Abstract
Background and Aims:
Magnetic resonance elastography (MRE) is an accurate biomarker of liver fibrosis, however, limited data characterize its association with clinical outcomes. We conducted individual participant data pooled meta-analysis (IPDMA) on patients with nonalcoholic fatty liver disease (NAFLD) to evaluate the association between liver stiffness (LS) on MRE and liver-related outcomes.
Methods:
A systematic search identified 6 cohorts of adults with NAFLD who underwent a baseline MRE and were followed for hepatic decompensation, hepatocellular carcinoma (HCC) and death. Cox- and logistic-regression were used to assess the association between LS on MRE and liver-related outcomes including a composite primary outcome defined as varices needing treatment, ascites and hepatic encephalopathy.
Results:
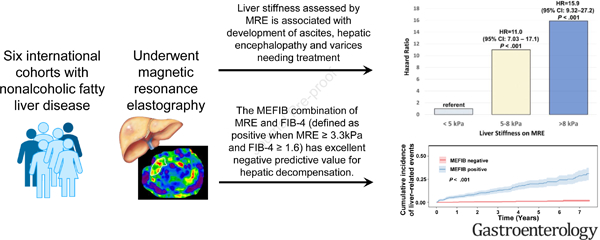
This IPDMA included 2018 patients (53% women) with a mean (± standard deviation) age of 57.8 (±14) years and MRE at baseline of 4.15 (±2.19) kPa, respectively. Among 1707 patients with available longitudinal data with a median (IQR) of 3 (4.2) years of follow-up, the hazard ratio (HR) for the primary outcome for MRE between 5–8 kPa was 11.0 (95%CI: 7.03–17.1, P < .001) and for ≥ 8 kPa was 15.9 (95%CI:9.32–27.2, P < .001), compared to those with MRE < 5 kPa. The MEFIB index (defined as positive when MRE ≥ 3.3kPa and FIB-4 ≥ 1.6) had a robust association with the primary outcome with a HR of 20.6 (95% CI: 10.4–40.8, P < .001) and a negative MEFIB had a high negative predictive value for the primary outcome, 99.1% at 5 years. The 3-year risk of incident HCC was 0.35% for MRE<5 kPa, 5.25% for 5–8 kPa, and 5.66% for MRE≥8 kPa, respectively.
Conclusion:
Liver stiffness assessed by MRE is associated with liver-related events and the combination of MRE and FIB-4 has excellent negative predictive value for hepatic decompensation. These data have important implications for clinical practice.
Keywords: nonalcoholic fatty liver disease, portal hypertension, cirrhosis, ascites, varices
Graphical Abstract
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) affects approximately one in four individuals globally1, 2, however, only a subset of patients develops progressive liver disease and are at an increased risk for liver-related and all-cause mortality 3, 4. Fibrosis stage is the strongest predictor of future outcomes 5, 6, however, histologic staging of fibrosis is impractical to scale to the affected population. Furthermore, liver biopsy is associated with a small risk of major complications, sampling error and inter- and intra-observer variability in interpretation 7, 8.
Multiple methods of non-invasive assessment of fibrosis in NAFLD have good diagnostic accuracy for advanced fibrosis including clinical prediction rules 9, proprietary blood based markers 10, 11 and ultrasound based liver stiffness measurements 12 but are limited in detecting earlier stages of fibrosis. Emerging data demonstrate the association of these non-invasive tests with liver-related outcomes and death 13–15. Magnetic resonance elastography (MRE) has excellent diagnostic accuracy for fibrosis, including at earlier fibrosis stages 16, 17. Longitudinal studies have demonstrated that an increase in liver stiffness on MRE is associated with histologic fibrosis progression18. Emerging studies also demonstrate a strong association between higher liver stiffness on MRE and hepatic decompensation and death 19–21.
Despite reproducing a strong association between MRE and liver-related outcomes these studies have primarily been single center with a limited number of outcomes precluding detailed analysis of longitudinal risk. A larger sample size is required to further investigate the association between liver stiffness on MRE and each component of hepatic decompensation. In addition, a larger sample will be required to evaluate the major unmet need of identifying a liver stiffness cut-point associated with a risk of hepatocellular carcinoma (HCC) that would warrant screening 22. Furthermore, the combination of MRE with other non-invasive tests including FIB-4 has demonstrated excellent diagnostic accuracy with high positive predictive value for NASH with significant fibrosis 23, 24 and a larger study would allow for the evaluation of the combination of non-invasive tests of liver-related outcomes. Therefore, we aimed to evaluate the association between liver stiffness on MRE and liver-related outcomes through a collaborative individual participant data meta-analysis (IPDMA).
MATERIALS AND METHODS
This collaborative IPDMA was conducted according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (Supplementary Table 1). Each individual site had local ethics approval and the current study was performed on de-identified data.
Search Strategy and Selection Criteria:
To identify all relevant articles evaluating the association between liver stiffness on MRE and liver-related outcomes in patients at risk for NAFLD a systematic literature search of several databases from each database’s inception to May 26, 2021 was conducted by an experienced medical librarian. The databases included Ovid MEDLINE(R, Epub Ahead of Print, In-Process & Other Non-Indexed Citations and Ovid MEDLINE(R) Daily, Ovid MEDLINE(R) Daily Update, EBM Reviews - Cochrane Central Register of Controlled Trials , EBM Reviews - Cochrane Database of Systematic Reviews , and Embase. (The actual search strategy is available in the supplementary material). Next, we consulted with experts in the field to identify additional published and unpublished primary studies. Studies were included if they met the following inclusion criteria: (1) liver stiffness assessment by MRE, (2) assessment for hepatic decompensation or death and (3) included adult patients (≥ 18 year of age) with NAFLD. NAFLD was defined as hepatic steatosis on imaging or historical liver biopsy in the absence of significant alcohol consumption, secondary causes of hepatic steatosis and other chronic underlying liver disease including viral hepatitis.
Two independent investigators (A.M.M., T.N.) reviewed the titles and abstracts of all citations identified by the search. Full-text manuscripts were retrieved for the included abstracts and were subsequently screened for eligibility by two independent investigators (A.M.M., T.N.). Disagreements at this level were resolved by consensus and a third reviewer if needed. Risk of bias was assessed by two independent investigators using the QUADAS-2 tool. QUADAS-2 tool consists of four key domains covering patient selection, index test, reference standard, and flow of patients through the study and timing of the index test(s) and reference standard (“flow and timing”). Each domain is assessed in terms of the risk of bias and the first three are also assessed in terms of concerns regarding applicability.25
Covariates:
The following data from each study was requested and abstracted. Demographic data including age at time of MRE, sex, race/ethnicity, BMI, defined as the body weight (in kilograms) divided by height (in meters) squared were included. Data regarding metabolic comorbidities including hypertension, hyperlipidemia and type 2 diabetes mellitus were requested. The following biochemical tests: glucose, albumin, hemoglobin A1c, alanine aminotransferase, aspartate aminotransferase, total bilirubin, alkaline phosphatase, fasting lipid panel, platelets, insulin, international normalized ratio, sodium and creatinine were requested. FIB-4 26, MELD-Na Score were calculated as described previously27.
Magnetic Resonance Imaging:
Liver stiffness data using 2D MRE was requested and the mean value was provided. NAFLD was diagnosed based upon imaging and clinical criteria consistent with the American Association for the Study of Liver Diseases NAFLD Practice Guidance as previously published 28. This study only included patients with NAFLD in this analysis.
Rationale for Choosing MRE Cut Points and MEFIB Index
Cut-points are helpful for clinicians and allow for ease in decision making and can be easily applied in clinical setting. The cut-point of 5 kPa has been demonstrated as having a high specificity (90%) for the presence of cirrhosis, therefore was chosen as the lower bound of a category with a high probability for an increased risk for hepatic decompensation.17 The ≥ 8 kPa cut-point recently demonstrated an association with a 20% 1-year risk of hepatic decompensation and was therefore chosen to evaluate a sub-group with the highest risk for hepatic decompensation.19
The MEFIB index, a combination of MRE and FIB-4, has identified a combined cut-point (defined as positive when MRE ≥ 3.3kPa and FIB-4 ≥ 1.6) that can identify patients with stage 2 fibrosis or higher with a high positive predictive value 23, 24 and may be incorporated into selection criteria for NAFLD treatment trials. Assessing the long-term risk associated with the MEFIB index and documenting low risk for those excluded is important to validate the use of the index and its association with long-term clinical outcomes will be helpful.
Outcome Measures and Follow up
The primary outcome was a composite endpoint including ascites, hepatic encephalopathy, varices needing treatment assessed by the local site investigator. Ascites was defined per American Association for the Study of Liver Diseases (AASLD) guidance by imaging or physical exam 29. Hepatic encephalopathy was defined as brain dysfunction cause by liver dysfunction and/or portosystemic shunting per practice guidelines 30. Varices needing treatment were defined as medium/large varices, small varices with high-risk stigmata, decompensated patients with small varices or variceal hemorrhage per AASLD guidance 31.
Secondary outcomes included developing HCC defined by histology or Liver Reporting and Data Systems (LI-RADS) for definite HCC, LI-RADS 5, death and each individual component of the primary outcome.
Patient Follow Up
Follow up time started at the time of the first MRE. For a subset (N= 311) of the total cohort there was no longitudinal follow up and only prevalent outcomes could be assessed. Participants (N=1707) with follow up time were followed until death or the last clinical encounter. Follow up assessment was performed by a retrospective chart review.
Statistical Analysis
Patient characteristics, including demographic, laboratory, imaging and outcome data are summarized with an ANOVA was performed on continuous variables presented as mean (SD), Kruskal-Wallis performed on those presented as median (IQR). Chi-square or Fisher’s exact test as appropriate on all categorical variables. Cumulative incidence curves were generated to evaluate for the cumulative incidence of liver-related events, HCC or death. Patients were censored at the time of death or liver transplantation. Logistic regression analyses were used to evaluate the association between liver stiffness measurement (LSM) on MRE and prevalent outcomes. Cox regression analyses were used to evaluate the association between LSM on MRE and the cumulative outcome as well as the secondary outcomes per 1 kPa increase in LSM. Three ordinal categories on MRE at cut-points of < 5 kPa, 5–8 kPa and ≥8 kPa were also evaluated. The combination of FIB-4 and MRE in the MEFIB score (MRE ≥ 3.3 kPa and FIB-4 ≥ 1.6) was evaluated for its association with outcomes. Multivariable models including age, sex and center were evaluated. All statistical analyses were performed using R version 4.0.5, and a P value less than 0.05 was considered statistically significant.
RESULTS
Study Selection and Characteristics
A total of three studies19, 32, 33 were identified using our primary search strategy and met our inclusion criteria. After contacting the primary or corresponding authors, all of these studies as well as three from additional sources (two subsequently published34, 35 and one unpublished) a total of six studies were included in the IPDMA. The study identification and selection process flow chart are show in Supplementary Figure 1. All studies were retrospective. Five studies included follow up time and one study only assessed for prevalent decompensation at the time of MRE. One study included 145 patients not meeting the study definition of NAFLD who were excluded and only 120 patients meeting the definition of NAFLD were included.35 Three studies were performed in the United States 19, 32, 35 at different centers while one was performed in Turkey and two in Japan33, 34. Four published studies underwent risk of bias assessment and had a Quality Assessment of Diagnostic Accuracy Studies assessment (Supplementary Table 2)
Characteristics of the Study Population
Two thousand and eighteen patients who underwent MRE were included. Participants had a mean age of 57.8 (±14) years and were predominantly female (53%). The mean BMI was 31(± 7) kg/m2. The mean (SD) liver stiffness on MRE was 4.15 (± 2.19), and three categories of liver stiffness < 5 kPa, 5–8 kPa and ≥ 8 kPa included 1484, 383, and 149 patients respectively. Higher liver stiffness was associated with older age, race/ethnicity, type II diabetes mellitus (DM), hypertension (HTN), higher HbA1c, AST, alkaline phosphatase, INR and total bilirubin as well as lower triglycerides, albumin, high density lipoprotein (HDL) and low density lipoprotein (LDL) cholesterol and platelet count. Higher FIB-4, NAFLD Fibrosis Score and MELD score were all associated with higher liver stiffness (Table 1).
Table 1.
Clinical, demographic, and imaging characteristics by liver stiffness on MRE
| MRE liver stiffness | Total N=2018 | < 5 kPa N=1486 | 5 – 8 kPa N=383 | ≥ 8 kPa N=149 | P |
|---|---|---|---|---|---|
| Demographic | |||||
| Age in years, mean (SD) | 57.84 (14.20) | 56.06 (14.38) | 62.79 (12.67) | 62.72 (11.86) | < .001 |
| Female, n (%) | 1076 (53.3%) | 787 (53.0%) | 206 (53.8%) | 83 (55.7%) | .787 |
| BMI (kg/m2), mean (SD) | 31.32 (7.42) | 31.3 (7.49) | 31.4 (7.19) | 31.5 (7.41) | .966 |
| Race | .002 | ||||
| White, n (%) | 1185 (58.7%) | 905 (61.0%) | 203 (53.0%) | 77 (51.7%) | |
| Hispanic, n (%) | 161 (8.0%) | 122 (8.2%) | 27 (7.0%) | 12 (8.1%) | |
| Asian, n (%) | 606 (30.0%) | 407 (27.4%) | 145 (37.9%) | 54 (36.2%) | |
| Other, n (%) | 66 (8.4%) | 52 (3.5%) | 8 (2.1%) | 6 (4.0%) | |
| Diabetes, n (%) | 736 (36.5%) | 415 (27.9%) | 226 (59.0%) | 95 (63.8%) | < .001 |
| Hypertension, n (%) | 624 (30.9%) | 367 (24.7%) | 183 (47.8%) | 74 (49.7%) | < .001 |
| Hyperlipidemia, n (%) | 294 (14.6%) | 217 (14.6%) | 62 (16.2%) | 15 (10.1%) | .20 |
| Biochemical profile | |||||
| HbA1c (%), median (IQR) | 6.2 (1.6) | 6.10 (1.5) | 6.60 (1.9) | 6.9 (1.9) | < .001 |
| AST (U/l), median (IQR) | 40.0 (31.3) | 37.0 (29.8) | 48.0 (34.0) | 56.5 (34.0) | < .001 |
| ALT (U/l), median (IQR) | 45.0 (47.0) | 45.0 (48.5) | 44.0 (41.3) | 44.5 (36.0) | .131 |
| Alkaline Phosphatase (U/l), median (IQR) | 109.0 (151.5) | 100.0 (128.0) | 139.0 (188.0) | 176.5 (184.3) | < .001 |
| Total bilirubin (mg/dl), median (IQR) | 0.60 (0.56) | 0.60 (0.40) | 0.70 (0.70) | 0.90 (0.77) | .001 |
| Albumin (g/dl), median (IQR) | 4.30 (0.60) | 4.34 (0.50) | 4.10 (0.80) | 3.80 (0.80) | < .001 |
| Triglycerides (mg/dl), median (IQR) | 144.0 (99.8) | 149.0 (105.0) | 132.0 (88.3) | 123.0 (81.5) | .002 |
| HDL (mg/dl), median (IQR) | 46.6 (19.0) | 47.0 (18.0) | 47.0 (20.3) | 43.2 (18.3) | .009 |
| LDL (mg/dl), median (IQR) | 106.0 (50.0) | 108.5 (51.8) | 101.0 (48.8) | 98.5 (38.3) | .003 |
| Platelet count (109/L), median (IQR) | 197 (112) | 214 (103) | 139 (90) | 135 (95) | < .001 |
| INR, median (IQR) | 1.02 (0.11) | 1.00 (0.13) | 1.10 (0.20) | 1.14 (0.23) | < .001 |
| Clinical scores | |||||
| FIB-4, median (IQR) | 1.83 (2.11) | 1.50 (1.35) | 3.40 (3.07) | 4.14 (3.28) | < .001 |
| NAFLD Fibrosis Score, median (IQR) | −0.59 (2.87) | −1.12 (2.34) | 0.91 (2.14) | 1.53 (2.22) | < .001 |
| MELD Score, median (IQR) | 7.0 (3.0) | 7.0 (2.0) | 8.0 (30) | 9.0 (5.0) | < .001 |
| Imaging | |||||
| MRE (kPa), mean (SD) | 4.15 (2.19) | 3.07 (0.88) | 6.20 (0.83) | 9.63 (1.61) | < .001 |
Abbreviations: HbA1c, Hemoglobin A1c; AST, Aspartate aminotransferase; ALT, Alanine aminotransferase; BMI, Body mass index; HDL, high-density lipoprotein; INR, International normalized ratio; IQR, interquartile range; LDL, low-density lipoprotein; FIB-4, Fibrosis index based on the 4 factor; MRE, Magnetic resonance elastography; SD, Standard deviation. ANOVA performed on continuous variables presented as mean (SD), Kruskal-Wallis performed on all other continuous variables. Chi-square or Fisher’s exact test as appropriate on all categorical variables
Factors Associated with Prevalent Ascites, Hepatic Encephalopathy and Varices Needing Treatment
At baseline among a total of 2018 patients 113 had the primary outcome of prevalent liver related-events (191 total events, ascites [n=98], hepatic encephalopathy [n=56], varices needing treatment [n=37]) and hepatocellular carcinoma was present in 30 participants. Higher LSM on MRE was associated with the primary outcome OR=1.50 (95% CI:1.40–1.61, P < .001). The OR for the primary outcome for MRE 5–8 kPa were OR=7.03 (95% CI: 4.39 – 11.45, P < .001) and for ≥ 8 kPa OR=16.6 (95% CI: 9.87 – 28.2, P < .001) respectively, compared to < 5 kPa (Table 2). The increased risk of the primary outcome remained significant on analysis adjusted for age, sex and center OR=7.95 (95% CI: 4.87 – 12.3, P < .001) for MRE 5–8 kPa and OR =22.9 (95% CI: 13.0 – 40.8, P < .001) for ≥ 8 kPa compared to < 5 kPa. In addition, a one-unit increase in FIB-4, (OR=1.33 [95% CI: 1.26 – 1.41, P < .001]), NAFLD Fibrosis Score (OR= 2.18 [95% CI: 1.89 – 2.53, P < .001]) and MELD score (OR= 1.21 [95% CI: 1.17 – 1.26, P < .001]) were associated with higher odds of liver-related events.
Table 2.
Factors Associated with the Primary Outcome (Ascites, Hepatic Encephalopathy or Varices Needing Treatment) at Baseline on Logistic Regression (N=2018)
| Liver-Related Outcomes OR (95% CI) | P-value | |
|---|---|---|
| MRE | ||
| < 5 kPa (referent) | ref | |
| 5 – 8 kPa | 7.03 (4.39 – 11.4) | < .001 |
| ≥ 8 kPa | 16.6 (9.87 – 28.2) | < .001 |
| Demographic & Biochemical | ||
| BMI (kg/m2) | 1.03 (1.00 – 1.05) | .053 |
| HbA1c (%) | 0.98 (0.81 – 1.15) | .772 |
| AST (U/l) (per 5-unit increase) | 1.01 (1.00 – 1.01) | .002 |
| ALT (U/l) (per 5-unit increase) | 0.99 (0.98 – 0.99) | < .001 |
| Alkaline Phosphatase (U/l) (per 5-unit increase) | 1.00 (0.997 – 1.00) | .155 |
| Total bilirubin (mg/dl) | 1.14 (1.04 – 1.27) | .014 |
| Albumin (g/dl) | 0.12 (0.09 – 0.17) | < .001 |
| Platelet count (109/L) (per 10-unit increase) | 0.99 (0.98 – 0.99) | < .001 |
| Clinical Score | ||
| FIB-4 (per 1-unit increase) | 1.33 (1.26 – 1.41) | < .001 |
| NAFLD Fibrosis Score (per 1-unit increase) | 2.18 (1.89 – 2.53) | < .001 |
| MELD score (per 1-unit increase) | 1.21 (1.17 – 1.26) | < .001 |
| Positive MEFIB | 19.5 (10.1 – 43.9) | < .001 |
Abbreviations: HbA1c, Hemoglobin A1c; AST, Aspartate aminotransferase; ALT, Alanine aminotransferase; BMI, Body mass index; HDL, high-density lipoprotein; INR, International normalized ratio; LDL, low-density lipoprotein; FIB-4, Fibrosis index based on the 4 factor; MRE, Magnetic resonance elastography
A positive MEFIB index, defined as a combination of MRE ≥ 3.3 kPa and FIB-4 ≥ 1.6, was present in 785 patients and had a strong association with prevalent hepatic decompensation compared to those with a negative MEFIB (12.1% vs 0.7%). The odds ratio for the hepatic decompensation was OR=19.5 [95% CI: 10.1 – 43.9, P < .001] for a positive MEFIB vs a negative MEFIB and remained significant in multivariable analysis adjusted for age, sex and center OR=24.4 [95%CI: 12.2 – 56.1, P < .001].
Predictors of Incident Ascites, Hepatic Encephalopathy and Varices Needing Treatment
Among 1707 patients with a median (IQR) follow up period of 3.0 (4.2) years an additional 120 patients had the primary outcome (total events 180, ascites [n=89], hepatic encephalopathy [n=54], varices needing treatment [n=37]) and 39 participants developed incident hepatocellular carcinoma. Over the study period 38 patients underwent incident liver transplantation and 169 died (Table 4).
Table 4.
Liver-Related Outcomes and Death by Liver Stiffness on MRE
| MRE Liver Stiffness | ||||
|---|---|---|---|---|
| Total N=2018 |
< 5 kPa N=1486 |
5 – 8 kPa N=383 |
≥ 8 kPa N=149 |
|
| Varices needing treatment– N (%) | 74 (3.7%) | 22 (1.5%) | 32 (8.4%) | 20 (13%) |
| Ascites– N (%) | 187 (9.3%) | 45 (3.0%) | 91 (24%) | 51 (34%) |
| Hepatic encephalopathy– N (%) | 110 (5.5%) | 20 (1.3%) | 58 (15%) | 32 (21%) |
| Hepatocellular carcinoma– N (%) | 69 (3.4%) | 21 (1.4%) | 32 (8.3%) | 16 (11%) |
| Liver transplant– N (%) | 38 (1.9%) | 10 (0.6%) | 17 (4.4%) | 11 (7.4%) |
| Death– N (%) | 169 (8.4%) | 87 (5.8%) | 50 (13%) | 32 (21%) |
The 1- and 3-year risk of the primary outcome was 0.68% and 1.64% for MRE < 5 kPa, 7.47% and 16.87% for 5–8 kPa and 8.88% and 19.14% for MRE ≥ 8 kPa respectively. The incident risk of the primary outcome and each of its individual components increased across the three liver stiffness categories, P < .001 (Figure 1) (MRE < 5 kPa vs. 5–8 kPa: p<0.001, MRE < 5 kPa vs. ≥ 8 kPa: P < .001, MRE 5–8 kPa vs ≥8 kPa: P = .002). Higher LSM on MRE was associated with incident hepatic decompensation HR=1.43 per 1 kPa increase in liver stiffness (95% CI: 1.36– 1.51, P < .001).The HR for the primary outcome for MRE 5–8 kPa were HR=11.0 (95% CI: 7.03 – 17.1, P < .001) and for ≥ 8 kPa HR=15.9 (95% CI: 9.32 – 27.2, P < .001) respectively, compared to < 5 kPa (Table 3). The increased risk of hepatic decompensation remained significant on analysis adjusted for age, sex and center HR=11.0 (95% CI: 6.96 – 17.3, P < .001) for MRE 5–8 kPa and HR =16.6 (95% CI: 9.61 – 28.7, P < .001) for ≥ 8 kPa compared to < 5 kPa. In addition, a one-unit increase in FIB-4, (HR=1.32 [95% CI: 1.27 – 1.36, P < .001]), NAFLD Fibrosis Score (HR= 2.00 [95% CI: 1.79 – 2.25, P < .001]) and MELD score (HR= 1.08 [95% CI: 1.04 – 1.13, P < .001]) were associated with higher odds of hepatic decompensation.
Figure 1:
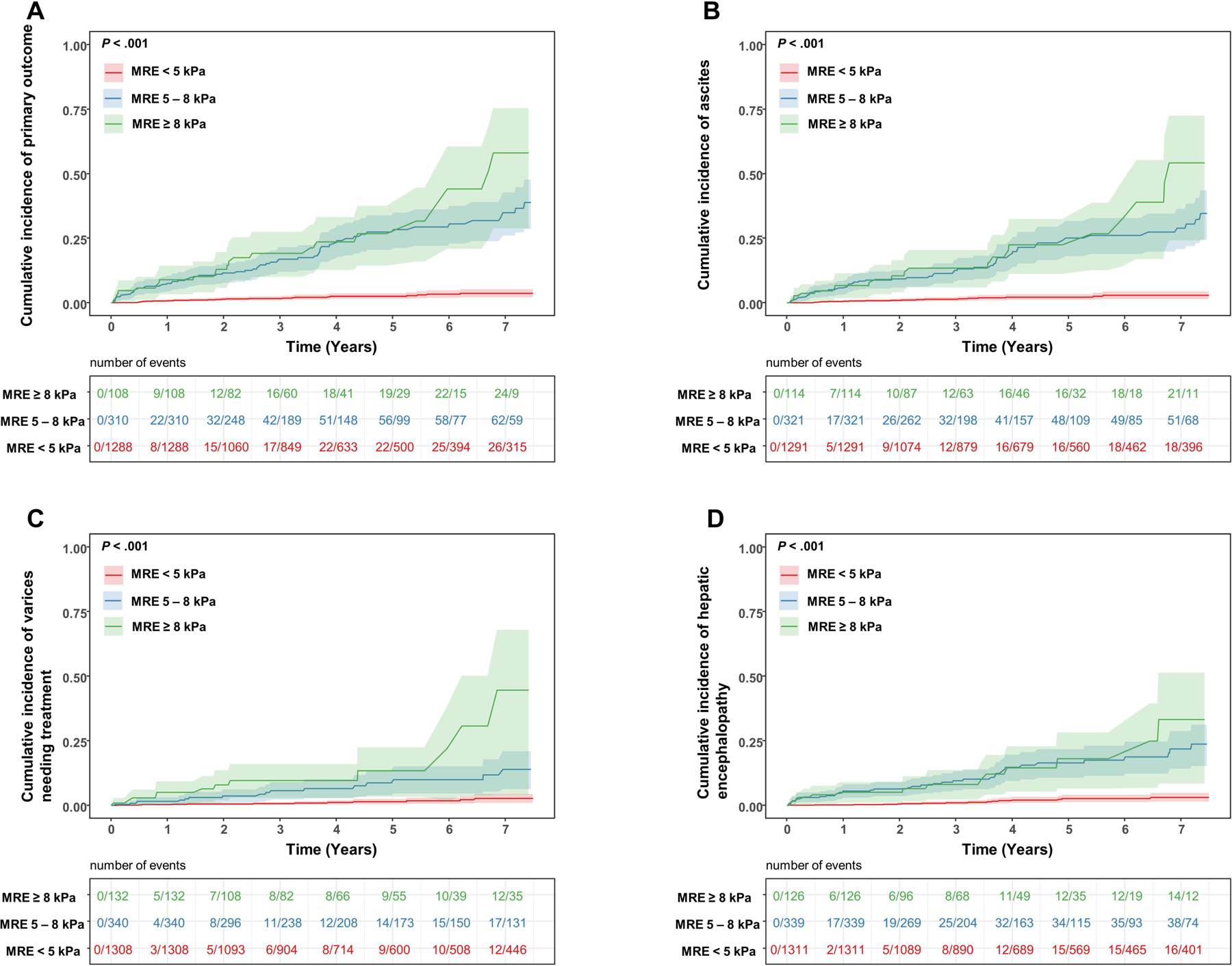
Cumulative incidence of (A) primary composite outcome, (B) ascites, (C) varices needing treatment and (D) hepatic encephalopathy by liver stiffness cut points on MRE < 5 kPa, 5–8 kPa and ≥ 8 kPa.
Table 3.
Factors Associated with Incident Development of the Primary Outcome (Ascites, Hepatic Encephalopathy or Varices Needing Treatment) on Cox-Proportional Hazards Regression (N=1707)
| Liver-Related Outcomes Hazard Ratios (95% CI) | P-value | |
|---|---|---|
| MRE | ||
| < 5 kPa (referent) | ||
| 5 – 8 kPa | 11.0 (7.03–17.1) | < .001 |
| ≥ 8 kPa | 15.9 (9.32–27.2) | < .001 |
| Demographic & Biochemical | ||
| BMI (kg/m2) | 1.02 (1.00–1.04) | .128 |
| HbA1c (%) | 1.25 (1.08–1.45) | .003 |
| AST (U/l) (per 5-unit increase) | 1.01 (1.00–1.01) | < .001 |
| ALT (U/l) (per 5-unit increase) | 1.00 (0.99–1.00) | .179 |
| Alkaline Phosphatase (U/l) (per 5-unit increase) | 1.001 (0.999–1.002) | .318 |
| Total bilirubin (mg/dl) | 1.31 (1.22–1.41) | < .001 |
| Albumin (g/dl) | 0.21 (0.16–0.28) | < .001 |
| Platelet count (109/L) (per 10-unit increase) | 0.98 (0.98–0.99) | < .001 |
| Clinical Score | ||
| FIB-4 | 1.32 (1.27–1.36) | < .001 |
| NAFLD Fibrosis Score | 2.00 (1.79–2.25) | < .001 |
| MELD score (per 1-unit increase) | 1.08 (1.04–1.13) | < .001 |
| Positive MEFIB | 20.6 (10.4–40.8) | < .001 |
Abbreviations: HbA1c– Hemoglobin A1c; AST– Aspartate aminotransferase; ALT– Alanine aminotransferase; BMI– Body mass index; HDL– high-density lipoprotein; INR– International normalized ratio; LDL– low-density lipoprotein; FIB-4– Fibrosis index based on the 4 factor
In sensitivity analysis of patients without overt evidence of cirrhosis and/or portal hypertension (N=1260) the results remained consistent (Supplemental Table 3). The HR for the primary outcome for MRE 5–8 kPa were HR=8.96 (95% CI: 4.20 – 19.1, P < .001) and for ≥ 8 kPa HR=19.9 (95% CI: 8.10 – 49.0, P < .001) respectively, compared to < 5 kPa. The increased risk of hepatic decompensation remained significant on analysis adjusted for age, sex and center HR=8.9 (95% CI: 4.1 – 19.3, P < .001) for MRE 5–8 kPa and HR =20.6 (95% CI: 8.1 – 52.3, P < .001) for ≥ 8 kPa compared to < 5 kPa.
A positive MEFIB index, had a strong association with incident hepatic decompensation compared to those with a negative MEFIB (HR=20.6 [95% CI: 10.4 – 40.8, P < .001]) and remained significant in multivariable analysis adjusted for age, sex and center HR=19.0 [95%CI: 9.45 – 38.1, P < .001]. Furthermore, among patients with indeterminate FIB-4 score (1.3 – 2.67), liver stiffness of < 5 kPa, 5–8 kPa and > 8kPa was associated with the primary outcome occurring in 2.3% (N=11 of 474 patients), 10.1% (N=9 of 89 patients) and 10.7% (N=3 of 28 patients) of patients respectively, P < .001.
Factors Associated with Incident Hepatocellular Carcinoma and Death
The 1- and 3-year risk of the incident HCC was 0.1% and 0.35% for MRE < 5 kPa, 3.71% and 5.25% for 5–8 kPa and 3.61% and 5.66% for MRE > 8 kPa respectively (Figure 3A). The 1- and 3-year risk of the incident HCC was 2.76% and 3.92% for a positive MEFIB index compared to <0.01% and 0.15% for participants with a negative MEFIB index (Figure 3B). The HR ratio for HCC for MRE 5–8 kPa and MRE ≥ 8 kPa compared to < 5 kPa were HR=23.4 (95%CI: 6.85–79.8, P < .001) and HR=33.8 (95%CI: 8.94–127.7, P < .001) respectively. The HR ratio for HCC for participants with a positive MEFIB index compared to a negative MEFIB index was HR=40.5 (95% CI: 5.48–298.9, P < .001).
Figure 3:
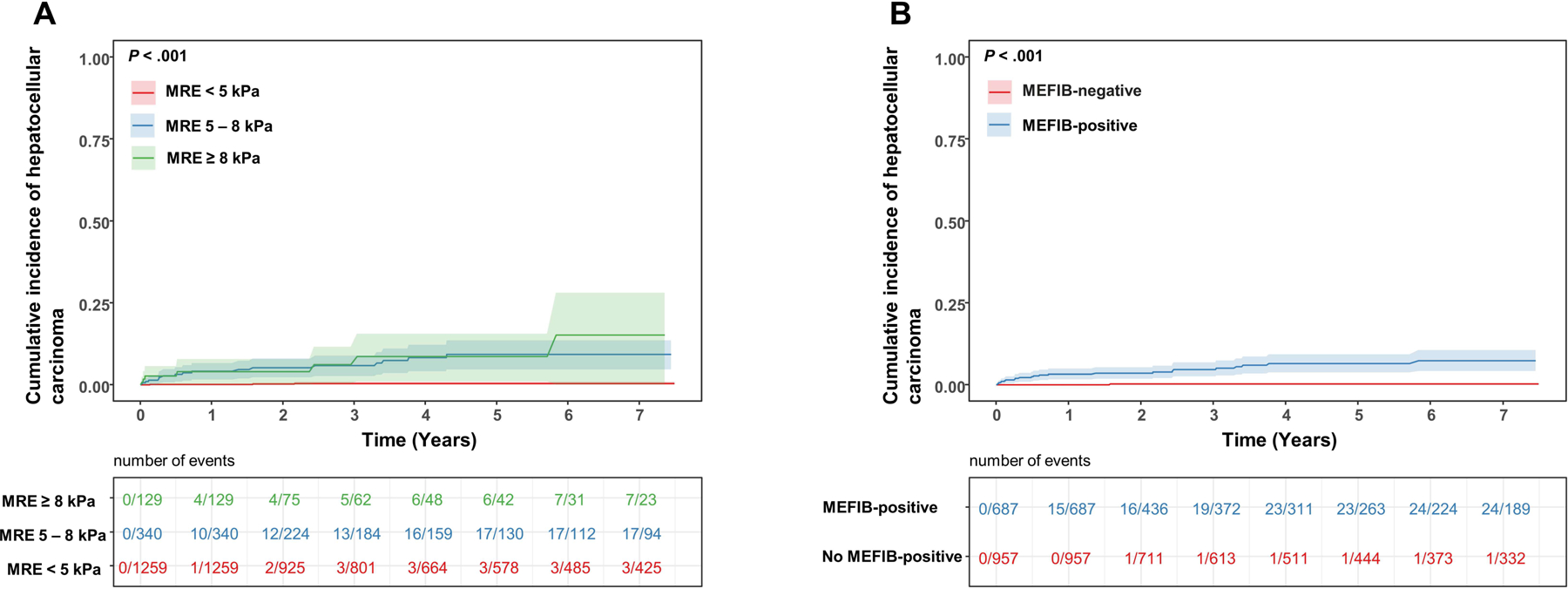
Cumulative incidence of hepatocellular carcinoma by (a) liver stiffness cut points on MRE < 5 kPa, 5–8 kPa and ≥ 8 kPa and (b) MEFIB index, with positive defined as a combination of MRE ≥ 3.3 kPa and FIB-4 ≥ 1.6.
Of the 169 deaths, 78 (46%) were liver-related and 91 (54%) were non-liver related. The 1- and 3-year risk of the death were 1.41% and 4.5% for MRE < 5 kPa, 4.51% and 10.18% for 5–8 kPa and 6.46% and 20.19% for MRE ≥ 8 kPa respectively (Supplemental Figure 2A). The 1- and 3-year risk of the death was 4.36% and 12.48% for a positive MEFIB index compared to 0.99% and 2.33% for participants with a negative MEFIB index (Supplemental Figure 2B). The HR ratio for death for MRE 5 −8 kPa and MRE ≥ 8 kPa compared to < 5 kPa were HR= 2.31 (95%CI:1.63–3.28, P < .001) and HR=4.78 (95%CI: 3.18–7.20, P < .001) respectively. The HR ratio for death for participants with a positive MEFIB index compared to a negative MEFIB index was HR=3.78 (95% CI: 2.68–5.34, P < .001).
Discussion
Using individual participant data from six, international centers with MRE and assessment of liver-related outcomes, we demonstrate that liver stiffness on MRE has a strong association with liver-related outcomes, hepatocellular carcinoma and death. The 3-year risk of the composite outcome of ascites, hepatic encephalopathy or varices needing treatment increased from 1.6% in those with MRE < 5 kPa to 19% among participants with MRE ≥ 8 kPa. Furthermore, the MEFIB combination of MRE and FIB-4 demonstrated strong associations with liver-related outcomes, hepatocellular carcinoma and death, and when negative was associated with a <1% risk of liver related outcomes at 3-years. This data and the association between liver stiffness on MRE and liver-related events among patients with indeterminate FIB-4 are of significant clinical value. Importantly, MRE ≥ 5 kPa is associated with a greater than 1.5% risk of HCC per year supporting the use of this MRE cut-point as an indication for HCC surveillance.
IN CONTEXT WITH PUBLISHED LITERATURE
Fibrosis stage and NASH histology have been adopted as surrogate markers for future liver-related outcomes in clinical trials based on longitudinal studies demonstrating the association between fibrosis stage on liver biopsy and liver-related outcomes and death3, 5. Recently, Hagstrom and colleagues evaluated clinical prediction rules of NAFLD fibrosis, including FIB-4, and demonstrated associations with a composite outcome of severe liver disease which included cirrhosis, HCC, liver transplantation or decompensated liver disease. However, the diagnostic accuracy of clinical prediction rules diminished over time and the study was limited in characterization of NAFLD status and outcome assessment, which was made by ICD codes.36 Petta and colleagues demonstrated the LSM on vibration controlled elastography > 21 kPa predicted the risk of liver decompensation among patients with F3-F4 NAFLD, however, baseline LSM did not predict mortality and the gradient of risk across different LSM thresholds was not clear.37
MRE is a quantitative biomarker of liver fibrosis17 and recent studies demonstrate a strong association with liver related outcomes. The largest study to date by Gidener and colleagues demonstrated that LSM on MRE was associated with the development of cirrhosis and with liver-related outcomes and death among 194 patients with compensated NAFLD cirrhosis and longitudinal follow-up.19 Smaller, single-center studies have defined multiple different optimal thresholds.32–34, 38 With this large, international, multi-center cohort, we are able to better define optimal cut-points for liver-related outcomes. This IPDMA combined published and unpublished data from six international centers creating a diverse cohort with individual patient data and provides important new data on the incident risk of liver-related events, HCC and death, which all demonstrated strong associations with MRE.
With the increasing importance of non-invasive tests (NITs) in clinical practice and clinical trials the findings from this manuscript will have immediate impact on (1) selection of patients for HCC screening based on NITs, (2) selection of patients for clinical trials based on MEFIB, (3) management of patients with indeterminate FIB-4, (4) frequency of liver stiffness assessment and (5) identification of patients without overt clinical evidence of cirrhosis who have compensated advanced chronic liver disease. This study identified that MRE ≥ 5 kPa is an appropriate cut-point to justify HCC screening, independent of a known diagnosis of cirrhosis based on the annual risk of incident HCC being higher than a cost-effectiveness threshold of ≥ 1.5%.22 Furthermore, the MEFIB combination of MRE and FIB-4 provides excellent negative predictive value for liver-related events, and patients who are negative are at low risk (<1%) for 3-years suggesting that this may be an appropriate interval between MRE assessments. These data also help define a strategy to address patients with an indeterminate FIB-4 score. If FIB-4 is greater than or equal to 1.6 a subsequent MRE less than 3.3 kPa places them at low risk for liver-related events. Coversely, an MRE ≥ 3.3 kPa places them at risk for future-liver related events with further increased risk for MRE 5–8 kPa and > 8 kPa. Importantly, in sensitivity analysis of patients without overt clinical evidence of cirrhosis, liver stiffness on MRE remained associated with liver-related events supporting its use to identify patients with compensated advanced chronic liver disease. Finally, LSM on MRE was clearly associated with death further supporting its importance as a biomarker of disease severity.
STRENGTHS AND LIMITATIONS
The large, diverse, international cohort with individual patient data is a substantial strength and included more than three times as many patients with suspected cirrhosis compared to a seminal recent study of the natural history NAFLD fibrosis on liver biopsy from Sanyal and colleagues.6 While the retrospective data collection and outcome assessment is a limitation, the expert assessment for the diagnosis of NAFLD and liver-related outcomes is a significant strength. Furthermore, to assess for variability between centers, multivariable logistic regression and Cox models adjusted for center and the findings remained consistent. An additional limitation is the lack of data on major adverse cardiovascular events; however, we did assess for all-cause mortality, with 54% of deaths in those without a liver-related event, and the findings supported a strong association between MRE and mortality. Future, multicenter, prospective studies will be required to clearly evaluate the association between liver stiffness and extra-hepatic events. An additional limitation is that we did not evaluate the impact of mild-moderate alcohol use and underlying cardiovascular disease on the association between MRE and liver-related events. Finally, MRE was only assessed at a single time point in this study. Currently, a limited number studies have demonstrated an association between change in MRE and change in liver histology in cohorts of 50–100 patients.18, 39 Furthermore, the implication of change in MRE over time on the risk of future liver-related events and mortality will require further study.
IMPLICATIONS FOR CLINICAL PRACTICE AND FUTURE RESEARCH
This study provides new data on the association between LSM on MRE and incident liver-related events and death that will be useful in clinical practice. MRE had a strong association with the development of meaningful liver-related outcomes including ascites, varices needing treatment, hepatic encephalopathy, HCC and death. These data have immediate implications for clinical practice, establishing very low risk in patients with a negative MEFIB index and justification for HCC screening in patients with NAFLD with MRE ≥ 5 kPa. Furthermore, MRE values can help stratify the gradient of risk for incident hepatic decompensation. MRE may be warranted in cases of greater likelihood of VCTE failure, BMI ≥ 35 kg/m2 40, ascites or when higher accuracy is needed, ie making a treatment decision. Previous studies have demonstrated the MRE has superior diagnostic accuracy for fibrosis risk stratification and head-to-head comparisons with VCTE for liver-related events should be evaluated to demonstrate if MRE has superior accuracy for this outcome. Future studies should evaluate the prospective risk of outcomes related to MRE and can consider other combinations of non-invasive tests associated with liver-related outcomes including the enhanced liver fibrosis, ELF, score.15 Furthermore, studies with repeated MRE assessments over time and evaluation of the impact of change in liver stiffness on liver-related outcomes will be critical to supporting its use as a biomarker of treatment response in NAFLD. In conclusion, liver stiffness on MRE has a strong association with liver-related events including incident HCC and the MEFIB combination of MRE with FIB-4 can be used to identify a population with a low-risk for liver-related events.
Supplementary Material
Figure 2:
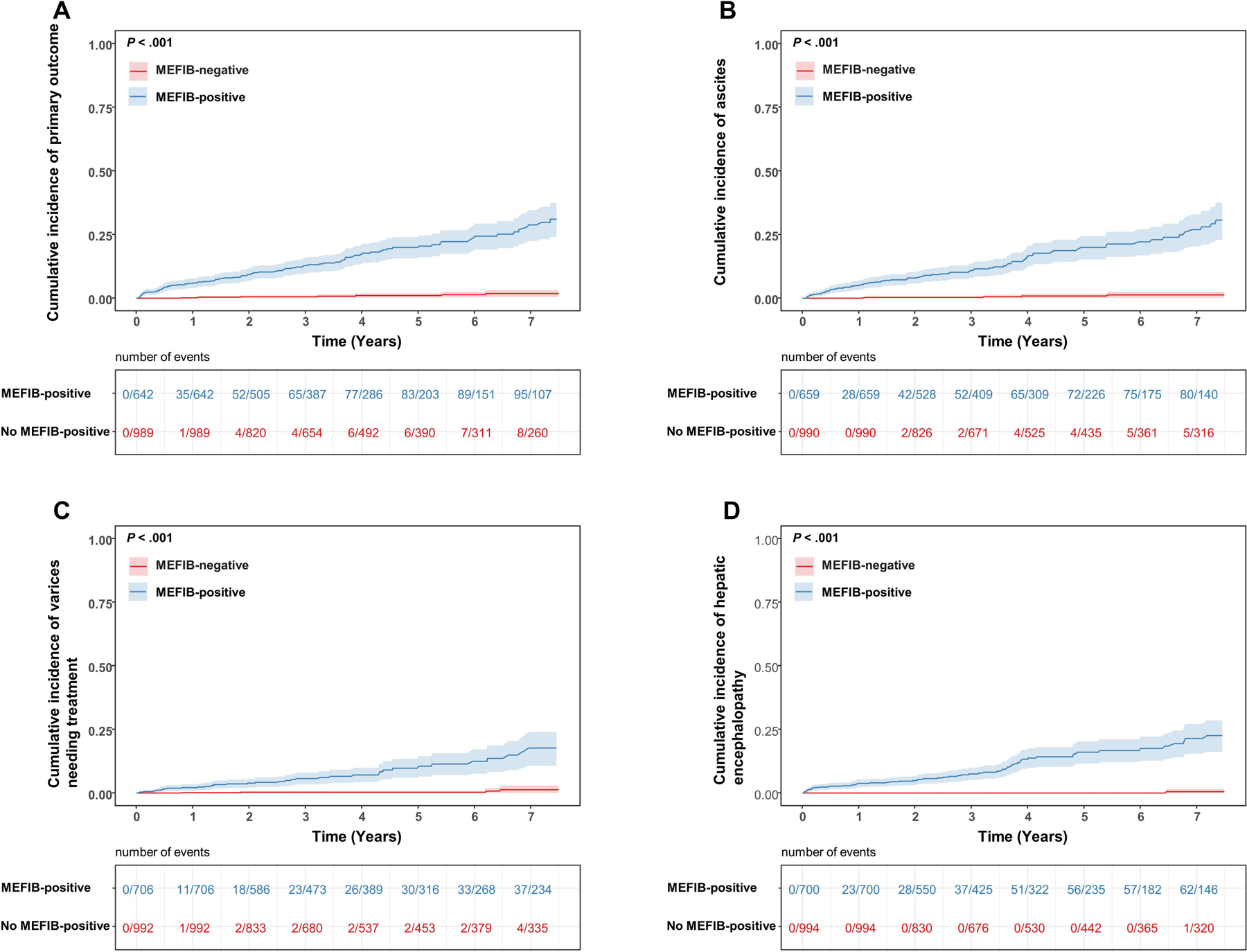
Cumulative incidence of (A) primary composite outcome, (B) ascites, (C) varices needing treatment and (D) hepatic encephalopathy by MEFIB index, with positive defined as a combination of MRE ≥ 3.3 kPa and FIB-4 ≥ 1.6.
FUNDING
VA is supported by NIDDK (K23DK119460). RL receives funding support from NCATS (5UL1TR001442), NIDDK (U01DK061734, U01DK130190, R01DK106419, R01DK121378, R01DK124318, P30DK120515), NHLBI (P01HL147835), and NIAAA (U01AA029019). AMA is supported by K23DK115594.
ABBREVIATIONS
- MRE
Magnetic resonance elastography
- IPDMA
individual participant data pooled meta-analysis
- NAFLD
nonalcoholic fatty liver disease
- LS
liver stiffness
- BMI
body mass index
- AASLD
American Association for the Study of Liver Diseases
- LI-RADS
Liver Reporting and Data Systems
- LSM
liver stiffness measurement
- SD
standard deviation
- IQR
interquartile range
- MELD
model for end-stage liver disease
- HCC
hepatocellular carcinoma
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Potential conflict of interests:
RL serves as a consultant to Aardvark Therapeutics, Altimmune, Anylam/Regeneron, Amgen, Arrowhead Pharmaceuticals, AstraZeneca, Bristol-Myer Squibb, CohBar, Eli Lilly, Galmed, Gilead, Glympse bio, Hightide, Inipharma, Intercept, Inventiva, Ionis, Janssen Inc., Madrigal, Metacrine, Inc., NGM Biopharmaceuticals, Novartis, Novo Nordisk, Merck, Pfizer, Sagimet, Theratechnologies, 89 bio, Terns Pharmaceuticals and Viking Therapeutics. In addition his institutions received research grants from Arrowhead Pharmaceuticals, Astrazeneca, Boehringer-Ingelheim, Bristol-Myers Squibb, Eli Lilly, Galectin Therapeutics, Galmed Pharmaceuticals, Gilead, Intercept, Hanmi, Intercept, Inventiva, Ionis, Janssen, Madrigal Pharmaceuticals, Merck, NGM Biopharmaceuticals, Novo Nordisk, Merck, Pfizer, Sonic Incytes and Terns Pharmaceuticals. Co-founder of LipoNexus Inc.
REFERENCES
- 1.Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73–84. [DOI] [PubMed] [Google Scholar]
- 2.Loomba R, Wong R, Fraysse J, et al. Nonalcoholic fatty liver disease progression rates to cirrhosis and progression of cirrhosis to decompensation and mortality: a real world analysis of Medicare data. Aliment Pharmacol Ther 2020;51:1149–1159. [DOI] [PubMed] [Google Scholar]
- 3.Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2015;149:389–97.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ekstedt M, Hagstrom H, Nasr P, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 2015;61:1547–54. [DOI] [PubMed] [Google Scholar]
- 5.Dulai PS, Singh S, Patel J, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology 2017;65:1557–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanyal AJ, Van Natta ML, Clark J, et al. Prospective Study of Outcomes in Adults with Nonalcoholic Fatty Liver Disease. N Engl J Med 2021;385:1559–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ratziu V, Charlotte F, Heurtier A, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology 2005;128:1898–906. [DOI] [PubMed] [Google Scholar]
- 8.Merriman RB, Ferrell LD, Patti MG, et al. Correlation of paired liver biopsies in morbidly obese patients with suspected nonalcoholic fatty liver disease. Hepatology 2006;44:874–880. [DOI] [PubMed] [Google Scholar]
- 9.Mózes FE, Lee JA, Selvaraj EA, et al. Diagnostic accuracy of non-invasive tests for advanced fibrosis in patients with NAFLD: an individual patient data meta-analysis. Gut 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loomba R, Jain A, Diehl AM, et al. Validation of Serum Test for Advanced Liver Fibrosis in Patients with Nonalcoholic Steatohepatitis. Clin Gastroenterol Hepatol 2018. [DOI] [PubMed] [Google Scholar]
- 11.Vali Y, Lee J, Boursier J, et al. Enhanced liver fibrosis test for the non-invasive diagnosis of fibrosis in patients with NAFLD: A systematic review and meta-analysis. J Hepatol 2020;73:252–262. [DOI] [PubMed] [Google Scholar]
- 12.Selvaraj EA, Mózes FE, Jayaswal ANA, et al. Diagnostic accuracy of elastography and magnetic resonance imaging in patients with NAFLD: A systematic review and meta-analysis. J Hepatol 2021. [DOI] [PubMed] [Google Scholar]
- 13.Hagström H, Nasr P, Ekstedt M, et al. Accuracy of Noninvasive Scoring Systems in Assessing Risk of Death and Liver-Related Endpoints in Patients With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol 2019;17:1148–1156.e4. [DOI] [PubMed] [Google Scholar]
- 14.Petta S, Sebastiani G, Viganò M, et al. Monitoring Occurrence of Liver-Related Events and Survival by Transient Elastography in Patients With Nonalcoholic Fatty Liver Disease and Compensated Advanced Chronic Liver disease. Clin Gastroenterol Hepatol 2020. [DOI] [PubMed] [Google Scholar]
- 15.Sanyal AJ, Harrison SA, Ratziu V, et al. The Natural History of Advanced Fibrosis Due to Nonalcoholic Steatohepatitis: Data From the Simtuzumab Trials. Hepatology 2019;70:1913–1927. [DOI] [PubMed] [Google Scholar]
- 16.Park CC, Nguyen P, Hernandez C, et al. Magnetic Resonance Elastography vs Transient Elastography in Detection of Fibrosis and Noninvasive Measurement of Steatosis in Patients With Biopsy-Proven Nonalcoholic Fatty Liver Disease. Gastroenterology 2017;152:598–607.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsu C, Caussy C, Imajo K, et al. Magnetic Resonance vs Transient Elastography Analysis of Patients With Nonalcoholic Fatty Liver Disease: A Systematic Review and Pooled Analysis of Individual Participants. Clin Gastroenterol Hepatol 2019;17:630–637.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ajmera VH, Liu A, Singh S, et al. Clinical Utility of an Increase in Magnetic Resonance Elastography in Predicting Fibrosis Progression in Nonalcoholic Fatty Liver Disease. Hepatology 2020;71:849–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gidener T, Ahmed OT, Larson JJ, et al. Liver Stiffness by Magnetic Resonance Elastography Predicts Future Cirrhosis, Decompensation and Death in NAFLD. Clin Gastroenterol Hepatol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han MAT, Vipani A, Noureddin N, et al. MR elastography-based liver fibrosis correlates with liver events in nonalcoholic fatty liver patients: A multicenter study. Liver International 2020;40:2242–2251. [DOI] [PubMed] [Google Scholar]
- 21.Tamaki N, Kurosaki M, Takahashi Y, et al. Liver fibrosis and fatty liver are independently associated with cardiovascular disease risk. J Gastroenterol Hepatol 2021. [DOI] [PubMed] [Google Scholar]
- 22.Loomba R, Lim JK, Patton H, et al. AGA Clinical Practice Update on Screening and Surveillance for Hepatocellular Carcinoma in Patients With Nonalcoholic Fatty Liver Disease: Expert Review. Gastroenterology 2020;158:1822–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tamaki N, Imajo K, Sharpton S, et al. MRE plus FIB-4 (MEFIB) versus FAST in detection of candidates for pharmacological treatment of NASH-related fibrosis. Hepatology 2021. [Google Scholar]
- 24.Jung J, Loomba RR, Imajo K, et al. MRE combined with FIB-4 (MEFIB) index in detection of candidates for pharmacological treatment of NASH-related fibrosis. Gut 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. QUADAS-2: Background Document. [Google Scholar]
- 26.Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006;43:1317–25. [DOI] [PubMed] [Google Scholar]
- 27.Kim WR, Biggins SW, Kremers WK, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med 2008;359:1018–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67:328–357. [DOI] [PubMed] [Google Scholar]
- 29.Biggins SW, Angeli P, Garcia-Tsao G, et al. Diagnosis, Evaluation, and Management of Ascites, Spontaneous Bacterial Peritonitis and Hepatorenal Syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2021;74:1014–1048. [DOI] [PubMed] [Google Scholar]
- 30.Vilstrup H, Amodio P, Bajaj J, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study Of Liver Diseases and the European Association for the Study of the Liver. Hepatology 2014;60:715–35. [DOI] [PubMed] [Google Scholar]
- 31.Garcia-Tsao G, Abraldes JG, Berzigotti A, et al. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology 2017;65:310–335. [DOI] [PubMed] [Google Scholar]
- 32.Han MAT, Vipani A, Noureddin N, et al. MR elastography-based liver fibrosis correlates with liver events in nonalcoholic fatty liver patients: A multicenter study. Liver Int 2020;40:2242–2251. [DOI] [PubMed] [Google Scholar]
- 33.Matsui N, Imajo K, Yoneda M, et al. Magnetic resonance elastography increases usefulness and safety of non-invasive screening for esophageal varices. J Gastroenterol Hepatol 2018;33:2022–2028. [DOI] [PubMed] [Google Scholar]
- 34.Tamaki N, Higuchi M, Kurosaki M, et al. Risk Difference of Liver-Related and Cardiovascular Events by Liver Fibrosis Status in Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ajmera V, Nguyen K, Tamaki N, et al. Prognostic utility of magnetic resonance elastography and MEFIB index in predicting liver-related outcomes and mortality in individuals at risk of and with nonalcoholic fatty liver disease. Therap Adv Gastroenterol 2022;15:17562848221093869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hagström H, Talbäck M, Andreasson A, et al. Ability of Noninvasive Scoring Systems to Identify Individuals in the Population at Risk for Severe Liver Disease. Gastroenterology 2020;158:200–214. [DOI] [PubMed] [Google Scholar]
- 37.Petta S, Sebastiani G, Viganò M, et al. Monitoring Occurrence of Liver-Related Events and Survival by Transient Elastography in Patients With Nonalcoholic Fatty Liver Disease and Compensated Advanced Chronic Liver Disease. Clin Gastroenterol Hepatol 2021;19:806–815.e5. [DOI] [PubMed] [Google Scholar]
- 38.Higuchi M, Tamaki N, Kurosaki M, et al. Longitudinal association of magnetic resonance elastography-associated liver stiffness with complications and mortality. Aliment Pharmacol Ther 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jayakumar S, Middleton MS, Lawitz EJ, et al. Longitudinal correlations between MRE, MRI-PDFF, and liver histology in patients with non-alcoholic steatohepatitis: Analysis of data from a phase II trial of selonsertib. J Hepatol 2019;70:133–141. [DOI] [PubMed] [Google Scholar]
- 40.Chen J, Yin M, Talwalkar JA, et al. Diagnostic Performance of MR Elastography and Vibration-controlled Transient Elastography in the Detection of Hepatic Fibrosis in Patients with Severe to Morbid Obesity. Radiology 2017;283:418–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


