Abstract
Purpose.
To evaluate the association of a history of infertility with long-term weight, body composition, and blood pressure.
Methods.
We studied 1,581 women from the prospective cohort Project Viva. History of infertility was based on self-reported time to pregnancy ≥12 months or use of medical treatment to conceive for the index or any prior pregnancy; a diagnosis of infertility; claims for infertility treatments/prescriptions abstracted from medical records. The outcomes were weight, waist circumference, and body fat assessed through 12 years postpartum; and blood pressure assessed through 3 years postpartum. We used linear mixed-effect models adjusted for age, race/ethnicity, income, education, marital status, parity, and age at menarche.
Results.
342 women (21.6%) had a history of infertility. In adjusted models, women with vs. without infertility, had higher average weight (3.29 kg, 95% CI: 1.35, 5.24), waist circumference (2.46 cm, 95% CI: 0.78, 4.13) and body fat (1.76 kg, 95% CI: 0.09, 3.43). Among younger (18–29 years), but not older (≥30 years) women, infertility was associated with higher systolic (4.08 mmHg, 95% CI: 0.93, 7.23) and diastolic blood pressure (2.16 mmHg, 95% CI: 0.11, 4.20).
Conclusions.
A history of infertility may serve as a marker to identify women at higher cardiometabolic risk.
Keywords: female infertility, body composition, weight, blood pressure, cardio-metabolic risk
Introduction
Infertility is a condition characterized by a failure to achieve a clinical pregnancy after ≥12 months of unaided attempts at conception [1]. Infertility encompasses a spectrum of reproductive dysfunction that includes secondary infertility, women who become pregnant without medical assistance, as well as women who require medical treatments to achieve a pregnancy [1]. Infertility affects approximately 15.5% of reproductive-aged women trying to conceive in the US [2]. Infertility has been associated with a range of long-term health outcomes, including future risk of type 2 diabetes and cardiovascular disease (CVD) [3,4].
Despite the known relationship between infertility and obesity-related chronic conditions, few studies have evaluated associations of infertility with subclinical indicators of disease progression, such as body composition, adiposity, and cardiovascular traits, for which results may be more relevant to the general-risk population and have potential to inform primary prevention. Studies of women with polycystic ovary syndrome (PCOS), a condition characterized by infertility [5], have shown that they are more likely to exhibit overweight and obesity, central adiposity, and metabolic alterations such as insulin resistance and dyslipidemia [6–8]. However, few studies outside of PCOS settings have tested the hypothesis of an association between infertility and precursors to clinical disease [9]. Such studies are critical because women with PCOS are likely not representative of the general population of reproductive-aged women who may experience infertility. In addition to the predominant focus on high-risk populations, most of the studies conducted to date have been cross-sectional or case-control in design [9], which leaves current evidence open to the possibility of troubling biases like reverse causation and selection bias [10,11]. Therefore, there is a need for prospective studies on the association between infertility and markers of cardiovascular health non-restricted to women with PCOS.
Considering the growing burden of infertility among women and the lack of literature on the relationship between infertility and long-term health, we aimed to evaluate the association of a history of infertility with long-term weight, body composition, and blood pressure in women from Project Viva.
Materials and methods
Study population
This study was a secondary analysis of data from women participating in Project Viva, an ongoing prospective cohort of mother-child pairs. Details on recruitment and eligibility are described elsewhere [12]. Briefly, Project Viva recruited pregnant women between 1999 and 2002 from Atrius Harvard Vanguard Medical Associates at around 10 weeks of gestation. Of the 2,100 women enrolled in Project Viva, 28 contributed two different singleton pregnancies (2,128 live births in total). For these women, we considered only the second pregnancy to avoid extraneous bias due to subsequent pregnancies. We further excluded women younger than 18 years at enrollment (n=28) and those with missing outcome data throughout the follow-up period (n=476). Women with intervening pregnancies during follow-up were not excluded because intervening pregnancies are likely on the causal pathway between fertility and long-term health. However, since pregnancy leads to acute changes in weight, adiposity, and blood pressure, we excluded outcome data at a specific visit if the woman reported being pregnant at the time of the visit. This resulted in the exclusion of 15 participants more, leaving 1,581 women in the analytical sample (n=1,577 with adiposity outcomes and n=1,464 with blood pressure) (Figure 1). Women included (n=1,581) vs. excluded (n=519) were older at enrollment (32.3 vs. 30.4 years), less likely to be Black (14.9 vs. 22.1%), single (7.6 vs. 11.7%), and smokers during the index pregnancy (11.3 vs. 16.9%). Women in the analytical sample were more highly educated (68.5 vs. 52.5% with college education), had a higher family income (62.5 vs. 55.5% >$70,000/year), a higher diet quality score during pregnancy (60.8 vs. 59.5 units), and a higher prevalence of infertility (7.8 vs. 4.6% with medically assisted reproduction [MAR], 13.8 vs. 10.2% without MAR) than women excluded from analysis (P <0.05 for all). Neither pre-pregnancy BMI, as a proxy for baseline BMI, nor first trimester blood pressure, as a proxy for baseline blood pressure, were associated with inclusion in the analytical sample.
Figure 1. Study flowchart.
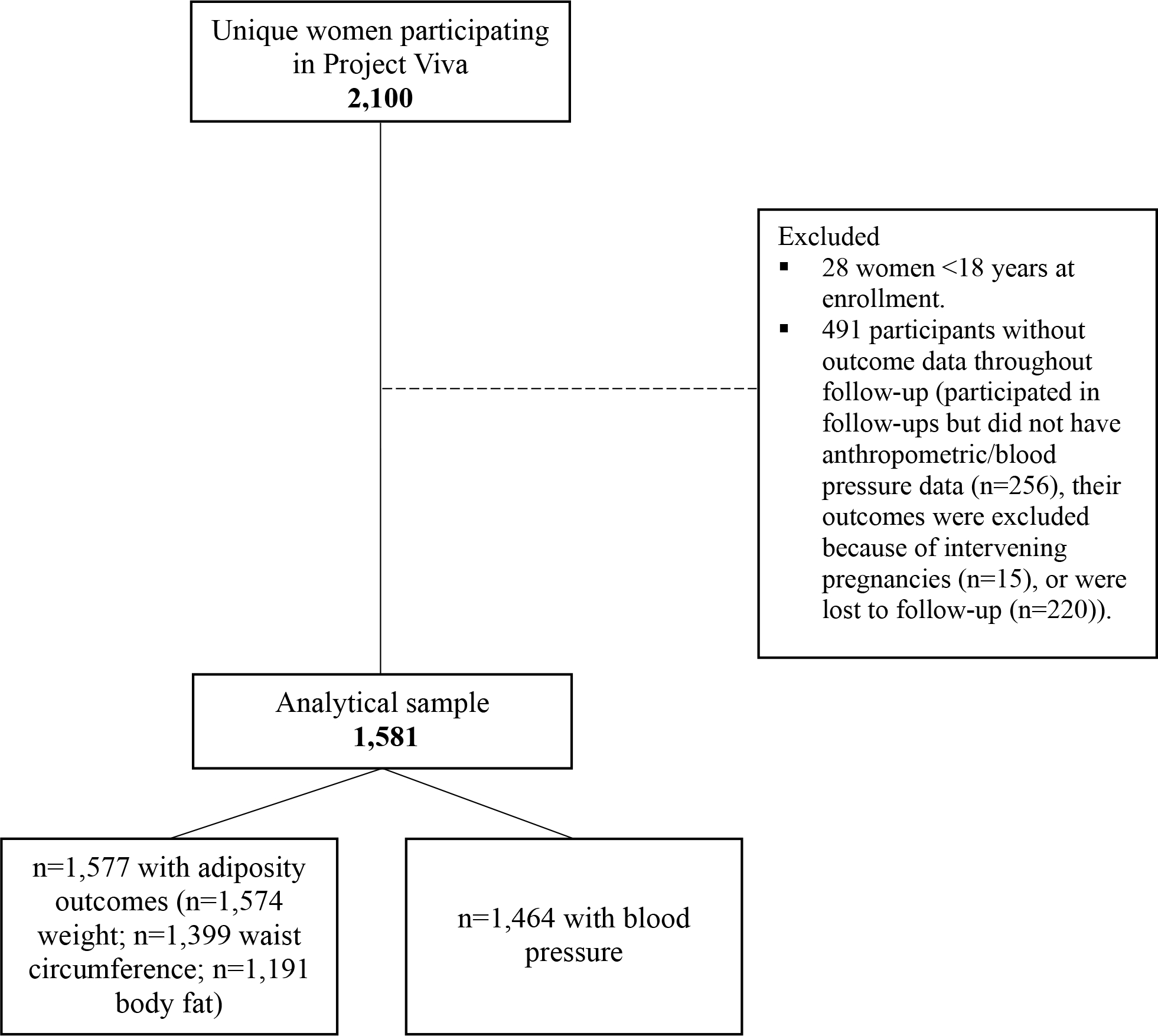
All participants provided written informed consent at enrollment and each study visit. The institutional review board of Harvard Pilgrim Health Care approved all study protocols.
History of infertility
We assessed infertility before the index pregnancy (yes/no) via three sources of information. First, we used questionnaire data from the first study visit inquiring on whether they were actively trying to become pregnant, and if so, the number of cycles it took them to become pregnant. Women reporting ≥12 cycles to become pregnant were classified as infertile. Second, we used information obtained from the women’s medical records on a history of infertility – specifically, whether they had a diagnosis of infertility (ICD-9 code 628.9 entries before the last menstrual period (LMP)+60 days), or had a claim for infertility consultation or services, including any use of assisted reproductive technologies, or prescriptions for fertility medications (e.g., clomiphene citrate, gonadotropins or gonadotropin-releasing hormone agonists before LMP+14 days). Lastly, on a follow-up questionnaire administered ~18 years after delivery, women were asked if it has taken them ≥12 months to become pregnant in any prior pregnancy or if they had used medical treatment for this purpose. If they responded “yes” to either question for a pregnancy before or including the index pregnancy, we classified them as having a history of infertility. The proportion of women identified with these criteria is described in Supplemental Table 1.
Weight, adiposity, and blood pressure
Weight was measured in light clothing on an electronic scale at 6 months, 3, 7, and 12 years postpartum. Waist circumference (WC) was measured at the umbilicus level to the nearest 0.1 cm with a non-stretchable measuring tape at 3, 7, and 12 years. We measured body fat (kg and %) through bioimpedance at 7 and 12 years. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured 5 times, 1 minute apart, with a Dinamap blood pressure monitor at 6 months and 3 years postpartum. We used the average for analysis.
Covariates
At the first study visit, women reported their age, race/ethnicity, education level, marital status, annual household income, and parity via a self-administered questionnaire. They also completed a food frequency questionnaire used to derive a metric of diet quality via the Alternate Healthy Eating Index modified for pregnancy (AHEI-P) [13] and reported on smoking habits (former, current, or never) and hours/week of light-to-moderate physical activity levels in the year before pregnancy [14]. We calculated pre-pregnancy body mass index (BMI, kg/m2) from self-reported pre-pregnancy weight and height. At a study visit conducted ~13 years after delivery, participants reported age at menarche.
Statistical analysis
We examined the longitudinal association of baseline infertility with weight, WC, body fat (kg and %), and blood pressure over a median of 12 years of follow-up using linear mixed-effects regression models [15]. In these models, the dependent variable was repeated measurements of each outcome over follow-up, and the independent variable of interest was infertility (yes/no). We included a random effect for individual ID and an unstructured correlation matrix. We noted a better model fit (i.e., lower AIC) for random intercept only models for blood pressure and random intercept plus random slope for adiposity outcomes, so subsequent models reflected this. Outcome trajectories were not different by infertility status (interaction between age at outcome assessment and infertility P >0.05); therefore, model estimates reflect the average difference in a given outcome during the follow-up period with respect to baseline infertility status.
We constructed a series of models to account for potential confounders, which we selected by a priori knowledge, and considering bivariate associations with the exposure/outcomes and their impact on the effect estimates. The unadjusted model included age at outcome assessment as a covariate. We additionally included age at enrollment, race/ethnicity, annual household income, education, marital status, and parity in model 1. Model 2 was further adjusted for age at menarche. In Model 3, we additionally adjusted for pre-pregnancy BMI (linear and quadratic terms) as a proxy for the woman’s baseline body size, acknowledging that the metric could be a post-exposure variable. Adjusting for smoking during pregnancy, physical activity, and the AHEI-P score did not influence the estimates; therefore, we did not include them in the final models.
For all outcomes, we tested for an interaction between parity, age at enrollment, and infertility. We detected a significant interaction between age and infertility for blood pressure (P <0.05). Hence, we conducted analyses stratified by age at enrollment (18–29, 30–34, and ≥35 years) for SBP/DBP.
Sensitivity analyses
First, we adjusted for height in models of adiposity outcomes to account for differential physiological implications of excess fat mass due to stature. Second, we included waist-to-height ratio (WC [cm]/height [cm]) as an alternative measure of central adiposity. These analyses yielded consistent results with the primary analyses (Supplemental Tables 2 and 3). Third, to assess the potential for selection bias due to missing covariate data (namely, age at menarche for which we had 32% missingness), we conducted chained equation multiple imputation to generate 50 imputed data sets. The imputation model included the exposure, outcomes, and covariates under study. We combined and analyzed the imputed data sets using MI ESTIMATE in Stata 16 and compared the results to complete case analysis (n=985 without missing covariates). The two analyses yielded similar results (data not shown), so the results included herein are based on imputed covariate data.
To assess the robustness of our findings, we conducted subgroup analyses. First, we restricted the sample to nulliparous women at enrollment (n=742). Second, for a more standard assessment of infertility status, we restricted the sample to women actively trying to become pregnant at study enrollment who reported their time to pregnancy (n=906 for adiposity outcomes; n=856 for blood pressure). Third, to reduce bias due to preexisting hypertensive disorders, we excluded 23 women with chronic hypertension at enrollment from models of blood pressure. MAR has been associated with a higher long-term risk of chronic conditions such as hypertension [16]. Therefore, we tried to isolate the effect of infertility by excluding women with a history of MAR (n=123 for adiposity outcomes; n=118 for blood pressure). MAR included women who had prescriptions for fertility medications abstracted from the medical records (e.g., clomiphene citrate, gonadotropins, or gonadotropin-releasing hormone agonists), or who, at the 18-year study visit, reported the use of fertility medications/treatments (e.g., medications to induce ovulation, intrauterine insemination, in-vitro fertilization, intracytoplasmic sperm injection, sperm, egg, or embryo donation). Finally, to rule out that PCOS drove the findings, we excluded 44 women diagnosed with this condition before the index pregnancy (based on medical records or self-reported PCOS diagnosis at the 18-year study visit).
We conducted all the analyses in Stata 16 (StataCorp L.P., College Station, Texas).
Results
Of the 1,581 women in this study, 21.6% had a history of infertility before the index pregnancy (n=123 [7.8%] with MAR). Women with infertility vs. those without were more likely to be ≥35 years of age at enrollment (44.7 vs. 25.5%), college-educated (75.7 vs. 66.5%), married or cohabiting (95.9 vs. 91.4%), high income (69.2 vs. 60.6%), and had a higher pre-pregnancy BMI (25.4±5.4 vs. 24.7±5.4 kg/m2). Other characteristics did not differ by infertility status (Table 1).
Table 1.
Background characteristics among 1,581 women in Project Viva, overall and stratified by a history of infertility*
| History of infertility | |||
|---|---|---|---|
| All women n=1,581 | Yes n=342 (21.6%) | No n=1,239 (78.4%) | |
| Sociodemographic characteristics | n (%) | n (%) | n (%) |
| Age at enrollment‡ | |||
| 18–24 years | 122 (7.7%) | 9 (2.6%) | 113 (9.1%) |
| 25–29 years | 314 (19.9%) | 45 (13.2%) | 269 (21.7%) |
| 30–34 years | 676 (42.8%) | 135(39.5%) | 541 (43.7%) |
| ≥35 years | 469 (29.7%) | 153 (44.7%) | 316 (25.5%) |
| Race/ethnicity | |||
| White | 1,078 (68.5%) | 249 (73.0%) | 829 (67.3%) |
| Asian | 87 (5.5%) | 20 (5.9%) | 67 (5.4%) |
| Black | 235 (14.9%) | 41 (12.0%) | 194 (15.7%) |
| Hispanic | 108 (6.9%) | 17 (5.0%) | 91 (7.4%) |
| Other | 65 (4.1%) | 14 (4.1%) | 51 (4.1%) |
| College graduate‡ | |||
| No | 496 (31.5%) | 83 (24.3%) | 413 (33.5%) |
| Yes | 1,077 (68.5%) | 258 (75.7%) | 819 (66.5%) |
| Married or cohabiting‡ | |||
| No | 120 (7.6%) | 14 (4.1%) | 106 (8.6%) |
| Yes | 1,453 (92.4%) | 327 (95.9%) | 1,126 (91.4%) |
| Household income >$70,000/year‡ | |||
| No | 540 (37.5%) | 96 (30.8%) | 444 (39.4%) |
| Yes | 900 (62.5%) | 216 (69.2%) | 684 (60.6%) |
| Maternal biological and lifestyle characteristics | |||
| Smoking status | |||
| Former | 308 (19.6%) | 75 (22.1%) | 233 (18.9%) |
| During pregnancy | 177 (11.3%) | 30 (8.8%) | 147 (11.9%) |
| Never | 1,086 (69.1%) | 235 (69.1%) | 851 (69.1%) |
| Pre-pregnancy BMI, kg/m2, mean ± SD‡ | 24.8 ± 5.4 | 25.4 ± 5.4 | 24.7 ± 5.4 |
| Diet quality, AHEI-P score, mean ± SD | 60.8 ± 10.2 | 61.0 ± 10.1 | 60.8 ± 10.2 |
| Physical activity prior to pregnancyϯ | |||
| <2.5 hours/week | 177 (13.3%) | 38 (13.1%) | 139 (13.4%) |
| ≥2.5 hours/week | 1,153 (86.7%) | 252 (86.9%) | 901 (86.6%) |
| Reproductive characteristics | |||
| Age at menarche | |||
| <12 years | 186 (17.4%) | 39 (15.9%) | 147 (17.9%) |
| 12–14 years | 766 (71.7%) | 180 (73.2%) | 586 (71.3%) |
| ≥15 years | 116 (10.9%) | 27 (11.0%) | 89 (10.8%) |
| Gravidity | |||
| 0 | 462 (29.2%) | 100 (29.2%) | 362 (29.2%) |
| ≥1 | 1,119 (70.8%) | 242 (70.8%) | 877 (70.8%) |
| Parity | |||
| 0 | 744 (47.1%) | 176 (51.5%) | 568 (45.8%) |
| 1 | 574 (36.3%) | 122 (35.7%) | 452 (36.5%) |
| ≥2 | 263 (16.6%) | 44 (12.9%) | 219 (17.7%) |
AHEI-P: alternate healthy eating index modified for pregnancy; BMI: body mass index.
Description conducted in the non-multiple imputed dataset; total may be less than 1,581 due to missing values. Variables described as n (%) unless otherwise indicated.
Statistical significance at alpha <0.05 for the differences in the distribution by history of infertility status, obtained by χ2 test or t-test.
The cut-off of ≥2.5 hours/week reflects the current guidelines for moderate-intensity physical activity.
Primary analyses
Women with a history of infertility had higher weight, body fat, WC, and SBP across follow-up, although only weight and SBP at six months were significantly different (Table 2). Compared to women with no history of infertility, those with infertility had 3.29 kg (95% CI: 1.35, 5.24) higher weight, 2.46 cm (95% CI: 0.78, 4.13) higher WC, and 1.76 kg (95% CI: 0.09, 3.43) higher body fat after accounting for sociodemographic characteristics, parity, and age at menarche (Table 3). We noted similar, albeit weaker, associations for percentage of fat mass. Adjusting for pre-pregnancy BMI (Model 3) attenuated all estimates towards the null.
Table 2.
Mean ± SD adiposity and blood pressure across 12 years of follow-up among women in Project Viva overall and stratified by a history of infertility
| History of infertility | |||||||
|---|---|---|---|---|---|---|---|
| All women n=1,581 | Yes n=342 (21.6%) | No n=1,239 (78.4%) | Mean difference* | ||||
| n | Mean ± SD | n | Mean ± SD | n | Mean ± SD | ||
| Outcome | |||||||
| Weight, kg | |||||||
| 6 months | 1,135 | 70.7 ± 15.8 | 257 | 72.8 ± 15.7 | 878 | 70.0 ± 15.8 | 2.8‡ |
| 3 years | 1,131 | 70.5 ± 16.3 | 248 | 72.1 ± 15.6 | 883 | 70.0 ± 16.5 | 2.0 |
| 7 years | 1,044 | 72.0 ± 17.8 | 235 | 73.4 ± 17.4 | 809 | 71.6 ± 17.9 | 1.8 |
| 12 years | 979 | 74.4 ± 18.6 | 222 | 75.3 ± 17.8 | 757 | 74.2 ± 18.8 | 1.2 |
| Waist circumference, cm | |||||||
| 3 years | 1,143 | 86.9 ± 12.7 | 254 | 88.0 ± 12.8 | 889 | 86.6 ± 12.6 | 1.4 |
| 7 years | 1,049 | 88.6 ± 14.4 | 238 | 89.9 ± 14.2 | 811 | 88.2 ± 14.5 | 1.7 |
| 12 years | 975 | 90.7 ± 15.1 | 220 | 91.3 ± 15.0 | 755 | 90.6 ± 15.1 | 0.7 |
| Fat mass, kg | |||||||
| 7 years | 1,043 | 25.9 ± 13.1 | 235 | 26.8 ± 12.7 | 808 | 25.7 ± 13.2 | 1.1 |
| 12 years | 971 | 27.7 ± 13.6 | 219 | 28.3 ± 13.4 | 752 | 27.5 ± 13.7 | 0.9 |
| Fat mass, % | |||||||
| 7 years | 1,043 | 34.0 ± 8.7 | 235 | 34.6 ± 8.6 | 808 | 33.9 ± 8.8 | 0.7 |
| 12 years | 971 | 35.2 ± 8.8 | 220 | 35.6 ± 8.9 | 751 | 35.1 ± 8.8 | 0.5 |
| Systolic blood pressure, mmHg | |||||||
| 6 months | 1,160 | 109.4 ± 11.1 | 263 | 110.8 ± 11.7 | 897 | 109.0 ± 10.9 | 1.8‡ |
| 3 years | 1,140 | 108.7 ± 11.6 | 255 | 108.8 ± 12.0 | 885 | 108.7 ± 11.5 | 0.2 |
| Diastolic blood pressure, mmHg | |||||||
| 6 months | 1,160 | 64.9 ± 7.8 | 263 | 65.5 ± 7.8 | 897 | 64.7 ± 7.8 | 0.8 |
| 3 years | 1,140 | 66.8 ± 8.5 | 255 | 66.7 ± 9.0 | 885 | 66.9 ± 8.3 | −0.2 |
Mean difference in outcomes between women with vs. without history of infertility obtained from a t-test.
Statistical significance at alpha <0.05.
Table 3.
Association between a history of infertility and adiposity measures through 12 years of follow-up among women in Project Viva (n=1,577)*
| Unadjusted | Model 1 | Model 2 | Model 3 | |
|---|---|---|---|---|
| β [95% CI] | β [95% CI] | β [95% CI] | β [95% CI] | |
| Weight, kg | 1.75 [−0.29, 3.78] | 3.32 [1.37, 5.27]‡ | 3.29 [1.35, 5.24]‡ | 0.61 [−0.45, 1.68] |
| WC, cm | 0.78 [−1.00, 2.55] | 2.50 [0.81, 4.19]‡ | 2.46 [0.78, 4.13]‡ | 0.15 [−0.87, 1.18] |
| Fat mass, kg | 0.38 [−1.43, 2.19] | 1.85 [0.16, 3.54]‡ | 1.76 [0.09, 3.43]‡ | −0.29 [−1.28, 0.70] |
| Fat mass, % | 0.23 [−0.94, 1.41] | 0.97 [−0.13, 2.08] | 0.91 [−0.17, 2.00] | −0.32 [−1.04, 0.40] |
WC: waist circumference.
Estimates represent β (95% CI) obtained from mixed-effects regression models where repeated assessments of weight or adiposity were the outcomes. The primary exposure was a history of infertility (yes vs. no [ref]). All models include a random intercept and slope for individual ID, and an unstructured covariance matrix.
Unadjusted model: age at weight or adiposity assessment.
Model 1: + age at enrollment (continuous), race/ethnicity (White, Asian, Black, Hispanic, other), annual household income (>$70,000, yes vs. no), education (college graduate, yes vs. no), marital status (married/cohabiting, yes vs. no), and parity (0, 1, ≥2).
Model 2: model 1 + age at menarche (<12, 12–14 or ≥15 years).
Model 3: model 2 + pre-pregnancy BMI and pre-pregnancy BMI squared.
Statistical significance at alpha <0.05.
Due to evidence of an interaction between age at enrollment and infertility status when the outcome was blood pressure, we conducted analyses stratified by baseline age. After accounting for sociodemographic characteristics, parity, and age at menarche, infertility was associated with higher SBP and DBP among women <30 years at enrollment, but not among older women (Figure 2). Accounting for pre-pregnancy BMI did not change the results for SBP but attenuated DBP estimates towards the null (Supplemental figure 1).
Figure 2. Association between a history of infertility and systolic blood pressure (SBP) and diastolic blood pressure (DBP) from six months through three years postpartum, stratified by age at enrollment among women in Project Viva.
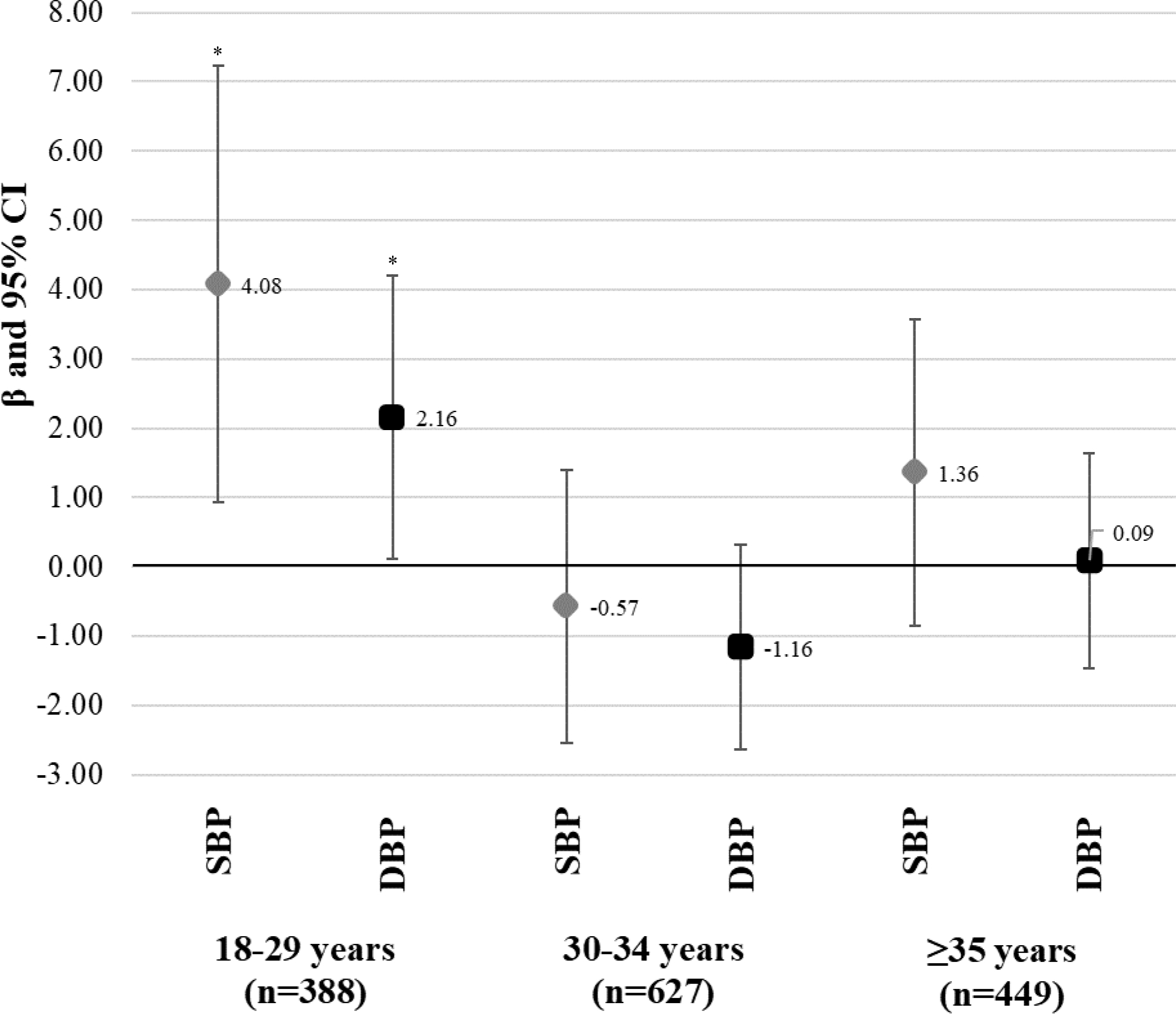
Estimates represent β (95% CI) from mixed-effects regression models where repeated assessments of blood pressure (median of 2 assessments per woman) were the outcome. The primary exposure was a history of infertility (yes vs. no [ref]). All models include a random intercept for individual ID, an unstructured covariance matrix, and are adjusted for age at blood pressure assessment, race/ethnicity (White, Black, Asian, Hispanic, other), income (>$70,000, yes vs. no), education (college graduate, yes vs. no), marital status (married/cohabiting, yes vs. no), parity (0, 1, ≥2), and age at menarche (<12, 12–14 or ≥15 years). * Statistical significance at alpha <0.05.
Sensitivity analyses
When we restricted the analysis to nulliparous women, the estimates were stronger and remained significant even after adjustment for pre-pregnancy BMI for both weight and WC (Supplemental Table 4). Similarly, in an analysis restricted to women planning their index pregnancy, infertility was associated with higher WC even after adjustment for pre-pregnancy BMI (Supplemental Table 5). We did not find different results for blood pressure among women planning their index pregnancy (Supplemental Table 6) or after excluding those with chronic hypertension (Supplemental Table 7). When we excluded women with MAR (Supplemental Tables 8 and 9) or PCOS (Supplemental Tables 10 and 11), the results were consistent with those of the primary analysis.
Discussion
In this prospective analysis of 1,581 women, 21.6% had experiences of infertility. This prevalence is higher than the 15.5% estimated in the National Survey of Family Growth [2] but comparable to the lifetime prevalence of infertility (~27%) reported in the Nurses’ Health Study II (NHS) [17]. We found that a history of infertility predicted higher weight, WC, and body fat through 12 years of follow-up. In addition, younger (<30 years) but not older (≥30 years) women with a history of infertility had higher blood pressure through three years postpartum than those without infertility. Our results align with findings from a cross-sectional analysis which found that self-reported infertility corresponded with higher BMI (1.03 kg/m2, 95% CI: 0.18, 1.89) and WC (3.08 in., 95% CI: 1.06, 5.09) [18]. A recent meta-analysis found that women with infertility had a 0.78 kg/m2 higher BMI (95% CI: 0.51, 1.04) than fertile women [9]. Importantly, the effect sizes we detected – i.e., differences of 2 kg body fat, 2 cm WC and 3 kg of total weight by fertility status – persisted after accounting for key confounders. Comparable gains in weight during adulthood (i.e., 2.5 to 10 kg) have been associated with nearly twice the risk of type 2 diabetes and 25% higher risk of hypertension and CVD in women [19]. Similarly, increments of 10 mmHg in SBP correspond with up to 2.5 times greater CVD risk [20].
Underlying causes of infertility may explain these associations. Women with PCOS, one of the leading causes of infertility [5,21], have a higher prevalence of the metabolic syndrome [22] and are more likely to have overweight/obesity and abdominal adiposity [7]. Additionally, hyperandrogenism and insulin resistance are key features of PCOS [23] that may contribute to weight gain and central fat accumulation [7,24]. While not all women with infertility had PCOS, the association we observed may be driven by subclinical PCOS characteristics that were either undiagnosed or failed to meet diagnostic criteria. Another explanation is that women with a history of infertility were already on a trajectory of higher adiposity [25,26] and metabolic dysfunction [27], and this analysis captured a snapshot of this relationship. This possibility is supported by the fact that adjustment for pre-pregnancy BMI attenuated the associations. However, in a sensitivity analysis restricted to nulliparous women, a subsample for whom pre-pregnancy BMI might be a better proxy of BMI status before assessment of infertility, the associations of infertility with weight and WC persisted after adjustment by pre-pregnancy BMI, suggesting that preexistent adiposity did not completely drive our findings.
Infertility was also associated with higher SBP and DBP, but only among women <30 years at enrollment. Most published studies have not found differences in blood pressure by infertility status [8,9,18], although an analysis in the NHS II found that the risk of hypertension was higher among women with infertility due to tubal disease (RR 1.15, 95% CI: 1.01, 1.31) [28]. In another study, Bilal et al. reported that Pakistani women with PCOS (n=100), of whom 72% experienced primary infertility, had higher mean arterial pressure than their age-matched controls (n=100): 99.8±1.26 vs. 85.11±1.31 mmHg [29]. Our findings add to the existing literature given its prospective design and granular assessment of age-specific associations and emphasize a need to monitor younger women with a history of infertility for later cardiovascular risk.
Possible mechanisms linking infertility to higher blood pressure include PCOS-related hyperandrogenism, which has been associated with increased blood pressure in human and animal studies [30,31], and/or infertility treatments, which increase the risk of hypertension [16]. However, excluding women with a history of MAR did not markedly change our findings.
Strengths of this study include the prospective design, repeated assessments of adiposity and blood pressure through 12 years postpartum, and enhanced generalizability owing to our study population comprised of parous women, as opposed to clinical studies of women with PCOS or specific conditions related to infertility [9].
Our study also has some limitations. First, there may be exposure misclassification due to recall bias or incomplete medical record data. Second, our measure of pre-pregnancy BMI, a key confounder to infertility and future adiposity, might be a post-exposure variable for women who experienced infertility before the index pregnancy. Third, we did not have information on causes of infertility, nor could we identify infertility caused solely by male factor infertility. Fourth, some background characteristics of participants included in the analysis differed from those excluded, including the prevalence of infertility. Therefore, we cannot rule out the possibility of selection bias. However, selection bias occurs when loss to follow-up, or selection into the sample, are affected by both the exposure and pathophysiological processes associated with the outcome. In the present study, although exposure status (infertility) influenced selection into the study and loss to follow-up, neither baseline BMI nor baseline blood pressure were related to inclusion into the analytical sample. Therefore, the probability of selection bias is likely minimal, and the most likely impact of data missingness is attenuation of the estimates towards the null. Fifth, women in our sample were predominantly white, highly educated, and recruited from multi-specialty clinics in Massachusetts that require health insurance providers to cover infertility services; therefore, these findings may not be applicable to women in other settings, especially those where access to fertility care is limited. Finally, since all participants were women recruited during pregnancy, results may not be generalizable to infertile women who never achieve pregnancy. Regardless, secondary infertility is more common than primary infertility [32], so our findings are still relevant to women experiencing infertility at any point in their lives.
Conclusions
Women with a history of infertility exhibit higher adiposity over a decade of follow-up and higher blood pressure through 3 years postpartum, although the latter association was only detected among participants <30 years at enrollment. These associations were of clinically relevant magnitude and have important implications for the long-term care of women with infertility. Identifying women with infertility, especially at younger ages, may represent an opportunity to prevent future CVD. One promising avenue could be to flag women with history of infertility for more frequent well-women visits to closely monitor (and manage, as needed) weight and blood pressure.
Supplementary Material
Acknowledgments:
We thank the participants and staff of Project Viva.
Source of funding:
This work was supported by grants from the US NIH R01 HD096032 and R01 HD034568, and the Harvard Pilgrim Health Care Institute. WP is supported by the Center for Clinical and Translational Sciences Institute KL2-TR002534. DCSC is currently supported by the National Research Service Award T32 HD 104612.
List of abbreviations
- AHEI-P
alternate healthy eating index modified for pregnancy
- BMI
body mass index
- CVD
cardiovascular disease
- DBP
diastolic blood pressure
- LMP
last menstrual period
- MAR
medically assisted reproduction
- NHS
Nurses’ Health Study
- PCOS
polycystic ovary syndrome
- SBP
systolic blood pressure
- SD
standard deviation
- US
United States
- WC
waist circumference
Footnotes
Conflicts of interest: None declared.
Data statement
The datasets analyzed during the current study are not publicly available due to privacy/ethical restrictions but are available from the corresponding author on reasonable request.
References
- [1].Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R, et al. The International Glossary on Infertility and Fertility Care, 2017. Hum Reprod 2017;32:1786–801. 10.1093/humrep/dex234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Thoma ME, McLain AC, Louis JF, King RB, Trumble AC, Sundaram R, et al. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil Steril 2013;99:1324–1331.e1. 10.1016/j.fertnstert.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Parikh NI, Cnattingius S, Mittleman MA, Ludvigsson JF, Ingelsson E. Subfertility and risk of later life maternal cardiovascular disease. Hum Reprod 2012;27:568–75. 10.1093/humrep/der400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tobias DK, Gaskins AJ, Missmer SA, Hu FB, Manson JE, Buck Louis GM, et al. History of infertility and risk of type 2 diabetes mellitus: a prospective cohort study. Diabetologia 2015;58:707–15. 10.1007/s00125-015-3493-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Joham AE, Teede HJ, Ranasinha S, Zoungas S, Boyle J. Prevalence of infertility and use of fertility treatment in women with polycystic ovary syndrome: data from a large community-based cohort study. J Womens Health (Larchmt) 2015;24:299–307. 10.1089/jwh.2014.5000. [DOI] [PubMed] [Google Scholar]
- [6].Hart R, Doherty DA. The potential implications of a PCOS diagnosis on a woman’s long-term health using data linkage. J Clin Endocrinol Metab 2015;100:911–9. 10.1210/jc.2014-3886. [DOI] [PubMed] [Google Scholar]
- [7].Lim SS, Davies MJ, Norman RJ, Moran LJ. Overweight, obesity and central obesity in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update 2012;18:618–37. 10.1093/humupd/dms030. [DOI] [PubMed] [Google Scholar]
- [8].Ghaffarzad A, Amani R, Mehrzad Sadaghiani M, Darabi M, Cheraghian B. Correlation of Serum Lipoprotein Ratios with Insulin Resistance in Infertile Women with Polycystic Ovarian Syndrome: A Case Control Study. Int J Fertil Steril 2016;10:29–35. 10.22074/ijfs.2016.4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mulder CL, Lassi ZS, Grieger JA, Ali A, Jankovic-Karasoulos T, Roberts CT, et al. Cardio-metabolic risk factors among young infertile women: a systematic review and meta-analysis. BJOG 2020. 10.1111/1471-0528.16171. [DOI] [PubMed] [Google Scholar]
- [10].Schulz KF, Grimes DA. Case-control studies: research in reverse. Lancet 2002;359:431–4. 10.1016/S0140-6736(02)07605-5. [DOI] [PubMed] [Google Scholar]
- [11].Wang X, Cheng Z. Cross-Sectional Studies: Strengths, Weaknesses, and Recommendations. Chest 2020;158:S65–71. 10.1016/j.chest.2020.03.012. [DOI] [PubMed] [Google Scholar]
- [12].Oken E, Baccarelli AA, Gold DR, Kleinman KP, Litonjua AA, De Meo D, et al. Cohort profile: project viva. Int J Epidemiol 2015;44:37–48. 10.1093/ije/dyu008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rifas-Shiman SL, Rich-Edwards JW, Kleinman KP, Oken E, Gillman MW. Dietary quality during pregnancy varies by maternal characteristics in Project Viva: a US cohort. J Am Diet Assoc 2009;109:1004–11. 10.1016/j.jada.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Oken E, Ning Y, Rifas-Shiman SL, Radesky JS, Rich-Edwards JW, Gillman MW. Associations of physical activity and inactivity before and during pregnancy with glucose tolerance. Obstet Gynecol 2006;108:1200–7. 10.1097/01.AOG.0000241088.60745.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Finucane MM, Samet JH, Horton NJ. Translational methods in biostatistics: linear mixed effect regression models of alcohol consumption and HIV disease progression over time. Epidemiol Perspect Innov 2007;4:8. 10.1186/1742-5573-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Westerlund E, Brandt L, Hovatta O, Wallén H, Ekbom A, Henriksson P. Incidence of hypertension, stroke, coronary heart disease, and diabetes in women who have delivered after in vitro fertilization: a population-based cohort study from Sweden. Fertil Steril 2014;102:1096–102. 10.1016/j.fertnstert.2014.06.024. [DOI] [PubMed] [Google Scholar]
- [17].Wang Yi-Xin, Farland Leslie V., Wang Siwen, Gaskins Audrey J., Wang Liang, Janet W. Rich-Edwards, Rulla Tamimi, Stacey A. Missmer, Jorge E. Chavarro. Association of infertility with premature mortality among US women: Prospective cohort study. Lancet Reg Heal – Am 2022;7:100122. 10.1016/j.lana.2021.100122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mahalingaiah S, Sun F, Cheng JJ, Chow ET, Lunetta KL, Murabito JM. Cardiovascular risk factors among women with self-reported infertility. Fertil Res Pract 2017;3:7. 10.1186/s40738-017-0034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zheng Y, Manson JE, Yuan C, Liang MH, Grodstein F, Stampfer MJ, et al. Associations of Weight Gain From Early to Middle Adulthood With Major Health Outcomes Later in Life. JAMA 2017;318:255–69. 10.1001/jama.2017.7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kannel WB, Vasan RS, Levy D. Is the relation of systolic blood pressure to risk of cardiovascular disease continuous and graded, or are there critical values? Hypertension 2003;42:453–6. 10.1161/01.HYP.0000093382.69464.C4. [DOI] [PubMed] [Google Scholar]
- [21].Hull MG. Epidemiology of infertility and polycystic ovarian disease: endocrinological and demographic studies. Gynecol Endocrinol 1987;1:235–45. 10.3109/09513598709023610. [DOI] [PubMed] [Google Scholar]
- [22].Moran LJ, Misso ML, Wild RA, Norman RJ. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update 2010;16:347–63. 10.1093/humupd/dmq001. [DOI] [PubMed] [Google Scholar]
- [23].Wang J, Wu D, Guo H, Li M. Hyperandrogenemia and insulin resistance: The chief culprit of polycystic ovary syndrome. Life Sci 2019;236:116940. 10.1016/j.lfs.2019.116940. [DOI] [PubMed] [Google Scholar]
- [24].Pennings N, Jaber J, Ahiawodzi P. Ten-year weight gain is associated with elevated fasting insulin levels and precedes glucose elevation. Diabetes Metab Res Rev 2018;34:e2986. 10.1002/dmrr.2986. [DOI] [PubMed] [Google Scholar]
- [25].Gesink Law DC, Maclehose RF, Longnecker MP. Obesity and time to pregnancy. Hum Reprod 2007;22:414–20. 10.1093/humrep/del400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].van der Steeg JW, Steures P, Eijkemans MJC, Habbema JDF, Hompes PGA, Burggraaff JM, et al. Obesity affects spontaneous pregnancy chances in subfertile, ovulatory women. Hum Reprod 2008;23:324–8. 10.1093/humrep/dem371. [DOI] [PubMed] [Google Scholar]
- [27].Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA 2003;289:76–9. 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- [28].Farland LV, Grodstein F, Srouji SS, Forman JP, Rich-Edwards J, Chavarro JE, et al. Infertility, fertility treatment, and risk of hypertension. Fertil Steril 2015;104:391–7. 10.1016/j.fertnstert.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Bilal M, Haseeb A, Rehman A. Relationship of Polycystic Ovarian Syndrome with Cardiovascular Risk Factors. Diabetes Metab Syndr 2018;12:375–80. 10.1016/j.dsx.2018.01.006. [DOI] [PubMed] [Google Scholar]
- [30].Reckelhoff JF. Androgens and Blood Pressure Control: Sex Differences and Mechanisms. Mayo Clin Proc 2019;94:536–43. 10.1016/j.mayocp.2018.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chen M-J, Yang W-S, Yang J-H, Chen C-L, Ho H-N, Yang Y-S. Relationship between androgen levels and blood pressure in young women with polycystic ovary syndrome. Hypertension 2007;49:1442–7. 10.1161/HYPERTENSIONAHA.106.083972. [DOI] [PubMed] [Google Scholar]
- [32].Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med 2012;9:e1001356. 10.1371/journal.pmed.1001356. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed during the current study are not publicly available due to privacy/ethical restrictions but are available from the corresponding author on reasonable request.


