hemodynamically significant > 50% stenosis or extracranial or intracranial vasculature. Therefore, it is unlikely to be related to > 50% stenosis on the left side. In addition, TOAST criteria include an “unknown” category. There is a small chance that some patients in this category had undocumented left carotid < 50% stenosis. In a recent subgroup analysis of a randomized clinical trial, index embolic stroke of unknown source and recurrent ischemic stroke were more frequently located in the left than in the right hemisphere (25). It is possible that this was the result of more sensitive recognition of left hemispheric symptoms rather than right. Alternatively, similar to our study, recurrent strokes in some cases may have originated from the same vascular, originally nonstenotic < 50% plaque in a carotid artery which may have progressed, resulting in recurrent stroke (26).
Although dominant hemisphere localization is the most likely explannation for the reported association, an alternative explannation might be that there is overalp between cardiovascular and neurodegenration overlap. Although IRIS excluded patients with dementia, it is possible that some patients had hidden early stage of Alzheimer’s disease (AD). In the early stages of AD, language impairment involves lexical retrieval problems, loss of verbal fluency, and breakdown in comprehension of higher order written and spoken languages (27). Criteria for the clinical diagnosis of AD include language impairment at onset in one of the subtypes (28). On the other hand, AD is associated with cerebral amyloid angiopathy, which carries higher risk of stroke (29, 30). This may also be an alternative explanation of increased risk of recurrent stroke in patients with language impairment (29, 30). In addition to language, post-stroke orientation impairment was independently associated with 41% increase in stroke recurrence. Orientation impairment can be localized in left temporo-parietal regions (31, 32, 33). An important disorder of orientation is AD, in which disorientation in space, time, and person is a hallmark because the default-mode network has a major involvement in the neuropathology of Alzheimer’s disease (34, 35). Similar to language impairment, cognitive impairment predicting stroke recurrence might be related to the crosstalk between neurodegeneration in AD and cerebrovascular pathology including cerebral amyloid angiopathy (29, 30).
In this study, impaired attention was associated with a 34% increase in risk of all-cause mortality. This is similar to our previous finding where, among patients with lacunar stroke, nonmemory domain impairment predicted mortality (16). Impaired attention might be a sign of executive dysfunction (36). Executive dysfunction has been regarded as the distinctive cognitive marker of subcortical vascular cognitive impairment (37). Again, as was mentioned above, the IRIS study excluded patients with dementia, while Model 4 was adjusted for etiology of stroke including small vessel disease. However, it is possible that white matter disease secondary to small vessel pathology was more prevalent in patients with impaired attention. Therefore, this subgroup might have had more aggressive small vessel disease with systemic features including renal and cardiac microangiopathies that increased the risk of all-cause mortality (38, 39).
Although risk-factor management in the IRIS trial including anti-hypertensive (67–73%), antithrombotic (95–99%), and statin (77–82%) medications, as well as smoking (85–88% nonsmokers) was well-controlled (18), there is a small chance that medication noncompliance was higher among patients with language, attention and orientation impairments resulting in higher risk of recurrent stroke and death. In IRIS, patients with better adherence to pioglitazone therapy had lower risk of stroke recurrence, MI and death (40).
This study has important clinical implications. It highlights the potential for cognitive testing in patients with recent stroke as those with cognitive impairment, including domain-specific abnormalities, may benefit from more aggressive secondary stroke prevention strategies that may decrease the risk not only for stroke recurrence but mortality as well. Simple cognitive testing at 3-month follow-up visit may identify patients at higher risk of stroke recurrence and death. Recent European Stroke Organization and European Academy of Neurology guidelines on PSCI support cognitive screening to be a part of the comprehensive assessment of stroke survivors (41). However, this document also states that there are insufficient data to make recommendations around the timing and the benefits of cognitive screening. The results of this and previous studies show that timing for cognitive screening is 3 to 6 months (3–13,16) after stroke, while potential benefit includes identification of population at risk of stroke recurrence and mortality. In patients with PSCI, further randomized clinical trials are needed to test different interventions to prevent recurrent stroke and death.
This study has strengths and limitations. The major strength of the study is that it represents large, well-defined, and closely monitored population. However, this can also be a limitation as the studied population represents a cohort of patients enrolled in the IRIS trial with stroke and IR with prespecified inclusion criteria. Therefore, results may not be generalizable to all patients with ischemic stroke and without IR. On the other hand, it adds new information to the risk stratification of the stroke recurrence in patients with IR. Furthermore, although we attempted to control for all possible relevant covariates in our analyses – including variables that were shown to be predictors of recurrent stroke, and death in IRIS (18), in addition to known risk factors for stroke recurrence as well as those identified in our previous studies – it is still possible that unmeasured confounders may bias our estimates. Another possible limitation is that 3MS does not include test for processing speed which is sensitive to detect small vessel disease (42).
Although domain-specific cognitive impairment after stroke in IR may represent passive bystander, given substantial overlap between neurodegenerative and cerebrovascular pathologies, causal relationship between PSCI and the risk of recurrent vascular events is plausible and requires further study. Even if PSCI is passive bystander it still may serve as useful biomarker predicting stroke recurrence and death in patients with stroke and IR. Development and validation of longitudinally tracked noninvasive markers of key vascular processes related to cognitive and neurologic impairment is listed among priorities of a National Plan to address AD and related dementias (43).
In conclusion, in patients with recent stroke/TIA and IR, post-stroke language and orientation impairments predict recurrent stroke, while attention deficit was associated with increased risk of all-cause mortality, suggesting that post-stroke cognitive screening at 3 months may identify subgroups of patients at higher risk.
Supplementary Material
Acknowledgements
We would like to acknowledge the IRIS Investigators and the National Institute of Health – National Institute of Neurological Disorders and Stroke (NINDS) for providing the data set.
Grant Support
IRIS was supported by NINDS/NIH grant #U01NS044876. ClinicalTrial Registration. clinicaltrials.gov Identifier: NCT00091949.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Statement of Ethics
All participants in the IRIS trial provided written informed consent and the IRIS trial was approved by the local institutional review boards of all participating centers. De-identified data from the study was acquired with approval from the NINDS, and the study was ruled not human subjects research by the George Washington University institutional review board.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
References
- 1.Pendlebury ST, Rothwell PM. Incidence and prevalence of dementia associated with transient ischaemic attack and stroke: analysis of the population-based Oxford Vascular Study. Lancet Neurol 2019; 18: 248–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacova C, Pearce LA, Costello R et al. Cognitive impairment in lacunar strokes: the SPS3 trial. Ann Neurol. 2012;72(3):351–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moroney JT, Bagiella E, Tatemichi TK et al. Dementia after stroke increases the risk of long-term stroke recurrence. Neurology 1997;48:1317–1325. [DOI] [PubMed] [Google Scholar]
- 4.Kwon HS, Lee D, Lee MH, et al. Post-stroke cognitive impairment as an independent predictor of ischemic stroke recurrence: PICASSO sub-study. J Neurol. 2020;267(3):688–693. [DOI] [PubMed] [Google Scholar]
- 5.Tatemichi T, Paik M, Bagiella E et al. Dementia after stroke is a predictor of long-term survival. Stroke 1994;25:1915–1919. [DOI] [PubMed] [Google Scholar]
- 6.Melkas S, Oksala NK, Jokinen H et al. Poststroke dementia predicts poor survival in long-term follow-up: influence of prestroke cognitive decline and previous stroke. J Neurol Neurosurg Psychiatry. 2009;80(8):865–70. [DOI] [PubMed] [Google Scholar]
- 7.Narasimhalu K, Ang S, De Silva DA et al. The prognostic effects of poststroke cognitive impairment no dementia and domain-specific cognitive impairments in nondisabled ischemic stroke patients. Stroke. 2011;42(4):883–8. [DOI] [PubMed] [Google Scholar]
- 8.Sibolt G, Curtze S, Melkas S et al. Poststroke dementia is associated with recurrent ischaemic stroke. J Neurol Neurosurg Psychiatry. 2013;84(7):722–6. [DOI] [PubMed] [Google Scholar]
- 9.Woo J, Kay R, Yuen Y, Nicholls M. Factors influencing long-term survival and disability among three-month stroke survivors. Neuroepidemiology 1992;11:143–150. [DOI] [PubMed] [Google Scholar]
- 10.Oksala NK, Jokinen H, Melkas S et al. Cognitive impairment predicts poststroke death in long-term follow-up. J Neurol Neurosurg Psychiatry. 2009;80(11):1230–5. [DOI] [PubMed] [Google Scholar]
- 11.Patel MD, Coshall C, Rudd AG et al. Cognitive impairment after stroke: clinical determinants and its associations with long-term stroke outcomes. J Am Geriatr Soc 2002; 50:700–706. [DOI] [PubMed] [Google Scholar]
- 12.Desmond DW, Moroney JT, Bagiella E et al. Dementia as a predictor of adverse outcomes following stroke: an evaluation of diagnostic methods. Stroke. 1998;29(1):69–74. [DOI] [PubMed] [Google Scholar]
- 13.Yaghi S, Cotsonis G, de Havenon A et al. Poststroke Montreal Cognitive Assessment and Recurrent Stroke in Patients With Symptomatic Intracranial Atherosclerosis. J Stroke Cerebrovasc Dis. 2020;29(4):104663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henon H, Vroylandt P, Durieu I et al. Leukoaraiosis more than dementia is a predictor of stroke recurrence. Stroke 2003;34:2935–2940. [DOI] [PubMed] [Google Scholar]
- 15.Béjot Y, Jacquin A, Rouaud O et al. One-year survival of demented stroke patients: data from the Dijon Stroke Registry, France (1985–2008). Eur J Neurol. 2012;19(5):712–7. [DOI] [PubMed] [Google Scholar]
- 16.Kwan A, Wei J, Dowling NM et al. Cognitive Impairment after Lacunar Stroke and the Risk of Recurrent Stroke and Death. Cerebrovasc Dis. 2021;50(4):383–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kernan WN, Inzucchi SE, Viscoli CM et al. Insulin resistance and risk for stroke. Neurology. 2002; 59: 809–15. [DOI] [PubMed] [Google Scholar]
- 18.Kernan WN, Viscoli CM, Furie KL et al. Pioglitazone after Ischemic Stroke or Transient Ischemic Attack. N Engl J Med. 2016;374(14):1321–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furie KL, Viscoli CM, Gorman M et al. Effects of pioglitazone on cognitive function in patients with a recent ischaemic stroke or TIA: a report from the IRIS trial. J Neurol Neurosurg Psychiatry. 2018;89(1):21–27. [DOI] [PubMed] [Google Scholar]
- 20.Viscoli CM, Brass LM, Carolei A et al. Pioglitazone for secondary prevention after ischemic stroke and transient ischemic attack: rationale and design of the Insulin Resistance Intervention after Stroke Trial. Am Heart J. 2014;168(6):823–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kernan WN, Viscoli CM, Dearborn JL et al. Targeting Pioglitazone Hydrochloride Therapy After Stroke or Transient Ischemic Attack According to Pretreatment Risk for Stroke or Myocardial Infarction. JAMA Neurol. 2017;74(11):1319–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teng EL, Chui HC. The Modified Mini Mental State (3MS) examination. J Clin Psychiatry 1987; 48: 314–8. [PubMed] [Google Scholar]
- 23.Espeland MA, Rapp SR, Robertson J et al. Benchmarks for designing two-stage studies using modified mini-mental state examinations: experience from the Women’s Health Initiative Memory Study. Clin Trials. 2006;3(2):99–106. [DOI] [PubMed] [Google Scholar]
- 24.van Buuren S, & Groothuis-Oudshoorn K mice: Multivariate Imputation by Chained Equations in R. Journal of Statistical Software. 2011. 45(3), 1–67. [Google Scholar]
- 25.Veltkamp R, Pearce LA, Korompoki E et al. Characteristics of Recurrent Ischemic Stroke After Embolic Stroke of Undetermined Source: Secondary Analysis of a Randomized Clinical Trial. JAMA Neurol. 2020;77(10):1233–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hyafil F, Schindler A, Sepp D et al. High-risk plaque features can be detected in nonstenotic carotid plaques of patients with ischaemic stroke classified as cryptogenic using combined (18)F-FDG PET/MR imaging. Eur J Nucl Med Mol Imaging. 2016;43(2):270–279. [DOI] [PubMed] [Google Scholar]
- 27.Ferris SH, Farlow M. Language impairment in Alzheimer’s disease and benefits of acetylcholinesterase inhibitors. Clin Interv Aging. 2013;8:1007–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKhann GM, Knopman DS, Chertkow H et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenberg SM, Bacskai BJ, Hernandez-Guillamon M et al. Cerebral amyloid angiopathy and Alzheimer disease - one peptide, two pathways. Nat Rev Neurol. 2020;16(1):30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shoamanesh A, Pearce LA, Bazan C et al. Microbleeds in the Secondary Prevention of Small Subcortical Strokes Trial: Stroke, mortality, and treatment interactions. Ann Neurol. 2017;82(2):196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peer M, Salomon R, Goldberg I et al. Brain system for mental orientation in space, time, and person. Proc Natl Acad Sci U S A. 2015; January;112(35):11072–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peer M, Lyon R, Arzy S. Orientation and disorientation: Lessons from patients with epilepsy. Epilepsy Behav 2014; 41:149–157 [DOI] [PubMed] [Google Scholar]
- 33.Spiers HJ, Burgess N, Maguire EA et al. Unilateral temporal lobectomy patients show lateralized topographical and episodic memory deficits in a virtual town. Brain. 2001;124(Pt 12):2476–89. [DOI] [PubMed] [Google Scholar]
- 34.Greicius MD, Srivastava G, Reiss AL et al. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: Evidence from functional MRI. Proc Natl Acad Sci USA 2004. 101(13):4637–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buckner RL, Snyder AZ, Shannon BJ et al. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25(34):7709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCabe DP, Roediger HL, McDaniel MA et al. The relationship between working memory capacity and executive functioning: evidence for a common executive attention construct. Neuropsychology. 2010;24(2):222–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Graham NL, Emery T, Hodges JR. Distinctive cognitive profiles in Alzheimer’s disease and subcortical vascular dementia. J Neurol Neurosurg Psychiatry. 2004;75(1):61–71 [PMC free article] [PubMed] [Google Scholar]
- 38.Berry C, Sidik N, Pereira AC et al. Small-Vessel Disease in the Heart and Brain: Current Knowledge, Unmet Therapeutic Need, and Future Directions. J Am Heart Assoc. 2019;8(3):e011104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toyoda K. Cerebral small vessel disease and chronic kidney disease. J Stroke. 2015; 17(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spence JD, Viscoli CM, Inzucchi SE et al. Pioglitazone Therapy in Patients With Stroke and Prediabetes: A Post Hoc Analysis of the IRIS Randomized Clinical Trial. JAMA Neurol. 2019;76(5):526–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quinn TJ, Richard E, Teuschl Y et al. European Stroke Organisation and European Academy of Neurology joint guidelines on post-stroke cognitive impairment. Eur Stroke J. 2021;6(3):1–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duering M, Gesierich B, Seiler S et al. Strategic white matter tracts for processing speed deficits in age-related small vessel disease. Neurology. 2014. Jun 3;82(22):1946–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corriveau RA, Koroshetz WJ, Gladman JT et al. Alzheimer’s Disease-Related Dementias Summit 2016: National research priorities. Neurology. 2017;89(23):2381–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


