Abstract
Objectives:
Neuroinflammation and secondary injury play a central role in the pathophysiology of intracerebral hemorrhage. The dual endothelin-1/VEGFsignal-peptide receptor (DEspR) has been reported to mediate the inflammatory response after acute brain injury in a rodent model. We performed a pilot study to assess the expression of DEspR on circulating leukocytes in patients who presented with spontaneous intracerebral hemorrhage (ICH).
Materials and Methods:
We performed a prospective observational study of patients presenting to two academic medical centers with ICH. Normal healthy volunteers (NHV) were also recruited for sample analysis. Whole blood was obtained, and flow cytometry was performed to examine DEspR expression on neutrophils, monocytes, and lymphocytes.
Results:
A total of 19 patients were included in analysis. Median ICH volume was 39cm3 [IQR 19cm3, 73cm3] and median ICH score was 2 [IQR 2, 3]. DEspR expression was more abundant on neutrophils (median 2.4% [IQR 0.5%, 5.8%], p=0.0064) and monocytes (median 4.4% [IQR 1.7%, 15.8%], p=0.003) relative to lymphocytes (median 0.9% [IQR 0.2%, 3.3%]). ICH patients had higher DEspR expression in all leukocytes relative to NHV (p<0.05 for all). Among ICH patients, those with a medical history of hypertension showed higher DEspR expression on neutrophils and monocytes (p = 0.018) compared to those without hypertension.
Conclusions:
In this pilot study, DEspR is expressed on circulating neutrophils and monocytes in humans after ICH, with higher levels of expression in those with hypertension. Future work in larger cohorts should examine the relationship of DEspR expression with neuroinflammatory endpoints and long-term outcome.
Keywords: Cerebral Hemorrhage, Hemorrhagic Stroke, Neutrophils, Monocytes
Introduction
Intracerebral hemorrhage (ICH) is one of the deadliest and most disabling forms of stroke, affecting more than 50,000 people in the United States each year.(1) Although ICH accounts for about 10 to 15% of all stroke cases, it is associated with higher mortality and disability compared to other stroke subtypes.(1) As no therapy has yet proven benefit, there is a need to identify novel targets for therapeutic development.
One potential opportunity lies within the role of the inflammatory cascade and secondary injury.(2) Brain injury following ICH typically activates local inflammatory factors, breakdown of the blood-brain barrier, and recruitment of circulating inflammatory cells.(3) While initial tissue damage often occurs within the first minutes and hours, the recruitment of peripheral immune cells and/or activation of the local immune response occurs in the hours and days thereafter, and can exacerbate the initial injury.(2) Multiple lines of evidence implicate neuroinflammation in contributing to neuronal death, perihematomal edema, and worse outcome after ICH.(4–8)
One recently identified pathway implicated in the neuroinflammatory response is the dual endothelin-1/VEGF signal-peptide receptor (DEspR).(9) DEspR was first identified as a putative receptor related to endothelin-1 and angiotensin-II, and implicated in hypertension.(10) We recently found that DEspR is upregulated in association with neuroinflammatory markers and may play a role in mediating neuronal injury in an animal model of spontaneous intracerebral hemorrhage.(11) To evaluate whether DEspR expression is also present in the setting of human ICH, we undertook an exploratory pilot study in patients presenting with spontaneous ICH. We examined whether peripheral leukocytes express DEspR following ICH, and what patient characteristics were associated with DEspR levels.
Methods
Patient Cohort
Patients presenting to Massachusetts General Hospital and Brigham and Women’s Hospital with spontaneous ICH were prospectively enrolled between October 2019 and March 2021. All patients or their surrogates provided informed consent following a protocol approved by the Mass General Brigham Institutional Review Board. Eligibility criteria were patients ≥18 years of age presenting with ICH of non-traumatic etiology. Patients or their surrogates were interviewed to obtain baseline demographic and clinical data, and further clinical variables were then extracted from the medical record. These data included admission Glasgow Coma Scale (GCS) score and history of hypertension (HTN). Approximately 90 days after enrollment, structured follow-up interviews were conducted by telephone with patients or their surrogates to assess functional outcome and mortality. Poor functional outcome was defined as 90-day modified Rankin Scale (mRS) ≥ 3.
Normal Healthy Volunteers
In addition to obtaining samples from enrolled ICH patients, samples were collected from normal healthy volunteers (NHVs; n=5) without hypertension or history of cerebrovascular disease. NHVs provided informed consent in accordance with the Mass General Brigham Institutional Review Board.
Flow Cytometry
Whole blood was collected in ethylenediaminetetraacetic acid (EDTA)-containing tubes at a mean ± standard deviation (SD) of 4.1 ± 2.2 days following symptom onset. Samples were stained with the following commercially available antibodies: anti-CD11b BV421 (Clone ICRF44, BD Biosciences); anti-CD45 BUV395 (Clone HI30, BD Biosciences); anti-CD14 PerCP (Clone HCD14, BioLegend); and anti-CD16 BV786 (Clone 3G8, BD Biosciences). To stain for cell surface DEspR, we further included one of two different custom anti-DEspR antibodies specific for different epitopes on the DEspR protein, hu6g8 (Lake Pharma, HV2-1h-hIgG4 S228P) or hu5g12, both at a final concentration of 10 μg/ml.(12) The respective epitopes for hu6g8 and hu5g12 are shown in Figure 1A. Anti-DEspR antibodies were covalently labeled with Alexa Fluor 568 or Alexa Fluor 647 (Thermo Fisher Scientific) following the manufacturer’s instructions. Cells were incubated for 30 minutes at 4°C in Hank’s balanced salt solution (HBSS) + 2% heat-inactivated fetal bovine serum (FBS), fixed with 1% paraformaldehyde (Alfa Aesar) for 10 minutes at 4°C, and lysed with 1X RBC lysis buffer (BioLegend) at room temperature for 10 minutes. IgG4b isotype control antibody was similarly labeled with Alexa Fluor 647 and included as an isotype control. In some sample runs, fluorescence-minus-one (FMO) (e.g., the flow cytometry panel without anti-DEspR antibody) was used, and it yielded the same results as isotype control. Flow cytometry was performed on an LSR Fortessa X-20 SORP (BD Bioscience) at the Massachusetts General Hospital Pathology Flow Cytometry Core Facility. Samples were analyzed in the flow cytometer at 5.9 ± 2.6 hours following blood draw.
Figure 1. DEspR is expressed on the surface of neutrophils and monocytes.
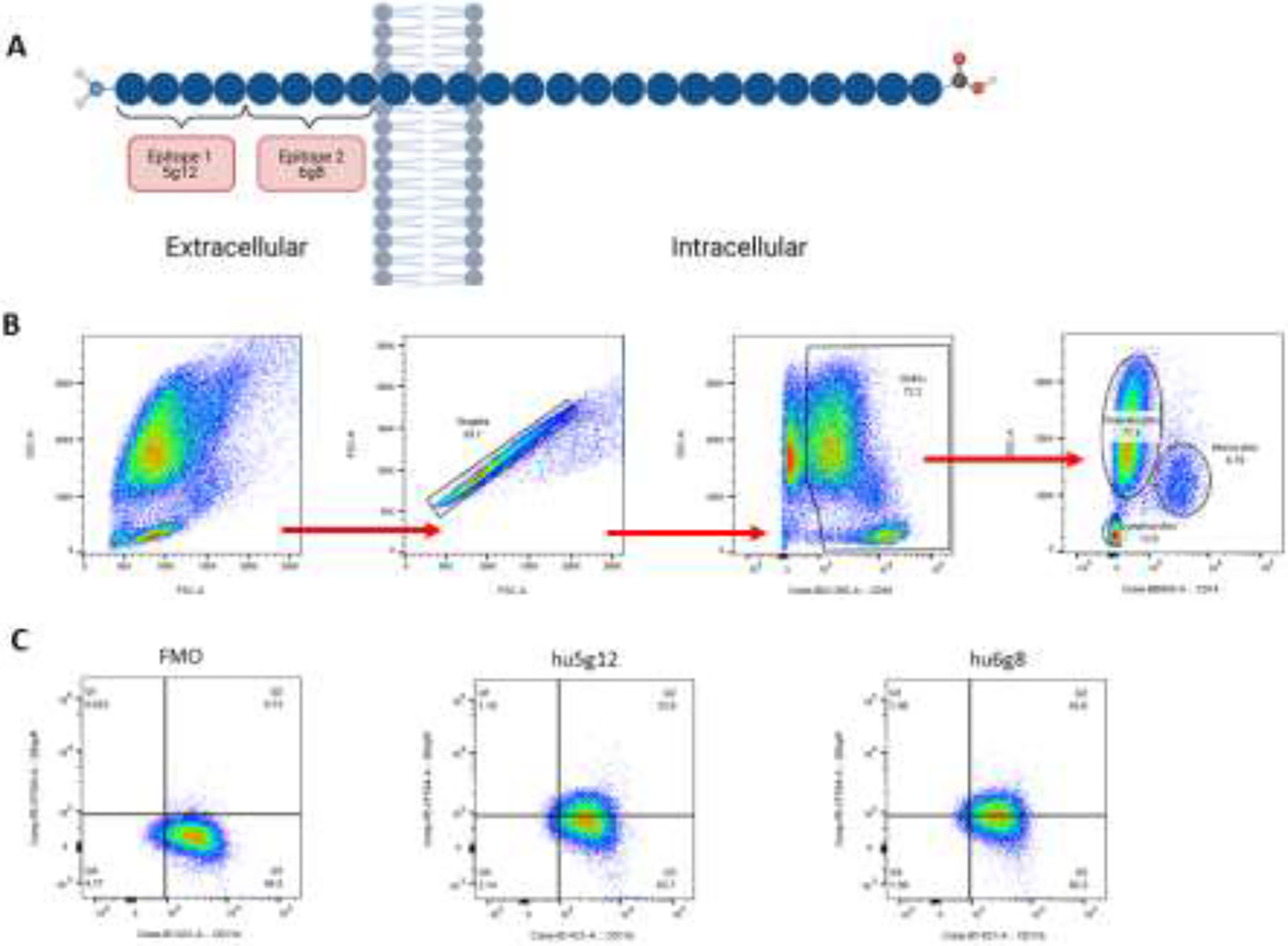
(A) Different epitopes on the DEspR protein – hu6g8 and hu5g12 – that were included in two custom anti-DEspR antibodies. (B) Manual gating strategy. Cells were sequentially selected using FSC-A, FSC-H, CD45, and CD14. The final subpopulations represented neutrophils, monocytes, and lymphocytes. (C) The presence of DEspR was defined as a positive signal above 1%. DEspR expression in patient samples was corroborated by the use of two independent antibodies, which showed similar positive signal from antibodies that targeted two different epitopes on the DEspR protein.
Analysis was conducted using FlowJo 10.6.1 (FlowJo, LLC). Samples were manually gated to identify singlets, and then further gated to select neutrophil, monocyte, and lymphocyte subpopulations. Figure 1B depicts the flow cytometry gating strategy. Changes in expression of DEspR+CD11b+ or DEspR+CD14+ cells were compared relative to the IgG4b isotype control or FMO.
Neuroimaging
Admission computed tomography (CT) scans were assessed by trained study staff for ICH location and volume (W.T.K. and R.L.S.). The presence of intraventricular extension was assessed and used to calculate the Graeb score, based on published methods.(13) The ICH score was calculated from these variables using a previously published method.(14)
Statistical Analysis
Study population characteristics were described by mean and SD for normally distributed, continuous variables or median with interquartile range [IQR] for non-normal data. Binary variables were reported as frequency and percentage. Univariate Wilcoxon Rank Sum tests were performed to assess the association of DEspR expression with binary outcome variables. Kruskal-Wallis tests with Dunn’s post-hoc test of pairwise comparisons was used to assess DEspR expression levels across cell types and between the ICH and NHV sub-populations. Statistical analyses were performed using STATA MP v15.1 (StataCorp LLC). All tests were two-sided with a significance threshold of p<0.05.
Data availability
Data from this study is available upon reasonable request, and in accordance with respective institutional data sharing policies.
Results
Patient Characteristics
Between October 2019 and March 2021, twenty-one patients with a clinical diagnosis of spontaneous ICH were enrolled. Two patients did not have blood samples drawn due to withdrawal of life-sustaining treatment or discharge from the hospital. The final study population included 19 patients, and Table 1 summarizes the patient characteristics. The final cohort had a mean age of 64±14 years and 53% (n=10) were female. A total of 13 patients (68%) had a history of hypertension. The cohort had a median ICH score of 2 [IQR 2, 3], a median ICH volume of 39cm3 [IQR 19cm3, 73cm3], and a median Graeb score of 0 [IQR 0, 7]. By the 90-day follow-up, patients had a median mRS score of 4 [IQR 2, 6] and 32% (n=6) had passed away.
Table 1.
Patient Characteristics
| n = 19 | |
|---|---|
| Age (years), mean ± SD | 64 ± 14 |
| Sex (F), n (%) | 10 (53%) |
| Admission GCS, median [IQR] | 8 [5, 15] |
| ICH Score, median [IQR] | 2 [2, 3] |
| Hypertension, n (%) | 13 (68%) |
| Radiographic Variables | |
| ICH Volume (cm3), median [IQR] | 39 [19, 73] |
| Deep location, n (%) | 10 (53%) |
| Graeb Score, median [IQR] | 0 [0, 7] |
| Onset to Sample Draw (days), mean ± SD | 4.1 ± 2.2 |
| Clinical Outcome | |
| mRS at 90 days, median [IQR] | 4 [2, 6] |
| Mortality at 90 days, n (%) | 6 (32%) |
DEspR is expressed on neutrophils and monocytes
Whole blood samples were collected at a mean ± SD of 4.1 ± 2.2 days after the onset of ICH and analyzed by flow cytometry 5.9 ± 2.6 hours following the blood draw. Samples stained with IgG4b isotype control or FMO were used as a control to define the absence of staining. DEspR expression by cell type is shown in Table 2. Among CD45+CD11b+ neutrophils, DEspR was detectable in 11 patients (58%) with a mean expression level of 7.5% ± 14.2%. DEspR expression was also detected on CD45+CD14+ monocytes in 18 patients (95%; mean expression level 9.2% ± 10.6%). To validate expression, a separate anti-DEspR antibody specific to a different epitope (5g12 clone; Figure 1A) was used to stain a parallel sample, which demonstrated a similar expression level to the 6g8 clone (Figure 1B).
Table 2.
DEspR Expression by Cell Type in ICH Patients and NHVs
| ICH Patients (n=19) | NHVs (n=5) | ||
|---|---|---|---|
| Cell Type | % Expression (Mean ± Std. Dev) | % Expression (Mean ± Std. Dev) | P-value |
| Neutrophils | 7.50 ± 14.2 | 1.17 ± 0.76 | 0.014 |
| Monocytes | 9.23 ± 10.6 | 0.52 ± 0.57 | 0.0025 |
| Lymphocytes | 2.60 ± 4.29 | 0.30 ± 0.38 | 0.040 |
In general, lymphocytes demonstrated lower DEspR expression (mean expression level 2.6% ± 4.3%) and on fewer patients (n=9 patients; 47%). DEspR expression level on lymphocytes was significantly lower than on monocytes (p=0.003) and trended towards a lower level compared to neutrophils (p=0.064). Moreover, NHV blood (n=5) had a mean DEspR expression level of 1.2% ± 0.8% on neutrophils and 0.5% ± 0.6% on monocytes (p=0.014 and p=0.0025 compared to ICH patient neutrophils and monocytes, respectively; see Table 2).
Clinical correlates with DEspR expression
We next explored patient characteristics based on the median value of DEspR expression (Table 3). There was no difference in DEspR expression based on ICH severity or time from LSW to sample draw (Table 3; p>0.05 for all). There was no difference in outcome in patients with high DEspR expression (p=0.09). We also examined associations with ICH risk factors. Patients with a history of hypertension had higher levels of DEspR compared to those patients without hypertension (23.8% ± 24.5% versus 4.4% ± 3.8%; p = 0.018). There was no association with DEspR expression and other known ICH risk factors, including age, male sex, current smoking status, history of alcohol abuse, Black or Hispanic race and ethnicity, history of Type II Diabetes, and presence of cerebral microbleeds on MRI (p>0.05 for all).
Table 3.
Patient Characteristics by DEspR Expression
| Low DEspR (n = 10) | High DEspR (n=9) | P-value | |
|---|---|---|---|
| Age (years), mean ± SD | 59.9 ± 14.1 | 67.8 ± 13.2 | 0.24 |
| Sex (F), n (%) | 4 (40%) | 5 (56%) | 0.50 |
| Admission GCS, median [IQR] | 11 [5, 15] | 8 [4, 14] | 0.41 |
| ICH Score, median [IQR] | 2 [2, 3] | 2 [2, 3] | 0.55 |
| ICH Volume (cm3), median [IQR] | 36.3 [32.3, 123] | 38.0 [19.1, 51.1] | 1.00 |
| Onset to Sample Draw (days), mean ± SD | 3.43 ± 1.66 | 4.85 ± 2.65 | 0.22 |
| mRS at 90 days, median [IQR] | 3 [2, 4] | 5 [4, 6] | 0.09 |
Discussion
In this study, we show that DEspR is detectable in humans following spontaneous intracerebral hemorrhage. This suggests a potential role for DEspR expression in ICH pathophysiology. Prior animal studies have implicated DEspR in non-ICH disease forms, including roles in cardiovascular disease, abnormal neural development, tumor proliferation, and impaired angiogenesis.(15,16) More recently, DEspR has been linked to neutrophil-mediated secondary injury in human patients with acute respiratory distress syndrome (ARDS).(17,18) Here, we build on this evidence by showing that DEspR is expressed on the surface of neutrophils and monocytes following ICH.
DEspR’s localization to leukocytes suggests that it may play a role in the inflammatory immune response. Given the critical roles neuroinflammation and secondary injury play in post-ICH mortality and morbidity,(19–23) this highlights a potential therapeutic opportunity. Specifically, inhibition of DEspR may mitigate the post-ICH inflammatory response and resulting secondary injury. Pilot data have shown that inhibition of DEspR in hypertensive stroke animal models has resulted in improved outcomes.(11) Our current work provides a foundation that supports further study of DEspR as a potential therapeutic target.
It is also notable that ICH patients with a medical history of hypertension expressed higher levels of DEspR on neutrophils and monocytes. This is consistent with prior studies, which showed that DEspR may play a role in the modulation of blood pressure.(24) It may be that DEspR plays a role specifically in the pathophysiology of ICH that is related to underlying hypertension.
Our study has limitations. This was an exploratory analysis with a small patient cohort, which had limited power to control for important covariates. A future study that includes more patients would be needed to assess relationships between DEspR expression and inflammatory markers such as perihematomal edema or long term poor functional outcome. Further prospective studies across multiple centers are likely needed to clarify the link between DEspR expression and outcome.
Overall, DEspR is expressed on leukocytes following ICH and may play an important and previously unrecognized role in ICH pathophysiology. These findings could clarify the contributing factors which impact patient outcomes after ICH and inform treatment opportunities to improve long-term function and independence. Future studies should work towards uncovering the mechanism of DEspR-mediated injury and examining the potentially neuroprotective effects of DEspR inhibition.
This manuscript complies with all Instructions for Authors for the Journal of Stroke and Cerebrovascular Diseases. All authorship requirements have been met and the final manuscript was approved by all authors. This manuscript has not been published elsewhere and is not under consideration at another journal. This study adhered to ethical guidelines and operated under a protocol approved by the Mass General Brigham Institutional Review Board (IRB). Informed consent was obtained from all individual participants (or their surrogates) included in the study. A STROBE checklist has been included as part of this manuscript submission.
Acknowledgments
We thank Victoria L. Herrera and Nelson Ruiz-Opazo for providing the anti-DEspR antibodies utilized for flow cytometric analysis.
Sources of Funding
This work was supported by a research grant provided by NControl Therapeutics (WTK). WTK was also supported by NIH R01 NS099209, and MBB by NIH K23 NS112447, American Academy of Neurology AI18-0000000062, and the Heitman Neurovascular Research Foundation (WTK, MBB). The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclosures
J.N.G. receives consulting and research fees from NControl Therapeutics, Inc., Cayuga, Pfizer, Takeda, Alexion, Octapharma, and CSL Behring. W.T.K. has received grant support and consulting fees from Biogen, Inc. and from NControl Therapeutics, Inc. W.T.K. serves as a scientific advisory board member for Biogen, Inc. and NControl Therapeutics, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hemphill JC, Greenberg SM, Anderson CS, et al. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Vol. 46, Stroke. 2015. p. 2032–60. [DOI] [PubMed] [Google Scholar]
- 2.Boltze J, Aronowski JA, Badaut J, et al. New Mechanistic Insights, Novel Treatment Paradigms, and Clinical Progress in Cerebrovascular Diseases. Front Aging Neurosci. 2021;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Askenase MH, Sansing LH. Stages of the Inflammatory Response in Pathology and Tissue Repair after Intracerebral Hemorrhage. Semin Neurol. 2016;36(3):288–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schreibman DL, Hong CM, Keledjian K, et al. Mannitol and Hypertonic Saline Reduce Swelling and Modulate Inflammatory Markers in a Rat Model of Intracerebral Hemorrhage. Neurocrit Care. 2018;29(2):253–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang J, Shi K, Li Z, et al. Organ- and cell-specific immune responses are associated with the outcomes of intracerebral hemorrhage. FASEB J. 2018;32(1):220–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen S, Peng J, Sherchan P, et al. TREM2 activation attenuates neuroinflammation and neuronal apoptosis via PI3K/Akt pathway after intracerebral hemorrhage in mice. J Neuroinflammation. 2020;17(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durocher M, Knepp B, Yee A, et al. Molecular Correlates of Hemorrhage and Edema Volumes Following Human Intracerebral Hemorrhage Implicate Inflammation, Autophagy, mRNA Splicing, and T Cell Receptor Signaling. Transl Stroke Res. 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xie J, Hong E, Ding B, et al. Inhibition of NOX4/ROS Suppresses Neuronal and Blood-Brain Barrier Injury by Attenuating Oxidative Stress After Intracerebral Hemorrhage. Front Cell Neurosci. 2020;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herrera VLM, Steffen M, Moran AM, et al. Confirmation of translatability and functionality certifies the dual endothelin1/VEGFsp receptor (DEspR) protein. BMC Mol Biol. 2016;17(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruiz-Opazo N, Hirayama K, Akimoto K, et al. Molecular characterization of a dual endothelin-1/angiotensin II receptor. Mol Med. 1998;4(2). [PMC free article] [PubMed] [Google Scholar]
- 11.Herrera V, Gromisch CM, Decano J, et al. Anti-DEspR antibody treatment after spontaneous intracerebral hemorrhage (ICH) in ICH-prone Dahl Salt-Sensitive hypertensive rate model increases overall survival, while pre-emptive treatment delays ICH onset. Eur Stroke J. 2021;6(1S):9. [Google Scholar]
- 12.Gromisch CM, Tan GLA, Pasion KA, et al. Humanized anti-DEspR IgG4S228P antibody increases overall survival in a pancreatic cancer stem cell-xenograft peritoneal carcinomatosis ratnu/nu model. BMC Cancer. 2021;21(1):1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graeb DA, Robertson WD, Lapointe JS, et al. Computed tomographic diagnosis of intraventricular hemorrhage. Etiology and prognosis. Radiology. 1982;143(1). [DOI] [PubMed] [Google Scholar]
- 14.Hemphill JC, Bonovich DC, Besmertis L, et al. The ICH score: A simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001;32(4). [DOI] [PubMed] [Google Scholar]
- 15.Herrera VLM, Ponce LRB, Bagamasbad PD, et al. Embryonic lethality in Dear gene-deficient mice: New player in angiogenesis. Physiol Genomics. 2005;23(3). [DOI] [PubMed] [Google Scholar]
- 16.Herrera VL, Decano JL, Tan GA, et al. DEspR roles in tumor vasculo-angiogenesis, invasiveness, CSC-survival and anoikis resistance: A “common receptor coordinator” paradigm. PLoS One. 2014;9(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herrera VLM, Walkey AJ, Nguyen MQ, et al. Increased Neutrophil-Subset Associated With Severity/Mortality In ARDS And COVID19-ARDS Expresses The Dual Endothelin-1/VEGFsignal-Peptide Receptor (DEspR): An Actionable Therapeutic Target. Res Sq. 2021; [Google Scholar]
- 18.deKay JT, Emery IF, Rud J, et al. DEspRhigh neutrophils are associated with critical illness in COVID-19. Sci Rep. 2021;11(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu H, Wang Z, Yu J, et al. Role and mechanisms of cytokines in the secondary brain injury after intracerebral hemorrhage. Vol. 178, Progress in Neurobiology. 2019. [DOI] [PubMed] [Google Scholar]
- 20.Urday S, Kimberly WT, Beslow LA, et al. Targeting secondary injury in intracerebral haemorrhage--perihaematomal oedema. Nat Rev Neurol. 2015;11(2):111–22. [DOI] [PubMed] [Google Scholar]
- 21.Haupenthal D, Kuramatsu JB, Volbers B, et al. Disability-Adjusted Life-Years Associated with Intracerebral Hemorrhage and Secondary Injury. JAMA Netw Open. 2021; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Volbers B, Giede-Jeppe A, Gerner ST, et al. Peak perihemorrhagic edema correlates with functional outcome in intracerebral hemorrhage. Neurology. 2018;90(12). [DOI] [PubMed] [Google Scholar]
- 23.Leasure AC, Steinschneider AF, Falcone GJ, et al. Abstract TP331: Plasma IL-6 Levels are Independently Associated With Functional Outcome and Markers of Secondary Injury in Spontaneous Intracerebral Hemorrhage. Stroke. 2018;49(Suppl_1). [Google Scholar]
- 24.Glorioso N, Herrera VLM, Didishvili T, et al. DEspR T/CATAAAA-box promoter variant decreases DEspR transcription and is associated with increased BP in Sardinian males. Physiol Genomics. 2011;43(21). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data from this study is available upon reasonable request, and in accordance with respective institutional data sharing policies.


