Abstract
The cell envelope of Gram-negative bacteria is composed of an inner membrane, outer membrane and an intervening periplasmic space. How the outer membrane lipids are trafficked and assembled there, and how the asymmetry of the outer membrane is maintained is an area of intense research. The Mla system has been implicated in the Maintenance of Lipid Asymmetry in the outer membrane, and is generally thought to drive the removal of mislocalized phospholipids from the outer membrane and their retrograde transport to the inner membrane. At the heart of the Mla pathway is an structurally unique ABC transporter complex in the inner membrane, called MlaFEDB. Recently, an explosion of cryo-EM studies has begun to shed light on the structure and lipid translocation mechanism of MlaFEDB, with many parallels to other ABC transporter families, including human ABCA and ABCG, as well as bacterial lipopolysaccharide and O-antigen transporters. Here we synthesize information from all available structures, and propose a model for lipid trafficking across the cell envelope by MlaFEDB.
Introduction
The outer membrane (OM) of Gram-negative bacteria plays a key role as a permeability barrier to protect the cell from antibiotics, detergents, and other environmental stressors. The OM is an asymmetric bilayer composed of an outer leaflet of lipopolysaccharide (LPS) and an inner leaflet of phospholipids. The pathways involved in OM assembly and maintenance, as well as in trafficking lipids between the inner membrane (IM) and OM are critical for cell survival, and are attractive targets for the development of new antibiotics [1–8]. Structural and biochemical work from many groups has led to a model for how LPS is trafficked across the cell envelope via the Lpt system [9–23]. Much less is understood about how phospholipids are transported to the OM, or how the asymmetry of the OM is maintained. Recently, several members of the MCE transporter family (containing a Mammalian Cell Entry domain), including the Mla, Pqi, and Let pathways [24–32], as well as YhdP/AsmA/TamB-like proteins [33,34] have been proposed to function in lipid import or export. For several of these less studied systems, the substrates and mechanisms remain to be discovered. Recent comprehensive reviews of lipid transport in bacteria highlight what is known about the MCE transporter family and LPS transport [35–38]. Here, we focus on the latest developments in our understanding of the Mla pathway, the best studied phospholipid transport system in Escherichia coli.
The Mla pathway (Maintenance of OM Lipid Asymmetry) is most widely thought to be involved in retrograde phospholipid transport from the OM back to the IM [24,25,39–42]. In the absence of flippases/floppases in the OM to maintain the asymmetric distribution of LPS and phospholipids, Mla may recognize mislocalized phospholipids that have strayed into the LPS-containing outer leaflet and remove them by re-importing them back to the IM. However, the Mla pathway has alternatively been proposed to drive anterograde phospholipid transport (IM to OM) [28,32,43], or even bidirectional transport [44], and this remains an area of active debate and research in the field. The Mla pathway consists of 3 main parts: 1) an OM complex of MlaA-OmpC/F; 2) an IM ABC transporter complex, MlaFEDB; and 3) a periplasmic lipid binding protein, MlaC, which ferries lipids between the OM and IM complexes (Fig. 1A). Crystal structures of MlaA-OmpC/F [27] and MlaC [28] suggested how lipids may move in/out of the OM via a channel through MlaA, and be ferried across the periplasm bound in a deep hydrophobic pocket within MlaC. Structures of MlaD [28] and MlaFB [45], together with computational structure predictions and low resolution cryo-EM maps [28,32], provided our first insights into the architecture of MlaFEDB at the IM. In 2020 and 2021, an explosion of publications on the structure of MlaFEDB at ~3–4 Å resolution has yielded key insights into the function of this important lipid transporter and revealed striking similarities to a diverse group of transporters from bacteria through humans.
Figure 1. Overview of the ABC transporter MlaFEDB.

A. MlaFEDB (PDB 6zy4) is a 12-subunit complex made of four proteins, located in the inner membrane (IM) of gram-negative bacteria. MlaA forms a complex with OmpF (PDB 5nuq) in the outer membrane (OM). MlaC (PDB 5uwa) is a soluble, periplasmic, lipid-binding protein, proposed to shuttle phospholipids (PL) between the IM and OM complexes. B. Cryo EM structures from three different species: A. baumannii (PDB 6z5u), E. coli (PDB 6xbd) and P. aeruginosa (PDB 7ch9). While MlaD of A. baumannii shows some helices at the periphery, the stoichiometry, secondary structure, and protein-interaction interfaces of the complex are well conserved across the species.
Structures of the MlaFEDB Complex
Six recent publications have yielded a wealth of new insights into the structure and function of the MlaFEDB complex from E. coli, Pseudomonas aeruginosa, and Acinetobacter baumannii (Table 1) [44,46–50]. The complex consists of 4 different proteins, MlaF, MlaE, MlaD, and MlaB, with a stoichiometry of 2:2:6:2 (Fig. 1B). At the heart of the complex is an ABC transporter that consists of a transmembrane subunit (MlaE) and an ABC ATPase (MlaF). Outside of this stereotypical ABC transporter core, the MlaFEDB complex differs from other structurally characterized ABC transporters studied to date in two key ways. First, MlaD, which is a ring-shaped homohexamer formed by 6 identical MCE domain proteins, sits atop the periplasmic end of the transporter, and is anchored in the IM by a single TM helix from each subunit. A hydrophobic tunnel runs through the center of this ring, and was previously proposed to serve as a pathway for lipid transport between the periplasm and the transmembrane region of the complex [28]. Intriguingly, in recent structures, the MlaD ring was trapped in several different conformations and positions relative to the rest of the transporter [46–48,50]. Thus, there appears to be a degree of flexibility in the MlaD ring and at the interface between MlaD and MlaE, which could be important for the transport mechanism. Second, a small Sulfate Transporter and Anti-Sigma factor antagonist (STAS) domain-containing protein, MlaB, binds to each of the two copies of the MlaF ATPase [28,32], and preliminary studies have indicated that MlaB may regulate MlaFEDB transporter activity [26,45]. While regulatory domains are often associated with the NBDs of ABC transporters [51–56], the binding of a STAS domain to this site on the NBDs was not previously described, and binding to the NBD involves a unique C-terminal tail from MlaF [45]. Typically, STAS domain proteins regulate the activity of their binding partners in a phosphorylation-dependent manner [57]; whether this is also the case for MlaB remains an open question. Overall, the structures of the MlaFEDB complexes from E. coli [44,46,48], P. aeruginosa [50], and A. baumannii [47,49] were the first high-resolution structures of the MlaFEDB complex, and revealed the structure of the MlaE subunit for the first time. The overall architecture of the MlaFEDB complex is conserved across species, though some key differences were noted and are discussed below. Through the efforts of multiple groups to trap the transporter in different states of the transport cycle, the new MlaFEDB structures also yielded information on the conformational plasticity of MlaFEDB and how these states may help to drive lipid transport, as well as insights into substrate recognition and substrate translocation between the MlaD pore and the ABC transporter core below.
Table 1.
List of available MlaFEDB structures.
| PDB | Species | Reported resolution (Å) | Nucleotide added | Lipids modeled in pocket? | MlaE Conformation | Membrane reconstitution | CC of pocket lipid densities | Ref. |
|---|---|---|---|---|---|---|---|---|
| 7CGE | E. coli | 2.9 | none | Yes | Outward-open | Lipid Nanodisc | 0.68 | [48] |
| 6ZY2 | E. coli | 3.6 | none | No | Outward-open | Detergent | NA | [44] |
| 6XBD | E. coli | 3.05 | none | Yes | Outward-open | Lipid Nanodisc | 0.74 | [46] |
| 6ZY3 | E. coli | 3.3 | none | Yes | Outward-open | Detergent + lipids | 0.54 | [44] |
| 7CGN | E. coli | 4.3 | ATP, E170Q in MlaF | No | Outward-open | Lipid Nanodisc | NA | [48] |
| 7CH0 | E. coli | 3.7 | ATP, E170Q in MlaF | No | Closed | Lipid Nanodisc | NA | [48] |
| 6ZY9 | E. coli | 3.3 | AMPPNP | No | Outward-open | Detergent | NA | [44] |
| 6ZY4 | E. coli | 4.1 | ADP | No | Outward-open | Detergent | NA | [44] |
| 7D06 | A. baumannii | 3.1 | none | Yes | Outward-open | Lipid Nanodisc | 0.73 | [47] |
| 7D08 | A. baumannii | 4.0 | ATP | No | Outward-open | Lipid Nanodisc | NA | [47] |
| 7D09 | A. baumannii | 3.6 | ATP | No | Outward-open | Lipid Nanodisc | NA | [47] |
| 7D0A | A. baumannii | 4.0 | ADP-vanadate | No | Closed | Lipid Nanodisc | NA | [47] |
| 6Z5U | A. baumannii | 3.9 | AppNHp (AMPPNP) | No | Outward-open | Detergent | NA | [49] |
| 7CH9 | P. aeruginosa | 3.5 | none | Yes | Outward-open | Detergent | 0.55 | [50] |
| 7CH8 | P. aeruginosa | 3.9 | ADP-vanadate | Yes | Outward-open | Detergent | 0.50 | [50] |
| 7CHA | P. aeruginosa | 3.9 | AMPPNP | Yes | Outward-open | Detergent | 0.48 | [50] |
Structures of the Transmembrane Domain, MlaE
Arguably, the greatest insights delivered by the recent MlaFEDB structures concern the structure and function of the transmembrane subunit, MlaE. MlaE functions as a homodimer, similar to many other ABC transporters which consist of a homo- or heterodimer of two homologous transmembrane domains along with a homo- or heterodimer of ABC domains. Until structures of MlaFEDB were available, it was unclear whether MlaE was homologous to known ABC transporter folds, and whether each MlaE subunit consisted of 5 or 6 TM helices. The recent structures reveal that MlaE has 5 TM helices (Fig. 2A) [44,46–50]. In addition, MlaE has a long interfacial helix at the N-terminus, called IF1 (Fig. 2A) [46] [44,46–50]. A clear counterpart of IF1 is not found in any structurally related ABC transporters, though TarG has a short N-terminal helix that is reminiscent of it [58]. IF1 plays a role in binding to 4 of 6 TM helices of the MlaD hexamer, which may explain why IF1 is unique to MlaE.
Figure 2. Comparison of MlaFEDB with other ABC transporters.
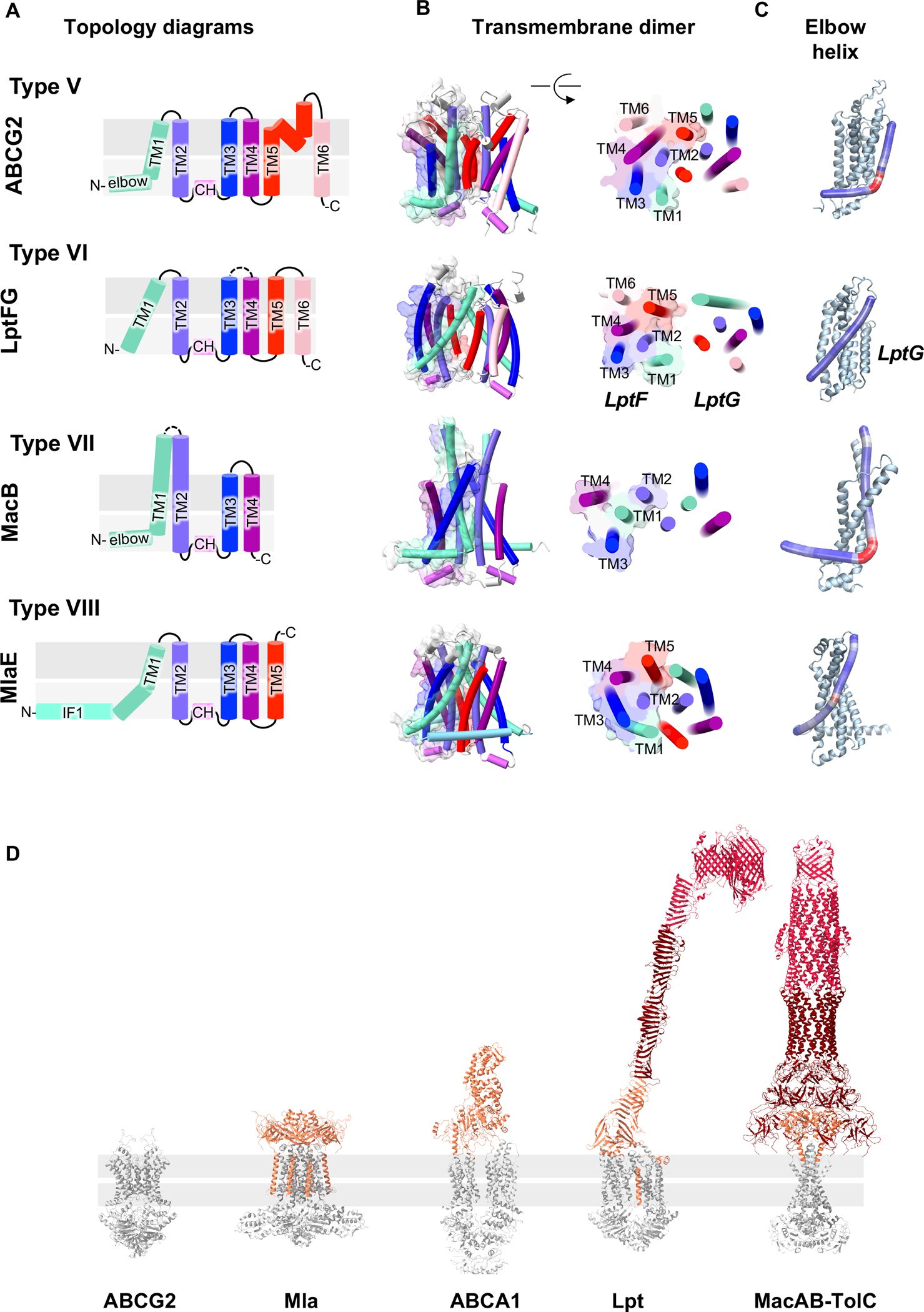
A-C. Comparison of the the transmembrane domain MlaE (PDB 6xbd) with transmembrane domains from type V (ABCG2, PDB 6hbu), type VI (LPS exporter transmembrane domains, LptFG, PDB 6mhu) and type VII (MacB, PDB 5gko) transporters. The topology diagram (A), the side and cross-section view of the dimer organization (B) and the elbow helix (C) are shown. Representations of the elbow helix were prepared using Bendix [80]. D. Overall structure of ABC transporters shows a wide variety of periplasmic domains: ABCG2 (PDB 6hbu) has only a few loops protruding in the periplasmic domain; the hexameric domain MlaD of MlaFEDB (PDB 6xbd) is relatively small compared to the torch-like domain of ABCA1 (PDB 5xjy), the bridge of the LPS exporter formed by LptA (PDB 2r19) between LptDE (PDB 5iv9) and LptB2FGC (PDB 6mit) or the tunnel formed by the MacAB-TolC complex (PDB 5nik). Note that the model of the LPS exporter depicted here is a composite of several different structures; a structure of the complete transporter remains to be determined. It is not known how many LptA molecules may oligomerize to form a bridge in the periplasm, which will determine the length of the bridge.
The recent structures of MlaE revealed striking similarity to a diverse group of ABC transporter transmembrane domains [44,46], including type V (e.g., ABCA, ABCG, Wzm-Wzt) [59–63], type VI (LPS exporter, LptFG) [17–21], and type VII (MacB and LolCE)[64–68] (Fig. 2A–B; for recent review of ABC transporter classification, see [69]). The vast majority of these related transporters have 6 transmembrane helices in their transmembrane domains (type V and VI), whereas MacB, the type VII ABC transporter subunit of the MacAB-TolC complex, has just 4 TM helices and lacks the equivalent of TM5 and TM6. In comparison, MlaE has 5 TM helices, lacking the equivalent of the TM6 found in all other type V and VI transporters, but with one additional TM relative to type VII. The first TM helix of MlaE is kinked within the membrane region [46]; the shorter N-terminal segment is amphipathic, and lies at a ~20° angle in the membrane near the interface with the cytoplasm. This segment is reminiscent of the “elbow helix”/”interfacial helix”, an N-terminal amphipathic helix that floats in the cytoplasmic leaflet of the membrane that is found in all structurally characterized type V and VII transporters [61,62,64–66,69] (Fig. 2C). In type V and VII transporters, a sharp turn clearly separates the elbow helix from TM1. In contrast, the first TM is unbroken in type VI transporters, which lack an elbow helix [17,18]. Thus, MlaE lies somewhere in between these two extremes. The similarity between type V (6 TMs), type VI (6 TMs) and type VII transporters (4 TMs) was previously noted [69], and MlaE (5 TMs) may represent a “missing link” in the evolution of this broader transporter type V/VI/VII group. While MlaE appears to be structurally and mechanistically most similar to the LPS exporter (type VI), based upon the criteria proposed for the classification of type V, VI, and VII transporters [69], MlaE may be different enough to be grouped separately based upon its transmembrane domain containing 5 TM helices, an elbow-like amphipathic helix, and an additional amphipathic helix, IF1. Thus, we propose that MlaE is the founding member of the type VIII group of ABC transporter transmembrane domains.
Substrate Binding
In the absence of nucleotide, structures of the E. coli, A. baumannii, and P. aeruginosa MlaFEDB complexes consistently revealed the presence of an outward-facing, V-shaped pocket at the MlaE dimer interface (Fig. 3A) [44,46–50]. As the hydrophobic pore through the MlaD hexamer lies immediately above this pocket, this organization creates a clear pathway from the periplasm into the transmembrane region within MlaE. It is noteworthy that several other type V/VI/VII/VIII transporters involved in the transport of lipids and other hydrophobic molecules also position accessory domains that create hydrophobic transport pathways immediately above the outward-open substrate-binding pocket. These include: the tunnel through the “torch” of ABCA1 [61], the transport bridge of the LPS exporter [9,10,19]; and MacA-TolC tunnel associated with MacB [65](Fig. 2D). Substrates of ABC transporters are usually bound along the interface between the two transmembrane domains, thus this pocket in the MlaE dimer likely represents a binding site for the phospholipid substrates transported by MlaFEDB. Densities for possible ligands in the MlaE pocket were described in many of the reported MlaFEDB structures [44,46–48,50], and have been interpreted in some cases to reflect the presence a single diacylphospholipid, or two diacylphospholipids, bound in the pocket in a variety of different conformations. It is worth noting that in some cases, the deposited EM density maps exhibit only exceedingly weak and fragmented density in the substrate-binding pocket. Clear validation metrics for ligands in cryo-EM structures are not yet well established, and there is no substitute for manual inspection of the maps and coordinates available from the PDB. However, per-residue real-space model-vs-map correlation coefficients (CC) can provide a rough measure of the quality of the EM density for the lipids in the pocket (Table 1). The lipid CC values reported here roughly correlate with our opinions following manual inspection of each model and map, with local CC > ~0.65 corresponding to reasonable EM density for ligand and general agreement between model and map; and local CC < ~0.65 indicating weak density and greater ambiguity as to the overall configuration of the ligand and its identity (e.g., lipid, vs detergent, vs water, vs other buffer components, which frequently are observed bound to proteins in high resolution structures [70]).
Figure 3. Substrate binding and possible mechanism of lipid transport in MlaFEDB.

A. MlaFEDB adopts an outward-open state in the absence of nucleotide (PDB 7cge), and a collapsed state in presence of ATP and a E170Q mutation, which inhibits ATP hydrolysis (PDB 7ch0). The cavities were defined and are represented using CASTp [81]. B. Two lipids bound in the cavity of MlaE, in the absence of nucleotide. At least one of lipid has one of the tails extended upwards to the MlaD pore, and the other downwards the MlaE dimer cavity (PDB 6xbd). C. The interfacial helices IF1 located at the N-terminus of each MlaE monomer (red) interact with transmembrane helices of MlaD, and form a cleft in which lipids may bind (PDB 7cge). D. Model for possible mechanism for retrograde and anterograde lipid transport. In the absence of ATP, MlaE is in the outward-open state, forming a continuous channel with MlaD, and the complex is able to accommodate lipids in its binding pocket. In the presence of ATP, the binding pocket collapses, and lipids may be extruded into the membrane (importer) or through the MlaD channel for handoff to MlaC (exporter). Model was created with BioRender.
Structures of MlaFEDB reconstituted into lipid nanodiscs have stronger and more continuous density for ligands in the MlaE pocket and corresponding higher ligand CC values [46–48] (Table 1). These densities were still relatively modest compared to generally accepted norms for structures determined by X-ray crystallography [71], but consistent with the shape and chemistry of bound phospholipids, and supported by biochemical experiments indicating the binding of phospholipids at that site [46,50]. In all of these structures, 2 phospholipids appear to be bound in the pocket, raising the possibility that MlaFEDB may be capable of transporting multiple diacylphospholipids each transport cycle. These structures also suggest that one or both of the bound lipids may adopt an extended conformation, with some of the fatty acid tails directed down into the pocket in MlaE while others extend upwards into the hydrophobic pore through MlaD (Fig. 3B). Additional densities for detergent, lipids, or other ligands were observed in multiple locations within the pore through the MlaD ring [44,46–50], including near the pore lining loops and within the barrel formed by the C-terminal helices of MlaD. As the features of these densities are less well defined, their identity and any functional relevance will require future investigation.
A second major site for bound lipids was observed in the cleft between the unique IF1 helix of MlaE and its adjacent TM helices (Fig. 3C). Here, tubular densities were observed in many of the detergent and nanodisc structures [44,46–50], and it seems likely that ordered phospholipids may occupy this site in native membranes. Thus, in addition to creating binding sites for the TM helices of MlaD, IF1 of MlaE was also proposed to play a possible role in substrate binding.
Transport Mechanism
Canonical ABC transporters move substrates across membranes using an ATP-dependent alternating-access mechanism, wherein access to the substrate binding pocket alternates between inward-open (cytoplasmic facing) and outward-open (extracytoplasmic facing) conformations of the transmembrane domain [72]. However, transporters structurally similar to MlaFEDB have been proposed to employ a range of different transport mechanisms, including alternating-access (ABCG [62,63,73–76]), extrusion (LPS exporter [19,20]), pumping (Wzm-Wzt and TarGH [58–60]) and other mechanisms that are not clearly understood (ABCA [61,77,78]). MlaFEDB adopts an outward-open conformation in the absence of nucleotide in all of the recent cryo-EM structures, regardless of whether lipids were bound in the pocket. Intriguingly, ATP-bound structures of E. coli MlaFEDB (Walker B mutation + ATP, in nanodiscs) [48] and A. baumannii MlaFEDB (ADP+Vanadate, in nanodiscs) [47] showed that the nucleotide binding domains were clamped tightly shut, sandwiching bound nucleotide, and the pocket between MlaE subunits was collapsed, preventing PL binding (Fig. 3A). Thus, MlaFEDB appears to cycle between outward-open and closed states, and may employ a transport mechanism reminiscent of the lipid extrusion reaction carried out by the LPS exporter [17–21]. In the resting state, the LPS exporter adopts an outward-open conformation, with a lateral gate open into the membrane environment to allow LPS to enter the substrate binding pocket. Closure of the ABC subunits upon ATP binding drives the collapse of the substrate binding pocket, squeezing the LPS molecule upwards and out of the membrane, into a proteinaceous bridge that creates a pathway to the outer membrane.
Conclusion
The clear homology between MlaE and LptF/G and the structural snapshots of MlaFEDB obtained thus far are consistent with an LPS exporter-like extrusion model (anterograde), wherein PLs bound in the pocket of MlaE are extruded up into the pore through the MlaD hexameric ring upon ATP binding (Fig. 3D). However, as Mla is most widely believed to drive lipid import (retrograde), based on available functional data, there is clearly still much that we do not understand about how MlaFEDB works, such as how the collapse of the lipid binding pocket could translocate lipids into the surrounding membrane (retrograde) instead of upwards into the periplasm (anterograde) (Fig. 3D). For example, it is unclear how IM lipids might enter or exit the pocket in MlaE in the first place, as thus far an open, lateral gate into the membrane environment has not been observed. One hypothesis is that lipids could conceivably enter from either the periplasmic leaflet (as in the LPS transporter) or cytoplasmic leaflet, and in the latter case, it has been suggested that lipids bound in the cleft formed by IF1 may represent substrates immediately before or after transport. While most type V/VI/VII/VIII transporters drive export of substrates (anterograde), it should also be noted that at least one type V transporter, ABCA4, is thought to drive import instead (retrograde) [79], and it is possible that MlaFEDB is another example of a type V/VI/VII/VIII transporter that transports in a non-canonical direction. If MlaFEDB indeed drives retrograde/import of PLs, the model supported by many in the field, it will be fascinating in the future to unravel how changes in the transport mechanism have enabled this reversal of transport direction to occur.
References
- 1.Alexander MK, Miu A, Oh A, Reichelt M, Ho H, Chalouni C, Labadie S, Wang L, Liang J, Nickerson NN, et al. : Disrupting Gram-Negative Bacterial Outer Membrane Biosynthesis through Inhibition of the Lipopolysaccharide Transporter MsbA. Antimicrob Agents Chemother 2018, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang G, Baidin V, Pahil KS, Moison E, Tomasek D, Ramadoss NS, Chatterjee AK, McNamara CW, Young TS, Schultz PG, et al. : Cell-based screen for discovering lipopolysaccharide biogenesis inhibitors. Proc Natl Acad Sci U S A 2018, 115:6834–6839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ho H, Miu A, Alexander MK, Garcia NK, Oh A, Zilberleyb I, Reichelt M, Austin CD, Tam C, Shriver S, et al. : Structural basis for dual-mode inhibition of the ABC transporter MsbA. Nature 2018, 557:196–201. [DOI] [PubMed] [Google Scholar]
- 4.Kaur H, Jakob RP, Marzinek JK, Green R, Imai Y, Bolla JR, Agustoni E, Robinson CV, Bond PJ, Lewis K, et al. : The antibiotic darobactin mimics a β-strand to inhibit outer membrane insertase. Nature 2021, 593:125–129. [DOI] [PubMed] [Google Scholar]
- 5.Hart EM, Mitchell AM, Konovalova A, Grabowicz M, Sheng J, Han X, Rodriguez-Rivera FP, Schwaid AG, Malinverni JC, Balibar CJ, et al. : A small-molecule inhibitor of BamA impervious to efflux and the outer membrane permeability barrier. Proc Natl Acad Sci U S A 2019, 116:21748–21757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Storek KM, Chan J, Vij R, Chiang N, Lin Z, Bevers J 3rd, Koth CM, Vernes J-M, Meng YG, Yin J, et al. : Massive antibody discovery used to probe structure-function relationships of the essential outer membrane protein LptD. Elife 2019, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Storek KM, Auerbach MR, Shi H, Garcia NK, Sun D, Nickerson NN, Vij R, Lin Z, Chiang N, Schneider K, et al. : Monoclonal antibody targeting the β-barrel assembly machine of Escherichia coli is bactericidal. Proc Natl Acad Sci U S A 2018, 115:3692–3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thélot FA, Zhang W, Song K, Xu C, Huang J, Liao M: Distinct allosteric mechanisms of first-generation MsbA inhibitors. Science 2021, 374:580–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suits MDL, Sperandeo P, Dehò G, Polissi A, Jia Z: Novel structure of the conserved gram-negative lipopolysaccharide transport protein A and mutagenesis analysis. J Mol Biol 2008, 380:476–488. [DOI] [PubMed] [Google Scholar]
- 10.Tran AX, Dong C, Whitfield C: Structure and functional analysis of LptC, a conserved membrane protein involved in the lipopolysaccharide export pathway in Escherichia coli. J Biol Chem 2010, 285:33529–33539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Z, Xiang Q, Zhu X, Dong H, He C, Wang H, Zhang Y, Wang W, Dong C: Structural and functional studies of conserved nucleotide-binding protein LptB in lipopolysaccharide transport. Biochem Biophys Res Commun 2014, 452:443–449. [DOI] [PubMed] [Google Scholar]
- 12.Malojčić G, Andres D, Grabowicz M, George AH, Ruiz N, Silhavy TJ, Kahne D: LptE binds to and alters the physical state of LPS to catalyze its assembly at the cell surface. Proc Natl Acad Sci U S A 2014, 111:9467–9472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong H, Xiang Q, Gu Y, Wang Z, Paterson NG, Stansfeld PJ, He C, Zhang Y, Wang W, Dong C: Structural basis for outer membrane lipopolysaccharide insertion. Nature 2014, 511:52–56. [DOI] [PubMed] [Google Scholar]
- 14.Sherman DJ, Lazarus MB, Murphy L, Liu C, Walker S, Ruiz N, Kahne D: Decoupling catalytic activity from biological function of the ATPase that powers lipopolysaccharide transport. Proc Natl Acad Sci U S A 2014, 111:4982–4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bollati M, Villa R, Gourlay LJ, Benedet M, Dehò G, Polissi A, Barbiroli A, Martorana AM, Sperandeo P, Bolognesi M, et al. : Crystal structure of LptH, the periplasmic component of the lipopolysaccharide transport machinery from Pseudomonas aeruginosa. FEBS J 2015, 282:1980–1997. [DOI] [PubMed] [Google Scholar]
- 16.Botos I, Majdalani N, Mayclin SJ, McCarthy JG, Lundquist K, Wojtowicz D, Barnard TJ, Gumbart JC, Buchanan SK: Structural and Functional Characterization of the LPS Transporter LptDE from Gram-Negative Pathogens. Structure 2016, 24:965–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo Q, Yang X, Yu S, Shi H, Wang K, Xiao L, Zhu G, Sun C, Li T, Li D, et al. : Structural basis for lipopolysaccharide extraction by ABC transporter LptB2FG. Nat Struct Mol Biol 2017, 24:469–474. [DOI] [PubMed] [Google Scholar]
- 18.Dong H, Zhang Z, Tang X, Paterson NG, Dong C: Structural and functional insights into the lipopolysaccharide ABC transporter LptB2FG. Nat Commun 2017, 8:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Owens TW, Taylor RJ, Pahil KS, Bertani BR, Ruiz N, Kruse AC, Kahne D: Structural basis of unidirectional export of lipopolysaccharide to the cell surface. Nature 2019, 567:550–553. * Structural and biochemical studies that suggest how LPS is translocated into the bridge of the LPS exporter.
- 20. Li Y, Orlando BJ, Liao M: Structural basis of lipopolysaccharide extraction by the LptB2FGC complex. Nature 2019, 567:486–490. ** One of two papers reporting the structure of the LPS exporter in a closed state, leading to the current model for LPS extrusion from the membrane and translocation to the periplasmic bridge. Also revealed how LPS binds to the transporter.
- 21. Tang X, Chang S, Luo Q, Zhang Z, Qiao W, Xu C, Zhang C, Niu Y, Yang W, Wang T, et al. : Cryo-EM structures of lipopolysaccharide transporter LptB2FGC in lipopolysaccharide or AMP-PNP-bound states reveal its transport mechanism. Nat Commun 2019, 10:4175. ** One of two papers reporting the structure of the LPS exporter in a closed state, leading to the current model for LPS extrusion from the membrane and translocation to the periplasmic bridge.
- 22.Okuda S, Freinkman E, Kahne D: Cytoplasmic ATP hydrolysis powers transport of lipopolysaccharide across the periplasm in E. coli. Science 2012, 338:1214–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sherman DJ, Xie R, Taylor RJ, George AH, Okuda S, Foster PJ, Needleman DJ, Kahne D: Lipopolysaccharide is transported to the cell surface by a membrane-to-membrane protein bridge. Science 2018, 359:798–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malinverni JC, Silhavy TJ: An ABC transport system that maintains lipid asymmetry in the gram-negative outer membrane. Proc Natl Acad Sci U S A 2009, 106:8009–8014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chong Z-S, Woo W-F, Chng S-S: Osmoporin OmpC forms a complex with MlaA to maintain outer membrane lipid asymmetry in Escherichia coli. Mol Microbiol 2015, 98:1133–1146. [DOI] [PubMed] [Google Scholar]
- 26.Thong S, Ercan B, Torta F, Fong ZY, Wong HYA, Wenk MR, Chng S-S: Defining key roles for auxiliary proteins in an ABC transporter that maintains bacterial outer membrane lipid asymmetry. Elife 2016, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abellón-Ruiz J, Kaptan SS, Baslé A, Claudi B, Bumann D, Kleinekathöfer U, van den Berg B: Structural basis for maintenance of bacterial outer membrane lipid asymmetry. Nat Microbiol 2017, 2:1616–1623. [DOI] [PubMed] [Google Scholar]
- 28.Ekiert DC, Bhabha G, Isom GL, Greenan G, Ovchinnikov S, Henderson IR, Cox JS, Vale RD: Architectures of Lipid Transport Systems for the Bacterial Outer Membrane. Cell 2017, 169:273–285.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Isom GL, Davies NJ, Chong Z-S, Bryant JA, Jamshad M, Sharif M, Cunningham AF, Knowles TJ, Chng S-S, Cole JA, et al. : MCE domain proteins: conserved inner membrane lipid-binding proteins required for outer membrane homeostasis. Scientific Reports 2017, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shrivastava R, X ‘er Jiang, Chng S-S: Outer membrane lipid homeostasis via retrograde phospholipid transport in Escherichia coli. Mol Microbiol 2017, 106:395–408. [DOI] [PubMed] [Google Scholar]
- 31.Nakayama T, Zhang-Akiyama Q-M: pqiABC and yebST, Putative mce Operons of Escherichia coli, Encode Transport Pathways and Contribute to Membrane Integrity. J Bacteriol 2017, 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kamischke C, Fan J, Bergeron J, Kulasekara HD, Dalebroux ZD, Burrell A, Kollman JM, Miller SI: The Acinetobacter baumannii Mla system and glycerophospholipid transport to the outer membrane. Elife 2019, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grimm J, Shi H, Wang W, Mitchell AM, Wingreen NS, Huang KC, Silhavy TJ: The inner membrane protein YhdP modulates the rate of anterograde phospholipid flow in Escherichia coli. Proc Natl Acad Sci U S A 2020, 117:26907–26914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ruiz N, Davis RM, Kumar S: YhdP, TamB, and YdbH Are Redundant but Essential for Growth and Lipid Homeostasis of the Gram-Negative Outer Membrane. MBio 2021, 12:e0271421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Low W-Y, Chng S-S: Current mechanistic understanding of intermembrane lipid trafficking important for maintenance of bacterial outer membrane lipid asymmetry. Curr Opin Chem Biol 2021, 65:163–171. [DOI] [PubMed] [Google Scholar]
- 36.Shrivastava R, Chng S-S: Lipid trafficking across the Gram-negative cell envelope. J Biol Chem 2019, 294:14175–14184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lundstedt E, Kahne D, Ruiz N: Assembly and Maintenance of Lipids at the Bacterial Outer Membrane. Chem Rev 2021, 121:5098–5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wong LH, Gatta AT, Levine TP: Lipid transfer proteins: the lipid commute via shuttles, bridges and tubes. Nat Rev Mol Cell Biol 2019, 20:85–101. [DOI] [PubMed] [Google Scholar]
- 39.Powers MJ, Trent MS: Phospholipid retention in the absence of asymmetry strengthens the outer membrane permeability barrier to last-resort antibiotics. Proc Natl Acad Sci U S A 2018, 115:E8518–E8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Powers MJ, Simpson BW, Trent MS: The Mla pathway in Acinetobacter baumannii has no demonstrable role in anterograde lipid transport. Elife 2020, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yeow J, Tan KW, Holdbrook DA, Chong Z-S, Marzinek JK, Bond PJ, Chng S-S: The architecture of the OmpC–MlaA complex sheds light on the maintenance of outer membrane lipid asymmetry in Escherichia coli. J Biol Chem 2018, 293:11325–11340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Low W-Y, Thong S, Chng S-S: ATP disrupts lipid-binding equilibrium to drive retrograde transport critical for bacterial outer membrane asymmetry. Proceedings of the National Academy of Sciences 2021, 118:e2110055118. * Describes development of a transport assay for Mla based upon radiolabeled lipid substrates, providing evidence that Mla drives retrograde phospholipid transport.
- 43. Hughes GW, Hall SCL, Laxton CS, Sridhar P, Mahadi AH, Hatton C, Piggot TJ, Wotherspoon PJ, Leney AC, Ward DG, et al. : Evidence for phospholipid export from the bacterial inner membrane by the Mla ABC transport system. Nature Microbiology 2019, 4:1692–1705. * Using a range of biochemical and physical techniques, these authors provide evidence that Mla facilitates anterograde transport.
- 44. Tang X, Chang S, Qiao W, Luo Q, Chen Y, Jia Z, Coleman J, Zhang K, Wang T, Zhang Z, et al. : Structural insights into outer membrane asymmetry maintenance in Gram-negative bacteria by MlaFEDB. Nat Struct Mol Biol 2021, 28:81–91. ** Reports structures of E. coli MlaFEDB in the outward-open state, and transport assay based on fluorescently labeled lipids, in which transport can occur bidirectionally, but is predominantly retrograde.
- 45. Kolich LR, Chang Y-T, Coudray N, Giacometti SI, MacRae MR, Isom GL, Teran EM, Bhabha G, Ekiert DC: Structure of MlaFB uncovers novel mechanisms of ABC transporter regulation. Elife 2020, 9:e60030. * Described the structure of MlaFB for the first time, suggesting a possible mechanism for post-translational regulation of MlaFEDB activity.
- 46. Coudray N, Isom GL, MacRae MR, Saiduddin MN: Structure of bacterial phospholipid transporter MlaFEDB with substrate bound. Elife. 2020. 9:e62518. ** Reported the structure of E. coli MlaFEDB in the outward-open state with two phospholipids bound, and showed that phospholipids are likely bound in the pocket in vivo.
- 47. Zhang Y, Fan Q, Chi X, Zhou Q, Li Y: Cryo-EM structures of Acinetobacter baumannii glycerophospholipid transporter. Cell Discov 2020, 6:86. ** Reported structures of A. baumannii MlaFEDB in both the outward-open state with two phospholipids bound, and a closed state with a collapsed lipid binding pocket.
- 48. Chi X, Fan Q, Zhang Y, Liang K, Wan L, Zhou Q, Li Y: Structural mechanism of phospholipids translocation by MlaFEDB complex. Cell Res 2020, 30:1127–1135. ** Reported structures of E. coli MlaFEDB in both the outward-open state with two phospholipids bound, and a closed state with a collapsed lipid binding pocket.
- 49.Mann D, Fan J, Somboon K, Farrell DP, Muenks A, Tzokov SB, DiMaio F, Khalid S, Miller SI, Bergeron JRC: Structure and lipid dynamics in the maintenance of lipid asymmetry inner membrane complex of A. baumannii. Commun Biol 2021, 4:817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou C, Shi H, Zhang M, Zhou L, Xiao L, Feng S, Im W, Zhou M, Zhang X, Huang Y: Structural Insight into Phospholipid Transport by the MlaFEBD Complex from P. aeruginosa. J Mol Biol 2021, 433:166986. [DOI] [PubMed] [Google Scholar]
- 51.Kadaba NS, Kaiser JT, Johnson E, Lee A, Rees DC: The high-affinity E. coli methionine ABC transporter: structure and allosteric regulation. Science 2008, 321:250–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gerber S, Comellas-Bigler M, Goetz BA, Locher KP: Structural basis of trans-inhibition in a molybdate/tungstate ABC transporter. Science 2008, 321:246–250. [DOI] [PubMed] [Google Scholar]
- 53.Deutscher J, Deutscher J, Francke C, Postma PW: How Phosphotransferase System-Related Protein Phosphorylation Regulates Carbohydrate Metabolism in Bacteria. Microbiology and Molecular Biology Reviews 2006, 70:939–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen S, Oldham ML, Davidson AL, Chen J: Carbon catabolite repression of the maltose transporter revealed by X-ray crystallography. Nature 2013, 499:364–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walter JD, Sawicka M, Dutzler R: Cryo-EM structures and functional characterization of murine Slc26a9 reveal mechanism of uncoupled chloride transport. Elife 2019, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ko SBH, Zeng W, Dorwart MR, Luo X, Kim KH, Millen L, Goto H, Naruse S, Soyombo A, Thomas PJ, et al. : Gating of CFTR by the STAS domain of SLC26 transporters. Nat Cell Biol 2004, 6:343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sharma AK, Rigby AC, Alper SL: STAS domain structure and function. Cell Physiol Biochem 2011, 28:407–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen L, Hou W-T, Fan T, Liu B, Pan T, Li Y-H, Jiang Y-L, Wen W, Chen Z-P, Sun L, et al. : Cryo-electron Microscopy Structure and Transport Mechanism of a Wall Teichoic Acid ABC Transporter. MBio 2020, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Caffalette CA, Zimmer J: Cryo-EM structure of the full-length WzmWzt ABC transporter required for lipid-linked O antigen transport. Proc Natl Acad Sci U S A 2021, 118. * The authors report the first full-length structure of the O-antigen exporter WzmWzt, providing insights into the mechanism of glycolipid translocation across the membrane.
- 60.Bi Y, Mann E, Whitfield C, Zimmer J: Architecture of a channel-forming O-antigen polysaccharide ABC transporter. Nature 2018, 553:361–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qian H, Zhao X, Cao P, Lei J, Yan N, Gong X: Structure of the Human Lipid Exporter ABCA1. Cell 2017, 169:1228–1239.e10. [DOI] [PubMed] [Google Scholar]
- 62.Lee J-Y, Kinch LN, Borek DM, Wang J, Wang J, Urbatsch IL, Xie X-S, Grishin NV, Cohen JC, Otwinowski Z, et al. : Crystal structure of the human sterol transporter ABCG5/ABCG8. Nature 2016, 533:561–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Manolaridis I, Jackson SM, Taylor NMI, Kowal J, Stahlberg H, Locher KP: Cryo-EM structures of a human ABCG2 mutant trapped in ATP-bound and substrate-bound states. Nature 2018, 563:426–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Crow A, Greene NP, Kaplan E, Koronakis V: Structure and mechanotransmission mechanism of the MacB ABC transporter superfamily. Proc Natl Acad Sci U S A 2017, 114:12572–12577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fitzpatrick AWP, Llabrés S, Neuberger A, Blaza JN, Bai X-C, Okada U, Murakami S, van Veen HW, Zachariae U, Scheres SHW, et al. : Structure of the MacAB-TolC ABC-type tripartite multidrug efflux pump. Nat Microbiol 2017, 2:17070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Murakami S, Okada U, Yamashita E: Crystal structure of tripartite-type ABC transporter, MacB from Acinetobacter baumannii. Nat Commun 2017. 8:1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sharma S, Zhou R, Wan L, Feng S, Song K, Xu C, Li Y, Liao M: Mechanism of LolCDE as a molecular extruder of bacterial triacylated lipoproteins. Nat Commun 2021, 12:4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tang X, Chang S, Zhang K, Luo Q, Zhang Z, Wang T, Qiao W, Wang C, Shen C, Zhang Z, et al. : Structural basis for bacterial lipoprotein relocation by the transporter LolCDE. Nat Struct Mol Biol 2021, 28:347–355. [DOI] [PubMed] [Google Scholar]
- 69.Thomas C, Aller SG, Beis K, Carpenter EP, Chang G, Chen L, Dassa E, Dean M, Duong Van Hoa F, Ekiert D, et al. : Structural and functional diversity calls for a new classification of ABC transporters. FEBS Lett 2020, 594:3767–3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kumar A, Chiu HJ, Axelrod HL, Morse A, Elsliger MA, Wilson IA, Deacon A: Ligands in PSI structures. Acta Crystallogr Sect F Struct Biol Cryst Commun 2010, 66:1309–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smart OS, Horský V, Gore S, Svobodová Vařeková R, Bendová V, Kleywegt GJ, Velankar S: Validation of ligands in macromolecular structures determined by X-ray crystallography. Acta Crystallogr D Struct Biol 2018, 74:228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thomas C, Tampé R: Structural and Mechanistic Principles of ABC Transporters. Annu Rev Biochem 2020, 89:605–636. [DOI] [PubMed] [Google Scholar]
- 73.Jackson SM, Manolaridis I, Kowal J, Zechner M, Taylor NMI, Bause M, Bauer S, Bartholomaeus R, Bernhardt G, Koenig B, et al. : Structural basis of small-molecule inhibition of human multidrug transporter ABCG2. Nat Struct Mol Biol 2018, 25:333–340. [DOI] [PubMed] [Google Scholar]
- 74.Taylor NMI, Manolaridis I, Jackson SM, Kowal J, Stahlberg H, Locher KP: Structure of the human multidrug transporter ABCG2. Nature 2017, 546:504–509. [DOI] [PubMed] [Google Scholar]
- 75.Skarda L, Kowal J, Locher KP: Structure of the Human Cholesterol Transporter ABCG1. J Mol Biol 2021, 433:167218. [DOI] [PubMed] [Google Scholar]
- 76.Sun Y, Wang J, Long T, Qi X, Donnelly L, Elghobashi-Meinhardt N, Esparza L, Cohen JC, Xie X-S, Hobbs HH, et al. : Molecular basis of cholesterol efflux via ABCG subfamily transporters. Proc Natl Acad Sci U S A 2021, 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu F, Lee J, Chen J: Molecular structures of the eukaryotic retinal importer ABCA4. Elife 2021, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xie T, Zhang Z, Fang Q, Du B, Gong X: Structural basis of substrate recognition and translocation by human ABCA4. Nat Commun 2021, 12:3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Quazi F, Lenevich S, Molday RS: ABCA4 is an N-retinylidene-phosphatidylethanolamine and phosphatidylethanolamine importer. Nat Commun 2012, 3:925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dahl ACE, Chavent M, Sansom MSP: Bendix: intuitive helix geometry analysis and abstraction. Bioinformatics 2012, 28:2193–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tian W, Chen C, Lei X, Zhao J, Liang J: CASTp 3.0: computed atlas of surface topography of proteins. Nucleic Acids Res 2018, 46:W363–W367. [DOI] [PMC free article] [PubMed] [Google Scholar]


