Abstract
Background & Aims:
The use of proton pump inhibitors (PPIs) has rapidly increased in the past two decades. Concerns about the regular use of PPIs contributing to mortality have been raised.
Methods:
We conducted a prospective cohort study using data collected from the Nurses’ Health Study (2004–2018) and the Health Professionals Follow-up Study (2004–2018). Cox proportional hazards models were used to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) for mortality according to PPI use. We utilized a modified lag-time approach to minimize reverse causation (i.e., protopathic bias).
Results:
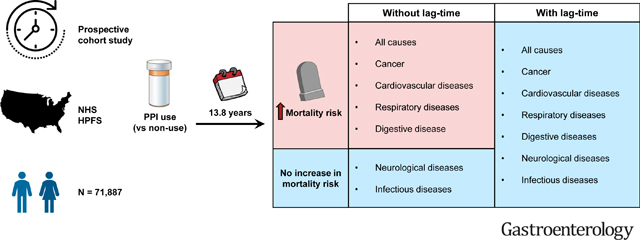
Among 50,156 women and 21,731 men followed for 831,407 person-years and a median of 13.8 years, we documented 22,125 deaths, including 4,592 deaths from cancer, 5,404 from cardiovascular diseases, and 12,129 deaths from other causes. Compared to non-users of PPIs, PPI users had significantly higher risks of all-cause mortality and mortality due to cancer, cardiovascular diseases, respiratory diseases, and digestive diseases. Upon applying lag-times of up to six years, the associations were attenuated and no longer statistically significant (all-cause: HR, 1.04; 95% CI, 0.97–1.11; cancer: HR, 1.07; 95% CI, 0.89–1.28; cardiovascular diseases: HR, 0.94; 95% CI, 0.81–1.10; respiratory diseases: HR, 1.20; 95% CI, 0.95–1.50; and digestive diseases: HR, 1.38; 95% CI, 0.88–2.18). Longer duration of PPI use did not confer higher risks of all-cause and cause-specific mortality.
Conclusions:
After accounting for protopathic bias, PPI use was not associated with higher risks of all-cause mortality and mortality due to major causes.
Keywords: Antacid, Death, Epidemiology, GERD, Medication
LAY SUMMARY
Proton pump inhibitor use was not associated with higher risks of death after accounting for reverse causation. Longer duration of PPI use did not confer higher mortality risks.
Graphical Abstract
INTRODUCTION
Proton pump inhibitors (PPIs) are commonly used medications, available either by prescription or over-the-counter in the United States (US). These agents inhibit gastric acid secretion and are often used to treat acid-mediated upper gastrointestinal disorders and for prophylaxis against stress ulcers.1 There has been a constant increase in the usage of PPIs over the past few decades. For instance, the use of prescription PPIs alone doubled from 1999 to 2012 in the US.2 Such growth is related to both the medication’s proven efficacy for approved indications and the overuse of potentially inappropriate prescriptions.3 Along with the increase in PPI use, there is growing concern about possible long-term adverse outcomes.
Accumulating evidence from numerous studies examining PPI-related adverseevents has gained attention over the past two decades.4 Some studies have suggested an association with mortality,5–18 although most have been restricted to selected patient populations.9–18 It is unclear whether PPI use is associated with higher mortality risk in the general population. Furthermore, one major challenge pharmacoepidemiologic studies often face is the susceptibility to protopathic bias. Protopathic bias occurs when a pharmaceutical agent is prescribed for an early manifestation of a disease which then appears to cause the disease when it is eventually diagnosed.19 In the case of PPIs and mortality, individuals who use PPIs in response to upper gastrointestinal symptoms are more likely to have comorbidities and, as a result, die from these medical conditions. Although randomized controlled trials may address this type of bias, they are often restricted by ethical concerns, sample size, cost, and length of follow-up. One approach to account for this bias in non-experimental studies is to incorporate lag-times into the exposure definition.19,20 Using this approach, any increased PPI use during the excluded period, which could be due to comorbid conditions prior to death, will not be considered in the quantification of the exposure, and thus, protopathic bias would be avoided.
Here, we utilized a modified lag-time approach to investigate the association between PPI use and mortality in the general population. Data were collected from two large prospective cohorts in which detailed information about medication use, lifestyle, and medical conditions has been periodically updated over long-term follow-up. These cohorts also provide a unique opportunity to examine PPI use over a range of durations in relation to the risks of all-cause and cause-specific mortality.
METHODS
Study population
We included participants from two ongoing US prospective cohorts. The Nurses’ Health Study (NHS) recruited 121,700 female registered nurses aged 30 to 55 years in 1976.21 The Health Professionals Follow-up Study (HPFS) enrolled 51,529 male health professionals aged 40 to 75 years in 1986.22 In both cohorts, questionnaires were mailed to participants at enrollment and every two years thereafter to obtain information on various lifestyle factors, medication use, and medical history. Diet was assessed using validated semi-quantitative food frequency questionnaires (SFFQs) beginning in 1980 in the NHS and 1986 in the HPFS, and updated every four years.23,24
For the current study, we used 2004 as the baseline for both cohorts when information on duration of prior PPI use was collected. We excluded participants who reported prior PPI use before the start of follow-up or had been diagnosed with upper gastrointestinal diseases, including gastroesophageal reflux disease, Barrett’s esophagus, peptic ulcer disease, and gastrointestinal bleeding. After these exclusions, the final analytic cohort included 50,156 women and 21,731 men. The study protocol was approved by the Institutional Review Boards of the Brigham and Women’s Hospital and the Harvard T.H. Chan School of Public Health.
Assessment of proton pump inhibitor use and histamine-2 receptor antagonist use
Information about PPI use was obtained from the biennial follow-up questionnaires starting in 2000 in the NHS and 2004 in the HPFS and updated every two years. Participants were asked about regular use of PPIs in the past two years. While examples of brand names were provided for reference (e.g., Prilosec, Nexium, Prevacid, Protonix, Aciphex), specific information on the brand, dose, and schedule was not specifically queried. Duration of prior use (0–2 years, 3–5 years, 6–9 years, ≥10 years) was asked in the 2004 questionnaire in both cohorts. Those who completed the medication section but did not report regular PPI use were considered non-users for that two-year period. If a participant did not return the questionnaire for a follow-up cycle, we carried forward PPI use status from the previous cycle. Duration of PPI use was additionally calculated through the sum of PPI use from baseline until the most recent cycle and categorized into non-users, 1–2 years, 3–4 years, 5–6 years, and ≥7 years. Similarly, participants were asked about regular use of histamine-2 receptor antagonists (H2RAs) in the past two years in both cohorts with examples of brand names provided for reference (e.g., Tagamet, Zantac, Pepcid).
Ascertainment of death
The main outcome was death from all and different causes, occurring after the return of the 2004 questionnaire and before June 1, 2018. Deaths were usually reported by next of kin, the postal system, or identified by searching the National Death Index.25,26 The cause of death was ascertained by review of death certificates and pertinent medical records. Deaths were grouped into several broad categories according to the International Classification of Diseases, Eighth Revision (ICD-8), including cancer (ICD-8 codes 140–209); cardiovascular diseases (ICD-8 codes 390–458); respiratory diseases (ICD-8 codes 460–519); digestive diseases (ICD-8 codes 520–577); renal diseases (ICD-8 codes 580–593); neurological diseases (ICD-8 codes 580–593) (most cases could be attributed to dementia); and infectious diseases (ICD-8 codes 0–140). Consistent with prior analysis, the cause of death due to cancer was further subdivided into common causes of cancer-specific death with sufficient sample size for analysis, including lung cancer, upper gastrointestinal cancer (including cancer of the esophagus, stomach, and small intestine), colorectal cancer, non-Hodgkin’s lymphoma, breast cancer, and ovarian cancer.27
Assessment of covariates
Information on demographic and lifestyle characteristics, including smoking status, body mass index (BMI), physical activity, non-steroidal anti-inflammatory drug (NSAID) use, and medical history, was assessed using the biennial questionnaires. Data on the Alternate Healthy Eating Index-2010 (AHEI-2010) and alcohol intake were collected using SFFQs.
Statistical analysis
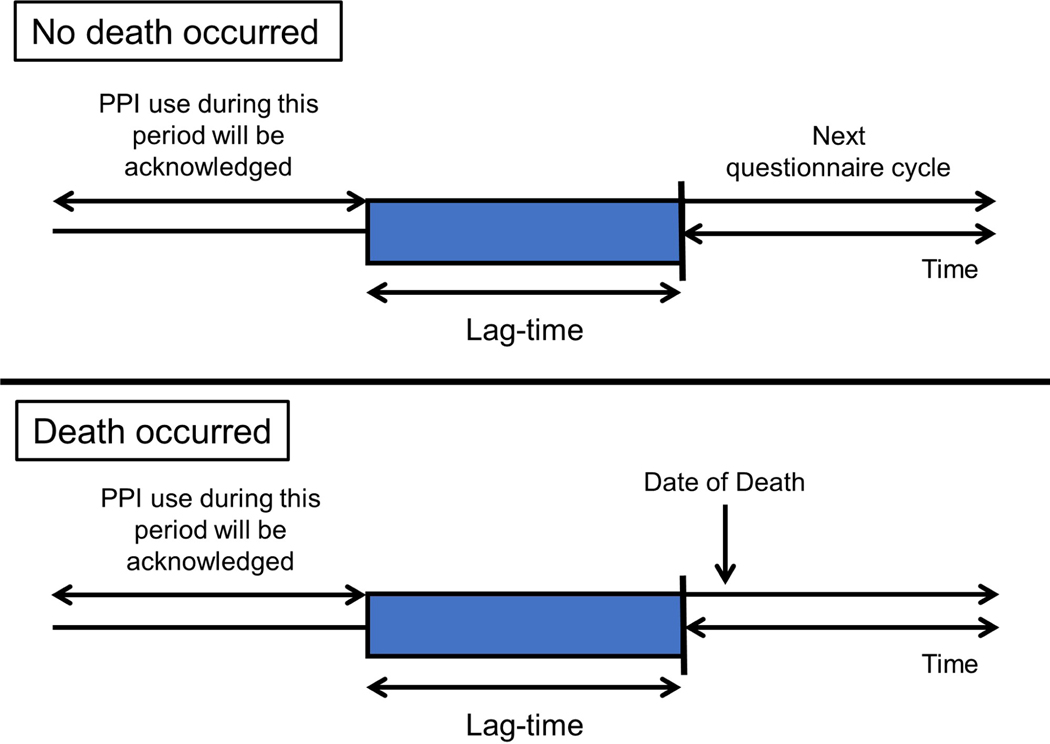
Person-years were accrued from the date of return of the baseline questionnaire to the date of death or the end of follow-up (June 1, 2018 for both cohorts), whichever occurred first. We employed Cox proportional hazards models stratified by age, cohort, and questionnaire cycle and adjusted for confounders to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) for mortality comparing PPI use and non-use. Confounders selected a priori included smoking status, BMI, physical activity, AHEI-2010, alcohol intake, regular NSAID use, H2RA use in the past, history of cancer, myocardial infarction, stroke, hypertension, diabetes mellites, hypercholesterolemia, gastroesophageal reflux disease, Barrett’s esophagus, peptic ulcer disease, gastrointestinal bleeding, and chronic obstructive pulmonary disease. To minimize the influence of protopathic bias, we utilized a modified lag-time approach which was adapted from prior studies.19,20 Instead of excluding from PPI use assessment the period preceding the index date, a period before each questionnaire cycle was excluded from the assessment of PPI use. We considered a two-year, a four-year, and a six-year lag-time based on the structure of our data (two-year interval). For example, in a two-year lag-time analysis, we used the exposure status in 2004 to model mortality risks starting in 2006; in a four-year lag-time analysis, we used the exposure status in 2004 to model mortality risks starting in 2008, and so on. Figure 1 illustrates this approach. To assess the cumulative exposure of PPIs, we examined the associations between duration of PPI use and mortality risks. The models were adjusted for the same confounders listed above.
Figure 1. The lag-time approach.
A period preceding each questionnaire cycle was excluded from the assessment of PPI use. Abbreviations: PPI, proton pump inhibitor.
For sensitivity analysis, we excluded participants who reported H2RA use prior to the start of follow-up and applied an active-comparator study design.28 First, we estimated the HRs and 95% CIs for mortality comparing PPI use, H2RA use, and non-use of both medications. Second, we compared the mortality risks between PPI users and H2RA users with H2RA users as the reference group and further employed the lag-time approach.
We conducted all analyses using the SAS software (SAS Institute, Inc., Version 9.4, Cary, NC). All statistical tests were two-sided with a P value less than 0.05 indicating statistical significance.
RESULTS
Study population
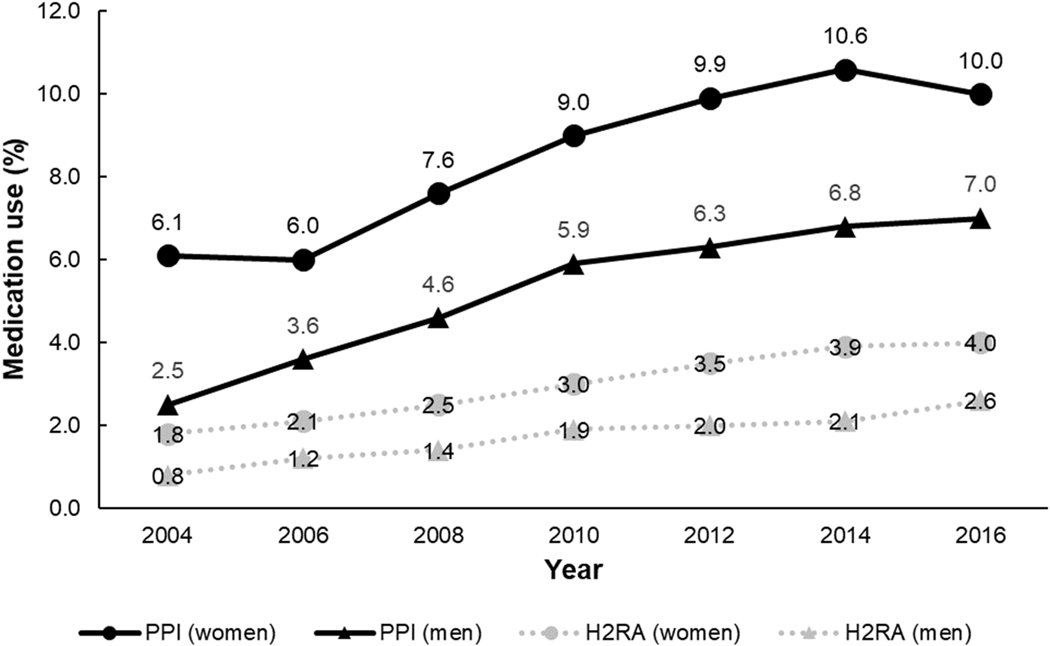
Our study included 50,156 women and 21,731 men contributing to 831,407 person-years over a median of 13.8 years of follow-up. We confirmed 22,125 deaths, including 4,592 deaths from cancer, 5,404 from cardiovascular diseases, and 12,129 deaths from other causes (including respiratory diseases, digestive diseases, renal diseases, neurological diseases, infectious diseases, and other less common medical conditions). Among study participants, 10,998 women (21.9%) and 2,945 men (13.6%) initiated PPI use at some point during the study period, and over that course, PPI use increased from 6.1% to 10.0% in women and from 2.5% to 7.0% in men (Figure 2). The age-standardized characteristics of study participants according to PPI use in the two cohorts are summarized in Table 1. The mean age at baseline for women and men was 68.9 years and 68.0 years, respectively. PPI use was associated with higher rates of H2RA use in the past. Compared to non-users of PPIs, PPI users were more likely to have cancer, cardiovascular diseases (myocardial infarction and stroke), and various medical conditions. Similar distributions could be observed when examining characteristics by H2RA use (Supplementary Table 1).
Figure 2. Proton pump inhibitor use and histamine-2 receptor antagonist use in women and men over the study period (2004–2018).
Abbreviations: H2RA, histamine-2 receptor antagonist; PPI, proton pump inhibitor.
Table 1.
Age-standardized characteristics of study participants according to proton pump inhibitor use over follow-upa
| Women (N=50,156) | Men (N=21,731) | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| All | PPI non-users | PPI users | All | PPI non-users | PPI users | |
| Age at baseline, years, mean (SD) | 68.9 (6.9) | 69.0 (6.9) | 67.8 (6.6) | 68.0 (8.0) | 68.0 (8.1) | 68.1 (7.7) |
| White, % | 91.3 | 91.4 | 90.4 | 95.0 | 94.9 | 96.0 |
| Smoking status, % | ||||||
| Never smokers | 49.3 | 49.5 | 47.0 | 54.4 | 54.6 | 49.6 |
| Past smokers | 47.4 | 47.0 | 50.7 | 42.7 | 42.4 | 48.1 |
| Current smokers | 3.3 | 3.5 | 2.3 | 2.9 | 3.0 | 2.3 |
| Body mass index, kg/m2, mean (SD) | 25.8 (5.3) | 25.7 (5.2) | 26.6 (5.4) | 25.9 (3.9) | 25.9 (3.9) | 26.3 (4.1) |
| Physical activity, MET-hrs/wk, mean (SD) | 18.6 (14.8) | 18.8 (14.9) | 16.6 (13.5) | 34.4 (23.2) | 34.6 (23.3) | 32.3 (21.6) |
| Alternate Healthy Eating Index-2010, mean (SD) | 49.6 (8.9) | 49.7 (8.9) | 48.9 (8.5) | 51 (9.4) | 51 (9.4) | 50.8 (9.1) |
| Alcohol intake, g/d, mean (SD) | 5.8 (8.6) | 5.8 (8.6) | 5.4 (8.2) | 11.3 (12.6) | 11.3 (12.6) | 11.5 (12.5) |
| Regular NSAID use, % | 67.2 | 66.9 | 69.6 | 55.7 | 55.5 | 59.6 |
| H2RA use in the past, % | 10.7 | 9.1 | 26.9 | 6.8 | 5.7 | 23.7 |
| Physical examination in the past two years, % | 76.5 | 76.8 | 74.0 | 69.1 | 69.2 | 68.1 |
| Medical condition | ||||||
| Cancer | 23.1 | 22.8 | 25.7 | 25.1 | 24.8 | 30.3 |
| Myocardial infarction | 5.4 | 5.3 | 7.1 | 10.2 | 10.0 | 12.9 |
| Stroke | 3.7 | 3.6 | 4.6 | 4.2 | 4.2 | 4.7 |
| Hypertension | 64.3 | 63.4 | 73.4 | 57.0 | 56.4 | 67.0 |
| Diabetes mellitus | 12.7 | 12.4 | 15.4 | 13.1 | 12.9 | 16.7 |
| Hypercholesterolemia | 72.1 | 71.4 | 79.2 | 64.9 | 64.4 | 73.0 |
| Chronic obstructive pulmonary disease | 9.3 | 8.9 | 13.2 | 3.8 | 3.8 | 5.2 |
Abbreviations: H2RA, histamine-2 receptor antagonist; MET, metabolic equivalent of task; NSAID, non-steroidal anti-inflammatory drug; PPI, proton pump inhibitor; SD, standard deviation.
Characteristics of study participants are presented by PPI use. All variables are standardized to the age distribution of the study population except for age. Mean (SD) is presented for continuous variables and percentage of participants for categorical variables. Values for all variables but age were calculated over follow-up.
PPI use and all-cause and cause-specific mortality
Compared to non-users of PPIs, PPI users had a significantly higher risk of all-cause mortality after adjusting for time-varying confounding (HR, 1.19; 95% CI, 1.13–1.24) (Table 2). PPI use was also associated with increased risks of cause-specific mortality, including due to cancer (HR, 1.30; 95% CI, 1.17–1.44), cardiovascular diseases (HR, 1.13; 95% CI, 1.02–1.26), respiratory diseases (HR, 1.32; 95% CI, 1.12–1.56), digestive diseases (HR, 1.50; 95% CI, 1.10–2.05), and renal diseases (HR, 2.09; 95% CI, 1.50–2.90). We did not observe an association between PPI use and mortality due to neurological diseases (HR, 1.01; 95% CI, 0.88–1.06) and infectious diseases (HR, 1.31; 95% CI, 0.96–1.78). When we applied successively longer lag-times, we found overall attenuation of the associations. Ultimately, a six-year lag-time resulted in reduced HRs for all-cause mortality (HR, 1.04; 95% CI, 0.97–1.11) and mortality due to cancer (HR, 1.07; 95% CI, 0.89–1.28), cardiovascular diseases (HR, 0.94; 95% CI, 0.81–1.10), respiratory diseases (HR, 1.20; 95% CI, 0.95–1.50), and digestive diseases (HR, 1.38; 95% CI, 0.88–2.18). In contrast, PPI users remained at a significantly elevated risk for mortality due to renal diseases (HR, 2.45; 95% CI, 1.59–3.78).
Table 2.
Hazard ratios (95% confidence intervals) for all-cause and cause-specific mortality according to proton pump inhibitor use
| Lag-timea | ||||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| No lag | 2 years | 4 years | 6 years | |||||
|
|
|
|
|
|||||
| Cause of death | No. of events | HR (95% CI)b | No. of events | HR (95% CI)b | No. of events | HR (95% CI)b | No. of events | HR (95% CI)b |
| All causes | ||||||||
| PPI non-users | 20092 | 1 (reference) | 18661 | 1 (reference) | 16634 | 1 (reference) | 14205 | 1 (reference) |
| PPI users | 2033 | 1.19 (1.13–1.24) | 1726 | 1.10 (1.04–1.15) | 1301 | 1.04 (0.98–1.11) | 950 | 1.04 (0.97–1.11) |
| Cancer | ||||||||
| PPI non-users | 4141 | 1 (reference) | 3594 | 1 (reference) | 2879 | 1 (reference) | 2136 | 1 (reference) |
| PPI users | 451 | 1.30 (1.17–1.44) | 323 | 1.17 (1.03–1.31) | 212 | 1.08 (0.94–1.26) | 132 | 1.07 (0.89–1.28) |
| Cardiovascular diseases | ||||||||
| PPI non-users | 4946 | 1 (reference) | 4554 | 1 (reference) | 4032 | 1 (reference) | 3412 | 1 (reference) |
| PPI users | 458 | 1.13 (1.02–1.26) | 388 | 1.03 (0.92–1.15) | 296 | 1.00 (0.88–1.13) | 207 | 0.94 (0.81–1.10) |
| Respiratory diseases | ||||||||
| PPI non-users | 1628 | 1 (reference) | 1499 | 1 (reference) | 1327 | 1 (reference) | 1122 | 1 (reference) |
| PPI users | 188 | 1.32 (1.12–1.56) | 178 | 1.32 (1.11–1.57) | 129 | 1.19 (0.98–1.45) | 96 | 1.20 (0.95–1.50) |
| Digestive diseases | ||||||||
| PPI non-users | 379 | 1 (reference) | 353 | 1 (reference) | 299 | 1 (reference) | 256 | 1 (reference) |
| PPI users | 54 | 1.50 (1.10–2.05) | 48 | 1.44 (1.04–2.00) | 39 | 1.67 (1.16–2.39) | 23 | 1.38 (0.88–2.18) |
| Renal diseases | ||||||||
| PPI non-users | 293 | 1 (reference) | 274 | 1 (reference) | 245 | 1 (reference) | 202 | 1 (reference) |
| PPI users | 51 | 2.09 (1.50–2.90) | 41 | 1.90 (1.32–2.73) | 35 | 1.88 (1.27–2.78) | 29 | 2.45 (1.59–3.78) |
| Neurological diseases | ||||||||
| PPI non-users | 3256 | 1 (reference) | 3174 | 1 (reference) | 3003 | 1 (reference) | 2728 | 1 (reference) |
| PPI users | 233 | 1.01 (0.88–1.16) | 206 | 0.87 (0.75–1.01) | 162 | 0.89 (0.77–1.04) | 135 | 0.83 (0.69–1.00) |
| Infectious diseases | ||||||||
| PPI non-users | 417 | 1 (reference) | 394 | 1 (reference) | 353 | 1 (reference) | 308 | 1 (reference) |
| PPI users | 54 | 1.31 (0.96–1.78) | 50 | 1.30 (0.94–1.79) | 39 | 1.33 (0.93–1.90) | 19 | 0.78 (0.48–1.28) |
Abbreviations: CI, confidence interval; H2RA, histamine-2 receptor antagonist; HR, hazard ratio; NSAID, non-steroidal anti-inflammatory drug; PPI, proton pump inhibitor.
For a modified lag-time approach, we considered a two-year, a four-year, and a six-year lag-time based on the structure of our data. For example, in a two-year lag-time analysis, we used the exposure status in 2004 to model mortality risk starting in 2006; in a four-year lag-time analysis, we used the exposure status in 2004 to model mortality risk starting in 2008, and so on.
Cox proportional hazards models stratified by age, cohort, and questionnaire cycle and adjusted for race, smoking status, body mass index, physical activity, Alternate Healthy Eating Index-2010, alcohol intake, regular NSAID use, H2RA use in the past, history of cancer, myocardial infarction, stroke, hypertension, diabetes mellitus, hypercholesterolemia, gastroesophageal reflux disease, Barrett’s esophagus, peptic ulcer disease, gastrointestinal bleeding, and chronic obstructive pulmonary disease.
For cancer-specific mortality, PPI use was associated with significantly increased risks of mortality due to lung cancer (HR, 1.38; 95% CI, 1.11–1.72), upper gastrointestinal cancer (HR, 1.68; 95% CI, 1.27–2.23), colorectal cancer (HR, 1.44; 95% CI, 1.00–2.08), and ovarian cancer (HR, 1.94; 95% CI, 1.32–2.84) (Table 3). Similar to the analysis of overall cancer, the associations with mortality by specific cancers were attenuated after applying successively longer lag-times. Risks of death from upper gastrointestinal cancer (HR, 1.06; 95% CI, 0.73–1.54) and colorectal cancer (HR, 1.27; 95% CI, 0.83–1.95) were attenuated upon applying a two-year lag-time while no significant association between PPI use and mortality due to lung cancer (HR, 1.16; 95% CI, 0.84–1.60) and ovarian cancer (HR, 0.57; 95% CI, 0.26–1.25) was found once a four-year lag-time was applied.
Table 3.
Hazard ratios (95% confidence intervals) for cancer-specific mortality according to proton pump inhibitor use
| Lag-timea | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| No lag | 2 years | 4 years | ||||
|
|
|
|
||||
| Cause of death | No. of events | HR (95% CI)b | No. of events | HR (95% CI)b | No. of events | HR (95% CI)b |
| Lung cancer | ||||||
| PPI non-users | 868 | 1 (reference) | 736 | 1 (reference) | 574 | 1 (reference) |
| PPI users | 106 | 1.38 (1.11–1.72) | 74 | 1.30 (1.01–1.68) | 45 | 1.16 (0.84–1.60) |
| Upper gastrointestinal cancer c | ||||||
| PPI non-users | 450 | 1 (reference) | 391 | 1 (reference) | 306 | 1 (reference) |
| PPI users | 64 | 1.68 (1.27–2.23) | 34 | 1.06 (0.73–1.54) | 29 | 1.32 (0.87–2.02) |
| Colorectal cancer | ||||||
| PPI non-users | 331 | 1 (reference) | 285 | 1 (reference) | 225 | 1 (reference) |
| PPI users | 37 | 1.44 (1.00–2.08) | 26 | 1.27 (0.83–1.95) | 19 | 1.24 (0.75–2.04) |
| Non-Hodgkin lymphoma | ||||||
| PPI non-users | 287 | 1 (reference) | 254 | 1 (reference) | 190 | 1 (reference) |
| PPI users | 22 | 1.09 (0.69–1.72) | 20 | 1.23 (0.76–1.98) | 14 | 1.28 (0.72–2.28) |
| Breast cancer (women) | ||||||
| PPI non-users | 368 | 1 (reference) | 314 | 1 (reference) | 251 | 1 (reference) |
| PPI users | 40 | 1.21 (0.86–1.71) | 33 | 1.22 (0.83–1.78) | 22 | 1.08 (0.69–1.71) |
| Ovarian cancer (women) | ||||||
| PPI non-users | 194 | 1 (reference) | 165 | 1 (reference) | 145 | 1 (reference) |
| PPI users | 36 | 1.94 (1.32–2.84) | 27 | 2.02 (1.31–3.12) | 7 | 0.57 (0.26–1.25) |
Abbreviations: CI, confidence interval; H2RA, histamine-2 receptor antagonist; HR, hazard ratio; NSAID, non-steroidal anti-inflammatory drug; PPI, proton pump inhibitor.
For a modified lag-time approach, we considered a two-year, a four-year, and a six-year lag-time based on the structure of our data. For example, in a two-year lag-time analysis, we used the exposure status in 2004 to model mortality risk starting in 2006; in a four-year lag-time analysis, we used the exposure status in 2004 to model mortality risk starting in 2008, and so on.
Cox proportional hazards models stratified by age, cohort, and questionnaire cycle and adjusted for race, smoking status, body mass index, physical activity, Alternate Healthy Eating Index-2010, alcohol intake, regular NSAID use, H2RA use in the past, history of cancer, myocardial infarction, stroke, hypertension, diabetes mellitus, hypercholesterolemia, gastroesophageal reflux disease, Barrett’s esophagus, peptic ulcer disease, gastrointestinal bleeding, and chronic obstructive pulmonary disease.
Upper gastrointestinal cancer includes cancer of the esophagus, stomach, and small intestine.
Duration of PPI use and all-cause and cause-specific mortality
Table 4 presents the associations between duration of PPI use and mortality risks. For all-cause mortality and mortality due to cancer, cardiovascular diseases, respiratory diseases, and digestive diseases, the greatest HRs were seen mostly in those who reported using PPIs for 1–2 years. Longer duration of PPI use did not confer higher risks of mortality for these endpoints. In contrast, a potential trend toward greater HRs with longer duration of PPI use was observed in mortality due to renal diseases, with the HR of 1.68 (95% CI, 1.19–2.38) for 1–2 years gradually increasing to 2.42 (95% CI, 1.23–4.77) for ≥7 years of PPI use. There were no apparent trends for mortality due to neurological diseases and infectious diseases.
Table 4.
Hazard ratios (95% confidence intervals) for all-cause and cause-specific mortality according to duration of proton pump inhibitor usea
| PPI use | |||||
|---|---|---|---|---|---|
|
|
|||||
| Cause of death | Non-users | 1–2 years | 3–4 years | 5–6 years | ≥7 years |
| All causes | 1 (reference) | 1.21 (1.15–1.27) | 1.03 (0.95–1.11) | 1.05 (0.94–1.17) | 0.94 (0.83–1.06) |
| Cancer | 1 (reference) | 1.37 (1.24–1.52) | 1.13 (0.94–1.36) | 0.91 (0.68–1.21) | 1.05 (0.77–1.44) |
| Cardiovascular diseases | 1 (reference) | 1.12 (1.01–1.24) | 0.92 (0.77–1.11) | 0.97 (0.76–1.24) | 0.87 (0.66–1.14) |
| Respiratory diseases | 1 (reference) | 1.46 (1.24–1.71) | 1.17 (0.89–1.54) | 1.29 (0.90–1.85) | 1.07 (0.70–1.63) |
| Digestive diseases | 1 (reference) | 1.56 (1.14–2.12) | 1.62 (1.04–2.54) | 1.09 (0.50–2.38) | 1.32 (0.63–2.76) |
| Renal diseases | 1 (reference) | 1.68 (1.19–2.38) | 1.45 (0.80–2.62) | 1.74 (0.83–3.65) | 2.42 (1.23–4.77) |
| Neurological diseases | 1 (reference) | 0.85 (0.73–1.00) | 0.89 (0.72–1.10) | 1.11 (0.87–1.42) | 1.11 (0.90–1.38) |
| Infectious diseases | 1 (reference) | 1.14 (0.82–1.58) | 1.38 (0.88–2.15) | 1.45 (0.81–2.60) | 0.74 (0.32–1.70) |
Abbreviations: H2RA, histamine-2 receptor antagonist; NSAID, non-steroidal anti-inflammatory drug; PPI, proton pump inhibitor.
Hazard ratios (95% confidence intervals) were calculated using Cox proportional hazards models stratified by age, cohort, and questionnaire cycle and adjusted for race, smoking status, body mass index, physical activity, Alternate Healthy Eating Index-2010, alcohol intake, regular NSAID use, H2RA use in the past, history of cancer, myocardial infarction, stroke, hypertension, diabetes mellitus, hypercholesterolemia, gastroesophageal reflux disease, Barrett’s esophagus, peptic ulcer disease, gastrointestinal bleeding, and chronic obstructive pulmonary disease.
Sensitivity analysis
We conducted sensitivity analyses to test whether our results were robust. Except for all-cause mortality and mortality due to other causes, PPI users and H2RA users showed similar HRs for mortality when compared to non-users of PPIs and H2RAs (Supplementary Table 2). We then used H2RA users as the reference group to directly compare the mortality risks between PPI users and H2RA users. Compared to H2RA users, PPI users were at higher risks of all-cause mortality (HR, 1.14; 95% CI, 1.03–1.27) and mortality due to other causes (HR, 1.21; 95% CI, 1.05–1.40) (Supplementary Table 3). Upon applying successively longer lag-times, we found that the associations with all-cause mortality and mortality due to other causes gradually attenuated to null.
DISCUSSION
In this large prospective study of women and men, PPI use was associated with an increase in the risks of all-cause and cause-specific mortality, including mortality due to cancer, cardiovascular diseases, respiratory diseases, digestive diseases, and renal diseases. However, these associations were largely attenuated after applying lag-times and the excess risk of mortality was not observed with longer duration of PPI use except for mortality due to renal diseases, a finding which should be interpreted with caution given the lack of information on potential confounders for renal diseases. Furthermore, we did not observe an increase in mortality risks associated with the use of PPIs compared with H2RAs with successively longer lag-times. Taken together, our results do not support positive associations between PPI use and all-cause mortality and mortality due to major causes.
Our null results are supported by one randomized controlled trial29 and several observational studies,30,31 in which no increased risk of death was found with PPI use. Nonetheless, the randomized controlled trial29 was restricted to a highly selected population (i.e., patients with stable cardiovascular disease and peripheral artery disease) and limited by short follow-up. The large cohort study of 1.9 million elderly Medicare enrollees30 only examined all-cause mortality but not cause-specific deaths. Although the prospective study of 0.44 million UK Biobank participants31 investigated all-cause and cause-specific mortality, it relied on adjustment for general health (overall health rating and longstanding illness), suggesting any positive associations were primarily due to residual confounding. This study did not properly account for protopathic bias or examine mortality due to renal diseases. As such, our study expands upon existing evidence and provides more robust evidence examining the associations between PPI use and mortality.
Findings from our initial analysis without considering lag-times are consistent with prior observational studies that showed associations of PPI use with higher risks for all-cause mortality6–8 and mortality due to cancer (especially lung cancer, upper gastrointestinal cancer, colorectal cancer, and ovarian cancer),8–10 cardiovascular diseases,5,11 respiratory diseases,12 digestive diseases,13–17 and renal diseases.8,18 Here, we conducted a prospective study of two nationwide cohorts with long-term follow-up of over ten years. Upon applying lag-times of up to six years, the excess mortality risks associated with PPI use were largely attenuated. This highlights the importance of carefully controlling for the influence of protopathic bias. Moreover, in our duration analysis, we found that the highest HRs were observed mostly among those who used PPIs for less than two years, while the magnitude of HRs steadily decreased with a longer duration of PPI use. This can be seen in mortality due to cancer and cardiovascular diseases, conditions in which PPIs are frequently used both therapeutically for gastrointestinal symptoms and prophylactically to prevent gastrointestinal injury due to chronic NSAID use.32,33 Thus, mortality due to these conditions within a few years of initiation of PPIs may be attributable to protopathic bias. Other medical conditions, although many of which are not PPI indications, were also more prevalent in PPI users which might result in increased risks for death from such conditions. Notably, in a sensitivity analysis, we implemented an active-comparator study design comparing the mortality risks among PPI users and H2RA users. Without lag-time, PPI users were at increased risks for all-cause mortality and mortality due to causes other than cancer and cardiovascular diseases compared to H2RA users. Decreased strengths of associations were observed after introducing a two-year and four-year lag-time. This confirmed our main findings and suggested PPIs might be preferred over H2RAs in sicker patients with comorbid conditions.
In contrast to our analyses of mortality due to other causes, associations between PPI use and the risk of death from renal diseases persisted despite applying lag-times. Additionally, the duration analysis showed a potential relationship between increasing duration of PPI use and higher risk of mortality due to renal diseases. Importantly, such increased mortality risk could not be established in this study since we did not have reliable data on renal diseases and therefore could not adjust for confounding in the models. Nonetheless, evidence from prior observational studies has linked PPI use to various renal endpoints, including acute interstitial nephritis,34,35 chronic kidney disease,36–39 end-stage renal disease,37 and mortality due to renal diseases.8,18 Taken together, although our study and prior observational studies cannot prove causation, they support the need for further studies examining the risk of mortality due to renal diseases in patients using PPIs.
This study has multiple strengths. First, our long-term follow-up of over ten years and repeated assessment of PPI use allowed analyses incorporating lag-times and increasing exposure duration to account for protopathic bias. Second, our cohort collected extensive information about potential confounding lifestyle factors that are often not available in administrative claims databases. Third, we further used an active-comparator study design to test if there was residual bias when using H2RA users as the reference group.
We acknowledge several limitations. First, we did not collect information on PPI brand, dosage, and schedule. However, most studies have suggested that any potential association is a class effect not related to any one specific drug.40 Second, the number of events for certain outcomes in PPI users was relatively small compared to other studies, especially when applying longer lag-times. Nonetheless, we examined the trend of the point estimates and not just the statistical significance. Moreover, there were still sufficient cases in non-PPI users. If an association truly existed, the number of cases in PPI users and non-PPI users should be balanced for the association to remain statistically significant in spite of the lag-times. Third, we did not have data on all potential confounding medical conditions. Inability to adjust for specific medical conditions might lead to residual confounding (bias away from the null). This is especially relevant to mortality due to renal diseases as the positive association with PPI use was a notable exception to our null findings for other causes of death. Finally, our study participants were mostly White health professionals. Further studies are needed for other racial, ethnic, and socioeconomic groups.
Patients with various medical conditions may use PPIs prior to death which leads to protopathic bias when evaluating mortality risk in PPI users. We utilized a modified lag-time approach and found no association between PPI use and all-cause mortality and mortality due to major causes after accounting for protopathic bias. Despite the lack of association with risk of death, it remains prudent to recommend the use of these agents to patients with appropriate indications and for the minimally effective duration.
Supplementary Material
WHAT YOU NEED TO KNOW.
Background and Context:
Proton pump inhibitor (PPI) use has been associated with a possible increased risk of death. However, prior studies vary widely with regard to study population, methodology, and endpoints.
New Findings:
PPI use was associated with higher risks of all-cause mortality and mortality due to cancer, cardiovascular diseases, respiratory diseases, and digestive diseases. Upon implementing lag-times of up to six years, the associations were attenuated and no longer significant.
Limitations:
We did not have information on PPI brand, dosage, and schedule. We were not able to adjust for specific medical conditions. Most of the study participants were White health professionals.
Impact:
Our study is among the first to assess the potential for protopathic bias across a range of endpoints by applying successively longer lag-times. Our results do not support positive associations between PPI use and mortality risks.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the contribution to this study from central cancer registries supported through the Centers for Disease Control and Prevention’s National Program of Cancer Registries (NPCR) and/or the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program. Central registries may also be supported by state agencies, universities, and cancer centers. Participating central cancer registries include the following: Alabama, Alaska, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Hawaii, Idaho, Indiana, Iowa, Kentucky, Louisiana, Massachusetts, Maine, Maryland, Michigan, Mississippi, Montana, Nebraska, Nevada, New Hampshire, New Jersey, New Mexico, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Puerto Rico, Rhode Island, Seattle SEER Registry, South Carolina, Tennessee, Texas, Utah, Virginia, West Virginia, Wyoming.
Grant Support:
This work was supported by the National Institutes of Health (UM1 CA186107 [NHS cohort infrastructure grant], U01 CA167552 [HPFS cohort infrastructure grant]; P30 CA091842 to YY; K23DK125838 to LHN; DK091417 to GCC; R35 CA253185 to ATC) and the Crohn’s and Colitis Foundation (Career Development Award and Research Fellowship Award to LHN). ATC is a Stuart and Suzanne Steele MGH Research Scholar and American Cancer Society Clinical Research Professor. The funders had no role in the design and conduct of the study. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders.
Abbreviations:
- AHEI-2010
Alternate Healthy Eating Index-2010
- BMI
body mass index
- CI
confidence interval
- H2RA
histamine-2 receptor antagonist
- HR
hazard ratio
- ICD
International Classification of Diseases
- NSAID
non-steroidal anti-inflammatory drug
- PPI
proton pump inhibitor
- SFFQ
semi-quantitative food frequency questionnaire
Footnotes
Disclosures: GCC is an employee of OM1, Inc and receives royalties from UpToDate as an author and Section Editor. ATC has consulted for OM1, Inc, Bayer Pharma AG, and Pfizer Inc for topics unrelated to this manuscript and Boehringer Ingelheim for litigation related to ranitidine and cancer. All remaining authors have declared no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Freedberg DE, Kim LS, Yang YX. The risks and benefits of long-term use of proton pump inhibitors: expert review and best practice advice from the American Gastroenterological Association. Gastroenterology. 2017;152(4):706–715. doi: 10.1053/j.gastro.2017.01.031 [DOI] [PubMed] [Google Scholar]
- 2.Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999–2012. JAMA. 2015;314(17):1818. doi: 10.1001/jama.2015.13766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lanas-Gimeno A, Hijos G, Lanas Á. Proton pump inhibitors, adverse events and increased risk of mortality. Expert Opinion on Drug Safety. 2019;18(11):1043–1053. doi: 10.1080/14740338.2019.1664470 [DOI] [PubMed] [Google Scholar]
- 4.Vaezi MF, Yang YX, Howden CW. Complications of proton pump inhibitor therapy. Gastroenterology. 2017;153(1):35–48. doi: 10.1053/j.gastro.2017.04.047 [DOI] [PubMed] [Google Scholar]
- 5.Shah NH, LePendu P, Bauer-Mehren A, et al. Proton pump inhibitor usage and the risk of myocardial infarction in the general population. Guo Y, ed. PLoS ONE. 2015;10(6):e0124653. doi: 10.1371/journal.pone.0124653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maggio M, Corsonello A, Ceda GP, et al. Proton pump inhibitors and risk of 1-year mortality and rehospitalization in older patients discharged from acute care hospitals. JAMA Intern Med. 2013;173(7):518. doi: 10.1001/jamainternmed.2013.2851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie Y, Bowe B, Li T, Xian H, Yan Y, Al-Aly Z. Risk of death among users of proton pump inhibitors: a longitudinal observational cohort study of United States veterans. BMJ Open. 2017;7(6):e015735. doi: 10.1136/bmjopen-2016-015735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xie Y, Bowe B, Yan Y, Xian H, Li T, Al-Aly Z. Estimates of all cause mortality and cause specific mortality associated with proton pump inhibitors among US veterans: cohort study. BMJ. Published online May 29, 2019:l1580. doi: 10.1136/bmj.l1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X, Liu Q, Halfdanarson ÓÖ, et al. Proton pump inhibitors and survival in patients with colorectal cancer: a Swedish population-based cohort study. Br J Cancer. 2021;125(6):893–900. doi: 10.1038/s41416-021-01480-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tvingsholm SA, Dehlendorff C, Østerlind K, Friis S, Jäättelä M. Proton pump inhibitor use and cancer mortality. Int J Cancer. 2018;143(6):1315–1326. doi: 10.1002/ijc.31529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charlot M, Ahlehoff O, Norgaard ML, et al. Proton-pump inhibitors are associated with increased cardiovascular risk independent of clopidogrel use: a nationwide cohort study. Ann Intern Med. 2010;153(6):378–386. doi: 10.7326/0003-4819-153-6-201009210-00005 [DOI] [PubMed] [Google Scholar]
- 12.Zirk-Sadowski J, Masoli JA, Delgado J, et al. Proton-pump inhibitors and long-term risk of community-acquired pneumonia in older adults. J Am Geriatr Soc. 2018;66(7):1332–1338. doi: 10.1111/jgs.15385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dultz G, Piiper A, Zeuzem S, Kronenberger B, Waidmann O. Proton pump inhibitor treatment is associated with the severity of liver disease and increased mortality in patients with cirrhosis. Aliment Pharmacol Ther. 2015;41(5):459–466. doi: 10.1111/apt.13061 [DOI] [PubMed] [Google Scholar]
- 14.Nardelli S, Gioia S, Ridola L, Farcomeni A, Merli M, Riggio O. Proton pump inhibitors are associated with minimal and overt hepatic encephalopathy and increased mortality in patients with cirrhosis. Hepatology. 2019;70(2):640–649. doi: 10.1002/hep.30304 [DOI] [PubMed] [Google Scholar]
- 15.Bettinger D, Martin D, Rieg S, et al. Treatment with proton pump inhibitors is associated with increased mortality in patients with pyogenic liver abscess. Aliment Pharmacol Ther. 2018;47(6):801–808. doi: 10.1111/apt.14512 [DOI] [PubMed] [Google Scholar]
- 16.Kwon JH, Koh SJ, Kim W, et al. Mortality associated with proton pump inhibitors in cirrhotic patients with spontaneous bacterial peritonitis. J Gastroenterol Hepatol. 2014;29(4):775–781. doi: 10.1111/jgh.12426 [DOI] [PubMed] [Google Scholar]
- 17.Hung TH, Tseng CW, Lee HF, Tsai CC, Tsai CC. Effect of proton pump inhibitors on mortality in patients with cirrhosis and spontaneous bacterial peritonitis. Ann Hepatol. 2018;17(6):933–939. doi: 10.5604/01.3001.0012.7193 [DOI] [PubMed] [Google Scholar]
- 18.Douwes RM, Gomes-Neto AW, Eisenga MF, et al. The association between use of proton-pump inhibitors and excess mortality after kidney transplantation: a cohort study. Taal MW, ed. PLoS Med. 2020;17(6):e1003140. doi: 10.1371/journal.pmed.1003140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tamim H, Monfared AAT, LeLorier J. Application of lag-time into exposure definitions to control for protopathic bias. Pharmacoepidem Drug Safe. 2007;16(3):250–258. doi: 10.1002/pds.1360 [DOI] [PubMed] [Google Scholar]
- 20.Arfè A, Corrao G. The lag-time approach improved drug-outcome association estimates in presence of protopathic bias. J Clin Epidemiol. 2016;78:101–107. doi: 10.1016/j.jclinepi.2016.03.003 [DOI] [PubMed] [Google Scholar]
- 21.Colditz GA, Manson JE, Hankinson SE. The Nurses’ Health Study: 20-year contribution to the understanding of health among women. J Womens Health. 1997;6(1):49–62. [DOI] [PubMed] [Google Scholar]
- 22.Rimm EB, Giovannucci EL, Willett WC, et al. Prospective study of alcohol consumption and risk of coronary disease in men. The Lancet. 1991;338(8765):464–468. doi: 10.1016/0140-6736(91)90542-W [DOI] [PubMed] [Google Scholar]
- 23.Willett WC, Sampson L, Browne ML, et al. The use of a self-administered questionnaire to assess diet four years in the past. American Journal of Epidemiology. 1988;127(1):188–199. doi: 10.1093/oxfordjournals.aje.a114780 [DOI] [PubMed] [Google Scholar]
- 24.Feskanich D, Rimm EB, Giovannucci EL, et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. Journal of the American Dietetic Association. 1993;93(7):790–796. doi: 10.1016/0002-8223(93)91754-E [DOI] [PubMed] [Google Scholar]
- 25.Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the National Death Index and Equifax Nationwide Death Search. American Journal of Epidemiology. 1994;140(11):1016–1019. doi: 10.1093/oxfordjournals.aje.a117191 [DOI] [PubMed] [Google Scholar]
- 26.Stampfer MJ, Willett WC, Speizer FE, et al. Test of the National Death Index. Am J Epidemiol. 1984;119(5):837–839. doi: 10.1093/oxfordjournals.aje.a113804 [DOI] [PubMed] [Google Scholar]
- 27.Charlton BM, Rich-Edwards JW, Colditz GA, et al. Oral contraceptive use and mortality after 36 years of follow-up in the Nurses’ Health Study: prospective cohort study. BMJ. 2014;349(oct31 7):g6356-g6356. doi: 10.1136/bmj.g6356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshida K, Solomon DH, Kim SC. Active-comparator design and new-user design in observational studies. Nat Rev Rheumatol. 2015;11(7):437–441. doi: 10.1038/nrrheum.2015.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moayyedi P, Eikelboom JW, Bosch J, et al. Safety of proton pump inhibitors based on a large, multi-year, randomized trial of patients receiving rivaroxaban or aspirin. Gastroenterology. 2019;157(3):682–691.e2. doi: 10.1053/j.gastro.2019.05.056 [DOI] [PubMed] [Google Scholar]
- 30.Baik SH, Fung KW, McDonald CJ. The mortality risk of Proton Pump Inhibitors in 1.9 Million US Seniors: an extended Cox survival analysis. Clinical Gastroenterology and Hepatology. Published online January 2021:S1542356521000173. doi: 10.1016/j.cgh.2021.01.014 [DOI] [PubMed] [Google Scholar]
- 31.He Q, Xia B, Meng W, et al. No associations between regular use of proton pump inhibitors and risk of all-cause and cause-specific mortality: a population-based cohort of 0.44 million participants. Am J Gastroenterol. 2021;116(11):2286–2291. doi: 10.14309/ajg.0000000000001377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Triadafilopoulos G, Roorda AK, Akiyama J. Indications and safety of proton pump inhibitor drug use in patients with cancer. Expert Opinion on Drug Safety. 2013;12(5):659–672. doi: 10.1517/14740338.2013.797961 [DOI] [PubMed] [Google Scholar]
- 33.Numico G, Fusco V, Franco P, Roila F. Proton pump inhibitors in cancer patients: how useful they are? A review of the most common indications for their use. Critical Reviews in Oncology/Hematology. 2017;111:144–151. doi: 10.1016/j.critrevonc.2017.01.014 [DOI] [PubMed] [Google Scholar]
- 34.Blank ML, Parkin L, Paul C, Herbison P. A nationwide nested case-control study indicates an increased risk of acute interstitial nephritis with proton pump inhibitor use. Kidney Int. 2014;86(4):837–844. doi: 10.1038/ki.2014.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Antoniou T, Macdonald EM, Hollands S, et al. Proton pump inhibitors and the risk of acute kidney injury in older patients: a population-based cohort study. CMAJ Open. 2015;3(2):E166–E171. doi: 10.9778/cmajo.20140074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lazarus B, Chen Y, Wilson FP, et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med. 2016;176(2):238. doi: 10.1001/jamainternmed.2015.7193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xie Y, Bowe B, Li T, Xian H, Balasubramanian S, Al-Aly Z. Proton pump inhibitors and risk of incident CKD and progression to ESRD. JASN. 2016;27(10):3153–3163. doi: 10.1681/ASN.2015121377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klatte DCF, Gasparini A, Xu H, et al. Association between proton pump inhibitor use and risk of progression of chronic kidney disease. Gastroenterology. 2017;153(3):702–710. doi: 10.1053/j.gastro.2017.05.046 [DOI] [PubMed] [Google Scholar]
- 39.Xie Y, Bowe B, Li T, Xian H, Yan Y, Al-Aly Z. Long-term kidney outcomes among users of proton pump inhibitors without intervening acute kidney injury. Kidney International. 2017;91(6):1482–1494. doi: 10.1016/j.kint.2016.12.021 [DOI] [PubMed] [Google Scholar]
- 40.Nguyen LH, Lochhead P, Joshi AD, et al. No significant association between proton pump inhibitor use and risk of stroke after adjustment for lifestyle factors and indication. Gastroenterology. 2018;154(5):1290–1297.e1. doi: 10.1053/j.gastro.2017.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.