Abstract
As rates of Cesarean delivery and common non-communicable disorders (NCDs) such as obesity, metabolic disease, and atopy/asthma have concomitantly increased in recent decades, investigators have attempted to discern a causal link. One line of research has led to a hypothesis that Cesarean birth disrupts the presumed normal process of colonization of the neonatal microbiome with vaginal microbes, yielding NCDs later-in-life. However, a direct link between a disrupted microbiota transfer at time of delivery and acute and/or chronic illness in infants born via Cesarean has not been causally established. Microbiota seeding from maternal vaginal or stool sources has been preliminarily evaluated as an intervention designed to compensate for the lack of (or limited) exposure to among Cesarean delivered neonates. However, to date, clinical trials have yet to show a clear health benefit with neonatal “vaginal seeding” practices. Until the long-term effects of these microbiome alterations can be fully determined, it is paramount to conduct parallel meaningful and mechanistic-minded interrogations of the impact of clinically modifiable maternal, nutritional, or environmental exposures on the functional microbiome over the duration of pregnancy and lactation to determine their role in the mitigation of childhood and adult NCDs.
Keywords: Microbiome, vaginal seeding, mode of delivery
The Developmental Origins of Health and Disease (DOHaD)
The ‘Developmental Origins of Health and Disease [DOHaD] Hypothesis’ [1,2] encompasses a substantial body of evidence which temporally and functionally links maternal exposures to adverse outcomes in her offspring (largely the non-communicable diseases [NCDs]) (See glossary) of obesity, metabolic disorders, cardiovascular disease, and behavioral outcomes. Initial mechanistic-minded studies in animal models demonstrated that maternal nutritional deprivation or high fat diet feeding brought about meaningful and persistent modulations in postnatal gene expression resulting from epigenomic changes in key metabolic pathways in the offspring [3,4]. More recently, others examined the similar temporal relationships through the lens of microbiome science, leading to the genesis of the ‘Hygiene Hypothesis.’ The Hygiene Hypothesis suggests that in addition to maternal exposure-driven fetal epigenetic variation, the lack of exposure to microbes early in life predisposes offspring to not only developing these same adverse outcomes, but also atopic and allergic diseases later-in-life [5]. However, despite nearly 100 years recognizing these links, we have failed to reveal meaningful mechanistic understandings of 1) how specific maternal elements contribute to functional fetal and early life developmental and 2) how to prevent infant morbidity and mortality.
As both the incidence and prevalence of Cesarean delivery and NCDs have increased regionally and temporally in parallel, a general acceptance of the DOHaD and Hygiene Hypotheses have lent to a line of logic that reasons that rises in Cesarean delivery are likely to be inextricably linked to rises in NCDs [8–13]. Some scientists, concerned about detrimental effects of Cesarean delivery on the neonatal microbiome, have called attention to the high rate of “elective” Cesarean deliveries and turned to experimental manipulation of the neonatal microbiome. This line of research focuses on seeding Cesarean born infants with either maternal vaginal or stool microbes in attempts to restore their microbiome to mirror those of their vaginally born counterparts [6,7]. As obstetricians, we are supportive of these scientists in their efforts to innovate, to potentially develop novel therapeutics, and to attempt to improve offspring outcomes in experimental seeding protocols conducted as part of approved research protocols. However, the clinical implications of seeding, especially the long-term effects, remain unknown and we and the American College of Obstetricians and Gynecologists (ACOG) acknowledge that they are not without risk. Thus, while microbiome seeding is a potentially promising trend within the field, it may not be a cure-all for the prevention of NCDs.
In this current clinical and translational science opinion piece, we will address some commonly-held misconceptions regarding rates of Cesarean delivery as well review the latest studies experimentally attempting to alter the microbiome of Cesarean born infants. As physician-scientists dedicated to bridging the basic sciences to clinical obstetrics, we hope to make the broader scientific community cognizant of the following points. First, we should be cautious not to imply that seeding of Cesarean born infants reduces childhood health disorders in the absence of data demonstrating such long-term efficacy. There is currently insufficient evidence of causal connections between Cesarean deliveries and child outcomes that account for potential confounders like maternal disease, and shared environmental and dietary exposures, nor lower duration of exclusive breastmilk feeding. Exercising such caution decreases the potential to cause implicit bias and harm by propagating a falsehood seen in the media that Cesarean deliveries are largely elective surgeries done for maternal or provider convenience--”[t]he mother-requested cesarean […] the fast, high-tech, hip celebrity way to have a child “[34]—and not medically indicated procedures reserved for maternal and fetal benefit. Second, in addition to focusing on exposures during the moment of delivery, we cannot neglect the importance of exposures over the duration of the known-to-be crucial periods of pregnancy and lactation. Third, there is the potential for seeding the neonate with maternal microbes (be they vaginal, fecal, or oral) to introduce or reinforce pathogens or pathobionts. While clearly some pathogens are more or less clinically screened for, pathobionts are not, and consequently, they can be transmitted at the time of both vaginal and Cesarean deliveries. Thus, if it is the transfer of these maternal microbiota--or their metabolites--which are the true culprits associated with NCDs, seeding may offer additional harm with no potential benefit. In the following sections, we will consider each of these three rational concerns and then identify opportunities for interrogating the range of maternal and neonatal exposures which frequently accompany Cesarean delivery. We suggest that these exposures—which are oftentimes inextricably linked to the surgery itself— should not be overlooked as potentially equally important drivers of variation in the early microbiome with resulting implications for child health and chronic disease risk.
Addressing misplaced concern with the rate of Cesarean delivery or occurrence of “elective” Cesarean birth
The rate of Cesarean deliveries performed in the United States (US) increased dramatically from ~20% in the mid-1990s to 30–35% by the early 2010s [9]. While clearly performed with the aim of reducing adverse outcomes in the patient or the fetus in a case-by-case basis, this increase in the Cesarean rate was not accompanied by the anticipated improvements in maternal and neonatal outcomes at a population-wide level [9,10]. Cesarean delivery is associated with higher maternal morbidity than successful vaginal delivery, including higher risk of blood loss, infection, organ injury, venous thromboembolism, and risk for morbidly adherent placenta in future pregnancies [9]. In efforts to lower the Cesarean delivery rate, ACOG has promoted novel protocols for labor management and changed its counseling regarding vaginal birth after Cesarean (VBAC) [9,11]. All such efforts reflect an earnest attempt to “do something” about the Cesarean delivery rate [10]. However, when discussing efforts to reduce the rate of Cesarean deliveries, it is important to be cognizant of three common erroneous assumptions or misconceptions.
First is the assumption that maternal preference is a significant driver of the escalated Cesarean delivery rate, a belief which likely stems from database-level data in which the indication for the surgery is listed as “elective.” A more appropriate descriptor for these cases would be “scheduled” or “unlabored” Cesarean delivery as these are most typically performed for medical indications such as having undergone a prior Cesarean delivery and not being a candidate for a vaginal birth after Cesarean, fetal malpresentation, abnormal placentation, or other new or chronic maternal medical conditions [12]. The appropriate and accepted term for Cesarean deliveries performed purely for maternal preference is “Cesarean delivery by maternal request” (CDMR), and, while difficult to determine, it is estimated that these deliveries account for between 1.05 and 2.5% of all primary Cesarean deliveries in the US or <1:1000 live births [13,14]. In contrast, the overwhelming majority of Cesarean deliveries are performed for clinical indications with the intent to preserve maternal or fetal well-being.
The second erroneous assumption is the belief that we can reduce the Cesarean delivery rate through the development and application of novel science and biomedical technology. Rather, we concur with our colleagues’ recently published perspective [9], and we too believe that the current rate of Cesarean delivery is the result of forces largely out of scientists’ or clinicians’ control including: 1) societal expectations of perfect obstetric outcomes, 2) a culture of blame, 3) medical-legal concerns, and 4) limitations of current technology and science to predict when, if, and how successful, intact vaginal deliveries will occur. It is indisputable that when given the singular concern of a current and existing pregnancy, Cesarean delivery offers a safe and appealing alternative when faced with the uncertainty of whether a “good” outcome (generally implying a live, healthy neonate) will occur with vaginal delivery. The unknowns are great and include whether (or not) there will be an obstructed labor, fetal intolerance to labor, chorioamnionitis, intrapartum stillbirth, or another rare but catastrophic outcome. While childbirth in the US is overall very safe, complications can have devastating outcomes for pregnant persons, their families, and their communities. As such, litigation concerns weigh heavily amongst obstetrician/gynecologists [15], making the certainty of a Cesarean delivery appealing. While there may be some room to reduce the Cesarean delivery rate with improvements in science and technology, we argue that such interventions likely fail to address the underlying forces driving the Cesarean delivery rate in the US [9, 20].
A third commonly held assumption is that Cesarean deliveries are a major contributor to our current population’s burden of chronic diseases, with differences in the microbiome of Cesarean vs vaginally born infants as a possible etiology for this increase in NCDs. Several observational studies have reported modest associations between Cesarean delivery and child obesity [16–18], Type II diabetes [19], chronic immune disorders [20], and asthma [21], while others show no link between Cesarean delivery and child health outcomes [22–25]. Overall, the evidence linking Cesarean delivery to adverse child health outcomes is heterogenous, conflicting, and unable to fully account for important potential confounding factors. For example, multiple previous studies have noted that the association between Cesarean delivery and obesity may be predominantly driven by unmeasured confounding [26–28]. As Almqvist et al. conclude in their study of rates of childhood asthma in neonates born via Cesarean delivery, which found that there was an increased risk of asthma medication use in those born via emergency Cesarean versus those delivered via Cesarean before the onset labor or those delivered vaginally, the indications for Cesarean delivery were driving differences rather than the surgery itself [29]. A recent study of 97,291 Swedish men found that there was a difference in rates of obesity in these men born via vaginal delivery (4.9%) versus nonelective Cesarean delivery (5.6%) but concluded it was the maternal factors driving these perceived differences including pre-pregnancy maternal BMI, maternal co-morbidities, and age [25]. Without data from randomized controlled trials (the gold standard in clinical research), it is impossible to control sufficiently for population differences and confidently state that mode of delivery is not a marker for differences in other exposures that bear a true causal link to long-term health outcomes. As randomizing patients to Cesarean vs vaginal mode of delivery would be unethical, some scientists have instead trialed seeding Cesarean born infants with maternal microbes in attempts to “restore” the microbiome of Cesarean born infants to mirror those of their vaginally born counterparts with the goal to ultimately improve short- and long-term child health outcomes. In the next section, we will review the latest approaches to microbial restoration with seeding practices, their findings and limitations, and their contributions to our understanding of the neonatal microbiome.
Trial by seeding: an alternative way to study the microbiota?
In response to evidence that neonatal and infant microbiota differ at least transiently by route of delivery, randomized clinical trials have been conducted which aim to determine whether different approaches can “restore” the natural microbiota, i.e. make the microbiota of infants born by Cesarean delivery more similar to the microbiota of those born by vaginal delivery. With such an approach, the hope is that seeding could re-establish vertical routes of microbial transmission that are interrupted by Cesarean delivery and consequently induce meaningful and lasting effects on the establishment or development of the infant microbiome. One widely known approach is “vaginal seeding”, which involves inoculating cotton gauze with vaginal fluids to transfer vaginal flora to the newborn infant (Figure 1). More recent studies have evaluated two alternative approaches to vaginal seeding: neonatal oral administration of maternal vaginal microbiota, or neonatal oral administration of maternal fecal microbiota. The idea of seeding through any of these routes has received substantial attention in both scientific and lay press [30–33], leading to expecting parents preparing for Cesarean delivery to inquire about seeding options at the hospital or at home [33]. At this time, recommendations from most medical societies including ACOG emphasize that practices attempting to alter the infant’s microbiota should not occur outside of IRB-approved research protocols until further safety and efficacy data are gathered [8]. However, given the relative simplicity of the process, parents may decide to carry out their own neonatal or infant seeding outside of research protocols, despite current recommendations [30]. Stinson et al. caution that the practice of vaginal seeding has become mainstream in some areas and is often performed without the oversight of health care providers [31]. These sentiments are reflected in popular press, with patients remarking “‘Our doctor is generally supportive of the idea, but he won’t do it himself […] That task will fall to my husband’” [35]. However, performing these seeding practices outside of strictly regulated protocols bear risk of unintended detrimental effects on neonatal health, as evidenced by a case of neonatal herpes simplex infection following vaginal seeding performed after an elective cesarean section of an asymptomatic woman [36]. Moreover, if in fact the risk of NCD is actually arising from maternal microbes and their metabolites, such practice maybe imparting greater harm. As such, it is crucial to understand the foundational work of neonatal microbiome transplantation and its implications. In this section, we will review the major studies attempting to alter the microbiota of infants born by Cesarean delivery.
Figure 1: Evaluating the effects of vaginal seeding on the development of the infant microbiome.
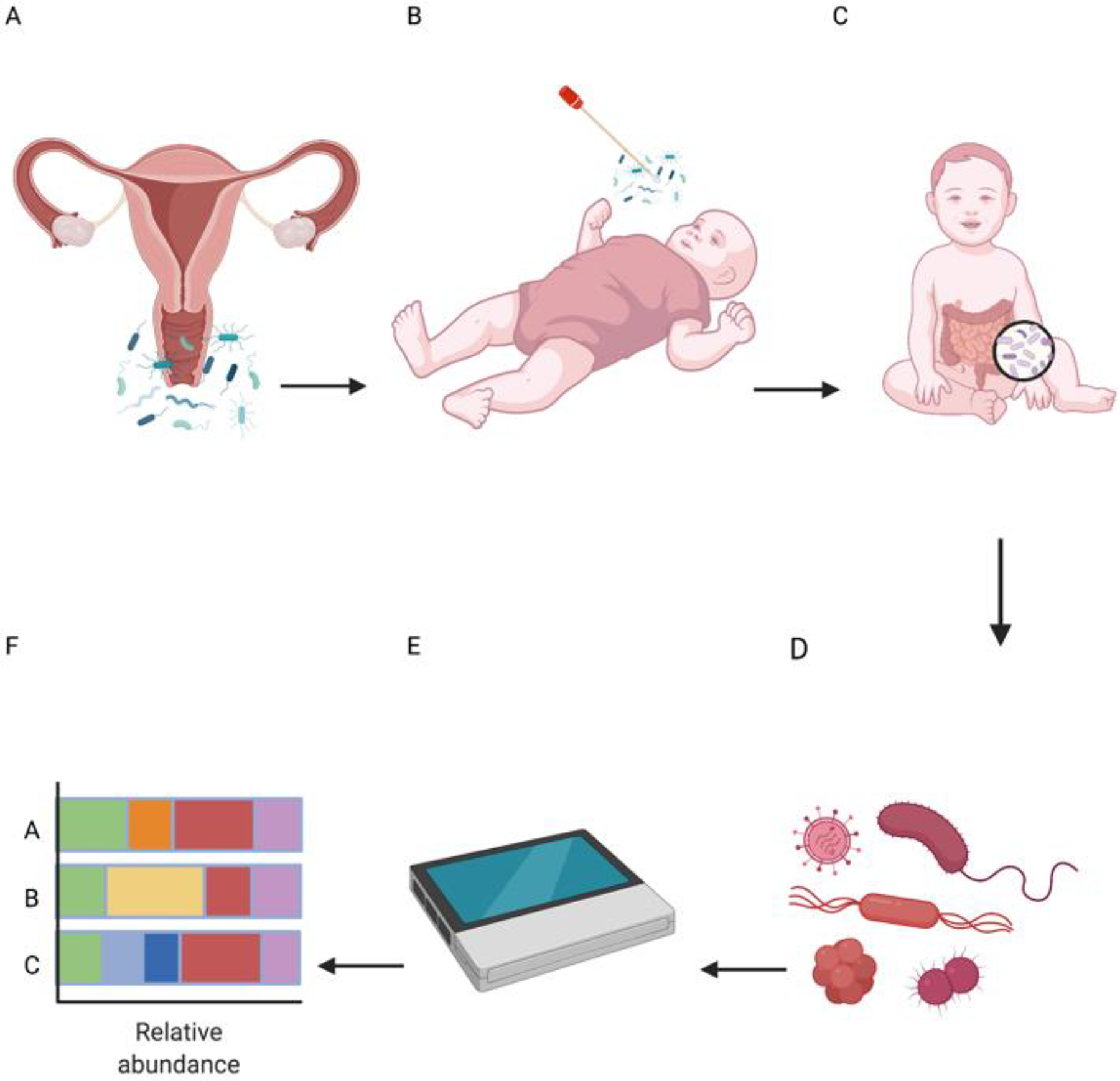
The process of vaginal seeding involves A) inoculating gauze with vaginal fluids (ie the maternal vaginal microbiome) and B) transferring the vaginal flora to the neonate either by direct swabbing or via oral administration immediately after birth. C) The microbiome of the neonate continues its nascent development and D) is sampled at multiple points throughout its first months of life. E) Samples are sent for sequencing studies to F) analyze the typically course (e.g., 16S based analysis of relative taxonomic abundance) composition of the neonatal (<30 days of life) or infant (>30 days to 1 year) microbiome.
The first study to propose vaginal seeding was published by Dominguez-Bellow et al. in 2016 [6]. This initial pilot study of 18 maternal/infant dyads aimed to restore the microbiota of infants born by Cesarean delivery using vaginal seeding. Of 11 infants born by Cesarean delivery, four were exposed to gauze inoculated by maternal vaginal fluids (vaginal seeding). Gravidae included in this study were screened for known vaginal pathogens, and serial samples were intermittently but inconsistently obtained during the first month of life. Bacterial source tracking revealed that the microbiomes of four neonates born by Cesarean delivery and exposed to vaginal seeding resembled the microbiomes of some neonates delivered vaginally, especially during the first week of life [6]. However, of these four Cesarean delivered neonates exposed to the vaginal seeding protocol, only one yielded microbiome specimen data at multiple study time points. Additionally, no Cesarean delivered neonates were exclusively breastfed. This study’s small size and inconsistent sampling data precludes any strong conclusions about the safety or efficacy of vaginal seeding, but as a proof-of-principle study, it introduced an innovative potential method to make a Cesarean delivered neonate bear some similarity to the microbiota of a vaginally delivered counterpart.
In 2020, Korpela et al. published a second innovative proof-of-concept study evaluating whether FMT with maternal inoculum could alter gut microbiota in her neonate when born via Cesarean delivery [7]. Seventeen gravidae were recruited and seven were ultimately selected after careful screening in accordance with European recommendations for fecal transplantation. The seven neonates received a diluted fecal sample from their own mothers’ stool, along with the first human milk feeding. The neonates and infants were followed closely for three months and demonstrated no immediately evident adverse effects. The fecal microbiota composition of the maternal FMT-treated Cesarean born infants resembled that of vaginally born infants through 12 weeks of life at which time data collection stopped. Notably, the authors also analyzed available microbiota datasets from theirs and several different cohorts and found that vaginal seeding was not effective in altering the microbiota in Cesarean born infants [7]. Overall, this study supports the potential value of FMT as an effective mechanism of altering gut microbiota of infants born via Cesarean delivery. Additionally, it comparatively demonstrates that vaginal seeding is a less effective approach. However, the long-term offspring outcomes were not assessed, and consequently the impact of the FMT— beneficial or not—on the health of the infant remains unknown at this time. Currently, there is one FMT trial on Cesarean-delivered neonates underway in Finland with planned follow up until 24 months of age (NCT04173208)I. This study is designed to fill gaps of knowledge and examine potentially clinically relevant immunologic outcomes by measuring differences in immunomarkers at multiple time points along the two-year follow up period, which were not examined in the Korpela et al. study.
Another study in this area was published by Wilson et al. in 2021 [37]. This pilot, single-blinded, randomized placebo-controlled trial evaluated whether oral administration of maternal vaginal microbiota to infants born via Cesarean delivery could alter their gut microbiome. Gravidae planning for scheduled, non-labored Cesarean delivery initially underwent comprehensive infectious disease screening to exclude those with known potential pathogens. At birth, neonates were randomized to receive a 3 mL solution of maternal vaginal microbes (Cesarean delivery-seeded, n = 12) or sterile water (Cesarean delivery-placebo, n = 13). Neonates born vaginally were included as the reference group (vaginal birth, n = 22), and offspring were assessed at birth and again at one and three months of age. Results showed no differences in the composition or function of the gut microbiome between Cesarean delivery-seeded and Cesarean delivery-placebo neonates nor between infants at either one or three months of age. There were no immediate serious adverse effects of treatment. Wilson et al. acknowledged limitations of their study including reduced participant numbers and limited colonization of the Cesarean-delivery seeded infant gut by maternal vaginal microbes [37]. Again, the authors conclude that the potential value of vaginal seeding should be questioned, and vaginal seeding alone does not have a significant impact on infant gut microbiome development. Finally, in the most recent study in 2021, Song et al. performed a large observational study on vaginal seeding after Cesarean to assess the effects of vaginal seeding at different body sites in offspring development in the first year of life [38]. The authors of this study were able to partially restore the microbiome features associated with vaginal delivery with seeding in Cesarean delivered neonates. However, they acknowledge that several genera (e.g. Bilophilia) do not appear to establish well in the Cesarean-seeded neonates [38]. They also observed that while there is often a significant association of Enterococcus with infants delivered via Cesarean, they did not appreciate a weakened association of this genus or most other Cesarean-associated genera with the Cesarean-seeded neonates [38]. Interestingly, they determined in their study population—“healthy” mothers undergoing scheduled Cesarean deliveries—that there is a notable taxonomic overlap between the maternal vagina and other maternal body sites, especially the gut, on the day of giving birth, which was not previously seen in women who were not pregnant [38]. We agree with the authors of this study that more research is needed to determine the basis of the differential uptake of certain taxa with seeding and the subsequent implications on the developing infant microbiome.
Despite a promising initial study from Dominguez-Bellow [6], subsequent work has not shown vaginal seeding to be an unequivocal effective approach to altering the neonatal microbiota [7,37]. Given that these initial studies were limited by small sample sizes and variations in seeding techniques, additional trials are underway. It is important to note, however, that none of the aforementioned studies or current ongoing clinical trials assess the effects of seeding on long-term offspring health outcomes nor on the onset nor occurrence of NCDs. A few studies have attempted to analyze differences in microbiota signatures in older children, finding the variance in microbiota composition explained by mode of delivery decreased with time to <2% at three and five years of age [39]. At this time, we only have proximal measures for comparing vaginally born neonates to Cesarean delivery-seeded neonates—likeness to the microbiome of a vaginally born infant. How this “restoration” ultimately affects ultimate health of the Cesarean delivered infant—whether positively, negatively, or neither—and for how long beyond 12 weeks of age the results persist still have yet to be determined.
We recognize the time that clinical trials take to conduct, and that there is often a long interval between concept and publication of data demonstrating efficacy. We were able to identify two vaginal seeding trials underway in the United States (NCT03298334)II, (NCT03567707)III and one in China (NCT03809390)IV, per ClinicalTrials.gov. As with others in the field, we look forward to their findings. In the interval, we propose two considerations remain at the forefront of research in this arena. First, based on the two most recent studies [7,37], there is a suggestion that vaginal seeding may be an ineffective approach, and we suggest that vaginal seeding may be “too little, too late.” We acknowledge that the data does support that there is a difference in a few taxa in the early-life microbiome (e.g., initial days to weeks) of neonates born via Cesarean and those born vaginally; our work and others support this conclusion [43]. However the evidence that these differences are (i) exclusively due to the mode of delivery itself and (ii) durable beyond the neonatal period is limited, lacking or shown to be to the contrary [29–33, 37–51]. Nevertheless, as Stinson et al. point out, popular perception is that Cesarean delivery deprives the neonate of exposure to vaginal microbiota, which leads to neonatal dysbiosis and subsequent risk of poorer health outcomes [31]. Consequently, attempts are developed to “correct the problem” even though the “problem” may not exist and the benefits of exposure to any individual bacterial species have not been established [31]. Given these limitations in evidence, it is important that we remain cognizant that maternal dysbiosis co-occurs with maternal medical comorbidities (for example, poorly controlled Type 2 or gestational diabetes), which themselves bear a higher risk of Cesarean delivery. In such instances, reinforcing with neonatal oral administration of maternal disrupted microbiota via fecal based seeding would not be “restorative” but might instead add “fuel to the fire.” Second, it is equally critical to examine how other clinically modifiable prenatal exposures (maternal comorbidities and maternal diet) and postnatal exposures (feeding via breastmilk or formula) shape the neonatal microbiota and may offer significant restorative potential in and of themselves.
Why maternal exposures and pregnancy matters: factors beyond Cesarean shaping the neonatal and infant microbiota
The practice of seeding Cesarean born infants with maternal microbes arose in response to observational studies indicating higher rates of chronic disease among Cesarean born infants; consequently, some purport that these differences are due to differences in the microbiome between infants delivered via Cesarean and those delivered vaginally [40–42]. We propose that these beliefs are based on two debatable assumptions: 1) true meaningful and lasting differences exist between the microbiomes of infants born via Cesarean and those born vaginally, and 2) these differences are due to differential exposure to maternal microbes primarily/only at time of delivery. However, these assumptions do not necessarily acknowledge the myriad of other factors known to influence the microbiome [43,44].
In a cohort chronicled by Yassour et al., 20% of the vaginally born infants had a “low Bacteroides” microbiome profile that more closely resembled the Cesarean born infants than their vaginally born counterparts; this finding suggests that differences between these two populations may not in fact be reliable [45]. In a longitudinally sampled cohort, Chu et al. examined oral, nares, and skin samples obtained at delivery from neonates born via Cesarean delivery and those born vaginally and found no differences between meconium samples of the two populations [43]. Importantly, there were no observed differences in any body site by mode of delivery that persisted at 6 weeks of age, and a “low Bacteroides” profile was also observed in approximately 1 in 5 vaginally born neonates and infants [43], similar to Yassour et al.’s findings [45]. As the gut microbiome has been more closely implicated with chronic disease risk [46,47], the significance of variations limited to oral, nares, and skin niches that disappear by 6 weeks of age coupled with the lack of demonstrable difference in meconium samples suggest that differences, when observed, are not durable and may not be physiologically relevant.
With respect to other potential drivers, there is ongoing and emerging evidence which supports the notion that gestational age, perinatal antibiotic exposure, neonatal and infant feeding practices, maternal diet, and environmental exposures have been shown to impact the neonatal and infant microbiome (Figure 2) [48].
Figure 2: Maternal and environmental factors that can affect the development of the infant microbiome.
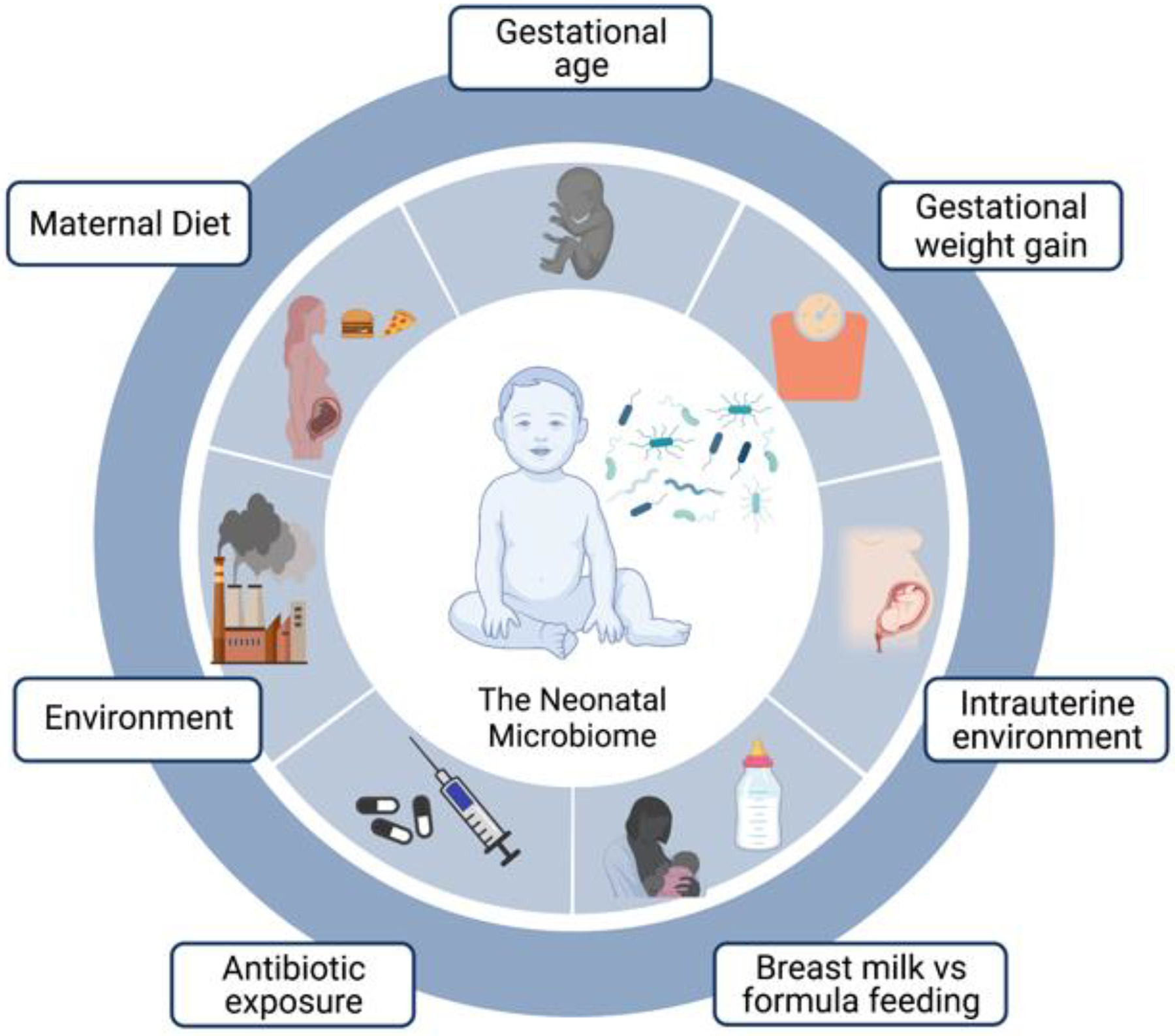
Several maternal and environmental factors h have been shown to have varying degrees of impact on the development of the neonatal, infant, and/or early childhood microbiome. Current evidence supports the notion that gestational age at delivery, environmental chemical exposures, perinatal antibiotic exposure, perturbations in the intrauterine environment, neonatal and infant feeding practices, and/or maternal diet all significantly impact the neonatal microbiome. Additionally, many factors are not independent and can combine for a cumulative effect (ie maternal diet can influence gestational weight gain which in turn can affect the breast milk microbiome and subsequently the neonatal microbiome).
Gestational age.
Firstly, gestational age (notably, being born preterm) significantly affects the neonatal microbiome both in the nascent microbial population as well as postnatal modifying factors related to Neonatal Intensive Care Unit (NICU) life that are more commonly experienced by preterm infants [48]. Preterm neonates (those born before 37 weeks gestational age) tend to experience delayed colonization with commensals in favor of pathobionts, particularly among neonates 23–30 weeks gestational age [49]. In a sequence-based meta-analysis of eight studies evaluating the neonatal microbiome in preterm infants, Pammi et al. observed patterns of increased operational taxonomic units (OTU) richness over time where the number of species increased significantly with gestational age [50]. As Pammi et al. point out, this finding may be representative of transition beyond the prenatally and vertically acquired microbiome and maybe more influenced by the NICU environment, feeding practices, and antibiotic exposure, among others [50].
Intrapartum antibiotic exposures.
Azad et al. demonstrated that intrapartum antibiotic prophylaxis (IAP) is associated with a disruption in the neonatal gut microbiota [51]. In their cohort of 198 healthy term deliveries, they noted a difference in the infant gut microbiota following a maternal IAP exposure, regardless of mode of delivery [51]. Moreover, these maternal IAP associations were independent of a ‘Cesarean delivery effect’ as a diminished microbiota richness and altered composition was similarly noted following antibiotic administration with vaginal delivery [51]. Additionally, they observed that these microbiota changes in IAP exposed infants persisted up to 12 months, particularly in the non-human breastmilk fed infants [51].
Neonatal and infant feeding.
Although neonates delivered vaginally have increased diversity in their intestinal microbiome in the immediate postnatal period [40], at 4 months of age, infants’ intestinal microbiomes could be differentiated primarily based on human milk vs formula feeding [41]. Chu et al. found that when controlling for the maternal indication for Cesarean delivery and other clinical factors, in robust computational modeling, the two factors that had a lasting impact on the infant microbiome at 6 weeks of age were formula feeding and the degree of fat in the maternal diet [43]. Breast milk contains its own unique microbiome that contributes to the bacterial communities noted in the neonatal gut microbiome [48]. Additionally, breast milk aids in colonization and development of the neonatal microbiome through a process of enchainment and tolerance as it contains fatty acids, immunoglobulins, and human milk oligosaccharides [48]. Murine studies have supported this understanding of the role of breast milk in the establishment of the offspring microbiome with evidence that maternal antibodies transferred via breast milk have a long-lasting impact on the infant intestinal microbiome [52].
Maternal diet.
Another factor important to the development of the neonatal intestinal microbiome is the maternal diet. Maternal diet impacts the maternal microbiome which in turn affects the offspring microbiome. For example, women who consume a high fat or Western-style diet (WSD) are noted to have decreased levels of the commensal enteric bacteria Bacteroides [53]. In animal models, Bacteroides is crucial for normal intestinal immune development, and consequently, it has been proposed that lacking these microbes during pregnancy may predispose the developing neonate to atopic and autoimmune disorders in later years of life [54]. In a non-human primate model, a maternal WSD imparts significant alterations to the juvenile microbiome that are irreversible, even when the juvenile primates are co-housed and reverted to a healthful diet after weaning [53]. Similar findings have been demonstrated in a study of a large human cohort, with differences in both the neonatal and infant microbiomes that persist for at least 4–6 weeks post-delivery associated with maternal WSD [55]. Jost et al. examined the bacterial loads between maternal and neonate stool and found that they were remarkably similar, suggesting that the maternal microbiome at birth and postpartum may be critical in the establishment and development of the neonatal microbiome [56].
Environmental exposures.
Just as a maternal WSD can confer a lasting footprint on the offspring gut microbiome as well as biologically relevant histone modifications [53], maternal environmental exposures are suspected to play a role in the developing neonatal microbiome. Studies have shown that environmental exposures interact with and modify the microbiome, and (conversely) the microbiome interacts and modifies environmental chemicals [57]. Thus, maternal exposure to environmental chemicals affects perinatal microbial health. Ingested xenobiotics like cadmium and polycyclic aromatic hydrocarbons are able to interfere with microbial enzymatic activity of the gut and have been shown to induce gut dysbiosis [58]. Given the changing fetal nutrient requirements during pregnancy, gravidae undergo dynamic metabolic adaptations modulated by the gut microbiota, which in turn direct fetal programming [59]. Thus, the resultant maternal gut dysbiosis from chemical exposure, much like the altered microbiome rendered by a high fat diet, has an avenue to affect fetal programming that potentially renders increased risk of chronic disease in the offspring.
Intrauterine environment.
When considering the factors influencing the formation of the fetal microbiome, it is also important to consider how the intrauterine environment itself may influence the microbiota of the developing fetus. Several scientists have challenged the notion of a sterile intrauterine environment in the absence of disease and have purported a distinct placental microbiome [60–71]. Evidence for a unique placental microbiome stems from a metagenomics study of 320 placentae which demonstrated a low-biomass microbial community of the placental parenchyma and chorionic villi [60]. This study demonstrated a unique placental microbiome niche composed of nonpathogenic commensal microbiota from the Firmicutes, Tenericutes, Proteobacteria, Bacteroides, and Fusobacteria phyla that was most akin to the human oral microbiome [60]. Other researchers have demonstrated a similarity between the placental microbiota and the neonatal meconium, suggesting that the microbiota may be transferred across the placenta into the fetus before being excreted into the amniotic fluid as fetal urine [62,72]. Gosalbes et al. postulate that because the neonatal meconium microbiota differs from the dominant bacterial groups found in the maternal skin, fecal, and vaginal niches, the neonatal microbiota is unlikely to originate in those maternal locations [73]. Instead, they argue that because meconium is formed starting at mid-gestation in fetal life (17 weeks and beyond), the microbiota detected in meconium is likely not simply due to contact with maternal habitus at time of delivery [73]. Indeed, it is well established that the meconium expressed within minutes to days of birth has been present in the small bowel since at least 20 weeks of gestation. Chen et al. hypothesize that maternal prenatal stress results in changes in the maternal intestinal, oral, and vaginal microbiomes that facilitate the translocation of bacteria to the intrauterine environment either hematogenously or through direct ascension, suggesting that the placenta is more conduit than barrier [74]. Recent work from Peterson et al. demonstrated that newborns who developed immunoglobulin E (IgE)-mediated allergic sensitization (atopy) by one year of age have a less diverse gut metabolome at birth as measured in the meconium, which begins forming in the fetal gut during the second trimester [75]. They argue that deficiency in microbiota maturation and immune development likely begins in utero [75] rather than at time of delivery.
It is important to acknowledge, however, that there is a debate regarding the existence of a placental microbiome [76–83]. In our own work, while we have consistently distinguished a metagenomic signal in the placenta from that of contaminant controls [45, 52, 54], we have also been explicit in noting it to be of low biomass, low abundance, and sparse. We have also remained consistently agnostic as to whether the placental or intrauterine microbiome is truly alive and colonizing, with a yet unclear functional role [8,68]. With further scientific advancement and continued curiosity, we are confident that investigators will determine whether these consistently observed low-biomass communities are alive and colonize the fetus or alternatively enable later colonization through processes of immune tolerance or colonization resistance.
Potential risks of “seeding” interventions
If exposures apart from mode of delivery are links to long-term disease risk, as we suggest, it is important to consider potential unintended consequences of administration of maternal dysbiotic microbial communities to the neonate, namely the potential to further propagate pathobionts in the neonate with an inoculum from seeding. Pathobionts are potentially pathogenic organisms that, under normal circumstances, live as non-harming symbionts. Even though pathobionts may be able to co-exist within the maternal host microbiome, their long-term effects on a developing neonate or infant are unknown, and may include potential to resist or prevent colonization by known beneficial commensal organisms. As we have discussed, the gut microbiome has been linked with various disease phenotypes including obesity and other cardiometabolic diseases, and it plays a role in immune response [46,84,85]. Additionally, such disease phenotypes may be transmissible via the microbiome; Turnbaugh and colleagues have shown that transplantation of the gut microbiome of obese mice to germ free mice leads to obesity in the germ-free mice [46]. This finding reinforces that the gut microbiome and chronic disease are linked and, importantly, shows that such disease phenotypes are potentially transmissible via transplantation of the microbiome [86].
It should be noted that studies have determined higher relative abundances of potential pathobionts immediately after birth in infants born via Cesarean delivery [87,88]. Mueller et al. found greater abundances of Clostridium perfringens and Clostridium neonatale in neonates born via Cesarean delivery compared to vaginal delivery at 3 months of age [87] while Shao et al. showed the mean relative abundance and frequency of six opportunistic pathogen species was enriched in neonates born via Cesarean delivery in the first 21 days of life compared to those born via vaginal delivery [88]. However, the significance difference in relative abundance of these potential pathobionts based on mode of delivery diminished with age in the Shao et al. study [88] and was noted to disappear by 12 months of age in the Mueller et al. study [87]. Additionally, the increased relative abundance of opportunistic bacteria in neonates born via Cesarean delivery does not necessarily indicate their bloom but rather may be a reflection of a lack of other intestinal microbes; any potential clinical outcomes have yet to be determined [33]. Thus, long-term clinical implications of this temporary increased relative abundance of potential pathobionts attributed to mode of delivery are unknown, and more research is needed to understand how decreasing the levels potential pathogens with FMT protocols—which was noted by Korpela et. al at 1 week and 12 weeks of age [7]—ultimately affects offspring development.
Additionally, when considering the transplant of maternal microbes via vaginal or stool seeding to Cesarean born neonates, it is important to consider that women undergoing Cesarean rate have higher rates of obesity, hypertension, and other cardiovascular co-morbidities [89,90]. If the transfer of maternal microbiota plays a role in driving up rates of NCDs, seeding may only offer harm with no potential benefit. In fact, seeding neonates with maternal microbes may serve to “double up” the bad exposure—giving the infant both the genetic predisposition for developing certain chronic diseases as well as the “disease phenotype” microbiome to go with it.
Indeed, observational studies have demonstrated that regardless of mode of delivery, neonates born to overweight (BMI > 25.0) mothers are more likely to become overweight at ages 1 (adjusted odds ratio [OR] 3.8; 95% CI 1.88–7.66) and 3 years of life (adjust OR, 3.79; 95% CI, 2.10–6.84), with neonates delivered via Cesarean to overweight mothers with the greatest odds of becoming overweight later in life [91]. Proponents of seeding Cesarean born infants with maternal microbes recognize the ability of these microbes to modify long-term health outcomes of the neonate. However, in the same vein, it is crucially important to recognize and be cognizant of the possibility of unforeseen negative consequences in the form of propagation of diseases or conditions that predisposed the mother to the Cesarean delivery in the first place. Manipulation of the microbiome without nuanced understanding of the mechanisms and long-term implications of various microbial profiles could produce both ill effects as well as beneficial ones [92]. One solution to the theoretical concerns regarding risks of transmitting a “disease phenotype” microbiome may be to perform these seeding procedures with a defined universal microbiome cocktail (so called ‘defined consortia’) when maternal co-morbidities are present. However, further research is needed to 1) define the inoculum that such a cocktail would include and 2) determine if it would be superior to a neonate’s own maternal microbiome via whatever source and route it naturally occurs.
Finally, there are risks with transplant of any microbiome, though particularly fecal. With proper processing procedures fecal transplant carries low risk of adverse events such as infection [93] though with widespread implementation it may be difficult to ensure appropriate safety standards are met. This concern is particularly worrisome when considering that the recipients are neonates with relatively immature immune systems and risks of compromised barrier integrity.
Rethinking our approach: three evidence-based targets for interventions
Until the long-term effects of the alteration of the microbiome of neonates delivered via Cesarean delivery can be fully determined, it is paramount to also perform meaningful and mechanistic-minded interrogations of the impact of exposures on the functional microbiome over the duration of pregnancy and lactation that have been associated with the mitigation of childhood and adult NCDs. We have identified three areas of attainable clinical intervention during pregnancy and postpartum that we believe are relevant targets for potentially impacting and reducing childhood and adult chronic disease (Figure 3).
Figure 3: Readily modifiable maternal factors which have been shown to impact the infant microbiome.
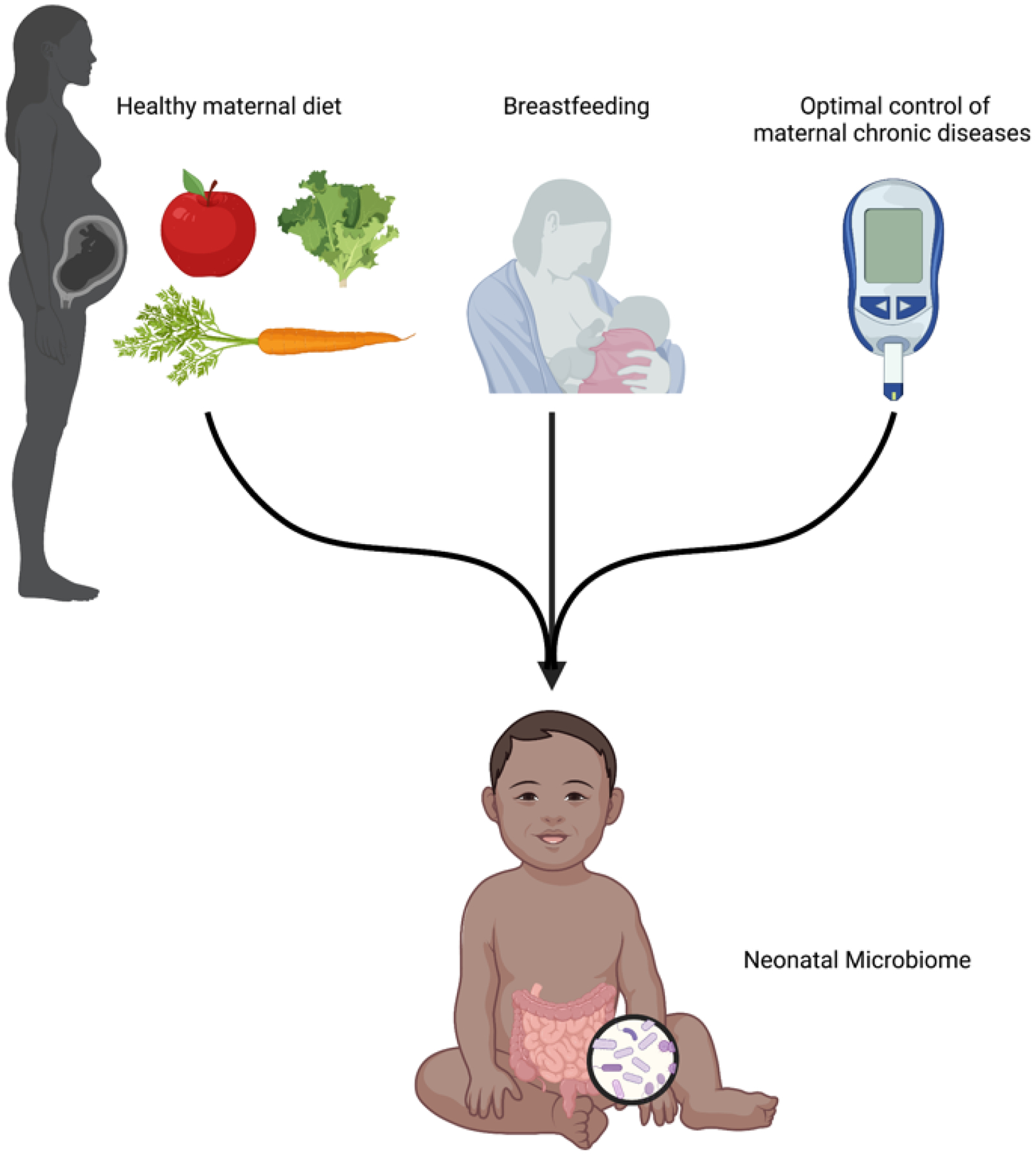
Three readily modifiable maternal factors have been shown to impact and affect the neonatal, infant, and/or early childhood microbiome community structure and function. Notably, some of these same modifiable factors have been shown to alter distinct microbiome communities during both pregnancy and the postpartum lactation period. For example, several lines of evidence in humans and primates have shown that that both a high fat maternal diet and poor glucose control alter the pregnancy and early offspring microbiome. Interestingly, one recent study [104] demonstrated that a high fat or glucose-enriched maternal diet during lactation impacted the milk microbiome function via alterations to human milk oligosaccharide composition.
Breastfeeding.
While there is still ambiguity regarding whether seeding practices at time of Cesarean delivery are beneficial or potentially harmful, there is clear evidence that breastfeeding and improved management of prenatal comorbidities have beneficial effects on the long-term health outcomes of offspring [94,95]. Breastfeeding has limited true medical contraindications in infants with classic galactosemia or in the U.S., mothers with human immunodeficiency virus (HIV) [96]. Some women may face barriers that impact their ability or desire to breastfeed but efforts have been made to reduce these challenges, including federally protected lactation break times and improved access to lactation consultation services [97]. Breastfeeding is linked to numerous long-term health outcomes including lower rates of offspring obesity [94]. There is also evidence that breastfeeding modifies the differential effects seen in the microbiomes of Cesarean and vaginally delivered infants [51] and therefore may neutralize the theoretical risks associated with Cesarean delivery. Women who deliver by Cesarean are less likely to breastfeed than women who deliver vaginally [98]. Thus, differential breastfeeding rates may not only serve as a potential contributor to the difference in chronic disease risk in these two populations but also as an evidence-based potential target of clinical intervention that needs further addressing.
Optimizing maternal nutrition.
In addition to breastfeeding, maternal diet (rather than obesity) itself is a potential target for intervention to mitigate the difference in disease risk seen in Cesarean vs vaginally born infants. A maternal high fat diet in the third trimester has been associated with differences in gut microbiota at delivery and within the first 6 weeks of life independent of mode of delivery, maternal obesity, or breastfeeding status [55]. Therefore, targeted prenatal interventions to support a healthy and nutritionally balanced diet among gravidae have the potential to support the development of a healthy gut microbiome from the neonatal period and beyond. As reviewed earlier, primate work has been crucial in establishing both the durability of the maternal WSD on offspring health, as well as its independence from maternal obesity.
Improved control of maternal co-morbidities and chronic diseases
Lastly, improved management of maternal comorbidities and chronic diseases confers long-term health benefits to both the parent and her offspring. Control of maternal co-morbidities such as diabetes, hypertension, thyroid disease, psychiatric illness, and weight gain both prior to conception and during pregnancy can improve newborn and child health outcomes [99]. Improved glycemic control in diabetic gravidae results in protection against metabolic syndrome in their offspring [100]. Clausen et al. report offspring risk of metabolic syndrome increases significantly with increasing maternal fasting glucose, making improved glucose control a worthwhile goal [95]. Additionally, improved control of maternal chronic conditions like obesity may allow practitioners to affect the Cesarean delivery rate in a meaningful way for both mother and baby. Morbidly obese women with a body mass index (BMI) > 40 kg/m2 are at significantly increased rate of Cesarean delivery [101]. Finally, interventions to support appropriate pregnancy weight gain have the potential to reduce the Cesarean delivery rate itself. Women who gain weight in excess of 2009 Institute of Medicine (IOM) guidelines [102] during their pregnancies have increased risk of Cesarean delivery [103]. Programs to support appropriate weight gain could reduce rates of Cesarean delivery and any associated harms, and we argue such programs are notable venues to direct time and efforts.
Concluding Remarks
While we and others have clearly shown that there is a transient difference in the microbiota of neonates born via Cesarean and vaginal delivery, these changes are limited to a few taxa, are not durable, and appear to resolve during infancy. As such, it is not surprising that meaningful lifelong impacts on the development of the offspring metabolic and immune system have yet to be causally linked. However, potential for reduction of harm readily resides within our reach (See Clinician’s corner). Specifically, if we can reduce the disproportionate disparities that contribute to increased Cesarean delivery risk. For example, with a lens on our diabetic or obese population, based on multiple lines of evidence, we can confidently anticipate three benefits of widened availability of optimized nutrition with relief of food scarcity and unrestricted access to medical care both preconception and prenatally. We would (1) improve glycemic control, optimizing fetal growth, (2) lower the Cesarean delivery rate and increase the duration of human milk feeding, and (3) potentially mitigate the longer-term risks from exposure to these conditions in utero. This heightened attention to maternal perinatal health—like improved glucose control and appropriate weight gain—can be attained with widened access to clinical and public health interventions and patient counseling without incurring any additional risk to mom or fetus. While there are many outstanding questions regarding the neonatal and infant microbiome (see Outstanding questions), we caution that societal focus needs to shift away from a narrow focus on reducing Cesarean delivery rates to meet a certain quota—which as we have demonstrated may be difficult to achieve in today’s society—to optimizing maternal healthcare before, during, and after pregnancy. Additionally, efforts to restore the neonatal and infant microbiome with vaginal or fecal seeding may not only fail to elucidate clearly beneficial outcomes but may in fact be harmful at both an individual and broader societal level. As physicians, our first responsibility is to do no harm. Despite growing popularity of neonatal seeding in the general press, health care practitioners and patients should only perform seeding practices within the confounds of strict scientific protocols to ensure safety [8]. To ultimately improve maternal and neonatal outcomes in association with presumptively beneficial alterations in the microbiome, attention should be directed to established beneficial realms of improved access and availability of preconception and prenatal care, nutritional counseling, lactation services, and limiting food scarcity and other health disparities.
Clinician’s Corner.
In the United States, Cesarean delivery is rarely performed as a purely elective procedure upon maternal request. Access to medically-indicated Cesarean delivery for situations of known maternal or fetal benefit are key to global efforts aimed at reducing health disparities and improving maternal and childhood morbidity and mortality.
In general, the “Cesarean delivery” effect on the offspring microbiome is not durable and involves relative differences in only a few taxa. In addition, initial clinical trials have failed to show the effectiveness or reproducibility of vaginal seeding practices aimed at restoring the neonatal microbiome but further trials are underway. Data on the effects of such practices on short and long-term offspring health outcomes are needed.
At this time, vaginal seeding should not be performed outside the context of an institutional review board-approved research protocol until sufficient data regarding the safety and benefits of the process are available.
In order to ultimately improve maternal and neonatal outcomes in association with presumptively beneficial alterations in the microbiome, attention should be directed to improving prenatal care and expanding access to women’s health care services during pregnancy and beyond.
With the reduction of the burden of comorbidities that contribute to increased Cesarean delivery risk, such as support for appropriate maternal weight gain, maternal dietary and nutritional counseling, and glucose control for diabetic mothers, we can potentially lower not only the Cesarean delivery rate but also mitigate the infant risk from exposure to these conditions in utero.
Additionally, while there remains ambiguity regarding whether seeding practices at time of Cesarean delivery are beneficial or potentially harmful based on currently available data, there is clear evidence that breastfeeding and improved management of prenatal comorbidities have beneficial effects on the long-term health outcome of offspring.
Outstanding Questions bullet points.
Are there true and lasting differences in the microbiomes of Cesarean and vaginally born infants and, if present, are such differences functionally meaningful?
How does the maternal microbiome and its metabolites influence the microbiota of the developing fetus?
Is the fetus merely exposed to microorganisms, or is the developing fetus truly colonized in utero?
How do low biomass communities, such as has been reported in the placenta, the amniotic fluid, the fetus, and the breastmilk, remain as low biomass? What keeps them “pruned”, and prevents them from developing into higher biomass communities? Is this important for developing fetal or neonatal immunity?
Are these low biomass communities important for preventing colonization with pathogens, including pathogenic bacteria or viruses? In the mom, the infant, or both?
At what time point in the developmental process does disruption to the microbiome result in a non-communicable disease state? Is this disease state later modifiable, or is it largely irreversible?
Highlights.
Increasing rates of Cesarean deliveries and common non-communicable disorders (NCDs) in children, such as obesity and metabolic disease, have resulted in attempts to uncover a causal link between the two.
One line of research argues that being born via Cesarean rather than vaginal delivery detrimentally alters the neonatal microbiome in a way that could potentially predispose the neonate to future NCDs.
In general, the effect on the offspring microbiome is not durable and involves relative differences in only a few taxa. In addition, initial clinical trials have failed to show the effectiveness or reproducibility of vaginal seeding practices aimed at restoring the neonatal microbiome but further trials are underway. Data on the effects of such practices on short and long-term offspring health outcomes are needed.
Only with a greater focus on the effects of maternal and fetal exposures during pregnancy and lactation will we discern true causal drivers of childhood and adult NCDs.
In the United States, Cesarean delivery is rarely performed as a purely elective procedure upon maternal request. Access to medically-indicated Cesarean delivery for situations of known maternal or fetal benefit are key to global efforts aimed at reducing health disparities and improving maternal and childhood morbidity and mortality.
Acknowledgements
Figures created with Biorender.com. Alexa Sassin is supported by the Women’s Reproductive Health Research program (WRHR) (K12 HD103087). Additional support is provided, in part, by NICHD Grant R01HD091731 (PI K.M. Aagaard)
Glossary
- ACOG
the American College of Obstetricians and Gynecologists, the professional membership organization providing evidence-based practice guidelines that serve as the standard of care in the field of Obstetrics and Gynecology.
- Chorioamnionitis
an acute inflammation of the membranes and chorion of the placenta, typically due to ascending bacterial infections, and associated with preterm labor and adverse neonatal outcomes.
- Fetal intolerance to labor
an abnormality in fetal heart rate and rhythm complicating labor and delivery that indicates fetal distress and is a frequent indication for Cesarean delivery.
- Gestational age
completed weeks of development in utero.
- Intrapartum antibiotic prophylaxis
administration of antibiotics (4 hours of penicillin or ampicillin is the gold standard) during labor and delivery to women colonized with group B Streptococci (GBS) or women with unknown GBS status who deliver preterm, have prolonged membrane rupture, or intrapartum fever.
- Intrapartum stillbirth
fetal death occurring after the onset of labor and prior to delivery.
- Metagenomics
sequencing of multiple small sections of microbial genomes followed by bioinformatics analysis to characterize the collection of genes in a given microbial community.
- Microbiota
a community formed by bacteria, fungi, and viruses of commensal, symbiotic, and pathogenic microorganisms that share similar spaces or body organs.
- Microbiome
all the genes and gene products like RNA, proteins, and metabolites produced by the microbiota.
- Neonatal Intensive Care Unit (NICU)
a hospital intensive care unit that specializes in providing medical care for high-risk newborns who are preterm, critically ill, or have a life-threatening health condition.
- Noncommunicable diseases (NCDs)
a group of conditions that are not transmissible directly from one person to another. They include most heart disease, stroke, most cancers, diabetes, chronic kidney disease, among others.
- Obstructed labor
labor where there is poor or no progress (in the form of maternal cervical dilation or fetal descent into the pelvis) despite adequate uterine contractions.
- Operational taxonomic units
an operational definition used to classify groups of closely related individuals, often used to classify bacteria based on sequence similarity of the 16S marker gene.
- Pathobionts
microbes with the potential to cause harm, or disease under certain circumstances. This term is often used for categorizing disease-associated taxa without proof of causality.
- Seeding
an experimental practice of inoculating a cotton gauze or a cotton swab with vaginal fluids to transfer the vaginal flora to the mouth, nose, or skin of a newborn infant; can also include oral administration of maternal vaginal or stool microbes.
- Xenobiotics
chemical substances that are foreign to the body or an ecological system
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
No interests to disclose
References
- 1.Barker D (1986) infant mortality, childhood nutrition, and ischaemic heart disease in england and wales. The Lancet 327, 1077–1081. 10.1016/s0140-6736(86)91340-1 [DOI] [PubMed] [Google Scholar]
- 2.Fleming TP et al. (2018) Origins of lifetime health around the time of conception: causes and consequences. Lancet 391, 1842–1852. 10.1016/S0140-6736(18)30312-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Safi-Stibler S and Gabory A (2020) Epigenetics and the Developmental Origins of Health and Disease: Parental environment signalling to the epigenome, critical time windows and sculpting the adult phenotype. Semin Cell Dev Biol 97, 172–180. 10.1016/j.semcdb.2019.09.008 [DOI] [PubMed] [Google Scholar]
- 4.Aagaard-Tillery KM et al. (2008) Developmental origins of disease and determinants of chromatin structure: maternal diet modifies the primate fetal epigenome. J Mol Endocrinol 41, 91–102. 10.1677/JME-08-0025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blaser MJ (2006) Who are we? Indigenous microbes and the ecology of human diseases. EMBO Rep 7, 956–960. 10.1038/sj.embor.7400812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dominguez-Bello MG et al. (2016) Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer. Nat Med 22, 250–253. 10.1038/nm.4039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Korpela K et al. (2020) Maternal Fecal Microbiota Transplantation in Cesarean-Born Infants Rapidly Restores Normal Gut Microbial Development: A Proof-of-Concept Study. Cell 183, 324–334 e325. 10.1016/j.cell.2020.08.047 [DOI] [PubMed] [Google Scholar]
- 8.(2017) ACOG Committee Opinion No 725: Vaginal Seeding, Committee on Obstetric Practice. Obstet Gynecol 130, e274. [DOI] [PubMed] [Google Scholar]
- 9.American College of, O. et al. (2014) Safe prevention of the primary cesarean delivery. Am J Obstet Gynecol 210, 179–193. 10.1016/j.ajog.2014.01.026 [DOI] [PubMed] [Google Scholar]
- 10.Clark SL et al. (2018) “Doing something” about the cesarean delivery rate. Am J Obstet Gynecol 219, 267–271. 10.1016/j.ajog.2018.04.044 [DOI] [PubMed] [Google Scholar]
- 11.(2019) ACOG Practice Bulletin No. 205: Vaginal Birth After Cesarean Delivery. Obstet Gynecol 133, e110–e127. 10.1097/AOG.0000000000003078 [DOI] [PubMed] [Google Scholar]
- 12.(2006) NIH State-of-the-Science Conference Statement on cesarean delivery on maternal request. NIH Consens State Sci Statements 23, 1–29 [PubMed] [Google Scholar]
- 13.(2019) ACOG Committee Opinion No. 761: Cesarean Delivery on Maternal Request. Obstet Gynecol 133, e73–e77. 10.1097/AOG.0000000000003006 [DOI] [PubMed] [Google Scholar]
- 14.Gossman GL et al. (2006) Trends in maternal request cesarean delivery from 1991 to 2004. Obstet Gynecol 108, 1506–1516. 10.1097/01.AOG.0000242564.79349.b7 [DOI] [PubMed] [Google Scholar]
- 15.Glaser LM et al. (2017) Trends in malpractice claims for obstetric and gynecologic procedures, 2005 through 2014. Am J Obstet Gynecol 217, 340 e341–340 e346. 10.1016/j.ajog.2017.05.037 [DOI] [PubMed] [Google Scholar]
- 16.Yuan C et al. (2016) Association Between Cesarean Birth and Risk of Obesity in Offspring in Childhood, Adolescence, and Early Adulthood. JAMA Pediatr 170, e162385. 10.1001/jamapediatrics.2016.2385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Darmasseelane K et al. (2014) Mode of delivery and offspring body mass index, overweight and obesity in adult life: a systematic review and meta-analysis. PLoS One 9, e87896. 10.1371/journal.pone.0087896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li HT et al. (2013) The impact of cesarean section on offspring overweight and obesity: a systematic review and meta-analysis. Int J Obes (Lond) 37, 893–899. 10.1038/ijo.2012.195 [DOI] [PubMed] [Google Scholar]
- 19.Chavarro JE et al. (2020) Association of Birth by Cesarean Delivery With Obesity and Type 2 Diabetes Among Adult Women. JAMA Netw Open 3, e202605. 10.1001/jamanetworkopen.2020.2605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sevelsted A et al. (2015) Cesarean section and chronic immune disorders. Pediatrics 135, e92–98. 10.1542/peds.2014-0596 [DOI] [PubMed] [Google Scholar]
- 21.Thavagnanam S et al. (2008) A meta-analysis of the association between Caesarean section and childhood asthma. Clin Exp Allergy 38, 629–633. 10.1111/j.1365-2222.2007.02780.x [DOI] [PubMed] [Google Scholar]
- 22.Masukume G et al. (2019) Caesarean section delivery and childhood obesity in a British longitudinal cohort study. PLOS ONE 14, e0223856. 10.1371/journal.pone.0223856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bruce A et al. (2014) Mode of delivery and risk of inflammatory bowel disease in the offspring: systematic review and meta-analysis of observational studies. Inflamm Bowel Dis 20, 1217–1226. 10.1097/MIB.0000000000000075 [DOI] [PubMed] [Google Scholar]
- 24.Koletzko S et al. (2018) Cesarean Section on the Risk of Celiac Disease in the Offspring: The Teddy Study. J Pediatr Gastroenterol Nutr 66, 417–424. 10.1097/MPG.0000000000001682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahlqvist VH et al. (2019) Elective and nonelective cesarean section and obesity among young adult male offspring: A Swedish population-based cohort study. Plos Med 16. ARTN e1002996 0.1371/journal.pmed.1002996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rifas-Shiman SL et al. (2018) Association of Cesarean Delivery With Body Mass Index z Score at Age 5 Years. JAMA Pediatr 172, 777–779. 10.1001/jamapediatrics.2018.0674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masukume G et al. (2018) The Impact of Caesarean Section on the Risk of Childhood Overweight and Obesity: New Evidence from a Contemporary Cohort Study. Sci Rep 8, 15113. 10.1038/s41598-018-33482-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masukume G et al. (2019) Association between caesarean section delivery and obesity in childhood: a longitudinal cohort study in Ireland. BMJ Open 9, e025051. 10.1136/bmjopen-2018-025051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Almqvist C et al. (2012) The impact of birth mode of delivery on childhood asthma and allergic diseases--a sibling study. Clin Exp Allergy 42, 1369–1376. 10.1111/j.1365-2222.2012.04021.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lokugamage AU and Pathberiya SDC (2019) The microbiome seeding debate - let’s frame it around women-centred care. Reprod Health 16, 91. 10.1186/s12978-019-0747-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stinson LF et al. (2018) A Critical Review of the Bacterial Baptism Hypothesis and the Impact of Cesarean Delivery on the Infant Microbiome. Front Med (Lausanne) 5, 135. 10.3389/fmed.2018.00135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelly JC et al. (2021) Vaginal seeding after cesarean birth: Can we build a better infant microbiome? Med (N Y) 2, 889–891. 10.1016/j.medj.2021.07.003 [DOI] [PubMed] [Google Scholar]
- 33.Fricke WF and Ravel J (2022) More data needed on neonatal microbiome seeding. Microbiome 10, 88. 10.1186/s40168-022-01282-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang L (2006). Elective Cesarean: Babies On Demand. Grow by WebMD [Google Scholar]
- 35.Molloy A (2015) Mothers facing C-sections look to vaginal ‘seeding’ to boost their babies’ health. The Guardian Published online 8/17/2015 [Google Scholar]
- 36.Huynh J et al. (2018) Potential Transmission of Herpes Simplex Virus Via Vaginal Seeding. Pediatr Infect Dis J 37, e278. 10.1097/INF.0000000000001965 [DOI] [PubMed] [Google Scholar]
- 37.Wilson BC et al. (2021) Oral administration of maternal vaginal microbes at birth to restore gut microbiome development in infants born by caesarean section: A pilot randomised placebo-controlled trial. EBioMedicine 69, 103443. 10.1016/j.ebiom.2021.103443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Song SJ et al. (2021) Naturalization of the microbiota developmental trajectory of Cesarean-born neonates after vaginal seeding. Med (N Y) 2, 951–964 e955. 10.1016/j.medj.2021.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roswall J et al. (2021) Developmental trajectory of the healthy human gut microbiota during the first 5 years of life. Cell Host Microbe 29, 765–776 e763. 10.1016/j.chom.2021.02.021 [DOI] [PubMed] [Google Scholar]
- 40.Biasucci G et al. (2008) Cesarean Delivery May Affect the Early Biodiversity of Intestinal Bacteria. The Journal of Nutrition 138, 1796S–1800S. 10.1093/jn/138.9.1796S [DOI] [PubMed] [Google Scholar]
- 41.Azad MB et al. (2013) Gut microbiota of healthy Canadian infants: profiles by mode of delivery and infant diet at 4 months. CMAJ 185, 385–394. 10.1503/cmaj.121189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murgas Torrazza R and Neu J (2011) The developing intestinal microbiome and its relationship to health and disease in the neonate. J Perinatol 31 Suppl 1, S29–34. 10.1038/jp.2010.172 [DOI] [PubMed] [Google Scholar]
- 43.Chu DM et al. (2017) Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat Med 23, 314–326. 10.1038/nm.4272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chu DM et al. (2019) The Development of the Human Microbiome. Gastroenterology Clinics of North America 48, 357–375. 10.1016/j.gtc.2019.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yassour M et al. (2016) Natural history of the infant gut microbiome and impact of antibiotic treatment on bacterial strain diversity and stability. Sci Transl Med 8, 343ra381. 10.1126/scitranslmed.aad0917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Turnbaugh PJ et al. (2009) A core gut microbiome in obese and lean twins. Nature 457, 480–484. 10.1038/nature07540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Round JL and Mazmanian SK (2009) The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol 9, 313–323. 10.1038/nri2515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Valentine G et al. (2019) The Neonatal Microbiome and Metagenomics: What Do We Know and What Is the Future? Neoreviews 20, e258–e271. 10.1542/neo.20-5-e258 [DOI] [PubMed] [Google Scholar]
- 49.Butel MJ et al. (2007) Conditions of bifidobacterial colonization in preterm infants: a prospective analysis. J Pediatr Gastroenterol Nutr 44, 577–582. 10.1097/MPG.0b013e3180406b20 [DOI] [PubMed] [Google Scholar]
- 50.Pammi M et al. (2017) Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: a systematic review and meta-analysis. Microbiome 5, 31. 10.1186/s40168-017-0248-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Azad MB et al. (2016) Impact of maternal intrapartum antibiotics, method of birth and breastfeeding on gut microbiota during the first year of life: a prospective cohort study. BJOG 123, 983–993. 10.1111/1471-0528.13601 [DOI] [PubMed] [Google Scholar]
- 52.Rogier EW et al. (2014) Secretory antibodies in breast milk promote long-term intestinal homeostasis by regulating the gut microbiota and host gene expression. Proc Natl Acad Sci U S A 111, 3074–3079. 10.1073/pnas.1315792111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ma J et al. (2014) High-fat maternal diet during pregnancy persistently alters the offspring microbiome in a primate model. Nat Commun 5, 3889. 10.1038/ncomms4889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Troy EB and Kasper DL (2010) Beneficial effects of Bacteroides fragilis polysaccharides on the immune system. Front Biosci (Landmark Ed) 15, 25–34. 10.2741/3603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chu DM et al. (2016) The early infant gut microbiome varies in association with a maternal high-fat diet. Genome Med 8, 77. 10.1186/s13073-016-0330-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jost T et al. (2012) New insights in gut microbiota establishment in healthy breast fed neonates. PLoS One 7, e44595. 10.1371/journal.pone.0044595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Banerjee S et al. (2020) Interactions between Environmental Exposures and the Microbiome: Implications for Fetal Programming. Curr Opin Endocr Metab Res 13, 39–48. 10.1016/j.coemr.2020.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Claus SP et al. (2016) The gut microbiota: a major player in the toxicity of environmental pollutants? NPJ Biofilms Microbiomes 2, 16003. 10.1038/npjbiofilms.2016.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jasarevic E and Bale TL (2019) Prenatal and postnatal contributions of the maternal microbiome on offspring programming. Front Neuroendocrinol 55, 100797. 10.1016/j.yfrne.2019.100797 [DOI] [PubMed] [Google Scholar]
- 60.Aagaard K et al. (2014) The placenta harbors a unique microbiome. Sci Transl Med 6, 237ra265. 10.1126/scitranslmed.3008599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bassols J et al. (2016) Gestational diabetes is associated with changes in placental microbiota and microbiome. Pediatric Research 80, 777–784. 10.1038/pr.2016.155 [DOI] [PubMed] [Google Scholar]
- 62.Collado MC et al. (2016) Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Sci Rep 6, 23129. 10.1038/srep23129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Doyle RM et al. (2014) Term and preterm labour are associated with distinct microbial community structures in placental membranes which are independent of mode of delivery. Placenta 35, 1099–1101. 10.1016/j.placenta.2014.10.007 [DOI] [PubMed] [Google Scholar]
- 64.Doyle RM et al. (2017) Bacterial communities found in placental tissues are associated with severe chorioamnionitis and adverse birth outcomes. PLOS ONE 12, e0180167. 10.1371/journal.pone.0180167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gomez-Arango LF et al. (2017) Contributions of the maternal oral and gut microbiome to placental microbial colonization in overweight and obese pregnant women. Scientific Reports 7, 2860. 10.1038/s41598-017-03066-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Parnell LA et al. (2017) Microbial communities in placentas from term normal pregnancy exhibit spatially variable profiles. Scientific Reports 7, 11200. 10.1038/s41598-017-11514-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Prince AL et al. (2016) The placental membrane microbiome is altered among subjects with spontaneous preterm birth with and without chorioamnionitis. American Journal of Obstetrics and Gynecology 214, 627.e621–627.e616. 10.1016/j.ajog.2016.01.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Satokari R et al. (2009) Bifidobacterium and Lactobacillus DNA in the human placenta. Lett Appl Microbiol 48, 8–12. 10.1111/j.1472-765X.2008.02475.x [DOI] [PubMed] [Google Scholar]
- 69.Seferovic MD et al. (2019) Visualization of microbes by 16S in situ hybridization in term and preterm placentas without intraamniotic infection. American Journal of Obstetrics and Gynecology 221, 146.e141–146.e123. 10.1016/j.ajog.2019.04.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tuominen H et al. (2018) HPV infection and bacterial microbiota in the placenta, uterine cervix and oral mucosa. Scientific Reports 8, 9787. 10.1038/s41598-018-27980-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zheng J et al. (2015) The Placental Microbiome Varies in Association with Low Birth Weight in Full-Term Neonates. Nutrients 7, 6924–6937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dong XD et al. (2015) Bacterial communities in neonatal feces are similar to mothers’ placentae. Can J Infect Dis Med Microbiol 26, 90–94. 10.1155/2015/737294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gosalbes MJ et al. (2013) Meconium microbiota types dominated by lactic acid or enteric bacteria are differentially associated with maternal eczema and respiratory problems in infants. Clin Exp Allergy 43, 198–211. 10.1111/cea.12063 [DOI] [PubMed] [Google Scholar]
- 74.Chen HJ and Gur TL (2019) Intrauterine Microbiota: Missing, or the Missing Link? Trends Neurosci 42, 402–413. 10.1016/j.tins.2019.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Petersen C et al. (2021) A rich meconium metabolome in human infants is associated with early-life gut microbiota composition and reduced allergic sensitization. Cell Rep Med 2. ARTN 100260 10.1016/j.xcrm.2021.100260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sterpu I et al. (2021) No evidence for a placental microbiome in human pregnancies at term. Am J Obstet Gynecol 224, 296 e291–296 e223. 10.1016/j.ajog.2020.08.103 [DOI] [PubMed] [Google Scholar]
- 77.Kuperman AA et al. (2020) Deep microbial analysis of multiple placentas shows no evidence for a placental microbiome. BJOG 127, 159–169. 10.1111/1471-0528.15896 [DOI] [PubMed] [Google Scholar]
- 78.de Goffau MC et al. (2019) Human placenta has no microbiome but can contain potential pathogens. Nature 572, 329–334. 10.1038/s41586-019-1451-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lager S et al. (2018) Detecting eukaryotic microbiota with single-cell sensitivity in human tissue. Microbiome 6, 151. 10.1186/s40168-018-0529-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lauder AP et al. (2016) Comparison of placenta samples with contamination controls does not provide evidence for a distinct placenta microbiota. Microbiome 4, 29. 10.1186/s40168-016-0172-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Leiby JS et al. (2018) Lack of detection of a human placenta microbiome in samples from preterm and term deliveries. Microbiome 6, 196. 10.1186/s40168-018-0575-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Leon LJ et al. (2018) Enrichment of Clinically Relevant Organisms in Spontaneous Preterm-Delivered Placentas and Reagent Contamination across All Clinical Groups in a Large Pregnancy Cohort in the United Kingdom. Appl Environ Microbiol 84. 10.1128/AEM.00483-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Theis KR et al. (2019) Does the human placenta delivered at term have a microbiota? Results of cultivation, quantitative real-time PCR, 16S rRNA gene sequencing, and metagenomics. American Journal of Obstetrics and Gynecology 220, 267.e261–267.e239. 10.1016/j.ajog.2018.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen X and Devaraj S (2018) Gut Microbiome in Obesity, Metabolic Syndrome, and Diabetes. Curr Diab Rep 18, 129. 10.1007/s11892-018-1104-3 [DOI] [PubMed] [Google Scholar]
- 85.Thaiss CA et al. (2016) The microbiome and innate immunity. Nature 535, 65–74. 10.1038/nature18847 [DOI] [PubMed] [Google Scholar]
- 86.Turnbaugh PJ et al. (2006) An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1031. 10.1038/nature05414 [DOI] [PubMed] [Google Scholar]
- 87.Mueller NT et al. (2021) Association of birth mode of delivery with infant faecal microbiota, potential pathobionts, and short chain fatty acids: a longitudinal study over the first year of life. BJOG 128, 1293–1303. 10.1111/1471-0528.16633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shao Y et al. (2019) Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth. Nature 574, 117–121. 10.1038/s41586-019-1560-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Declercq E et al. (2006) Maternal risk profiles and the primary cesarean rate in the United States, 1991–2002. Am J Public Health 96, 867–872. 10.2105/AJPH.2004.052381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Spong CY et al. (2012) Preventing the First Cesarean Delivery: Summary of a Joint: Eunice Kennedy Shriver: National Institute of Child Health and Human Development, Society for Maternal-Fetal Medicine, and American College of Obstetricians and Gynecologists Workshop. Obstetrics & Gynecology 120, 1181–1193. http:// 10.1097/AOG.0b013e3182704880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tun HM et al. (2018) Roles of Birth Mode and Infant Gut Microbiota in Intergenerational Transmission of Overweight and Obesity From Mother to Offspring. JAMA Pediatr 172, 368–377. 10.1001/jamapediatrics.2017.5535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Aagaard K and Hohmann E (2019) Regulating microbiome manipulation. Nat Med 25, 874–876. 10.1038/s41591-019-0451-1 [DOI] [PubMed] [Google Scholar]
- 93.Cammarota G et al. (2019) International consensus conference on stool banking for faecal microbiota transplantation in clinical practice. Gut 68, 2111. 10.1136/gutjnl-2019-319548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ma J et al. (2020) Breastfeeding and childhood obesity: A 12-country study. Matern Child Nutr 16, e12984. 10.1111/mcn.12984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Clausen TD et al. (2009) Overweight and the metabolic syndrome in adult offspring of women with diet-treated gestational diabetes mellitus or type 1 diabetes. J Clin Endocrinol Metab 94, 2464–2470. 10.1210/jc.2009-0305 [DOI] [PubMed] [Google Scholar]
- 96.(AAP), A.A.o.P. (2021). Breastfeeding Overview.
- 97.Prevention, C.f.D.C.a. (2022). Breastfeeding Strategies.
- 98.Hobbs AJ et al. (2016) The impact of caesarean section on breastfeeding initiation, duration and difficulties in the first four months postpartum. BMC Pregnancy Childbirth 16, 90. 10.1186/s12884-016-0876-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lassi ZS et al. (2014) Preconception care: screening and management of chronic disease and promoting psychological health. Reprod Health 11 Suppl 3, S5. 10.1186/1742-4755-11-S3-S5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Aerts L and Van Assche FA (2006) Animal evidence for the transgenerational development of diabetes mellitus. Int J Biochem Cell Biol 38, 894–903. 10.1016/j.biocel.2005.07.006 [DOI] [PubMed] [Google Scholar]
- 101.Machado LS (2012) Cesarean section in morbidly obese parturients: practical implications and complications. N Am J Med Sci 4, 13–18. 10.4103/1947-2714.92895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.(2009) In Weight Gain During Pregnancy: Reexamining the Guidelines (Rasmussen KM and Yaktine AL, eds), [PubMed] [Google Scholar]
- 103.Goldstein RF et al. (2017) Association of Gestational Weight Gain With Maternal and Infant Outcomes: A Systematic Review and Meta-analysis. JAMA 317, 2207–2225. 10.1001/jama.2017.3635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Seferovic MD et al. (2020) Maternal diet alters human milk oligosaccharide composition with implications for the milk metagenome. Sci Rep 10, 22092. 10.1038/s41598-020-79022-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Resources
- I). This study is registered with ClinicalTrials.gov. For more information please see https://clinicaltrials.gov/ct2/show/NCT04173208.
- II). This study is registered with ClinicalTrials.gov. For more information please see https://clinicaltrials.gov/ct2/show/NCT03298334.
- III). This study is registered with ClinicalTrials.gov. For more information please see https://clinicaltrials.gov/ct2/show/NCT03567707.
- IV). This study is registered with ClinicalTrials.gov. For more information please see https://clinicaltrials.gov/ct2/show/NCT03809390.


