Abstract
The remodeling of the cervix from a closed rigid structure to one that can open sufficiently for passage of a term infant is achieved by a complex series of molecular events that in large part are regulated by the steroid hormones progesterone and estrogen. Among hormonal influences, progesterone exerts a dominant role for most of pregnancy to initiate a loss of tissue strength yet maintain competence in a phase termed softening. Equally important are the molecular events that abrogate progesterone function in late pregnancy to allow a loss of tissue competence and strength during cervical ripening and dilation. In this review, we focus on current understanding by which progesterone receptor signaling for the majority of pregnancy followed by a loss/shift in progesterone receptor action at the end of pregnancy, collectively ensure cervical remodeling as necessary for successful parturition.
INTRODUCTION
Throughout pregnancy, the cervix must remain closed to ensure protection of the fetus through pregnancy (competence) yet simultaneously undergo progressive softening for successful parturition at term (compliance). The intricate balance between the competence and compliance is tightly controlled by action of endocrine factors such as steroids (i.e., progesterone, P4; estrogen, E2), and non-steroidal hormones (i.e., relaxin, oxytocin). In recent years, our current understanding of endocrine regulation in pregnancy and parturition has expanded. Among hormonal influences, P4 exerts a dominant role for most of pregnancy to achieve cervical softening. Equally important are the molecular events that abrogate P4 function in late pregnancy to allow cervical ripening and dilation. In this review, we focus on current understanding by which progesterone signaling regulates aspects of cervical competence and compliance during softening and how a loss of progesterone signaling the final steps to achieve maximal compliance during ripening and dilation.
CERVICAL REMODELING
The cervix is comprised of a stromal region that harbors fibroblasts, smooth muscle cells, blood vessels and immune cells. The fibroblasts synthesize a complex extracellular matrix (ECM) in which collagen and elastic fiber structure influences cervical function. Smooth muscle cells are interspersed between the fibroblasts in rodents and women and in the human cervix are in greater density in the region of the internal os compared to the external os [1,2]. Lining the cervical stroma is a cervical epithelium. Epithelial cells provide a physical and immunological barrier against external insults such as harmful pathogens. The remodeling of the cervix in preparation for parturition begins in early pregnancy and is divided into distinct phases termed cervical softening, ripening, dilation and postpartum repair. The softening period is a progesterone dominant phase and can be further divided into an early and late softening period based on a distinct transcriptional landscape and distinct mechanical properties as described in the mouse [3,4,5,6]. Cervical ripening and dilation are accelerated phases of remodeling that encompass the end of pregnancy and parturition. This is an estrogen dominant phase of remodeling. The ripening and dilation phases are referred collectively in this review as there are no molecular markers that distinguish these rapid overlapping phases. The demonstration that the cervix softens in the first trimester of pregnancy in women, that cervical length as visualized by vaginal ultrasound gradually shortens in a term pregnancy and that morphological changes associated with cervical ripening/dilation occur in late pregnancy suggest conservation of these phases in the human [7,8].
FACTORS REGULATING CERVICAL REMODELING
The steroid hormones progesterone and estrogen play a central role in the molecular events that ensure appropriate and timely cervical remodeling for safe delivery of a term baby. In most species with the exception of humans, non-human primates and guinea pigs, progesterone synthesis is high throughout pregnancy and circulating levels decline in late gestation to allow onset of parturition [9,10,11,12,13,14]. While progesterone synthesis remains high in women until birth due to placental production, loss of progesterone function is achieved by numerous pathways resulting in similar changes to the uterus and cervix as reported in other species [15,16,17]. Estrogen biosynthesis is lower in early pregnancy and gradually increases in the latter half of pregnancy. The relationship between progesterone and estrogen is complex and tissue specific, though the necessity of estrogen signaling to induce progesterone receptor synthesis and the ability of progesterone to suppress estrogen actions is generally conserved [18].
In addition to the steroid hormones, peptide hormones also contribute to the regulation of reproductive tract function. Relaxin (Rln) secreted by the ovary, binds to a membrane receptor, Rxfp1, has numerous proposed roles such as tissue growth and development, vasodilation, inhibition of myometrial contractions, softening of the cervix, development of mammary gland, and inhibition of tissue fibrosis [19,20,21,22,23]. The primary role of relaxin in pregnancy and parturition is to facilitate cervical growth and remodeling [24,25,26,27].
Prostaglandins are eicosanoids that mediate numerous inflammatory events associated with uterine contractions and fetal membrane remodeling [28]. The prostaglandin, PGE is used clinically to induce cervical softening and has long been considered to be a key driver of cervical remodeling in women and animal models [29]. In recent years, comparisons between term cervical remodeling and inflammation-mediated premature ripening demonstrate an increase in prostaglandin synthesis and reduced prostaglandin metabolism in inflammation mediated preterm birth, but not in term cervical remodeling [28]. Supporting clinical evidence calls into question the role of prostaglandins to physiologic cervical remodeling [30].
PROGESTERONE AND ITS ACTIONS IN THE CERVIX
Progesterone binds the progesterone receptor, a nuclear receptor that mediates transcriptional control. The complexity of progesterone-mediated transcriptional regulation is in part driven by the fact that the PR gene encodes for two isoforms PR-A and PR-B with an extra coding sequence at the N-terminal of the PR-B gene. PR-A and PR-B display similar DNA and hormone binding affinities, can bind to DNA as homo- or heterodimers but are transcribed by different promoters [31]. Progesterone can transactivate distinct transcriptional programs dependent on the relative abundance of PR-A/PR-B isoforms [32,33]. Studies in isoform specific KO models demonstrate that PR-A is necessary and sufficient for fertilization, implantation and maintenance of pregnancy to term [34,35]. Both in vitro and ex vivo uterine studies demonstrate a role of PR-B to maintain uterine quiescence and suppress inflammation during pregnancy. Transitions to increased levels of PR-A in late pregnancy, termed the progesterone receptor isoform switch, promote the activation of contractility and inflammatory processes [36,37,38].
The influence of P4’s actions on cervical remodeling are demonstrated by the ability of progesterone receptor antagonists to induce cervical ripening (onapristone or RU486) and the ability of P4 agonists (e.g. R5020, promegestone) to prevent cervical ripening in women and animal models [28,39,40,41,42,43]. In the context of cervical remodeling, evidence for PR isoform specific transcriptional programs exist but are not well understood. The global PGR knockout mouse demonstrates impaired ovarian function and implantation defects that limit the utility of this genetic model to ascertain isoform specific actions in the cervix during pregnancy [15]. Several in vitro studies demonstrate an antagonistic role of PR-A on the function of PR-B resulting in functional P4 withdrawal [44,45,46]. A shift in the PR-B to PR-A ratio is not observed in the mouse cervix at term and markers of cervical ripening at term are reported to occur normally in the PR-B KO mouse [34]. This observation suggests that in addition to antagonizing PR-B function, PR-A is able to activate gene targets necessary for cervical remodeling. Further in vivo studies in the cervix are needed to clarify the isoform specific functions in cervical remodeling.
PROGESTERONE RECEPTOR EXPRESSION IN CERVICAL CELLS
Multiple studies demonstrate cervix cellular proliferation, differentiation and turnover is regulated by hormones, especially estrogen and progesterone during the nonpregnant cycle, pregnancy and postpartum [47]. Both cervical stromal and epithelial cells express progesterone receptors [48]. In the nonpregnant mouse cervix, epithelial PR is required for apoptosis and for suppressing epithelial proliferation. This function is distinct from the uterine epithelia in which epithelial PR is not required to induce apoptosis or suppress proliferation. The studies described by Mehta et al. highlight cell and tissue specificity of PR’s actions that differ between the nonpregnant uterus and cervix. During pregnancy both the stroma and epithelia continue to express PR. As seen in Figure 1, while all layers of epithelia express PR, at specific time points the basal epithelia have reduced expression compared to the luminal layers (gestation days 6, 12 and 18). Stromal PR expression appears robust and constant during pregnancy and postpartum. Prior studies demonstrate PR expression in the stroma with an absence of PR expression in macrophages [43]. Similar PR patterns are described in the rat cervix during pregnancy [47]. In addition, a constant high expression of ERα was observed in the cervical epithelia and stroma throughout pregnancy and decline postpartum.
Figure 1: Progesterone receptor (PR) localization in non-pregnant and pregnant cervix.
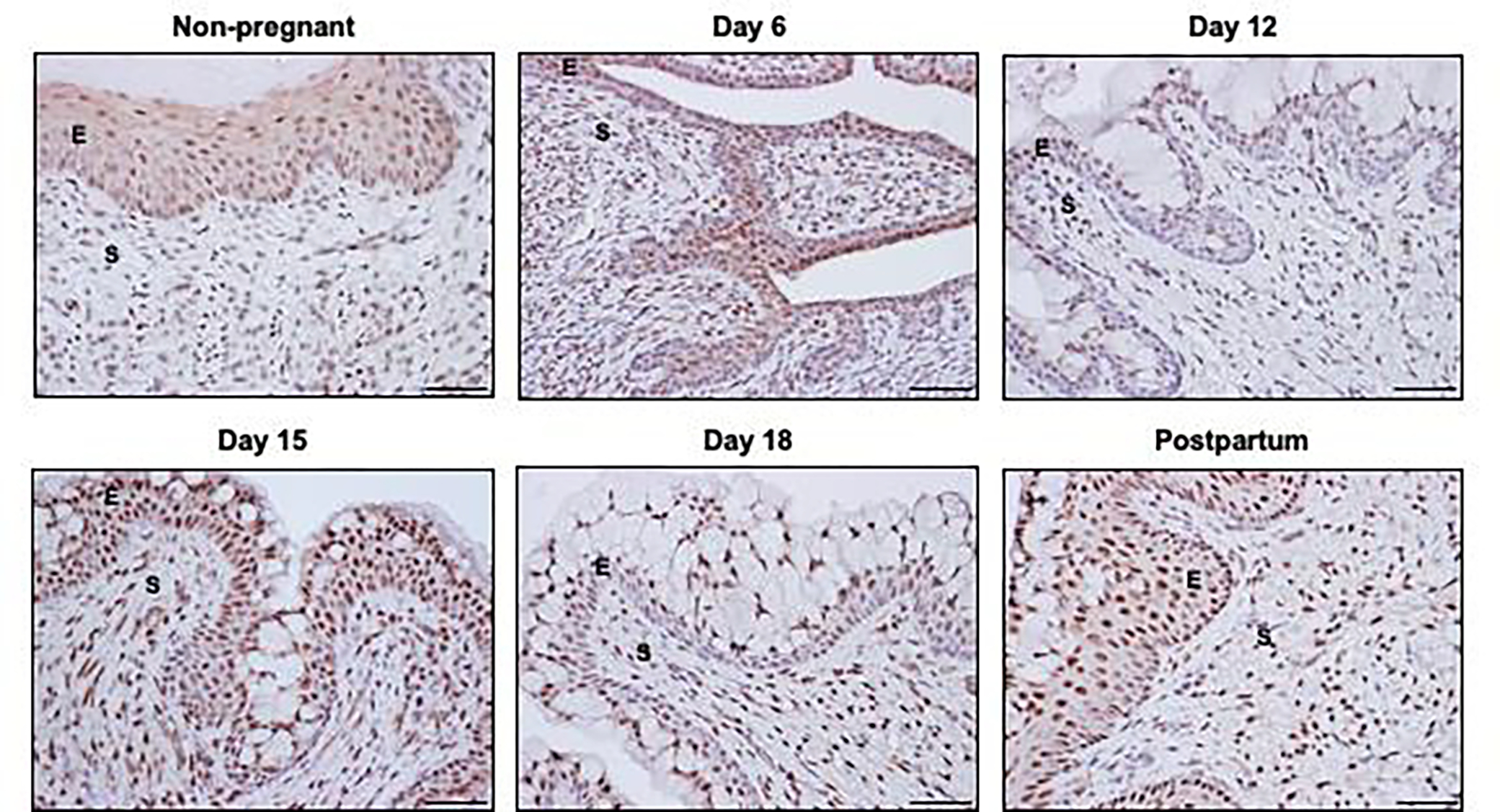
Immunohistochemical localization of PR in cervical sections of non-pregnant (NP), pregnant and postpartum (PP) mice. Epithelial PR is localized throughout the basal and luminal layers of squamous epithelia in the NP and PP cervix. Similar patterns are noted in cervical sections from gestation days 6 and 15 while basal layers have reduced PR expression relative to the luminal secretory cells on days 12 and 18. Stromal PR remains high throughout pregnancy and postpartum compared to non-pregnant cervix. Images are taken at 20X. E= Epithelia; S= Stroma.
PROGESTERONE ACTION IN THE SOFTENING PHASE
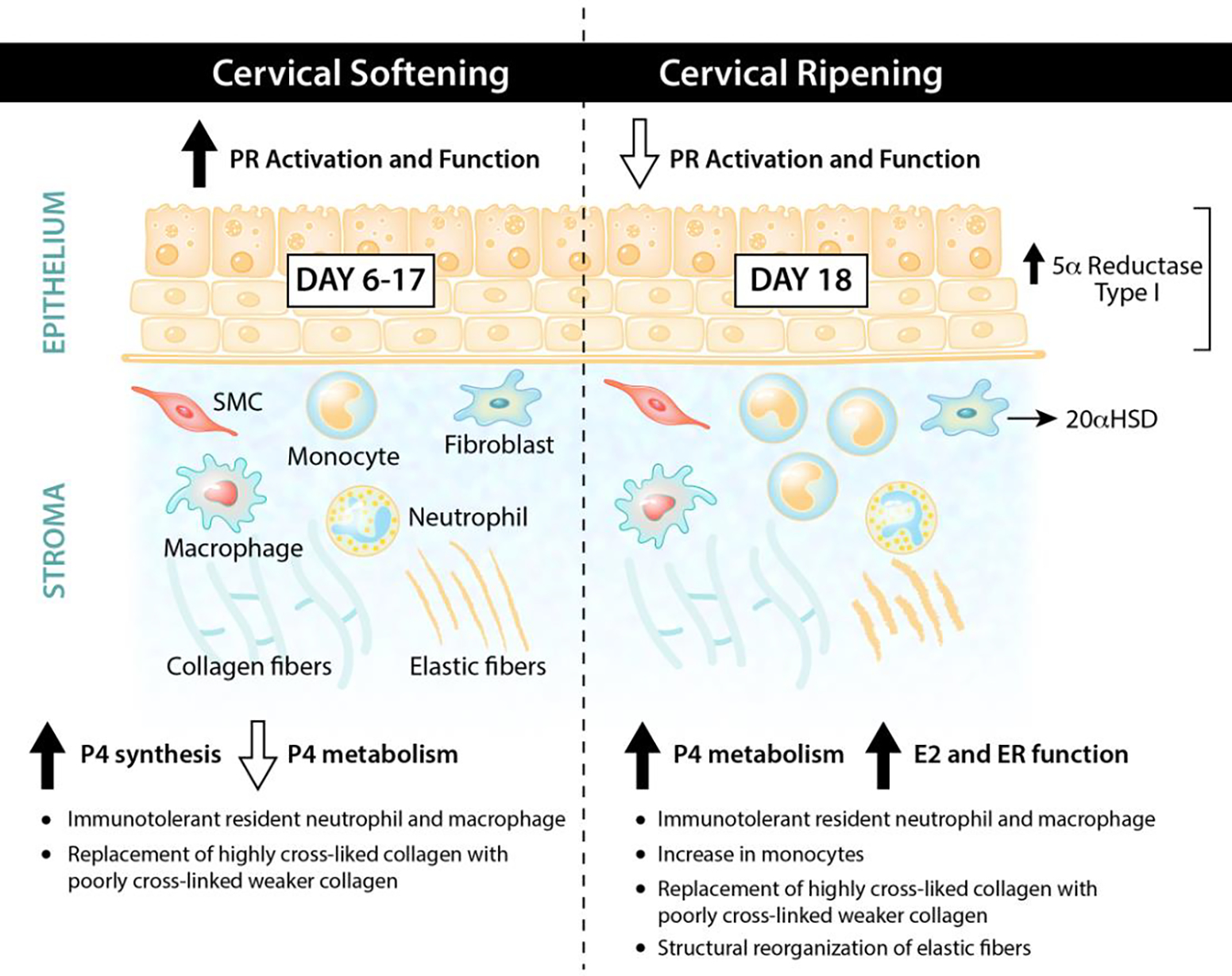
Advances in our understanding of the molecular events that drive cervical remodeling set the stage to better understand the regulatory roles of steroid hormones to this process. Starting in early pregnancy, the cervix initiates a slow progressive remodeling phase termed softening. Key hallmarks of this phase are the increase in tissue compliance with maintenance of tissue competence and immunotolerance [49]. This phase requires progesterone receptor function [40,41]. The softening period overlaps in late pregnancy with the accelerated phases of ripening and dilation, in which the cervix loses its mechanical strength, yet maintains an immunoprotected barrier. This phase is characterized by reduced progesterone receptor function and increased action of estrogen signaling [4,50].
Changes in the composition and structure of the cervical extracellular matrix dictate the balance between compliance and competence [51]. Collagen fibers are the major structural protein made by cervical stroma. During softening, collagen content remains constant yet collagen turnover is high [52]. This allows the replacement of mature, highly cross-linked collagen with newly synthesized, less cross-linked collagen [3,53]. It is achieved by high synthesis of fibrillar collagens, and a decline in expression of enzymes that determine the type and degree of collagen cross-links (Plod2 and Lox, respectively) which further correlate with the decline in tissue stiffness during cervical softening. However, molecules in the ECM that are necessary for assembly of collagen fibrils such as the small leucine rich proteoglycans, decorin, biglycan and lumican are expressed at steady levels in the pregnant and nonpregnant cervix. This ensures seamless processing, assembly and deposition of collagen with appropriate strength required for cervical tissue function at different stages of pregnancy [4, 52]. Structural changes to elastic fibers are also observed in cervical remodeling and similar to fibrillar collagen, their assembly also requires decorin or other SLRP family members [4,54]. While collagen structure regulates tissue strength, elastic fibers are likely to contribute to tissue elasticity and resilience [5]. Nonstructural ECM proteins that likely modulate the function of collagen and/or elastic fibers are suppressed at the level of transcription during softening. These include thrombospondin 2 and tenascin C [53]. The stepwise changes in structure and function of the cervical ECM identified in rodent models is likely conserved in the nonhuman primate (NHP) as determined by shear wave elasticity imaging to assess softening [55,56]. Similar changes in cervical ECM in women is supported by the increased risk of preterm birth due to cervical insufficiency in women with Ehler-Danlos syndrome that results from genetic defects in proteins necessary for assembly of collagen and elastic fibers [57].
The regulatory role of progesterone and estrogen to modulate ECM structure and function are well documented in the cervix, uterus, pelvic floor and breast [4,51,58,59,60]. Cell and tissue differences in regulation however; complicate current understanding and limit our ability to extrapolate findings. For example, the cross-link forming enzyme lysyl oxidase is regulated by estrogen (E2) in the mouse cervix while progesterone (P4) suppresses E2-induced lysyl oxidase expression in the vagina [61,62].
Our prior studies in decorin null mice with cervical defects in assembly of collagen fibrils and elastic fibers suggested a dominant role of progesterone in collagen homeostasis and estrogen in elastic fiber homeostasis [4]. Collagen fibril defects in ovariectomized decorin null mice treated with P4 for 15 days were resolved while estrogen treatment in this model rescued elastic fiber defects. Gene expression studies in cervices of ovariectomized wild type mice demonstrate greater overlap and complexity in P4 and E2 regulation, as either P4 or E2 treatment can induce transcription of one or more fibrillar collagens as well as regulate components of elastic fibers (Eln, Fbn1, Fbn2, Mfap). Extrapolating the findings in an ovariectomized system to pregnancy indicate the dynamic changes in P4:E2 levels and cell type specific expression of their nuclear receptors allow for dynamic changes in cervical ECM structure and function during cervical softening and ripening. Interestingly, P4 was shown to inhibit collagen synthesis in human cervical fibroblast 3-D cultures though this may not reflect in loss of collagen content in the cervix during pregnancy [63,64].
Adding to the richness and diversity of cervical ECM structure-function regulation in pregnancy is the expression of the peptide hormone relaxin (Rln) and its receptor, Rxfp1 [65]. Increased levels of relaxin in mid to late pregnancy contribute to ECM remodeling, though specific gene targets have not been identified. Mice lacking Rxfp1 or Rln have parturition defects that in part can be attributed to a failure of cervical remodeling [66]. Improved understanding of the molecular changes driving ECM reorganization that begin in early pregnancy and the contributions of specific ECM components to distinct mechanical parameters (e.g., stiffness, strength, resilience, etc.) of the cervix emphasize the need of future studies to understand the role of PR signaling in regulation of these processes during pregnancy and parturition [51].
Consistent with progesterone’s role in limiting pro-inflammatory responses, the softening period is one of immunotolerance and high P synthesis [67]. The concept that pregnancy is a period of immune suppression has shifted in recent years as evidence builds in support of an immune environment that is functional yet tolerant [68]. Immunotolerance in the cervical microenvironment during pregnancy in women is supported by the observation that cervicovaginal secretions from pregnant women are capable of eliciting similar immune mediators in response to HIV exposure as compared to cervicovaginal secretions from nonpregnant women [69]. Cervical studies in mice demonstrate the steady presence of resident leukocytes (neutrophils, macrophages) throughout pregnancy [70,71]. These cells appear quiescent based on low myeloperoxidase activity of neutrophils and gene expression patterns that include low or absent proinflammatory gene marker expression [52,70,67]. The cervical epithelia also provide immunoprotection through a physical and immune barrier [72].
Gene expression patterns during pregnancy in the softening period are altered to induce mucin biosynthesis and transport, expression of protease inhibitors (Spink5), and epithelial restitution factors (Tff1) [67]. While not well-studied during pregnancy, mucosal synthesis and secretion, as well as epithelial cell proliferation, differentiation and tight junction permeability are regulated by nonpregnant cyclic shifts in progesterone and estrogen [73,74]. A deeper understanding of the steroid hormone regulation of immune tolerance by immune and epithelial cells during cervical softening is necessary to identify mis-regulation that contributes to prematurity.
PROGESTERONE ACTION IN THE RIPENING PHASE
The transition from the period of cervical softening to cervical ripening at the end of pregnancy is largely regulated by the decline in progesterone function and increased responsiveness to estrogen. In mice and numerous other species, circulating progesterone levels decline in late pregnancy while in the human, progesterone synthesis by the placenta continues until birth [10,12]. Despite this important distinction, reduced progesterone signaling is a common aspect of parturition initiation in humans and most mammalian species. Decades of research by many laboratories have uncovered the diverse mechanisms by which progesterone function is abrogated to alleviate uterine quiescence, and initiate cervical ripening. In addition to the PR isoform switch that has been discussed earlier, local metabolism of P4 was identified as a key driver of cervical ripening.
Numerous enzymes convert progesterone to less inactive progestins. These include aldoketoreductases (Akr1c1, Akr1c2, Akr1c3) and steroid 5α-reductase which metabolize P4 into 20α-hydroxyprogesterone and 5α-dihydroprogesterone, respectively [75,76]. Transcripts encoding 5alpha-reductase type 1 (5α-R1) are induced in the cervical epithelia on gestation day 15 in mice with peak expression on days 18 and 19 [77]. Mice lacking steroid 5α-R1 demonstrated a parturition defect in 70% of pregnancies due to a failure of cervical ripening. While circulating levels of progesterone declined on gestation day 18, tissue levels of P4 remain elevated in the 5α-R1 null mice and prevented cervical ripening. Uterine contractility was close to normal despite the lack of cervical ripening in the 5α-R1 deficient mice [50]. While the expression of 5α-R1 is not induced in the human cervix in late pregnancy, studies by Andersson et al. demonstrated a conserved role of local steroid metabolism to achieve cervical ripening during parturition in women [16].
Consistent with its ability to modulate immunoprotection, the decline in progesterone during cervical ripening alters epithelial barrier properties and the cervical immune cell composition. The epithelia modulate barrier functions through changes in composition of tight junction proteins, desmosomes and mucus synthesis. Immunoprotection is provided through formation of a mucus barrier and production of antimicrobials such as secretory leukocyte protease inhibitor (SLPI), IgA and IgG [78,79]. While combined and independent actions of estrogen and progesterone via binding their nuclear receptors to regulate the described functions of cervical epithelia is supported by studies in the non-pregnant cervix, pregnancy-specific regulation of epithelial functions by PR remain limited.
The reduced availability of ligand to bind PR leads to a significant alteration in cervical immune cell composition during cervical ripening in mice with an influx of tissue monocytes and increase in eosinophils [80]. While the cell-specific regulatory events remain to be defined, the link with progesterone and progesterone receptor is supported by the failed influx of monocytes in the 5a-R1 knockout mice during cervical ripening and the premature increase in monocyte in the cervices of mice treated with the progesterone receptor antagonist, RU486 [70]. During labor or shortly postpartum, numbers or activity of neutrophils and macrophages are increased. Gene expression patterns identify macrophage heterogeneity with markers of proinflammatory macrophages (M1) and immunosuppressive/tissue repair macrophages (M2). While loss of progesterone function during cervical ripening results in the influx of tissue monocytes, this does not support a role of physiological inflammation to achieve cervical ripening but rather the postpartum maturation to M1/M2 macrophages to allow for rapid tissue repair after birth [70,80]. While numerous studies suggest leukocytes and physiologic inflammation play a key role to drive cervical ripening/dilation via release of proteases that breakdown the cervical ECM [81,82,83] there is substantial evidence to oppose this historical paradigm. For example, in women, gene expression microarray patterns from term pregnant cervical tissue collected before and after birth do not identify increases in proinflammatory genes until after birth [84]. Similarly in mice, transcriptional patterns identified by RNA-seq demonstrate suppression of innate immune and inflammatory pathways during cervical softening (gestations days 6, 12 and 15) and ripening (day 18) as compared to non-pregnant [52]. Depletion of neutrophils in pregnant mice does not prevent cervical ripening or a term birth [70,85]. The necessity of macrophages to cervical ripening is difficult to interpret from depletion studies due to compromised placental function and a high incidence of infant mortality [86].
Thus, the actions of progesterone receptor are diminished during cervical ripening. Increased P4 metabolism within the cervical microenvironment in conjunction with alterations in PR isoforms and reduced P4 synthesis ensure timely changes in the cervix. This may relieve repression of genes regulated by PR directly or indirectly through PRs ability to regulate other signaling molecules. In addition, since PR can reduce estrogen receptor signaling, reduced PR activity may allow increased transcription of E2 regulated genes. Important to this phase of cervical remodeling, loss of P4 action induces an influx of inflammatory cells whose activation in the postpartum period suggests a role in postpartum repair of the cervix.
PREGNANCY COMPLICATIONS AND THE CERVIX
Preterm birth (PTB) is the leading cause of neonatal morbidity and mortality worldwide and affects, approximately 1 out of every 10 births in the United States [87]. The majority of PTBs are spontaneous (not medically induced) and caused by multiple etiologies, some of which remain undetermined. While premature cervical remodeling precedes preterm birth in most cases, cervical dysfunction as the initiating factor is established in cervical insufficiency as well as in ascending infection [88]. In cervical insufficiency, structural defects in the cervix lead to premature opening in the absence of uterine contractions [57,89,90]. In ascending infection, a breach in the cervical barrier allows for the ascension of harmful pathogens which can result in inflammation-mediated premature birth. Ascending infection accounts for roughly 25–40% of spontaneous PTBs in women [88].
CERVICAL INSUFFICIENCY
The molecular events that drive cervical insufficiency remain to be identified though genetic evidence targets ECM dysfunction as a key contributor [57]. Pregnant women with gene defects in molecules required for collagen or elastic fiber synthesis or assembly have a greater incidence of preterm birth due to cervical insufficiency or preterm premature rupture of membranes (PPROM) [89]. Given the demonstrated regulatory role of progesterone, estrogen and relaxin in regulation of ECM, future studies to define how altered hormone signaling may contribute to ECM dysfunction in cervical insufficiency is warranted.
INFLAMMATION AND PRETERM BIRTH
Loss of PR signaling is necessary to induce myometrial contraction, relieve suppression of inflammatory processes and reorganize the cervical ECM to ensure maximal tissue compliance during the ripening phase. Given this accepted paradigm, researchers have sought to understand how PR controls these diverse processes and how perturbations in PR signaling or PR-targeted pathways contribute to PTB. Administration of progesterone receptor antagonists such as mifepristone (RU486) or onapristone to mice induces PTB within hours. Features of cervical ripening at term are prematurely induced with RU486 treatment on gestation day 15 demonstrating that loss of PR functions are necessary and sufficient to achieve ripening [28,39]. Scientists have studied several models of inflammation-mediated PTB via ascending infection of E. coli, intraperitoneal, intrauterine or intra-amniotic application of lipopolysaccharide, and viral exposure [6,91]. In contrast to term or RU486-induced PTB, premature ripening induced by intrauterine LPS-induced inflammation models are characterized by a distinct transcriptional program that includes a robust pro-inflammatory response and increased protease expression [6,92]. Furthermore, prostaglandin levels are increased and necessary for inflammation-mediated ripening but not term ripening. Assessment of ECM ultrastructure suggests degradation that is consistent with reports of impaired mechanical function [5, 6]. Collectively, these studies demonstrate a pathway of cervical remodeling with infection/inflammation that is distinct from term ripening. A decline in circulating levels of P4 before the onset of inflammation-mediated PTB is observed (28,93). Studies conducted in human cervical fibroblast cells report the ability of the proinflammatory cytokine, Il-1β to induce the aldoketoreductases (Akr1c1, Akr1c2, Akr1c3) and steroid 5α-reductase type 1 at both mRNA and protein levels which collectively metabolize P4 to less active progestins [94]. Studies carried out by Kniss and Summerfield [95] in human cervical stromal fibroblasts demonstrate P4-mediated changes in cytokine induced gene expression. P4 selectively modulated the global cytokine-induced gene expression profile with both synergistic (Irak3, Hsd11b1, Fkbp5) and antagonistic (Has2, Il-1β, IL6, Mmp10, Ptgs2) effects on genes with proposed functions in the cervical stroma [95]. Collectively, both mouse in vivo and human cell data suggest a decline in PR signaling precedes the activation of inflammatory responses. Further, mechanistic studies are warranted to demonstrate this connection.
Equally important to understanding the impact of uncontrolled inflammation to cervical remodeling, is the need to understand how host response mechanisms fail to allow unmitigated inflammation. The cervical epithelia are the first line of defense against bacterial and viral induced damage. Mice with a disrupted cervical epithelial barrier due to gene targeted loss of hyaluronan or pathogen exposure (e.g., Group B Streptococcus or Ureaplasma parvum exposure after chemical disruption) provide insight as to how a compromise in host protection contributes to PTB with exposure to ascending infection [91,96,97]. The regulatory role of PR and ER on epithelial cell proliferation, apoptosis, barrier and immune protection has been well studied in the non-pregnant cervix, yet in the context of term pregnancy and premature birth the signaling pathways in both the stroma and epithelia that regulate pregnancy-specific changes and responses is an understudied area that warrants investigation.
PROGESTERONE THERAPY FOR PRETERM BIRTH
Given the necessity of progesterone for maintenance of pregnancy, numerous clinical studies report the benefits of progestin (17-hydroxyprogesterone caproate or progesterone) therapy for prevention of preterm birth [98,99]. In particular, vaginal progesterone was reported to benefit women with a sonographically detected short cervix, one risk factor for PTB. However, two recent large randomized clinical trials failed to demonstrate efficacy [100,101,102]. This subsequently led to recommendations to withdraw FDA approval of the drug Makena (17-hydroxyprogesterone caproate) [103]. Consistent with in the lack of efficacy of progestin therapy in women, a similar outcome was observed in a mouse model of inflammation-mediated PTB with intrauterine LPS administration. Recent studies by Zierdan et al. [104] report the development of a vaginal nano delivery system for optimized delivery of P4. They demonstrate that P4 alone cannot prevent LPS-induced PTB but co-administration of P4 with a histone deacetylation inhibitor effectively reduced the occurrence of PTB resulting in birth of viable pups. Collectively, these studies suggest loss of P4 may be necessary but not sufficient to induce PTB mediated by inflammation and that benefits of P4 therapy, if any, will require identification of specific PTB etiologies that are responsive.
CURRENT UNDERSTANDING AND FUTURE DIRECTIONS
As summarized in Figure 2, during the first 95% of pregnancy, progesterone receptor signaling events are regulators of the key processes necessary for the success of cervical softening. This includes an extensive ECM reorganization that increases tissue compliance yet keeps the cervix closed and competent and the generation of an immuno-protective environment. Equally critical to the success of cervical remodeling is the reduced PR function during the ripening phase that is collectively achieved by the PR isoform switch, local metabolism of P4 and in most species other than human, by a decline in P4 synthesis. This is superseded with increased ER signaling that regulates further changes to achieve maximal tissue compliance with the onset of uterine contractions during parturition. A major gap in our understanding are the molecular details by which PRA, PRB and ER regulate cell specific events through the softening and ripening period and how these are modulated by peptide hormones or growth factors. For example, a functional role of smooth muscle cells in cervical remodeling is unknown. Defining the potential hormone-regulated transitions between synthetic and contractile SMC phenotypes during cervical softening and ripening may provide functional insights. Several priority questions are: What is the cell- type specific direct gene targets of both PR isoforms in the cervix? What are the paracrine regulatory signals by which for example PR may regulate transcription of a secreted stromal factor that in turn activates signaling pathways in the cervical epithelia? Do perturbations in PR regulated signaling pathways giving rise to PTB of a specific etiology and is this PTB subtype a potential target of P4 therapies? New advances in genomics, spatial transcriptomics and single cell analysis will allow researchers the tools to tackle some of these fascinating questions and ultimately advance knowledge relevant to a term birth and preterm births of specific etiologies.
Figure 2: Progesterone and progesterone receptor signaling during cervical remodeling.
Acknowledgements:
This work was supported by the National Institutes of Health Grant R01 HD088481 (MM).
We thank Angela Diehl for illustration design.
References
- [1].Ferland DJ, Darios ES, Watts SW, The persistence of active smooth muscle in the female rat cervix through pregnancy, Am J Obstet Gynecol, 212 (2015) 244 e241–248. 10.1016/j.ajog.2014.08.001. [DOI] [PubMed] [Google Scholar]
- [2].Vink JY, Qin S, Brock CO, Zork NM, Feltovich HM, Chen X, Urie P, Myers KM, Hall TJ, Wapner R, Kitajewski JK, Shawber CJ, Gallos G, A new paradigm for the role of smooth muscle cells in the human cervix, Am J Obstet Gynecol, 215 (2016) 478 e471–478 e411. 10.1016/j.ajog.2016.04.053. [DOI] [PubMed] [Google Scholar]
- [3].Yoshida K, Jiang H, Kim M, Vink J, Cremers S, Paik D, Wapner R, Mahendroo M, Myers K, Quantitative evaluation of collagen crosslinks and corresponding tensile mechanical properties in mouse cervical tissue during normal pregnancy, PLoS One, 9 (2014) e112391. 10.1371/journal.pone.0112391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Nallasamy S, Yoshida K, Akins M, Myers K, Iozzo R, Mahendroo M, Steroid Hormones Are Key Modulators of Tissue Mechanical Function via Regulation of Collagen and Elastic Fibers, Endocrinology, 158 (2017) 950–962. 10.1210/en.2016-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jayyosi C, Lee N, Willcockson A, Nallasamy S, Mahendroo M, Myers K, The mechanical response of the mouse cervix to tensile cyclic loading in term and preterm pregnancy, Acta Biomater, 78 (2018) 308–319. 10.1016/j.actbio.2018.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Willcockson AR, Nandu T, Liu CL, Nallasamy S, Kraus WL, Mahendroo M, Transcriptome signature identifies distinct cervical pathways induced in lipopolysaccharide-mediated preterm birth, Biol Reprod, 98 (2018) 408–421. 10.1093/biolre/iox180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zhang Y, Akins ML, Murari K, Xi J, Li MJ, Luby-Phelps K, Mahendroo M, Li X, A compact fiber-optic SHG scanning endomicroscope and its application to visualize cervical remodeling during pregnancy, Proc Natl Acad Sci U S A, 109 (2012) 12878–12883. 10.1073/pnas.1121495109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Badir S, Mazza E, Zimmermann R, Bajka M, Cervical softening occurs early in pregnancy: characterization of cervical stiffness in 100 healthy women using the aspiration technique, Prenat Diagn, 33 (2013) 737–741. 10.1002/pd.4116. [DOI] [PubMed] [Google Scholar]
- [9].Bartholomeusz RK, Bruce NW, Martin CE, Hartmann PE, Serial measurement of arterial plasma progesterone levels throughout gestation and parturition in individual rats, Acta Endocrinol (Copenh), 82 (1976) 436–443. 10.1530/acta.0.0820436. [DOI] [PubMed] [Google Scholar]
- [10].Virgo BB, Bellward GD, Serum progesterone levels in the pregnant and postpartum laboratory mouse, Endocrinology, 95 (1974) 1486–1490. 10.1210/endo-95-5-1486. [DOI] [PubMed] [Google Scholar]
- [11].Walsh SW, Stanczyk FZ, Novy MJ, Daily hormonal changes in the maternal, fetal, and amniotic fluid compartments before parturition in a primate species, J Clin Endocrinol Metab, 58 (1984) 629–639. 10.1210/jcem-58-4-629. [DOI] [PubMed] [Google Scholar]
- [12].Tulchinsky D, Hobel CJ, Yeager E, Marshall JR, Plama estradiol, estriol, and progesterone in human pregnancy. II. Clinical applications in Rh-isoimmunization disease, Am J Obstet Gynecol, 113 (1972) 766–770. 10.1016/0002-9378(72)90556-x. [DOI] [PubMed] [Google Scholar]
- [13].Boroditsky RS, Reyes FI, Winter JS, Faiman C, Maternal serum estrogen and progesterone concentrations preceding normal labor, Obstet Gynecol, 51 (1978) 686–691. [PubMed] [Google Scholar]
- [14].Lewis PR, Galvin PM, Short RV, Salivary oestriol and progesterone concentrations in women during late pregnancy, parturition and the puerperium, J Endocrinol, 115 (1987) 177–181. 10.1677/joe.0.1150177. [DOI] [PubMed] [Google Scholar]
- [15].Renthal NE, Williams KC, Montalbano AP, Chen CC, Gao L, Mendelson CR, Molecular Regulation of Parturition: A Myometrial Perspective, Cold Spring Harb Perspect Med, 5 (2015). 10.1101/cshperspect.a023069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Andersson S, Minjarez D, Yost NP, Word RA, Estrogen and progesterone metabolism in the cervix during pregnancy and parturition, J Clin Endocrinol Metab, 93 (2008) 2366–2374. 10.1210/jc.2007-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Henkelmann GC, Hagemeister FB, Fuller LM, Two cycles of MOPP and radiotherapy for stage III1A and stage III1B Hodgkin’s disease, J Clin Oncol, 6 (1988) 1293–1302. 10.1200/JCO.1988.6.8.1293. [DOI] [PubMed] [Google Scholar]
- [18].Kraus WL, Katzenellenbogen BS, Regulation of progesterone receptor gene expression and growth in the rat uterus: modulation of estrogen actions by progesterone and sex steroid hormone antagonists, Endocrinology, 132 (1993) 2371–2379. 10.1210/endo.132.6.8504742. [DOI] [PubMed] [Google Scholar]
- [19].Marshall SA, Senadheera SN, Jelinic M, O’Sullivan K, Parry LJ, Tare M, Relaxin Deficiency Leads to Uterine Artery Dysfunction During Pregnancy in Mice, Front Physiol, 9 (2018) 255. 10.3389/fphys.2018.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Vodstrcil LA, Tare M, Novak J, Dragomir N, Ramirez RJ, Wlodek ME, Conrad KP, Parry LJ, Relaxin mediates uterine artery compliance during pregnancy and increases uterine blood flow, FASEB J, 26 (2012) 4035–4044. 10.1096/fj.12-210567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bennett RG, Relaxin and its role in the development and treatment of fibrosis, Transl Res, 154 (2009) 1–6. 10.1016/j.trsl.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yao L, Agoulnik AI, Cooke PS, Meling DD, Sherwood OD, Relaxin acts on stromal cells to promote epithelial and stromal proliferation and inhibit apoptosis in the mouse cervix and vagina, Endocrinology, 149 (2008) 2072–2079. 10.1210/en.2007-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Parry LJ, Vodstrcil LA, Relaxin physiology in the female reproductive tract during pregnancy, Adv Exp Med Biol, 612 (2007) 34–48. 10.1007/978-0-387-74672-2_4. [DOI] [PubMed] [Google Scholar]
- [24].Weiss G, Relaxin and the control of primate parturition, Ital J Anat Embryol, 118 (2013) 17–18. [PubMed] [Google Scholar]
- [25].Soh YM, Tiwari A, Mahendroo M, Conrad KP, Parry LJ, Relaxin regulates hyaluronan synthesis and aquaporins in the cervix of late pregnant mice, Endocrinology, 153 (2012) 6054–6064. 10.1210/en.2012-1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sherwood OD, Relaxin’s physiological roles and other diverse actions, Endocr Rev, 25 (2004) 205–234. 10.1210/er.2003-0013. [DOI] [PubMed] [Google Scholar]
- [27].Lenhart JA, Ryan PL, Ohleth KM, Palmer SS, Bagnell CA, Relaxin increases secretion of tissue inhibitor of matrix metalloproteinase-1 and −2 during uterine and cervical growth and remodeling in the pig, Endocrinology, 143 (2002) 91–98. 10.1210/endo.143.1.8562. [DOI] [PubMed] [Google Scholar]
- [28].Timmons BC, Reese J, Socrate S, Ehinger N, Paria BC, Milne GL, Akins ML, Auchus RJ, McIntire D, House M, Mahendroo M, Prostaglandins are essential for cervical ripening in LPS-mediated preterm birth but not term or antiprogestin-driven preterm ripening, Endocrinology, 155 (2014) 287–298. 10.1210/en.2013-1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Romero R, Munoz H, Gomez R, Parra M, Polanco M, Valverde V, Hasbun J, Garrido J, Ghezzi F, Mazor M, Tolosa JE, Mitchell MD, Increase in prostaglandin bioavailability precedes the onset of human parturition, Prostaglandins Leukot Essent Fatty Acids, 54 (1996) 187–191. 10.1016/s0952-3278(96)90015-0. [DOI] [PubMed] [Google Scholar]
- [30].MacDonald PC, Casey ML, The accumulation of prostaglandins (PG) in amniotic fluid is an aftereffect of labor and not indicative of a role for PGE2 or PGF2 alpha in the initiation of human parturition, J Clin Endocrinol Metab, 76 (1993) 1332–1339. 10.1210/jcem.76.5.8496326. [DOI] [PubMed] [Google Scholar]
- [31].Conneely OM, Mulac-Jericevic B, Lydon JP, Progesterone-dependent regulation of female reproductive activity by two distinct progesterone receptor isoforms, Steroids, 68 (2003) 771–778. 10.1016/s0039-128x(03)00126-0. [DOI] [PubMed] [Google Scholar]
- [32].Merlino AA, Welsh TN, Tan H, Yi LJ, Cannon V, Mercer BM, Mesiano S, Nuclear progesterone receptors in the human pregnancy myometrium: evidence that parturition involves functional progesterone withdrawal mediated by increased expression of progesterone receptor-A, J Clin Endocrinol Metab, 92 (2007) 1927–1933. 10.1210/jc.2007-0077. [DOI] [PubMed] [Google Scholar]
- [33].Peavey MC, Wu SP, Li R, Liu J, Emery OM, Wang T, Zhou L, Wetendorf M, Yallampalli C, Gibbons WE, Lydon JP, DeMayo FJ, Progesterone receptor isoform B regulates the Oxtr-Plcl2-Trpc3 pathway to suppress uterine contractility, Proc Natl Acad Sci U S A, 118 (2021). 10.1073/pnas.2011643118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Heuerman AC, Hollinger TT, Menon R, Mesiano S, Yellon SM, Cervix Stromal Cells and the Progesterone Receptor A Isoform Mediate Effects of Progesterone for Prepartum Remodeling, Reprod Sci, 26 (2019) 690–696. 10.1177/1933719118820462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Fernandez-Valdivia R, Mukherjee A, Mulac-Jericevic B, Conneely OM, DeMayo FJ, Amato P, Lydon JP, Revealing progesterone’s role in uterine and mammary gland biology: insights from the mouse, Semin Reprod Med, 23 (2005) 22–37. 10.1055/s-2005-864031. [DOI] [PubMed] [Google Scholar]
- [36].Mesiano S, Welsh TN, Steroid hormone control of myometrial contractility and parturition, Semin Cell Dev Biol, 18 (2007) 321–331. 10.1016/j.semcdb.2007.05.003. [DOI] [PubMed] [Google Scholar]
- [37].Nadeem L, Shynlova O, Matysiak-Zablocki E, Mesiano S, Dong X, Lye S, Molecular evidence of functional progesterone withdrawal in human myometrium, Nat Commun, 7 (2016) 11565. 10.1038/ncomms11565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hardy DB, Janowski BA, Corey DR, Mendelson CR, Progesterone receptor plays a major antiinflammatory role in human myometrial cells by antagonism of nuclear factor-kappaB activation of cyclooxygenase 2 expression, Mol Endocrinol, 20 (2006) 2724–2733. 10.1210/me.2006-0112. [DOI] [PubMed] [Google Scholar]
- [39].Holt R, Timmons BC, Akgul Y, Akins ML, Mahendroo M, The molecular mechanisms of cervical ripening differ between term and preterm birth, Endocrinology, 152 (2011) 1036–1046. 10.1210/en.2010-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hegele-Hartung C, Chwalisz K, Beier HM, Elger W, Ripening of the uterine cervix of the guinea-pig after treatment with the progesterone antagonist onapristone (ZK 98.299): an electron microscopic study, Hum Reprod, 4 (1989) 369–377. 10.1093/oxfordjournals.humrep.a136909. [DOI] [PubMed] [Google Scholar]
- [41].Chwalisz K, The use of progesterone antagonists for cervical ripening and as an adjunct to labour and delivery, Hum Reprod, 9 Suppl 1 (1994) 131–161. 10.1093/humrep/9.suppl_1.131. [DOI] [PubMed] [Google Scholar]
- [42].Kuon RJ, Shi SQ, Maul H, Sohn C, Balducci J, Maner WL, Garfield RE, Pharmacologic actions of progestins to inhibit cervical ripening and prevent delivery depend on their properties, the route of administration, and the vehicle, Am J Obstet Gynecol, 202 (2010) 455 e451–459. 10.1016/j.ajog.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Yellon SM, Dobyns AE, Beck HL, Kurtzman JT, Garfield RE, Kirby MA, Loss of progesterone receptor-mediated actions induce preterm cellular and structural remodeling of the cervix and premature birth, PLoS One, 8 (2013) e81340. 10.1371/journal.pone.0081340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Vegeto E, Shahbaz MM, Wen DX, Goldman ME, O’Malley BW, McDonnell DP, Human progesterone receptor A form is a cell- and promoter-specific repressor of human progesterone receptor B function, Mol Endocrinol, 7 (1993) 1244–1255. 10.1210/mend.7.10.8264658. [DOI] [PubMed] [Google Scholar]
- [45].Sartorius CA, Melville MY, Hovland AR, Tung L, Takimoto GS, Horwitz KB, A third transactivation function (AF3) of human progesterone receptors located in the unique N-terminal segment of the B-isoform, Mol Endocrinol, 8 (1994) 1347–1360. 10.1210/mend.8.10.7854352. [DOI] [PubMed] [Google Scholar]
- [46].Conneely OM, Mulac-Jericevic B, Lydon JP, De Mayo FJ, Reproductive functions of the progesterone receptor isoforms: lessons from knock-out mice, Mol Cell Endocrinol, 179 (2001) 97–103. 10.1016/s0303-7207(01)00465-8. [DOI] [PubMed] [Google Scholar]
- [47].Ramos JG, Varayoud J, Bosquiazzo VL, Luque EH, Munoz-de-Toro M, Cellular turnover in the rat uterine cervix and its relationship to estrogen and progesterone receptor dynamics, Biol Reprod, 67 (2002) 735–742. 10.1095/biolreprod.101.002402. [DOI] [PubMed] [Google Scholar]
- [48].Mehta FF, Son J, Hewitt SC, Jang E, Lydon JP, Korach KS, Chung SH, Distinct functions and regulation of epithelial progesterone receptor in the mouse cervix, vagina, and uterus, Oncotarget, 7 (2016) 17455–17467. 10.18632/oncotarget.8159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Nallasamy S, Mahendroo M, Distinct Roles of Cervical Epithelia and Stroma in Pregnancy and Parturition, Semin Reprod Med, 35 (2017) 190–200. 10.1055/s-0037-1599091. [DOI] [PubMed] [Google Scholar]
- [50].Mahendroo MS, Porter A, Russell DW, Word RA, The parturition defect in steroid 5alpha-reductase type 1 knockout mice is due to impaired cervical ripening, Mol Endocrinol, 13 (1999) 981–992. 10.1210/mend.13.6.0307. [DOI] [PubMed] [Google Scholar]
- [51].Yoshida K, Jayyosi C, Lee N, Mahendroo M, Myers KM, Mechanics of cervical remodelling: insights from rodent models of pregnancy, Interface Focus, 9 (2019) 20190026. 10.1098/rsfs.2019.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Nallasamy S, Palacios HH, Setlem R, Caraballo MC, Li K, Cao E, Shankaran M, Hellerstein M, Mahendroo M, Transcriptome and proteome dynamics of cervical remodeling in the mouse during pregnancy, Biol Reprod, (2021). 10.1093/biolre/ioab144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Akins ML, Luby-Phelps K, Bank RA, Mahendroo M, Cervical softening during pregnancy: regulated changes in collagen cross-linking and composition of matricellular proteins in the mouse, Biol Reprod, 84 (2011) 1053–1062. 10.1095/biolreprod.110.089599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].House M, Kaplan DL, Socrate S, Relationships between mechanical properties and extracellular matrix constituents of the cervical stroma during pregnancy, Semin Perinatol, 33 (2009) 300–307. 10.1053/j.semperi.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Rosado-Mendez IM, Carlson LC, Woo KM, Santoso AP, Guerrero QW, Palmeri ML, Feltovich H, Hall TJ, Quantitative assessment of cervical softening during pregnancy in the Rhesus macaque with shear wave elasticity imaging, Phys Med Biol, 63 (2018) 085016. 10.1088/1361-6560/aab532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Carlson LC, Hall TJ, Rosado-Mendez IM, Mao L, Feltovich H, Quantitative assessment of cervical softening during pregnancy with shear wave elasticity imaging: an in vivo longitudinal study, Interface Focus, 9 (2019) 20190030. 10.1098/rsfs.2019.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Volozonoka L, Rots D, Kempa I, Kornete A, Rezeberga D, Gailite L, Miskova A, Genetic landscape of preterm birth due to cervical insufficiency: Comprehensive gene analysis and patient next-generation sequencing data interpretation, PLoS One, 15 (2020) e0230771. 10.1371/journal.pone.0230771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Hattar R, Maller O, McDaniel S, Hansen KC, Hedman KJ, Lyons TR, Lucia S, Wilson RS Jr., Schedin P, Tamoxifen induces pleiotrophic changes in mammary stroma resulting in extracellular matrix that suppresses transformed phenotypes, Breast Cancer Res, 11 (2009) R5. 10.1186/bcr2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Balgobin S, Montoya TI, Shi H, Acevedo JF, Keller PW, Riegel M, Wai CY, Word RA, Estrogen alters remodeling of the vaginal wall after surgical injury in guinea pigs, Biol Reprod, 89 (2013) 138. 10.1095/biolreprod.113.112367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Smith OW, Kaltreider NB, Collagen Content of the Nonpregnant Rat Uterus as Related to the Functional Responses to Estrogen and Progesterone, Endocrinology, 73 (1963) 619–628. 10.1210/endo-73-5-619. [DOI] [PubMed] [Google Scholar]
- [61].Ozasa H, Tominaga T, Nishimura T, Takeda T, Lysyl oxidase activity in the mouse uterine cervix is physiologically regulated by estrogen, Endocrinology, 109 (1981) 618–621. 10.1210/endo-109-2-618. [DOI] [PubMed] [Google Scholar]
- [62].Drewes PG, Yanagisawa H, Starcher B, Hornstra I, Csiszar K, Marinis SI, Keller P, Word RA, Pelvic organ prolapse in fibulin-5 knockout mice: pregnancy-induced changes in elastic fiber homeostasis in mouse vagina, Am J Pathol, 170 (2007) 578–589. 10.2353/ajpath.2007.060662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].House M, Tadesse-Telila S, Norwitz ER, Socrate S, Kaplan DL, Inhibitory effect of progesterone on cervical tissue formation in a three-dimensional culture system with human cervical fibroblasts, Biol Reprod, 90 (2014) 18. 10.1095/biolreprod.113.112540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].House M, Kelly J, Klebanov N, Yoshida K, Myers K, Kaplan DL, Mechanical and Biochemical Effects of Progesterone on Engineered Cervical Tissue, Tissue Eng Part A, 24 (2018) 1765–1774. 10.1089/ten.TEA.2018.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Winn RJ, Baker MD, Sherwood OD, Individual and combined effects of relaxin, estrogen, and progesterone in ovariectomized gilts. I. Effects on the growth, softening, and histological properties of the cervix, Endocrinology, 135 (1994) 1241–1249. 10.1210/endo.135.3.8070369. [DOI] [PubMed] [Google Scholar]
- [66].Zhao L, Roche PJ, Gunnersen JM, Hammond VE, Tregear GW, Wintour EM, Beck F, Mice without a functional relaxin gene are unable to deliver milk to their pups, Endocrinology, 140 (1999) 445–453. 10.1210/endo.140.1.6404. [DOI] [PubMed] [Google Scholar]
- [67].Read CP, Word RA, Ruscheinsky MA, Timmons BC, Mahendroo MS, Cervical remodeling during pregnancy and parturition: molecular characterization of the softening phase in mice, Reproduction, 134 (2007) 327–340. 10.1530/REP-07-0032. [DOI] [PubMed] [Google Scholar]
- [68].Racicot K, Kwon JY, Aldo P, Silasi M, Mor G, Understanding the complexity of the immune system during pregnancy, Am J Reprod Immunol, 72 (2014) 107–116. 10.1111/aji.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Hughes BL, Dutt R, Raker C, Barthelemy M, Rossoll RM, Ramratnam B, Wira CR, Cu-Uvin S, The impact of pregnancy on anti-HIV activity of cervicovaginal secretions, Am J Obstet Gynecol, 215 (2016) 748 e741–748 e712. 10.1016/j.ajog.2016.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Timmons BC, Mahendroo MS, Timing of neutrophil activation and expression of proinflammatory markers do not support a role for neutrophils in cervical ripening in the mouse, Biol Reprod, 74 (2006) 236–245. 10.1095/biolreprod.105.044891. [DOI] [PubMed] [Google Scholar]
- [71].Kirby MA, Heuerman AC, Custer M, Dobyns AE, Strilaeff R, Stutz KN, Cooperrider J, Elsissy JG, Yellon SM, Progesterone Receptor-Mediated Actions Regulate Remodeling of the Cervix in Preparation for Preterm Parturition, Reprod Sci, 23 (2016) 1473–1483. 10.1177/1933719116650756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].De Tomasi JB, Opata MM, Mowa CN, Immunity in the Cervix: Interphase between Immune and Cervical Epithelial Cells, Journal of Immunology Research, 2019 (2019) 7693183. 10.1155/2019/7693183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Timmons BC, Mitchell SM, Gilpin C, Mahendroo MS, Dynamic changes in the cervical epithelial tight junction complex and differentiation occur during cervical ripening and parturition, Endocrinology, 148 (2007) 1278–1287. 10.1210/en.2006-0851. [DOI] [PubMed] [Google Scholar]
- [74].Chan LN, Tsang LL, Rowlands DK, Rochelle LG, Boucher RC, Liu CQ, Chan HC, Distribution and regulation of ENaC subunit and CFTR mRNA expression in murine female reproductive tract, J Membr Biol, 185 (2002) 165–176. 10.1007/s00232-001-0117-y. [DOI] [PubMed] [Google Scholar]
- [75].Hevir N, Vouk K, Sinkovec J, Ribic-Pucelj M, Rizner TL, Aldo-keto reductases AKR1C1, AKR1C2 and AKR1C3 may enhance progesterone metabolism in ovarian endometriosis, Chem Biol Interact, 191 (2011) 217–226. 10.1016/j.cbi.2011.01.003. [DOI] [PubMed] [Google Scholar]
- [76].Penning TM, Wangtrakuldee P, Auchus RJ, Structural and Functional Biology of Aldo-Keto Reductase Steroid-Transforming Enzymes, Endocr Rev, 40 (2019) 447–475. 10.1210/er.2018-00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Mahendroo MS, Cala KM, Russell DW, 5 alpha-reduced androgens play a key role in murine parturition, Mol Endocrinol, 10 (1996) 380–392. 10.1210/mend.10.4.8721983. [DOI] [PubMed] [Google Scholar]
- [78].Lacroix G, Gouyer V, Gottrand F, Desseyn JL, The Cervicovaginal Mucus Barrier, Int J Mol Sci, 21 (2020). 10.3390/ijms21218266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Wira CR, Grant-Tschudy KS, Crane-Godreau MA, Epithelial cells in the female reproductive tract: a central role as sentinels of immune protection, Am J Reprod Immunol, 53 (2005) 65–76. 10.1111/j.1600-0897.2004.00248.x. [DOI] [PubMed] [Google Scholar]
- [80].Timmons BC, Fairhurst AM, Mahendroo MS, Temporal changes in myeloid cells in the cervix during pregnancy and parturition, J Immunol, 182 (2009) 2700–2707. 10.4049/jimmunol.0803138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Yellon SM, Contributions to the dynamics of cervix remodeling prior to term and preterm birth, Biol Reprod, 96 (2017) 13–23. 10.1095/biolreprod.116.142844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Yellon SM, Immunobiology of Cervix Ripening, Front Immunol, 10 (2019) 3156. 10.3389/fimmu.2019.03156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Tantengco OAG, Menon R, Breaking Down the Barrier: The Role of Cervical Infection and Inflammation in Preterm Birth, Front Glob Womens Health, 2 (2021) 777643. 10.3389/fgwh.2021.777643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Hassan SS, Romero R, Tarca AL, Nhan-Chang CL, Vaisbuch E, Erez O, Mittal P, Kusanovic JP, Mazaki-Tovi S, Yeo L, Draghici S, Kim JS, Uldbjerg N, Kim CJ, The transcriptome of cervical ripening in human pregnancy before the onset of labor at term: identification of novel molecular functions involved in this process, J Matern Fetal Neonatal Med, 22 (2009) 1183–1193. 10.3109/14767050903353216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Filipovich Y, Agrawal V, Crawford SE, Fitchev P, Qu X, Klein J, Hirsch E, Depletion of polymorphonuclear leukocytes has no effect on preterm delivery in a mouse model of Escherichia coli-induced labor, Am J Obstet Gynecol, 213 (2015) 697 e691–610. 10.1016/j.ajog.2015.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Yellon SM, Greaves E, Heuerman AC, Dobyns AE, Norman JE, Effects of macrophage depletion on characteristics of cervix remodeling and pregnancy in CD11b-dtr mice, Biol Reprod, 100 (2019) 1386–1394. 10.1093/biolre/ioz002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Martin JA, Hamilton BE, Osterman MJK, Driscoll AK, Births: Final Data for 2019, Natl Vital Stat Rep, 70 (2021) 1–51. [PubMed] [Google Scholar]
- [88].Cunningham FG, Leveno KJ, Bloom SL, Dashe JS, Hoffman BL, Casey BM, Spong CY, Preterm Birth, Williams Obstetrics, 25e, McGraw-Hill Education, New York, NY, 2018. [Google Scholar]
- [89].Anum EA, Hill LD, Pandya A, Strauss JF 3rd, Connective tissue and related disorders and preterm birth: clues to genes contributing to prematurity, Placenta, 30 (2009) 207–215. 10.1016/j.placenta.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Warren JE, Silver RM, Dalton J, Nelson LT, Branch DW, Porter TF, Collagen 1Alpha1 and transforming growth factor-beta polymorphisms in women with cervical insufficiency, Obstet Gynecol, 110 (2007) 619–624. 10.1097/01.AOG.0000277261.92756.1a. [DOI] [PubMed] [Google Scholar]
- [91].Akgul Y, Word RA, Ensign LM, Yamaguchi Y, Lydon J, Hanes J, Mahendroo M, Hyaluronan in cervical epithelia protects against infection-mediated preterm birth, J Clin Invest, 124 (2014) 5481–5489. 10.1172/JCI78765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Gonzalez JM, Xu H, Chai J, Ofori E, Elovitz MA, Preterm and term cervical ripening in CD1 Mice (Mus musculus): similar or divergent molecular mechanisms?, Biol Reprod, 81 (2009) 1226–1232. 10.1095/biolreprod.108.075309. [DOI] [PubMed] [Google Scholar]
- [93].Filipovich Y, Lu SJ, Akira S, Hirsch E, The adaptor protein MyD88 is essential for E coli-induced preterm delivery in mice, Am J Obstet Gynecol, 200 (2009) 93 e91–98. 10.1016/j.ajog.2008.08.038. [DOI] [PubMed] [Google Scholar]
- [94].Fathian A, Hyatt K, Knudtson E, Mesiano S, Myers D, 690: Regulation of PR-A and PR-B target genes in myometrial cells by progesterone (P4) and select synthetic progestins, 17α-hydroxyprogesterone capropate (17OHPC) and nomegestrol acetate (NOMAC), American Journal of Obstetrics & Gynecology, 214 (2016) S364. 10.1016/j.ajog.2015.10.737. [DOI] [Google Scholar]
- [95].Kniss DA, Summerfield TL, Progesterone Receptor Signaling Selectively Modulates Cytokine-Induced Global Gene Expression in Human Cervical Stromal Cells, Front Genet, 11 (2020) 883. 10.3389/fgene.2020.00883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Pavlidis I, Spiller OB, Sammut Demarco G, MacPherson H, Howie SEM, Norman JE, Stock SJ, Cervical epithelial damage promotes Ureaplasma parvum ascending infection, intrauterine inflammation and preterm birth induction in mice, Nat Commun, 11 (2020) 199. 10.1038/s41467-019-14089-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Vornhagen J, Quach P, Boldenow E, Merillat S, Whidbey C, Ngo LY, Adams Waldorf KM, Rajagopal L, Bacterial Hyaluronidase Promotes Ascending GBS Infection and Preterm Birth, mBio, 7 (2016). 10.1128/mBio.00781-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Meis PJ, Klebanoff M, Thom E, Dombrowski MP, Sibai B, Moawad AH, Spong CY, Hauth JC, Miodovnik M, Varner MW, Leveno KJ, Caritis SN, Iams JD, Wapner RJ, Conway D, O’Sullivan MJ, Carpenter M, Mercer B, Ramin SM, Thorp JM, Peaceman AM, Gabbe S, National H Institute of Child, N. Human Development Maternal-Fetal Medicine Units, Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate, N Engl J Med, 348 (2003) 2379–2385. 10.1056/NEJMoa035140. [DOI] [PubMed] [Google Scholar]
- [99].Hassan SS, Romero R, Vidyadhari D, Fusey S, Baxter JK, Khandelwal M, Vijayaraghavan J, Trivedi Y, Soma-Pillay P, Sambarey P, Dayal A, Potapov V, O’Brien J, Astakhov V, Yuzko O, Kinzler W, Dattel B, Sehdev H, Mazheika L, Manchulenko D, Gervasi MT, Sullivan L, Conde-Agudelo A, Phillips JA, Creasy GW, Trial P, Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter, randomized, double-blind, placebo-controlled trial, Ultrasound Obstet Gynecol, 38 (2011) 18–31. 10.1002/uog.9017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Norman JE, Progesterone and preterm birth, Int J Gynaecol Obstet, 150 (2020) 24–30. 10.1002/ijgo.13187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Blackwell SC, Gyamfi-Bannerman C, Biggio JR Jr., Chauhan SP, Hughes BL, Louis JM, Manuck TA, Miller HS, Das AF, Saade GR, Nielsen P, Baker J, Yuzko OM, Reznichenko GI, Reznichenko NY, Pekarev O, Tatarova N, Gudeman J, Birch R, Jozwiakowski MJ, Duncan M, Williams L, Krop J, 17-OHPC to Prevent Recurrent Preterm Birth in Singleton Gestations (PROLONG Study): A Multicenter, International, Randomized Double-Blind Trial, Am J Perinatol, 37 (2020) 127–136. 10.1055/s-0039-3400227. [DOI] [PubMed] [Google Scholar]
- [102].Norman JE, Marlow N, Messow CM, Shennan A, Bennett PR, Thornton S, Robson SC, McConnachie A, Petrou S, Sebire NJ, Lavender T, Whyte S, Norrie J, O.s. group, Vaginal progesterone prophylaxis for preterm birth (the OPPTIMUM study): a multicentre, randomised, double-blind trial, Lancet, 387 (2016) 2106–2116. 10.1016/S0140-6736(16)00350-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Chang CY, Nguyen CP, Wesley B, Guo J, Johnson LL, Joffe HV, Withdrawing Approval of Makena — A Proposal from the FDA Center for Drug Evaluation and Research, New England Journal of Medicine, 383 (2020) e131. 10.1056/NEJMp2031055. [DOI] [PubMed] [Google Scholar]
- [104].Zierden HC, Ortiz JI, DeLong K, Yu J, Li G, Dimitrion P, Bensouda S, Laney V, Bailey A, Anders NM, Scardina M, Mahendroo M, Mesiano S, Burd I, Wagner G, Hanes J, Ensign LM, Enhanced drug delivery to the reproductive tract using nanomedicine reveals therapeutic options for prevention of preterm birth, Sci Transl Med, 13 (2021). 10.1126/scitranslmed.abc6245. [DOI] [PMC free article] [PubMed] [Google Scholar]


