Abstract
Background
Little is known about the long-term outcomes and patterns of disease progression in patients with unresectable, liver-confined HCC who had complete response (CR) to transarterial embolization and do not undergo subsequent resection or liver transplantation (LT).
Methods
A retrospective review of patients who participated in a randomized phase II trial comparing bland hepatic artery embolization (HAE) or drug-eluting bead transarterial chemoembolization (DEB-TACE) with doxorubicin-loaded microspheres and had an overall CR by modified RECIST criteria to embolization was performed. The overall survival (OS), incidence of recurrence, patterns of recurrence, and factors associated with recurrence were assessed.
Results
Of the 101 patients analyzed in the randomized phase II trial, 37 with a CR were included in this study. Within the study cohort, 17 patients were treated with HAE (46%) and 20 with DEB-TACE (54%). The median age was 67 years (range: 42–82). Most patients were male (86.5%) and Caucasian (78%). The median pre-treatment MELD score was 10, 70% had BCLC Stage B or C and 38% had extrahepatic disease. The median follow-up was 49 months (95%CI: 9–108) with a median OS of 25 months (95%CI: 18.9–30.9). Three-year and 5-year survival were 31% (95%CI: 16.7–45.9%) and 18% (95%CI: 6.8–32.1%). Six-month, 1-year, and 2-year cumulative incidence of recurrence were 51% (95%CI: 34.0–66.2%), 76% (95%CI: 57.7–86.8%), and 92% (95%CI: 74.5–97.6%), respectively. For the first recurrence, the most common site was the previously treated hepatic site or local only (32%, 12/37) followed by a new hepatic site not previously treated or regional (24%, 9/37). Three-year cumulative incidence of recurrence was 65% (95%CI: 46.4–78.4%) for local, 76% (95%CI: 56.7–87.2%) for regional, and 46% (95%CI: 29.1–69.2%) for extrahepatic or distant recurrence. Patients with higher alpha fetoprotein (AFP) values had a higher hazard of death (HR: 1.36, 95%CI: 1.16–1.59, p<0.001) and a higher hazard of any recurrence (HR: 1.20, 95%CI: 1.04–1.38, p=0.012). Patients who received DEB-TACE had a higher hazard of death (HR: 2.46, 95%CI: 1.13–5.35, p=0.023) compared to patients who received HAE. However, no significant association was found between embolization type and recurrence (HR: 0.82, p=0.54).
Conclusion
Patients with advanced-stage HCC and complete response to embolization do not have durable responses and have inevitable disease progression. Most patients with recurrence have liver-confined disease and should be evaluated for additional consolidative treatments.
I. Introduction
Hepatocellular carcinoma (HCC), the most common primary liver cancer worldwide, has a high mortality rate and its incidence continues to rise. HCC is the fifth most common cancer worldwide and the second most common cause of cancer-related deaths1–4. Furthermore, it is estimated that the 5-year survival from the time of diagnosis for resected HCC is approximately 30%, 9.5% for unresectable/liver-confined HCC, and 3.5% for metastatic HCC6.
Partial hepatectomy and liver transplantation (LT) are considered potentially curative treatments for those with HCC within resection and transplantation criteria. These treatments are also associated with improvement in overall survival. Five-year recurrence-free survival rates of up to 83% have been reported after LT in well selected early stage patients7–12. Percutaneous tumor ablation and complete resection also have been shown to be associated with prolonged overall survival in early stage HCC11, 12.
For patients with advanced or unresectable disease who are not surgical candidates, there are other treatment options which can slow disease progression and prolong survival. Locoregional therapy (LRT), such as bland hepatic artery embolization (HAE), transarterial chemoembolization (TACE), drug-eluting bead transarterial chemoembolization (DEB-TACE) and Yttrium-90 radioembolization (RAE), have been widely used treatment modalities for unresectable liver-confined disease13. Of these, TACE has become the preferred treatment after it was demonstrated to provide a survival advantage in randomized trials compared to best supportive care 14, 15. However, a systematic review from 2007 found that that there was no evidence that TACE was more effective than HAE alone 16. As part of a grant supported by the National Cancer Institute, we have demonstrated in a randomized controlled trial (RCT) that there is no difference in response, progression-free survival (PFS), or overall survival (OS) after treatment with HAE as compared to DEB-TACE with doxorubicin-loaded microspheres17.
In recent years, some centers have proposed LT after downstaging with LRT. Similar survival rates have been reported after LT in downstaged patients and those who initially presented within the Milan criteria18–22. Others have investigated the role of resection after downstaging with LRT and have demonstrated promising OS rates23–26. These findings suggest a role for resection and LT following downstaging with LRT in patients with good response. There is a paucity of published literature describing the prognosis of patients with unresectable, liver-confined HCC who had radiologic complete response (CR) to LRT and do not undergo subsequent resection or LT. One small retrospective study evaluated patients treated with TACE who had a CR and found that the median time to recurrence was 12 months and the 3-year survival rates was 49%. In this study, the small group of patients who underwent LT had significantly prolonged survival27. However, this study included patients with early-stage HCC that did not undergo surgery for various reasons including old age, inaccessible tumor location for ablation or resection, or long waiting time for LT. Furthermore, while previous studies have reported the prognosis of patients with CR to LRT, they do not characterize the patterns of disease progression or recurrence in this patient population27–30.
The outcomes for patients with unresectable, liver-confined disease who are not offered resection or LT but respond well to LRT are not well described. The primary aim of this study was to analyze the outcomes, patterns of disease progression, and prognosis of patients who presented with locally advanced, liver-confined HCC treated with either HAE or TACE with doxorubicin-eluting beads (DEB-TACE) in a prospective randomized trial17 who had radiologic CR and did not undergo subsequent resection or LT.
II. Methods
Study Design and Patient Population
This study is a retrospective analysis of data collected from at prospective, single-center, single-blind, randomized phase II trial conducted at our center. The study protocol and outcomes have been previously described in detail17. In brief, patients with biopsy or radiologically proven HCC that is resectable/early stage, unresectable/liver confined (includes patients with multiple nodules with or without vascular invasion), or advanced stage (Barcelona Clinic Liver Cancer [BCLC] Stage C)31 were eligible. Patients who had resectable HCC or met the Milan or UCSF criteria for transplantation but were poor surgical candidates due to age, performance status, other comorbidities or patient refusal were included in the study. Portal vein invasion was allowed as long as liver function was preserved. Patients could have limited extrahepatic disease, such as a few small lung nodules or enlarged regional lymph nodes, at enrollment. Patients from the trial who had radiologic CR following embolization as defined by the mRECIST criteria were included in the present study32.
Statistical Analysis
The objective of this study was to describe and analyze survival and disease progression among complete responders in this trial. Disease progression was assessed using multiphase computed tomography. Each multiphase CT was interpreted by an experienced hepatobiliary radiologist. Sites of progression were defined as “local” if recurrence occurred at a treated hepatic site, “regional” if the disease progression occurred at a hepatic site not previously treated, and “distant” if the progression occurred in any extrahepatic site. Liver-confined progression was defined as any local, regional, or combined locoregional site of progression.
OS was calculated from time of radiologic CR until death from any cause; patients alive at last follow up were censored. TTR was measured from CR until first progression as determined by imaging and/or biopsy. Death was treated as a competing risk and patients alive without recurrence were censored. Kaplan Meier methods and competing risk analyses with Fine & Grey methods were used to calculate estimates, where appropriate. We used event history plots to illustrate timing of each type of recurrence33. Univariable Cox and competing risks regression assessed the relationship between baseline patient characteristics and clinical outcomes.
Two-sided p-values less than 0.05 were considered statistically significant. All analyses were performed using SAS 9.4 TS1M6 (The SAS Institute, Cary, NC).
III. Results
Clinical Characteristics
Of the 101 treated in the randomized trial, 38 with a radiologic CR were included. One patient was excluded from the final analysis due to missing follow-up data. The baseline clinical characteristics of the 37 patients are summarized in Table 1. The median age was 67 years (range: 42–82). Most patients were male (87%) and Caucasian (78%). The median Charlson Comorbidity Index (CCI)34 was 6 (range 4–13), and viral hepatitis was present in 21 patients (57%). The median pre-treatment MELD score was 10 and 70% were classified as BCLC Stage B or C.
Table 1.
Patient Characteristics
| N (%)* | ||
|---|---|---|
|
| ||
| # Patients | 37 | |
| Age at Treatment, years | Median (Range) | 67 (42–82) |
| Gender | Male | 32 (86.5) |
| Female | 5 (13.5) | |
| Race | White | 29 (78.4) |
| Asian | 4 (10.8) | |
| Black | 3 (8.1) | |
| Other | 1 (2.7) | |
| BMI | Median (Range) (N=35) | 26.9 (17.9–37.0) |
| Extrahepatic Disease | Yes | 14 (37.8) |
| Extrahepatic Site | Lymph Nodes | 8 (21.6) |
| Lungs | 6 (16.2) | |
| None | 23 (62.2) | |
| Portal Vein Thrombus | Yes | 4 (10.8) |
| UCSF Criteria for Transplant | Yes | 8 (21.6) |
| Milan Criteria for Transplant | Yes | 3 (8.1) |
| Pre-Treatment Meld Score | Median (Range) (N=37) | 10 (6–20) |
| BCLC Stage | A | 11 (29.7) |
| B | 12 (32.4) | |
| C | 14 (37.8) | |
| AFP | Median (Range) (N=36) | 12.9 (2.3–51123) |
| History/Comorbidities | ||
| Charlson Comorbidity Index | Median (Range) | 6 (4–13) |
| Prior Cancer | Yes | 8 (21.6) |
| Family History of Cancer | Yes | 16 (43.2) |
| History of Smoking | Yes | 25 (67.6) |
| History of Alcohol Use | Yes | 14 (37.8) |
| Hepatitis B | Yes | 9 (24.3) |
| Hepatitis C | Yes | 13 (35.1) |
| Hepatitis B or C | Yes | 21 (56.8) |
| Prior Liver Ablation | Yes | 4 (10.8) |
| Prior Liver Surgery | Yes | 4 (10.8) |
Numbers represent frequency with percent of total in parentheses unless otherwise stated.
UCSF=University of California, San Francisco; BCLC=Barcelona Clinic Liver Cancer
Treatment characteristics are summarized in Table 2. Among our study cohort, 17 patients were treated with HAE (46%) and 20 with DEB-TACE (54%). The median total number of embolization procedures was 2 (range 1–8). The median time to CR was 2.6 weeks (range: 0.1–29.1). Three (8%) patients had received prior systemic therapy. Fifteen (41%) patients received post-CR systemic treatment at the time of progression, of which 13 received sorafenib, 1 received pegylated arginine deiminase (ADI-PEG20) and another received lenvatinib. The median time to post-CR systemic treatment was 22.6 months (range 2.9–102.3). One patient (2.7%) received post-CR radiation at an outside institution.
Table 2.
Treatment Characteristics
| N (%)* | ||
|---|---|---|
|
| ||
| Prior Systemic Therapy | Yes | 2 (5.4) |
| No | 35 (94.6) | |
| Prior Systemic Therapy Drug | None | 35 (94.6) |
| Sorafenib | 2 (5.4) | |
| Treatment Arm | DEB-TACE | 20 (54.1) |
| HAE | 17 (45.9) | |
| Weeks between Treatment Start and CR | Median (Range) (N=37) | 2.6 (0.1–29.1) |
| # Lesions | Median (Range) (N=37) | 3 (1–10) |
| 1 | 13 (35.1) | |
| 2 | 4 (10.8) | |
| 3 | 7 (18.9) | |
| 4 | 5 (13.5) | |
| 5 | 1 (2.7) | |
| 5+‡ | 7 (18.9) | |
| # Target Lesions | 1 | 23 (62.2) |
| 2 | 9 (24.3) | |
| 3 | 3 (8.1) | |
| 4 | 2 (5.4) | |
| Sum Lesion Size, cm† | Median (Range) | 7.1 (1.1–15.2) |
| Total # Embolizations | Median (Range) | 2 (1–8) |
| Post-CR Systemic Therapy | Yes | 15 (40.5) |
| No | 22 (59.5) | |
| Post-CR Systemic Therapy Drug | None | 22 (59.5) |
| Sorafenib | 13 (35.1) | |
| Other/Trial§ | 2 (5.4) | |
| Months between CR and Adj. Systemic Therapy | Median (Range) (N=15) | 22.6 (2.9–102.3) |
| Post-CR Radiation | Yes | 1 (2.7) |
| No | 36 (97.3) | |
DEB-TACE=chemoembolization with doxorubicin-loaded microspheres; HAE=hepatic artery embolization; CR=complete response
Numbers represent frequency with percent of total in parentheses unless otherwise stated
Sum of all the sizes in diameter of all lesions prior to embolization.
Total number of lesions too many to count
For post-CR systemic therapy, one patient received ADI-PEG20 and another received Lenvatinib.
Recurrence and Survival
At a median follow up time for survivors of 49 months (range 9 – 108), 32 patients (86%) had died with a median OS of 25 months (95%CI: 18.9–30.9). Three-year and 5-year survival were 31% (95%CI: 16.7–45.9%) and 18% (95%CI: 6.8–32.1%), respectively. At last follow-up, 35 of the 37 patients had disease progression. Six-month, 1-year, and 2-year cumulative incidence of progression were 51% (95%CI: 34.0–66.2%),76% (95%CI: 57.7–86.8%), and 92% (95%CI: 74.5–94.6%), respectively (Figure 1). For the first progression, the most common site was local only (32%, 12/37) followed by regional (24%, 9/37). Twenty-six of 37 patients (70%) had liver-confined first recurrence sites. Three of 37 patients (8%) had a distant site as a first site of progression (Table 3). One-year and 2-year cumulative incidence of progression was 38% (95%CI: 22.3–53.3%) and 60% (95%CI: 41.4–73.6%) for local; 51% (95%CI: 34.0–66.2%) and 70% (95%CI: 52–82.7%) for regional; and 27% (95%CI: 13.9–42.0%) and 43% (95%CI: 26.8–58.6%) for distant progression. Three-year cumulative incidence of progression was 65% (95%CI: 46.4–78.4%) for local, 76% (95%CI: 56.7–87.2%) for regional, and 46% (95%CI: 29.1–69.2%) for distant progression (Figure 2).
Figure 1.
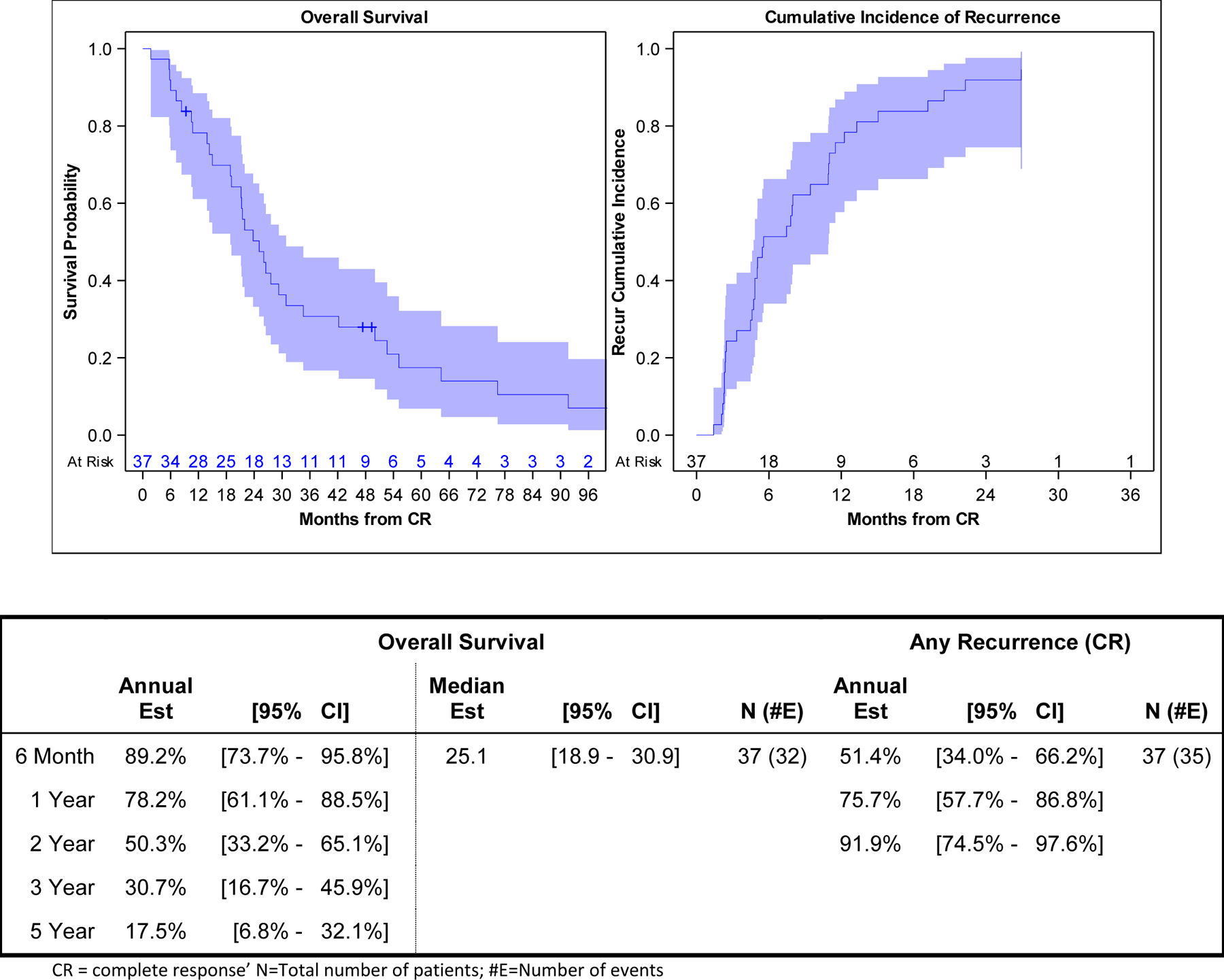
Kaplan-Meier Plot of Overall Survival and Cumulative Incidence of First Recurrence/Disease Progression
Table 3.
Survival and Disease Progression
| N (%) | ||
|---|---|---|
|
| ||
| Follow up Time for Survivors, months | Median (Range) (N=5) | 49 (9–108) |
| Last Follow up Status | AWD | 5 (13.5) |
| DOD | 30 (81.1) | |
| DOU | 2 (5.4) | |
| First Progression Type | Local Only | 12 (32.4) |
| Regional Only | 9 (24.3) | |
| Locoregional | 5 (13.5) | |
| Regional and Distant | 5 (13.5) | |
| Distant Only | 3 (8.1) | |
| Locoregional and Distant | 2 (5.4) | |
| Local and Distant | 1 (2.7) | |
| Any Local Progression | Yes | 24 (64.9) |
| No | 13 (35.1) | |
| Any Regional Progression | Yes | 28 (75.7) |
| No | 9 (24.3) | |
| Any Distant Progression | Yes | 18 (48.6) |
| No | 19 (51.4) | |
AWD = alive with disease; DOD = died of disease; DOU = died of unknown cause
Figure 2.
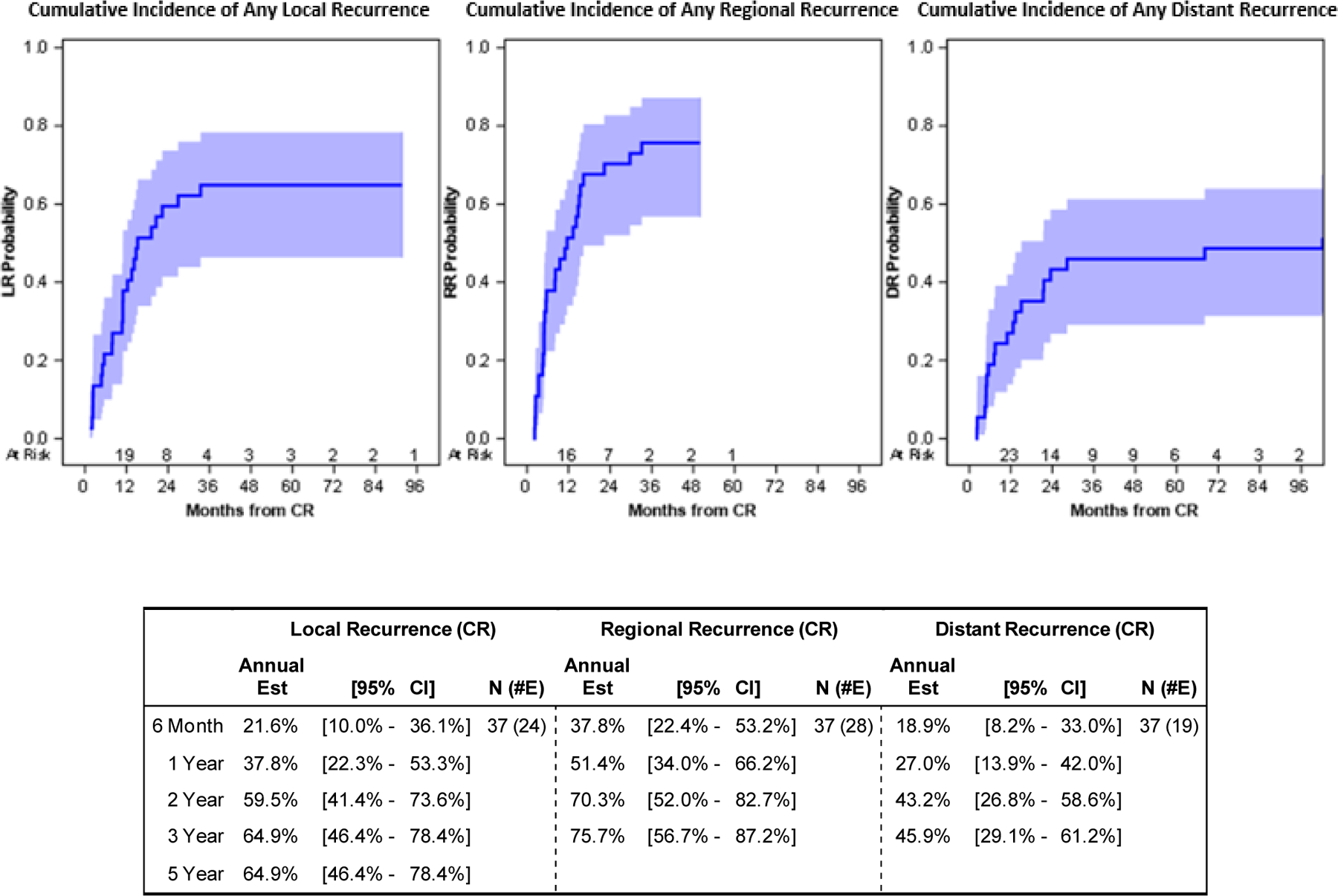
First Disease Progression Plots and Estimates
Local progression after radiologic CR frequently appeared first. A few patients had distant progression followed by a local progression later (Figure 3).
Figure 3.
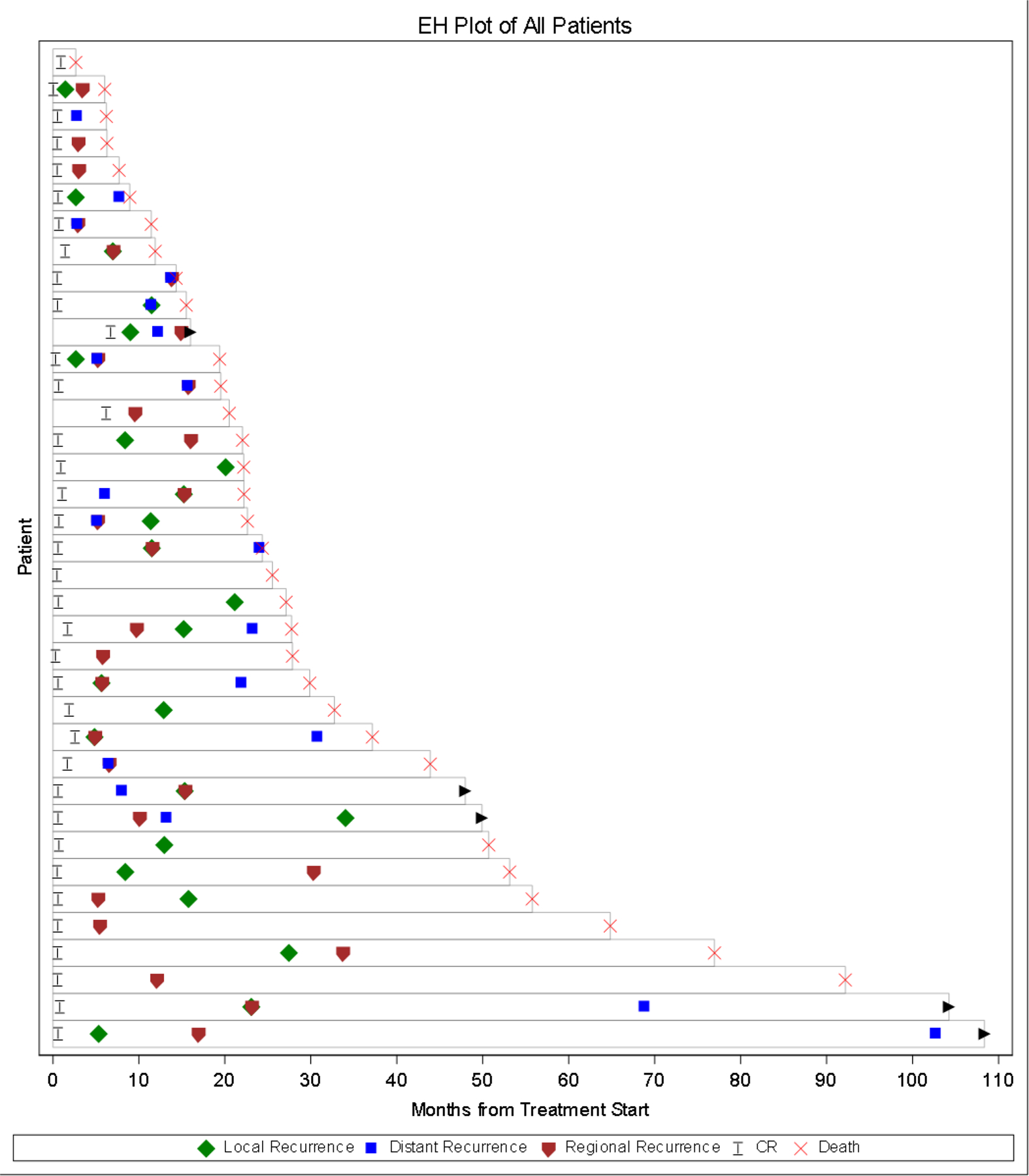
Event History Plot from Treatment Start
There were few significant associations between patient factors and OS or TTR. Patients with higher AFP values had a higher hazard of death (HR: 1.36, 95%CI: 1.16–1.59, p<0.001) and a higher hazard of any disease progression (HR: 1.20, 95%CI: 1.04–1.38, p=0.012). No significant association was found between AFP and distant progression (HR: 0.98, p=0.82). Patients who received DEB-TACE had a higher hazard of death (HR: 2.46, 95%CI: 1.13–5.35, p=0.023) compared to patients who received HAE (Table 4). No significant association was found between embolization type and disease progression (HR: 0.82, p=0.54). There were two marginal associations where patients with extrahepatic disease had a lower risk of death (HR: 0.46, 95%CI: 0.21–1.00, p=0.051) and patients with higher CCI had a lower risk of death (HR: 0.86, 95%CI: 0.74–1.00, p=0.046).
Table 4.
Univariate Analyses: Predictive Factors for Overall Survival and Disease Progression
| Outcome | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall Survival | Any Progression | Distant Progression | |||||||||||
| N(#E) | HR | [95% CI] | p-value | N(#E) | HR | [95% CI] | p-value | N(#E) | HR | [95% CI] | p-value | ||
|
| |||||||||||||
| Age at Treatment, years | 37 (32) | 0.97 | [0.93 – 1.01] | 0.10 | 37 (35) | 0.98 | [0.95 – 1.02] | 0.38 | 37 (19) | 0.95 | [0.90 – 1.02] | 0.14 | |
| Gender | Female | 5 (4) | 0.84 | [0.29 – 2.43] | 0.75 | 5 (5) | 0.92 | [0.49 – 1.73] | 0.79 | 5 (4) | 2.29 | [0.83 – 6.33] | 0.11 |
| Male | 32 (28) | REF | 32 (30) | REF | 32 (15) | REF | |||||||
| BMI | 35 (30) | 0.95 | [0.88 – 1.03] | 0.21 | 35 (34) | 0.97 | [0.91 – 1.04] | 0.47 | 35 (18) | 0.97 | [0.87 – 1.08] | 0.52 | |
| Extrahepatic Disease | Yes | 14 (10) | 0.46 | [0.21 – 1.00] | 0.051 | 14 (14) | 1.11 | [0.58 – 2.12] | 0.76 | 14 (9) | 1.77 | [0.74 – 4.22] | 0.20 |
| No | 23 (22) | REF | 23 (21) | REF | 23 (10) | REF | |||||||
| Charlson Comorbidity Index | 37 (32) | 0.86 | [0.74 – 1.00] | 0.046 | 37 (35) | 0.96 | [0.85 – 1.09] | 0.51 | 37 (19) | 1.08 | [0.93 – 1.24] | 0.31 | |
| Prior Cancer | Yes | 8 (8) | 0.94 | [0.42 – 2.10] | 0.88 | 8 (7) | 0.78 | [0.34 – 1.79] | 0.56 | 8 (2) | 0.37 | [0.08 – 1.82] | 0.22 |
| No | 29 (24) | REF | 29 (28) | REF | 29 (17) | REF | |||||||
| Family History of Cancer | Yes | 16 (14) | 0.63 | [0.31 – 1.28] | 0.20 | 16 (15) | 0.73 | [0.38 – 1.43] | 0.37 | 16 (7) | 0.59 | [0.24 – 1.41] | 0.23 |
| No | 21 (18) | REF | 21 (20) | REF | 21 (12) | REF | |||||||
| History of Smoking | Yes | 25 (23) | 1.69 | [0.77 – 3.68] | 0.19 | 25 (24) | 0.80 | [0.36 – 1.76] | 0.58 | 25 (13) | 1.09 | [0.43 – 2.77] | 0.86 |
| No | 12 (9) | REF | 12 (11) | REF | 12 (6) | REF | |||||||
| History of Alcohol Use | Yes | 14 (13) | 1.26 | [0.61 – 2.61] | 0.53 | 14 (13) | 0.97 | [0.50 – 1.88] | 0.92 | 14 (5) | 0.43 | [0.17 – 1.12] | 0.08 |
| No | 23 (19) | REF | 23 (22) | REF | 23 (14) | REF | |||||||
| Hepatitis B | Yes | 9 (7) | 1.00 | [0.43 – 2.33] | >0.95 | 9 (9) | 0.98 | [0.49 – 1.93] | 0.94 | 9 (5) | 1.13 | [0.43 – 2.99] | 0.81 |
| No | 28 (25) | REF | 28 (26) | REF | 28 (14) | REF | |||||||
| Hepatitis C | Yes | 13 (11) | 0.90 | [0.43 – 1.88] | 0.78 | 13 (13) | 0.95 | [0.53 – 1.71] | 0.86 | 13 (9) | 1.69 | [0.71 – 4.01] | 0.24 |
| No | 24 (21) | REF | 24 (22) | REF | 24 (10) | REF | |||||||
| Hepatitis B/C | Yes | 21 (17) | 0.84 | [0.42 – 1.70] | 0.63 | 21 (21) | 1.02 | [0.51 – 2.05] | >0.95 | 21 (13) | 1.66 | [0.60 – 4.59] | 0.33 |
| No | 16 (15) | REF | 16 (14) | REF | 16 (6) | REF | |||||||
| Treatment Arm | DEB-TACE | 20 (18) | 2.46 | [1.13 – 5.35] | 0.023 | 20 (18) | 0.82 | [0.44 – 1.55] | 0.54 | 20 (11) | 1.52 | [0.64 – 3.62] | 0.35 |
| HAE | 17 (14) | REF | 17 (17) | REF | 17 (8) | REF | |||||||
| UCSF Criteria for Transplant | Yes | 8 (8) | 1.05 | [0.47 – 2.35] | 0.90 | 8 (7) | 0.73 | [0.31 – 1.70] | 0.47 | 8 (4) | 1.12 | [0.35 – 3.63] | 0.85 |
| No | 29 (24) | REF | 29 (28) | REF | 29 (15) | REF | |||||||
| Milan Criteria for Transplant | Yes | 3 (3) | 1.12 | [0.34 – 3.74] | 0.85 | 3 (3) | 1.01 | [0.36 – 2.86] | >0.95 | 3 (2) | 2.38 | [0.34 – 16.67] | 0.38 |
| No | 34 (29) | REF | 34 (32) | REF | 34 (17) | REF | |||||||
| Pre-Treatment Meld Score | 37 (32) | 1.07 | [0.98 – 1.17] | 0.14 | 37 (35) | 1.01 | [0.90 – 1.12] | 0.93 | 37 (19) | 0.95 | [0.80 – 1.12] | 0.55 | |
| AFP (log-transformed) | 36 (31) | 1.36 | [1.16 – 1.59] | <.001 | 36 (34) | 1.20 | [1.04 – 1.38] | 0.012 | 36 (18) | 0.98 | [0.82 – 1.17] | 0.82 | |
N=Total number of patients; #E=Number of events; HR=Hazard Ratio; 95%CI = 95% confidence interval; DEB-TACE = transarterial -chemoembolization with doxorubicin-loaded microspheres; HAE = hepatic artery embolization; AFP = alpha fetal protein
IV. Discussion:
In this study, we used data from a previous RCT at our institution, which showed no difference in response, PFS or OS after treatment with HAE vs embolization with doxorubicin-loaded microspheres17. This present study indicates that while embolization is effective in achieving radiologic CR and is a reasonable treatment option in patients with advanced-stage or unresectable, liver-confined HCC, HAE or DEB-TACE alone is palliative with eventual disease progression in everyone. The fact that there was no difference in progression rate or pattern suggests that the addition of a chemotherapeutic agent alone to HAE does not improve PFS.
Our study showed that by the end of follow up, 32 patients had died with a median OS of 25 months. Overall, the 3-year and 5-year cumulative survival rates of 31% and 18%, respectively, in our cohort were lower compared to what have been reported in previous studies of patients with radiologic CR after embolization27, 37. It is important to note that most reports exclude patients who are BCLC C or have portal vein tumor thrombus. A prospective study that investigated the risk factors for recurrence and survival in 134 patients with inoperable HCC who underwent TACE and had CR reported a 3-year survival rate of 40% and 5-year survival rate of 27% among those who had local recurrence within a year36. Another study in 168 treatment-naïve patients with HCC who underwent TACE as a first line treatment reported a cumulative survival rate of 49% after 3 years among those who had CR27. However, these differences could be attributed to the different patient selection criteria in these studies. For instance, the study by Cerban et27 al excluded patients who had any extrahepatic disease and had prior surgeries or treatments for HCC while 38% of our study population had limited extrahepatic disease, such as a few small lung nodules or enlarged regional lymph nodes, and approximately 11% of our cohort had either a previous liver ablation or liver surgery prior to embolization, which suggest more advanced disease at baseline. Furthermore, in the study by Rou et al36, only 1.5% of the patients had extrahepatic disease and 66% of the patients had solitary tumors with a median maximal diameter or 2.5cm while only 35% of our cohort had solitary lesions and 19% had more than 5 tumors, suggesting more advanced disease in our cohort.
In our patients, local disease progression is common within 6 months of radiologic CR. The cumulative incidence of progression was more than 50% at six months and 1 year after radiologic CR, and over 90% at 2 years after radiologic CR. Similar rates of disease progression within a year have been reported in previous studies of patients with radiologic CR after embolization, which ranged from 39% to 58%27, 35, 36. The timing and pattern of progression in our cohort illustrate that local progression after radiologic CR frequently appears first, sometimes overlapping with regional progression. Most of our patients had liver-confined first recurrence sites, with 32% recurring first locally in the index lesion that was targeted for embolization, which suggests that radiologic CR does not equal pathologic CR. This also points to the limitation of the mRECIST criteria in assessing treatment effects of LRT on HCC, and the potential utility of other response evaluation criteria and incorporating other factors, such as AFP, AFP-L3 and des-gamma-carboxy protein (DCP) levels in assessing overall treatment response37. In our study, distant progression often appeared later, and the majority of patients had liver-confined disease, which presents a potential opportunity for more aggressive treatment, such as resection or transplant in a subset of patients.
Recent studies have demonstrated the effectiveness of downstaging advanced-stage HCC with TACE to meet Milan criteria for liver transplantation or surgical resection and excellent long-term outcomes associated with surgery in these patients, similar to those presenting with early stage HCC18–26. These studies report that patients with advanced-stage HCC who had radiologic CR from TACE and subsequently underwent LT had significantly improved survival, with five-year post-transplant survival and recurrence-free probabilities up to 77.8% and 90.8%, respectively18–26. However, these studies seldom report the outcomes of those that did not undergo LT. To our knowledge, this present study is the only study that assessed disease progression and survival in patients with advanced-stage HCC who had radiologic CR after HAE versus those who had CR after DEB-TACE and did not undergo subsequent surgery. Our study found that the majority of the first site of disease progression after radiologic CR to embolization remain confined to the liver with half having liver-only disease at three years after radiologic CR. As such, patients treated with good responses to embolization should be evaluated for liver resection or transplantation when feasible, or should be evaluated for additional treatments, such as retreatment with LRT and systemic therapy usually reserved for advanced disease.
This study was conducted before the emergence of data for various systemic therapies for HCC. Studies have demonstrated that molecularly targeted agents, such as sorafenib and lenvatinib improve survival in HCC38,39. More recently, the combination of atezolizumab, a checkpoint inhibitor, plus bevacizumab, a monoclonal antibody that inhibits angiogenesis and tumor growth, has been shown to improve survival over sorafenib alone in HCC37. However, it is important to note that these systemic treatments have been reserved for patients with advanced stage and unresectable HCC41. Furthermore, locoregional intra-arterial therapies are still the ‘preferred’ treatment in National Comprehensive Cancer Network (NCCN) guidelines for the management of liver-confined disease42. Our study shows that a subset of patients who achieved radiologic CR with liver-confined disease remain at risk for disease progression after embolization in the short term and decreased survival without resection or transplantation. This subset of patients with liver-confined HCC should be evaluated for consolidative treatments, such as modern systemic therapy, additional LRT, liver resection, or transplantation.
We did not find many significant associations between patient factors and OS or any progression after radiologic CR with the exception of pre-treatment AFP levels, which has been reported in previous studies27,35. Our study indicates that higher AFP values are associated with increased risk of death and increased risk of any type of disease progression. However, no significant association was found between pre-treatment AFP levels and distant disease progression after radiologic CR, suggesting that AFP is not a true biomarker. AFP levels are more likely to increase as the disease progresses, and its effect on prognosis seems dependent on when it is measured43.
Limitations
There are a few limitations to the current study. First, this study is retrospective analysis of a trial looking at secondary endpoints, which by nature is subject to some degree of inherent bias. The second limitation is related to its small sample size given that this was an analysis of the long-term outcomes in a subgroup of 37 patients from an RCT who had radiologic CR to embolization based on the mRECIST criteria. As a result, there were unexpected marginal associations where patients with extrahepatic disease had a lower risk of death and patients with higher CCI also had a lower risk of death. Both findings are counterintuitive and may be a function of the small sample size.
Previous studies also have demonstrated that advanced age, multiple nodules, higher pre-treatment MELD score, and initial pre-treatment AFP > 25 ng/ml were predictive factors for recurrence and disease progression after radiologic CR from TACE27,35. However, our study did not find significant associations between patient factors, such age, gender, comorbidities, or pre-treatment MELD score, with any recurrence or OS, which could also be attributed to the small sample size. Nevertheless, the study cohort itself represents long-term follow up of patients with advanced-stage HCC who had radiologic CR to embolization based on a robust RCT comparing HAE to DEB-TACE and provides long-term outcomes and prognosis for patients with liver-confined HCC who had radiologic CR to LRT and do not undergo subsequent resection or LT.
V. Conclusion
Patients with advanced-stage HCC and complete response to embolization do not have durable responses and have inevitable disease progression. Those with liver-confined disease should be evaluated for additional consolidative treatments, including repeated LRT, systemic therapy, resection or transplantation when feasible, to improve their survival. Additional multicenter studies are needed to identify which patients with advanced-stage HCC who had radiologic CR to embolization would benefit most from subsequent liver resection or transplantation.
FUNDING:
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
DISCLOSURES: GAA reports research support from Arcus, Agios, Astra Zeneca, BioNtech, BMS, Celgene, Flatiron, Genentech/Roche, Genoscience, Incyte, Polaris, Puma, QED, Silenseed, Yiviva; and consulting support from Adicet, Alnylam, Astra Zeneca, Autem, Bayer, Beigene, Berry Genomics, Celgene, Cend, CytomX, Eisai, Eli Lilly, Exelixis, Flatiron, Genentech/Roche, Genoscience, Helio, Incyte, Ipsen, Legend Biotech, Merck, Nerviano, QED, Redhill, Rafael, Servier, Silenseed, Sobi, Surface Oncology, Therabionics, Vector, and Yiviva. Other authors have no relevant conflicts of interest to report.
References:
- 1.World Health Organization. Available at http://globocan.iarc.fr/old/FactSheets/cancers/liver-new.asp. Accessed on November 16, 2020.
- 2.European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu, & European Association for the Study of the Liver (2018). EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. Journal of hepatology, 69(1), 182–236. 10.1016/j.jhep.2018.03.019 [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016; 66:7–30. [DOI] [PubMed] [Google Scholar]
- 4.White DL, Thrift AP, Kanwal F, Davila J, El-Serag HB. Incidence of Hepatocellular Carcinoma in All 50 United States, From 2000 Through 2012. Gastroenterology 2017;152(4):812–820.e5. doi: 10.1053/j.gastro.2016.11.0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention, Division of Cancer Prevention and Control, U.S. Cancer Statistics. Available at https://www.cdc.gov/cancer/liver/index.htm. Accessed on November 16, 2020.
- 6.Momin BR, Pinheiro PS, Carreira H, Li C, Weir HK. Liver cancer survival in the United States by race and stage (2001–2009): Findings from the CONCORD-2 study. Cancer 2017;123 Suppl 24(Suppl 24):5059–5078. doi: 10.1002/cncr.30820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mazzaferro V, Regalia E, Doci R, et al.Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med, 334 (1996), pp. 693–699 [DOI] [PubMed] [Google Scholar]
- 8.UpToDate. Liver transplantation for hepatocellular carcinoma Available at: https://www.uptodate.com/contents/liver-transplantation-for-hepatocellular-carcinoma. Accessed November 30, 2020.
- 9.Clavien PA, Lesurtel M, Bossuyt PM, et al.Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol, 13 (2012), pp. e11–e22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazzaferro, Bhoori S, Sposito C, et al.Milan criteria in liver transplantation for hepatocellular carcinoma: an evidence-based analysis of 15 years of experience, Liver Transpl, 17 (Suppl 2) (2011), pp. S44–S57 [DOI] [PubMed] [Google Scholar]
- 11.Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018;67(1):358–380. doi: 10.1002/hep.29086 [DOI] [PubMed] [Google Scholar]
- 12.Kulik L, El-Serag HB. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology 2019;156(2):477–491.e1. doi: 10.1053/j.gastro.2018.08.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018. Jan;67(1):358–380. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 14.Llovet JM, Real MI, Montaña X, et al. ; Barcelona Liver Cancer Group: Arterial embolisation or chemoembolization versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: A randomized controlled trial. Lancet 359:1734–1739, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Lo CM, Ngan H, Tso WK, et al. : Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 35:1164–1171, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Marelli L, Stigliano R, Triantos C, et al. Transarterial therapy for hepatocellular carcinoma: Which technique is more effective? A systematic review of cohort and randomized studies. Cardiovasc Intervent Radiol 30:6–25, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Brown KT, Do RK, Gonen M, et al. Randomized Trial of Hepatic Artery Embolization for Hepatocellular Carcinoma Using Doxorubicin-Eluting Microspheres Compared With Embolization With Microspheres Alone. J Clin Oncol 2016;34(17):2046–2053. doi: 10.1200/JCO.2015.64.0821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yao FY, Xiao L, Bass NM, et al.Liver transplantation for hepatocellular carcinoma: validation of the UCSF-expanded criteria based on preoperative imaging. Am J Transplant, 7 (2007), pp. 2587–2596 [DOI] [PubMed] [Google Scholar]
- 19.Chapman WC, Majella Doyle MB, Stuart JE, et al.Outcomes of neoadjuvant transarterial chemoembolization to downstage hepatocellular carcinoma before liver transplantation. Ann Surg, 248 (2008), pp. 617–625 [DOI] [PubMed] [Google Scholar]
- 20.Yao FY, Kerlan RK Jr, Hirose R, et al. Excellent outcome following down-staging of hepatocellular carcinoma prior to liver transplantation: an intention-to-treat analysis. Hepatology 2008;48(3):819–827. doi: 10.1002/hep.22412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yao FY, Mehta N, Flemming J, et al. Downstaging of hepatocellular cancer before liver transplant: long-term outcome compared to tumors within Milan criteria. Hepatology 2015;61(6):1968–1977. doi: 10.1002/hep.27752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chapman WC, Garcia-Aroz S, Vachharajani N, et al. Liver Transplantation for Advanced Hepatocellular Carcinoma after Downstaging Without Up-Front Stage Restrictions. J Am Coll Surg 2017;224(4):610–621. doi: 10.1016/j.jamcollsurg.2016.12.020 [DOI] [PubMed] [Google Scholar]
- 23.Tang ZY, Zhou XD, Ma ZC, et al. Downstaging followed by resection plays a role in improving prognosis of unresectable hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int 2004;3(4):495–498. [PubMed] [Google Scholar]
- 24.Lau WY, Lai EC. Salvage surgery following downstaging of unresectable hepatocellular carcinoma–a strategy to increase resectability. Ann Surg Oncol 2007;14:3301–3309. [DOI] [PubMed] [Google Scholar]
- 25.Chen J, Lai L, Lin Q, et al. Hepatic resection after transarterial chemoembolization increases overall survival in large/multifocal hepatocellular carcinoma: a retrospective cohort study. Oncotarget 2017;8(1):408–417. doi: 10.18632/oncotarget.13427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y, Huang G, Wang Y, Liang L, Peng B, Fan W, Yang J, Huang Y, Yao W, Li J. Is Salvage Liver Resection Necessary for Initially Unresectable Hepatocellular Carcinoma Patients Downstaged by Transarterial Chemoembolization? Ten Years of Experience. Oncologist 2016;21:1442–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cerban R, Ester C, Iacob S, et al. Predictive Factors of Tumor Recurrence and Survival in Patients with Hepatocellular Carcinoma treated with Transarterial Chemoembolization. J Gastrointestin Liver Dis 2018;27(4):409–417. doi: 10.15403/jgld.2014.1121.274.fcr. [DOI] [PubMed] [Google Scholar]
- 28.Kim BK, Kim SU, Kim KA, et al. Complete response at first chemoembolization is still the most robust predictor for favorable outcome in hepatocellular carcinoma. J Hepatol 2015;62(6):1304–1310. doi: 10.1016/j.jhep.2015.01.022 [DOI] [PubMed] [Google Scholar]
- 29.Young S, Sanghvi T, Sharma S, et al. Local recurrence following complete radiologic response in patients treated with transarterial chemoembolization for hepatocellular carcinoma. Diagn Interv Imaging 2022;103(3):143–149. doi: 10.1016/j.diii.2022.01.006 [DOI] [PubMed] [Google Scholar]
- 30.Haywood N, Gennaro K, Obert J, et al. Does the Degree of Hepatocellular Carcinoma Tumor Necrosis following Transarterial Chemoembolization Impact Patient Survival?. J Oncol 2016;2016:4692139. doi: 10.1155/2016/4692139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reig M, Forner A, Rimola J, Ferrer-Fábrega J, Burrel M, Garcia-Criado A, Kelley R, Galle P, Mazzaferro V, Salem R, Sangro B, Singal A, Vogel A, Fuster J, Ayuso C, Bruix J, BCLC strategy for prognosis prediction and treatment recommendation Barcelona Clinic Liver Cancer (BCLC) staging system. The 2022 update Journal of Hepatology (2021), doi: 10.1016/j.jhep.2021.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010;30:52–60. [DOI] [PubMed] [Google Scholar]
- 33.Gray RJ (1988) A class of K-sample tests for comparing the cumulative incidence of a competing risk, Annals of Statistics, 16:1141–1154. [Google Scholar]
- 34.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 35.Nakano MM, Yamamoto A, Nishida N, et al. Risk factors for local recurrence of hepatocellular carcinoma after transcatheter arterial chemoembolization with drug-eluting beads (DEB-TACE). Jpn J Radiol 2019;37(7):543–548. doi: 10.1007/s11604-019-00840-4 [DOI] [PubMed] [Google Scholar]
- 36.Rou WS, Lee BS, Moon HS, Lee ES, Kim SH, Lee HY. Risk factors and therapeutic results of early local recurrence after transcatheter arterial chemoembolization. World J Gastroenterol 2014;20(22):6995–7004. doi: 10.3748/wjg.v20.i22.6995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kudo M, Kubo S, Takayasu K, Sakamoto M, Tanaka M, Ikai I, Furuse J, Nakamura K, Makuuchi M; Liver Cancer Study Group of Japan (Committee for Response Evaluation Criteria in Cancer of the Liver, Liver Cancer Study Group of Japan). Response Evaluation Criteria in Cancer of the Liver (RECICL) proposed by the Liver Cancer Study Group of Japan (2009 Revised Version). Hepatol Res 2010. Jul;40(7):686–92. doi: 10.1111/j.1872-034X.2010.00674.x. [DOI] [PubMed] [Google Scholar]
- 38.Abou-Alfa GK, Qin S, Ryoo BY, Lu SN, Yen CJ, Feng YH, Lim HY, Izzo F, Colombo M, Sarker D, Bolondi L, Vaccaro G, Harris WP, Chen Z, Hubner RA, Meyer T, Sun W, Harding JJ, Hollywood EM, Ma J, Wan PJ, Ly M, Bomalaski J, Johnston A, Lin CC, Chao Y, Chen LT. Phase III randomized study of second line ADI-PEG 20 plus best supportive care versus placebo plus best supportive care in patients with advanced hepatocellular carcinoma. Ann Oncol 2018. Jun 1;29(6):1402–1408. doi: 10.1093/annonc/mdy101. [DOI] [PubMed] [Google Scholar]
- 39.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 2018. Mar 24;391(10126):1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 40.TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL; IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med 2020. May 14;382(20):1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 41.Gordan JD, Kennedy EB, Abou-Alfa GK, Beg MS, Brower ST, Gade TP, Goff L, Gupta S, Guy J, Harris WP, Iyer R, Jaiyesimi I, Jhawer M, Karippot A, Kaseb AO, Kelley RK, Knox JJ, Kortmansky J, Leaf A, Remak WM, Shroff RT, Sohal DPS, Taddei TH, Venepalli NK, Wilson A, Zhu AX, Rose MG. Systemic Therapy for Advanced Hepatocellular Carcinoma: ASCO Guideline. J Clin Oncol 2020. Dec 20;38(36):4317–4345. doi: 10.1200/JCO.20.02672. Epub 2020 Nov 16. [DOI] [PubMed] [Google Scholar]
- 42.National Comprehensive Cancer Network (NCCN). Hepatobiliary Cancers (Version 1.2022) https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf. Accessed May 1, 2022.
- 43.Abou-Alfa GK. Ramucirumab and the controversial role of α-fetoprotein in hepatocellular carcinoma. Lancet Oncol 2019. Feb;20(2):177–179. doi: 10.1016/S1470-2045(19)30009-9. Epub 2019 Jan 18. [DOI] [PubMed] [Google Scholar]


